1. Introduction
Chicken coccidiosis is a parasitic protozoonosis caused by one or more of the seven species of
Eimeria that are parasitic within the epithelial cells of the chicken intestinal tract [
1,
2]. Coccidiosis is characterized by intestinal injury, diarrhea, or bloody stools [
3].
Eimeria tenella is the most virulent, widely distributed, and most harmful of the seven chicken coccidia species, mainly attacking the cecum[
4]. The highest incidence rate was found in 3~6-week-old chicks. The symptoms of excreting blood, stool, or even blood appear within 4~5 days of infection. Large numbers of deaths begin one to two days after the appearance of bloody stools, with a mortality rate of up to 80% in severe cases [
5,
6,
7].
Drug control has been the main method for chicken coccidiosis for a long time. However, many problems such as the emergence of drug resistant strains, drug residues, and increased treatment costs during the prevention and treatment process have greatly limited drug control [
8]. Therefore, the key strategy and measure for effective prevention and control of this disease is to develop a safe, effective, low toxicity and environmental protection vaccine against coccidiosis in chickens. Currently, live coccidian oocysts vaccines with virulent and precocious virulence are widely used. However, traditional coccidiosis vaccines have shortcomings which limit their wide application such as high production costs, cumbersome production processes, environmental pollution caused by the spread of pathogens to the outside world, and the improper use of wild virus live worm vaccines can cause outbreaks of coccidiosis [
9]. Recent studies have demonstrated that recombinant protein vaccines can be used as effective control measures against coccidiosis [
10].
Rhoptry proteins (ROPs) are conservative and immune-protective, and some members are among the main virulence factors of
Toxoplasma gondii [
11]. Therefore, this protein family is considered a potential candidate molecule in the development of anti-
toxoplasmosis vaccines. As one of the key virulence factors of the
T. gondii type I strain, ROP18 participates in multiple mechanisms to escape host immune response. ROP5 controls the activity of ROP18 and affects IFN- γ and Irgm3 dependent other effectors to regulate the acute toxicity of
T. gondii [
12]. Similarly, ROPs play an important role in the process of
E. tenella invading host cells. It has the property of a protective antigen, and the corresponding antibody can inhibit the infection of coccidia to the host [
13]. Research has proven that
EtROP17 can inhibit host cell apoptosis [
14]. In ROPs such as ROP14, ROP30, ROP52, and ROP53,
E. tenella was detected in the proteome at different developmental stages; they have some degree of homology with the ROPs of
T. gondii [
14], but their functions are unclear. Zhang Li used RNA-Seq to detect and compare the transcriptome of the precocious strain and the virulent strain at the mitotic stage. q PCR was used to verify and screen the pseudopathogenic-related gene ROP27 of
E. tenella, which is presumed to have good immunogenicity [
15,
16]. However, it is not yet known whether the recombinant protein can effectively prevent and treat
E. tenella disease.
Therefore, this study aims to investigate whether chicken EtROP27 can serve as a potential candidate factor for anti E.tenella disease by expressing the recombinant protein ROP27 and detecting its immune protective effect. The research results provide new ideas for the prevention of E. tenella disease and the development of vaccines, which have important scientific significance.
2. Materilas and Methods
2.1. Ethics statement
All experiments involving animals were carried out in accordance with national regulations on the protection of animal welfare and strictly follow the guidelines. Additionally, it was approved by the Animal Protection and Utilization Committee of Guangxi University, China.
2.2. Experimental animals and parasite
Firstly, 15-day-old SPF chicken embryos were obtained from Beijing Meri Avigon Laboratory Animal Technology Co., Ltd. (Beijing, China). The E. tenella Shanxi virulent and precocious strains were used in this experiment, which were donated by the Veterinary Pathology Laboratory of the College of Veterinary Medicine, Shanxi Agricultural University.
2.3. Reagents
PrimeSTAR® Max DNA Polymerase, DL2,000 DNA Marker, DL5,000 DNA Marker, pMD™ 18-T Vector Cloning Kit, E.coli BL21(DE3) Competent Cells, E.coli DH5α Competent Cells, QuickCut™ EcoR I, QuickCut™ Xho I, T4 DNA Ligase, TritonX-100, Tris, Glycine, SDS, Acrylamide, SYBR Green qPCR Kit were purchased from TaKaRa (Ōsaka shi, Japan); pET-28a (+) DNA was purchased from Sangon Biotech (Shanghai, China); isopropyl β-D-thiogalactoside (IPTG), LB broth powder, LB agar powder, Ampicillin, Kanamycin were purchased from Solarbio (Beijing, China); 6*His, His-Tag Monoclonal antibody, HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H+L), HRP-conjugated Affinipure Goat Anti-Mouse IgG(H+L), Fluorescein (FITC)–conjugated Affinipure Goat Anti-Rabbit IgG(H+L), ECL detection kit were purchased from Proteintech (Chicago, IL).
2.4. Plasmid construction
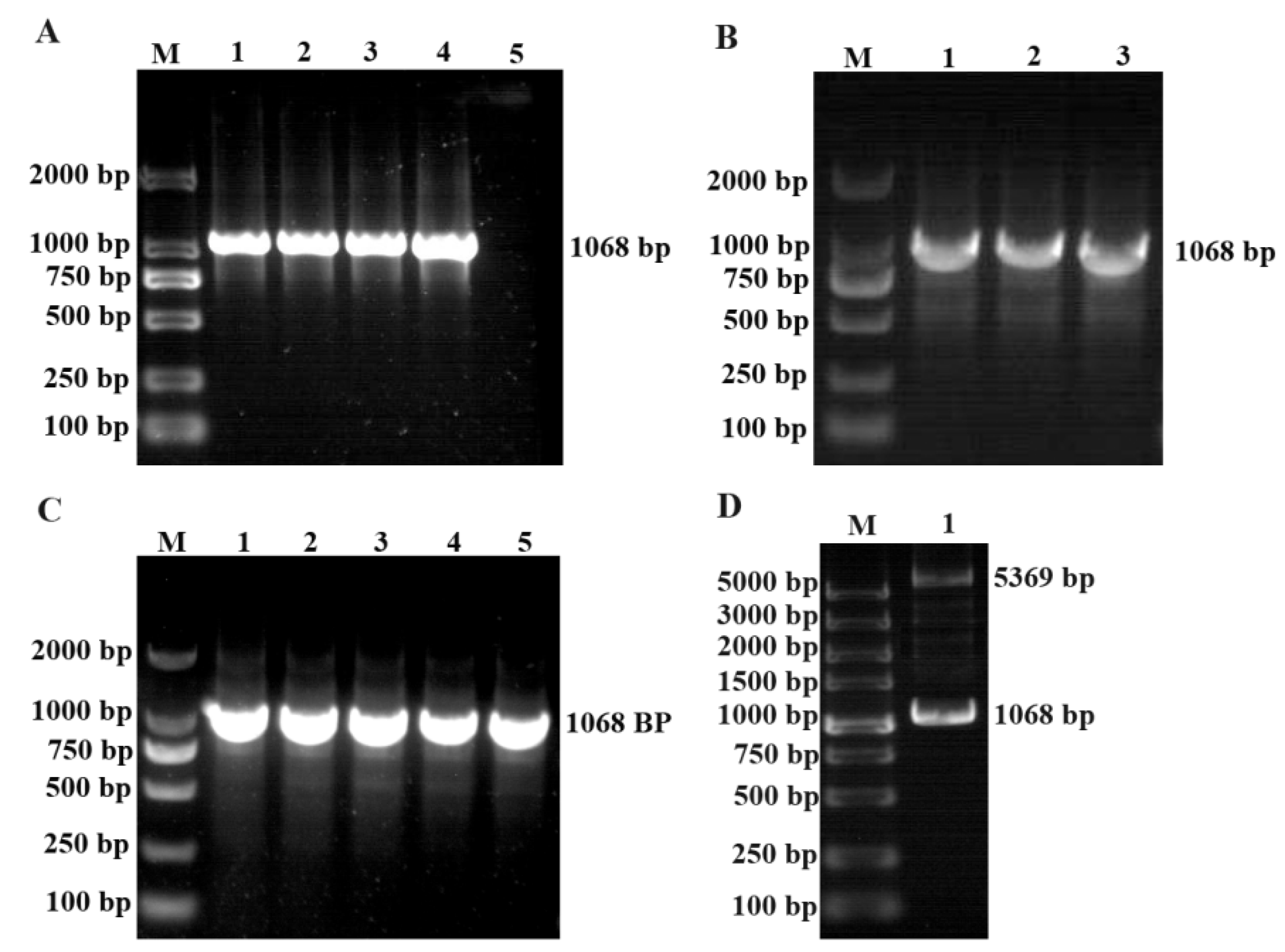
Design specific primers to measure the ROP27 sequence (Cluster-10347.5943) based on transcriptome and send it to the company for synthesis. Primer sequences were as follows: ROP27 sense primer, 5′-GAATTCAGGTAACGAGTCTCTGC-3; and anti-sense primer, 5′-TTGCCAGAATTGGCTCTACTACG-3’. The PCR product was purified and cloned into the pMD18-T vector according to the operation steps of the gel extraction kit. A single bacterial colony was selected for amplification, culture, and sequencing, and the bacterial solution with the most correct sequencing was selected for plasmid extraction. The plasmid and pET-28a (+) DNA were digested using EcoR I and Xho I endonucleases, and the digested products were purified. The target fragment was cloned into the eukaryotic expression vector, and then a single colony was selected for amplification, culture, and sequencing. The plasmid with the correct sequencing was named pET-EtROP27.
2.5. Protein expression and purification
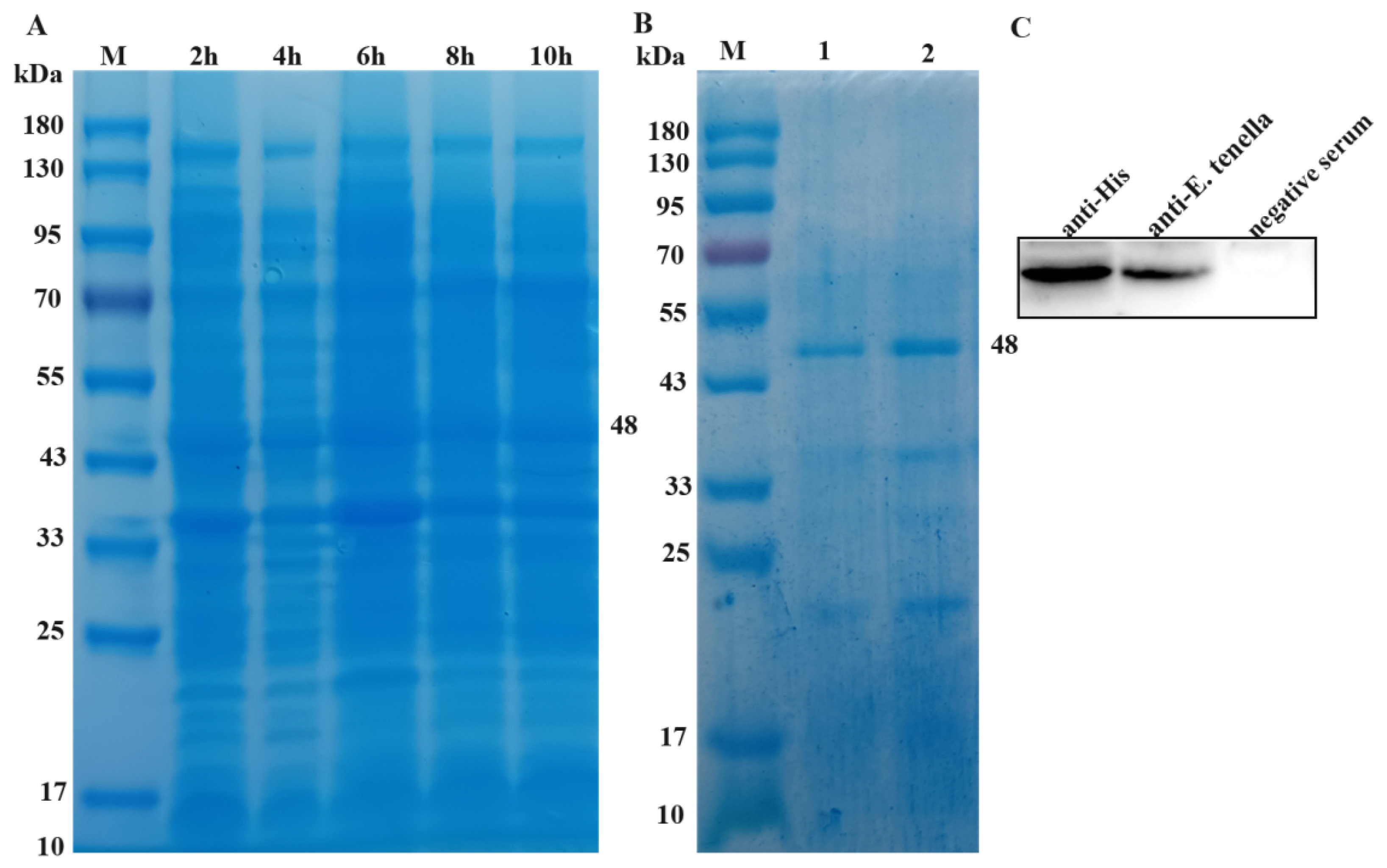
The recombinant plasmid pET-EtROP27 was transformed into BL21 (DE3) Competitive Cells. Subsequently, positive colonies were selected for enrichment. IPTG with a final concentration of 1 mmol/L was added to induce expression for 8 hours when the OD600 value of the bacterial solution was about 0.5. The heavy suspension liquid was placed on ice for ultrasonic crushing treatment, then the sediment and supernatant were collected. We followed the method of protein purification as described by Barkhordari F. Protein purity and concentration were determined through 12% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the BCA protein determination kit. The purified protein was stored at - 80℃ and used for subsequent experiments.
2.6. Preparation of anti-E. tenella and anti-rEtROP27 positive serum
1×104 sporulated E. tenella oocysts were intragastrically administered to 2-week-old SPF chickens. After 3 days, each chicken was infected with 5000 sporulated oocysts every 3 days for a total of four times. Blood was collected by cardiac puncture and centrifuged to prepare anti-E. tenella positive serum; this was then stored at -80℃ for standby.
Three 2-month-old New Zealand white rabbits were immunized with rEtROP27 which emulsified with Freund's adjuvant at a dose of 200 µg per rabbit three times, once every two weeks. Before each immunization, 30 µL of rabbit blood was taken from the ear vein, and the serum was isolated to detect the antibody titer. The heart took blood and centrifuged to collect serum after the third immunization. The collected serum was deactivated in a water bath at 56℃ for 30 min and stored at - 80℃ for standby.
2.7. Indirect ELISA determination of antibody titer
Rabbit anti-EtROP27 serum is the sample to be tested, and the non-immunized serum is negative. The purified
EtROP27 recombinant protein was used as the detection antibody. We used PBST to test the serum according to 1:500, 1:1000, 1:2000, 1:4000, 1: 8000, 1:16000, and 1:32000 gradient dilution. Serum antibody titer testing was performed according to the method reported by Dong-chao Zhang [
17].
2.8. Western blotting analysis
The protein samples were separated using a 12% polyacrylamide gels and were transferred to 0.22 µm polyvinylidene fluoride membranes using the Bio-Rad wet transfer system. After blocking at 37°C with 5% BSA in PBS, the membranes were probed with anti-E. tenella (1:400), anti-rEtROP27(1:500), anti-HIS (1:2000), respectively, with an overnight incubation at 4°C. Next, the membranes were washed with PBS containing tween-20 (PBST) four times and were incubated with horseradish peroxidase-linked secondary anti-chicken, anti-rabbit or anti-mouse IgG antibodies for 1 h at 37°C. After washing with PBST, the signal was visualized using an ECL detection kit.
2.9. Primary culture of chicken embryo caecal epithelial cells
The cecum was removed from 15-day-old SPF embryos and striped the mesentery carefully; the cecum was fully washed by the PBS buffer, then cut into 1 mm3 size tissue and washed; the tissue was resuspended and mixed by thermolysin (50 mg/L), and digested at 41℃ for 2 h; PBS solution was used to stop the digestion, centrifugation at 1200 r/min for 5 min, and PBS and enzyme solution were discarded; the cell pellet was resuspended in 10% FBS low-glycemic DMEM cell culture medium, seeded in a cell culture flask and adhered to the culture for 70 min; the liquid in the flask was collected, centrifuged at 1200 r/min for 5 min, and then resuspended and counted in DMEM/F12 culture medium containing 2.5% fetal bovine serum (FBS); the cells were seeded in a cell culture plate and could be used for subsequent experiments when the cell attachment rate was reached about 85%.
2.10. E. tenella sporozoite preparation
E. tenella sporulated oocysts were centrifuged at 2000 r/min for 5 min, the supernatant was discarded, and PBS was added to wash thoroughly. The oocysts were suspended in 2 mL PBS and ground with a homogenizer until the decapsulation rate reached 80%. The above solution was centrifuged at 1800 r/min for 5min. Precipitates were then resuspended with sporocyst digestion solution which contains 0.75% trypsin and 10% chicken bile and digest with shaking at 41℃ (150 r/min) until 80% of the sporozoites are released. After filtration, the digestion solution was removed by centrifugation at 3000 r/min for 10 min, and the precipitate was suspended in DMEM solution. The amount of sporozoites used in this experiment was 1×104.
2.11. RT-PCR analysis
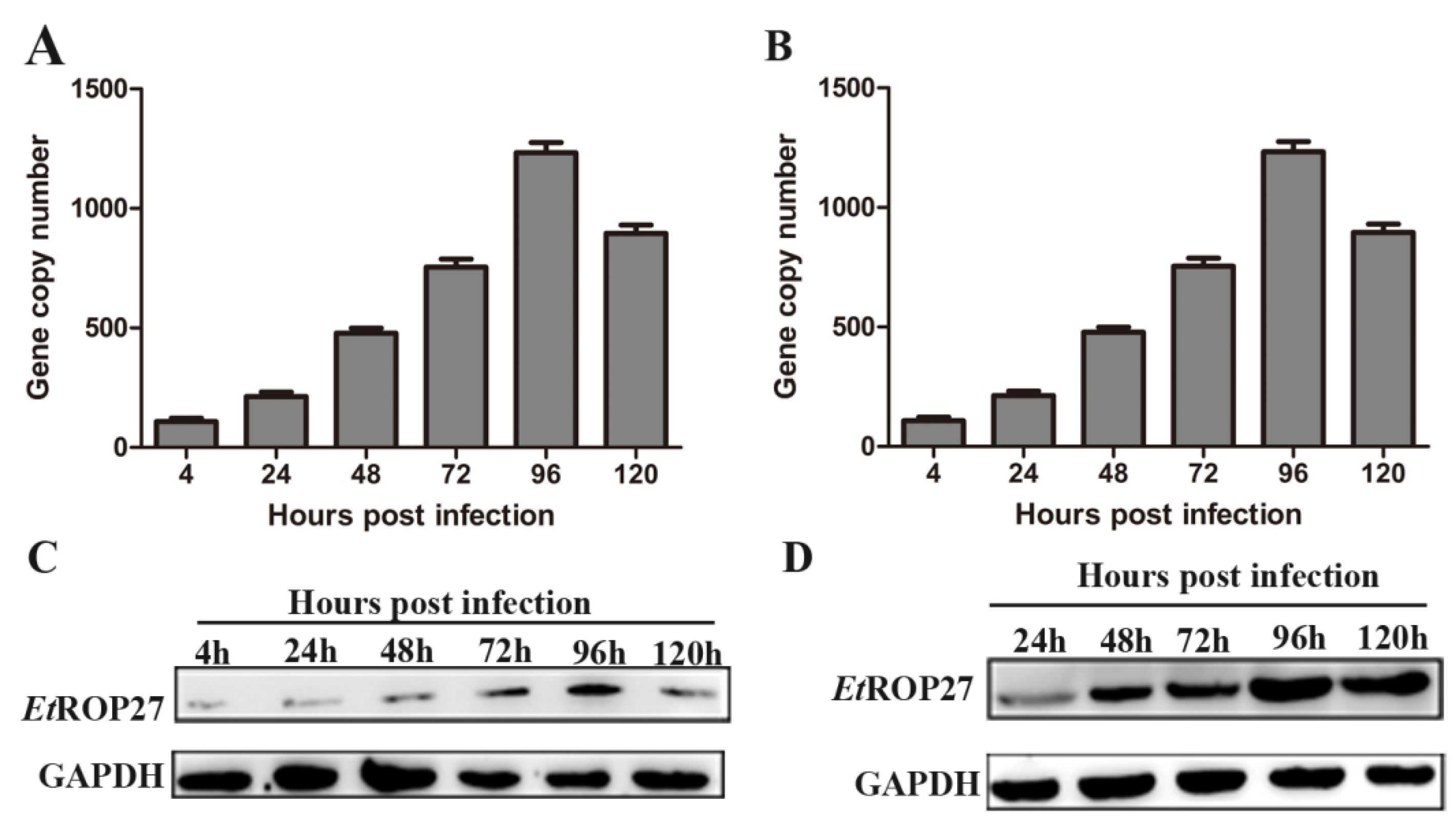
Total RNA from chicken cecum or primary cecal epithelial cells of chicken embryo cells infected with E. tenella virulent and precocious strains was extracted using TRIzol Reagent according to the manufacturer’s instructions. The cDNA was generated by reverse transcription using PrimeScript reverse transcriptase according to the manufacturer’s instructions. ROP27 mRNA was assessed by using quantitative qPCR with a TaKaRa SYBR Green. qPCR Kit on a CFX96 Touch real-time PCR detection system. Primer sequences were as follows: ROP27 sense primer, 5′-AGCTACGACACTCCTGTTGC-3′; and anti-sense primer, 5′-ACTCAAGACGGAGTTGCTGG-3′. PCR products were purified using a Gel Extraction Kit and cloned into the pMD18-T vector. Plasmids were diluted serially and used as standards for quantitative analysis. The initial copy number of ROP27 gene in each group was calculated using the following formula: X = -K log Ct + b, where X is the initial copy number and K, Ct, and b refer to the slope rate, cycle threshold, and constant, respectively.
2.12. Animal experiment
Firstly, 14-day-old chickens were randomly divided into 5 groups, with 10 chickens in each group. Three experimental groups of chicken legs were subcutaneously injected with recombinant proteins emulsified using Freund's adjuvant (50 μg, 100 μg, 150 μg, respectively). The infection control group and the uninfection control group were injected with PBS emulsified with complete Freund's adjuvant. After 7 days, emulsify with incomplete Freund's adjuvant and undergo a second immunization at the same dose. After 7 days, each chicken in the experimental group and the infection control group received oral administration of 5×104 sporulation oocysts of E. tenella were obtained, and PBS was given to the uninfection control group.
2.13. Concentration of serum antibody
Concentration of IgY antibody level in serum were detected by utilizing an indirect enzyme-linked immunosorbent assay (ELISA) commercial kits named "chick cytokine ELISA Quantitation Kits" (catalog numbers: CSB-E11635Ch for IgY CUSABIO, Wuhan, China), according to manufacturer’s instructions.
2.14. Immunoprotective parameters in vivo
In vivo immunoprotective parameters of the r
EtROP27 protein include clinical (weight, survival rate and mortality), pathological (cecum lesion score), and parasitological (ACI and oocyst output) factors [
18]. Weight gain and survival rate were calculated directly. Cecum lesion score is a continuous number from 0 (none) to 4 (severe) representing different levels of cecum lesion, according to Johnson and Reid [
19], which is evaluated by three independent observers. Furthermore, cecal content was collected in order to calculate oocysts per gram (OPG) using McMaster
,s counting technique [
20]. ACI as a synthetic criterion, which responded to holistic health conditions of chickens, and represents various degrees of vaccine protection: ACI > 180 is good protection, 160 < ACI < 179 is moderate protection, 120 < ACI < 159 is limited protection, and ACI < 120 was no protection [
21].
2.15. Image and statistical analyses
Values are presented as the arithmetic mean ± standard error. Each experiment was repeated at least three times. The SPSS 17.0 statistical software package (Chicago, USA) was used to perform ANOVA analysis of all data. Histograms were prepared via GraphPad Prism 5.0 software (San Diego, CA, USA). Fluorescence intensity was analyzed using ImageJ software (National Institutes of Health, USA). All results were considered statistically significant at a p-value < 0.05.
4. Discussion
Chicken coccidiosis is one of the most important diseases threatening the health and welfare of poultry caused by
Eimeria. In poultry, there are mainly seven different types of
Eimeria, of which
E. tenella is the most pathogenic and widely distributed species [
22]. The life cycle of Eimeria tenella is relatively complex, and can be generally divided into two developmental stages: the exogenous stage (spore-stage reproduction) and the endogenous stage (schizogeny and gametogenesis). The predation of host cell nutrients by worms, and the formation and release of merozoites and gametophytes destroy cell structures and functions, causing hemorrhagic inflammation, emaciation, and death in the cecum during
E. tenella schizogeny and gametogenesis [
22]. At present, the control of coccidiosis mainly relies on anti coccidiosis drugs, but there are many shortcomings. Therefore, there is an urgent need to develop a safe and effective vaccine against chicken coccidia to effectively prevent and control this disease.
E. tenella is a member of the apicomplexan protozoa [
23]. The protozoa of the
apicomplexan have conservative and unique secretory organelles, including rod-shaped bodies, dense particles, and microlines, which have relatively conservative invasion mechanisms against host cells [
24]. Rod-shaped bodies are rod-shaped organelles and large vesicles containing dense substances, and are composed of osmiophilic substances. Secretion of rhoptry neck protein (RON) related to parasite invasion in the early stage is crucial for the formation and function of mobile connections between parasites and host cell membranes, such as RON1, RON2, and RON3 [
25]. The release of ROPs from the base of the rod-shaped body into the PV changes the environment of the PV and enters the host cell [
26,
27]. They interact with the host cell through signal transduction, causing a series of downstream effects, becoming a key determinant of protozoan virulence. After entering the cell, ROPs can disrupt host cell signaling and defense mechanisms, and assist in recruiting host organelles [
28].
EtROP plays an important role in parasitic invasion of host cells, modification of host vacuoles, hijacking, and regulation of host cells, and has been proven to be a key virulence factor. It also has the properties of a protective antigen, and corresponding antibodies can inhibit the infection of coccidia to the host [
28]. Studies have confirmed the expression of
EtROP17 in
E. tenella merozoites. Western blotting analysis showed that
EtROP17 can be recognized by the chicken immune system and induce antibody responses. Vaccination of animals with r
EtROP17 can significantly reduce egg sac output and reduce cecal lesions [
29]. These indicate that
EtROP17 can be used as an effective candidate vaccine for
E. tenella. Similarly, studies have shown that
EtROP30 and
EtROP35 are natural antigens of coccidia, which have good immunogenicity and can be used as the basis for candidate vaccine proteins [
30,
31].
EtROP27 is a differential gene detected by the transcriptome which is presumed to be a pathogenic-related gene [
15,
16]. However, its expression and function are still unclear. The different expression levels of secreted proteins at different stages of the parasite's life cycle are often closely related to their function. High expression proteins in the mitotic stage of invading host cells are usually associated with invading host cells, or with parasite development and host–parasite interactions [
32]. The extracellular stage (sporozoite and merozoite) of
Eimeria is immunologically fragile, but it is crucial for the invasion and development of the parasite [
33]. The high expression of proteins in the sporozoite and/or merozoite stage makes it an ideal candidate vaccine. The highly expressed ROP
Et ROPK Eten5-A of
E. tenella during the sporozoite stage has good immunogenicity [
34]. In this study, we first conducted bioinformatics analysis on
EtROP27. The analysis results indicate that the protein is a secreted protein with potential as an antigen. Subsequently, a prokaryotic expression recombinant plasmid for
EtROP27 was successfully constructed and purified, and polyclonal antibodies against
EtROP27 were successfully obtained. RT-PCR and Western blotting results indicate that
EtROP27 protein is naturally present and expressed at all stages of coccidian development, with the highest expression level in the first generation of schizogenesis. Therefore, it may be a good candidate protein for vaccines.
Subunit vaccines are expected to become an alternative control strategy for avoiding defects in anti coccidian drugs and live egg sac vaccines, and the screening of effective immune protective proteins is a key focus of subunit vaccine research. Animal experiments showed that the rEtROP27 protein could cause increased average body weight gain, increased IgY titer in serum, decreased oocyst output and bloody stool, and lower mean lesion scores compared with the infection control. The ACI value of rEtROP27 protein 100 μg or 150 μg reached more than 160. These results indicate that EtROP27 can effectively induce immune protection against E. tenella in chickens.
This study provides a basis for determining the key genes that dominate or regulate the pathogenicity of coccidia, for searching for drug action targets that control the pathogenicity of coccidia, for developing gene deletion vaccines with strong fecundity and immunogenicity but weak adverse reactions, and for further reducing the adverse effects of precocious strains. They will also provide new ideas and scientific bases for further revealing the pathogenic mechanism of coccidia against the host.