1. Introduction
Currently, the world is 1 °C hotter than it used to be before the Industrial Revolution (1850-1900), and at our current pace, it could reach a 1.5 °C increase by 2040. While seemingly small, this value is an average taken from multiple measurements around the world, and as such, many regions have already exceeded this value, affecting a fifth of the world population. This warming leads to rising sea levels, a higher risk of wildfires, and lower crop yields, alongside many other consequences that lead to the destruction of ecosystems and accentuate poverty [
1]. Currently, the cement industry contributes 6~8% to global carbon emissions, a rather significant value that will grow accordingly with the ever-increasing demand of the construction sector, unless proper action is taken [
2,
3].
To summarize cement production, limestone (CaCO
3) is extracted and crushed, and some materials like iron ore or clay may be added to add necessary minerals. The mix is then ground more finely and goes through the precalciner, a tower of four to six cyclones where carbonation occurs and quicklime (CaO) is formed, as shown in Equation (1). The mix then enters a rotary kiln, reaching temperatures up to 1,450 °C, where important minerals are formed, creating clinker. The clinker is then cooled and blended into a fine powder with additives like gypsum, becoming cement [
4].
During cement production, 590 kg of carbon dioxide (CO
2) are released for every ton of cement produced [
2]. 40% comes from burning fossil fuels, usually pulverized coal, and 60% are released during calcination. To reduce fuel emissions, the most reliable method is altering the fuel mix; however, this is a balancing act to keep the burning conditions in the kiln close to those of coal, or at least without affecting cement quality. For example, using a high biomass content in the fuel mix would raise the moisture content, reducing the maximum temperature in the kiln and not reaching the threshold necessary for forming certain minerals. The remaining 60% of CO
2 emissions come from calcination, a crucial part of the process, so these emissions must be captured or mitigated in some other way.
2. Cement Industry Decarbonization Strategies
The simplest one, albeit already done in most of the world, would be to shift from the old vertical shaft kiln to the rotating kiln. All modern countries have made the switch, and China, responsible for over half of the world’s cement production, has also done so. Other major producers like India and Vietnam could also perform the switch. For them, it remains the best decarbonization method despite the high costs of replacing a major piece of equipment. While reducing emissions might not be a priority for these countries, these kilns have lower energy consumption and can produce higher-quality cement, justifying the investment [
5].
For the rest of the world, decarbonization will most likely have to be implemented by retrofitting the process. One method could be lowering the clinker content in cement from the usual 0.7 kg clinker/kg cement, which can be done by replacing clinker with waste materials like copper tailings and sugar cane bagasse, reducing the amount of limestone needed and thus the emissions from carbonation. One promising recipe is limestone calcinated clay cement (LC3), which claims to reduce CO2 emissions by up to 40% while having a near-equal performance as standard cement.
Since the process operates at relatively high temperatures and has significant heat losses (35-40%), waste heat recovery is also an option. Using the Rankine cycle, waste heat can be converted into electricity, mitigating emissions by requiring less electricity from the grid. A modern cement plant is expected to have an electrical consumption of over 20 MWh, so this technology would only slightly mitigate it and require a significant capital investment. The last issue is that the waste heat might already be used for parts of the process, like the pre-heating cyclones [
6,
7].
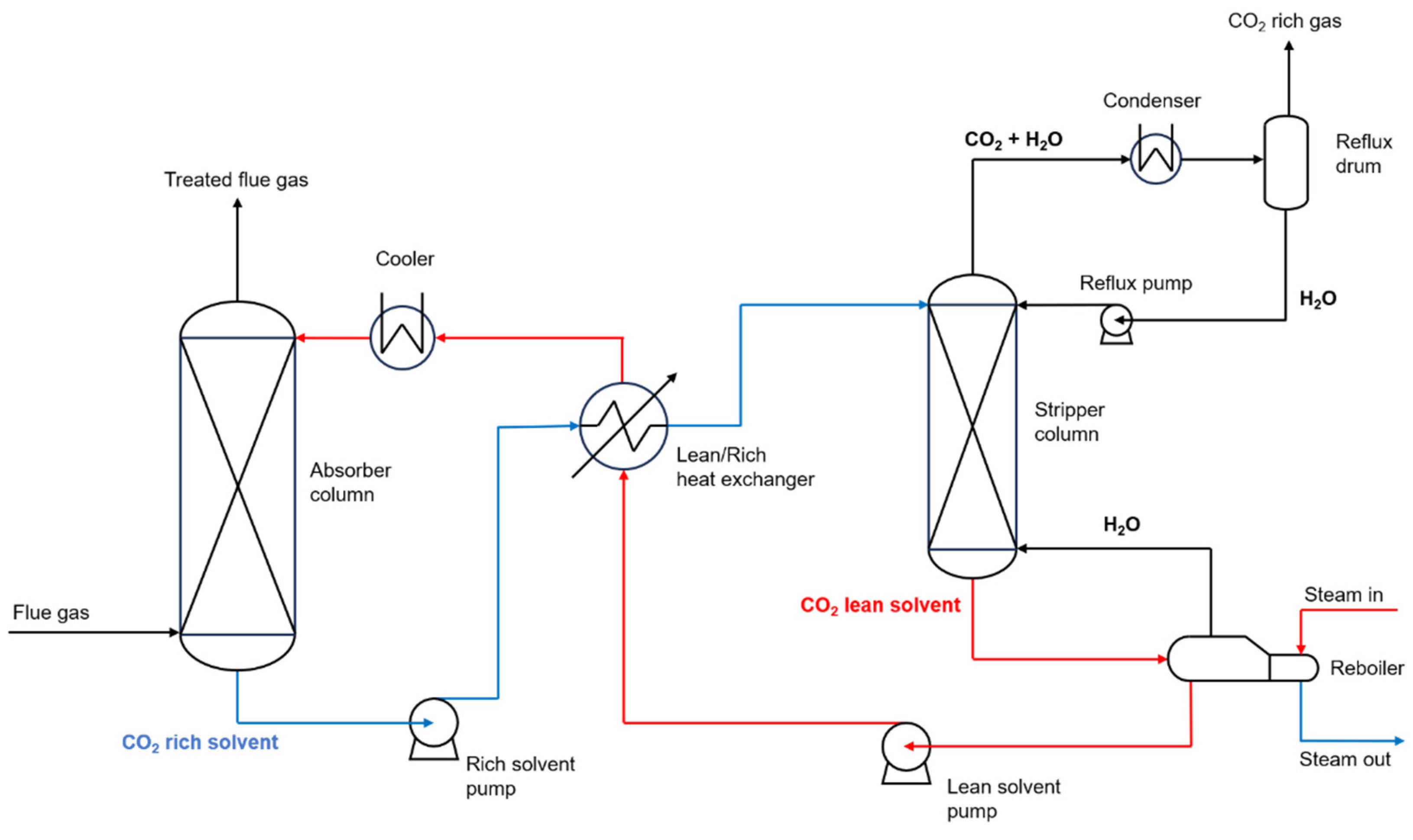
Nevertheless, what most have in mind for this topic would be carbon capture and storage (CCS), an end-of-pipe treatment that captures the CO
2 at the end of the process. This method can have different configurations, but a common one is the reactive absorption-stripping method, which uses amines, such as ethanolamine (C
2H
7NO), to react with water and CO
2 in an absorption column. In this method, the flue gas enters from the bottom of the column, counter-current to the solvent, and has its CO
2 removed by the solvent, composed of water and amines. It then exits by the top of the column and is sent to the stack. The solvent, now rich in CO
2, is then heated and sent to the top of a stripping column, counter-current to the steam generated by the reboiler. At the top of this column, CO
2 and water are sent to a condenser, where the water is recovered back into the column, and the CO
2 is purified and ready to be compressed for storage and transportation. At the bottom, the regenerated solvent is used to heat the stream from the bottom of the absorption column and sent back to the top of the absorber, where the cycle begins again [
8].
Figure 1 shows a diagram of this process.
While ideal on paper, as it would afford time to switch to less polluting energy sources, this is an expensive addition to the process, costing around 124
$/ton CO
2 at a 90% capture rate [
7]. Besides more investment in research, another way to improve its viability is to complement it with other methods, like oxyfuel, where nitrogen (N
2) is removed from the primary combustion air. The nearly pure oxygen (>95 vol% O
2) allows for improved burning efficiency and dramatically increases CO
2 concentration in the flue gas, which makes it much easier for CCS units to remove it. The downsides of oxyfuel include a high cost and the risk of damaging the kilns’ inner lining due to the higher than normal temperatures.
To further complicate matters, all this CO
2 gas must be stored or used somewhere. Currently, its production far outweighs that needed in industries like metallurgy or beverage production, but that could change as more businesses see an opportunity in a waste product. As for storing CO
2, it can be compressed and injected again into deep reservoirs of porous rock under an impermeable layer of rock, similar to the formation from where we extracted oil and gas. Studies also show an abundance of storage sites, exceeding what is expected to be needed, but further analysis is necessary to determine their commercial viability [
9].
There are, however, two interesting strategies whose viability is analyzed more in-depth in this study: oxygen (O2) enrichment, a more moderate use of oxyfuel, which increases the O2 concentration in the air while only partially removing N2; and the addition of hydrogen (H2) to the fuel mix to achieve a more complete combustion and allow for the use of alternative fuels while maintaining close to normal combustion conditions.
3. Oxygen enrichment (OXE)
Air is volumetrically composed of 21% O2 and 78% N2, with the remaining 1% consisting of other gases like CO2 and argon. Only O2 is consumed during combustion, while the remaining components are inert, adding unnecessary volume and lowering the temperature during combustion. OXE uses an air separator, usually through pressure swing adsorption (PSA) or cryogenic distillation, to obtain a stream of high-purity O2 (90-95%) that can then be added to the primary burning air, outside air that is directly injected into the kiln burner, raising the overall O2 concentration. This reduces the volume of outside air that must be drawn, improves fuel efficiency, and increases the maximum flame temperature while keeping the same shape and only slightly increasing the average temperature of the kiln.
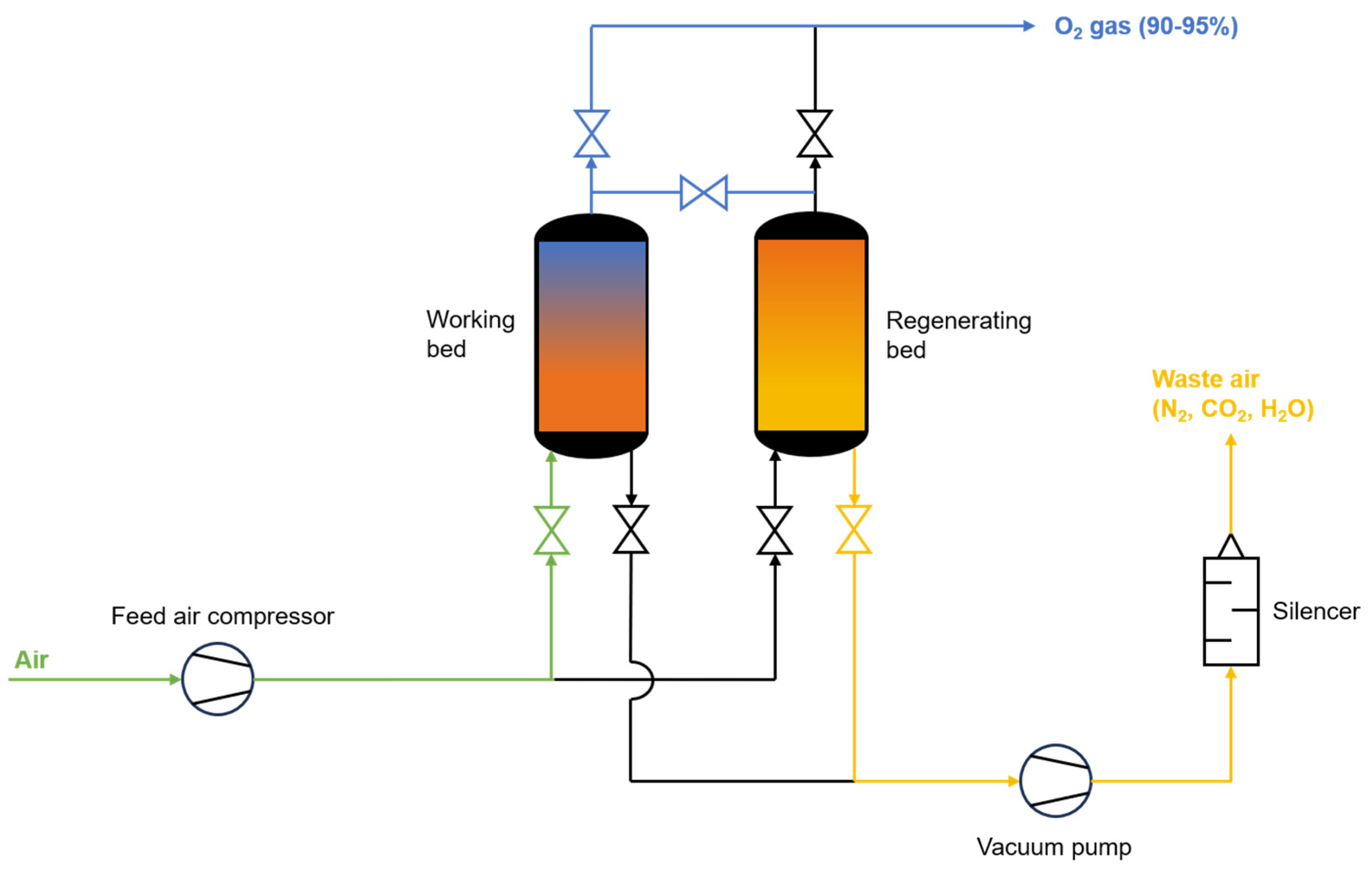
PSA usually comprises two packed beds containing zeolites, such as 5A and 13X, made from aluminum and silica and, in this case, able to adsorb O
2 at high pressures (5 to 10 bar). The process alternates between the two beds; when one bed is pressurized with the feed stream, the other purges the remaining gases, lowering its pressure. The valve opens once the pressure is high enough in the working bed. O
2 is then extracted, with a portion used to help regenerate the other bed. Then, both beds are connected to equalize the pressure, which lowers energy consumption as it reduces the amount of pressurization needed. This cycle repeats with the beds switching roles to provide a continuous oxygen flow. An alternative to regular PSA is the vacuum PSA, the chosen method to assess the process costs. The latter uses a vacuum pump during desorption of the regenerating bed operating below 0.6 barg for improved regeneration, only requiring some feed pressurization [
10].
Figure 2 shows a schematic diagram of the vacuum PSA system.
Using the average production of a European cement line of 3,000 tons of clinker per day, an air intake of around 2.25 kg/s or 6,265 Nm
3/h will be necessary. According to Wang et al., increasing O
2 concentration from 21% to 27% is optimal, improving the heat transfer rate, increasing temperature, and decreasing CO emissions, meaning a more complete combustion is occurring [
11]. At higher O
2 concentrations, NO
x production spikes, as thermal NO
x production becomes far more relevant after a certain temperature threshold.
Using a purity of 90% for the enriched air, usually considered a reasonable value, 545 Nm
3/h of enriched air will have to be injected alongside 5,720 Nm
3/h of regular air, as to maintain the same volume of primary air as normal operating conditions [
12]. A cost of 200
$/ton per annum CAPEX of O
2 production capacity, and a consumption of 60 kWh/ton of air used can be employed to calculate investment costs [
13]. While this source appears reliable and recent, there are no dates for this information, so a margin of 50% will be added to the implementation costs. With all of this taken into account, implementing OXE should cost around 1.85 M€ and carry an additional electrical consumption of 217 kWh, a small amount compared to the >20 MWh necessary to operate the plant.
Table 1 shows a summary of the costs and benefits of this implementation.
4. Addition of hydrogen into the fuel mix
Adding H2 to the fuel mix can prove beneficial, both to help decarbonize the process by using a fuel that only releases water when burned and that improves combustion, making it more complete. This is achieved due to H2′s high energetic content, burning temperature, and speed, which increases fuel efficiency, with less carbon being used to form other pollutants like CO and NOx, thus reducing fuel consumption. This can also prove beneficial to companies aiming to use a fuel mix containing more moisture, like biomass or waste-based fuels, as these tend to have lower burning temperatures. For this case, a 5% H2 thermal content in the fuel was considered to evaluate the economic viability of using a significant quantity of H2.
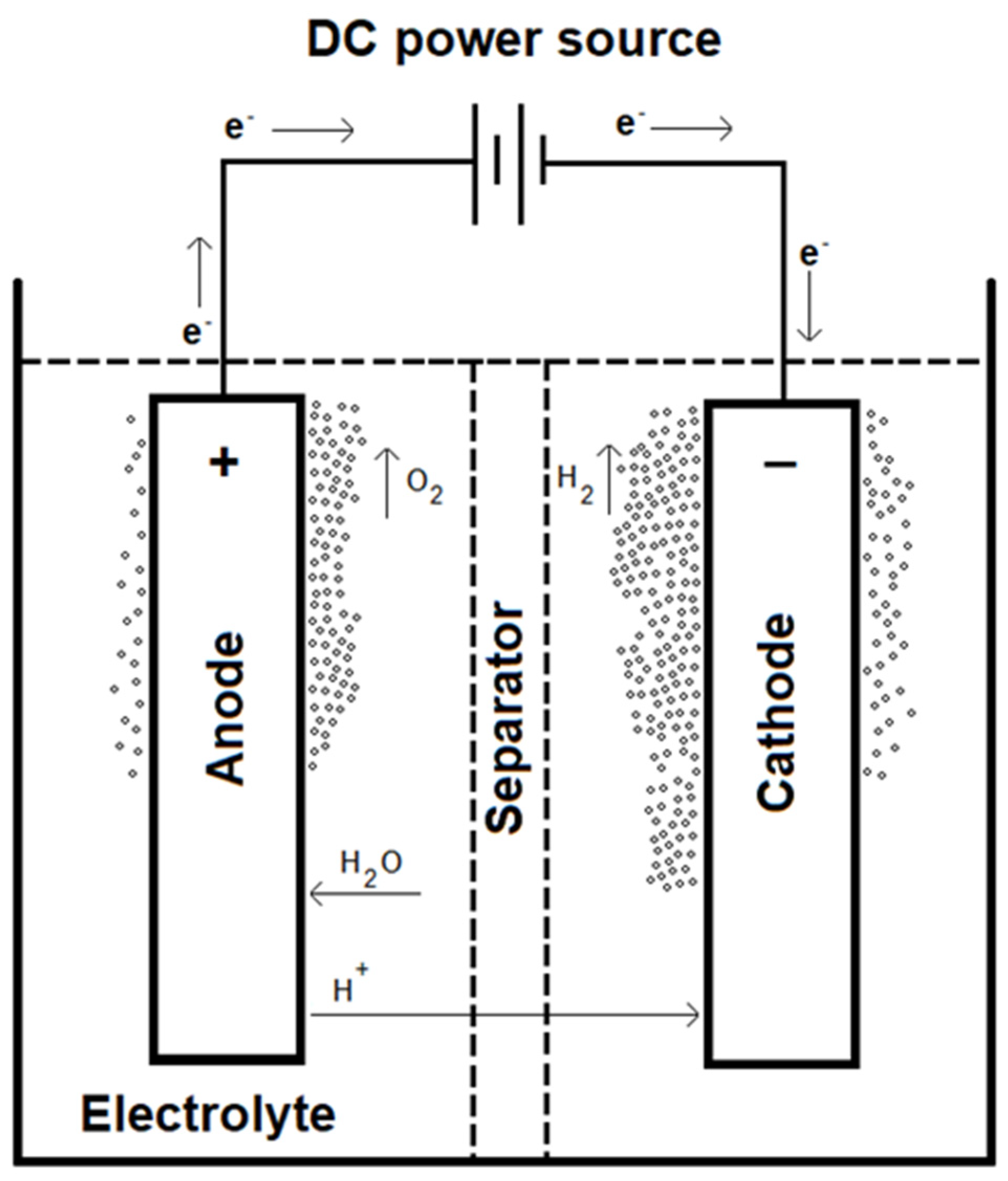
However, any environmental benefits from using H
2 as a fuel are invalidated if its production is not equally low in emissions. One of the best ways to do this is through electrolysis: by applying an electrical current through water, it is possible to split it into H
2 and O
2 gases. If the electricity used is from renewable sources or nuclear, then there are no carbon emissions associated with H
2 production through this method. It should be noted that this is one of the most expensive methods at 3.2 - 7.7
$/kg H
2, due to both its high electricity consumption and the materials necessary to build the cell [
15].
Figure 3 shows one such cell.
There are four main types of electrolyzers, but the ones most likely to be seen in a current industrial setting are alkaline water electrolysis (AWE), a cell similar to the one in
Figure 3 but in alkaline solution (usually of potassium hydroxide) and using nickel and stainless steel electrodes, or the proton-exchange membrane (PEM) electrolysis, where the aqueous electrolyte is replaced by a solid polymer electrolyte membrane, usually Nafion
®, with noble-metal based electrode materials. PEM electrolyzers are more recent, and despite costing more than AWE, their price is expected to drop as they offer some advantages like better efficiency and higher H
2 purity; thus, PEM implementation will be considered [
16].
To calculate costs for the same production line (3000 tons clinker/day), with a specific fuel consumption of 3280 MJ/ton clinker [
17] and an H
2 HHV of 142 MJ/kg or 39.4 kWh/kg [
18], an H
2 flow of 144 kg/h or 1606 m
3/h will be needed to meet our criteria. Two sources were considered to calculate the equipment cost: a report from the US Department of Energy (DOE) [
19] and a report from the British Department for Business, Energy and Industrial Strategy (BEIS) [
20].
According to the DOE, the PEM electrolyzer CAPEX costs depend on the electrical capacity required, costing between 940 and 1400 €/kW. Assuming an input of 48 kWh per kg of H
2 [
21], an electrolyzer capacity of 6.93 MWh will be required, resulting in an equipment cost between 6.44 and 9.67 M€. The BEIS calculates the CAPEX using the heating capacity of the H
2 produced (HHV). From there, it can be estimated that a conservative interval for PEM electrolyzer costs would be 930-1400 €/kWh H
2. Then, the equipment necessary to produce 5.6 MWh of H
2 will cost between 5.22 and 7.83 M€.
Since these sources only consider the equipment price, to obtain the final implementation, the following steps were taken [
17]:
+5% for reconstruction of the nearby area/equipment;
Assume the equipment costs make up 48% of installation costs, with 37% for civil and steel work and 15% for erecting it;
+10% for contingency and fees;
+20% for interest, working capital, and owner’s costs.
The calculated costs are summarized in
Table 2, with LB and HB standing for the costs’ lower and higher bounds according to each source.
Averaging the results, the equipment needed to ramp up H
2 production would cost 21.05 M€. This would also carry an additional consumption of approximately 6.9 MWh, a rather significant increase in consumption. As for the additional costs, assuming a PEM electrolysis stack is used over a 10-year lifetime period, there should be no need to replace the membranes, considering that the BEIS report states that PEM membranes only need replacement every 11 years [
20].
5. Discussion
These two alternatives are currently possible to implement and show potential to help decarbonize the cement industry. However, they have greatly varying costs, with 5% H
2 being elevn times more expensive than the OXE approach. Naturally, a smaller percentage could be used, as even 1% would have a similar effect as kindling, allowing for a higher biomass content and reduced emissions. Still, this case was studied to examine the economic viability of a higher H
2 content, and currently, the cost is simply too high for its benefits. Additionally, this H
2 content should not be enough to achieve a completely net-zero fuel mix composed of only H
2 and biomass, as a study found it would need something closer to 7.5% of the thermal fraction in H
2 to improve burning conditions compared to the base case [
22].
As for downsides, both would raise the kiln temperature, with the H2 injection producing a higher increase than the OXE, and while more modern kilns are likely to have no issues with this, older models may have their inner lining damaged. For OXE, the higher O2 concentration also means a higher risk of ignition, so some additional safety control would be necessary. While H2 injection is undoubtedly helpful in reducing emissions, it is still quite expensive, and there is little to no information on its effects on clinker production or reducing fuel consumption. So, unlike OXE, this method’s direct benefits to production are unclear.
As for how cement quality could be affected by these methods, for OXE there should be no issues, and there could even be some slight improvements [
14]. As for the 5% H
2 feed, effectiveness will depend on the fuel composition. If the temperature is close to or slightly higher than normal functioning conditions, it should pose no complications.
6. Conclusions
There is a wide offer of decarbonization methods at the disposal of the cement industry. However, their cost can be quite prohibitive and provide no additional benefits to the process, discouraging their adoption. The use of H2 as fuel and O2 enrichment have been outlined as good choices, with the latter showing potential for reducing both emissions and fuel consumption. In contrast, H2 production by water electrolysis still proves too expensive to be used at a significant scale. Regardless, with improvements to both these technologies and others, they could be combined to achieve the carbon emission reduction goals set worldwide.
Author Contributions
Conceptualization, D.M.F.S., M.M., D.C; methodology, D.C.; software, D.C. and B.C.D.; validation, D.C.; formal analysis, D.C. and B.C.D.; investigation, B.C.D.; resources, M.M. and D.C.; data curation, B.C.D. and D.C.; writing—original draft preparation, B.C.D.; writing—review and editing, D.M.F.S., M.M. and D.C; visualization, B.C.D.; supervision, D.M.F.S. and D.C.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
D.M. Cecílio acknowledge the funding support of the P2020 Clean Cement Line project (LIS-BOA-01-0247-FEDER-027500). The support of the Foundation for Science and Technology (FCT, Portugal) is gratefully acknowledged for funding a contract in the scope of programmatic funding UIDP/04540/2020 (D.M.F. Santos).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- V. Masson-Delmotte, P. Z. et. al. Framing and Context. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. https://www.ipcc.ch/site/assets/uploads/sites/2/2018/12/SR15_FAQ_Low_Res.pdf (2018).
- IEA. Cement. https://www.iea.org/reports/cement (2022).
- TIMPERLEY, J. Q&A: Why cement emissions matter for climate change. CarbonBrief https://www.carbonbrief.org/qa-why-cement-emissions-matter-for-climate-change/ (2018).
- Vikström, A. Separate Calcination in cement clinker production. (Umeå University, 2021).
- Xu, X. et al. Modernizing cement manufacturing in China leads to substantial environmental gains. Commun Earth Environ 3, (2022). [CrossRef]
- Fennell, P. S., Davis, S. J. & Mohammed, A. Decarbonizing cement production. Joule 5, 1305–1311 (2021). [CrossRef]
- Pisciotta, M. et al. Current state of industrial heating and opportunities for decarbonization. Prog Energy Combust Sci 91, 100982 (2022). [CrossRef]
- Madeddu, C., Errico, M., Baratti, R. CO2 Capture by Reactive Absorption-Stripping (2019). ISBN: 978-3-030-04578-4.
- Malischek, R. & McCulloch, S. The world has vast capacity to store CO2: Net zero means we’ll need it. IEA https://www.iea.org/commentaries/the-world-has-vast-capacity-to-store-co2-net-zero-means-we-ll-need-it (2021).
- Wood, K., Liu, Y. Yu, Y. Design, Simulation and Optimization of Adsorptive and Chromatographic Separations (2018). [CrossRef]
- Wang, M. et al. Numerical simulation of oxy-coal combustion in a rotary cement kiln. Appl Therm Eng 103, 491–500 (2016). [CrossRef]
- Tsiliyannis, C.A. Cement manufacturing using alternative fuels: Enhanced productivity and environmental compliance via oxygen enrichment. Energy, (2016), 1202-1218, 113. [CrossRef]
- THUNDER SAID ENERGY. Cryogenic air separation: Costs and energy economics? https://thundersaidenergy.com/downloads/cryogenic-air-separation-the-economics/ (2023).
- Liu, Y. Q. et al. Experimental study on improving cement quality with oxygen-enriched combustion technology (IOP, 2015). [CrossRef]
- IEA. Global average levelised cost of hydrogen production by energy source and technology, 2019 and 2050. IEA https://www.iea.org/data-and-statistics/charts/global-average-levelised-cost-of-hydrogen-production-by-energy-source-and-technology-2019-and-2050 (2022).
- Chatenet, M. et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem Soc Rev 51, 4583–4762 (2022). [CrossRef]
- IEAGHG. Deployment of CCS in the Cement Industry. (2013).
- Rodrigue, J.-P. The Geography of Transport Systems. 5th Edition (Routledge, London, 2020). [CrossRef]
- Vickers, J. et al. Cost of Electrolytic Hydrogen Production with Existing Technology (2020).
- Department for Business, Energy and Industrial Strategy. Hydrogen Production Costs 2021 (2021).
- Chang, Y. Phoumin, H. Curtailed electricity and hydrogen in Association of Southeast Asian Nations and East Asia Summit: Perspectives form an economic and environmental analysis. International Journal of Hydrogen Energy, (2022), 24548-24557, 47(58). [CrossRef]
- Hercog, J. et al. Pilot testing and numerical simulations of the multifuel burner for the cement kiln. Fuel 342, 127801 (2023). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).