1. Introduction
Approximately 37 million Americans (11.3% of the United States [US] population) have diabetes mellitus [
1]. It is the 8
th leading cause of death nationwide and in 2021 alone was responsible for 103,294 deaths [
2]. The vast majority (90%-95%) of Americans with diabetes mellitus have type 2 diabetes, a condition which inhibits the body from processing blood sugar due to insulin resistance [
3]. Type 2 diabetes (hereafter referred to as diabetes) most often develops in people over the age of 45 and its prevalence increases with age [
3,
4].
In the US, more than 25% of adults over the age of 65 have diabetes [
5]. The higher prevalence of diabetes among this population has been attributed to age-related changes in organ function and body composition. Among older adults, diabetes has traditionally been associated with an increased risk of vascular complications such as coronary heart disease, stroke, diabetic kidney disease, retinopathy, and peripheral neuropathy [
6]. In recent years, research has also identified emerging complications affecting older adults with diabetes, including various cancers, infections, and diseases of the liver, as well as dementia and cognitive impairment [
6].
Dementia is an umbrella term for changes in cognition due to physiological conditions such as Alzheimer’s disease, vascular dementia, and various other illnesses affecting the brain [
6]. Numerous studies have found individuals with diabetes are at an increased risk of developing dementia [
6,
7] Furthermore, research has shown that diabetics with mild cognitive impairment have an increased risk of their condition progressing to dementia [
6]. Clinical pathways that may contribute to this relationship include impaired insulin signaling, inflammation, and hypoglycemia [
7].
Research has dedicated considerable attention to the burden and cost of diabetes-related hospital care with a focus on potentially avoidable hospitalizations (PAHs) for ambulatory care sensitive conditions. Diabetes hospitalization costs have been increasing over the past two decades, which has been attributed, in part, to PAHs for uncontrolled diabetes, short- and long-term diabetic complications, and lower extremity amputations [
8]. From 2001-2014, the national cost of diabetes related PAHs for these conditions alone increased from
$4.5 billion to
$5.9 billion. The majority (75%) of this increase was due to a rise in diabetes-related PAHs, with the remainder being attributed to an increase in mean cost per admission.
Hospitalizations have also played a significant role in driving the healthcare costs of dementia over the years [
9,
10]. Lin and colleagues found that in 2013 alone, the total cost of hospitalizations for Medicare beneficiaries with dementia was
$4.7 billion [
10]. They also found nearly one in ten of these patients were hospitalized for a potentially avoidable condition, and that nearly 1 in 5 had an unplanned readmission within 30 days. A state-wide matched analysis of Medicare costs in Tennessee by Husaini and colleagues, found that hospitalization costs were 14% higher among dementia patients as compared to patients without dementia; those patients with dementia also had a significantly higher frequency of diabetes (36% vs 32%,
p<.001) [
9].
People with both diabetes and dementia have been shown to be at an increased risk for PAHs. A 2017 study by Lin et al., which examined Medicare claims data, found that persons with dementia were more likely to have PAHs for short- and long-term diabetic complications than patients without dementia [
11]. Another study by Zaslavsky et al. found that incident dementia was associated with increased rates of hospitalization among persons with diabetes, both for diabetic complications and non-diabetic complications, such as dehydration or urinary tract infections [
12]. While there is a growing body of research focused on the risk for and outcomes of PAHs among individuals with comorbid diabetes and dementia, few studies provide in depth comparisons of the demographic and health characteristics among patients with diabetes, patients with dementia, and patients with comorbid diabetes and dementia. To our knowledge, no studies have examined the differences in cost and duration of hospitalization for persons with these distinct health profiles.
To address this gap in the literature, we analyzed Los Angeles County hospital discharge data from 2019-2021 to better understand the factors affecting hospitalizations of persons with diabetes, persons with dementia, and persons with both of these conditions. The present study sought to: (1) describe the differences in demographic and hospitalization characteristics between these groups; (2) examine variables effecting total charges and length of stay; and (3) compare the odds of diabetic complications for individuals with comorbid diabetes and dementia, as compared to individuals with diabetes only.
2. Materials and Methods
2.1. Data Source and Study Population
Hospitalizations were examined using patient discharge data (PDD) for the years 2019-2021 derived from the California Department of Health Care Access and Information (HCAI) [
13]. HCAI maintains non-public, limited data sets consisting of patient-level inpatient discharge data collected from all state-licensed hospitals in California. Licensed hospitals include general acute care, acute psychiatric, chemical dependency recovery, and psychiatric health facilities. PDD contains demographic, clinical, payer, and facility data for all inpatient records.
All hospitalizations of Los Angeles County residents aged 50 years and older at the time of admission were included in the present analysis (n=1,472,688), reflecting 47% of all hospitalization records during the study period (n=3,160,404). The analysis sample was stratified into four mutually exclusive groups based on disease status: (i) diabetes, (ii) dementia, (iii) diabetes and dementia, and (iv) no diabetes or dementia. Disease status was classified using International Classification of Diseases, Tenth Revision, Clinical Modifications (ICD-10-CM) codes (see Supplemental
Table S1) [
14]. Diagnosis of diabetes and/or dementia was established based on the listing of a diabetes or dementia ICD-10-CM code as (a) the chief cause of admission of the patient for hospital care or (b) a coexisting condition at time of admission that developed subsequently during the hospital stay, or that affected the treatment received and/or the length of stay on the hospitalization record.
2.2. Variables
To understand the characteristics of hospitalizations by disease status, descriptive and univariate statistics were generated for the following variables: length of stay—the total number of days from admission to discharge date; total charges—the total charges for services rendered based on the hospital’s full established rates, in US dollars; age; sex; race/ethnicity; year of hospital admission; disposition—the consequent arrangement or event ending the patient’s stay in the hospital; type of care—the licensure of the bed occupied by the patient; expected source of payment—the entity or organization expected to pay the greatest share of the patient’s bill such as Medicare or private coverage; type of admission, such as emergency or elective; source of admission—site where the patient originated such as a non-health care facility of different hospital facility.
Length of stay, total charges, and age were examined as continuous variables. Race/ethnicity was categorized as: White, Black, Hispanic, Asian, and Other (inclusive of Other, American Indian/Alaskan Native, Native Hawaiian or Other Pacific Islander, and Multiracial). Disposition was categorized in alignment with categories established in the Agency for Healthcare Research and Quality’s Healthcare Cost & Utilization Project as: routine discharge, transfer to a short-term hospital, transfer to other (inclusive of skilled nursing facilities [SNF], intermediate care facilities [ICF], and other types of facilities), home health care, against medical advice, and died [
15]. Type of care was categorized as acute care or other types of care (inclusive of skilled nursing care/intermediate care, psychiatric care, chemical dependency recovery care, and physical rehabilitation care) for this analysis. Expected source of payment was categorized as Medicare, Medi-Cal (California’s Medicaid program), private coverage, and other (inclusive of workers’ compensation, county indigent programs, other government, other indigent, self-pay, and other). Type of admission was categorized as emergency, urgent, elective, and trauma. Finally, source of admission was categorized as non-health care facility, clinic/physician’s office, different hospital facility, SNF/ICF/assisted living facility (ALF), or other (inclusive of court/law enforcement sites, one distinct unit to another distinct unit of the same hospital, ambulatory surgery centers, hospices, and designated disaster alternate care sites). Additionally, because the study period coincided with the onset and peak of the coronavirus disease 2019 (COVID-19) pandemic which had a significant impact on hospitalizations across the US, diagnosis of COVID-19 was also examined [
16,
17,
18].
2.3. Total Charges
A multiple linear regression model was constructed for total charges incurred for all services rendered during the patient’s hospitalization. To satisfy the normality assumption for linear regression modelling, the dependent variable was log-transformed, and subsequently, 1 was added to stabilize the log-transformed variable to account for instances of zero values for total charges. The total charge variable was regressed on disease status, age, sex, race/ethnicity, year of hospital admission, disposition, type of care, expected source of payment, type of admission, source of admission, and COVID-19 diagnosis.
Best subsets, backwards, and stepwise selection algorithms were utilized with Akaike information criterion (AIC), Bayesian information criterion (BIC), and adjusted R-squared noted for all approaches to determine the best fitted model; the practical and clinical relevancy of the variables to be included were also considered. In the model building, all three algorithms selected all variables of interest for inclusion in the final model. The model was then checked for potentially influential outliers utilizing statistical measures of influence; based on results no observations were deleted. Normality of residuals was also assessed by histogram, overlayed with a kernel density and normal plot. Finally, homoskedasticity was assessed using the studentized residuals vs. fitted values plot. No violations of any model assumptions were detected.
Discharge records with any missing or invalid data for the log total charge variable or any of the covariates were excluded from the analysis. The total sample size included in the final linear model was n=1,450,843. Parameter estimates and 95% confidence intervals (CIs) were generated, and the statistical significance threshold was set at p ≤ 0.05.
2.4. Length of Stay
A multinomial logistic regression model was built for length of stay, which was categorized by quartiles: 0-1 days, 2-3 days, 4-6 days, and 7+ days. Despite the ordinal nature of the length of stay variable, it was analyzed as a non-ordered multinomial variable due to a violation of the proportional odds assumption. Length of stay was regressed on disease status, age category (50-59 years, 60-69 years, 70-79 years, 80-89 years, and 90+ years), sex, race/ethnicity, year of hospital admission, disposition, type of care, expected source of payment, type of admission, source of admission, and COVID-19 diagnosis.
Forward and backward selection algorithms were used to test variable selection. The model fit was assessed using objective functions like likelihood ratio test measures, AIC, and BIC; as with the multiple linear regression model, practical and clinical relevancy of identified variables were also considered. In the model building, both algorithms selected all variables of interest for inclusion in the final model. Model checking included an assessment of multicollinearity by measuring the Cramer’s V for each pair of categorical variables. The absence of complete separation was ascertained by checking standard errors and parameter estimates for extremely large and/or infinite values. No violations of any multinomial logistic models were detected.
Discharges that had any missing or invalid data for the length of stay variable or any of the covariates were excluded from analysis. The total sample size included in the final multinomial model was n=1,450,843. Adjusted odds ratios (AORs) and 95% CIs were generated, and the statistical significance threshold was set at p ≤ 0.05.
2.5. Matched-Case Control Analysis
To explore the differences in odds of diabetic complications experienced by those with diabetes alone compared to those with comorbid diabetes and dementia, a 1:4 matched, nested case-control analysis was employed. From the pool of discharge records with complete data (n=1,450,843), cases (those with diabetes and dementia) and controls (those with diabetes) were selected and matched based on year of hospital admission, sex, age, and race/ethnicity. The final matched sample size consisted of 1,492 cases and 5,968 controls.
Univariate and descriptive statistics for cases and controls were examined for length of stay, total charges, age, sex, race/ethnicity, year of hospital admission, dispositions, type of care, expected sources of payment (categorized as Medicare, Medi-Cal, and other), type of admission (categorized as emergency, urgent, and other), and source of admission.
Conditional logistic regression models were employed to assess the likelihood of hospitalization for five diabetic complications for cases compared to controls. Complications examined included: ophthalmic complications; kidney complications; neurological complications; hypertensive diseases; and lower-extremity amputations (based on ICD-10-Procedural Coding System classification; see Supplemental
Table S1). Matched odds ratios (MORs) and 95% CIs were generated for each model; the statistical significance threshold was set at
p ≤ 0.05.
After evaluating for potential confounders, no sizable impacts to the crude MOR estimates were detected and therefore, regression models were unadjusted. Model checking included an assessment of the linearity between the log-odds of the diabetic complications and age; outlier influence assessment and case-wise deletion procedures; and confirmation of the efficiency of the matching process.
All statistical analyses were conducted using SAS analytical software version 9.4 (SAS Institute Inc., Cary, NC). This study was considered exempt as non-human subjects research by the Los Angeles County Department of Public Health Institutional Review Board.
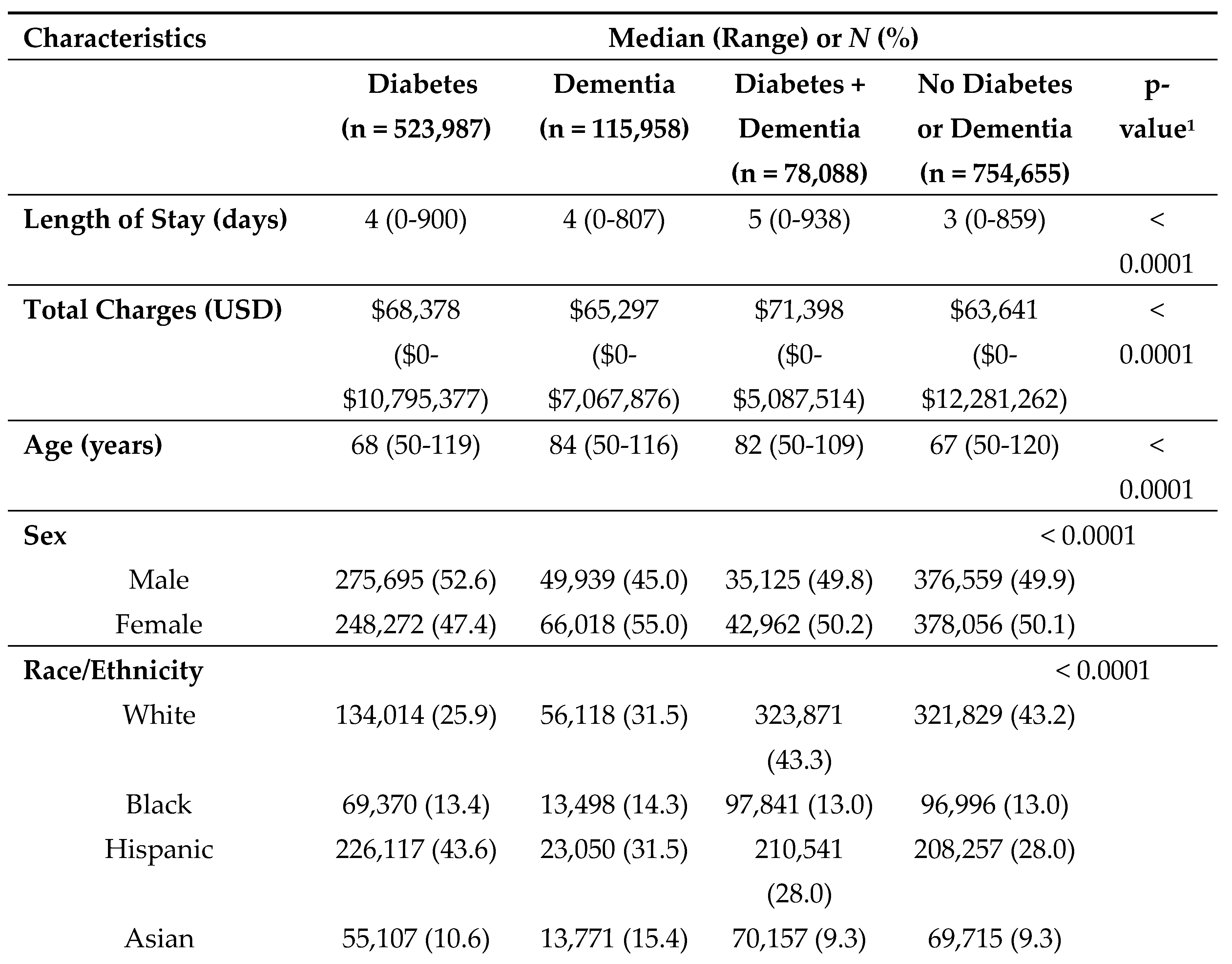
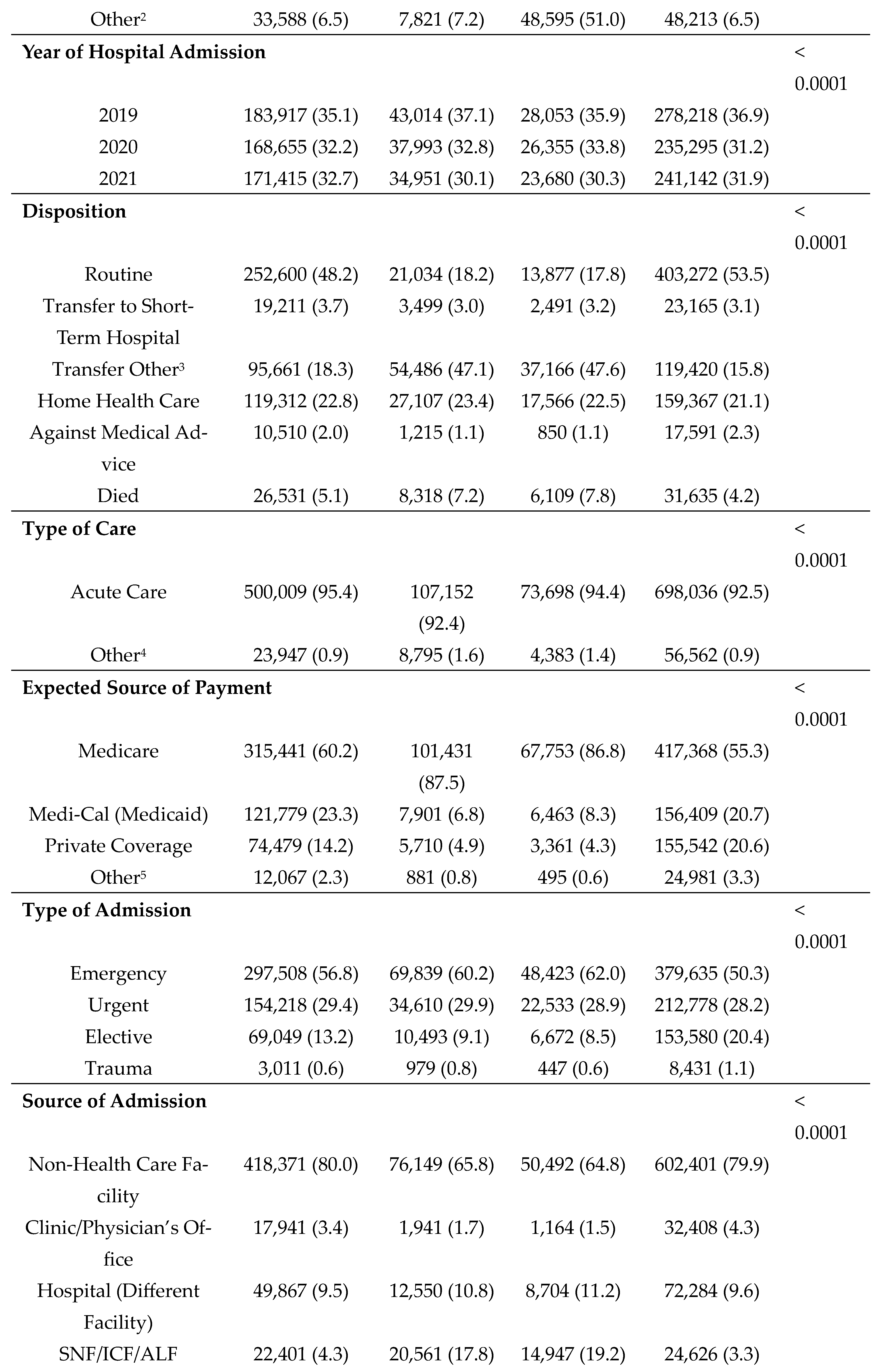
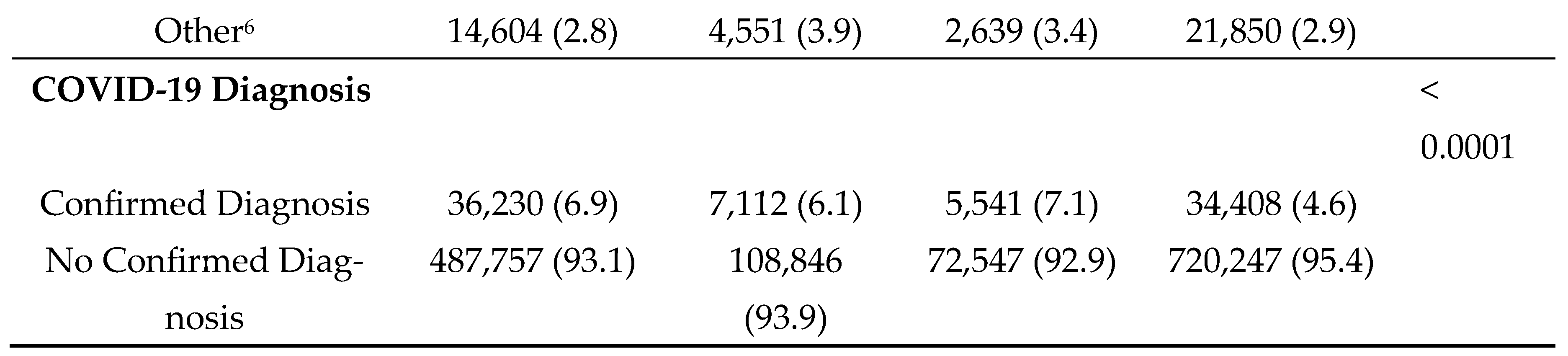
3. Results
During the 3-year study period, a total of 523,987 discharges (36.7%) were indicated as having diabetes and incurred a total of
$62.5 billion in total charges; 32,376 of these discharges listed diabetes as the chief cause of admission. A total of 115,958 (8.1%) had dementia and incurred a total of
$11.8 billion in total charges; of these approximately 5% (5,849) listed dementia as the chief cause of admission. Approximately 5.5% of patients had both (n=78,088) diabetes and dementia and incurred a total of
$8.7 billion in hospital charges. Median length of stay and total charges were highest among those with comorbid diabetes and dementia–5 days and
$71,398, respectively (
Table 1). Median age at admission was found to be highest among patients with dementia alone (84 years). Differences by sex were most pronounced among those with dementia alone, with females being more frequently diagnosed with dementia (55.0%) as compared to males (45.0%). Race/ethnicity patterns varied significantly by disease status. For example, among those with diabetes alone, Hispanics comprised the largest group (43.6%); and among those with comorbid diabetes and dementia, those classified as Other made up the largest group (51.0%). Notably, disposition upon discharge varied substantially, with far fewer patients with dementia (alone or in conjunction with diabetes) having a routine discharge (e.g., 18.2% of those with dementia compared to 48.2% of those with diabetes). Across all disease categories, Medicare was the primary source of payment for the majority of inpatients and most admissions were characterized as emergency admissions originating from non-healthcare facility sources (e.g., patients' homes). The frequency of COVID-19 diagnoses was highest among individuals with comorbid diabetes and dementia (7.1%).
Multivariable linear regression revealed that individuals with diabetes alone exhibited 8.9% [exp(β
1)-1=exp(-0.115)-1] higher total charges when compared to individuals without dementia or diabetes, while holding all other variables constant (p<0.0001), see
Table 2. Conversely, those with dementia or comorbid diabetes and dementia had lower total charges (-10.9% and -2.9%, respectively; p<0.0001).
Multinomial logistic regression examining length of stay revealed that relative to those who were hospitalized for 0-1 days, patients with comorbid diabetes and dementia had the highest odds of a 2-3 day length of stay (AOR=1.20, 95% CI=1.17-1.24), with other predictor variables in the model held constant, see
Table 3. Model estimates for a length of stay of 4-6 days and 7+ days revealed parallel patterns.
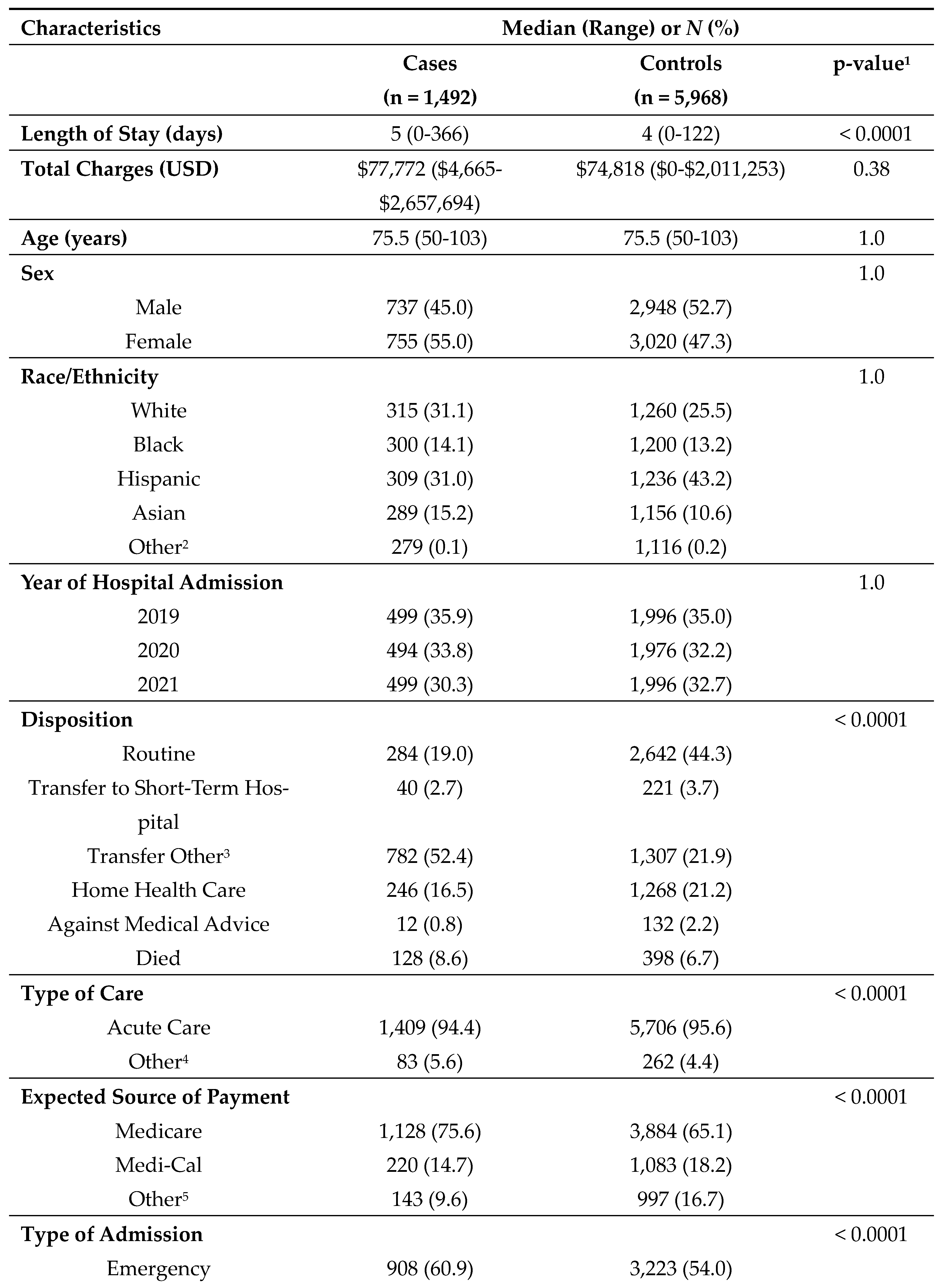
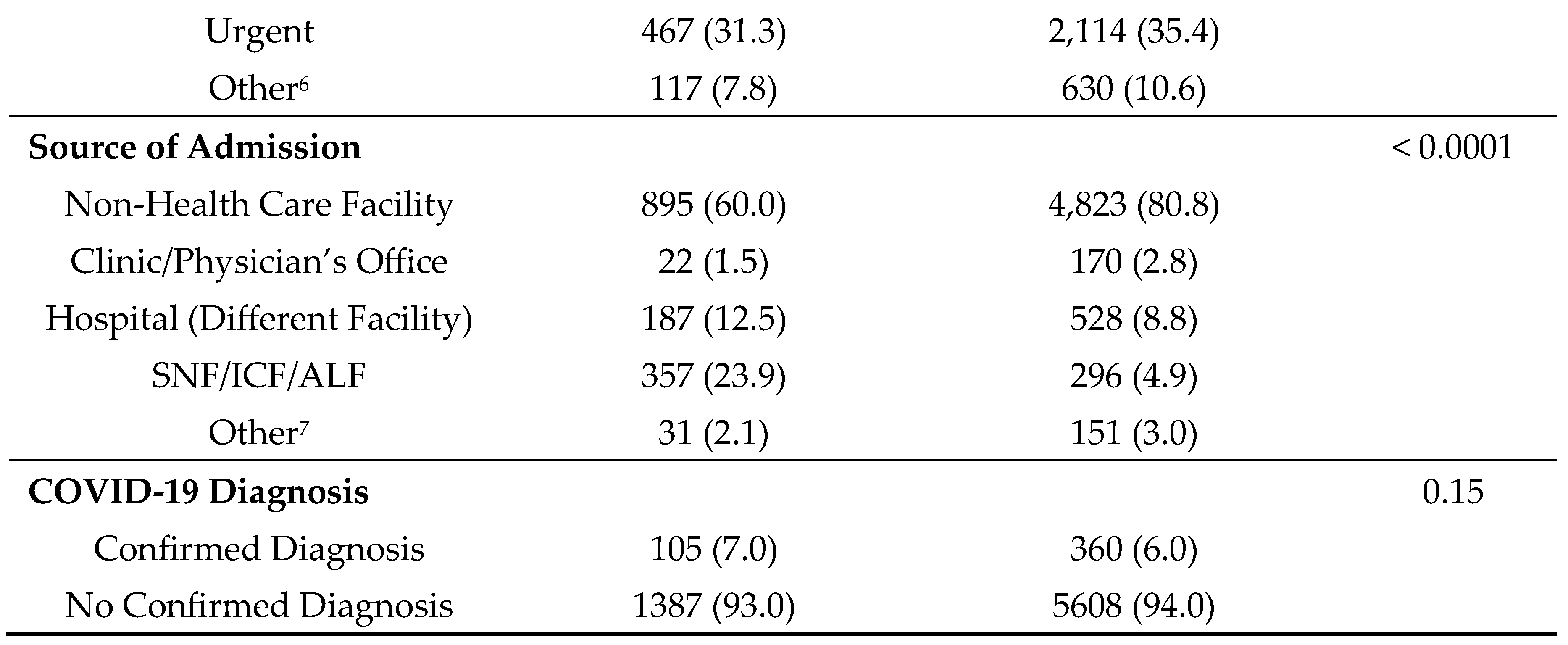
After matching, patients with comorbid diabetes and dementia exhibited significant differences across all variables of interest, with the exception of total charges (
Table 4).
Conditional logistic regression analyses revealed that there were minimal differences in diabetic complications among those with comorbid diabetes and dementia as compared to those with dementia, with the exception of hypertensive diseases (
Table 5). Comorbid diabetes and dementia was associated with a decreased risk of hypertensive diseases (OR=0.81, 95% CI=0.67-0.97).
4. Discussion
Previous studies have explored the economic burden of and factors influencing hospitalizations among patients with diabetes, dementia, and their associated comorbidities [
8,
9,
19,
20]. However, these studies have primarily focused on a single disease profile, such as diabetes or dementia, or examined the two as part of a more general description of comorbidities. Our study specifically sought to build upon this research by providing a more comprehensive understanding of the health profiles of hospitalized patients in Los Angeles County who have comorbid diabetes and dementia. Four takeaways can be gleaned from these hospitalization data.
First, patients with comorbid diabetes and dementia experienced longer hospital stays compared to the other groups. This is in keeping with previous research which shows that patients with dementia may require more time to assess and manage comorbidities before discharge, have longer recovery periods more generally, or have increased disease severity, and as such, are often moved to skilled nursing facilities and other post-hospitalization venues earlier than diabetics [
19]. Interestingly, despite patients with diabetes generally having a shorter length of stay, they had the highest total charges, after adjusting for covariates, when compared to other disease groups. This may be attributed, in part, to high-cost care procedures being implemented in the first days of admission. For example, a study by Fine et al. found that cost of care for hospitalized patients with pneumonia was lowest on the day of discharge and the 2 days preceding discharge [
21]. Furthermore, caregivers of patients with comorbid diabetes and dementia may need further support in learning how to manage their condition, which could extend length of stay with minimal relative added costs [
22].
Second, our study showed that there were differences in racial and ethnic disparities across the different disease profiles in the HCAI data. This may suggest that health inequities in access to and the delivery of care are present in how hospitals detect and manage patients with comorbid diabetes and dementia. Prior research indicates that documentation of a dementia diagnosis is likely distorted in hospitalization records, as dementia diagnoses are often missed or delayed among non-White racial and ethnic groups [
23]. For example, non-Hispanic Black adults and Hispanics have been shown to be 27% and 84% more likely, respectively, to have a missed or delayed diagnosis of dementia compared to non-Hispanic whites.
Third, patients with dementia and those with comorbid diabetes and dementia were more frequently transferred to specialized long-term care facilities (e.g., SNF), whereas patients with diabetes alone had more routine charges. This finding aligns with existing literature showing that transitions to a skilled nursing facility are especially common among patients with dementia [
24]. Patients with dementia are more likely to have multiple coexisting chronic conditions, often requiring more complex and intensive care [
25]. It could also be that the community resources that are geared toward diabetes care are more organized and reimbursable than dementia care – an area of need where better health policies and interventions should be implemented [
26,
27,
28].
Finally, results from the matched case-control analysis suggest that the presence of hypertensive complications were lower among patients with comorbid diabetes and dementia than patients with diabetes alone. While somewhat counterintuitive since hypertension is a risk factor for dementia, this finding, nonetheless, aligns with other studies that have found an inverse association between dementia and late-life hypertension [
29,
30].
As previously noted, this study coincides with the COVID-19 pandemic which had significant impact on hospitalizations, especially for older adults [
31]. Age is considered to be the most important risk factor for severe COVID-19 symptoms, with the risk of severe outcomes increasing with advancing age [
32]. Diabetes has also been identified as a significant risk factor for severe COVID-19 and subsequent hospitalization [
33,
34].
4.1. Limitations
While this study has as number of strengths, including its large sample size and low rate of missing data, it has several limitations. First, discharge records represent unique hospitalizations rather than individuals and do not allow for examination of readmissions after discharge or recurrent hospitalizations. Second, recorded diagnoses related to a prior episode that have no bearing on the current hospital stay are excluded. This may have resulted in an underestimation of patients with diabetes and dementia. Third, use of ICD-10-CM codes alone to determine presence of dementia likely also contributed to an underestimation of dementia. Research has shown that many of those who meet the diagnostic criteria for dementia are not diagnosed by a physician [
35]. Finally, detailed information on patient risk factors, medical history, and socioeconomic status, which can increase severity of disease and correspondingly hospitalization outcomes, were not available. Without such information, we were unable to adequately adjust for these confounding factors in our analyses.
5. Conclusions
Study findings highlight the substantial healthcare burden associated with comorbid diabetes and dementia. This burden is expected to grow as the number of Americans with these conditions is projected to increase substantially over the next few decades [
35,
36,
37,
38]. Our results underscore the need for a multi-faceted approach to address both diabetes and dementia, in many instances, concurrently, especially for high-risk populations that lack adequate access to health services, are vulnerable to socioeconomic challenges, and have historically experienced health inequities and barriers to care, including systemic racism [
39,
40]. Investments in strategies such as preventative care, care coordination, advance care planning, personalized care plans, and community supports for improving self-management and caregiver assistance will be critical for mitigating the financial and social impacts of these prevailing and costly chronic diseases [
41,
42,
43,
44,
45,
46,
47]. These are all areas of health policy research, strategy intervention, and program implementation that will be required to address the comorbidity of diabetes and dementia in Los Angeles County and elsewhere across the US [
48,
49,
50].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: ICD-10-CM and ICD-10-PCS Codes Used.
Author Contributions
Conceptualization, DMR, NCB, TK; Formal Analysis, DMR and NCB; Writing – Original Draft Preparation, DMR, DRS, MAR; Writing – Review & Editing, DMR, DRS, MAR, TK, NCB; Fund Acquisition – NCB.
Funding
This work was supported in part by funding from the Alzheimer’s Association through a cooperative agreement with the Centers for Disease Control and Prevention [NU58DP006744].
Institutional Review Board Statement
This present analysis was considered exempt as non-human subjects research by the Los Angeles County Department of Public Health Institutional Review Board.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from the California Department of Health Care Access and Information and are available at
https://hcai.ca.gov/data-and-reports/request-data/ to eligible hospitals and health departments with the permission of the California Department of Health Care Access and Information.
Acknowledgments
The findings and conclusions presented in this article are those of the authors and do not necessarily represent the views of the funder, grantee, or any of the other organizations referenced in the text. The authors would like to thank the California Department of Health Care Access and Information for providing access to patient discharge data.
Conflicts of Interest
The authors have no relevant financial relationships or other conflicts of interest pertaining to this subject matter to disclose.
References
- American Diabetes Association. Statistics About Diabetes. Available online: https://diabetes.org/about-us/statistics/about-diabetes (accessed on 27 September 2023).
- Centers for Disease Control and Prevention. National Center for Health Statistics. Diabetes. Available online: https://www.cdc.gov/nchs/fastats/diabetes.htm (accessed on 27 September 2023).
- Centers for Disease Control and Prevention. Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/basics/type2.html (accessed on 27 September 2023).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019, 157, 107843:1–107843:10. [CrossRef]
- Sesti, G.; Antonelli Incalzi, R.; Bonora, E.; Consoli, A.; Giaccari, A.; Maggi, S.; Paolisso, G.; Purrello, F.; Vendemiale, G.; Ferrara, N. Management of Diabetes in Older Adults. Nutr Metab Cardiovasc Dis. 2018, 28, 206–218. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The Burden and Risks of Emerging Complications of Diabetes Mellitus. Nat Rev Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Simó, R.; Ciudin, A.; Simó-Servat, O.; Hernández, C. Cognitive Impairment and Dementia: A New Emerging Complication of Type 2 Diabetes-The Diabetologist's Perspective. Acta Diabetol. 2017, 54, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.S.; Zhang, P.; Hora, I.; Geiss, L.S.; Luman, E.T.; Gregg, E.W. Factors Contributing to Increases in Diabetes-Related Preventable Hospitalization Costs among U.S. Adults During 2001-2014. Diabetes Care. 2019, 42, 77–84. [CrossRef]
- Husaini, B.; Gudlavalleti, A.S.; Cain, V.; Levine, R.; Moonis, M. Risk Factors and Hospitalization Costs of Dementia Patients: Examining Race and Gender Variations. Indian J Community Med. 2015, 40, 258–263. [Google Scholar] [CrossRef]
- Lin, P.J.; Zhong, Y.; Fillit, H.M.; Cohen, J.T.; Neumann, P.J. Hospitalizations for Ambulatory Care Sensitive Conditions and Unplanned Readmissions among Medicare Beneficiaries with Alzheimer's Disease. Alzheimers Dement. 2017, 13, 1174–1178. [Google Scholar] [CrossRef]
- Lin, P.J.; Fillit, H.M.; Cohen, J.T.; Neumann, P.J. Potentially Avoidable Hospitalizations among Medicare Beneficiaries with Alzheimer's Disease and Related Disorders. Alzheimers Dement. 2013, 9, 30–38. [Google Scholar] [CrossRef]
- Zaslavsky, O.; Yu, O.; Walker, R.L.; Crane, P.K.; Gray, S.L.; Sadak, T.; Borson, S.; Larson, E.B. Incident Dementia, Glycated Hemoglobin (HbA1c) Levels, and Potentially Preventable Hospitalizations in People Aged 65 and Older with Diabetes. J Gerontol A Biol Sci Med Sci. 2021, 76, 2054–2061. [Google Scholar] [CrossRef]
- California Department of Health Care Access and Information. Limited Data Request Information. Available online: https://hcai.ca.gov/data-and-reports/request-data/limited-data-request-information/ (accessed on 27 September 2023).
- Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Available online: https://www.cdc.gov/nchs/icd/icd-10-cm.htm (accessed on 27 September 2023).
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project User Support. Available online: https://hcup-us.ahrq.gov/db/vars/dispuniform/nisnote.jsp (accessed on 27 September 2023).
- DeLaroche, A.M.; Rodean, J.; Aronson, P.L.; Fleegler, E.W.; Florin, T.A.; Goyal, M.; Hirsch, A.W.; Jain, S.; Kornblith, A.E.; Sills, M.R.; et al. Pediatric Emergency Department Visits at US Children's Hospitals During the COVID-19 Pandemic. Pediatrics. 2021, 147, e2020039628;1–e2020039628;11. [CrossRef]
- Birkmeyer, J.D.; Barnato, A.; Birkmeyer, N.; Bessler, R.; Skinner, J. The Impact of the COVID-19 Pandemic on Hospital Admissions in the United States. Health Aff (Millwood). 2020, 39, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Nourazari, S.; Davis, S.R.; Granovsky, R.; Austin, R.; Straff, D.J.; Joseph, J.W.; Sanchez, L.D. Decreased Hospital Admissions through Emergency Departments during the COVID-19 Pandemic. Am J Emerg Med. 2021, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.; Massano, J.; Freitas, A. Hospital Admissions 2000-2014: A Retrospective Analysis of 288 096 Events in Patients with Dementia. Arch Gerontol Geriatr. 2018, 77, 150–157. [Google Scholar] [CrossRef]
- Sherzai, D.; Sherzai, A.; Lui, K.; Pan, D.; Chiou, D.; Bazargan, M.; Shaheen, M. The Association Between Diabetes and Dementia among Elderly Individuals: A Nationwide Inpatient Sample Analysis. J Geriatr Psychiatry Neurol. 2016, 29, 120–125. [Google Scholar] [CrossRef]
- Fine, M.J.; Pratt, H.M.; Obrosky, D.S.; Lave, J.R.; McIntosh, L.J.; Singer, D.E.; Coley, C.M.; Kapoor, W.N. Relation between Length of Hospital Stay and Costs of Care for Patients with Community-Acquired Pneumonia. Am J Med. 2000, 109, 378–385. [Google Scholar] [CrossRef]
- Lebrec, J.; Ascher-Svanum, H.; Chen, Y.; Reed, C.; Kahle-Wrobleski, K.; Hake, A.M.; Raskin, J.; Naderali, E.; Schuster, D.; Heine, R.J.; et al. Effect of Diabetes on Caregiver Burden in an Observational Study of Individuals with Alzheimer’s Disease. BMC Geriatr. 2016, 16, 93:1–93:14. [Google Scholar] [CrossRef]
- Lin, P.J.; Daly, A.T.; Olchanski, N.; Cohen, J.T.; Neumann, P.J.; Faul, J.D.; Fillit, H.M.; Freund, K.M. Dementia Diagnosis Disparities by Race and Ethnicity. Med Care. 2021, 59, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Block, L.; Hovanes, M.; Gilmore-Bykovskyi, AL. Written Discharge Communication of Diagnostic and Decision-Making Information for Persons Living with Dementia during Hospital to Skilled Nursing Facility Transitions. Geriatr Nurs. 2022, 45, 215–222. [Google Scholar] [CrossRef]
- Sadarangani, T.; Perissinotto, C.; Boafo, J.; Zhong, J.; Yu, G. Multimorbidity Patterns in Adult Day Health Center Clients with Dementia: A Latent Class Analysis. BMC Geriatr. 2022, 22, 514:1–514:11. [CrossRef]
- Hodges, K.; Fox-Grage, W.; Tewarson, H. The National Academy for State Health Policy. The Future of Aging Policy: A Snapshot of State Priorities. Available online: https://nashp.org/the-future-of-aging-policy-a-snapshot-of-state-priorities/ (accessed 27 September 2023).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023, 46 (Suppl. 1), S19–S40. [Google Scholar] [CrossRef]
- Gaugler, J.E.; Borson, S.; Epps, F.; Shih, R.A.; Parker, L.J.; McGuire, L.C. The Intersection of Social Determinants of Health and Family Care of People Living with Alzheimer's Disease and Related Dementias: A Public Health Opportunity. Alzheimers Dement. 2023, 10.1002/alz.13437:1–10.1002/alz.13437:10. [CrossRef]
- Bauer, K.; Schwarzkopf, L.; Graessel, E.; Holle, R. A Claims Data-Based Comparison of Comorbidity in Individuals with and without Dementia. BMC Geriatr. 2014, 14, 10:1–10:13. [CrossRef]
- Power, M.C.; Weuve, J.; Gagne, J.J.; McQueen, M.B.; Viswanathan, A.; Blacker, D. The Association between Blood Pressure and Incident Alzheimer Disease: A Systematic Review and Meta-Analysis. Epidemiology. 2011, 22, 646–659. [Google Scholar] [CrossRef]
- Singhal, S.; Kumar, P.; Singh, S.; Saha, S.; Dey, AB. Clinical Features and Outcomes of COVID-19 in Older Adults: A Systematic Review and Meta-Analysis. BMC Geriatr. 2021, 21, 321:1–321:9. [CrossRef]
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 27 September 2023).
- Khunti, K.; Del Prato, S.; Mathieu, C.; Kahn, S.E.; Gabbay, R.A.; Buse, J.B. COVID-19, Hyperglycemia and New-Onset Diabetes. Diabetes Care. 2021, 44, 2645–2655. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Benahmed, A.G.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjørklund, G. Interrelations between COVID-19 and Other Disorders. Clin Immunol. 2021, 224, 108651:1–108651:12. [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 27 September 2023).
- Lin, J.; Thompson, T.J.; Cheng, Y.J.; Zhuo, X.; Zhang, P.; Gregg, E.; Rolka, D.B. Projection of the Future Diabetes Burden in the United States through 2060. Popul Health Metr. 2018, 16, 9:1–9:9. [CrossRef]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and Ethnic Estimates of Alzheimer's Disease and Related Dementias in the United States (2015-2060) in Adults Aged ≥65 years. Alzheimers Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer Disease in the United States (2010-2050) Estimated Using the 2010 Census. Neurology. 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Braveman, P.; Arkin, E.; Proctor, D.; Kauh, T.; Holm, N.; Robert Wood Johnson Foundation. Systemic Racism and Health Equity. Available online: https://www.rwjf.org/en/insights/our-research/2021/12/systemic-racism-and-health-equity.html (accessed on 27 September 2023).
- Yearby, R.; Clark, B.; Figueroa, J.F. Structural Racism in Historical and Modern US Health Care Policy. Health Aff (Millwood). 2022, 41, 187–194. [Google Scholar] [CrossRef]
- Bosisio, F.; Sterie, A.C.; Rubli Truchard, E.; Jox, R.J. Implementing Advance Care Planning in Early Dementia Care: Results and Insights from a Pilot Interventional Trial. BMC Geriatr. 2021, 21, 573:1–573:11. [CrossRef]
- Dunning, T.L. Palliative and End-of-Life Care: Vital Aspects of Holistic Diabetes Care of Older People with Diabetes. Diabetes Spectr. 2020, 33, 246–254. [Google Scholar] [CrossRef]
- Srikanth, V.; Sinclair, A.J.; Hill-Briggs, F.; Moran, C.; Biessels, G.J. Type 2 Diabetes and Cognitive Dysfunction-towards Effective Management of Both Comorbidities. Lancet Diabetes Endocrinol. 2020, 8, 535–545. [Google Scholar] [CrossRef]
- Vuohijoki, A.; Mikkola, I.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Winell, K.; Frittitta, L.; Timonen, M.; Hagnäs, M. Implementation of a Personalized Care Plan for Patients with Type 2 Diabetes is Associated with Improvements in Clinical Outcomes: An Observational Real-World Study. J Prim Care Community Health. 2020, 11, 2150132720921700:1–2150132720921700:7. [CrossRef]
- Bunn, F.; Goodman, C.; Jones, P.R.; Russell, B.; Trivedi, D.; Sinclair, A.; Bayer, A.; Rait, G.; Rycroft-Malone, J.; Burton, C. Managing Diabetes in People with Dementia: A Realist Review. Health Technol Assess. 2017, 21, 1–140. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, M. Effectiveness of Person-Centered Care on People with Dementia: A Systematic Review and Meta-Analysis. Clin Interv Aging. 2017, 12, 381–397. [Google Scholar] [CrossRef]
- Hopkins, R.; Shaver, K.; Weinstock, R.S. Management of Adults with Diabetes and Cognitive Problems. Diabetes Spectr. 2016, 29, 224–237. [Google Scholar] [CrossRef]
- Fulmer, T.; Reuben, D.B.; Auerbach, J.; Fick, D.M.; Galambos, C.; Johnson, K.S. Actualizing Better Health and Health Care for Older Adults. Health Aff (Millwood). 2021, 40, 219–225. [Google Scholar] [CrossRef]
- Super, N. Three Trends Shaping the Politics of Aging in America. Public Policy & Aging Report. 2020, 30, 39–45. [CrossRef]
- National Institute on Aging. National Institute on Aging: Strategic Directions for Research: 2020-2025. Available online: https://www.nia.nih.gov/about/aging-strategic-directions-research (accessed on 27 September 2023).
Table 1.
Hospital Discharges by Disease Status, Los Angeles County, 2019-2021.
Table 1.
Hospital Discharges by Disease Status, Los Angeles County, 2019-2021.
Table 2.
Multivariable Linear Regression Analysis of Disease Status on Log Total Charges Billed for Hospitalizations, Los Angeles County, 2019-2021.
Table 2.
Multivariable Linear Regression Analysis of Disease Status on Log Total Charges Billed for Hospitalizations, Los Angeles County, 2019-2021.
| Covariate |
Parameter Estimate |
95% Confidence Interval |
p-value |
| Disease Status (Referent: No Diabetes or Dementia) |
|---|
| Diabetes |
0.085 |
(0.082, 0.089) |
< 0.0001 |
| Dementia |
-0.115 |
(-0.121, -0.109) |
< 0.0001 |
| Diabetes + Dementia |
-0.029 |
(-0.036, -0.022) |
< 0.0001 |
Table 3.
Multinomial Logistic Regression Analysis of Hospital Discharges by Disease Status on Length of Stay, Los Angeles County, 2019-2021.
Table 3.
Multinomial Logistic Regression Analysis of Hospital Discharges by Disease Status on Length of Stay, Los Angeles County, 2019-2021.
| Length of Stay in Days Categorized by Quartiles (Referent Group: 0-1 Days)
|
|---|
| Length of Stay Groups |
2-3 Days |
4-6 Days |
7+ Days |
| |
Adjusted OR (95% CI) |
Adjusted OR (95% CI) |
Adjusted OR (95% CI) |
| Disease Status (Referent: No Diabetes or Dementia) |
|---|
| Diabetes |
1.12 (1.11-1.13)* |
1.22 (1.21-1.24)* |
1.33 (1.31-1.34)* |
| Dementia |
1.15 (1.13-1.18)* |
1.25 (1.22-1.28)* |
1.22 (1.20-1.26)* |
| Diabetes + Dementia |
1.20 (1.17-1.24)* |
1.42 (1.38-1.46)* |
1.49 (1.44-1.53)* |
Table 4.
Hospital Discharge Characteristics of Matched Cases and Controls for Hospitalizations, Los Angeles County, 2019-2021.
Table 4.
Hospital Discharge Characteristics of Matched Cases and Controls for Hospitalizations, Los Angeles County, 2019-2021.
Table 5.
Crude Conditional Logistic Regression Analyses – Association Between Disease Status and Diabetic Complications for Hospitalizations, Los Angeles County, 2019-2021.
Table 5.
Crude Conditional Logistic Regression Analyses – Association Between Disease Status and Diabetic Complications for Hospitalizations, Los Angeles County, 2019-2021.
| Diabetic Complications |
Matched Odds Ratio |
95% Confidence Interval |
p-value |
| Kidney Complications |
1.04 |
0.92-1.18 |
0.49 |
| Ophthalmic Complications |
0.69 |
0.47-1.02 |
0.06 |
| Neurologic Complications |
1.00 |
0.80-1.27 |
0.98 |
| Hypertensive Diseases |
0.81 |
0.67-0.97 |
0.02 |
| Low-Extremity Amputation Procedures |
1.36 |
0.73-2.55 |
0.33 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).