Submitted:
10 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search strategy
2.2. Inclusion and exclusion criteria
2.3. Article selection
2.4. Data extraction
2.5. Evaluation of methodological quality
2.6. Data analysis and synthesis
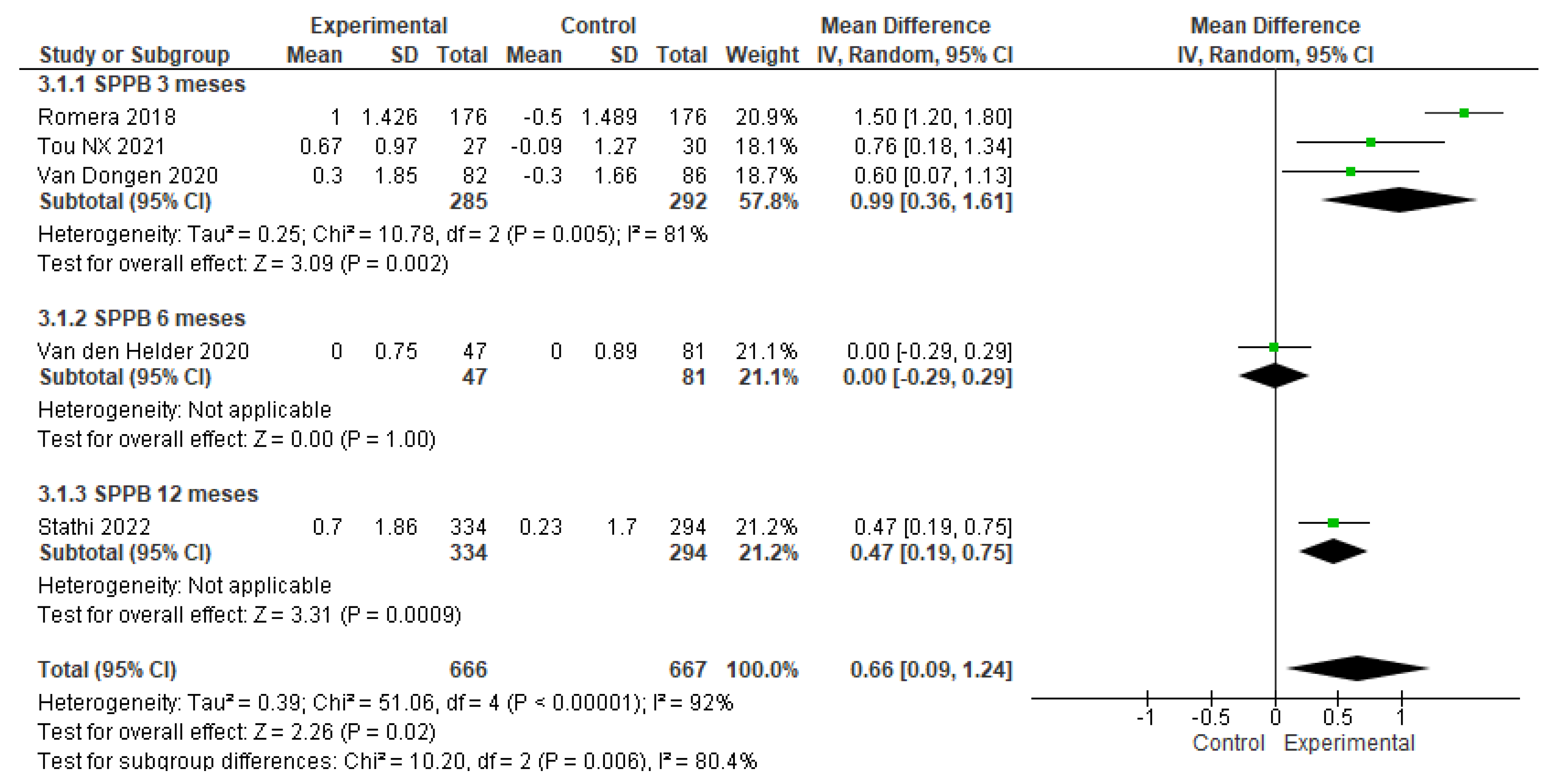
3. Results
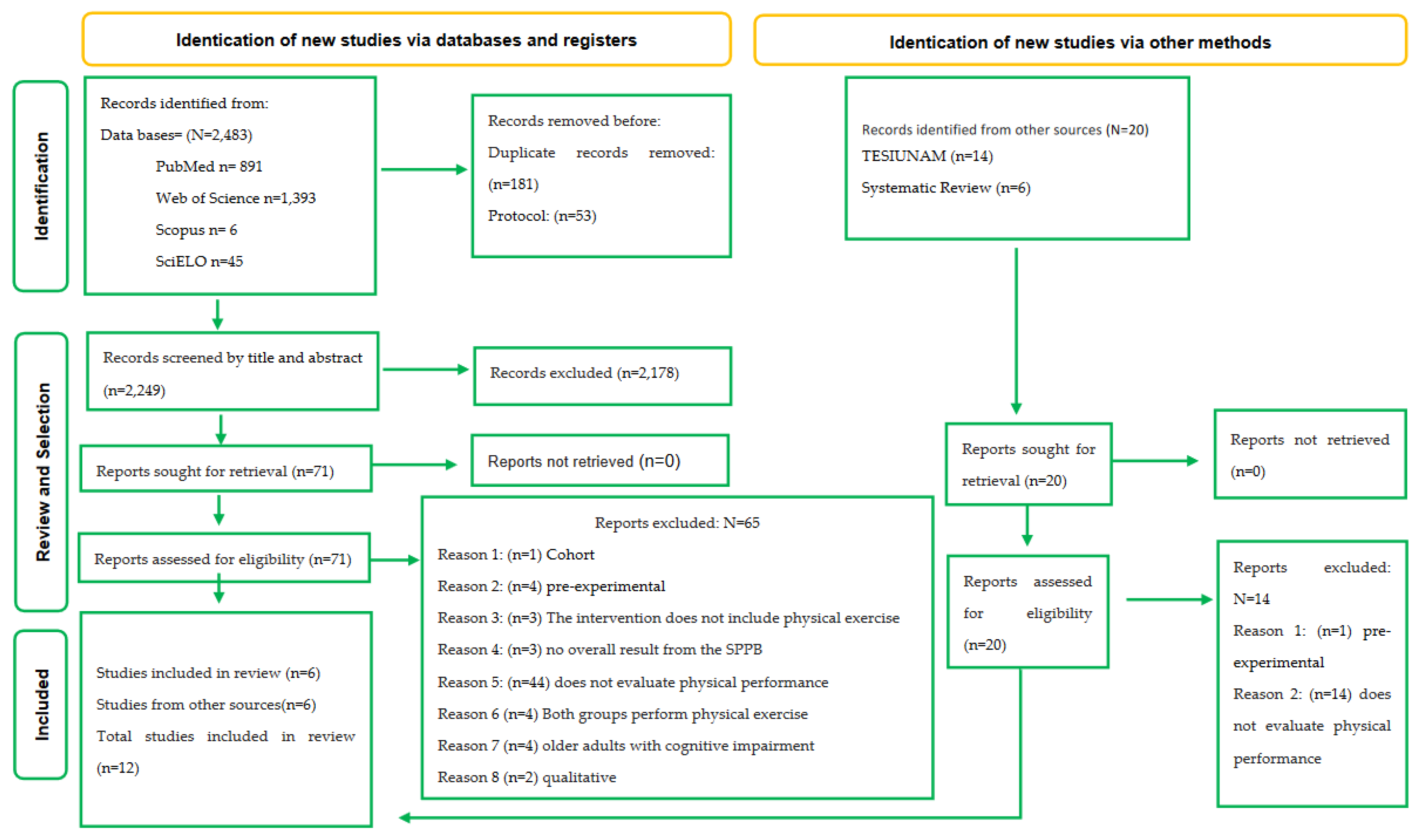
3.1. Literature search
3.2. Study characteristics
3.2.2. Intervention period
3.2.3. Intervention components
3.2.4. Intervention venues
3.2.5. Measuring results
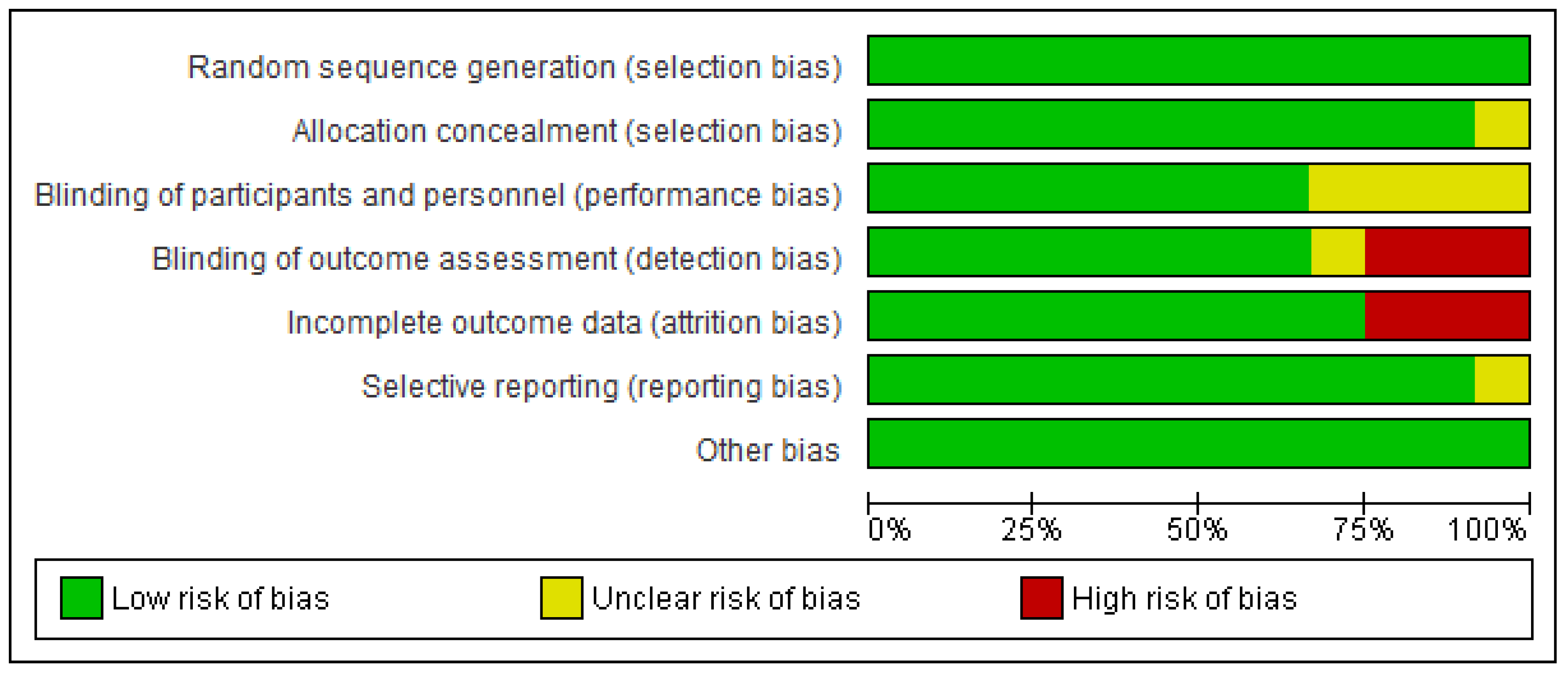
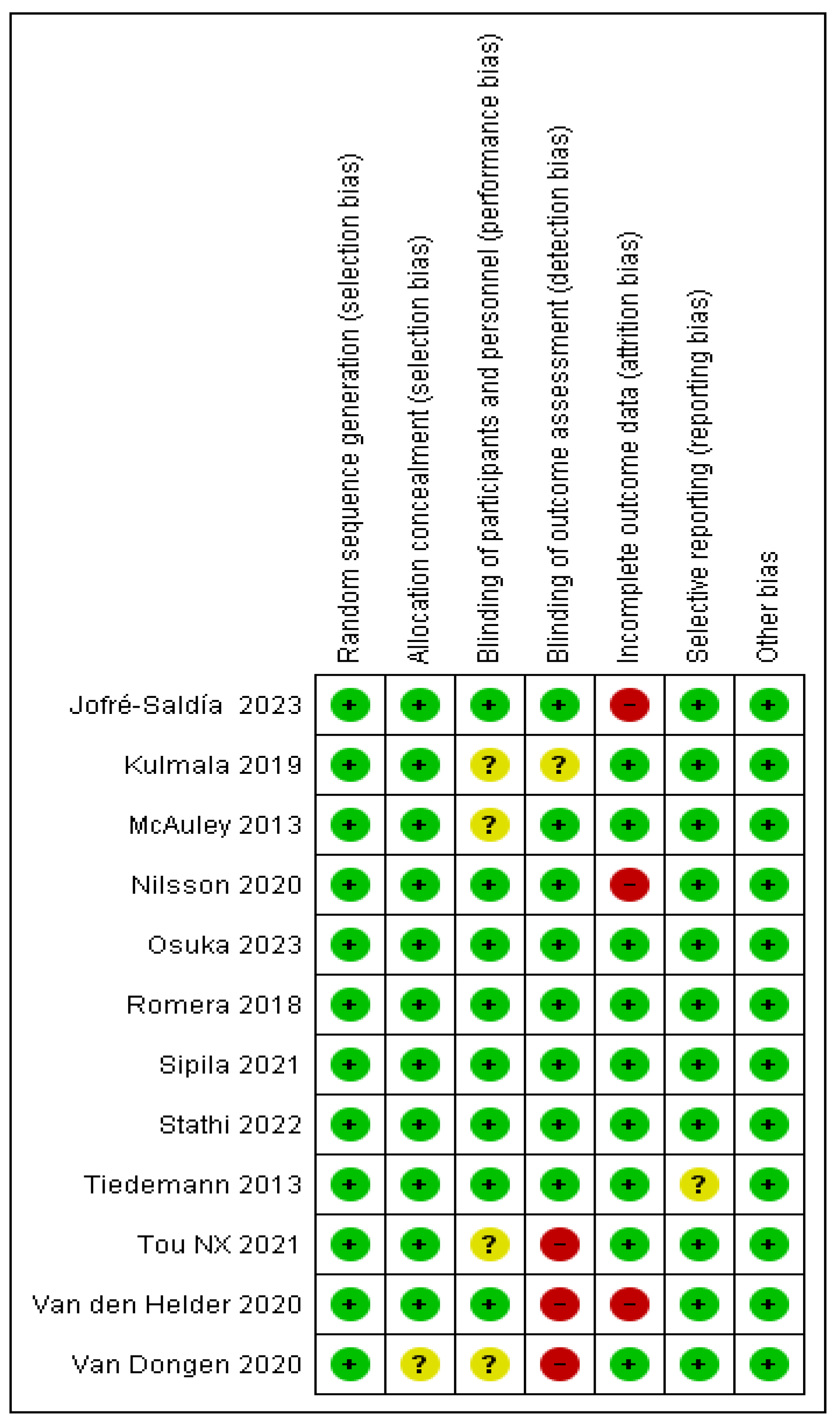
3.3. Assessing the quality of the studies
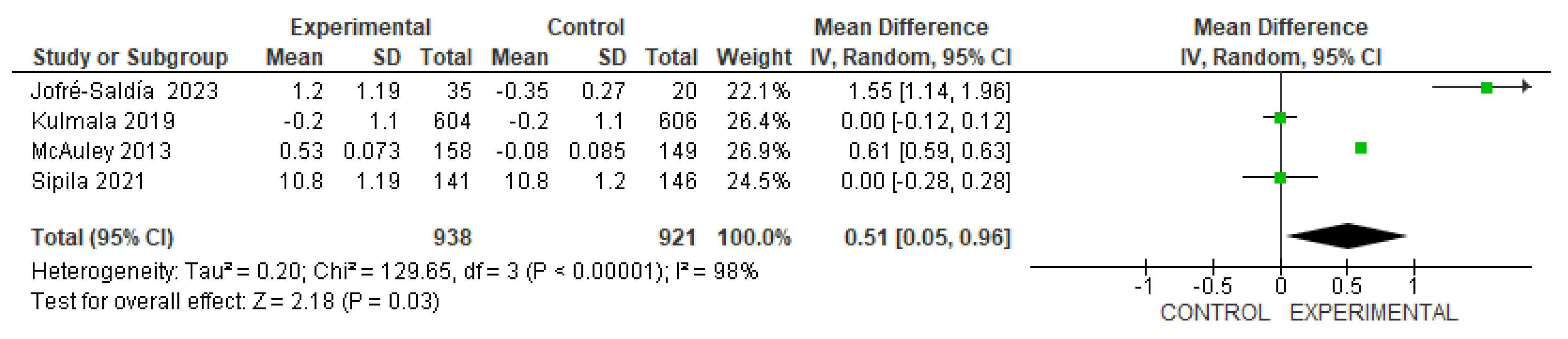
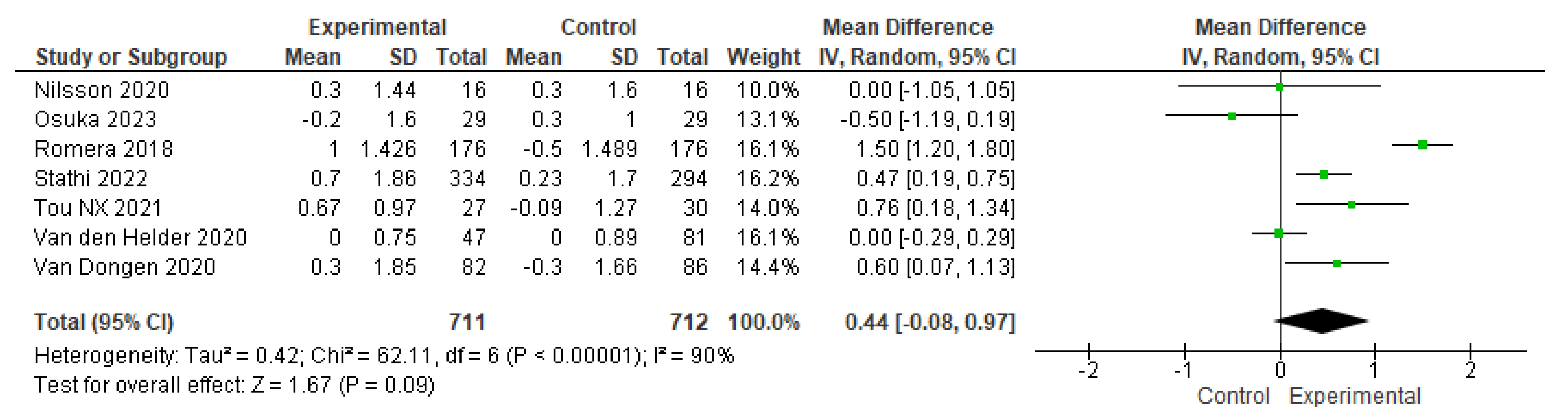
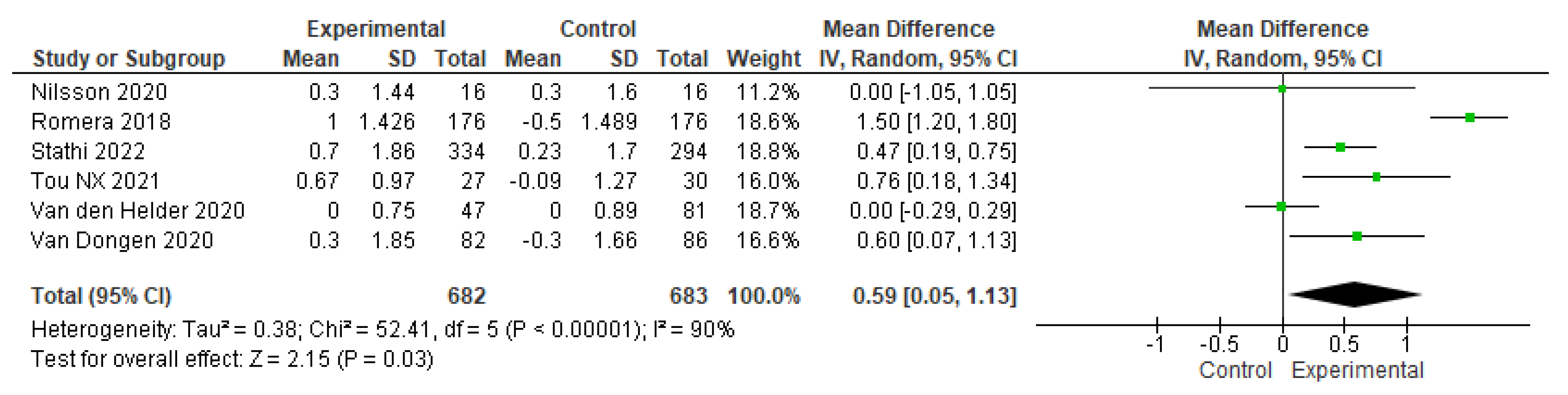
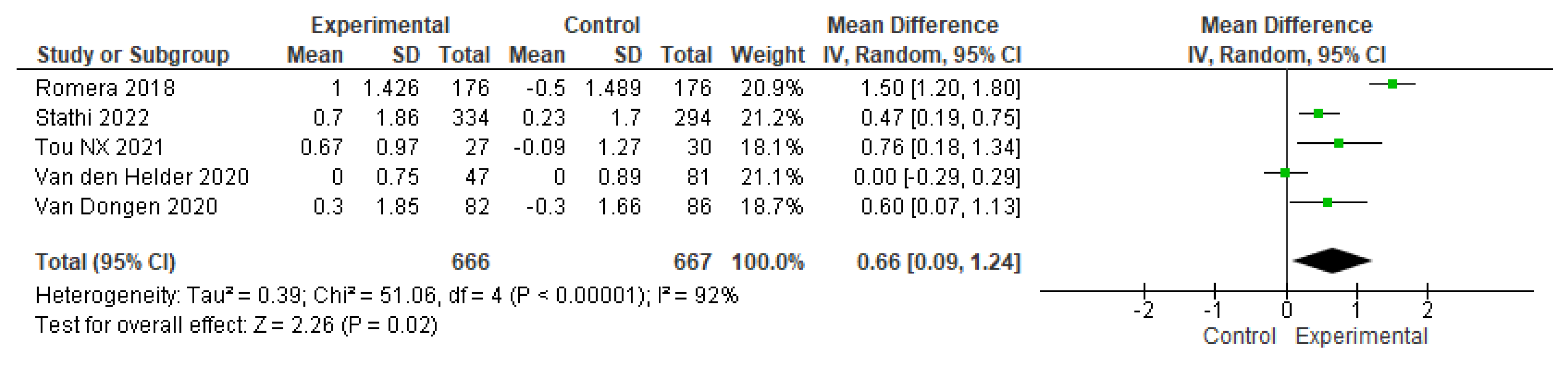
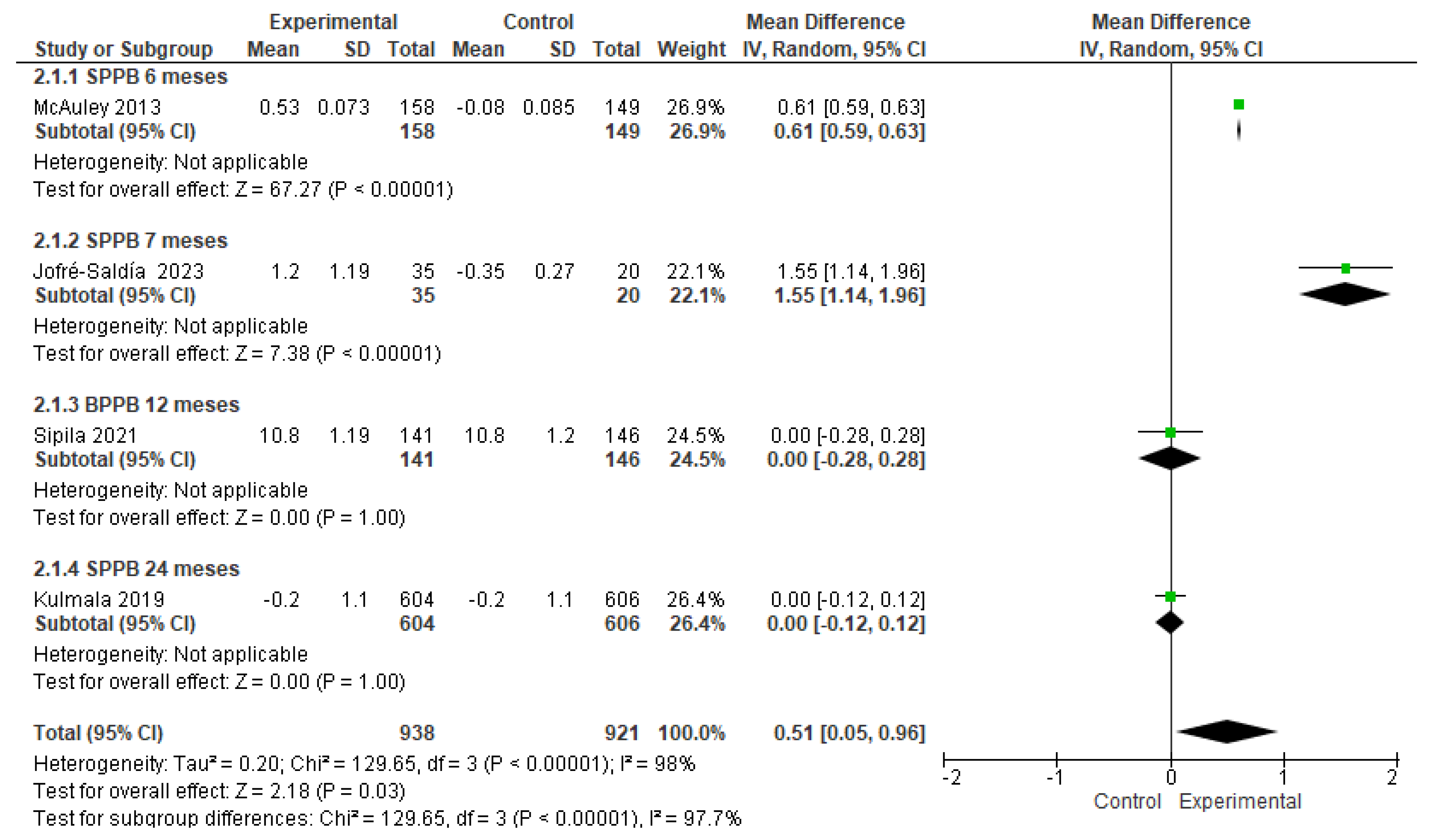
3.4. The effects of physical-training interventions on the performance of older adults
3.4.1. The effect of exercise on the physical performance of older adults without frailty
3.4.2. The effect of exercise on the physical performance of older adults with frailty
3.4.3. The effect of exercise training time on the physical performance of older adults without frailty
3.4.4. The effect of exercise training time on the physical performance of older adults with frailty
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Falck, R.S.; Percival, A.G.; Tai, D.; et al. International depiction of the cost of functional independence limitations among older adults living in the community: a systematic review and cost-of-impairment study. BMC Geriatr. 2022, 22, 815. [Google Scholar] [CrossRef]
- World Health Organization. World report on ageing and health. Geneva: World Health Organization, 2015. Available online: https://www.who.int/publications/i/item/9789241565042.
- World Health Organization. Integrated care for older people (ICOPE): Guidance for person-centred assessment and pathways in primary care. Geneva World Health Organization, 2019. Available online: https://www.who.int/publications/i/item/WHO-FWC-ALC-19.1.
- World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization, 2020. Available online: https://www.who.int/publications/i/item/9789240015128.
- Mosqueda-Fernández, A. Importancia de la realización de actividad física en la tercera edad. Dilemas contemporáneos: Educación, Política y Valores 2021, 9, 00036. [CrossRef]
- Acosta-Benito, M.Á.; Martín-Lesende, I. Fragilidad en atención primaria: diagnóstico y manejo multidisciplinar. Aten. Primaria 2022, 54, 102395. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- de Fátima Ribeiro Silva, C.; Ohara, D. G.; Matos, A. P.; et al. Short Physical Performance Battery as a Measure of Physical Performance and Mortality Predictor in Older Adults: A Comprehensive Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 10612. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Shen, J.; Li, M. Effects of multi-domain intervention on intrinsic capacity in older adults: A systematic review of randomized controlled trials (RCTs). Exp. Gerontol. 2023, 174, 112112. [Google Scholar] [CrossRef]
- Xi, P.; Ding, J.; Wan, S.; et al. A Meta-Analysis to Detect Efficacy of Physical Activity Interventions to Enhance Effects Related to the Fragility among Older Adults. Comput. Math. Methods Med. 2022, 3424972. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P. M.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, 790–799. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane 2023, Available online: https://training.cochrane.org/handbooks.
- Cochrane Training. ReviewManager (RevMan) Cochrane’s custom software for writing Cochrane reviews. Available online: https://training.cochrane.org/online-learning/core-software/revman.
- McAuley, E.; Wójcicki, T. R.; Learmonth, Y. C.; et al. Effects of a DVD-delivered exercise intervention on physical function in older adults with multiple sclerosis: A pilot randomized controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Jofré-Saldía, E.; Villalobos-Gorigoitía, Á.; Cofré-Bolados, C.; et al. Multicomponent Training in Progressive Phases Improves Functional Capacity, Physical Capacity, Quality of Life, and Exercise Motivation in Community-Dwelling Older Adults: A Randomized Clinical Trial. Int. J. Environ. Res. Public. Health 2023, 20, 2755. [Google Scholar] [CrossRef]
- Sipilä, S.; Tirkkonen, A.; Savikangas, T.; et al. Effects of physical and cognitive training on gait speed and cognition in older adults: A randomized controlled trial. Scand. J. Med. Sci. Sports 2021, 31, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, J.; Ngandu, T.; Havulinna, S.; et al. The Effect of Multidomain Lifestyle Intervention on Daily Functioning in Older People. J. Am. Geriatr. Soc. 2019, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, A.; O’Rourke, S.; Sesto, R.; et al. A 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2013, 68, 1068–1075. [Google Scholar] [CrossRef]
- Osuka, Y.; Sasai, H.; Kojima, N.; et al. Adherence, safety and potential effectiveness of a home-based Radio-Taiso exercise program in older adults with frailty: A pilot randomized controlled trial. Geriatr. Gerontol. Int. 2023, 23, 32–37. [Google Scholar] [CrossRef]
- Tou, N. X.; Wee, S. L.; Seah, W. T.; et al. Effectiveness of Community-Delivered Functional Power Training Program for Frail and Pre-frail Community-Dwelling Older Adults: a Randomized Controlled Study. Prev. Sci. 2021, 22, 1048–1059. [Google Scholar] [CrossRef]
- Stathi, A.; Greaves, C. J.; Thompson, J. L.; et al. Effect of a physical activity and behaviour maintenance programme on functional mobility decline in older adults: the REACT (Retirement in Action) randomised controlled trial. Lancet Public Health 2022, 7, e316–e326. [Google Scholar] [CrossRef]
- Romera-Liebana, L.; Orfila, F.; Segura, J. M.; et al. Effects of a Primary Care-Based Multifactorial Intervention on Physical and Cognitive Function in Frail, Elderly Individuals: A Randomized Controlled Trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2018, 73, 1688–1674. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M. I.; Mikhail, A.; Lan, L.; et al. A Five-Ingredient Nutritional Supplement and Home-Based Resistance Exercise Improve Lean Mass and Strength in Free-Living Elderly. Nutrients 2020, 12, 2391. [Google Scholar] [CrossRef]
- van den Helder, J.; Mehra, S.; van Dronkelaar, C.; et al. Blended home-based exercise and dietary protein in community-dwelling older adults: a cluster randomized controlled trial. J. Cachexia Sarcopenia Muscle 2020, 11, 1590–1602. [Google Scholar] [CrossRef]
- van Dongen, E.J.I.; Haveman-Nies, A.; Doets, E. L.; et al. Effectiveness of a Diet and Resistance Exercise Intervention on Muscle Health in Older Adults: ProMuscle in Practice. J. Am. Med. Dir. Assoc. 2020, 21, 1065–1072. [Google Scholar] [CrossRef]
- Salas-Groves, E.; Childress, A.; Albracht-Schulte, K.; et al. Effectiveness of Home-Based Exercise and Nutrition Programs for Senior Adults on Muscle Outcomes: A Scoping Review. Clin. Interv. Aging 2023, 18, 1067–1091. [Google Scholar] [CrossRef]
- Astrone, P.; Perracini, M.R.; Martin, F.C.; et al. The potential of assessment based on the WHO framework of intrinsic capacity in fragility fracture prevention. Aging Clin. Exp. Res. 2022, 34, 2635–2643. [Google Scholar] [CrossRef]
- Chhetri, J.K.; Xue, Q.L.; Ma, L.; et al. Intrinsic Capacity as a Determinant of Physical Resilience in Older Adults. J. Nutr. Health Aging 2021, 25, 1006–1011. [Google Scholar] [CrossRef]
- Belloni, G.; Cesari, M. Frailty and Intrinsic Capacity: Two Distinct but Related Constructs. Front. Med. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Blackwell, J.; Boraxbekk, C. J.; et al. Copenhagen Consensus statement 2019: physical activity and ageing. Br. J. Sports. Med. 2019, 53, 856–858. [Google Scholar] [CrossRef]
- Fien, S.; Linton, C.; Mitchell, J.S.; et al. of community-based exercise programs for community-dwelling older adults in rural/regional areas: a scoping review. Aging Clin. Exp. Res. 2022, 34, 1511–1528. [Google Scholar] [CrossRef]
- Haider, S.; Grabovac, I.; Dorner, T.E. Effects of physical activity interventions in frail and prefrail community-dwelling people on frailty status, muscle strength, physical performance and muscle mass-a narrative review. Wien. Klin. Wochenschr. 2019, 131, 244–254. [Google Scholar] [CrossRef]
- Blancafort Alias, S.; Cuevas-Lara, C.; Martínez-Velilla, N.; et al. A Multi-Domain Group-Based Intervention to Promote Physical Activity, Healthy Nutrition, and Psychological Wellbeing in Older People with Losses in Intrinsic Capacity: AMICOPE Development Study. Int. J. Environ. Res. Public Health 2021, 18, 5979. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, A.D.; Makwana, N.; Luscombe-Marsh, N.; et al. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI. Database System. Rev. Implement. Rep. 2018, 16, 752–775. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Bae, J. Correction: Does the combination of resistance training and a nutritional intervention have a synergic effect on muscle mass, strength, and physical function in older adults? A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 531. [Google Scholar] [CrossRef] [PubMed]
- Kis, O.; Buch, A.; Stern, N.; et al. Minimally supervised home-based resistance training and muscle function in older adults: A meta-analysis. Arch. Gerontol. Geriatr. 2019, 84, 103909. [Google Scholar] [CrossRef] [PubMed]
- Mahjur, M.; Norasteh, A. A. The Effect of Unsupervised Home-Based Exercise Training on Physical Functioning Outcomes in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2021, 23, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; et al. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Objective | Study design | Study population | Results/comparison |
|---|---|---|---|---|
| MacAuley et al. 2013 [15] | To Test the Effectiveness of a Home Exercise Program Provided on DVD on the Physical Function of Older Adults. | Randomized controlled trial | 307 community-dwelling older adults |
SPPB GE: Pre 10.38±0.118 vs. post 10.91± 0.13 GC: Pre 10.46 ± 0.14 vs. post 10.38 ± 0.13 p = 0.005 |
| Jofré-Saldía et al. 2023 [16] | To evaluate the effect of a progressive multicomponent training program on functional capacity in a group of older adults in the community. | Randomized controlled trial | 55 community-dwelling older adults. |
SPPB GE: Pre 10.60± 1.67 vs. post 11.80± O.47 GC: Pre 9.45± 2.63 vs. post 9.10 ± 2.90 p = 0.000 |
| Sipilä et al. 2021 [17] | To test whether the combination of physical and cognitive exercise has greater effects on walking speed compared to physical exercise training alone. | Randomized controlled trial | 287community-dwelling older adults |
SPPB GE: Pre 10.2± 0.1 vs. post 10.8±1.19 GC: Pre 10.1± 0.1 vs. Post 10.8 ± 1.2 p = 0.52 |
| Kulmala et al. 2019 [18] | To investigate the effect of a multidomain lifestyle intervention on the daily functioning of older people. | Randomized controlled trial | 1,260 community-dwelling older adults |
SPPB GE: Pre 10.8± 1.4 vs. post 10.8±1.4 GC: Pre 10.8± 1.4 vs. post 10.8±1.4 p = 0.00 |
| Tiedemann et al. 2013 [19] | To determine the effect of an Iyengar yoga program on balance and mobility in community-dwelling older people. | Randomized controlled trial | 54 community-dwelling older adults |
SPPB Equilibrio de pie GE: Pre 38.9 ± 2.8 vs. post 39.7 ± 0.99 GC: Pre 38.9 ± 3.1 vs. post 38.2 ± 5.2 p = 0.04 Sentarse y pararse GE: Pre 10.1 ± 3.8 vs. post 8.8 ± 2.6 GC: Pre 11.9 ± 5.2 vs. post 13.6 ± 6.1 p = 0.001 Caminata 4 metros GE: Pre 2.6 ± 0.6 vs. post 2.4 ± 0.4 GC: Pre 2.5 ± 0.6 vs. post 2.8 ± 0.6 p = 0.001 |
| Author (Year) | Objective | Study design | Study population | Results/comparison |
|---|---|---|---|---|
| Osuka et al. 2023 [20] | To Determine the Effectiveness of the Home-Based Radio-Taiso Exercise Program in Frail Older Adults. | Randomized controlled trial | 58 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: pre 6.2± 1.9 vs. post 6.0 ± 2.0 GC: pre 6.7 ± 2.1 vs. post 7.0 ± 1.8 p = 0.337 |
| Tou et al. 2021 [21] | To examine the effectiveness of a functional power exercise (FPT) program for community-dwelling pre-frail and frail older adults. | Randomized controlled trial | 57 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: pre 10.85 ± 1.46 vs. post 11.52 ± O.73 GC: pre 10.90 ± 1.65 vs. post 10.81 ± 2.00 p = 0.043 |
| Stathi et al. 2022 [22] | To establish whether a community-based active aging intervention can prevent declines in lower extremity physical functioning in older adults at risk of mobility limitation. | Randomized controlled trial | 628 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: pre 7.38± 1.58 vs. post 8.08 ± 2.87 GC: pre 7.36± 1.54 vs. post 7.59 ± 2.61 p = 0.014 |
| Romera-Liébana et al. 2018 [23] | To evaluate the effectiveness of a multidisciplinary intervention to modify physical and cognitive frailty parameters in older people. | Randomized controlled trial | 352 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: pre 7.1± 2.3 vs. post 8.1 ± 2.2 GC: pre 7.3± 2.4 vs. post 6.8 ± 2.3 p = 0.001 |
| Nilsson et al. 2020 [24] | To examine the effects of HBRE/MIS on muscle mass, strength, and function in community-dwelling older men | Randomized controlled trial | 45 varones community-dwelling older adults with pre-frailty and frailty |
SPPB GE: Pre 10.3± 0.3 vs. post 10.6±0.4 GC: Pre 11.0 ± 0.4 vs. post 11.3±0.4 p = 0.00 |
| Van den Helder et al. 2020 [25] | To determine the effectiveness of combined exercise (e-health + coaching) at home and a dietary protein intervention on physical performance in community-dwelling older adults. | Randomized controlled trial | 128 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: Pre 11.19± 1.2 vs. post 11.2±0.1 GC: Pre 11.26 ± 1.3 vs. post 11.3±0.1 p = 0.09 |
| Van Dongen et al. 2020 [26] | To evaluate the effectiveness of a dietary protein intervention combined with resistance exercise on physical functioning in older adults. | Randomized controlled trial | 168 community-dwelling older adults with pre-frailty and frailty |
SPPB GE: Pre 10.1 vs. post 10.4 GC: Pre 10.2 vs. post 9.9 p = 0.04 |
| AUTHOR (YEAR) | CHARACTERISTICS | TIME |
|---|---|---|
| MacAuley et al. 2013 [15] | Multicomponent training program Progressive strengthening exercises delivered on DVD disc to do at home, focused on flexibility, strength and balance (FlexToBa). • Balance (standing on one foot while holding a chair, triceps extension while balancing on one leg) • Flexibility (hamstring stretch). • Strengthening (bicep curls and shoulder press), resistance bands are used |
Time: 24 weeks Frequency: 3 days a week Duration: Does not mention |
| Jofré-Saldía et al. 2023 [16] | Multicomponent training program Balance and flexibility: through exercises with bosu, mini bosu, minitramp, and fitball Resistance (elastic bands and medicine ball) Cardiorespiratory capacity: walking training in a room with dimensions of 20 m long by 10 m wide |
Time: 27 weeks divided into 3 phases of 9 weeks each Frequency: 2 days a week Duration: 1st phase: Strength exercises: 45 minutes 2nd phase: Exercises for cardiorespiratory endurance: 50 minutes 3rd phase: exercises for balance and flexibility 60 minutes |
| Sipilä et al. 2021 [17] | Multicomponent training program Supervised training sessions and home exercises. Training periods had variations in training specificity, volume, and intensity. Pneumatic resistance training machines were used for resistance exercises. • Postural balance •Muscular strength •Endurance |
Time: 12 months Frequency: 2 days per week supervised training sessions and home exercise 2 to 3 times per week. -Walking and balance: 1 day per week -Stamina and balance: 1 day per week Duration: -Walk 150 minutes per week -Balance: 45 minutes -Stamina and balance: 1 hour |
| Kulmala et al. 2019 [18] | Multicomponent training program: • Postural balance. • Strength: exercises for the eight major muscle groups (knee extension and flexion, abdominal and back muscles, rotation, upper back and arm muscles, using bench press for lower extremity muscles. • Individual and group aerobics such as Nordic walking, aqua gymnastics, jogging and gymnastics. |
Time: 24 months Frequency: -Progressive muscle strength training: 1-3 times per week -Aerobic exercise: 2-5 times per week Duration: 40 -60 minutes |
| Tiedemann et al. 2013 [19] | Group Iyengar yoga sessions. focused on standing postures to improve flexibility and muscle strength. The balance challenge increased over time by gradually increasing the difficulty of the postures performed. 1. “Utkatasana” Chair Pose 2. “Trikonasana” Triangle Pose Modification: A block or chair is placed under the lower hand if required or the pose can be performed with back to the wall for support where needed 3. “Virabhadrasana 1” Warrior 1 4. “Virabhadarasana 2” Warrior 2 5. “Virabhadrasana 3” Warrior 3Modification: Pose performed next to a wall for support if needed 6. “Vriksasana” Tree Pose Modification: Pose performed next to a wall for support if needed 7. “Adha Chandrasana” Half Moon Pose Modification: A block or chair is placed under the lower hand if required or the pose can be performed with back to the wall for support where needed |
Time: 12 weeks Frequency: 2 days per week Duration: 1 hour group session |
| AUTHOR (YEAR) | CHARACTERISTICS | TIME |
|---|---|---|
| Osuka et al. 2023 [20] | Multicomponent physical exercise: Balance, strength, endurance, flexibility and coordination, broadcast daily on public radio and television. From 8 to 13 rhythmic movements with music: • Radio-Taiso at home. • Radio-Taiso no. 1 • Radio-Taiso no. 2 • Minna no Taiso |
Time: 12 weeks Frequency: 1-4 times a day Duration: 10 minutes |
| Tou et al. 2021 [21] | Progressive power and balance exercises targeting upper and lower body muscles Sit to Stand/Squat. Knee Ups (Hip Flexion) Calf + Toe Raises Knee Extension Seated Heel Drag/Hamstring Curl (Knee Flexion) Hip Extension Hip Abduction For the power training, body weight and/or resistance bands were used as resistance and participants were instructed to move as fast as they can during the concentric phase and slowly during the eccentric phase (approximately 3s) of the exercise movements. |
Time: 12 weeks Frequency: 2 sessions per week Duration: 60 minutes |
| Stathi et al. 2022 [22] | Multicomponent training program • Balance • Lower extremity muscle strength • Cardiorespiratory Capacity • Coordination and flexibility |
Time: 52 weeks Frequency: 2 sessions per week Duration: 60 minutes |
| Romera-Liébana et al. 2018 [23] | Aerobic exercise program | Time: 12 weeks Frequency: 2 sessions per week Duration: 60 minutes |
| Nilsson et al. 2020 [24] | Strengthening for the lower and upper body. Home resistance bands, biceps curl, triceps extension, lateral raise, seated row, bench press, sit-ups, calf raise, chair squat, knee extension, knee flexion, knee flexion were used for training. hip, and back flexion and walk at least 5,000 steps on exercise days and 10,000 steps on rest days |
Time: 12 weeks Frequency: 3 days per week Duration: does not refer |
| Van den Helder et al. 2020 [25] | Multicomponent training program Progressive functional training at home. Focused on improving the frequency and intensity of functional activities of daily living (climbing stairs, getting up from a chair, and shopping). For its application, a tablet PC is provided with the personalized training program. - Domain 1. Strength (strength in torso and extremities) - Domain 2. Endurance (cardiorespiratory fitness) - Domain 3. Flexibility (flexibility and range of motion of the torso and extremities) - Domain 4. Balance and coordination (neuromotor skills) |
Time: 6 months Frequency: 2 times a week Duration 45 minutes |
| Van Dongen et al. 2020 [26] | Progressive resistance exercises • Leg press, leg extension, lat pulldown, upright row and chest press using leg and chest press machines to work muscle groups |
Time: 3 months Frequency: 2 times a week Duration: 60 minutes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





