Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Lignin extraction and color reduction process
2.3. Moisture, ashes, total lignin, and carbohydrates content
2.4. Color
2.5. Structural characterization
2.5.1. Attenuated Total Reflectance – Fourier-transform Infrared spectroscopy (ATR-FTIR)
2.6. Differential scanning calorimetry (DSC)
2.7. Particle size
2.8. Gel Permeation Chromatography (GPC)
2.9. Solubility
2.10. Biological activity
2.10.1. Total phenolic content (TPC)
2.10.2. Antioxidant activity
2.11. Emulsion stability index (ESI)
2.12. Safety assessment
2.12.1. Cytotoxicity
2.12.2. Mutagenicity
2.12.3. Skin sensitization
2.13. Accelerated stability
2.13.1. Oil-in-water (o/w) emulsion preparation
2.13.2. Organoleptic evaluation
2.13.3. Physico-chemical evaluation
2.13.4. Antioxidant activity
2.13.5. Microbial contamination
3. Results and discussion
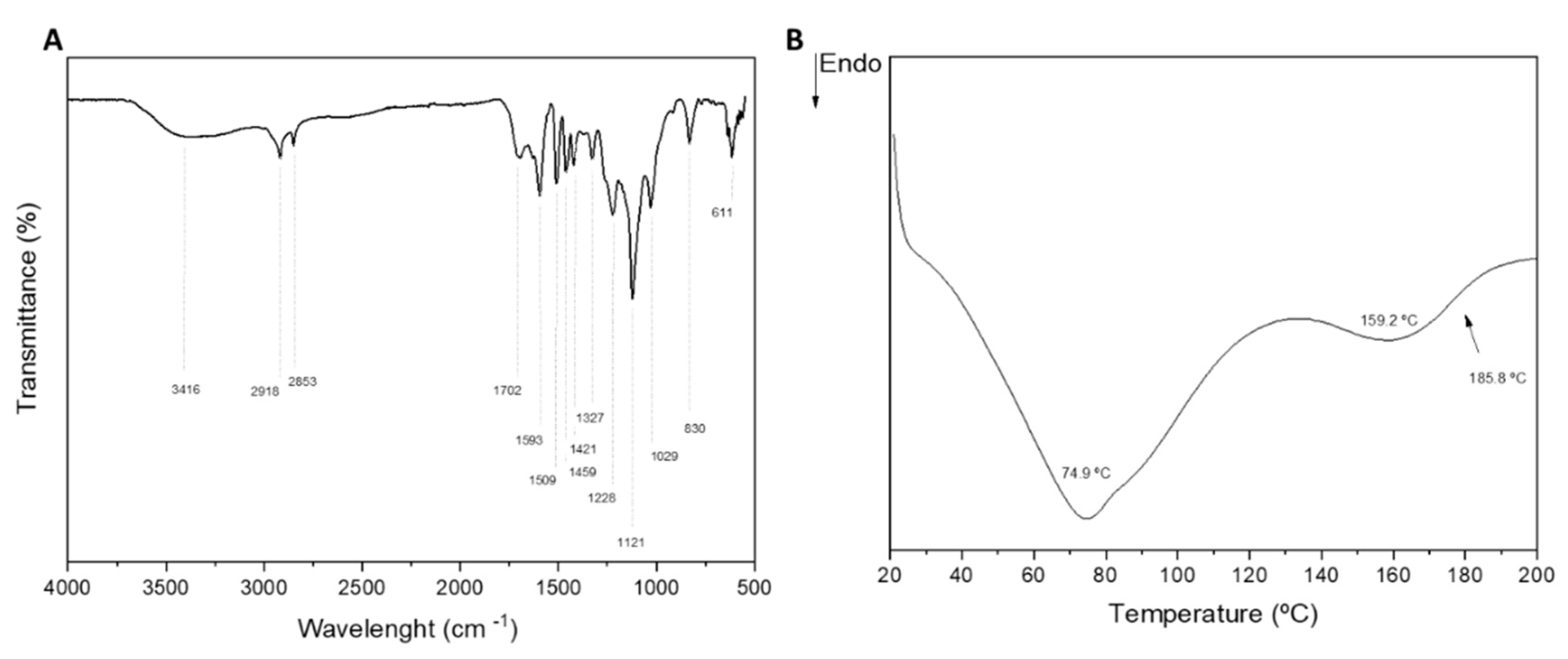
3.1. Physico-chemical, structural, and thermal analyses
3.2. Particle size and molecular weight
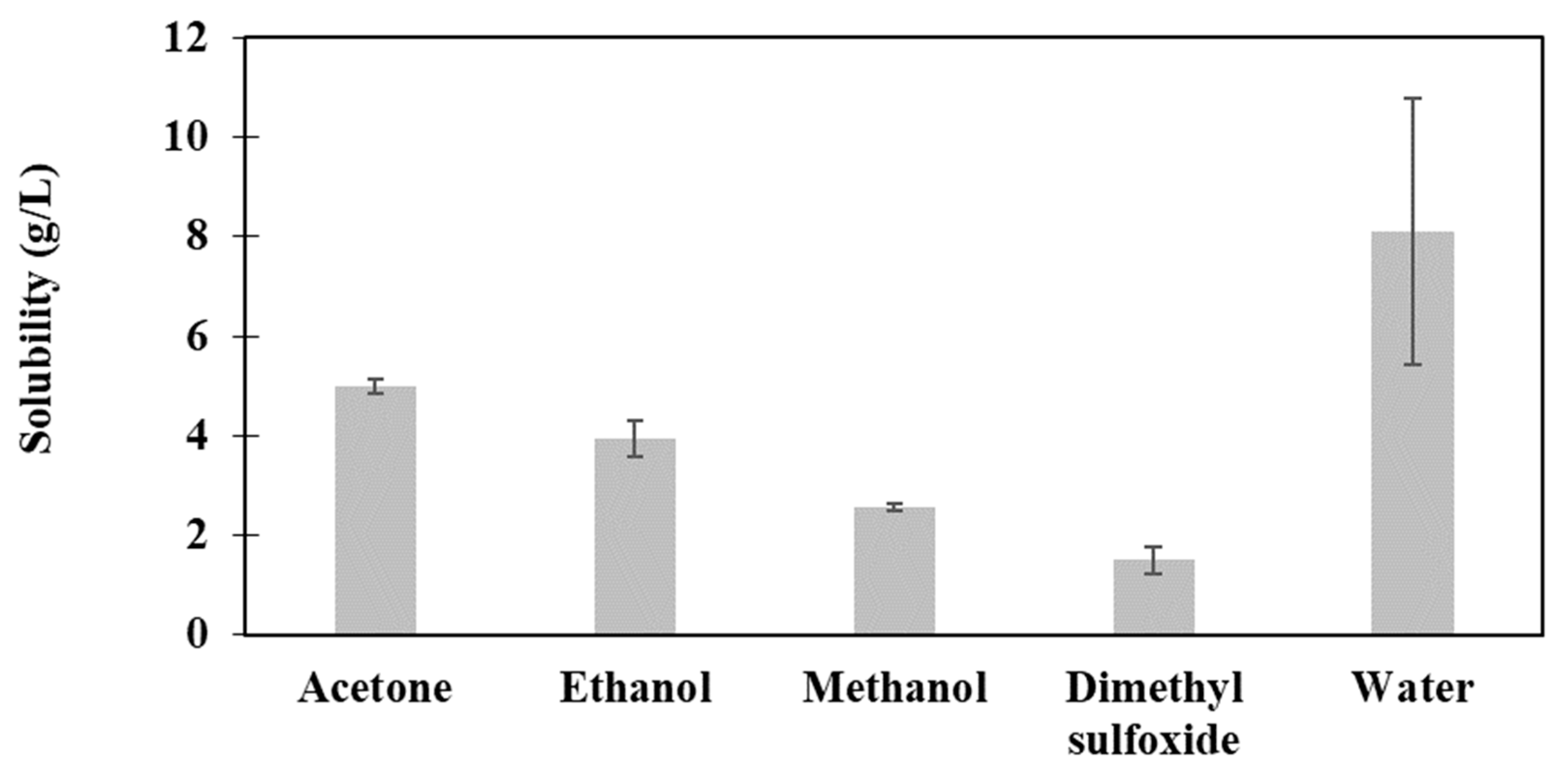
3.3. Solubility
3.4. Total phenolic compounds (TPC) and antioxidant potential
3.5. Emulsion stability index (ESI)
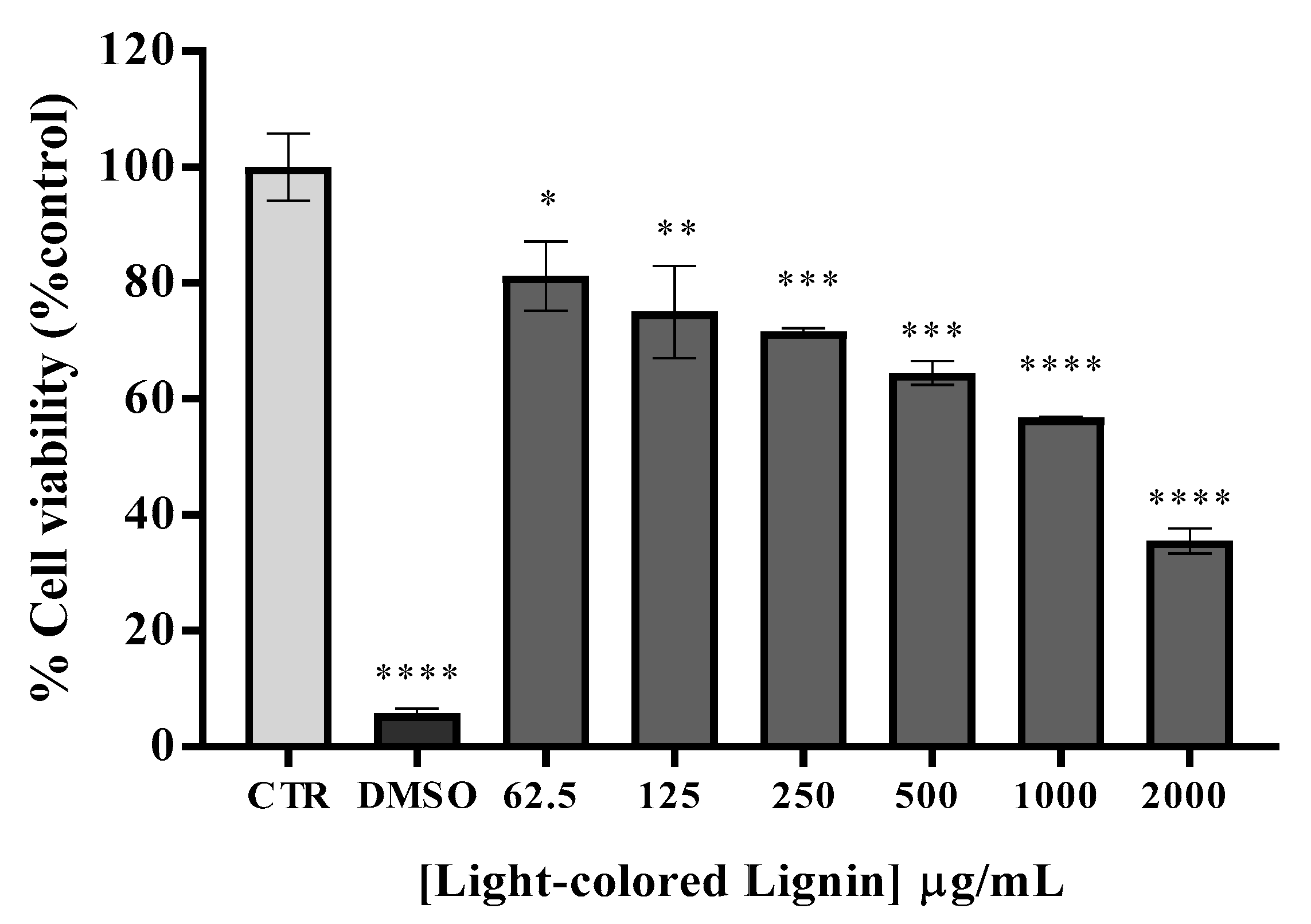
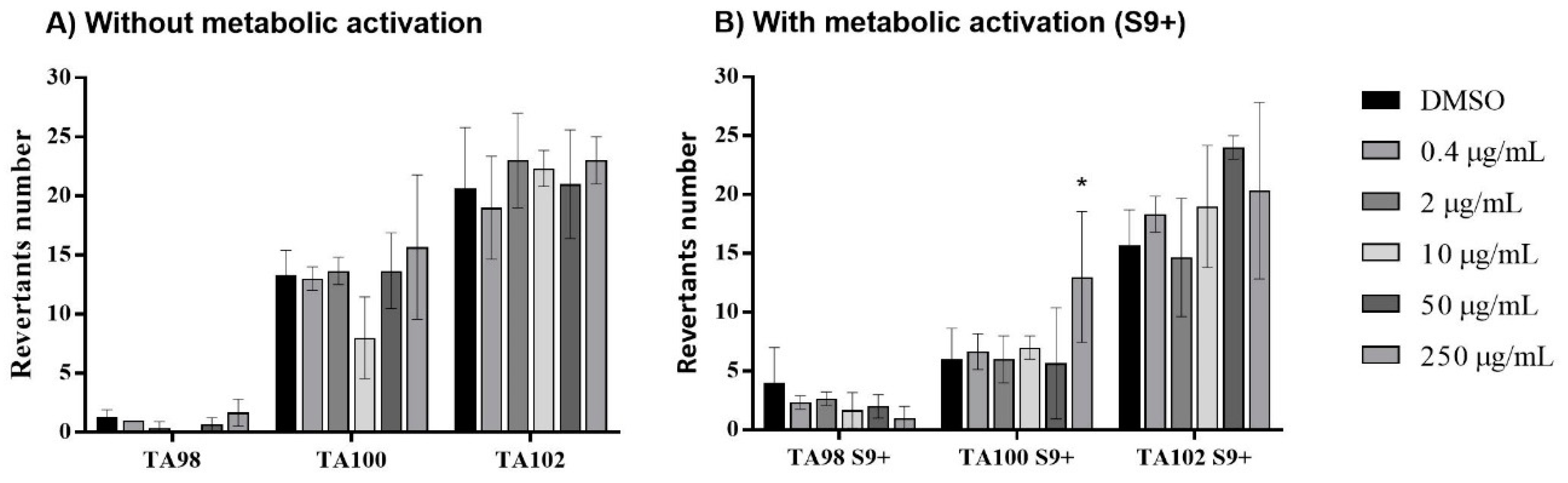
3.6. Cytotoxicity, mutagenicity, and skin sensitization
3.7. Accelerated stability
4. Conclusion
CRediT authorship contribution statement
Acknowledgments
References
- G.J. Gil-Chávez, S.S.P. Padhi, C. V. Pereira, J.N. Guerreiro, A.A. Matias, I. Smirnova, Cytotoxicity and biological capacity of sulfur-free lignins obtained in novel biorefining process, Int J Biol Macromol. 136 (2019) 697–703. [CrossRef]
- P.M.A. Vinardell, M. Montserrat, Lignins and Their Derivatives with Beneficial Effects on Human Health, Int J Mol Sci. 18 (2017) 1219. [CrossRef]
- J.L. Espinoza-Acosta, P.I. Torres-Chávez, B. Ramírez-Wong, C.M. López-Saiz, B. Montaño-Leyva, Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications, Bioresources. 11 (2016) 5452–5481. [CrossRef]
- M.P. Vinardell, V. Ugartondo, M. Mitjans, Potential applications of antioxidant lignins from different sources, Ind Crops Prod. 27 (2008) 220–223. [CrossRef]
- N. Ratanasumarn, P. Chitprasert, Cosmetic potential of lignin extracts from alkaline-treated sugarcane bagasse: Optimization of extraction conditions using response surface methodology, Int J Biol Macromol. 153 (2020) 138–145. [CrossRef]
- Ajao, J. Jeaidi, M. Benali, A. Restrepo, N. El Mehdi, Y. Boumghar, Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications, Molecules. 23 (2018) 377. [CrossRef]
- J. Zheng, L. Chen, X. Qiu, Y. Liu, Y. Qin, Structure investigation of light-colored lignin extracted by Lewis acid-based deep eutectic solvent from softwood, Bioresour Technol. 385 (2023) 129458. [CrossRef]
- S. Falkehag, Modified lignin surfactants, US Patent US3763139A, 02 October 1973, 1973. https://patentimages.storage.googleapis.com/25/3b/5a/d99a7c889348dd/US3763139.pdf (accessed May 4, 2023).
- S.I. Falkehag, H.H. Moorer, C.W. Bailey, Alkylene chlorohydrin,oxide or carbonate modified sulfonated lignins in a disperse or vat dye cake, US Patent 3672817A, 27 June 1972, 1972. https://patentimages.storage.googleapis.com/6d/48/24/516ce9e694a38f/US3672817.pdf (accessed May 4, 2023).
- S.Y. Lin, Process for Reduction of Lignin Color, US Patent 4184845A, 22 January 1980, 1980. https://patentimages.storage.googleapis.com/f4/0a/fe/816bfc1b2194bb/US4184845.pdf (accessed May 4, 2023).
- P. Dilling, P. Sarjeant, Reduction of lignin color, US Patent 4454066, 12 June 1984, 1972. https://patentimages.storage.googleapis.com/77/fd/89/204cb7b9726ae3/US4454066.pdf (accessed May 4, 2023).
- H. Zhang, Y. Bai, W. Zhou, F. Chen, Color reduction of sulfonated eucalyptus kraft lignin, Int J Biol Macromol. 97 (2017) 201–208. [CrossRef]
- R. Li, D. Huang, S. Chen, L. Lei, Y. Chen, J. Tao, W. Zhou, G. Wang, Insight into the self-assembly process of bamboo lignin purified by solvent fractionation to form uniform nanospheres with excellent UV resistance, Colloids Surf A Physicochem Eng Asp. 642 (2022) 128652. [CrossRef]
- H. Zhang, S. Fu, Y. Chen, Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations, Int J Biol Macromol. 147 (2020) 607–615. [CrossRef]
- H. Zhang, Y. Bai, B. Yu, X. Liu, F. Chen, A practicable process for lignin color reduction: fractionation of lignin using methanol/water as a solvent, Green Chemistry. 19 (2017) 5152–5162. [CrossRef]
- J. Wang, Y. Deng, Y. Qian, X. Qiu, Y. Ren, D. Yang, Reduction of lignin color via one-step UV irradiation, Green Chemistry. 18 (2016) 695–699. [CrossRef]
- V. Saritha, Y.A. Maruthi, K. Mukkanti, Biological Decolourization of Higher Concentrations of Synthetic Lignin by Native Fungi, 1 (2010) 1–4.
- B. Jiang, Y. Zhang, L. Gu, W. Wu, H. Zhao, Y. Jin, Structural elucidation and antioxidant activity of lignin isolated from rice straw and alkali-oxygen black liquor, Int J Biol Macromol. 116 (2018) 513–519. [CrossRef]
- H. Sadeghifar, Lignin as A Natural Sunscreen-An Overview, (2020) 20389–20391. [CrossRef]
- M.H. Tran, D.-P. Phan, E.Y. Lee, Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens, Green Chemistry. 23 (2021) 4633–4646. [CrossRef]
- H. Zhang, X. Liu, S. Fu, Y. Chen, High-value utilization of kraft lignin: Color reduction and evaluation as sunscreen ingredient, Int J Biol Macromol. 133 (2019) 86–92. [CrossRef]
- Z. Ahmad, W.W. Al Dajani, M. Paleologou, C. Xu, Sustainable process for the depolymerization/oxidation of softwood and hardwood kraft lignins using hydrogen peroxide under ambient conditions, Molecules. 25 (2020) 1–19. [CrossRef]
- S. Tripathi, N. Sharma, I. Alam, N.K. Bhardwaj, Effectiveness of different green chemistry approaches during mixed hardwood bamboo pulp bleaching and their impact on environment, International Journal of Environmental Science and Technology. 16 (2019) 4327–4338. [CrossRef]
- K. Dölle, A. Honig, Laboratory Bleaching System for Oxygen and Ozone Bleaching, Asian Journal of Chemical Sciences. 4 (2018) 1–12. [CrossRef]
- J.F. Kadla, H. Chang, The Reactions of Peroxides with Lignin and Lignin Model Compounds, in: Oxidative Delignification Chemistry, American Chemical Society, 2001: pp. 108–129. [CrossRef]
- Y. Li, Q. Fu, R. Rojas, M. Yan, M. Lawoko, L. Berglund, Lignin-Retaining Transparent Wood, ChemSusChem. 10 (2017) 3445–3451. [CrossRef]
- P. Bisht, K.K. Pandey, H.C. Barshilia, Photostable transparent wood composite functionalized with an UV-absorber, Polym Degrad Stab. 189 (2021) 109600. [CrossRef]
- Wachter, S. Tomas, P. Rantuch, J. Martinka, A. Pastierova, Effect of UV Radiation on Optical Properties and Hardness of Transparent Wood, Polymers (Basel). 13 (2021) 2067. [CrossRef]
- Y. Li, Z. Cai, M. Liao, J. Long, W. Zhao, Y. Chen, X. Li, Catalytic depolymerization of organosolv sugarcane bagasse lignin in cooperative ionic liquid pairs, Catal Today. 298 (2017) 168–174. [CrossRef]
- C.A.E. Costa, P.C.R. Pinto, A.E. Rodrigues, Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin, Ind Crops Prod. 61 (2014) 479–491. [CrossRef]
- I.F. Mota, P.R. Pinto, A.M. Ribeiro, J.M. Loureiro, A.E. Rodrigues, Downstream processing of an oxidized industrial kraft liquor by membrane fractionation for vanillin and syringaldehyde recovery, Sep Purif Technol. 197 (2018). [CrossRef]
- A.A. Vilas-Boas, D.A. Campos, C. Nunes, S. Ribeiro, J. Nunes, A. Oliveira, M. Pintado, Polyphenol extraction by different techniques for valorisation of non-compliant portuguese sweet cherries towards a novel antioxidant extract, Sustainability (Switzerland). 12 (2020). [CrossRef]
- F. Antunes, I.F. Mota, J.F. Fangueiro, G. Lopes, M. Pintado, P.S. Costa, From sugarcane to skin: Lignin as a multifunctional ingredient for cosmetic application, Int J Biol Macromol. 234 (2023) 123592. [CrossRef]
- M. del M. Contreras, B. Hernández-Ledesma, L. Amigo, P.J. Martín-Álvarez, I. Recio, Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology, Lwt. 44 (2011) 9–15. [CrossRef]
- B. Goncalves, V. Falco, J. Moutinho-Pereira, E. Bacelar, F. Peixoto, C. Correia, Effects of elevated CO2 on grapevine (Vitis vinifera L.): volatile composition, phenolic content, and in vitro antioxidant activity of red wine., J Agric Food Chem. 57 (2009) 265–273. [CrossRef]
- S.J. Choi, J.W. Won, K.M. Park, P.-S. Chang, A New Method for Determining the Emulsion Stability Index by Backscattering Light Detection, J Food Process Eng. 37 (2014) 229–236. [CrossRef]
- International Organization for Standardization (ISO) 17516:2014 — Microbiological limits for cosmetics, 2014.
- X. Lu, X. Gu, Y. Shi, A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications, Int J Biol Macromol. 210 (2022) 716–741. [CrossRef]
- E. Cequier, Extraction and characterization of lignin from olive pomace : a comparison study among ionic liquid, sulfuric acid, and alkaline treatments, (2019) 241–252.
- C. Heitner, D. Dimmel, J. Schmidt, Lignin and Lignans:Advances in Chemistry, 1st ed., CRC Press, 2010. [CrossRef]
- L. An, C. Si, G. Wang, W. Sui, Z. Tao, Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis, Ind Crops Prod. 128 (2019) 177–185. [CrossRef]
- R. Kaur, S.K. Uppal, Structural characterization and antioxidant activity of lignin from sugarcane bagasse, Colloid Polym Sci. 293 (2015) 2585–2592. [CrossRef]
- X.-F. Sun, H. Wang, G. Zhang, P. Fowler, M. Rajaratnam, Extraction and characterization of lignins from maize stem and sugarcane bagasse, J Appl Polym Sci. 120 (2011) 3587–3595. [CrossRef]
- L. Oliveira, D. Evtuguin, N. Cordeiro, A.J.D. Silvestre, Structural characterization of stalk lignin from banana plant, Ind Crops Prod. 29 (2009) 86–95. [CrossRef]
- S.Y. Lin, C.W. Dence, eds., Methods in Lignin Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 1992. [CrossRef]
- H. Yang, Z. Dong, B. Liu, Y. Chen, M. Gong, S. Li, H. Chen, A new insight of lignin pyrolysis mechanism based on functional group evolutions of solid char, Fuel. 288 (2021) 119719. [CrossRef]
- Mihai Brebu, C. Vasile, Thermal Degradation of Lignin – A Review, Cellulose Chem. Technol. 44 (2010) 353–363.
- J.C. Domínguez, M. Oliet, M. V Alonso, M.A. Gilarranz, F. Rodríguez, Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus, Ind Crops Prod. 27 (2008) 150–156. [CrossRef]
- N. Ramezani, M. Sain, Thermal and Physiochemical Characterization of Lignin Extracted from Wheat Straw by Organosolv Process, J Polym Environ. 26 (2018) 3109–3116. [CrossRef]
- W.G. Glasser, About Making Lignin Great Again—Some Lessons From the Past, Front Chem. 7 (2019) 1–17. [CrossRef]
- C. Cheng, J. Wang, D. Shen, J. Xue, S. Guan, S. Gu, K. Luo, Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review, Polymers (Basel). 9 (2017) 240. [CrossRef]
- V. Ponnuchamy, O. Gordobil, R.H. Diaz, A. Sandak, J. Sandak, Fractionation of lignin using organic solvents: A combined experimental and theoretical study, Int J Biol Macromol. 168 (2021) 792–805. [CrossRef]
- E.I. Evstigneyev, S.M. Shevchenko, Structure, chemical reactivity and solubility of lignin: a fresh look, Wood Sci Technol. 53 (2019) 7–47. [CrossRef]
- F. Antunes, I.F. Mota, J.F. Fangueiro, G. Lopes, M. Pintado, P.S. Costa, From sugarcane to skin: Lignin as a multifunctional ingredient for cosmetic application, Int J Biol Macromol. 234 (2023) 123592. [CrossRef]
- L. Cesari, F. Mutelet, L. Canabady-Rochelle, Antioxidant properties of phenolic surrogates of lignin depolymerisation, Ind Crops Prod. 129 (2019) 480–487. [CrossRef]
- R. Kaur, S.K. Uppal, P. Sharma, Antioxidant and Antibacterial Activities of Sugarcane Bagasse Lignin and Chemically Modified Lignins, Sugar Tech. 19 (2017) 675–680. [CrossRef]
- L.B. Brenelli, L.R.B. Mariutti, R. Villares Portugal, M.A. de Farias, N. Bragagnolo, A.Z. Mercadante, T.T. Franco, S.C. Rabelo, F.M. Squina, Modified lignin from sugarcane bagasse as an emulsifier in oil-in-water nanoemulsions, Ind Crops Prod. 167 (2021) 113532. [CrossRef]
- L. Bai, L.G. Greca, W. Xiang, J. Lehtonen, S. Huan, R.W.N. Nugroho, B.L. Tardy, O.J. Rojas, Adsorption and Assembly of Cellulosic and Lignin Colloids at Oil/Water Interfaces, Langmuir. 35 (2019) 571–588. [CrossRef]
- R. Kreiling, H. Gehrke, T.H. Broschard, B. Dreeßen, D. Eigler, D. Hart, V. Höpflinger, M. Kleber, J. Kupny, Q. Li, P. Ungeheuer, U.G. Sauer, In chemico, in vitro and in vivo comparison of the skin sensitizing potential of eight unsaturated and one saturated lipid compounds, Regulatory Toxicology and Pharmacology. 90 (2017) 262–276. [CrossRef]
| Commercial name | INCI | Function | Blank (%) | LCLig (%) |
|---|---|---|---|---|
| Part A (aqueous phase) | ||||
| Deionized water | Aqua | Solvent | 79.6 | 74.6 |
| Glycerin | Glycerin | Humectant | 5 | 5 |
| SolagumTM AX | Acacia Senegal Gum; Xanthan Gum | Thickening and stabilizing agent | 0.9 | 0.9 |
| LCLig | - | Active ingredient | - | 5 |
| Part B (oily phase) | ||||
| Lanette | Cetyl stearyl alcohol | Emulsifier | 2.5 | 2.5 |
| Tego® Care PBS 6 MB | Polyglyceryl-6 Distearate; Polyglyceryl-6 Behenate | Emulsifier | 4 | 4 |
| Shea butter | Butyrospermum parkii butter | Emollient | 2 | 2 |
| Squalane | Squalane | Emollient | 5 | 5 |
| Part C | ||||
| Euxyl PE 9010 | Phenoxyethanol and Ethylhexylglycerin | Preservative | 1 | 1 |
| Sample | Total lignin (wt%) | Carbohydrates(wt%) | Ash (wt%) |
Color CIELAB L*/ a* / b* |
Appearance |
|---|---|---|---|---|---|
| LCLig | 81.60 ± 3.60 | 3.50 ± 0.40 | 6.03 ± 0.01 | 73 / 2.5 / 24.5 |  |
| Aliphatic OH (mmol/glignin) | Carboxylic acids (mmol/glignin) | Phenolic units (mmol/glignin) | |||||
| Condensed | Non condensed | Total | |||||
| S | G | H | |||||
| δ 146.4 - 150.8 mg/L | δ 135.6 - 133.6 mg/L | δ 145.8-143.8 and 142.2-140.2 mg/L | δ 143.8 -142.2 mg/L | δ 140.2 -137.4 mg/L | δ 137.4 - 136.9 mg/L | ||
| LCLig | 3.73 | 1.76 | 0.13 | 0.10 | 0.63 | 0.04 | 0.89 |
| Sample | Peak max (°C) | Enthalpy (J/g) | Peak Height (mW/mg) |
|---|---|---|---|
| LCLig | 74.9 159.2 |
-65.76 -8.367 |
-0.6585 -8.367 |
| Sample | Particle size (mm) | Mw (g/mol) | Mn (g/mol) | PD | ||
|---|---|---|---|---|---|---|
| Dv(10) | Dv(50) | Dv(90) | ||||
| LCLig | 2.83 ± 0.01 | 8.07 ± 0.02 | 27.24 ± 0.32 | 14300 ± 2059 | 10598 ± 1577 | 1.35 |
| Lignin | TPC (mg GAE/g) |
ORAC (µmol TE/g) |
ABTS * IC50 (mg/mL) |
|---|---|---|---|
| LCLig | 169.3 ± 40.9 | 2571.5 ± 826 | 1.28 ± 0.13 |
| LCLig (wt%) | ESI (%) |
|---|---|
| 1.0 | 57.39 ± 1.33 |
| 2.5 | 65.49 ± 0.04 |
| 5.0 | 79.59 ± 2.56 |
| 7.5 | 85.52 ± 6.34 |
| 10.0 | 100.00 ± 0.00 |
| Sample | Conc. (mg/mL) | Cys % depletion |
Cys and lys % depletion |
Reactivity (cys) |
Reactivity class | DPRA Prediction |
|---|---|---|---|---|---|---|
| Cynamaldehyde (Positive control) |
13.2 | 76 ± 3 | 66 ± 1 | Moderate | High reactivity | Sensitizer |
| LCLig | 1.40 | 6.0 ± 0.8 | 3.4 ± 0.4 | Minimal | Minimal | No Sensitizer |
| 0.75 | 5.7 ± 0.6 | 2.8 ± 0.3 | ||||
| 0.35 | 5.9 ± 0.4 | 2.8 ± 0.2 |
| Parameter | T (°C) | Blank o/w emulsion | 5 wt% LCLig o/w emulsion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 1 Month | 2 Months | 3 Months | Initial | 1 Month | 2 Months | 3 Months | ||
| Physical appearance | 4 | Homogeneous and smooth consistency; good spreadability | Homogeneous and smooth consistency | ||||||
| 25 | |||||||||
| 40 | |||||||||
| ColorL*a*b* | 4 | 90.42 -0.682.88 | 81.65-0.843.32 | 82.92-0.372.00 | 87.43-0.682.46 | 55.17.425.7 | 53.005.7020.46 | 52.545..3823.29 | 53.137.1923.48 |
| 25 | 83.53-0.964.05 | 84.99-0.482.85 | 86.81-0.63.51 | 52.545.3820.38 | 52.607.0223.29 | 52.746.7621.47 | |||
| 40 | 81.87-0.833.68 | 84.43-0.532.89 | 88.07-0.973.42 | 51.745.3319.66 | 52.136.8821.28 | 52.776.8420.87 | |||
| pH | 4 | 5.33 ± 0.02 | 3.89 ± 0.02 | 4.13 ± 0.02 | 4.40 ± 0.02 | 5.4 ± 0.07 | 5.34 ± 0.07 | 5.26 ± 0.07 | 5.41 ± 0.07 |
| 25 | 3.74 ± 0.02 | 3.87 ± 0.02 | 4.28 ± 0.02 | 5.31 ± 0.07 | 5.26 ± 0.07 | 5.33 ± 0.07 | |||
| 40 | 3.73 ± 0.02 | 3.87 ± 0.02 | 4.59 ± 0.02 | 5.34 ± 0.07 | 5.27 ± 0.07 | 5.43 ± 0.07 | |||
| Viscosity (mPa s) | 4 | 1561 ± 8 | 1957 ± 93 | 1961 ± 83 | 1628 ± 54 | 1414 ± 17 | 1507 ±1 | 1870 ± 14 | 1491 ± 13 |
| 25 | 1991 ± 95 | 1968 ± 43 | 1883 ± 06 | 1768 ±1 | 1730 ± 2 | 1815 ± 4 | |||
| 40 | 1715 ± 127 | 1961 ± 83 | 1628 ± 54 | 1507 ± 1 | 1870 ± 14 | 1491 ± 13 | |||
| Antioxidant activity ABTS, IC50 (mg/mL) | 4 | Not detected | 0.64 ± 0.07 | 0.91 ± 0.03 | 0.91 ± 0.15 | 0.67 ± 0.03 | |||
| 25 | 0.89 ± 0.05 | 0.83 ± 0.05 | 0.73 ± 0.09 | ||||||
| 40 | 0.93 ± 0.04 | 0.55 ± 0.04 | 0.60 ± 0.03 | ||||||
| Total counts of yeast and mold (CFU/g) | 4 | Absent | <10 | ||||||
| 25 | |||||||||
| 40 | |||||||||
| Total viable aerobic count (CFU/g) | 4 | <10 | <10 | ||||||
| 25 | |||||||||
| 40 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





