Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
Preface.
1. Plausibility of the protophotosynthesis models depends on the definition of photosynthesis.
- 1)
- light absorption / influence of light;
- 2)
- carbon dioxide assimilation / conversion;
- 3)
- synthesis of organic chemical compounds;
- 4)
- release of oxygen / production of oxygen from water.
- ⮚
- only light-assisted adsorption / assimilation of carbon dioxide (in the "technological" limit - not only CO2, but also of other atmospheric agents, in particular, pollutants);
- ⮚
- only photoinduced redox processes (photocatalytic processes used for photodisinfection, especially those based on dispersed semiconductors usually associated with modeling of the photosynthesis elementary stages);
- ⮚
- only photocatalysis and photo-assisted chemical synthesis;
- ⮚
- only on photoinduced purification and oxygenation of the atmosphere (this is also a common practice, from the terrestrial conditions to various technological models of the "space biospheres" developed since the last quarter of the 20th century);
- ⮚
- only on obtaining energy (this aspect can be clearly seen in the design of the biomimetic solar cells based on the principles of the natural photosynthesis in the understanding of technologists and engineers).
2. Basic principles of the reliable reconstruction of protophotosynthesis: From physical and geochemical selection criteria to evolutionary consistency.
3. Coupling between the light harvesting, charge separation and catalysis in a minimal singular model of protophotosynthetic machinery.
4. Integration of minimal protophotosynthetic functions in a single structure as a criterion for the unity of their emergence.
5. What is the minimal set of functions sufficient for (proto)photosynthesis modeling from the standpoint of mathematical biophysics?
5.1. Photochemistry.
5.2. Redox processes.
5.2.1. The need for redox-catalytic agents for the evolution of redox states of carriers.
5.2.2. The need for electrostatic interactions in redox-evolution of photosynthesis.
5.3. Catalysis and macrokinetics.
5.3.1. Protophotosynthetic catalysis should be photoredox catalysis.
5.3.2. Protophotosynthetic catalysis should be membrane / membrane mimetic catalysis.
5.3.3. The need for charge separation and reversible charging-discharging cycles in protomenranes.
5.3.4. Photoelectrocatalytic acceleration of processes in early photosynthesis.
5.3.5. Indifference to the charge carrier nature, similarity of equivalent circuits and the presence of reversible non-covalent interactions.
5.3.6. The search for enzyme-mimetic catalytic pathways for prebiological photocatalysis.
5.3.7. The need to reproduce the kinetics of multienzyme complexes in models of complex photosynthetic and protophotosynthetic systems.
5.4. Membrane processes.
5.4.1. The need for phase separation and compartmentalization for matching the kinetic model of primary photosynthetic processes.
5.4.2. Double electric layer is necessary for reversible charging-discharging and electrochemical / photoelectrochemical oxidation-reduction processes.
5.4.3. Accounting for the kinetics of membrane processes.
5.4.4. Accounting for the membrane geometry and diffusion limitations.
5.4.5. The need for a reaction-diffusion approach to the emergence of photosynthesis.
5.4.6. Accounting for the chemiosmotic coupling.
5.4.7. Accounting for the kinetics of the membrane potential formation.
5.5. Chemical synthesiss.
6. A unified geochemical basis of prebiotic photo- and chemosynthesis: The possible native mineral constituents of protophotosynthesis.
7. Mineral photosensitive catalytic semiconductors as the basic actors and components of abiotic protophotosynthesis.
8. Geochemical consistency and "semiconductor worlds" in abiogenesis.
9. Self-organization in “semiconductor worlds” under the solar energy pumping: Energy supply for the processes of protophotosynthesis and abiogenesis.
10. From semiconductor-based artificial photosynthesis to abiotic photosynthesis / protophotosynthesis modeling.
11. Photoinduced redox processes and chemical gradients in the coevolution of protorespiration and prothotosynthesis: Is it possible to model redox catalysis in photosynthesis without multienzyme complexes?
12. Inseparable complex of photoinduced phenomena on a semiconductor protophotosynthetic interface as a set of reaction-diffusion processes.
13. Towards the formation of precursors of the photosensitive systems and photoenzymes at mineral heterojunctions: From membrane mimetic interfaces in “semiconductor worlds” to “heteroepitaxial worlds”.
14. Similarity between the dynamics of processes in the electric double layer of membrane mimetic semiconductor surfaces and photoinduced membrane potential oscillations in biological cells.
15. Similarity between the equivalent circuits of semiconductor protophotosynthetic photocatalytic interfaces and photosynthetic membranes.
16. The independence of formal kinetics of oscillations from the substrate as a prerequisite of the possible evolutionary transition from mineral semiconductor kinetics / dynamics to photosynthetic kinetics / dynamics.
17. From the formal kinetics of elementary excitations in semiconductors to quasiparticle-assisted biophysics of the primary forms of (proto)photosynthesis.
18. A fundamental role of the phase boundaries / interfaces in the evolution of protophotosynthesis (charge carrier transport, surface reaction kinetics, etc.)
18.1. Photophysical and photochemical activity of surfaces and interfaces
18.2. Electrophysical and electrochemical activity
18.3. (Chemi-)sorption and catalytic activity.
18.4. Surface kinetic activity, in particular, catalytic chemical oscillations.
18.5. Redox activity.
18.6. Reaction-diffusion activity.
18.7. Structure-forming surface activity.
19. Evolution of the active surface as a way of transition from chemical to biological evolution.
- a)
- which variable or which physiological agent demonstrates this or that kinetic curve (it is inadmissible to identify mechanisms with the equivalent kinetics of different variables or objects that do not correspond to each other);
- b)
- is a function / kinetics-preserving transition possible between the carriers of two formally close kinetic curves (it is obvious that if in one case we are talking about magnetic hysteresis, in the other case - about phenomena specific to ferroelectrics, in the third case - about the physics of semiconductors, and in the fourth case - about photosynthesis, as in a number of just cited works [760,761,762], it is impossible to draw a line of substitution between them);
- c)
- what evolutionary reasons cause the introduction of this or that structure (or one or another member of the equation or an agent formally involved in kinetics) into photosynthesis or another physiological process: if physical introduction does not result in the assimilation, or is rejected by natural selection or is not inherited as “ exotic new acquisition", physically unsupported by the environment, then it is hardly rational to talk about its interpretation in the framework of evolutionary (proto)biology.
20. The inevitability of the membrane participation in the development of protophotosynthesis.
Acknowledgments
References
- Kuhn, H. Model consideration for the origin of life. Environmental structure as stimulus for the evolution of chemical systems. Naturwissenschaften 1976, 2, 68–80, [https://pubmed.ncbi.nlm.nih.gov/934343/]. [Google Scholar] [CrossRef]
- Bartsev, S.I.; Mezhevikin, V.V. Natural selection in a flow as a universal mechanism of evolution of prebiological autocatalytic systems. Doklady Biochemistry and Biophysics 2003, 388, No. 1, 35–38, [https://pubmed.ncbi.nlm.nih.gov/12741130/]. [Google Scholar] [CrossRef]
- Oxford English Dictionary, 2nd ed. Clarendon Press: Oxford, 1989.
- Oxford Dictionary of Biochemistry and Molecular Biology. Oxford University Press: Oxford, 1997; 508 p. [ISBN 978-0-19-854768-6]. Oxford Dictionary of Biochemistry and Molecular Biology (2nd ed.) Oxford University Press: Oxford, 2006. [https://www.oxfordreference.com/display/10.1093/acref/9780198529170.001.0001/acref-9780198529170-e-15515].
- Concise Medical Dictionary (8th ed.) Oxford University Press: Oxford, 2010. [https://www.oxfordreference.com/display/10.1093/acref/9780199557141.001.0001/acref-9780199557141-e-7777].
- A Dictionary of Food and Nutrition (3rd ed.) Oxford University Press: Oxford, 2009. [https://www.oxfordreference.com/display/10.1093/acref/9780199234875.001.0001/acref-9780199234875-e-4170].
- Oxford Dictionary of Biology (6th edition). Oxford University Press: Oxford - New York, 2008. [https://www.oxfordreference.com/display/10.1093/acref/9780199204625.001.0001/acref-9780199204625-e-3399].
- Barnes, C.R. On the food of green plants. Bot. Gaz. 1893, 18, 403–411, [http://www.jstor.org/stable/2464454]. [Google Scholar] [CrossRef]
- Gest, H. History of the word photosynthesis and evolution of its definition. Photosynthesis Research, 2002, 73, 7–10. [Google Scholar] [CrossRef]
- Pfeffer, W. The physiology of plants; a treatise upon the metabolism and sources of energy in plants (Ed. and Transl. by A.J. Ewart.) Clarendon Press: Oxford, 1900. [CrossRef]
- Kamen, M.D. Primary Processes in Photosynthesis. Academic Press: New York, 1963. [https://shop.elsevier.com/books/primary-processes-in-photosynthesis/kamen/978-1-4832-2959-1].
- Gest, H. Photosynthetic and quasi-photosynthetic bacteria. FEMS Microbiol. Lett. 1993, 112, 1–6. [Google Scholar] [CrossRef]
- Gest, H. Evolution of knowledge encapsulated in scientific definitions. Persp. Biol. Med. 2001, 44, 556–564. [Google Scholar] [CrossRef]
- Gest, H. History of concepts of the comparative biochemistry of oxygenic and anoxygenic photosyntheses. Photosyn. Res. 1993, 35, 87–96. [Google Scholar] [CrossRef]
- Badyaev, A.V. Evolution despite natural selection? Emergence theory and the ever elusive link between adaptation and adaptability. Acta Biotheoretica 2008, 56, 249–255. [Google Scholar] [CrossRef]
- Van der Steen, W. J. Methodological problems in evolutionary biology. X. Natural selection without selective agents. Acta Biotheoretica 1998, 46, 99–107. [Google Scholar] [CrossRef]
- Sharma, V.; Annila, A. Natural process–Natural selection. Biophysical Chemistry 2007, 127, 123–128, [https://pubmed.ncbi.nlm.nih.gov/17289252/]. [Google Scholar] [CrossRef] [PubMed]
- Oro, J.; Armangue, G.; Mar, A. The principle of cooperation and life's origin and evolution. NASA, Washington Second Symposium on Chemical Evolution and the Origin and Evolution of Life; 1986, p. 78.
- Shnoll, S. Physico-chemical factors of biological evolution. In Soviet Scientific Reviews Supplement Series. Physicochemical Biology (Revised Edition); Routledge, 1981; Volume 1, 280 p.
- Volkenstein, M.Y. Physical Approaches to Biological Evolution (reprint of the original 1st ed. 1994); Springer: Berlin, Heidelberg, 2011; 420 p. [Google Scholar] [CrossRef]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy conversion in natural and artificial photosynthesis. Chem. Biol. 2010, 17, 434–447, [https://pubmed.ncbi.nlm.nih.gov/20534342/]. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Shkuropatov, A.Y.; Shuvalov, V.A. Femtosecond nuclear oscillations under charge separation in reaction centers of photosynthesis. Biochemistry (Moscow) 2003, 68, 541–550, [https://pubmed.ncbi.nlm.nih.gov/12882636/]. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Shuvalov, V.A. Modeling of reversible charge separation in reaction centers of photosynthesis: an incoherent approach. J. Theor. Biol. 2014, 343, 92–101, [https://pubmed.ncbi.nlm.nih.gov/24270095/]. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Shuvalov, V.A. Reversible charge separation in reaction centers of photosynthesis: a classical model. Doklady Biochemistry and Biophysics 2013, 450, 143–146, [https://pubmed.ncbi.nlm.nih.gov/23824456/]. [Google Scholar] [CrossRef]
- Slovacek, R.E.; Hind, G. Correlation between photosynthesis and the transthylakoid proton gradient. Biochim. Biophys. Acta 1981, 635, 393–404, [https://pubmed.ncbi.nlm.nih.gov/7236671/]. [Google Scholar] [CrossRef]
- Enser, U.; Heber, U. Metabolic regulation by pH gradients. Inhibition of photosynthesis by indirect proton transfer across the chloroplast envelope. Biochim. Biophys. Acta 1980, 592, 577–591, [https://pubmed.ncbi.nlm.nih.gov/6251871/]. [Google Scholar] [CrossRef]
- Geacintov, N.E. Tracing charge separation events in photosynthesis: anomalous photovoltage polarity events explained. Biophys. J. 1993, 65, 11–12, [https://pubmed.ncbi.nlm.nih.gov/8369419/]. [Google Scholar] [CrossRef] [PubMed]
- LeBard, D.N.; Kapko, V.; Matyushov, D.V. Energetics and kinetics of primary charge separation in bacterial photosynthesis. J. Phys. Chem. B. 2008, 112, 10322–10342, [https://pubmed.ncbi.nlm.nih.gov/18636767/]. [Google Scholar] [CrossRef] [PubMed]
- Makri, N.; Sim, E.; Makarov, D.E.; Topaler, M. Long-time quantum simulation of the primary charge separation in bacterial photosynthesis. Proc. Nat. Acad. Sci. USA 1996, 93, 3926–3931, [https://pubmed.ncbi.nlm.nih.gov/8632991/]. [Google Scholar] [CrossRef] [PubMed]
- Fajer, J.; Brune, D.C.; Davis, M.S.; Forman, A.; Spaulding, L.D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin (reduced bacteriopheophytin / transient electron acceptor). Proc. Nat. Acad. Sci. USA 1975, 72, 4956–4960, [https://pubmed.ncbi.nlm.nih.gov/174084/]. [Google Scholar] [CrossRef] [PubMed]
- Parson, W.W.; Chu, Z.T.; Warshel, A. Electrostatic control of charge separation in bacterial photosynthesis. Biochim. Biophys. Acta 1990, 1017, 251–272, [https://pubmed.ncbi.nlm.nih.gov/2196939/]. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F. Arsenic microbes and contaminated aquifers. Trends Microbiol. 2005, 13, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Wadas, T.J.; Hester, H.; Schmehl, R.; Eisenberg, R. Platinum chromophore-based systems for photoinduced charge separation: a molecular design approach for artificial photosynthesis. Inorg. Chem. 2005, 44, 6865–6878. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Boixel, J.; Pellegrin, Y.; Blart, E.; Becker, H.C.; Odobel, F.; Hammarström, L. Accumulative charge separation inspired by photosynthesis. J. Am. Chem. Soc. 2010, 132, 17977–17979. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Boixel, J.; Pellegrin, Y.; Blart, E.; Becker, H.C.; Odobel, F.; Hammarström, L. Accumulative electron transfer: multiple charge separation in artificial photosynthesis. Faraday Discuss. 2012, 155, 233–252, [https://pubmed.ncbi.nlm.nih.gov/22470977/]. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Ohkubo, K.; Suenobu, T. Long-lived charge separation and applications in artificial photosynthesis. Acc. Chem. Res. 2014, 47, 1455–1464, [https://pubmed.ncbi.nlm.nih.gov/24793793/]. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xiao, T.; Zhang, Q.; Liu, Z. Photosynthesis-inspired bifunctional energy-harvesting devices that convert light and salinity gradients into electricity. Chem. Commun. 2018, 54, 12310–12313, [https://pubmed.ncbi.nlm.nih.gov/30272063/]. [Google Scholar] [CrossRef] [PubMed]
- Altamura, E.; Milano, F.; Tangorra, R.R.; Trotta, M.; Omar, O.H.; Stano, P.; Mavelli, F. Highly oriented photosynthetic reaction centers generate a proton gradient in synthetic protocells. Proc. Nat. Acad. Sci. 2017, 114, 3837–3842, [https://pubmed.ncbi.nlm.nih.gov/28320948/]. [Google Scholar] [CrossRef]
- Katsoukis, G.; Frei, H. Heterobinuclear light absorber coupled to molecular wire for charge transport across ultrathin silica membrane for artificial photosynthesis. ACS Appl. Mater. Interfaces 2018, 10, 31422–31432, [https://pubmed.ncbi.nlm.nih.gov/30146876/]. [Google Scholar] [CrossRef]
- Matyushov, D.V. Reorganization asymmetry of electron transfer in ferroelectric media and principles of artificial photosynthesis. J. Phys. Chem. B. 2006, 110, 10095–10104, [https://pubmed.ncbi.nlm.nih.gov/16706471/]. [Google Scholar] [CrossRef]
- Leuchtag, H.R. Indications of the existence of ferroelectric units in excitable-membrane channels. J. Theor. Biol.; 1987, 127, 321–340, [https://pubmed.ncbi.nlm.nih.gov/2448549/]. [Google Scholar] [CrossRef] [PubMed]
- Leuchtag, H.R. Phase transitions and ion currents in a model ferroelectric channel unit. J. Theor. Biol.; 1987, 127, 341–359, [https://pubmed.ncbi.nlm.nih.gov/2448550/]. [Google Scholar] [CrossRef] [PubMed]
- Leuchtag, H.R. A proposed physical explanation of the activation of sodium channels. Ferroelectrics 1988, 86, 105–113. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Leuchtag, H.R. Bioferroelectricity: Modeling the transitions of the sodium channel. Ferroelectrics 1994, 155, 19–24. [Google Scholar] [CrossRef]
- Leuchtag, H.R. Fit of the dielectric anomaly of squid axon membrane near heat-block temperature to the ferroelectric Curie-Weiss law. Biophysical Chemistry 1995, 53, 197–205, [https://pubmed.ncbi.nlm.nih.gov/17020847/]. [Google Scholar] [CrossRef] [PubMed]
- Leuchtag, H.R.; Bystrov, V.S. Theoretical models of conformational transitions and ion conduction in voltage-dependent ion channels: Bioferroelectricity and superionic conduction. Ferroelectrics 1999, 220, 157–204. [Google Scholar] [CrossRef]
- Fendler, J.H. Artificial photosynthesis–an example of membrane mimetic chemistry. BioEssays 1984, 1, 165–167. [Google Scholar] [CrossRef]
- Brown, K.A.; King, P.W. Coupling biology to synthetic nanomaterials for semi-artificial photosynthesis. Photosynth. Res. 2020, 143, 193–203, [https://pubmed.ncbi.nlm.nih.gov/31641988/]. [Google Scholar] [CrossRef]
- Hafizi, B. Nonlinear evolution equations, recurrence and stochasticity. The Physics of Fluids 1981, 24, 1791–1798. [Google Scholar] [CrossRef]
- Saccone, C.; Preparata, G.; Lanave, C.; Quagliariello, E.; Bernardi, G.; Ullmann, A. Chance, stochasticity and evolution: the Markov clock. In Enzyme Adaptation to Natural Philosophy: Heritage from Jacques Monod.; Elsevier Science Publishers BV (Biomedical Division): Amsterdam – New York, Netherlands – USA, 1987; pp. 159–172. [Google Scholar]
- Lenormand, T.; Roze, D.; Rousset, F. Stochasticity in evolution. Trends in Ecology and Evolution 2009, 24, 157–165, [https://pubmed.ncbi.nlm.nih.gov/19178980/]. [Google Scholar] [CrossRef]
- Rosenfeld, S. Mathematical descriptions of biochemical networks: stability, stochasticity, evolution. Progress in Biophysics and Molecular Biology 2011, 106, 400–409, [https://pubmed.ncbi.nlm.nih.gov/21419158/]. [Google Scholar] [CrossRef]
- Pradas, M.; Schmuck, M.; Pavliotis, G.; Kalliadasis, S. Understanding the evolution of complex multiscale systems: Dynamic renormalization, non-equilibrium entropy and stochasticity. In Bulletin of the American Physical Society (66th Annual Meeting of the APS Division of Fluid Dynamics), 2013, 58, H35.00008.
- Egel, R. “Parabiotic Evolution”: From Stochasticity in Geochemical and Subsequent Processes to Genes, Genomes and Modular Cells 2017, MDPI Preprint. [CrossRef]
- Danino, M.; Kessler, D.A.; Shnerb, N.M. Environmental stochasticity and the speed of evolution. Journal of Statistical Physics 2018, 172, 126–142. [Google Scholar] [CrossRef]
- Hyun, J.S.; Park, C.J. Classification of contradiction relations and their solving dimensions based on the butterfly model for contradiction solving for physical contradiction of TRIZ. Knowledge Management Research 2014, 15, 15–34. [Google Scholar] [CrossRef]
- Choi, S.W. Review and application of creative problem-solving processes for technical and physical contradictions using cause-and-effect contradiction tree and integrated principles of TRIZ. Journal of the Korea Safety Management and Science 2015, 17, 215–228. [Google Scholar] [CrossRef]
- Zhang, X.P.; Qiu, M.; Pi, Y.M. Discussion on applying the ideal final result of TRIZ in the simplified physical problems. Journal of Heihe University 2010, 2, 10. [Google Scholar]
- Kim, J.; Kim, J.; Lee, Y.; Lim, W.; Moon, I. Application of TRIZ creativity intensification approach to chemical process safety. Journal of Loss Prevention in the Process Industries 2009, 22, 1039–1043. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhao, H.Y.; Jiang, T.; Zhang, Q.M.; Huang, Y.B. The inventing principles of 40 TRIZ reflected in the chemical industry. Guangzhou Chemical Industry, 2010, 30, 8. [Google Scholar]
- Tunuli, M.S.; Fendler, J.H. Aspects of artificial photosynthesis. Photosensitized electron transfer across bilayers, charge separation, and hydrogen production in anionic surfactant vesicles. J. Amer. Chem. Soc. 1981, 103, 2507–2513. [Google Scholar] [CrossRef]
- Infelta, P.P.; Graetzel, M.; Fendler, J.H. Aspects of artificial photosynthesis. Photosensitized electron transfer and charge separation in cationic surfactant vesicles. J. Amer. Chem. Soc. 1980, 102, 1479–1483. [Google Scholar] [CrossRef]
- Kurihara, K.; Tundo, P.; Fendler, J.H. Aspects of artificial photosynthesis. Photosensitized electron transfer and charge separation in redox active surfactant aggregates. J. Phys. Chem. 1983, 87, 3777–3782. [Google Scholar] [CrossRef]
- Tunuli, M.S.; Fendler, J.H. Aspects of artificial photosynthesis: the role of potential gradients in promoting charge separation in the presence of surfactant vesicles. In Inorganic Reactions in Organized Media; American Chemical Society, Washington, D.C., USA, 1982; Volume 177, pp. 53-70. [CrossRef]
- Macnaughtan, M.L.; Frei, H.M. Synthesis of heterobimetallic charge transfer chromophores and coupled oxidation catalysts for artificial photosynthesis. In Preprints of Symposia – Division of Fuel Chemistry, American Chemical Society, CD ROM Edition; American Chemical Society, Washington, D.C., USA, 2011; Volume 56 (Issue 1), 149.
- Lee, S.H.; Kim, J.H.; Park, C.B. Coupling photocatalysis and redox biocatalysis toward biocatalyzed artificial photosynthesis. Chem. Eur. J. 2013, 19, 4392–4406, [https://pubmed.ncbi.nlm.nih.gov/23436280/]. [Google Scholar] [CrossRef]
- Đokić, M.; Soo, H.S. Artificial photosynthesis by light absorption, charge separation, and multielectron catalysis. Chem. Commun. 2018, 54, 6554–6572. [Google Scholar] [CrossRef]
- Pannwitz, A.; Wenger, O.S. Proton-coupled multi-electron transfer and its relevance for artificial photosynthesis and photoredox catalysis. Chem. Commun. 2019, 55, 4004–4014, [https://pubmed.ncbi.nlm.nih.gov/30810148/]. [Google Scholar] [CrossRef] [PubMed]
- Moberg, S. Artificial photosynthesis-4-aminobenzoic acids effect on charge transfer in a photo catalytic system. PhD Thesis, Uppsala University (Department of Physics and Astronomy), Uppsala, 2019. [Google Scholar]
- Rasmussen, S.; Chen, L.; Deamer, D.; Krakauer, D.C.; Packard, N.H.; Stadler, P.F.; Bedau, M.A. Transitions from nonliving to living matter. Science 2009, 303, 963–965, [https://pubmed.ncbi.nlm.nih.gov/14963315/]. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Chen, L.; Nilsson, M.; Abe, S. Bridging nonliving and living matter. Artificial Life 2003, 9, 269–316, [https://pubmed.ncbi.nlm.nih.gov/14556688/]. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Grigalavicius, M.; Krisciukaitis, S.; Medzevicius, G. Quantum processes in 8-oxo-guanine-Ru (bipyridine)32+ photosynthetic systems of artificial minimal cells. Open Physics 2011, 9, 775–791. [Google Scholar] [CrossRef]
- Tamulis, A. Quantum mechanical investigations of photosynthetic systems of artificial minimal cells based on 8-oxo-guanine-Ru (bipyridine) 2+3. Journal of Computational and Theoretical Nanoscience 2011, 8, 624–636. [Google Scholar] [CrossRef]
- Tamulis, A.; Grigalavicius, M. The emergence and evolution of life in a “fatty acid world” based on quantum mechanics. Origins Life Evol. Biospheres 2011, 41, 51–71, [https://pubmed.ncbi.nlm.nih.gov/20443139/]. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Tamulis, V. Question 9: Quantum self-assembly and photoinduced electron tunneling in photosynthetic systems of artificial minimal living cells. Origins Life Evol. Biospheres 2007, 37, 473–476, [https://pubmed.ncbi.nlm.nih.gov/17610046/]. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Tamulis, V. Quantum self-assembly and photoinduced electron tunneling in photosynthetic system of minimal living cell. Viva Origino 2007, 35, 66–72. [Google Scholar] [CrossRef]
- Tamulis, A.; Berteska, L.; Grigalavicius, M.; Baltrusaitis, J. Quantum dynamics of self-assembly of minimal photosynthetic cells. Quantum Matter 2016, 5, 5–18. [Google Scholar] [CrossRef]
- Tamulis, A.; Grigalavicius, M. Molecular spintronics control of photosynthesis in artificial cell. J. Comput. Theor. Nanosci. 2013, 10, 989–995. [Google Scholar] [CrossRef]
- Tamulis, A.; Grigalavicius, M. Quantum entanglement in photoactive prebiotic systems. Systems and Synthetic Biology 2014, 8, 117–140, [https://pubmed.ncbi.nlm.nih.gov/24799958/]. [Google Scholar] [CrossRef]
- Tamulis, A.; Grigalavicius, M.; Baltrusaitis, J. Phenomenon of quantum entanglement in a system composed of two minimal protocells. Origins Life Evol. Biospheres 2013, 43, 49–66, [https://pubmed.ncbi.nlm.nih.gov/23242832/]. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Grigalavicius, M.; Serbenta, J.; Plausinaitis, K. Quantum entangled single bioorganic supramolecules as light absorbing and light emitting logical devices. J. Comput. Theor. Nanosci. 2015, 12, 1827–1840. [Google Scholar] [CrossRef]
- Rubin, A.; Riznichenko, G. Mathematical Biophysics; Springer: New York, Heidelberg, Dordrecht, London, 2014; 274 p. [Google Scholar]
- Rubin, A.B. Fundamentals of Biophysics; John Wiley & Sons, Inc.: Hoboken, New Jersey, and Scrivener Publishing LLC: Salem, Massachusetts, 2014; 212 p. [Google Scholar]
- Blumenfeld, L.A. Problems of Biological Physics; Springer: Berlin, Heidelberg, 1981; 224 p. [Google Scholar]
- Armstrong, E.F. Studies on enzyme action. II.--The rate of the change, conditioned by sucroclastic enzymes, and its bearing on the law of mass action. Proceedings of the Royal Society of London 1904, 73, 500–516. [Google Scholar] [CrossRef]
- Armstrong, E.F.; Hilditch, T.P. A study of catalytic actions at solid surfaces. V.—The rate of change conditioned by a nickel catalyst and its bearing on the law of mass action. Proceedings of the Royal Society of London, Series A 1920, 98, 27–40. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Catalysis by polymer-immobilized metal complexes. Gordon Breach Sci. Publ.: Amsterdam, 1998; 424 p. [CrossRef]
- Skulachev, V.P. The sodium cycle: a novel type of bacterial energetics. J. Bioenergetics Biomembranes 1989, 21, 635–647, [https://pubmed.ncbi.nlm.nih.gov/2687258/]. [Google Scholar] [CrossRef]
- Skulachev, V.P. The latest news from the sodium world. Biochimica et Biophysica Acta – Bioenergetics 1994, 1187, 216–221. [Google Scholar] [CrossRef]
- Dibrova, D.; Mulkidjanian, A. Reconstruction of the primordial “Sodium World”. Biochimica et Biophysica Acta – Bioenergetics 2014, 1837, e84. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Dibrov, P.; Galperin, M.Y. The past and present of sodium energetics: may the sodium-motive force be with you. Biochimica et Biophysica Acta –Bioenergetics 2008, 1777, 985–992. [Google Scholar] [CrossRef]
- Henry, V. Théorie générale de l'action de quelques diastases (présentée par M. Roux.). Comptes rendus hebdomadaires des séances de l'Académie des sciences 1902, 135, 916–919. [Google Scholar]
- Michaelis, L.; Menten, M.L. The kinetics of the inversion effect. Biochem. Z. 1913, 49, 333–369. [Google Scholar]
- Michaelis, L.; Menten, M.L. The kinetics of invertin action. FEBS Letters 2013, 587, 2712–2720, [https://pubmed.ncbi.nlm.nih.gov/23867202/]. [Google Scholar] [CrossRef]
- Kwak, J.; Kim, M.C.; Lee, S.Y. An enzyme-coupled artificial photosynthesis system prepared from antenna protein-mimetic tyrosyl bolaamphiphile self-assembly. Nanoscale 2016, 8, 15064–15070. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, L.A. The evolution of biocatalysts. Russ. Chem. Rev. 1961, 30, 117–133. [Google Scholar] [CrossRef]
- Nikolaev, L.A. The principles of biocatalyst modeling. Russ. Chem. Rev. 1964, 33, 275–286. [Google Scholar] [CrossRef]
- Purmal, A.P.; Nikolaev, L.A. The modelling of biological catalysts. Russ. Chem. Rev. 1985, 54, 466–475. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.; Li, X. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Shang, C.; Zhang, Z.; Dong, S. Triple-enzyme mimetic activity of nickel–palladium hollow nanoparticles and their application in colorimetric biosensing of glucose. Chem. Commun. 2016, 52, 5410–5413. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, G.; Sun, F.; Lin, Y. Heterogeneous nanostructure design based on the epitaxial growth of spongy MoSx on 2D Co(OH)2 nanoflakes for triple-enzyme mimetic activity: experimental and density functional theory studies on the dramatic activation mechanism. ACS Appl. Mater. Interfaces 2018, 10, 32567–32578. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, H.; Liu, C.; Bing, W.; Wang, Z.; Qu, X. A multinuclear metal complex based DNase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angew. Chem. Int. Ed. 2016, 55, 10732–10736, [https://pubmed.ncbi.nlm.nih.gov/27484616/]. [Google Scholar] [CrossRef] [PubMed]
- Nagiev, T.M. Mimetic simulation of enzyme catalysis. Russ. J. Phys. Chem. 1996, 70, 895–903. [Google Scholar]
- Nagiev, T. The Theory of Conjugate Reactions in the Context of Modern Ideas. Advances in Chemical Engineering and Science 2019, 10, 52–68. [Google Scholar] [CrossRef]
- Chang, T.M.S.; Yu, Y.T.; Grunwald, J. Artificial cell immobilized multienzyme systems and cofactors. In Enzyme Engineering? Vol. 6; Springer: Boston, MA, 1982; pp. 451–456. [Google Scholar] [CrossRef]
- Chang, T.M.S.; Kuntarian, N. Galactose conversion using a microcapsule immobilized multienzyme cofactor recycling system. In Enzyme engineering, Vol. 4; Springer: Boston, MA, 1978; pp. 193–197. [Google Scholar] [CrossRef]
- Campbell, J.; Chang, T.M.S. Immobilized multienzyme systems and coenzyme requirements: perspectives in biomedical applications. Biomedical Applications of Immobilized Enzymes and Proteins 1977, 2, 281–302. [Google Scholar]
- Sun, G.; Shi, J.; Jia, S.; Luo, Y.; Jiang, Z.; Yuan, X. General model for artificial photosynthesis with capsule-immobilized enzyme. AIChE Journal 2022, 68, E17409. [Google Scholar] [CrossRef]
- Sarma, R.; Islam, M.; Running, M.P.; Bhattacharyya, D. Multienzyme immobilized polymeric membrane reactor for the transformation of a lignin model compound. Polymers 2018, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Yotova, L.; Medhat, N. Optical biosensor with multienzyme system immobilized onto hybrid membrane for pesticides determination. Int. J. Bioautomation 2011, 15, 267–276. [Google Scholar]
- Ho, S.P.; Kostin, M.D. Kinetics of immobilized multienzyme systems. J. Chem. Phys. 1974, 61, 918–920. [Google Scholar] [CrossRef]
- Fernandes, P.M. Mathematical modeling of immobilized multienzyme systems. Doctoral dissertation, Rutgers University, 1977.
- Gu, K.F.; Chang, T.M.S. Conversion of α-ketoglutarate into L-glutamic acid with urea as ammonium source using multienzyme systems and dextran-NAD+ immobilized by microencapsulation within artificial cells in a bioreactor. Biotechnol. Bioeng. 1988, 32, 59–62. [Google Scholar] [CrossRef]
- Chang, T.M.S. Recycling of NAD(P) by multienzyme systems immobilized by microencapsulation in artificial cells. In Methods in Enzymology; Academic Press, 1987; Volume 136, pp. 67-82. [CrossRef]
- Chang, T. Biotechnological approach using artificial cells immobilized multienzyme systems and hepatocytes for bioartificial liver. Biomaterials Artificial Cells and Artificial Organs 1988, 16, 844. [Google Scholar]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biological Reviews 1966, 41, 445–501. [Google Scholar] [CrossRef]
- Slater, E.C. An evaluation of the Mitchell hypothesis of chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Eur. J. Biochem. 1967, 1, 317–326, [https://pubmed.ncbi.nlm.nih.gov/4293928/]. [Google Scholar] [CrossRef]
- Telfer, A.; Evans, M.C.W. Evidence for chemiosmotic coupling of electron transport to ATP synthesis in spinach chloroplasts. Biochimica et Biophysica Acta – Bioenergetics 1972, 256, 625–637, [https://pubmed.ncbi.nlm.nih.gov/5020234/]. [Google Scholar] [CrossRef]
- Hangarter, R.P.; Good, N.E. Energy thresholds for ATP synthesis in chloroplasts. Biochimica et Biophysica Acta – Bioenergetics 1982, 681, 397–404. [Google Scholar] [CrossRef]
- Jakobsson, E. Interactions of cell volume, membrane potential, and membrane transport parameters. American Journal of Physiology-Cell Physiology 1980, 238, C196–C206, [https://pubmed.ncbi.nlm.nih.gov/7377338/]. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Dunham, P.B. Membrane mechanisms and intracellular signalling in cell volume regulation. International Review of Cytology 1995, 161, 173–262, [https://pubmed.ncbi.nlm.nih.gov/7558691/]. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Mills, J.W. Membrane events involved in volume regulation. In Current Topics in Membranes; Academic Press, 1999; Volume 48, pp. 123-196. [CrossRef]
- Marrink, S.J.; Sok, R.M.; Berendsen, H.J.C. Free volume properties of a simulated lipid membrane. Journal of Chemical Physics 1996, 104, 9090–9099. [Google Scholar] [CrossRef]
- Smith, D.C.; Bassham, J.A.; Kirk, M. Dynamics of the photosynthesis of carbon compounds II. Amino acid synthesis. Biochimica et Biophysica Acta 1961, 48, 299–313. [Google Scholar] [CrossRef]
- Heber, U. Protein synthesis in chloroplasts during photosynthesis. Nature 1962, 195, 91–92, [https://pubmed.ncbi.nlm.nih.gov/13905812/]. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Morris, I. Synthesis of lipid during photosynthesis by phytoplankton of the Southern Ocean. Science 1980, 207, 197–199, [https://pubmed.ncbi.nlm.nih.gov/17809104/]. [Google Scholar] [CrossRef]
- Weber, A.P. Synthesis, export and partitioning of the end products of photosynthesis. In The structure and function of plastids; Springer: Dordrecht, 2007; pp. 273–292. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harbor perspectives in biology 2010, 2, a002162, [https://pubmed.ncbi.nlm.nih.gov/20452963/]. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A perspective on the role of minerals in prebiotic synthesis. AMBIO: A Journal of the Human Environment 2004, 33, 539–551, [https://pubmed.ncbi.nlm.nih.gov/15666687/]. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Evolution of the first metabolic cycles. Proceedings of the National Academy of Sciences 1990, 87, 200–204, [https://pubmed.ncbi.nlm.nih.gov/2296579/]. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Groundworks for an evolutionary biochemistry: the iron-sulfur world. Progress in Biophysics and Molecular Biology 1992, 58, 85–201, [https://pubmed.ncbi.nlm.nih.gov/1509092/]. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philosophical Transactions of the Royal Society, B, Biological Science, 2006, 361, 1787–1806, [https://pubmed.ncbi.nlm.nih.gov/17008219/]. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Pyrite formation, the first energy source for life: a hypothesis. Systematic and Applied Microbiology 1988, 10, 207–210. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Before enzymes and templates: theory of surface metabolism. Microbiological reviews 1988, 52, 452. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Wächtershäuser, G. α-Hydroxy and α-Amino Acids Under Possible Hadean, Volcanic Origin-of-Life Conditions. Science 2006, 314, 630–632, [https://pubmed.ncbi.nlm.nih.gov/17068257/]. [Google Scholar] [CrossRef]
- White, L.M.; Bhartia, R.; Stucky, G.D.; Kanik, I.; Russell, M.J. Mackinawite and greigite in ancient alkaline hydrothermal chimneys: identifying potential key catalysts for emergent life. Earth and Planetary Science Letters 2015, 430, 105–114. [Google Scholar] [CrossRef]
- Wu, M.; John, S.T.; Pan, Y. Electronic structures of greigite (Fe3S4): A hybrid functional study and prediction for a Verwey transition. Scientific Reports 2016, 6, 21637. [Google Scholar] [CrossRef]
- Mielke, R.E.; Robinson, K.J.; White, L.M.; McGlynn, S.E.; McEachern, K.; Bhartia, R.; Kanik, I,; Russell, M. J. Iron-sulfide-bearing chimneys as potential catalytic energy traps at life's emergence. Astrobiology 2011, 11, 933–950, [https://pubmed.ncbi.nlm.nih.gov/22111762/]. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 2003, 358, 59–85, [https://pubmed.ncbi.nlm.nih.gov/12594918/]. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philosophical Transactions of the Royal Society B: Biological Sciences 2007, 362, 1887–1926, [https://pubmed.ncbi.nlm.nih.gov/17255002/]. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371, [https://pubmed.ncbi.nlm.nih.gov/20572872/]. [Google Scholar] [CrossRef] [PubMed]
- Macleod, G.; McKeown; C. ; Hall; A. J.; Russell, M.J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Origins of Life and Evolution of the Biosphere 1994, 24, 19–41, [https://pubmed.ncbi.nlm.nih.gov/11536657/]. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Daniel, R.M.; Hall, A.J. On the emergence of life via catalytic iron-sulphide membranes. Terra Nova 1993, 5, 343–347. [Google Scholar] [CrossRef]
- Russell, M.J.; Martin, W. The rocky roots of the acetyl-CoA pathway. Trends in Biochemical Sciences 2004, 29, 358–363, [https://pubmed.ncbi.nlm.nih.gov/15236743/]. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Hall, A.J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. Journal of the Geological Society 1997, 154, 377–402, [https://pubmed.ncbi.nlm.nih.gov/11541234/]. [Google Scholar] [CrossRef]
- Russell, M.J. Green rust: The simple organizing ‘seed’of all life? Life 2018, 8, 35. [Google Scholar] [CrossRef]
- Russell, M.J.; Ponce, A. Six ‘must-have’minerals for life’s emergence: Olivine, pyrrhotite, bridgmanite, serpentine, fougerite and mackinawite. Life 2020, 10, 291. [Google Scholar] [CrossRef]
- Kizilstein, L.Ya. Framboidal pyrite involved in the revival of life on The Earth? Priroda 2007, 1, 49–54. (in Russian). [Google Scholar]
- Kizilshtein, L.Ya.; Minaeva, L.G. Origin of the framboidal pyrite. Dokl. Akad. Nauk SSSR 1972, 206, 1187–1189. [Google Scholar]
- Liu, A.G. Framboidal pyrite shroud confirms the ‘death mask'model for moldic preservation of ediacaran soft-bodied organismsediacaran taphonomy. Palaios 2016, 31, 259–274. [Google Scholar] [CrossRef]
- Retallack, G.J. Comment to Liu. Framboidal pyrite shroud confirms the ‘death mask'model for moldic preservation of ediacaran soft-bodied organisms. Palaios 2017, 32, 195–196. [Google Scholar] [CrossRef]
- Kalliokoski, J. Framboids—macrocrystals of colloidal pyrite. Econ. Geol. 1965, 60, 1562. [Google Scholar]
- Sawłowicz, Z. Framboids: from their origin to application. Prace Mineralogiczne (Mineralogical Transactions, Polska Akademia Nauk - Komisja Nauk Mineralogicznych) 2000, 88, 3–58. [Google Scholar]
- Granick, S. Speculations on the origins and evolution of photosynthesis. Annals of the New York Academy of Sciences 1957, 69, 292–308, [https://pubmed.ncbi.nlm.nih.gov/13479007/]. [Google Scholar] [CrossRef]
- Granick, S. Evolution of heme and chlorophyll. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, N. Y.; 1965; pp. 67–88. [Google Scholar]
- Mauzerall, D. Light, iron, Sam Granick and the origin of life. Photosynthesis research 1992, 33, 163–170, [https://pubmed.ncbi.nlm.nih.gov/24408576/]. [Google Scholar] [CrossRef] [PubMed]
- Shnoll, S. Physico-chemical factors of biological evolution; Nauka: Moscow, USSR, 1979; 263 p. (in Russian) [Google Scholar]
- Grätzel, M. Energy resources through photochemistry and catalysis. Acad. Press: New York; 1983, 632 p. [CrossRef]
- Ferreira, D.L.; Sousa, J.C.L.; Maronesi, R.N.; Bettini, J.; Schiavon, M.A.; Teixeira, A.V.; Silva, A.G. Size-dependent bandgap and particle size distribution of colloidal semiconductor nanocrystals. Journal of Chemical Physics 2017, 147, 154102. [Google Scholar] [CrossRef]
- Volkenstein, F.F. Semiconductors as catalysts for chemical reactions; Publishing House of Moscow State University: Moscow, 1968; pp. 1–40. [Google Scholar]
- Emeline, A.V.; Otroshchenko, V.A.; Ryabchuk, V.K.; Serpone, N. Abiogenesis and photostimulated heterogeneous reactions in the interstellar medium and on primitive earth: relevance to the genesis of life. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2003, 3, 203–224. [Google Scholar] [CrossRef]
- Parmon, V.N.; Zakharenko, V.S. Photocatalysis and photosorption in the Earth's atmosphere. Cattech, 2001 5, 96-115. [CrossRef]
- Parmon, V.N. Abiogenic catalysis in Nature. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1999, 151, 351–365. [Google Scholar] [CrossRef]
- Xia, D.; Wang, W.; Wong, P.K. Visible-light-driven photocatalytic treatment by environmental minerals. In Advances in Photocatalytic Disinfection; Springer: Berlin, Heidelberg, 2017; pp. 41–61. [Google Scholar] [CrossRef]
- Nikandrov, V.V. Inorganic semiconductors as photosensitizers in biochemical redox reactions. Membr. Cell Biol. 1998, 12, 755–769. [Google Scholar]
- Nikandrov, V.V.; Grätzel, C.K.; Moser, J.E.; Grätzel, M. Light induced redox reactions involving mammalian ferritin as photocatalyst. Journal of Photochemistry and Photobiology B: Biology 1997, 41, 83–89, [https://pubmed.ncbi.nlm.nih.gov/9440316/]. [Google Scholar] [CrossRef]
- Shumilin, I.A.; Nikandrov, V.V.; Popov, V.O.; Krasnovsky, A.A. Photogeneration of NADH under coupled action of CdS semiconductor and hydrogenase from Alcaligenes eutrophus without exogenous mediators. FEBS letters 1992, 306, 125–128, [https://pubmed.ncbi.nlm.nih.gov/1633866/]. [Google Scholar] [CrossRef]
- Nikandrov, V.V.; Shlyk, M.A.; Zorin, N.A.; Gogotov, I.N.; Krasnovsky, A.A. Efficient photoinduced electron transfer from inorganic semiconductor TiO2 to bacterial hydrogenase. FEBS Letters 1988, 234, 111–114. [Google Scholar] [CrossRef]
- Krasnovsky, A.A.; Nikandrov, V.V. The photobiocatalytic system: Inorganic semiconductors coupled to bacterial cells. FEBS letters 1987, 219, 93–96. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Shi, Z.; Wang, J.; Yang, J.; Li, X.; Song, H. Optimized design of three-dimensional multi-shell Fe3O4/SiO2/ZnO/ZnSe microspheres with type II heterostructure for photocatalytic applications. Applied Catalysis B: Environmental 2018, 227, 61–69. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M. Magnetite hybrid photocatalysis: advance environmental remediation. Reviews in Inorganic Chemistry 2016, 36, 135–151. [Google Scholar] [CrossRef]
- Fakhri, A.; Naji, M.; Nejad, P.A. Adsorption and photocatalysis efficiency of magnetite quantum dots anchored tin dioxide nanofibers for removal of mutagenic compound: toxicity evaluation and antibacterial activity. Journal of Photochemistry and Photobiology B: Biology 2017, 173, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lee, Z.; Wei, M.; Chang, C.C.; Chu, K.W. Photocatalytic hydrogen production by magnetically separable Fe3O4@ZnS and NiCo2O4@ZnS core–shell nanoparticles. International Journal of Hydrogen Energy 2015, 40, 11436–11443. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nanoparticles with ZnS and CdS. International Journal of Hydrogen Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Pati, S.P.; Kumar, S.; Das, D. Effects of magnetite nanoparticles on optical properties of zinc sulfide in fluorescent-magnetic Fe3O4/ZnS nanocomposites. Powder technology 2014, 254, 583–590. [Google Scholar] [CrossRef]
- Atla, S.B.; Lin, W.R.; Chien, T.C.; Tseng, M.J.; Shu, J.C.; Chen, C.C.; Chen, C.Y. Fabrication of Fe3O4/ZnO magnetite core shell and its application in photocatalysis using sunlight. Materials Chemistry and Physics 2018, 216, 380–386. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Mayoral, A.; García-Hernández, M.; Amara, A.B.H.; Ruiz-Hitzky, E. Sepiolite nanoplatform for the simultaneous assembly of magnetite and zinc oxide nanoparticles as photocatalyst for improving removal of organic pollutants. Journal of Hazardous Materials 2017, 340, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and photocatalytic properties of magnetically reusable Fe3O4@ZnO core/shell nanoparticles. Physica E: Low-dimensional Systems and Nanostructures 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Huang, S.; Gu, L.; Zhu, N.; Feng, K.; Yuan, H.; Lou, Z.; Li, Y.; Shan, A. Heavy metal recovery from electroplating wastewater by synthesis of mixed-Fe3O4@SiO2/metal oxide magnetite photocatalysts. Green Chemistry 2014, 16(5), 2696–2705. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvoy, S. Novel photocatalyst: titania-coated magnetite. Activity and photodissolution. The Journal of Physical Chemistry B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Low-temperature preparation of anatase titania-coated magnetite. Journal of Physics and Chemistry of Solids 2008, 69, 1980–1984. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, K.; Lu, N.; Xu, H.; Zhang, S.; Takeuchi, N.; Kobayashi, H.; Li, R. The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification. Applied Catalysis B: Environmental 2017, 218, 20–31. [Google Scholar] [CrossRef]
- Darabi, R.R.; Jahanshahi, M.; Peyravi, M. A support assisted by photocatalytic Fe3O4/ZnO nanocomposite for thin-film forward osmosis membrane. Chemical Engineering Research and Design 2018, 133, 11–25. [Google Scholar] [CrossRef]
- Hu, J.S.; Ren, L.L.; Guo, Y.G.; Liang, H.P.; Cao, A.M.; Wan, L.J.; Bai, C.L. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angewandte Chemie International Edition 2005, 44, 1269–1273, [https://pubmed.ncbi.nlm.nih.gov/15651014/]. [Google Scholar] [CrossRef]
- Nasi, L.; Calestani, D.; Besagni, T.; Ferro, P.; Fabbri, F.; Licci, F.; Mosca, R. ZnS and ZnO nanosheets from ZnS(en)0.5 precursor: nanoscale structure and photocatalytic properties. The Journal of Physical Chemistry C 2012, 116, 6960–6965. [Google Scholar] [CrossRef]
- Hitkari, G.; Singh, S.; Pandey, G. Structural, optical and photocatalytic study of ZnO and ZnO–ZnS synthesized by chemical method. Nano-Structures Nano-Objects 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Park, J.M.; Oh, S.H.; Kim, Y. ZnS–ZnO heterostructure nanorings grown under a possible early Earth atmosphere. Crystal Growth Design 2020, 20, 1196–1202. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Li, S.; Li, H.; Yan, L. Synthesis and adsorption/photocatalysis performance of pyrite FeS2. Applied surface science 2013, 268, 213–217. [Google Scholar] [CrossRef]
- Morales-Gallardo, M.V.; Ayala, A.M.; Pal, M.; Jacome, M.C.; Antonio, J.T.; Mathews, N.R. Synthesis of pyrite FeS2 nanorods by simple hydrothermal method and its photocatalytic activity. Chemical Physics Letters 2016, 660, 93–98. [Google Scholar] [CrossRef]
- Liu, L.; Kankam, I.; Zhuang, H.L. Single-layer antiferromagnetic semiconductor CoS2 with pentagonal structure. Physical Review B 2018, 98, 205425. [Google Scholar] [CrossRef]
- Faber, M.S.; Park, K.; Caban-Acevedo, M.; Santra, P.K.; Jin, S. Earth-abundant cobalt pyrite (CoS2) thin film on glass as a robust, high-performance counter electrode for quantum dot-sensitized solar cells. Journal Of Physical Chemistry Letters 2013, 4, 1843–1849. [Google Scholar] [CrossRef]
- Anand, J.S.; Rajan, R.K.; Zaidan, A.A.M. Electrosynthesized NiS2 thin films and their optical and semiconductor studies. Reports in Electrochemistry 2013, 3, 25–29. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N. Growth and characterization of semiconducting nickel sulfide nanocrystals from air-stable single-source metal organic precursors. Cogent Chemistry 2015, 1, 1030195. [Google Scholar] [CrossRef]
- Mitsui, T.; Môri, N.; Yomo, S.; Ogawa, S. Semiconductor-metal phase diagram of Co-doped NiS2. Solid State Communications 1974, 15, 917–920. [Google Scholar] [CrossRef]
- Jarrett, H.S.; Bouchard, R.J.; Gillson, J.L.; Jones, G.A.; Marcus, S.M.; Weiher, J.F. The métal-semiconductor phase diagram for NiS2− xSex. Materials Research Bulletin 1973, 8, 877–882. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J.; Lu, Z.; Xia, H. Hierarchical FeS2 nanosheet@Fe2O3 nanosphere heterostructure as promising electrode material for supercapacitors. Materials Letters 2016, 166, 223–226. [Google Scholar] [CrossRef]
- Yan, S.; Wang, K.; Zhou, F.; Lin, S.; Song, H.; Shi, Y.; Yao, J. Ultrafine Co:FeS2/CoS2 heterostructure nanowires for highly efficient hydrogen evolution reaction. ACS Applied Energy Materials 2020, 3, 514–520. [Google Scholar] [CrossRef]
- Wang, K.; Song, H.; Lin, Z.; Gao, Y.; Wu, H.; Yan, S.; Wang, J.; Shi, Y. Improving hydrogen evolution performance of Co:FeS2/CoS2 nano-heterostructure at elevated temperatures. Materials Express 2019, 9, 786–791. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, X.; Chen, Y.; Zhang, P.; Sui, M.; Liu, H.; Sun, X. NiS2@MoS2 nanospheres anchored on reduced graphene oxide: a novel ternary heterostructure with enhanced electromagnetic absorption property. Nanomaterials 2019, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kang, M. Physicochemical properties of core/shell structured pyrite FeS2/anatase TiO2 composites and their photocatalytic hydrogen production performances. Current Applied Physics 2013, 13, 1482–1489. [Google Scholar] [CrossRef]
- Rashid, J.; Saleem, S.; Awan, S.U.; Iqbal, A.; Kumar, R.; Barakat, M.A.; Arshad, M.; Zaheer, M.; Rafique, M.; Awad, M. Stabilized fabrication of anatase-TiO2/FeS2 (pyrite) semiconductor composite nanocrystals for enhanced solar light-mediated photocatalytic degradation of methylene blue. RSC Advances 2018, 8, 11935–11945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Meng, Z.D.; Ghosh, T.; Oh, W.C. Enhanced photocatalytic efficiency of nanoscale NiS2/TiO2 catalysts synthesized by hydrothermal and sol-gel method. Journal of the Korean Ceramic Society 2012, 49, 135–141. [Google Scholar] [CrossRef]
- Zeda, M.E.N.G.; Wonchun, O.H. Photodegradation of organic dye by CoS2 and carbon (C60, Graphene, CNT)/TiO2 composite sensitizer. Chinese Journal of Catalysis 2012, 33, 1495–1501. [Google Scholar] [CrossRef]
- Zhu, L.; Jo, S.B.; Ye, S.; Ullah, K.; Meng, Z.D.; Oh, W.C. A green and direct synthesis of photosensitized CoS2–graphene/TiO2 hybrid with high photocatalytic performance. Journal of Industrial and Engineering Chemistry 2015, 22, 264–271. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, Y.; Hu, Z.; Xiao, B. Investigation on preparation of pyrite tailings-based mineral admixture with photocatalytic activity. Construction and Building Materials 2017, 138, 26–34. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y. On the origin of life in the zinc world: 1. Photosynthesizing, porous edifices built of hydrothermally precipitated zinc sulfide as cradles of life on Earth. Biology Direct 2009, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Galperin, M.Y. On the origin of life in the zinc world. 2. Validation of the hypothesis on the photosynthesizing zinc sulfide edifices as cradles of life on Earth. Biology direct 2009, 4, 27. [Google Scholar] [CrossRef]
- Rao, H.; Lu, Z.; Liu, X.; Ge, H.; Zhang, Z.; Zou, P.; He, H.; Wang, Y. Visible light-driven photocatalytic degradation performance for methylene blue with different multi-morphological features of ZnS. RSC Advances 2016, 6, 46299–46307. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O.P. Photocatalytic degradation of organic dyes under UV–Visible light using capped ZnS nanoparticles. Solar Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Mani, S.K.; Saroja, M.; Venkatachalam, M.; Rajamanickam, T. Antimicrobial activity and photocatalytic degradation properties of zinc sulfide nanoparticles synthesized by using plant extracts. Journal of Nanostructures 2018, 8, 107–118. [Google Scholar] [CrossRef]
- Rafiq, A.; Imran, M.; Ikram, M.; Naz, M.; Aqeel, M.; Majeed, H.; Hussain, S.G.; Ali, S. Photocatalytic and catalytic degradation of organic dye by uncapped and capped ZnS quantum dots. Materials Research Express 2019, 6, 055801. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proceedings of the National Academy of Sciences 2012, 109, E821–E830, [https://pubmed.ncbi.nlm.nih.gov/22331915/]. [Google Scholar] [CrossRef]
- Guzman, M.I. Abiotic photosynthesis: from prebiotic chemistry to metabolism. In Origins of Life: The Primal Self-Organization; Springer: Berlin, Heidelberg; 2011; pp. 85–105. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Galperin, M.Y. On the abundance of zinc in the evolutionarily old protein domains. Proceedings of the National Academy of Sciences 2010, 107, E137–E137, [https://pubmed.ncbi.nlm.nih.gov/20693418/]. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Yang, B.; Liu, X.; Yang, Y.; Su, W. Photocatalytic reversible amination of α-keto acids on a ZnS surface: implications for the prebiotic metabolism. Chemical Communications 2012, 48, 2146–2148, [https://pubmed.ncbi.nlm.nih.gov/22237955/]. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Liu, X.; Yang, Y.; Su, W. Enhanced photocatalytic performance of ZnS for reversible amination of α-oxo acids by hydrothermal treatment. Origins of Life and Evolution of Biospheres 2012, 42, 263–273, [https://pubmed.ncbi.nlm.nih.gov/22638837/]. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guzman, M.I. CO2 reduction under periodic illumination of ZnS. Journal of Physical Chemistry C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. Photocatalytic reduction of fumarate to succinate on ZnS mineral surfaces. Journal of Physical Chemistry C 2016, 120, 7349–7357. [Google Scholar] [CrossRef]
- Zhang, X.V.; Martin, S.T. Driving parts of Krebs cycle in reverse through mineral photochemistry. Journal of the American Chemical Society 2006, 128, 16032–16033. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.I.; Martin, S.T. Prebiotic metabolism: production by mineral photoelectrochemistry of α-ketocarboxylic acids in the reductive tricarboxylic acid cycle. Astrobiology 2009, 9, 833–842, [https://pubmed.ncbi.nlm.nih.gov/19968461/]. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.I.; Martin, S.T. Photo-production of lactate from glyoxylate: how minerals can facilitate energy storage in a prebiotic world. Chemical Communications 2010, 46, 2265–2267. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.I.; Martin, S.T. Oxaloacetate-to-malate conversion by mineral photoelectrochemistry: implications for the viability of the reductive tricarboxylic acid cycle in prebiotic chemistry. International Journal of Astrobiology 2008, 7, 271–278. [Google Scholar] [CrossRef]
- Zhang, X.V.; Ellery, S.P.; Friend, C.M.; Holland, H.D.; Michel, F.M.; Schoonen, M.A.; Martin, S.T. Photodriven reduction and oxidation reactions on colloidal semiconductor particles: Implications for prebiotic synthesis. Journal of Photochemistry and Photobiology A: Chemistry 2007, 185, 301–311. [Google Scholar] [CrossRef]
- Mamajanov, I.; Caudan, M.; Jia, T.Z. Protoenzymes: The case of hyperbranched polymer-scaffolded ZnS nanocrystals. Life 2020, 10, 150, [https://pubmed.ncbi.nlm.nih.gov/32823487/]. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Influence of Fe, Ni, and Cu doping on the photocatalytic efficiency of ZnS: implications for prebiotic chemistry, 2016, arXiv preprint arXiv:1610.00859.
- Doane, T.A. A survey of photogeochemistry. Geochemical transactions 2017, 18, 1, [https://pubmed.ncbi.nlm.nih.gov/28246525/]. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G. From light to life. Origins of Life and Evolution of Biospheres 2015, 45, 347–350, [https://pubmed.ncbi.nlm.nih.gov/26105723/]. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.R. Denitrification in sunlight. Nature 1934, 134, 572–573. [Google Scholar] [CrossRef]
- Rao, G.G.; Varadanam, C.I. Photo-ammonification of organic nitrogenous compounds in the soil. Nature 1938, 142, 618. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Strampach, N.; Hui, L.N.; Palmer, M.R.; Salehi, J. Nitrogen photoreduction on desert sands under sterile conditions. Proceedings of the National Academy of Sciences 1983, 80, 3873–3876, [https://pubmed.ncbi.nlm.nih.gov/16593330/]. [Google Scholar] [CrossRef]
- Kim, J.D. The evolution of biological geochemical electron transfer reactions. PhD Thesis, State University of New Jersey, New Brunswick, 2013. [Google Scholar]
- Jelen, B.I.; Giovannelli, D.; Falkowski, P.G. The role of microbial electron transfer in the coevolution of the biosphere and geosphere. Annual Review of Microbiology 2016, 70, 45–62, [https://pubmed.ncbi.nlm.nih.gov/27297124/]. [Google Scholar] [CrossRef]
- Shuey, R.T. Semiconducting ore minerals; Elsevier Scientific Publishing Company: Amsterdam – Oxford – New York; 1975; pp. 1-415. [CrossRef]
- Borutzky, B.Y. Essays on Fundamental and Genetic Mineralogy: 1. What is the Mineral and Mineral Species? New Data on Minerals 2005, 40, 159–166. [Google Scholar]
- Borutzky, B.Y. Essays on Fundamental and Genetic Mineralogy: 2. The practive of working out “natural genetic” systematics of minerals. New Data on Minerals 2006, 41, 162-171.
- Barawi, M.; Ferrer, I.J.; Flores, E.; Yoda, S.; Ares, J.R.; Sánchez, C. Hydrogen photoassisted generation by visible light and an earth abundant photocatalyst: pyrite (FeS2). Journal of Physical Chemistry C 2016, 120, 9547–9552. [Google Scholar] [CrossRef]
- Mateo-Marti, E.; Galvez-Martinez, S.; Gil-Lozano, C.; Zorzano, M.P. Pyrite-induced UV-photocatalytic abiotic nitrogen fixation: implications for early atmospheres and life. Scientific Reports 2019, 9, 15311. [Google Scholar] [CrossRef]
- Puthussery, J.; Seefeld, S.; Berry, N.; Gibbs, M.; Law, M. Colloidal iron pyrite (FeS2) nanocrystal inks for thin-film photovoltaics. Journal of the American Chemical Society 2011, 133, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Kirkeminde, A.; Gong, M.; Ren, S. The renaissance of iron pyrite photovoltaics: progress, challenges, and perspectives. In Low-cost Nanomaterials; Springer: London; 2014; pp. 137-166. [CrossRef]
- Macpherson, H.A.; Stoldt, C.R. Iron pyrite nanocubes: size and shape considerations for photovoltaic application. Acs Nano 2012, 6, 8940–8949. [Google Scholar] [CrossRef]
- Dasbach, R.; Willeke, G.; Blenk, O. Iron sulfide for photovoltaics. MRS Bulletin 1993, 18, 56–60. [Google Scholar] [CrossRef]
- Li, W.; Döblinger, M.; Vaneski, A.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Pyrite nanocrystals: shape-controlled synthesis and tunable optical properties via reversible self-assembly. Journal of Materials Chemistry 2011, 21, 17946–17952. [Google Scholar] [CrossRef]
- Tian, A.; Xu, Q.; Shi, X.; Yang, H.; Xue, X.; You, J.; Wang, X.; Dong, C.; Yan, X.; Zhou, H. Pyrite nanotube array films as an efficient photocatalyst for degradation of methylene blue and phenol. RSC Advances 2015, 5, 62724–62731. [Google Scholar] [CrossRef]
- Moradi, M.; Kalantary, R.R.; Esrafili, A.; Jafari, A.J.; Gholami, M. Visible light photocatalytic inactivation of Escherichia coli by natural pyrite assisted by oxalate at neutral pH. Journal of Molecular Liquids 2017, 248, 880–889. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Singh, S. Temperature and pressure behaviour of narrow-gap semiconductors including galena. Current Applied Physics 2014, 14, 496–507. [Google Scholar] [CrossRef]
- Schuhmann, D.; Vanel, P.; Talib, A. influence of the semiconductor character of some minerals upon the adsorption of surfactants-application to the galena xanthate system. Journal de Chimie Physique et de Physico-Chimie Biologique 1988, 85, 551–554. [Google Scholar] [CrossRef]
- Thompson, K.C.; Simkovich, G.; Aplan, F.F. Flotation and electrokinetic properties of the semiconductor, galena. Journal of The Electrochemical Society 1984, 131, c99. [Google Scholar] [CrossRef]
- Martínez, M.D.C.L. Influencia del carácter semiconductor de la galena sobre su potencial de electrodo y sobre la adsorción del xantato. Doctoral dissertation, Universidad Complutense de Madrid, Italy, 1975.
- Dimitrova, S.; Moldovanova, M. Semiconductor properties of pure galena crystals. Physica Status Solidi 1965, 8, 173–176. [Google Scholar]
- Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Harris, J.; Korgel, B.A. Pyrite nanocrystal solar cells: promising, or fool’s gold? The Journal of Physical Chemistry Letters 2012, 3, 2352–2356. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, Y.; Exstrom, C.L.; Darveau, S.A.; Huang, J. Air stable, photosensitive, phase pure iron pyrite nanocrystal thin films for photovoltaic application. Nano Letters 2011, 11, 4953–4957. [Google Scholar] [CrossRef]
- Du, H.; Yang, C.; Pu, W.; Zeng, L.; Gong, J. Enhanced electrochemical reduction of N2 to ammonia over pyrite FeS2 with excellent selectivity. ACS Sustainable Chemistry and Engineering 2020, 8, 10572–10580. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy positions of oxide semiconductors and photocatalysis with iron complex oxides. Journal of Solid State Chemistry 1996, 126, 227–234. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons; 2003; 793 p.
- Cartwright, J.H.; García-Ruiz, J.M.; Novella, M.L.; Otálora, F. Formation of chemical gardens. Journal of Colloid and Interface Science 2002, 256, 351–359. [Google Scholar] [CrossRef]
- Barge, L.M.; Cardoso, S.S.; Cartwright, J.H.; Cooper, G.J.; Cronin, L.; De Wit, A.; Doloboff, I.J.; Escribano, B.; Goldstein, R.E.; Haudin, F.; Jones, D.E.; Mackay, A.L.; Maselko, J.; Pagano, J.J.; Pantaleone, J.; Russel, M.J.; Sainz-Díaz, C.I.; Steinbock, O.; Stone, D.A.; Tanimoto, Y.; Thomas, N.L. From chemical gardens to chemobrionics. Chemical reviews 2015, 115, 8652–8703. [Google Scholar] [CrossRef]
- Allamandola, L.J.; Sandford, S.A.; Wopenka, B. Interstellar polycyclic aromatic hydrocarbons and carbon in interplanetary dust particles and meteorites. Science 1987, 237, 56–59, [https://pubmed.ncbi.nlm.nih.gov/17813622/]. [Google Scholar] [CrossRef] [PubMed]
- Lovas, F.J.; McMahon, R.J.; Grabow, J.U.; Schnell, M.; Mack, J.; Scott, L.T.; Kuczkowski, R.L. Interstellar chemistry: a strategy for detecting polycyclic aromatic hydrocarbons in space. Journal of the American Chemical Society 2005, 127, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, H.; Koretsune, T.; Hotta, C.; Takeya, J.; Kimura, T.; Wakabayashi, Y. Large surface relaxation in the organic semiconductor tetracene. Nature Communications 2014, 5, 5400. [Google Scholar] [CrossRef] [PubMed]
- Hepp, A.; Heil, H.; Weise, W.; Ahles, M.; Schmechel, R.; von Seggern, H. Light-emitting field-effect transistor based on a tetracene thin film. Physical Review Letters 2003, 91, 157406. [Google Scholar] [CrossRef] [PubMed]
- Blasberger, A.; Behar, E.; Perets, H.B.; Brosch, N.; Tielens, A.G. Observational evidence linking interstellar UV absorption to PAH molecules. The Astrophysical Journal 2017, 836, 173. [Google Scholar] [CrossRef]
- Koch, N. Organic electronic devices and their functional interfaces. Chem. Phys. Chem 2007, 8, 1438–1455. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takeya, J. Organic field-effect transistors using single crystals. Science and Technology of Advanced Materials 2009, 10, 024314. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y. Organic semiconductors for organic field-effect transistors. Science and Technology of Advanced Materials 2009, 10, 024313. [Google Scholar] [CrossRef]
- Zaia, D.A.M. A review of adsorption of amino acids on minerals: was it important for origin of life? Amino Acids 2004, 27, 113–118, [https://pubmed.ncbi.nlm.nih.gov/15309580/]. [Google Scholar] [CrossRef]
- Klabunovskii, E.I. Can enantiomorphic crystals like quartz play a role in the origin of homochirality on earth? Astrobiology 2001, 1, 127–131, [https://pubmed.ncbi.nlm.nih.gov/12467116/]. [Google Scholar] [CrossRef]
- Fedo, C.M.; Whitehouse, M.J. Metasomatic origin of quartz-pyroxene rock, Akilia, Greenland, and implications for Earth's earliest life. Science 2002, 296, 1448–1452, [https://pubmed.ncbi.nlm.nih.gov/12029129/]. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Rasmussen, S.; Cleaves, J.; Chen, L. Experimentally tracing the key steps in the origin of life: the aromatic world. Astrobiology 2006, 6, 490–520, [https://pubmed.ncbi.nlm.nih.gov/16805704/]. [Google Scholar] [CrossRef]
- Menor-Salván, C.; Ruiz-Bermejo, M.; Osuna-Esteban, S.; Muñoz-Caro, G.; Veintemillas-Verdaguer, S. Synthesis of polycyclic aromatic hydrocarbons and acetylene polymers in ice: a prebiotic scenario. Chemistry Biodiversity 2008, 5, 2729–2739, [https://pubmed.ncbi.nlm.nih.gov/19089832/]. [Google Scholar] [CrossRef]
- Groen, J.; Deamer, D.W.; Kros, A.; Ehrenfreund, P. Polycyclic aromatic hydrocarbons as plausible prebiotic membrane components. Origins of Life and Evolution of Biospheres 2012, 42, 295–306, [https://pubmed.ncbi.nlm.nih.gov/22798228/]. [Google Scholar] [CrossRef]
- Morowitz, H.J. Beginnings of cellular life: metabolism recapitulates biogenesis. Yale University Press: New Haven – London; 1993; 210 p.
- Girerd, J.J.; Philouze, C.; Anxolabehere-Mallart, E.; Sainton, J.; Blondin, G.; Frapart, Y. Manganese models for photosynthesis: from self-assembly to design. Journal of Inorganic Biochemistry 1995, 59, 610. [Google Scholar] [CrossRef]
- Hansen, M, Troppmann, S, König, B. Artificial photosynthesis at dynamic self-assembled interfaces in water. Chemistry – A European Journal, 2016; 22 , 58-72. [CrossRef]
- Hsin, J.; Chandler, D.E.; Gumbart, J.; Harrison, C.B.; Sener, M.; Strumpfer, J.; Schulten, K. Self-assembly of photosynthetic membranes. Chem. Phys. Chem. 2010, 11, 1154–1159. [Google Scholar] [CrossRef]
- Lee, J.S.; Nam, D.H.; Kuk, S.K.; Park, C.B. Near-infrared-light-driven artificial photosynthesis by nanobiocatalytic assemblies. Chemistry, 2014, 20, 3584–3588, [https://pubmed.ncbi.nlm.nih.gov/24615772/]. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.; Lee, J.S.; Park, C.B. Self-assembled light-harvesting peptide nanotubes for mimicking natural photosynthesis. Angewandte Chemie International Edition 2012, 51, 517–520, [https://pubmed.ncbi.nlm.nih.gov/21976303/]. [Google Scholar] [CrossRef] [PubMed]
- Cardona, T. Reconstructing the origin of oxygenic photosynthesis: do assembly and photoactivation recapitulate evolution? Frontiers in Plant Science 2016, 7, 257, [https://pubmed.ncbi.nlm.nih.gov/26973693/]. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Zhang, R.; Lan, D.; Ge, J. Synthesis of magnetite–semiconductor–metal trimer nanoparticles through functional modular assembly: a magnetically separable photocatalyst with photothermic enhancement for water reduction. ACS Applied Materials Interfaces 2018, 10, 4929–4936, [https://pubmed.ncbi.nlm.nih.gov/29345458/]. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Dékány, I.; Fendler, J.H. Ultrathin graphite oxide–polyelectrolyte composites prepared by self-assembly: Transition between conductive and non-conductive states. Advanced Materials 1996, 8, 637–641. [Google Scholar] [CrossRef]
- Graetzel, M. Artificial photosynthesis, very efficient visible light energy harvesting, and conversion by spectral sensitization of fractal oxide semiconductor films. In Photochemical Energy Conversion (Proc. Int. Conf. Photochem. Convers. Storage Solar Energy), 1989.
- Guijarro, N.; Formal, F.L.; Sivula, K. Artificial photosynthesis with semiconductor–liquid junctions. CHIMIA International Journal for Chemistry 2015, 69, 30–40, [https://pubmed.ncbi.nlm.nih.gov/26507086/]. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Domen, K. Introductory lecture: sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis. Faraday Discussions 2017, 198, 11–35, [https://pubmed.ncbi.nlm.nih.gov/28272623/]. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Long, J.; Yang, L.; Chen, W.; Dai, W.; Fu, X.; Wang, X. Organic semiconductor for artificial photosynthesis: water splitting into hydrogen by a bioinspired C3N3S3 polymer under visible light irradiation. Chemical Science 2011, 2, 1826–1830. [Google Scholar] [CrossRef]
- Zhou, H.; Li, P.; Liu, J.; Chen, Z.; Liu, L.; Dontsova, D.; Yan, R.; Fan, T.; Zhang, D.; Ye, J. Biomimetic polymeric semiconductor based hybrid nanosystems for artificial photosynthesis towards solar fuels generation via CO2 reduction. Nano Energy 2016, 25, 128–135. [Google Scholar] [CrossRef]
- Pang, H.; Masuda, T.; Ye, J. Semiconductor-based photoelectrochemical conversion of carbon dioxide: stepping towards artificial photosynthesis. Chemistry–an Asian Journal 2018, 13, 127-142. [CrossRef]
- Hoffmann, M.R.; Moss, J.A.; Baum, M.M. Artificial photosynthesis: semiconductor photocatalytic fixation of CO2 to afford higher organic compounds. Dalton Transactions 2011, 40, 5151–5158, [https://pubmed.ncbi.nlm.nih.gov/21373667/]. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Clayton, R.K. The first step in photosynthesis: evidence for its electronic nature. Proceedings of the National Academy of Sciences of the USA 1960, 46, 769–776, [https://pubmed.ncbi.nlm.nih.gov/16590669/]. [Google Scholar] [CrossRef] [PubMed]
- Graetzel, M. Artificial photosynthesis: water cleavage into hydrogen and oxygen by visible light. Accounts of Chemical Research 1981, 14, 376–384. [Google Scholar] [CrossRef]
- Duret, A.; Grätzel, M. Visible light-induced water oxidation on mesoscopic α-Fe2O3 films made by ultrasonic spray pyrolysis. Journal of Physical Chemistry B 2005, 109, 17184–17191. [Google Scholar] [CrossRef]
- Le Formal, F.; Grätzel, M.; Sivula, K. Controlling photoactivity in ultrathin hematite films for solar water-splitting. Advanced Functional Materials 2010, 20, 1099–1107. [Google Scholar] [CrossRef]
- Ravirajan, P.; Peiró, A.M.; Nazeeruddin, M.K.; Graetzel, M.; Bradley, D.D.; Durrant, J.R.; Nelson, J. Hybrid polymer/zinc oxide photovoltaic devices with vertically oriented ZnO nanorods and an amphiphilic molecular interface layer. The Journal of Physical Chemistry B 2006, 110, 7635–7639. [Google Scholar] [CrossRef]
- Mershin, A.; Matsumoto, K.; Kaiser, L.; Yu, D.; Vaughn, M.; Nazeeruddin, M.K.; Bruce, B.D.; Graetzel, M.; Zhang, S. Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO. Scientific Reports 2012, 2, 234. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Chandiran, A.K.; Nazeeruddin, M.; Grätzel, M. Low temperature dye-sensitized solar cells based on conformal thin zinc oxide overlayer on mesoporous insulating template by atomic layer deposition. Scientia Iranica - Transactions on Nanotechnology (F) 2014, 21, 2479–2484. [Google Scholar]
- Kumar, M.H.; Yantara, N.; Dharani, S.; Graetzel, M.; Mhaisalkar, S.; Boix, P.P.; Mathews, N. Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chemical Communications 2013, 49, 11089–11091, [https://pubmed.ncbi.nlm.nih.gov/24141601/]. [Google Scholar] [CrossRef]
- Nguyen, M.; Tran, P.D.; Pramana, S.S.; Lee, R.L.; Batabyal, S.K.; Mathews, N.; Wong, L.H.; Graetzel, M. In situ photo-assisted deposition of MoS2 electrocatalyst onto zinc cadmium sulphide nanoparticle surfaces to construct an efficient photocatalyst for hydrogen generation. Nanoscale 2013, 5, 1479–1482. [Google Scholar] [CrossRef]
- Lima-de-Faria, A. Evolution without selection: Form and function by autoevolution. Elsevier: Amsterdam, Netherlands; 1988; 372 p.
- Dyer, B.D; Schuster, P.; Holm, N.G. A. Lima-de-Faria, evolution without selection form and function by autoevolution. Origins of Life and Evolution of Biospheres 1989, 19, 645–652. [Google Scholar] [CrossRef]
- Hughes, A.L. Evolution without selection: form and function in autoevolution. By A. Lima-de-Faria. Molecular Biology and Evolution 1990, 7, 634. [Google Scholar]
- Gerischer, H.; Michel-Beyerle, M.E.; Rebentrost, F.; Tributsch, H. Sensitization of charge injection into semiconductors with large band gap. Electrochimica Acta 1968, 13, 1509–1515. [Google Scholar] [CrossRef]
- Tributsch, H.; Calvin, M. Electrochemistry of excited molecules: photo-electrochemical reactions of chlorophylls. Photochemistry and Photobiology 1971, 14, 95–112. [Google Scholar] [CrossRef]
- Yi, C.; Giordano, F.; Cevey-Ha, N.L.; Tsao, H.N.; Zakeeruddin, S.M.; Grätzel, M. Influence of structural variations in push–pull zinc porphyrins on photovoltaic performance of dye-sensitized solar cells. Chem. Sus. Chem. 2014, 7, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Grätzel, M. Light induced redox reactions of water soluble porphyrins, sensitization of hydrogen generation from water by zinc porphyrin derivatives. Helvetica Chimica Acta 1980, 63, 478–485. [Google Scholar] [CrossRef]
- Pileni, M.P.; Graetzel, M. Zinc porphyrin sensitized reduction of simple and functional quinones in micellar systems. Journal of Physical Chemistry 1980, 84, 1822–1825. [Google Scholar] [CrossRef]
- Hurst, J.K.; Lee, L.Y.; Graetzel, M. Photoredox behavior of zinc(II) porphyrins in vesicle assemblies. Journal of the American Chemical Society 1983, 105, 7048–7056. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Vlachopoulos, N.; Krishnan, V.; Monnier, A.; Graetzel, M. Sensitization of titanium dioxide in the visible light region using zinc porphyrins. Journal of Physical Chemistry 1987, 91, 2342–2347. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Shelnutt, J.A.; Graetzel, M. Sensitization and photoredox reactions of zinc(II) and antimony(V) uroporphyrins in aqueous media. Inorganic Chemistry 1988, 27, 2820–2825. [Google Scholar] [CrossRef]
- Yum, J.H.; Jang, S.R.; Humphry-Baker, R.; Grätzel, M.; Cid, J.J.; Torres, T.; Nazeeruddin, M.K. Effect of coadsorbent on the photovoltaic performance of zinc pthalocyanine-sensitized solar cells. Langmuir 2008, 24, 5636–5640, [https://pubmed.ncbi.nlm.nih.gov/18435553/]. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, L.; Kumar, C.V.; Reddy, P.Y.; Yum, J.H.; Grätzel, M.; Nazeeruddin, M.K. Unsymmetrical extended π-conjugated zinc phthalocyanine for sensitization of nanocrystalline TiO2 films. Journal of Chemical Sciences 2009, 121, 75. [Google Scholar] [CrossRef]
- Ince, M.; Cardinali, F.; Yum, J.H.; Martínez-Díaz, M.V.; Nazeeruddin, M.K.; Grätzel, M.; Torres, T. Convergent synthesis of near-infrared absorbing,“push–pull”, bisthiophene-substituted, zinc(II) phthalocyanines and their application in dye-sensitized solar cells. Chemistry–A European Journal 2012, 18, 6343–6348, [https://pubmed.ncbi.nlm.nih.gov/22473900/]. [Google Scholar] [CrossRef]
- Molina, D.; Ruiz-Preciado, M. A.; Sadegh, F.; Álvaro-Martins, M.J.; Grätzel, M.; Hagfeldt, A.; Sastre-Santos, Á. p-Phenylene-bridged zinc phthalocyanine-dimer as hole-transporting material in perovskite solar cells. Journal of Porphyrins and Phthalocyanines 2019, 23, 546–553. [Google Scholar] [CrossRef]
- Xiong, J.; Bauer, C.E. A cytochrome b origin of photosynthetic reaction centers: an evolutionary link between respiration and photosynthesis. Journal of Molecular Biology 2002, 322, 1025–1037, [https://pubmed.ncbi.nlm.nih.gov/12367526/]. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. A redox switch hypothesis for the origin of two light reactions in photosynthesis. FEBS Letters 2005, 579, 963–968, [https://pubmed.ncbi.nlm.nih.gov/15710376/]. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, J.; Wang, X. Semiconductor–redox catalysis promoted by metal–organic frameworks for CO2 reduction. Physical Chemistry Chemical Physics 2014, 16, 14656–14660, [https://pubmed.ncbi.nlm.nih.gov/24921181/]. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, M. Catalysis of redox processes at illuminated semiconductor electrodes. Journal of the Electrochemical Society 1983, 130, C124. [Google Scholar]
- Mei, B.; Han, K.; Mul, G. Driving surface redox reactions in heterogeneous photocatalysis: the active state of illuminated semiconductor-supported nanoparticles during overall water-splitting. ACS Catalysis 2018, 8, 9154–9164. [Google Scholar] [CrossRef]
- Annadhasan, M.; Selvam, K.; Swaminathan, M. A combined-redox synthesis of 2-alkylbenzimidazoles from 2-nitroanilines by semiconductor photocatalysis. Synthetic Communications 2012, 42, 1500–1508. [Google Scholar] [CrossRef]
- Zhou, R. Semiconductor photocatalysis: mechanisms, photocatalytic performances and lifetime of redox carriers. PhD Thesis, University of Kentucky, Lexington, Kentucky, USA, 2017. [Google Scholar]
- Dukovic, G. Excited state processes in semiconductor nanocrystals and their relationships with light-driven multi-electron catalysis. ECS Meeting Abstracts 2019, 41, 1956. [Google Scholar] [CrossRef]
- Hongbo, L. Auto-catalysis and cross-catalysis in mineralization of enriched ore of anshan-type iron deposits. Journal of Northeastern University 1995, P618.310.1 [in Chineese].
- Tóth, J. Gradient systems are cross-catalytic. Reaction Kinetics and Catalysis Letters 1979, 12, 253–257. [Google Scholar] [CrossRef]
- Basza, G.; Beck, M.T. Autocatalysis, cross-catalysis, self-inhibition and crosswise inhibition: Pathways into exotic chemical kinetics. Acta Chim. Hung 1972, 73, 26–37. [Google Scholar]
- Rastogi, R.P.; Mathur, P. Complex Dynamics in Systems Involving Both Cross Catalytic and Autocatalytic Processes. Proceedings of the Indian National Science Academy-Part A: Physical Sciences 2009, 75, 159. [Google Scholar]
- Wu, Y.; Shi, J.; Ding, F.; Zhao, J.; Zou, X.; Wang, M.; Zhang, S.; Tong, Z.; Zhang, S.; Jiang, Z. Integrated enzyme-photocatalysis system for carbon dioxide conversion. Scientia Sinica Chimica 2016, 47, 315–329. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.L.; Guan, Z.; He, Y.H. Concurrent asymmetric reactions combining photocatalysis and enzyme catalysis: direct enantioselective synthesis of 2,2-disubstituted indol-3-ones from 2-arylindoles. Angewandte Chemie 2019, 131, 124–130, [https://pubmed.ncbi.nlm.nih.gov/30421485/]. [Google Scholar] [CrossRef]
- Ju, E.; Dong, K.; Wang, Z.; Zhang, Y.; Cao, F.; Chen, Z.; Pu, F.; Ren, J.; Qu, X. Confinement of reactive oxygen species in an artificial-enzyme-based hollow structure to eliminate adverse effects of photocatalysis on UV filters. Chemistry–A European Journal, 2017, 23, 13518-13524; [https://pubmed.ncbi.nlm.nih.gov/28741846/]. [Google Scholar] [CrossRef]
- Yi, H.; Yan, M.; Huang, D.; Zeng, G.; Lai, C.; Li, M.; Huo, X.; Qin, L.; Liu, S.; Liu, X.; Li, B.; Wang, H.; Shen, M.; Fu, Y.; Guo, X. Synergistic effect of artificial enzyme and 2D nano-structured Bi2WO6 for eco-friendly and efficient biomimetic photocatalysis. Applied Catalysis B: Environmental 2019, 250, 52-62. [CrossRef]
- Yadav, R.K.; Baeg, J.O.; Oh, G.H.; Park, N.J.; Kong, K.J.; Kim, J.; Hwang, D.W.; Biswas, S.K. A photocatalyst–enzyme coupled artificial photosynthesis system for solar energy in production of formic acid from CO2. Journal of the American Chemical Society 2012, 134, 11455–11461, [https://pubmed.ncbi.nlm.nih.gov/22769600/]. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Wang, Y.; Guan, Y.; Deng, K.; Lv, K.; Sun, J.; Li, Z.; Li, M. Photocatalytic properties and electrochemical characteristic of a novel biomimetic oxygenase enzyme photocatalyst iron(II) tetrahydroxymethyltetra(1,4-dithiin)porphyrazine for the degradation of organic pollutants. Journal of Molecular Catalysis A: Chemical 2013, 372, 114–120. [Google Scholar] [CrossRef]
- Li, W.; Pei, X.; Deng, F.; Luo, X.; Li, F.; Xiao, Y. Bio-inspired artificial functional photocatalyst: biomimetic enzyme-like TiO2/reduced graphene oxide nanocomposite with excellent molecular recognition ability. Nanotechnology 2015, 26, 175706. [Google Scholar] [CrossRef] [PubMed]
- Crocker, L.; Koehler, P.; Bernhard, P.; Kerbs, A.; Euser, T.; Fruk, L. Enzyme-inspired flavin–polydopamine as a biocompatible nanoparticle photocatalyst. Nanoscale Horizons 2019, 4, 1318–1325. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Artemkina, S.B.; Poltarak, P.A.; Fedorov, V.E. Metal free MoS2 2D sheets as a peroxidase enzyme and visible-light-induced photocatalyst towards detection and reduction of Cr(VI) ions. New Journal of Chemistry 2018, 42, 16919–16929. [Google Scholar] [CrossRef]
- Inoue, H. Fixation of carbon-dioxide using photocatalyst and enzyme. Denki Kagaku 1993, 61 (1), 113–114. [Google Scholar]
- Shen, X.; Zhu, L.; Liu, G.; Tang, H.; Liu, S.; Li, W. Photocatalytic removal of pentachlorophenol by means of an enzyme-like molecular imprinted photocatalyst and inhibition of the generation of highly toxic intermediates. New Journal of Chemistry 2009, 33, 2278–2285. [Google Scholar] [CrossRef]
- Habibi, N.; Etemadifari, Z.; Dianati, M. Magnetic nanocomposite thin film photocatalyst and cell extract enzyme biocatalyst in application of nanobiotechnology for development of a photo-bio desulfurization system. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 2016, 46, 857–860. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Wang, X.; Ding, H.; Zeng, C.; Yang, X.; Hao, R.; Wang, C.; Santosh, M. Photoelectrons from minerals and microbial world: A perspective on life evolution in the early Earth. Precambrian Research 2013, 231, 401–408. [Google Scholar] [CrossRef]
- Varfolomeev, S.D.; Bachurin, S.O.; Osipov, I.V.; Aliev, K.V.; Berezin, I.V.; Kabanov, V.A. Bioelectro-catalysis-enzyme active-center-semiconductor matrix electron-transfer. Doklady Akademii Nauk SSSR 1978, 239, 348–351. [Google Scholar]
- Zhang, H.; Wu, J.; Han, J.; Wang, L.; Zhang, W.; Dong, H.; Li, C.; Wang, Y. Photocatalyst/enzyme heterojunction fabricated for high-efficiency photoenzyme synergic catalytic degrading Bisphenol A in water. Chemical Engineering Journal 2020, 385, 123764. [Google Scholar] [CrossRef]
- Wang, F.X.; Ye, C.; Mo, S.; Liao, L.L.; Luo, H.Q.; Li, N.B. A novel photoelectrochemical sensing platform based on Fe2O3@Bi2S3 heterojunction for an enzymatic process and enzyme activity inhibition reaction. Sensors and Actuators B: Chemical 2019, 288, 202–209. [Google Scholar] [CrossRef]
- Koike, K.; Takagi, D.; Hashimoto, M.; Hashimoto, T.; Inoue, T.; Ogata, K.I.; Sasa, S.; Inoue, M.; Yano, M. Characteristics of enzyme-based ZnO/Zn0.7Mg0.3O heterojunction field-effect transistor as glucose sensor. Japanese Journal of Applied Physics 2009, 48, 04C081. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kobayashi, K.; Saito, T. (1996). Martian Soil Analysis. Its implication for life on mars.In: Chemical Evolution: Physics of the Origin and Evolution of Life (Proceedings of the Fourth Trieste Conference on Chemical Evolution, Trieste, Italy, 4-8 September 1995); Kluwer Academic Publishers, Dordrecht – Boston – London, Netherlands – USA – UK, 1996; pp. 389-398. [CrossRef]
- Fox, A. C.; Eigenbrode, J. L.; Freeman, K. H. Radiolysis of macromolecular organic material in Mars-relevant mineral matrices. Journal of Geophysical Research: Planetsn 2019, 124, 3257–3266. [Google Scholar] [CrossRef]
- Ochiai, E.I. Inorganic Chemistry of Earliest Sediments: Bioinorganic Chemical Aspects of the Origin and Evolution of Life. In Proceedings of the NATO Advanced Study Institute held at Maratea (Italy, June 1–12 , 1981); Ponnamperuma, C., Ed.; D.Reidel Publishing Company, Dordrecht : Hollanf / Boston : USA / London: England, 1981, 235-276. [CrossRef]
- Dai, H.; Zhang, S.; Xu, G.; Peng, Y.; Gong, L.; Li, X.; Li, Y.; Lin, Y.; Chen, G. Highly photoactive heterojunction based on gC3N4 nanosheets decorated with dendritic zinc (II) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of choline. RSC Advances 2014, 4, 58226–58230. [Google Scholar] [CrossRef]
- Giersch, C. Stationary diffusion gradients associated with photosynthetic carbon flux—a study of compartmental versus diffusion–reaction models. Journal of Theoretical Biology 2003, 224, 385–397, [https://pubmed.ncbi.nlm.nih.gov/12941596/]. [Google Scholar] [CrossRef]
- Wang, H.; Lin, S.; Allen, J.P.; Williams, J.C.; Blankert, S.; Laser, C.; Woodbury, N.W. Protein dynamics control the kinetics of initial electron transfer in photosynthesis. Science 2007, 316, 747–750, [https://pubmed.ncbi.nlm.nih.gov/17478721/]. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.; Petroff, A.P.; Liang, B.; Sim, M.; Bosak, T. Reaction-diffusion, early photosynthesis, and the spatial organization of conical stromatolites. In American Geophysical Union, Fall Meeting 2009, San Francisco, California, 2009; NG42A-04.
- Bard, A.J.; Memming, R.; Miller, B. Terminology in semiconductor electrochemistry and photoelectrochemical energy conversion (Recommendations 1991). Pure and Applied Chemistry 1991, 63, 569–596. [Google Scholar] [CrossRef]
- Wagner, E.; Tetzner, J.; Haertle, U.; Deitzer, G.F. Endogenous rhythmicity and energy transduction VIII. Kinetics in enzyme activity, redox state and energy charge as related to photomorphogenesis in seedlings of Chenopodium rubrum L. 1. Berichte der Deutschen Botanischen Gesellschaft 1974, 87, 291–302. [Google Scholar] [CrossRef]
- Deitzer, G.; Hopkins, D.; Wagner, E. Analysis of ultradian rhythms of enzyme-activity in chenopodium rubrum during photomorphogenesis. Plant Physiology 1976, 57, 19. [Google Scholar]
- Petrov, R.; Popov, V. Regulation of the catalytic properties of NAD-dependent hydrogenase-influence of the redox potential of the medium. Biochemistry-Moscow, 1988, 53, 1466-1470. [Google Scholar]
- Kim, J.Y.; Park, H.S.; Im Kang, S.; Choi, E. J.; Kim, I.Y. Redox regulation of cytosolic glycerol-3-phosphate dehydrogenase: Cys102 is the target of the redox control and essential for the catalytic activity. Biochimica et Biophysica Acta-General Subjects 2002, 1569, 67–74, [https://pubmed.ncbi.nlm.nih.gov/11853959/]. [Google Scholar] [CrossRef]
- Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Current Opinion in Chemical Biology 2017, 37, 115–121, [https://pubmed.ncbi.nlm.nih.gov/28282633/]. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chai, J.; Ou, X.; Li, M.; Liu, Z. Structural insights into substrate selectivity, catalytic mechanism, and redox regulation of rice photosystem II core phosphatase. Molecular Plant 2019, 12, 86–98, [https://pubmed.ncbi.nlm.nih.gov/30453087/]. [Google Scholar] [CrossRef] [PubMed]
- Grabov, A.; Bottger, M. Are redox reactions involved in regulation of K+ channels in the plasma membrane of Limnobium stoloniferum root hairs? Plant Physiology 1994, 105, 927–935, [https://pubmed.ncbi.nlm.nih.gov/12232255/]. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, M.P.; Callegaro, M.T.; Barzon, E.; Benetti, M.; Bindoli, A. Purification of mitochondrial thioredoxin reductase and its involvement in the redox regulation of membrane permeability. Free Radical Biology and Medicine 1998, 24, 370–376, [https://pubmed.ncbi.nlm.nih.gov/9433913/]. [Google Scholar] [CrossRef] [PubMed]
- Bindoli, A.; Rigobello, M.P. Redox regulation of mitochondrial membrane permeability transition. Free Radical Biology and Medicine 2002, 33, S96. [Google Scholar]
- Horton, P.; Allen, J.F.; Black, M.T.; Bennett, J. Regulation of phosphorylation of chloroplast membrane polypeptides by the redox state of plastoquinone. FEBS Letters 1981, 125, 193–196. [Google Scholar] [CrossRef]
- Sies, H.; Dafré, A.L.; Ji, Y.; Akerboom, T.P. Protein S-thiolation and redox regulation of membrane-bound glutathione transferase. Chemico-Biological Iinteractions 1998, 111, 177–185, [https://pubmed.ncbi.nlm.nih.gov/9679553/]. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Li, H.; Grinkova, Y.; Sibhatu, H.; Jamal, J.; Poulos, T.L.; Sligar, S.G. Understanding redox regulation in membrane associated cytochrome P450s and the FMN domain of nitric oxide synthase. Chemical Research in Toxicology 2010, 23, 268–269. [Google Scholar]
- Lu, Y.; Wang, H.R.; Li, H.; Cui, H.R.; Feng, Y.G.; Wang, X.Y. A chloroplast membrane protein LTO1/AtVKOR involving in redox regulation and ROS homeostasis. Plant cell reports 2013, 32, 1427–1440, [https://pubmed.ncbi.nlm.nih.gov/23689258/]. [Google Scholar] [CrossRef]
- Spinello, A.; Ritacco, I.; Magistrato, A. The catalytic mechanism of steroidogenic cytochromes P450 from all-atom simulations: entwinement with membrane environment, redox partners, and post-transcriptional regulation. Catalysts 2019, 9, 81. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhang, J.Z.; Sakimoto, K.K.; Yang, P.; Reisner, E. Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat. Nanotechnol. 2018, 13, 890–899, [https://pubmed.ncbi.nlm.nih.gov/30291349/]. [Google Scholar] [CrossRef]
- McCormick, T.M.; Calitree, B.D.; Orchard, A.; Kraut, N.D.; Bright, F.V.; Detty, M.R.; Eisenberg, R. Reductive side of water splitting in artificial photosynthesis: new homogeneous photosystems of great activity and mechanistic insight. J. Am. Chem. Soc. 2010, 132, 15480–15483, [https://pubmed.ncbi.nlm.nih.gov/20945839/]. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 4. A direct biomimetic approach to [4, 6]-and [4, 8]-biflavanoids. Journal of the Chemical Society, Perkin Transactions 1981, 1, 1235–1245. [Google Scholar] [CrossRef]
- Roux, D.G.; Ferreira, D. The direct biomimetic synthesis, structure and absolute configuration of angular and linear condensed tannins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer, Vienna, 1982; pp. 47-76. [CrossRef]
- Pelter, A.; Satchwell, P.; Ward, R.S.; Blake, K. Effective, direct biomimetic synthesis of dibenzocyclooctene lignans by hypervalent iodine oxidation of phenolic dibenzylbutyrolactones. Journal of the Chemical Society, Perkin Transactions 1995, 1, 2201–2202. [Google Scholar] [CrossRef]
- Fuchino, Y.; Amao, Y. Photochemical and photophysical properties of carotenoid immobilized on a surfactant micellar medium including chlorophyll as an artificial photosynthesis system. Biophysics 2006, 2, 57–61, [https://pubmed.ncbi.nlm.nih.gov/27857560/]. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Sartorel, A.; Toma, F.M.; Puntoriero, F.; Scandola, F.; Campagna, S.; Prato, M.; Bonchio, M. Artificial photosynthesis challenges: water oxidation at nanostructured interfaces. Top. Curr. Chem. 2011, 303, 121–150, [https://pubmed.ncbi.nlm.nih.gov/21547686/]. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.; Duwe, H. P.; Pfeiffer, W. On the biomembranes as composite lamellae of smectic A liquid crystal and macromolecular network: elastic properties, local and collective dynamics. Physica Scripta 1989, 1989, 107–113. [Google Scholar] [CrossRef]
- Kajiyama, T.; Kumano, A.; Takayanagi, M.; Okahata, Y.; Kunitake, T. Crystal-liquid crystal phase transformation and water permeability of artificial amphiphiles as biomembrane model. Chemistry Letters 1979, 8, 645–648. [Google Scholar] [CrossRef]
- Smieja, J.M.; Benson, E.E.; Kumar, B.; Grice, K.A.; Seu, C.S.; Miller, A.J.; Mayer, J.M.; Kubiak, C.P. Kinetic and structural studies, origins of selectivity, and interfacial charge transfer in the artificial photosynthesis of CO. Proc. Natl. Acad. Sci. U S A 2012, 109, 15646–50, [https://pubmed.ncbi.nlm.nih.gov/22652573/]. [Google Scholar] [CrossRef] [PubMed]
- Schaming, D.; Hatay, I.; Cortez, F.; Olaya, A.; Méendez, M.A.; Ge, P.Y.; Deng, H.; Voyame, P.; Nazemi, Z.; Girault, H. Artificial photosynthesis at soft interfaces. Chimia 2011, 65, 356–359, [https://pubmed.ncbi.nlm.nih.gov/21744694/]. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Xue, B.; Frere, S.; Slutsky, I.; Cao, Y.; Wang, W.; Gazit, E. Multiporous supramolecular microspheres for artificial photosynthesis. Chem. Mater. 2017, 29, 4454–4460, [https://pubmed.ncbi.nlm.nih.gov/28572704/]. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Antonietti, M.; Liu, J. Bio-inspired carbon nitride mesoporous spheres for artificial photosynthesis: photocatalytic cofactor regeneration for sustainable enzymatic synthesis. Journal of Materials Chemistry A 2014, 2, 7686–7693. [Google Scholar] [CrossRef]
- Bacsa, R.R.; de Parseval, P.; Martin, F.; Serp, P. Geomimetic catalysis: From volcanic stones to ultra-selective Fe–Mo/Al2O3–TiO2 catalysts for few-walled carbon nanotube production. Carbon 2013, 64, 219–224. [Google Scholar] [CrossRef]
- Poe, S.L.; Kobašlija, M.; McQuade, D.T. Microcapsule enabled multicatalyst system. Journal of the American Chemical Society 2006, 128, 15586–15587, [https://pubmed.ncbi.nlm.nih.gov/17147357/]. [Google Scholar] [CrossRef] [PubMed]
- Poe, S.L.; Kobašlija, M.; McQuade, D.T. Mechanism and application of a microcapsule enabled multicatalyst reaction. Journal of the American Chemical Society 2007, 129, 9216–9221, [https://pubmed.ncbi.nlm.nih.gov/17602626/]. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Occurrence and prevention of photodissolution at the phase junction of magnetite and titanium dioxide. Journal of Molecular Catalysis A: Chemical 2002, 180, 193–200. [Google Scholar] [CrossRef]
- Krupp, H.; Schnabel, W. Light-modulated electrostatic double layer adhesion. Journal of Adhesion 1973, 5, 269–277. [Google Scholar] [CrossRef]
- Barker, G.C.; Cloke, G. Electrical double layer perturbation by light absorption at the interface. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 1974, 52, 468–473. [Google Scholar] [CrossRef]
- Donners, W.A.B.; Rijnbout, J.B.; Vrij, A. Light scattering from soap films: I. Determination of double-layer repulsion forces. Journal of Colloid and Interface Science 1977, 6, 249–260. [Google Scholar] [CrossRef]
- Plieth, W.J. Light absorption and light scattering in the field of the electrochemical double layer. In “Nonlinear behaviour of molecules, atoms, and ions in electric, magnetic, or electromagnetic fields”: Proceedings of the 31st International Meeting of The Société de Chimie Physique (25-28 September, 1978); Elsevier: Amsterdam – New York, Netherlands – USA, 1979; p. 251. [Google Scholar]
- Joosten, J.G.H. Electrical double layer and London—van der Waals forces in soap films studied by laser light scattering. Berichte der Bunsengesellschaft für Physikalische Chemie 1984, 88, 1153–1161. [Google Scholar] [CrossRef]
- Semenov, S.N. Electrophoresis and field flow fractionation in electric double layer observed by dynamic light scattering as possible analytical instrument. Jpn. J. Electroph. 1999, 43, 8. [Google Scholar]
- Plieth, W.J.; Gruschinske, P.; Hensel, H.J. Electrochromic changes of light absorption by the electric field of the electrolytic double layer. Berichte der Bunsengesellschaft für physikalische Chemie 1978, 82, 615–620. [Google Scholar] [CrossRef]
- De Grooth, B.G.; Van Gorkom, H.J.; Meiburg, R.F. Electrochromic absorbance changes in spinach chloroplasts induced by an external electrical field. Biochimica et Biophysica Acta-Bioenergetics 1980, 589, 299–314. [Google Scholar] [CrossRef]
- Schlodder, E.; Witt, H.T. Electrochromic absorption changes of a chloroplast suspension induced by an external electric field. FEBS Letters 1980, 112, 105–113. [Google Scholar] [CrossRef]
- De Grooth, B.G.; Amesz, J. Electrochromic absorbance changes of photosynthetic pigments in Rhodopseudomonas sphaeroides. I. Stimulation by secondary electron transport at low temperature. Biochimica et Biophysica Acta-Bioenergetics 1977, 462, 237–246. [Google Scholar] [CrossRef] [PubMed]
- De Grooth, B.G.; Amesz, J. Electrochromic absorbance changes of photosynthetic pigments in Rhodopseudomonas sphaeroides II. Analysis of the band shifts of carotenoid and bacteriochlorophyll. Biochimica et Biophysica Acta-Bioenergetics 1977, 462, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Plieth, W. Dependence of light-absorption of adsorbed molecules on the electric-field of the double-layer in the visible and ultraviolet spectral region. Abstracts of Papers of the American Chemical Society 1985, 190, 38. [Google Scholar]
- Bullard, T.; Freudenthal, J.; Avagyan, S.; Kahr, B. Test of Cairns-Smith’s ‘crystals-as-genes’ hypothesis. Faraday Discussions 2007, 136, 231–245, [https://pubmed.ncbi.nlm.nih.gov/17955812/]. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, S.; Jie, J.; Li, S.; Zheng, S.; Weng, M.; Yu, C.; Li, S.; chen, D.; Pan, F. A descriptor of “material genes”: Effective atomic size in structural unit of ionic crystals. Science China Technological Sciences 2019, 62, 849–855. [Google Scholar] [CrossRef]
- Hanf, R.; Fey, S.; Schmitt, M.; Hermann, G.; Dietzek, B.; Popp, J. Catalytic efficiency of a photoenzyme—an adaptation to natural light conditions. Chem. Phys. Chem. 2012, 13, 2013–2015, [https://pubmed.ncbi.nlm.nih.gov/22505323/]. [Google Scholar] [CrossRef] [PubMed]
- Björn, L.O. Comment on “Catalytic efficiency of a photoenzyme—an adaptation to natural light conditions” by J. Popp et al. Chem. Phys. Chem. 2013, 14, 2595–2597, [https://pubmed.ncbi.nlm.nih.gov/23712896/]. [Google Scholar] [CrossRef]
- Hermann, G.; Schmitt, M.; Dietzek, B.; Popp, J. Response to the comments by L.O. Björn on our paper “catalytic efficiency of a photoenzyme—an adaptation to natural light conditions”. Chem. Phys. Chem. 2013, 14, 2598–2600, [https://pubmed.ncbi.nlm.nih.gov/23712948/]. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.; Shen, Y.; Yang, X.; Qu, J.; Du, F.; Sun, W.; Li, C.M. Photoenzymatic catalytic cascade system of a pyromellitic diimide/g-C3N4 heterojunction to efficiently regenerate NADH for highly selective CO2 reduction toward formic acid. ACS Applied Materials & Interfaces 2021, 13, 46650–46658. [Google Scholar] [CrossRef]
- Ertl, M.; Reichl, E.; Knör, G. Multielectron redox catalysis with efficient tyrosinase activity based on a visible-light controlled artificial photoenzyme. European Journal of Organic Chemistry 2020, 2020, 3077–3080. [Google Scholar] [CrossRef]
- Sakaushi, K.; Lyalin, A.; Tominaka, S.; Taketsugu, T.; Uosaki, K. Two-dimensional corrugated porous carbon-, nitrogen-framework/metal heterojunction for efficient multielectron transfer processes with controlled kinetics. ACS Nano 2017, 11, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Kitazumi, Y.; Tsujimura, S.; Shirai, O.; Yamamoto, M.; Kano, K. Electrostatic interaction between an enzyme and electrodes in the electric double layer examined in a view of direct electron transfer-type bioelectrocatalysis. Biosensors and Bioelectronics 2015, 63, 138–144, [https://pubmed.ncbi.nlm.nih.gov/25078712/]. [Google Scholar] [CrossRef] [PubMed]
- Higson, S.P.; Vadgama, P. A study of electrical double layer effects in the pretreatment of two-electrode cells for enzyme electrodes. Electroanalysis 1994, 6, 431–436. [Google Scholar] [CrossRef]
- Urbakh, M.; Brodskii, A. Effect of the double-layer structure on surface-plasmon frequencies and light-reflection at a metal-electrolyte interface. Soviet Electrochemistry 1979, 15, 726–731. [Google Scholar]
- Willner, I.; Zahavy, E.; Heleg-Shabtai, V. Eosin-modified reconstituted Co(II) protoporphyrin IX Mmoglobin: a semisynthetic photoenzyme for H2 evolution and hydrogenation. Journal of the American Chemical Society 1995, 117, 542–543. [Google Scholar] [CrossRef]
- Ghosh, I.; König, B. Chromoselective photocatalysis: controlled bond activation through light-color regulation of redox potentials. Angewandte Chemie Int. Ed. 2016, 55, 7676–7679, [https://pubmed.ncbi.nlm.nih.gov/27198967/]. [Google Scholar] [CrossRef]
- Mairanovskii, S.G.E.; Klyukina, L.D.; Frumkin, A.N. The polarographic catalytic surface waves of hydrogen as affected by the structure of the double layer. Doklady Akademii Nauk 1961, 141, 147–150. [Google Scholar]
- Mairanovskii, S.G.; Frumkin, A.N. Effect of the catalyst adsorption and of the double layer structure on the catalytic waves of hydrogen evolution. Review of Polarography 1963, 11, 96–101. [Google Scholar] [CrossRef]
- Pohoaţa, V.; Popa, G.; Schrittwieser, R.; Ionita, C.; Cercek, M. Properties and control of anode double layer oscillations and related phenomena. Physical Review E 2003, 68, 016405, [https://pubmed.ncbi.nlm.nih.gov/12935256/]. [Google Scholar] [CrossRef]
- Kondo, T.; Yanagisawa, M.; Fujihira, M. Effect of electrical double layers on photoinduced electron transfer quenching of an amphiphilic Ru(II)(bpy)2+3 derivative in Langmuir—Blodgett films. Electrochimica Acta 1991, 36, 1793–1798. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, C.Y.; Zhou, C.T.; Yan, X.Q.; Yu, M.Y.; He, X.T. Suppressing longitudinal double-layer oscillations by using elliptically polarized laser pulses in the hole-boring radiation pressure acceleration regime. Physics of Plasmas 2013, 20, 023102. [Google Scholar] [CrossRef]
- Kondo, T. ; Effect of electrical double layers on photoinduced electron transfer in heterogeneous Langmuir-Blodgett films. Doctoral dissertation, PhD Thesis, Tokyo Institute of Technology, 1993.
- Nair, V.; Ananthoju, B.; Mohapatra, J.; Aslam, M. Photon induced non-linear quantized double layer charging in quaternary semiconducting quantum dots. Journal of Colloid and Interface Science 2018, 514, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Kaler, K.V.; Jones, T. A nonequilibrium statistical mechanical calculation of the surface conductance of the electrical double layer of biological cells and its application to dielectrophoresis. Journal of Physical Chemistry 1993, 97, 4745–4755. [Google Scholar] [CrossRef]
- Saphier, S.; Piran, R.; Keinan, E. Photoenzymes and photoabzymes. In Catalytic Antibodies; Keinan, E., Ed.; Wiley-WCH: Weinheim, Germany; 2005; pp. 350–369. [Google Scholar] [CrossRef]
- Pennline, J.A.; Rosenbaum, J.S.; Desimone, J.A.; Mikulecky, D.C. A nonlinear boundary value problem arising in the structure of the double layer at an enzymatic surface. Mathematical Biosciences 1977, 37, 1–17. [Google Scholar] [CrossRef]
- Dukhin, S.S. Non-equilibrium (dynamic) electrical double layer. In Encyclopedia of Surface and Colloid Science; CRC Press: Boca Raton, USA, 2015; pp. 4969–4975. [Google Scholar]
- Wang, H.; Adeleye, A.S.; Huang, Y.; Li, F.; Keller, A.A. Heteroaggregation of nanoparticles with biocolloids and geocolloids. Advances in Colloid and Interface Science 2015, 226, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.; Felmy, A.R.; Chilakapati, A. Non-equilibrium thermodynamic simulation of metal uptake in the bacterial electrical double-layer. Colloids and Surfaces B: Biointerfaces 2000, 18, 19–29. [Google Scholar] [CrossRef]
- Dreyer, W.; Guhlke, C.; Müller, R. Modeling of electrochemical double layers in thermodynamic non-equilibrium. Physical Chemistry Chemical Physics 2015, 17, 27176–27194. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Shilov, V.N. Kinetic aspects of electrochemistry of disperse systems. Part II. Induced dipole moment and the non-equilibrium double layer of a colloid particle. Advances in Colloid and Interface Science 1980, 13, 153–195. [Google Scholar] [CrossRef]
- Lyklema, J. Non-equilibrium double layers in connection with colloid stability. In The Structure, Dynamics and Equilibrium Properties of Colloidal Systems; Springer: Dordrecht, 1990; pp. 789–799. [Google Scholar] [CrossRef]
- Baca, J.M.; Hernandez, F.R.; De las Nieves Lopez, F.J.; Hidalgo-Alvarez, R. Calculation of ζ-potential by non-equilibrium double layer theory in positive polystyrene model colloids. Journal of Non-Equilibrium Thermodynamics 1991, 16, 187–199. [Google Scholar] [CrossRef]
- Abarzhi, I.; Malkin, E.; Dukhin, S. Non-equilibrium frontal ion adsorption dynamics in a long bed when the double-layer is taken into account. Colloid Journal of the USSR 1978, 40, 351–356. [Google Scholar]
- Anishchenko, D.V.; Levin, O.V.; Malev, V.V. Double layer structural effects in cyclic voltammetry curves complicated with non-equilibrium injection of charge carriers into redox polymer films. Electrochimica Acta 2017, 241, 375–385. [Google Scholar] [CrossRef]
- Manzanares, J.A.; Murphy, W.D.; Mafe, S.; Reiss, H. Numerical simulation of the nonequilibrium diffuse double layer in ion-exchange membranes. Journal of Physical Chemistry 1993, 97, 8524–8530. [Google Scholar] [CrossRef]
- Haran, S.O. Non-equilibrium electric double layer and electroosmosis at ion-exchange membranes. PhD Thesis, Ben Gurion University, Negev, 2006. [Google Scholar]
- Thom, A. Electrostatic double layer interactions in the transport modelling of reverse osmosis. PhD Thesis, McMaster University, Hamilton, Ontario, 1993. [Google Scholar]
- Rubinstein, M.; Papoian, G.A. Polyelectrolytes in biology and soft matter. Soft Matter 2012, 8, 9265–9267. [Google Scholar] [CrossRef]
- Markley, L.L.; Bixler, H.J.; Cross, R.A. Utilization of polyelectrolyte complexes in biology and medicine. Journal of Biomedical Materials Research 1968, 2, 145–155, [https://pubmed.ncbi.nlm.nih.gov/5708002/]. [Google Scholar] [CrossRef] [PubMed]
- Ennis, J.; Sjöström, L.; Åkesson, T.; Jönsson, B. Attractive osmotic pressure in an electric double layer with grafted polyelectrolytes. Journal of Physical Chemistry B 1998, 102, 2149–2164. [Google Scholar] [CrossRef]
- Parsegian, V.A.; Rand, R.P.; Fuller, N.L. Direct osmotic stress measurements of hydration and electrostatic double-layer forces between bilayers of double-chained ammonium acetate surfactants. Journal of Physical Chemistry 1991, 95, 4777–4782. [Google Scholar] [CrossRef]
- Moon, G.J.; Ahn, M.M.; Kang, I.S. Osmotic pressure of ionic liquids in an electric double layer: Prediction based on a continuum model. Physical Review E 2015, 92, 063020, [https://pubmed.ncbi.nlm.nih.gov/26764817/]. [Google Scholar] [CrossRef]
- Levich, V.G. Theory of the nonequilibrium double layer. Dokl. Akad. Nauk SSSR 1949, 67, 309. [Google Scholar]
- Sparnaay, M.J. Non-equilibrium diffuse double-layer. Transactions of the Faraday Society 1957, 53, 306–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.J.; Barber, R.W.; Emerson, D.R. Influence of the electric double layer on induced pressure fields and development lengths in electro-osmotic flows. Modern Physics Letters B 2005, 19, 1655–1658. [Google Scholar] [CrossRef]
- Sadr, R.; Yoda, M.; Gnanaprakasam, P.; Conlisk, A.T. Velocity measurements inside the diffuse electric double layer in electro-osmotic flow. Applied Physics Letters 2006, 89, 044103. [Google Scholar] [CrossRef]
- van der Wouden, E.J.; Gardeniers, J.G.; van den Berg, A. Transient charging of the electric double layer in field effect-flow. In Proc. 10th International Conference on Miniaturized Systems for Chemistry and Life Sciences, µTAS – 2006 (5 Nov 2006 – 9 Nov 2006); Kitamori, T., Fujita, H., Hasebe, S., Eds.; Japan Academic Association: Tokyo, Japan, 2006; pp. 83–85. [Google Scholar]
- Ramos, A.; Castellanos, A. Travelling wave electro-osmosis: nonlinear double layer analysis and application to pumping of liquid. In American Physical Society March Meeting (March 21-25, 2005); 2005; N37.004.
- Ogawa, T. Simple oscillations in photosynthesis of higher plants. Biochimica et Biophysica Acta -Bioenergetics 1982, 681, 103–109. [Google Scholar] [CrossRef]
- Slvak, M.N.; Walker, D.A. Oscillations in photosynthesis. In Hungarian-USA Binational Symposium on Photosynthesis: a conference held at Salve Regina College, Newport, Rhode Island (August 15-18, 1986); Salve Regina College: Newport, Rhode Island, 1986; p. 105. [Google Scholar]
- Zvalinskii, V.I.; Litvin, F.F. Modelling the oscillations of the evolution of oxygen during photosynthesis. Biophysics 1990; 35, 288-293.
- Hennessey, T.L. , Field, C.B. Circadian rhythms in photosynthesis: oscillations in carbon assimilation and stomatal conductance under constant conditions. Plant Physiology 1991, 96(3), 831–836, [https://pubmed.ncbi.nlm.nih.gov/16668261/]. [Google Scholar] [CrossRef]
- Lakhno, V.D. Oscillations in the primary charge separation in bacterial photosynthesis. Physical Chemistry Chemical Physics 2002, 4, 2246–2250. [Google Scholar] [CrossRef]
- Siebke, K.; Yin, Z.H.; Raghavendra, A.S.; Heber, U. Vacuolar pH oscillations in mesophyll cells accompany oscillations of photosynthesis in leaves: Interdependence of cellular compartments, and regulation of electron flow in photosynthesis. Planta 1992, 186, 526–531, [https://pubmed.ncbi.nlm.nih.gov/24186782/]. [Google Scholar] [CrossRef]
- Anjum, S.A. , Ashraf, U.; Khan, I.; Tanveer, M.; Saleem, M.F.; Wang, L. Aluminum and chromium toxicity in maize: implications for agronomic attributes, net photosynthesis, physio-biochemical oscillations, and metal accumulation in different plant parts. Water, Air, and Soil Pollution 2016, 227, 326. [Google Scholar] [CrossRef]
- Laisk, A.; Walker, D.A. Control of phosphate turnover as a rate-limiting factor and possible cause of oscillations in photosynthesis: a mathematical model. Proceedings of the Royal society of London. Series B. Biological Sciences 1986, 227, 281–302. [Google Scholar] [CrossRef]
- Barber, J.; Laisk, A.; Schreiber, U. Towards understanding oscillations: A mathematical model of the biochemistry of photosynthesis: Discussion. Philosophical Transactions of the Royal Society of London Series B 1989, 323, 383–384. [Google Scholar] [CrossRef]
- Dubinsky, A.Y.; Ivlev, A.A.; Igamberdiev, A.U. Theoretical analysis of the possibility of existence of oscillations in photosynthesis. Biophysics 2010, 55, 55–58. [Google Scholar] [CrossRef]
- Barber, J.; Mills, J.; Love, A. Electrical diffuse layers and their influence on photosynthetic processes. FEBS Letters 1977, 74, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Membrane surface charges and potentials in relation to photosynthesis. Biochimica et Biophysica Acta-Reviews on Bioenergetics 1980, 594, 253–308, [https://pubmed.ncbi.nlm.nih.gov/7018576/]. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, F.B.; Gu, G.B.; Wang, L.Y.; Zheng, S.J.; Zhang, Q. Photocatalytic reaction kinetics model based on electrical double layer theory. II. Infrared spectroscopic characterization of methyl orange adsorption on TiO2 surface. Transactions of the Nonferrous Metals Society of China 2002, 12, 1187–1190. [Google Scholar]
- Venkatesh, D.; Pavalamalar, S.; Anbalagan, K. Photocatalytic, gas-sensing and double layer capacitance properties of nanoscale SnO2 obtained from template free solution phase synthesis. Journal of Materials Science: Materials in Electronics 2019, 30, 9245–9258. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Kruk, J.; Górecka, M.; Karpińska, B.; Karpiński, S. Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. The Plant Cell 2010, 22, 2201–2218, [https://pubmed.ncbi.nlm.nih.gov/20639446/]. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.S. Quantum coherence in photosynthesis. Procedia Chemistry 2011, 3, 222–231. [Google Scholar] [CrossRef]
- Dickinson, E.J.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite element software for electrochemical analysis. A mini-review. Electrochemistry communications 2017, 40, 71–74. [Google Scholar] [CrossRef]
- Kaffash, A.; Rostami, K.; Zare, H.R. Modeling of an electrochemical nanobiosensor in COMSOL Multiphysics to determine phenol in the presence of horseradish peroxidase enzyme. Enzyme and Microbial Technology 2019, 121, 23–28. [Google Scholar] [CrossRef]
- Li, A.; Lin, Z.J. Efficient mass transport and electrochemistry coupling scheme for reliable multiphysics modeling of planar solid oxide fuel cell stack. Chinese Journal of Chemical Physics 2017, 30, 139. [Google Scholar] [CrossRef]
- Sugimoto, T.; Kobayashi, M.; Adachi, Y. Orthokinetic aggregation of charged colloidal particles in the presence of repulsive double layer force: a trajectory analysis with the solution of non-linear Poisson–Boltzmann equation. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2015, 483, 321–327. [Google Scholar] [CrossRef]
- Tawari, S. L.; Koch, D. L.; Cohen, C. Electrical double-layer effects on the Brownian diffusivity and aggregation rate of Laponite clay particles. Journal of Colloid and Interface Science 2001, 240, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chiew, Y.C.; Valentini, J.E. The study of surface dilational properties of nonionic surfactant solutions by propagation of electrocapillary waves. Journal of Colloid and Interface Science 1993, 155, 8–15. [Google Scholar] [CrossRef]
- Zubarev, N.M. A nonlinear dispersion relationship for electrocapillary waves on the charged surface of a dielectric liquid. Technical Physics Letters 2001, 27, 689–691. [Google Scholar] [CrossRef]
- Belonozhko, D.F.; Grigor’ev, A.I. Nonlinear electrocapillary waves on a charged surface of the ideal liquid. Technical Physics Letters 2003, 29, 768–770. [Google Scholar] [CrossRef]
- Zubarev, N.M.; Zubareva, O.V. Nonlinear dispersion relation for electrocapillary waves on the surface of a dielectric liquid. Technical Physics Letters 2006, 32, 1027–1029. [Google Scholar] [CrossRef]
- Daikhin, L.I.; Kornyshev, A.A.; Urbakh, M. Effect of capillary waves on the double layer capacitance of the interface between two immiscible electrolytes. Electrochimica Acta 1999, 45, 685–690. [Google Scholar] [CrossRef]
- Budroni, M.A. Cross-diffusion-driven hydrodynamic instabilities in a double-layer system: General classification and nonlinear simulations. Physical Review E 2015, 92, 063007, [https://pubmed.ncbi.nlm.nih.gov/26764804/]. [Google Scholar] [CrossRef]
- Budroni, M.A.; De Wit, A. Dissipative structures: From reaction-diffusion to chemo-hydrodynamic patterns. Chaos: An Interdisciplinary Journal of Nonlinear Science 2017, 27, 104617. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Dukhin, S.S.; Matijevic, E. Nonequilibrium double layer and electrokinetic phenomena. Surface and Colloid Science 1974, 7, 273–335. [Google Scholar] [CrossRef]
- Paquin-Lefebvre, F.; Xu, B.; DiPietro, K.L.; Lindsay, A.E.; Jilkine, A. Pattern formation in a coupled membrane-bulk reaction-diffusion model for intracellular polarization and oscillations. Journal of Theoretical Biology 2020, 110242. [Google Scholar] [CrossRef]
- Yochelis, A. Catalytic membrane reactor model as a laboratory for pattern emergence in reaction-diffusion-advection media. Israel Journal of Chemistry 2018, 58, 722–732. [Google Scholar] [CrossRef]
- Xu, N.; Riley, J. Nonlinear analysis of a classical system: The double-layer capacitor. Electrochemistry Communications 2011, 13, 1077–1081. [Google Scholar] [CrossRef]
- Maenhout, G.; Schulenberg, T. Linear and non-linear interface model based on the electric double layer theory. Wissenschaftliche Berichte FZKA 2002, 6669, 3–54. [Google Scholar]
- Stigter, D. The charged colloidal cylinder with a Gouy double layer. Journal of Colloid and Interface Science 1975, 53, 296–306. [Google Scholar] [CrossRef]
- Hsu, L. Y.; Keh, H. J. Diffusioosmosis of electrolyte solutions around a circular cylinder at arbitrary zeta potential and double-layer thickness. Industrial & Engineering Chemistry Research 2009, 48, 2443-2450. [CrossRef]
- Ohshima, H. Diffuse double layer interaction between two parallel plates with constant surface charge density in an electrolyte solution IV. Numerical calculation of the interaction between similar plates using the non-linear Poisson-Boltzmann equation. Colloid and Polymer Science 1976, 254, 484–491. [Google Scholar] [CrossRef]
- Posey, F.A.; Morozumi, T. Theory of potentiostatic and galvanostatic charging of the double layer in porous electrodes. Journal of the Electrochemical Society 1966, 113, 176. [Google Scholar] [CrossRef]
- Chang, N.; Zhang, H.; Shi, M.S.; Li, J.; Yin, C.J.; Wang, H.T.; Wang, L. Regulation of the adsorption affinity of metal-organic framework MIL-101 via a TiO2 coating strategy for high capacity adsorption and efficient photocatalysis. Microporous and Mesoporous Materials 2018, 266, 47–55. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Izotov, V.Y.; Kornyshev, A.A. Differential capacitance of the electric double-layer in dilute-solutions of surface-inactive electrolytes and upon the specific adsorption of ions-nonlocal and non-linear effects. Soviet Electrochemistry 1983, 19, 364–368. [Google Scholar]
- Hurwitz, H.; Botte, P.; Mulenga, M. Status of ion adsorption investigations at the Hg electrode-related aspects of double-layer phenomena at biomembrane surfaces. Abstracts of Papers of the American Chemical Society 1984, 187, 120. [Google Scholar]
- Godoy, S.; García-Colín, L.S.; Micenmacher, V. Generalized Landauer equation: Absorption-controlled diffusion processes. Physical Review E 1999, 59, 6180. [Google Scholar] [CrossRef]
- Godoy, S.; Garcı́a-Colı́n, L.S.; Micenmacher, V. Multiple-scattering coefficients and absorption controlled diffusive processes. Journal of Chemical Physics 1999, 111, 9389–9392. [Google Scholar] [CrossRef]
- Shichi, A.; Satsuma, A.; Hattori, T. Adsorption-controlled diffusion in catalytic reduction of NO with hydrocarbons over zeolite catalysts. Catalysis Today 2004, 93, 777–781. [Google Scholar] [CrossRef]
- Wantanabe, T.; Maeda, H. Adsorption controlled redox activity. Surface enhanced Raman investigation of cystine versus cystein on silver electrodes. J. Phys. Chem. 1989, 93, 3258–3260. [Google Scholar] [CrossRef]
- Rouxhet, P.G. Lysozyme on apatites: a model of protein adsorption controlled by electrostatic interactions. Colloids and Surfaces 1989, 37, 339–355. [Google Scholar] [CrossRef]
- Dunwell, M.; Yan, Y.; Xu, B. Understanding the influence of the electrochemical double-layer on heterogeneous electrochemical reactions. Current Opinion in Chemical Engineering 2018, 20, 151–158. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Saito, N.; Hirai, T. Adsorption-driven photocatalytic activity of mesoporous titanium dioxide. Journal of the American Chemical Society 2005, 127, 12820–12822. [Google Scholar] [CrossRef]
- Jinnan, C.; Jiong, P. Velocity of droplet with sorption-controlled surfactant in electrolyte solution. Journal of Chemical Industry and Engineering (China) 2000, 51, 120–125. [Google Scholar]
- Chan, K.Y.; Borhan, A. Spontaneous spreading of surfactant-bearing drops in the sorption-controlled limit. Journal of Colloid and Interface Science 2006, 302, 374–377, [https://pubmed.ncbi.nlm.nih.gov/16860811/]. [Google Scholar] [CrossRef]
- Miklavcic, S.J.; Said, E. Electrostatic potential and double layer force in a semiconductor–electrolyte-semiconductor heterojunction. Physical Review E 2006, 74, 061606. [Google Scholar] [CrossRef]
- Böer, K.W. Heterojunction interface double layer and consequences for photovoltaic cells, specifically CdS(z)ZnS(1-z)S/Cu2S. Physica Status Solidi. A, Applied Research 1978, 49, 455–462. [Google Scholar] [CrossRef]
- Antonini, G.; Deschrijver, D.; Dhaene, T. Broadband rational macromodeling based on the adaptive frequency sampling algorithm and the partial element equivalent circuit method. IEEE Transactions on Electromagnetic Compatibility 2008, 50, 128–137. [Google Scholar] [CrossRef]
- Li, R.S.; Liu, Q.H. Sustained oscillations in isothermal, heterogeneously catalyzed reactions with the simplest Langmuir-type kinetics. Chemical Engineering Science 1992, 47, 3156–3158. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Kasemo, B. Surface Restructuring, Kinetic Oscillations, and Chaos in Heterogeneous Catalytic Reactions. Journal of Statistical Physics 2000, 101, 631–647. [Google Scholar] [CrossRef]
- Kuzovkov, V.N.; Kortlüke, O.; von Niessen, W. Comment on “Surface restructuring, kinetic oscillations, and chaos in heterogeneous catalytic reactions”. Physical Review E 2001, 63, 023101, [https://pubmed.ncbi.nlm.nih.gov/11308525/]. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Reply to “Comment on ‘Surface restructuring, kinetic oscillations, and chaos in heterogeneous catalytic reactions’”. Physical Review E 2001, 63, 023102, [https://pubmed.ncbi.nlm.nih.gov/11308526/]. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P. Simulation of kinetic oscillations in catalytic reactions accompanied by adsorbate-induced surface restructuring. Surface Science 1999, 426, 345–357. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Simulation of kinetic oscillations in catalytic reactions accompanied by oxide formation. Surface Review and Letters 1999, 6, 347–353. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Kasemo, B. Kinetic oscillations on nm-sized catalyst particles: oxide model. Surface Science 2002, 511, 23–33. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Kasemo, B. Fluctuations in kinetic oscillations on nm-sized catalyst particles. Surface Science 2005, 588, L220–L226. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Surface restructuring and aperiodic kinetic oscillations in heterogeneous catalytic reactions. Physica D: Nonlinear Phenomena 2000, 144, 87–96, [https://pubmed.ncbi.nlm.nih.gov/11970707/]. [Google Scholar] [CrossRef]
- Zolotarev, P.P.; Starov, V.M. Effect of oscillations of adsorptive concentration in adsorbent grain surface on adsorption process in case of nonlinear isotherm and mixed kinetics. Zhurnal Fizicheskoi Khimii 1975, 49, 2437–2439. [Google Scholar]
- Zolotarev, P.P.; Starov, V. Effect of random temperature oscillations on physical adsorption-kinetics. Zhurnal Fizicheskoi Khimii 1974, 48, 2598–2600. [Google Scholar]
- Chen, S.; Noles, T.; Schell, M. Differences in oscillations and sequences of dynamical states caused by anion adsorption in the electrochemical oxidation of formic acid. Journal of Physical Chemistry A 2000, 104, 6791–6798. [Google Scholar] [CrossRef]
- Wang, X.J.; Gaspard, P.; Gray, P.; Nicolis, G.; Baras, F.; Borckmans, P.; Scott, S. Homoclinicity and multimodal periodic or chaotic oscillations in chemical kinetics. In Spatial Inhomogeneities and Transient Behavior in Chemical Kinetics; Gray, P., Ed.; Manchester University Press: Manchester, England, 1990; pp. 687–690. [Google Scholar]
- Kulginov, D.; Zhdanov, V.P.; Kasemo, B. Oscillatory surface reaction kinetics due to coupling of bistability and diffusion limitations. Journal of Chemical Physics 1997, 106, 3117–3128. [Google Scholar] [CrossRef]
- Thames Jr, H.D.; Elster, A.D. Equilibrium states and oscillations for localized two-enzyme kinetics: A model for circadian rhythms. Journal of Theoretical Biology 1976, 59, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Selegny, E.; Vincent, J. Chemical oscillations in homogeneous Michaelian multi-enzyme systems. 1. Analytical kinetic treatment. Journal de Chimie Physique et de Physico-Chimie Biologique 1980, 77, 1083–1091. [Google Scholar] [CrossRef]
- Hau, S.S. Frequency-domain enzyme kinetics in the context of artificial calcium oscillations. PhD Thesis, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA, 1996. [Google Scholar]
- Fernández, J.M.; Bezanilla, F.; Taylor, R.E. Distribution and kinetics of membrane dielectric polarization. II. Frequency domain studies of gating currents. The Journal of General Physiology 1982, 79, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Gokhshtein, A.Y. Electron density oscillations in the double-layer field. Doklady Physical Chemistry 1996, 351, 292–295. [Google Scholar]
- Gokhshtein, A.Y. Oscillations of the electron density in the double layer field. Doklady Akademii Nauk 1996, 351, 59–63. [Google Scholar]
- Evstigneev, A.; Sachenko, A. Effect of Friedel oscillations on the capacity of a double electric layer. Fizika Tverdogo Tela 1992, 34, 2287–2290. [Google Scholar]
- Evstigneev, A.M.; Sachenko, A.V. Influence of Friedel oscillations on the capacitance of a double electrical layer. Soviet Physics. Solid State 1992, 34, 1224–1225. [Google Scholar]
- Gradov, O.V.; Gradova, M.A. Photoinduced spatiotemporal oscillations and self-organization of dissipative structures in polymer-immobilized dispersed semiconductors. Journal of Nano- and Electronic Physics 2018, 10, 04022-1–04022-8. [Google Scholar] [CrossRef] [PubMed]
- Gradov, O.V.; Gradova, M.A. Synchronization of photochemical processes and photoinduced self-organization in dispersed semiconductors under optical pumping. In Abstract Book of the 3rd International Symposium “Molecular Photonics", St. Petersburg – Repino (June 24- 29 2012); 2012; p. 156. [CrossRef]
- Schmidtnaake, G.; Pippel, W. Modeling of diffusion controled reaction and adsorption processes by means of Markov-chains. Chemische Technik 1984, 36, 411–415. [Google Scholar]
- Goldstein, B.N.; Aksirov, A.M.; Zakrjevskaya, D.T. A new kinetic model for biochemical oscillations: Graph-theoretical analysis. Biophysical Chemistry 2009, 145, 111–115, [https://pubmed.ncbi.nlm.nih.gov/19837504/]. [Google Scholar] [CrossRef]
- Mincheva, M. Oscillations in non-mass action kinetics models of biochemical reaction networks arising from pairs of subnetworks. Journal of Mathematical Chemistry 2012, 50, 1111–1125. [Google Scholar] [CrossRef]
- Peusner, L.; Mikulecky, D.C.; Bunow, B.; Caplan, S.R. A network thermodynamic approach to Hill and King–Altman reaction–diffusion kinetics. Journal of Chemical Physics 1985, 83, 5559–5566. [Google Scholar] [CrossRef]
- Rieckmann, C.; Keil, F.J. Multicomponent diffusion and reaction in three-dimensional networks: General kinetics. Industrial and Engineering Chemistry Research 1997, 36, 3275–3281. [Google Scholar] [CrossRef]
- Fuchs, O. Kinetik physikalisch-chemischer oszillationen. Colloid and Polymer Science 1980, 258, 985–986. [Google Scholar] [CrossRef]
- Skorobogatov, G. A minimal kinetic scheme providing the effect of chemical oscillations. Doklady Akademii Nauk SSSR 1986, 290, 403–409. [Google Scholar]
- Kolthoff, I.M.; Yamashita, K.; Hie, T.B.; Kanbe, A. Characteristics of polarographic catalytic waves observed with bovine-serum albumin: kinetic or diffusion control. Proceedings of the National Academy of Sciences 1973, 70, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Mattern, K.; Felderhof, B.U. Self-consistent cluster expansion for wave propagation and diffusion-controlled reactions in a random medium. Physica A: Statistical Mechanics and its Applications 1987, 143, 21–39. [Google Scholar] [CrossRef]
- Assel, M.; Höfer, T.; Laubereau, A.; Kaiser, W. Diffusion-controlled intermolecular electron transfer studied by transient absorption and degenerate four-wave mixing measurements. Chemical Physics Letters 1995, 234, 151–158. [Google Scholar] [CrossRef]
- Lebiedz, D.; Brandt-Pollmann, U. Manipulation of self-aggregation patterns and waves in a reaction-diffusion system by optimal boundary control strategies. Physical Review Letters 2003, 91, 208301, [https://pubmed.ncbi.nlm.nih.gov/14683405/]. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; Nirdosh, I.; Sedahmed, G.H. Intensification of the rate of electropolishing and diffusion controlled electrochemical machining by workpiece oscillation. Journal of the Taiwan Institute of Chemical Engineers 2014, 45, 840–845. [Google Scholar] [CrossRef]
- Walz, D.; Caplan, S.R. Chemical oscillations arise solely from kinetic nonlinearity and hence can occur near equilibrium. Biophysical Journal 1995, 69, 1698–1707, [https://pubmed.ncbi.nlm.nih.gov/8580313/]. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, Y.M. Chemical oscillations and instabilities. Non-linear chemical kinetics. Zeitschrift für Physikalische Chemie 1995, 192, 138. [Google Scholar] [CrossRef]
- Barragán, D. Essentials of kinetics and thermodynamics for understanding chemical oscillations. Foundations of Chemistry 2015, 17, 93–106. [Google Scholar] [CrossRef]
- Sagdeev R., Z.; Usikov D., A.; Zaslavsky G., M. Nonlinear Physics: From the Pendulum to Turbulence and Chaos. Harwood Academic Publishers (Gordon and Breach): New York, USA, 1988; 656 p.
- Schöll, E. Theoretical approaches to nonlinear and chaotic dynamics of generation-recombination processes in semiconductors. Applied Physics A 1989, 48, 95–106. [Google Scholar] [CrossRef]
- Landsberg, P.T.; Robbins, D.J.; Schöll, E. Threshold switching as a generation-recombination induced non-equilibrium phase transition. Physica Status Solidi A 1978, 50, 423–426. [Google Scholar] [CrossRef]
- Schöll, E. Nonequilibrium Phase Transitions in Semiconductors: Self-Organization Induced by Generation and Recombination Processes; Haken, H., Ed.; Springer-Verlag: Springer, Berlin, Heidelberg, 1987; 313 p. [Google Scholar] [CrossRef]
- Schöll, E. Continuous bifurcation and dissipative structures associated with a soft mode recombination instability in semiconductors. In Dynamical System and Chaos – Proceedings of the Sitges Conference on Statistical Mechanics Sitges, Barcelona, Spain (September 5 – 11, 1982); Garrido, L., Ed.; Springer: Berlin, Heidelberg, 1983; pp. 204–211. [Google Scholar] [CrossRef]
- Schöll, E. Stability of generation-recombination induced dissipative structures in semiconductors. Zeitschrift für Physik B Condensed Matter 1983, 52, 321–334. [Google Scholar] [CrossRef]
- Klaassen, F.M.; Van Vliet, K.M.; Fassett, J.R. Generation-recombination noise in various photoconductive semiconductors. Journal of Physics and Chemistry of Solids 1961, 22, 391–399. [Google Scholar] [CrossRef]
- Long, D. Generation-recombination noise limited detectivities of impurity and intrinsic photoconductive 8–14μ infrared detectors. Infrared Physics 1967, 7, 121–128. [Google Scholar] [CrossRef]
- Beck, W.A. Photoconductive gain and generation-recombination noise in multiple-quantum-well infrared detectors. Applied Physics Letters 1993, 63, 3589–3591. [Google Scholar] [CrossRef]
- Shadrin, V.D.; Mitin, V.V.; Kochelap, V.A.; Choi, K.K. Photoconductive gain and generation-recombination noise in quantum well infrared photodetectors. Journal of Applied Physics 1995, 77, 1771–1775. [Google Scholar] [CrossRef]
- Rudenko, T.; Gerz, S.; Nikitenko, V.; Makarov, A. Frequency-spectrum of chloroplast cross-section oscillations. Biofizika 1983, 28, 445–450. [Google Scholar]
- Sivak, M.N.; Walker, D.A. Oscillations and other symptoms of limitation of in vivo photosynthesis by inadequate phosphate supply to the chloroplast. Plant Physiology and Biochemistry 1987, 25, 635–648. [Google Scholar]
- Sayeed, S.A.; Mohanty, P. Oscillations in wheat chloroplast photochemical activity: Effect of uncouplers. In Membrane Receptors, Dynamics, and Energetics; Wirtz, K.W.A., Ed.; Springer: Boston, 1987; pp. 311–318. [Google Scholar] [CrossRef]
- Sayeed, S.A.; Mohanty, P. Rhythmic oscillations in wheat chloroplast photochemical activity. I. Oscillations in whole chain, photosystem II and photosystem I electron transport activities. Proceedings: Plant Sciences 1988, 98, 157–174. [Google Scholar] [CrossRef]
- Sayeed, S.A.; Mohanty, P. Oscillations in wheat chloroplast photochemical activity: Part III--Characterization of the possible oscillators in electron transport chain. Indian Journal of Biochemistry and Biophysics 1988, 25, 625–630. [Google Scholar]
- Sayeed, S.A.; Mohanty, P. Rhythmic oscillations in wheat chloroplast photochemical activity. II. Further characterization of the rhythm in photosystem II photoelectron transport activity. Proceedings: Plant Sciences 1988, 98, 175–181. [Google Scholar] [CrossRef]
- Kocks, P.; Ross, J. Kinetic model for (damped) oscillations of transthylakoid pH in plants. Journal of Physical Chemistry 1995, 99, 16490–16497. [Google Scholar] [CrossRef]
- Fritz, L.; Stringher, C.G.; Colepicolo, P. Imaging oscillations in Gonyaulax: a chloroplast rhythm of nitrate reductase visualized by immunocytochemistry. Brazilian journal of medical and biological research 1996, 29, 111–117. [Google Scholar]
- Smrčinová, M.; Sørensen, P.G.; Krempasky, J.; Ballo, P. Chaotic oscillations in a chloroplast system under constant illumination. International Journal of Bifurcation and Chaos 1998, 8, 2467–2470. [Google Scholar] [CrossRef]
- Shchepetov, D.S.; Chernavsky, D.S.; Gorokhov, V.V.; Grishanova, N.P.; Pashchenko, V.Z.; Rubin, A.B. The nature of oscillations in the kinetics of electron transfer in the reaction center of purple bacteria. Doklady. Biochemistry and Biophysics 2009, 425, 87–90, [https://pubmed.ncbi.nlm.nih.gov/19496329/]. [Google Scholar] [CrossRef] [PubMed]
- Shchepetov, D.S.; Chernavsky, D.S.; Gorokhov, V.V.; Paschenko, V.Z.; Rubin, A.B. Application of the standard theory of electronic transitions to the description of oscillations in the kinetics of electron transfer in reaction centers of purple bacteria. Biophysics 2009, 54, 691–698, [https://pubmed.ncbi.nlm.nih.gov/20067182/]. [Google Scholar] [CrossRef]
- Karageorgiy, P.M.; Leiderman, A.Y. Theory of kinetic oscillations in semiconductors. Fizika Tverdogo Tela 1967, 9, 2151–2156. [Google Scholar]
- Karageorgiy, P.M.; Leiderman, A.Y. Forced kinetic oscillations in semiconductor diode structures Soviet Physics: Semiconductors 1967, 1, 617. 1.
- Parfenev, R. V.; Sologub, V. V.; Goltsman, B. M. (1968). Quantum oscillations of the kinetic and photo- electric coefficients of n-type bismuth telluride. Fizika Tverdogo Tela 1968, 10, 3087. [Google Scholar]
- Cebrián, E.; Bonilla, L.L.; Carpio, A. Self-sustained current oscillations in the kinetic theory of semiconductor superlattices. Journal of Computational Physics 2009, 228, 7689–7705. [Google Scholar] [CrossRef]
- Firsov, I.; Lang, I. Kinetic theory of semiconductors with low mobility developed for strong coupling between the current carriers and lattice oscillations. Soviet Physics-JETP 1963, 16, 1301–1312. [Google Scholar]
- Budagyan, B.G.; Aivazov, A.A.; Stanovov, O.N. Oscillations of the photoconductivity and characteristic features of the relaxation kinetics of a-Si:H. Semiconductors 1993, 27, 822–825. [Google Scholar]
- Pavlyuk, S.P.; Kushnyirenko, V.V. The kinetics of onset of oscillations in n+-n-n+ transistors and resistors under influence of high density pulses of current. Vyisnyk Kyivskogo Universytetu. Fyiziko-Matematichnyi Nauki 2007, 280-283.
- Walker, D.A. Concerning oscillations. Photosynthesis research 1992, 34, 387–395, [https://pubmed.ncbi.nlm.nih.gov/24408834/]. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, M.; Gimmler, H.; Schreiber, U.; Heber, U. Relative insensitivity of photosynthesis to the dissipation of a transthylakoid proton gradient in intact chloroplasts. Physiologie Végétale 1985, 23, 801–812. [Google Scholar]
- Peterson, R.B.; Sivak, M.N.; Walker, D.A. Carbon dioxide-induced oscillations in fluorescence and photosynthesis: role of thylakoid membrane energization in regulation of photosystem II activity. Plant Physiology 1988, 88, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, K.H.; Kolbowski, J.; Hansen, U.P. Further evidence for the relationship between light-induced changes of plasmalemma transport and transthylakoid proton uptake. Journal of Experimental Botany 1989, 40, 239–245. [Google Scholar] [CrossRef]
- Laasch, H.; Ihle, C.; Günther, G. Detecting localized proton currents in photophosphorylation by procaine inhibition of the transthylakoid pH-gradient. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1993, 1140, 251–261. [Google Scholar] [CrossRef]
- Cruz, J.A.; Sacksteder, C.A.; Kanazawa, A.; Kramer, D.M. Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry 2001, 40, 1226–1237, [https://pubmed.ncbi.nlm.nih.gov/11170448/]. [Google Scholar] [CrossRef]
- Cruz, J.A.; Kanazawa, A.; Treff, N.; Kramer, D.M. Storage of light-driven transthylakoid proton motive force as an electric field (Δψ) under steady-state conditions in intact cells of Chlamydomonas Reinhardtii. Photosynthesis Research 2005, 85, 221–233, [https://pubmed.ncbi.nlm.nih.gov/16075322/]. [Google Scholar] [CrossRef]
- Radenović, Č.; Jeremić, M.; Maksimov, G.; Beljanski, M.; Filipović, M.; Čamdžija, Z. Mechanisms and parameters of transients and oscillations of delayed chlorophyll fluorescence induction processes in the excited thylakoid membrane of the maize intact leaf. Journal of Scientific Agricultural Research 2008, 69, 5–21. [Google Scholar]
- Radenović, Č.N.; Jeremić, M.G.; Maximov, G.V.; Beljanski, M.V.; Radojčić, A.R. Mechanisms and parameters of transients and oscillations of delayed chlorophyll fluorescence in the thylakoid membrane of the intact maize leaf. Russian Journal of Physical Chemistry A 2009, 83, 1582–1591. [Google Scholar] [CrossRef]
- Radenović, Č.; Marković, K.; Radojčić, A.; Anđelković, V.; Kalauzi, A.J. Interdependence between oscillations and transients of delayed fluorescence induction processes in the thylakoid membrane of the intact maize leaf: Responses to effects of increased temperatures and drought. Zbornik Matice Srpske za Prirodne Nauke 2010, 118, 7–26. [Google Scholar] [CrossRef]
- Maynard, S.N. Determining the origin of a Ca2+ Wave released in Arabidopsis Thaliana upon photostimulation of the ER-Chloroplast Nexus. PhD Thesis, Texas A&M University, College Station, Texas, 2017. [Google Scholar]
- Elber, R. ; A new paradigm for atomically detailed simulations of kinetics in biophysical systems. Quarterly reviews of biophysics 2017, 50, e8, [https://pubmed.ncbi.nlm.nih.gov/29233220/]. [Google Scholar] [CrossRef]
- Arnold, W. ; An Electron-Hole Picture of Photosynthesis. The Journal of physical chemistry 1965, 69, 788–791, [https://pubmed.ncbi.nlm.nih.gov/14296949/]. [Google Scholar] [CrossRef]
- Pearlstein, R.M. ; Photosynthetic exciton theory in the 1960s. Photosynthesis Research 2002, 73, 119–126, [https://pubmed.ncbi.nlm.nih.gov/16245112/]. [Google Scholar] [CrossRef]
- Campillo, A.J.; Shapiro, S.L.; Kollman, V.H.; Winn, K.R.; Hyer, R.C. Picosecond exciton annihilation in photosynthetic systems. Biophysical journal 1976, 16, 93–97, [https://pubmed.ncbi.nlm.nih.gov/1244893/]. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, R. ; Exciton percolation in mixed molecular crystals and aggregates: from naphthalene to photosynthesis. The Journal of Physical Chemistry 1976, 80, 2191–2195. [Google Scholar] [CrossRef]
- Pearlstein, R.M. ; Exciton migration and trapping in photosynthesis. Photochemistry and Photobiology 1982, 35, 835–844. [Google Scholar] [CrossRef]
- Pearlstein, R.M. Structure and exciton effects in photosynthesis. New Comprehensive Biochemistry 1987, 15, 299–317. [Google Scholar] [CrossRef]
- Lavergne, J.; Trissl, H.W. Theory of fluorescence induction in photosystem II: derivation of analytical expressions in a model including exciton-radical-pair equilibrium and restricted energy transfer between photosynthetic units. Biophysical journal 1995, 68, 2474–2492, [https://pubmed.ncbi.nlm.nih.gov/7647250/]. [Google Scholar] [CrossRef]
- Novoderezhkin, V.I.; Razjivin, A.P. ; Exciton dynamics in circular aggregates: application to antenna of photosynthetic purple bacteria. Biophysical journal 1995, 68, 1089–1100, [https://pubmed.ncbi.nlm.nih.gov/7756528/]. [Google Scholar] [CrossRef]
- Monshouwer, R.; Abrahamsson, M.; Van Mourik, F.; Van Grondelle, R. Superradiance and exciton delocalization in bacterial photosynthetic light-harvesting systems. The Journal of Physical Chemistry B 1997, 101, 7241–7248. [Google Scholar] [CrossRef]
- Chachisvilis, M.; Kühn, O.; Pullerits, T.; Sundström, V. ; Excitons in photosynthetic purple bacteria: wavelike motion or incoherent hopping? The Journal of Physical Chemistry B 1997, 101, 7275–7283. [Google Scholar] [CrossRef]
- Renger, T.; May, V. ; Multiple exciton effects in molecular aggregates: Application to a photosynthetic antenna complex. Physical review letters 1997, 78, 3406. [Google Scholar] [CrossRef]
- Renger, T.; May, V. ; Ultrafast exciton motion in photosynthetic antenna systems: the FMO-complex. The Journal of Physical Chemistry A 1998, 102, 4381–4391. [Google Scholar] [CrossRef]
- Lee, H.; Cheng, Y.C.; Fleming, G.R. ; Coherence dynamics in photosynthesis: protein protection of excitonic coherence. Science 2007, 316, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Abramavicius, D.; Mukamel, S. ; Quantum oscillatory exciton migration in photosynthetic reaction centers. The Journal of chemical physics 2010, 133, 08B603, [https://pubmed.ncbi.nlm.nih.gov/20707578/]. [Google Scholar] [CrossRef] [PubMed]
- Abramavicius, D.; Voronine, D.V.; Mukamel, S. ; Double-quantum resonances and exciton-scattering in coherent 2D spectroscopy of photosynthetic complexes. Proceedings of the National Academy of Sciences 2008, 105, 8525-8530, [https://pubmed.ncbi.nlm.nih.gov/18562293/]. [Google Scholar] [CrossRef]
- Bode, S.; Quentmeier, C.C.; Liao, P.N.; Hafi, N.; Barros, T.; Wilk, L.; Bittner, F.; Walla, P.J. ; On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proceedings of the National Academy of Sciences 2009, 106, 12311-12316, [https://pubmed.ncbi.nlm.nih.gov/19617542/]. [Google Scholar] [CrossRef]
- Mostame, S.; Rebentrost, P.; Eisfeld, A.; Kerman, A.J.; Tsomokos, D.I.; Aspuru-Guzik, A. ; Quantum simulator of an open quantum system using superconducting qubits: exciton transport in photosynthetic complexes. New Journal of Physics 2012, 14, 105013. [Google Scholar] [CrossRef]
- Westenhoff, S.; Palecek, D.; Edlund, P.; Smith, P.; Zigmantas, D. ; Coherent picosecond exciton dynamics in a photosynthetic reaction center. Journal of the American Chemical Society 2012, 134, 16484–16487, [https://pubmed.ncbi.nlm.nih.gov/23009768/]. [Google Scholar] [CrossRef]
- Scholes, G.D.; Smyth, C. ; Perspective: detecting and measuring exciton delocalization in photosynthetic light harvesting. The Journal of Chemical Physics 2014, 140, 03B201_1, [https://pubmed.ncbi.nlm.nih.gov/24655162/]. [Google Scholar] [CrossRef]
- Warshel, A.; Chu, Z.T.; Parson, W.W. ; Dispersed polaron simulations of electron transfer in photosynthetic reaction centers. Science 1989, 246, 112–116, [https://pubmed.ncbi.nlm.nih.gov/2675313/]. [Google Scholar] [CrossRef] [PubMed]
- Damjanović, A.; Kosztin, I.; Kleinekathöfer, U.; Schulten, K. ; Excitons in a photosynthetic light-harvesting system: a combined molecular dynamics, quantum chemistry, and polaron model study. Physical Review E 2002, 65, 031919, [https://pubmed.ncbi.nlm.nih.gov/11909121/]. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, A.; Rätsep, M.; Timpmann, K.; Trinkunas, G. ; Excitonic polarons in quasi-one-dimensional LH1 and LH2 bacteriochlorophyll a antenna aggregates from photosynthetic bacteria: A wavelength-dependent selective spectroscopy study. Chemical Physics 2009, 357, 102–112. [Google Scholar] [CrossRef]
- Qin, M.; Shen, H.Z.; Zhao, X.L.; Yi, X.X. ; Effects of system-bath coupling on a photosynthetic heat engine: A polaron master-equation approach. Physical Review A 2017, 96, 012125. [Google Scholar] [CrossRef]
- Zhang, Z.; Saurabh, P.; Dorfman, K.E.; Debnath, A.; Mukamel, S. ; Monitoring polariton dynamics in the LHCII photosynthetic antenna in a microcavity by two-photon coincidence counting. The Journal of chemical physics 2018, 148, 074302, [https://pubmed.ncbi.nlm.nih.gov/29471638/]. [Google Scholar] [CrossRef]
- Coles, D.; Flatten, L.C.; Sydney, T.; Hounslow, E.; Saikin, S.K.; Aspuru-Guzik, A.; Vedral, V.; Tang, J.K.H.; Taylor, R.A.; Smith, J.M.; Lidzey, D.G. ; A nanophotonic structure containing living photosynthetic bacteria. Small 2017, 13, 1701777. [Google Scholar] [CrossRef]
- Coles, D.M.; Flatten, L.C.; Sydney, T.; Hounslow, E.; Saikin, S.K.; Aspuru-Guzik, A.; Vedral, V.; Tang, J.K.H.; Taylor, R.A.; Smith, J.M.; Lidzey, D.G. Polaritons in living systems: modifying energy landscapes in photosynthetic organisms using a photonic structure. arXiv preprint arXiv:1702.01705, 2017.
- Squire, R.; March, N.; Ingles, J. Coherent exciton-polariton model for photosynthetic energy transfer. Bulletin of the American Physical Society 2015, 60, BB1–000019. [Google Scholar]
- Hayes, J.M.; Small, G.J. ; Photochemical hole burning and strong electron-phonon coupling: primary donor states of reaction centers of photosynthetic bacteria. The Journal of Physical Chemistry 1986, 90, 4928–4931. [Google Scholar] [CrossRef]
- Jankowiak, R.; Reppert, M.; Zazubovich, V.; Pieper, J.; Reinot, T. Site selective and single complex laser-based spectroscopies: A window on excited state electronic structure, excitation energy transfer, and electron–phonon coupling of selected photosynthetic complexes. Chem. Rev. 2011, 111, 4546–4598, [https://pubmed.ncbi.nlm.nih.gov/21595428/]. [Google Scholar] [CrossRef]
- Kell, A.; Feng, X.; Reppert, M.; Jankowiak, R. On the shape of the phonon spectral density in photosynthetic complexes. J. Phys. Chem. B 2013, 117, 7317–7323, [https://pubmed.ncbi.nlm.nih.gov/23718713/]. [Google Scholar] [CrossRef]
- Pajusalu, M.; Rätsep, M.; Freiberg, A. Temperature dependent electron–phonon coupling in chlorin-doped impurity glass and in photosynthetic FMO protein containing bacteriochlorophyll a. J.Luminescence 2014, 152, 79–83. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Fang, A.P.; Li, H.R. Phonon-assisted excitation energy transfer in photosynthetic systems. Chinese Physics B 2014, 25(9), 098201. [Google Scholar] [CrossRef]
- Pavlovich, V.S. Model for primary electron transfer and coupling of electronic states at reaction centers of purple bacteria. Journal of Applied Spectroscopy 2006, 73, 328–339. [Google Scholar] [CrossRef]
- Pavlovich, V.S. Hystons, new quasi-particles, and electron transfer in bacterial photosynthesis. Physica E: Low-dimensional Systems and Nanostructures 2002, 14, 282–288. [Google Scholar] [CrossRef]
- Pavlovich, V.S. Conception of hystons in bacterial photosynthesis: spectra, exciton dynamics, and electron transfer. Proc SPIE 2007, 6727, 67271T. [Google Scholar] [CrossRef]
- Lee, M.K.; Huo, P.; Coker, D.F. Semiclassical path integral dynamics: Photosynthetic energy transfer with realistic environment interactions. Ann. Rev. Phys. Chem. 2016, 67, 639–668, [https://pubmed.ncbi.nlm.nih.gov/27090842/]. [Google Scholar] [CrossRef]
- Onizhuk, M.; Sohoni, S.; Galli, G.; Engel, G.S. Spatial Patterns of Light-Harvesting Antenna Complex Arrangements Tune the Transfer-to-Trap Efficiency of Excitons in Purple Bacteria. The Journal of Physical Chemistry Letters 2021, 12, 6967–6973. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Liu, Z.; Miyatake, T.; Tamiaki, H.; Kobayashi, T.; Zhang, Z.; Du, J.; Leng, Y. Ultrafast dynamics of multi-exciton state coupled to coherent vibration in zinc chlorin aggregates for artificial photosynthesis. Optics Express 2017, 25, 29667–29675, [https://pubmed.ncbi.nlm.nih.gov/29221004/]. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Chen, X.; Lin, L.; Fang, Y.; Wang, X. Biomimetic donor–acceptor motifs in conjugated polymers for promoting exciton splitting and charge separation. Angew. Chem. Int. Ed. 2018, 57, 8729–8733, [https://pubmed.ncbi.nlm.nih.gov/29797759/]. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.S.; Lloyd, L.T.; Sohail, S.H.; Allodi, M.A.; Otto, J.P.; Saer, R.G.; Wood, R.E.; Massey, S.C.; Ting, P.C.; Blankenship, R.E.; Engel, G.S. Photosynthesis tunes quantum-mechanical mixing of electronic and vibrational states to steer exciton energy transfer. Proceedings of the National Academy of Sciences 2021, 118, E2018240118, [https://pubmed.ncbi.nlm.nih.gov/33688046/]. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chen, Z.; Brennaman, M.K.; Concepcion, J.J.; Patrocinio, A.O.T.; Iha, N.Y.M.; Meyer, T.J. Making solar fuels by artificial photosynthesis. Pure and Applied Chemistry 2011, 83, 749–768. [Google Scholar] [CrossRef]
- Stock, M.; Dunn, S. LiNbO3 - A new material for artificial photosynthesis. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 2011, 58, 1988–1993, [https://pubmed.ncbi.nlm.nih.gov/21937336/]. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.K.; Zain, M.M.; Kadhum, A.A.H. Artificial photosynthesis using LiNbO3 as photocatalyst for sustainable and environmental friendly construction and reduction of global warming: A review. Catalysis Reviews 2014, 56, 175–186. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M. Artificial photosynthesis in concrete surface by using LiNbO3. Advances in Environmental Biology 2015, 9, 1–9, [https://pubmed.ncbi.nlm.nih.gov/24376384/]. [Google Scholar] [CrossRef]
- Cortecchia, D.; Yin, j.; Bruno, A.; Lo, S.-Z.A.; Gurzadyan, G.G.; Mhaisalkar, S.; Brédas, J.-L.; Soci, C. Polaron self-localization in white-light emitting hybrid perovskites. J. Mat. Chem. C 2017, 5, 2771–2780. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, L.W. Large polaron formation and its effect on electron transport in hybrid perovskites. Energy & Environmental Science 2019, 12, 1219–1230. [Google Scholar] [CrossRef]
- Chen, Y.C.; Song, B.; Leggett, A.J.; Ao, P.; Zhu, X. Resonant confinement of an excitonic polariton and ultraefficient light harvest in artificial photosynthesis. Phys. Rev. Lett. 2019, 122, 257402, [https://pubmed.ncbi.nlm.nih.gov/31347870/]. [Google Scholar] [CrossRef]
- Liu, S.; Weng, B.; Tang, Z.R.; Xu, Y.J. Constructing one-dimensional silver nanowire-doped reduced graphene oxide integrated with CdS nanowire network hybrid structures toward artificial photosynthesis. Nanoscale 2015, 7, 861–866. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhou, S.S.; Chen, Z.G.; Liu, C.B.; Chen, F.; Wu, Z.Y. An artificial photosynthesis system based on CeO2 as light harvester and N-doped graphene Cu(II) complex as artificial metalloenzyme for CO2 reduction to methanol fuel. Cat. Commun. 2016, 73, 7–11. [Google Scholar] [CrossRef]
- Guiglion, P.; Berardo, E.; Butchosa, C.; Wobbe, M.C.; Zwijnenburg, M.A. Modelling materials for solar fuel synthesis by artificial photosynthesis; predicting the optical, electronic and redox properties of photocatalysts. Journal of Physics: Condensed Matter 2016, 28, 074001, [https://pubmed.ncbi.nlm.nih.gov/26808228/]. [Google Scholar] [CrossRef]
- Jena, N.; Rawat, A.; De Sarkar, A. Strain and pH facilitated artificial photosynthesis in monolayer MoS2 nanosheets. J. Mat. Chem. A 2017, 5, 22265–22276. [Google Scholar] [CrossRef]
- Jain, P.K. Plasmonic Photosynthesis. ECS Meeting Abstracts 2019, 41, 1949. [Google Scholar] [CrossRef]
- Yu, S.; Jain, P.K. Plasmonic photosynthesis of C1–C3 hydrocarbons from carbon dioxide assisted by an ionic liquid. Nature Commun. 2019, 10, 1–7, [https://pubmed.ncbi.nlm.nih.gov/31043604/]. [Google Scholar] [CrossRef]
- Yu, S.; Jain, P.K. Selective branching of plasmonic photosynthesis into hydrocarbon production and hydrogen generation. ACS Energy Letters 2019, 4, 2295–2300. [Google Scholar] [CrossRef]
- Yu, S.; Jain, P.K. Isotope effects in plasmonic photosynthesis. Angew. Chem. 2020, 132, 22666–22669, [https://pubmed.ncbi.nlm.nih.gov/32898311/]. [Google Scholar] [CrossRef]
- Yu, S.; Jain, P.K. Plasmonic catalysis, photoredox chemistry, and photosynthesis. Plasmonic Catalysis: From Fundamentals to Applications 2021 (in press). [CrossRef]
- Ueno, K.; Oshikiri, T.; Shi, X.; Zhong, Y.; Misawa, H. Plasmon-induced artificial photosynthesis. Interface Focus 2015, 5, 20140082, [https://pubmed.ncbi.nlm.nih.gov/26052419/]. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ueno, K.; Mori, Y.; Shi, X.; Oshikiri, T.; Murakoshi, K.; Inoue, H.; Misawa, H. Plasmon-assisted water splitting using two sides of the same SrTiO3 single-crystal substrate: conversion of visible light to chemical energy. Angew. Chem. Int. Ed. 2014, 53, 10350–10354, [https://pubmed.ncbi.nlm.nih.gov/24988943/]. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.D.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-enhanced photocatalytic CO2 conversion within metal–organic frameworks under visible light. J. Am. Chem. Soc. 2017, 139, 356–362, [https://pubmed.ncbi.nlm.nih.gov/28004911/]. [Google Scholar] [CrossRef]
- Glushko, E.Y. Nonuniform kinetics of quasiparticles in a steady-state PACKET representation. Ukrainskii Fizicheskii Zhurnal 1981, 26, 2037–2043. [Google Scholar]
- Seminozhenko, V.P. Kinetics of interacting quasiparticles in strong external fields. Physics Reports 1982, 91, 103–182. [Google Scholar] [CrossRef]
- Sinisky, I.; Golosov, A.; Men, A. The kinetics of the reactions of solid-phases in the crystalline quasiparticles method. Berichte der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics 1982, 86, 482–482. [Google Scholar]
- Glushko, E.Ya. Nonhomogeneous kinetics of quasiparticles in steady-state packet representation. Physica Status Solidi B 1982, 114, 685–694. [Google Scholar] [CrossRef]
- Rawat, A.; Jena, N.; De Sarkar, A. A comprehensive study on carrier mobility and artificial photosynthetic properties in group VI B transition metal dichalcogenide monolayers. J. Mater. Chem. A 2018, 6, 8693–8704. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Jin, S.; Zhang, X.; Xie, Y. Low-dimensional semiconductors in artificial photosynthesis: An outlook for the interactions between particles/quasiparticles. ACS Central Science 2020, 6, 1058–1069, [https://pubmed.ncbi.nlm.nih.gov/32724841/]. [Google Scholar] [CrossRef]
- Dorfman, K.E.; Voronine, D.V.; Mukamel, S.; Scully, M.O. Photosynthetic reaction center as a quantum heat engine. Proceedings of the National Academy of Sciences 2013, 110, 2746–2751, [https://pubmed.ncbi.nlm.nih.gov/23365138/]. [Google Scholar] [CrossRef]
- Ioffe I., I.; Reshetov, V.A.; Dobrotvorsky, A.M. Heterogeneous catalysis: Physical and chemical bases; Chemistry: Leningrad, 1985; 224 p. [Google Scholar]
- Ioffe, I.I.; Dobrotvorskii, A.M.; Belozerskikh, V.A. Prediction and analysis of heterogeneous catalysis mechanisms by pattern recognition methods with a computer. Russ. Chem. Rev. 1983, 52, 229–241. [Google Scholar] [CrossRef]
- Dobrotvorskii, A.M. A quasi-fermion model of electronic-structure and its applications in the chemosorption and heterogeneous catalysis. Doklady Akademii Nauk SSSR 1984, 279, 915–919. [Google Scholar]
- Dobrotvorskii, A.M. , Afanasjeva, O.V. A quasifermion approach to modelling interatomic interactions in solids. Journal of Physics: Condensed Matter 1993, 5, 8839–8848. [Google Scholar]
- Kiselev, V.F.; Plotnikov, G.S.; Bespalov, V.A.; Zoteev, A.V.; Fomin, Y.D. Elementary excitations in a semiconductor-adsorbed-molecule system. Kinetics and Catalysis 1987, 28, 14–27. [Google Scholar]
- Kiselev, V.F.; Krylov, O.V. Electronic phenomena in adsorption and catalysis on semiconductors and dielectrics., 2nd ed.; Springer: Berlin, 2012; 287 p. [Google Scholar]
- McCarroll, J.J. Surface physics and catalysis. Surface Science 1975, 53, 297–316. [Google Scholar] [CrossRef]
- Lundström, I.; Armgarth, M.; Petersson, L.G. Physics with catalytic metal gate chemical sensors. Critical Reviews in Solid State and Material Sciences 1989, 15, 201–278. [Google Scholar] [CrossRef]
- Zamaraev, K.I. Chemical physics and catalysis. Pure and Applied Chemistry 1997, 69, 865–876. [Google Scholar] [CrossRef]
- Sirin, S.; Pearlman, D.A.; Sherman, W. Physics-based enzyme design: Predicting binding affinity and catalytic activity. Proteins: Structure, Function, and Bioinformatics 2014, 82, 3397–3409, [https://pubmed.ncbi.nlm.nih.gov/25243583/]. [Google Scholar] [CrossRef] [PubMed]
- Fodor, É.; Marchetti, M.C. The statistical physics of active matter: From self-catalytic colloids to living cells. Physica A: Statistical Mechanics and its Applications 2018, 504, 106–120. [Google Scholar] [CrossRef]
- Brandt N., B.; Kulbachinsky, V.A. Quasiparticles in condensed matter physics, 3rd ed.; Fizmatlit: Moscow, 2016. [Google Scholar]
- Fan, T.Y.; Sun, J.J. Four-phonon model of soft-matter quasicrystals for studying thermodynamics. Philosophical Magazine Letters 2014, 94, 112–117. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Sharma, P. Geometrically nonlinear deformation and the emergent behavior of polarons in soft matter. Soft Matter 2015, 11, 8042–8047, [https://pubmed.ncbi.nlm.nih.gov/26345397/]. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.; Wecker, A.; Yuan, J. Advanced optical materials in the new decade: From metamaterials, smart soft matter, and lasers to terahertz, polaritons, and AIE. Advanced Optical Materials 2020, 8, 1901916. [Google Scholar] [CrossRef]
- Yuan, H. Single molecules in soft matter: A study of biomolecular conformation, heterogeneity and plasmon enhanced fluorescence. PhD Thesis, Leiden Institute of Physics, Leiden University, 2013. [Google Scholar]
- Matsko, N.; Letofsky-Papst, I.; Mittal, V. What is hidden in the volume plasmon? EFTEM plasmon to carbon map for soft matter characterization. Imaging & Microscopy 2014, 16, 2–4. [Google Scholar]
- Miller, T. Multiscale dynamics in soft-matter systems: Enzyme catalysis, sec-facilitated protein translocation, and ion-conduction in polymers. Bulletin of the American Physical Society 2016, 61, S22–009. [Google Scholar]
- Khokhlov, A.R. Water solutions of amphiphilic polymers: Nanostructure formation and possibilities for catalysis (soft matter as structured materials). Physical Characteristics Research 2005, 84, 832. [Google Scholar]
- Dornhaus, R.; Benner, R.E.; Chang, R.K.; Chabay, I. Surface plasmon contribution to SERS. Surface Science 1980, 101, 367–373. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Wu, Y.; Xu, Y.; Su, Y.; Zhang, L.; Qi, Y.; Wen, X.; Yang, H. Hybrid surface plasmon effect and SERS characterization in a heterogeneous composite structure of Au nano-array and Ag film. Results in Physics 2020, 17, 103175. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sensors and Actuators B: Chemical 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Yamamoto, M. Surface plasmon resonance (SPR) theory: Tutorial. Review of Polarography 2002, 48, 209–237. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Optics Express 2009, 17, 16505–16517, [https://pubmed.ncbi.nlm.nih.gov/19770865/]. [Google Scholar] [CrossRef]
- Herrmann, F.H.; Börner, T.; Hagemann, R. Biosynthesis of thylakoids and the membrane-bound enzyme systems of photosynthesis. Results and Problems in Cell Differentiation 1980, 10, 147–177, [https://pubmed.ncbi.nlm.nih.gov/6999569/]. [Google Scholar] [CrossRef] [PubMed]
- Latzko, E.; Kelly, G.J. Photosynthesis control of carbon metabolism through enzyme regulation and membrane-mediated metabolite transport. In Thirty Years of Photosynthesis 1974–2004; Springer: Berlin, Heidelberg, 2006; pp. 33–52. [Google Scholar] [CrossRef]
- Miller, K.; Staeheli, L.A. Direct identification of photosynthetic enzymes on membrane surfaces revealed by deep-etching. Journal of Cell Biology 1973, 59, A226–A226. [Google Scholar]
- Anderson, L.E.; Avron, M. Light modulation of enzyme activity in chloroplasts: Generation of membrane-bound vicinal-dithiol groups by photosynthetic electron transport. Plant Physiology 1976, 57, 209–213, [https://pubmed.ncbi.nlm.nih.gov/16659452/]. [Google Scholar] [CrossRef] [PubMed]
- Krogmann, D.W. The organization of photosynthetic enzymes on the chloroplast membrane. In The Enzymes of Biological Membranes; Martonosi, A., Ed.; Springer: Boston, MA, 1976; pp. 143-162. [Google Scholar] [CrossRef]
- Cuendet, P.; Gratzel, M. Biophotocatalysis based on semiconducting powders. Experientia 1984, 40, 604–604. [Google Scholar]
- López-Vidal, M.G.; Gamboa, G.; Oksdath-Mansilla, G.; Bisogno, F.R. Photobiocatalysis. In Biocatalysis for Practitioners: Techniques, Reactions and Applications 2021, 317-359. [CrossRef]
- Lee, S.H.; Choi, D.S.; Kuk, S.K.; Park, C.B. Photobiocatalysis: Activating redox enzymes by direct or indirect transfer of photoinduced electrons. Angew. Chem. Int. Ed. 2018, 57, 7958–7985, [https://pubmed.ncbi.nlm.nih.gov/29194901/]. [Google Scholar] [CrossRef] [PubMed]
- Maciá Agulló, J.A.; Corma Canós, A.; García Gómez, H. Photobiocatalysis: The power of combining photocatalysis and enzymes. Chemistry-a European Journal 2015, 21, 10940–10959, [https://pubmed.ncbi.nlm.nih.gov/26014675/]. [Google Scholar] [CrossRef] [PubMed]
- Drbohlavová, J. Preparation of photocatalytically active surfaces. Ph. D. Thesis, FCH VUT – IRCELYON, Brno – Lyon, France, 2008.
- Schmidt, H.K.; Akarsu, M.; Naumann, M.; Müller, T.S. Doped nanoparticles for photocatalytically active surfaces. Transactions of the Materials Research Society of Japan 2004, 29, 2717–2724. [Google Scholar] [CrossRef]
- Böttger, M.; Graumann, T.; Boughaled, R.; Neumann, F.; Kowalsky, W.; Johannes, H.H. Development of a new qualification method for photocatalytically active surfaces based on a solid state luminescent dye. J. Photochem. Photobiol. A: Chem. 2013, 253, 7–15. [Google Scholar] [CrossRef]
- Schlettwein, D. Light-induced charge transfer using phthalocyanines in active interfaces: Photoredox interaction or semiconductor junction? J.f Porphyrins Phthalocyanines 2008, 12, 337. [Google Scholar]
- Irie, H.; Hashimoto, K. Photocatalytic active surfaces and photo-induced high hydrophilicity/high hydrophobicity. In Environmental Photochemistry, Part II; Boule, P., Bahnemann, D. W., Robertson, P. K. J., Eds.; Springer: Berlin, Heidelberg, 2005; pp. 425–450. [Google Scholar] [CrossRef]
- Prins, R.; Schildenberger, M.; Bonetti, Y.C.; Gobrecht, J. Nanotechnology and model catalysis: The use of photolithography for creating active surfaces. CHIMIA International Journal for Chemistry 2000, 54, 63–65. [Google Scholar] [CrossRef]
- Kurz, J.; Eberle, F.; Graumann, T.; Kaschel, M.-E.; Sähr, A.; Neumann, F.; Dalpke, A.H.; Erdinger, L. Inactivation of LPS and RNase A on photocatalytically active surfaces. Chemosphere 2011, 84, 1188–1193, [https://pubmed.ncbi.nlm.nih.gov/21762949/]. [Google Scholar] [CrossRef]
- Hamedani Golshan, N. Understanding electrically active interface formation on wide bandgap semiconductors through molecular beam epitaxy using Fe₃O₄ for spintronics as a base case. PhD Thsesis, Northeastern University, Boston, 2017.
- Dimoulas, A. Electrically active interface and bulk semiconductor defects in high-k/Germanium structures. In Defects in High-k Gate Dielectric Stacks; Gusev, E., Ed.; Springer: Dordrecht, 2006; pp. 237-248. [Google Scholar] [CrossRef]
- Raynaud, C.; Autran, J.L.; Balland, B.; Guillot, G.; Jaussaud, C.; Billon, T. Electrical characterization of instabilities in 6H silicon carbide metal-oxide-semiconductor capacitors. Journal of Applied Physics 1994, 76, 993–997. [Google Scholar] [CrossRef]
- Gomes, H.L.; Stallinga, P.; Cölle, M.; De Leeuw, D.M.; Biscarini, F. Electrical instabilities in organic semiconductors caused by trapped supercooled water. Applied Physics Letters 2006, 88, 082101. [Google Scholar] [CrossRef]
- Di Pietro, R.; Sirringhaus, H. High resolution optical spectroscopy of air-induced electrical instabilities in n-type polymer semiconductors. Advanced Materials 2012, 24, 3367–3372, https://pubmed.ncbi.nlm.nih.gov/22605674/. [Google Scholar] [CrossRef]
- Jones, B.L.; Beaudet, P.R. Negative photoconductivity and electrical instabilities in semiconductors. Canadian Journal of Physics 1967, 45, 4091–4101. [Google Scholar] [CrossRef]
- Hajto, J.P. Optical and electrical instabilities in amorphous semiconductors. PhD Thesis, University of Edinburgh, Edinburgh, 1993. [Google Scholar]
- Hurley, P.K.; Cherkaoui, K.; Groenland, A. Electrically active interface defects in the (100) Si/SiOx/HfO2/TiN system: Origin, instabilities and passivation. ECS Transactions 2006, 3, 97. [Google Scholar] [CrossRef]
- Djara, V.; O’Regan, T.P.; Cherkaoui, K.; Schmidt, M.; Monaghan, S.; O’Connor, E.; Povey, I.M.; O’Connell, D.; Pemble, M.E.; Hurley, P.K. Electrically active interface defects in the In0.53Ga0.47As MOS system. Microelectronic Engineering 2013, 109, 182–188. [Google Scholar] [CrossRef]
- Rubio-Gimenez, V.; Tatay, S.; Volatron, F.; Martinez-Casado, F.J.; Martí-Gastaldo, C.; Coronado, E. High-quality metal–organic framework ultrathin films for electronically active interfaces. Journal of the American Chemical Society, 2016, 138, 2576–2584, [https://pubmed.ncbi.nlm.nih.gov/26847507/]. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.S.; Mulfort, K.L. Redox-active MOF with bio-mimetic cobalt nodes: Toward artificial photosynthesis in framework architectures. In Abstracts of Papers of The American Chemical Society 2012, 244. [Google Scholar]
- Xu, J.; He, S.; Zhang, H.; Huang, J.; Lin, H.; Wang, X.; Long, J. Layered metal–organic framework/graphene nanoarchitectures for organic photosynthesis under visible light. J. Mater. Chem. A 2012, 3, 24261–24271. [Google Scholar] [CrossRef]
- Heidary, N.; Harris, T.G.; Ly, K.H.; Kornienko, N. Artificial photosynthesis with metal and covalent organic frameworks (MOFs and COFs): Challenges and prospects in fuel-forming electrocatalysis. Physiologia Plantarum 2019, 166, 460–471, [https://pubmed.ncbi.nlm.nih.gov/30706497/]. [Google Scholar] [CrossRef]
- Malyshev, V.V. Response of semiconducting metal oxides to water vapor as a result of water molecules chemical transformations on catalytically active surfaces. Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82, 2329–2339. [Google Scholar] [CrossRef]
- O’Mullane, A.P. Creating active interfaces as a strategy to improve electrochemical water splitting reactions. Journal of Physics: Energy 2020, 2, 041001. [Google Scholar] [CrossRef]
- Greuter, F. Electrically active interfaces in ZnO varistors. Solid State Ionics 1995, 75, 67–78. [Google Scholar] [CrossRef]
- Ling, Z.; Russell, J.D.; Leach, C. The effect of variations in sintering temperature on the structure of electrically active interfaces in zinc oxide varistors. Key Engineering Materials 1997, 132, 1305–1308. [Google Scholar] [CrossRef]
- Leach, C.; Ling, Z.; Freer, R. The effect of sintering temperature variations on the development of electrically active interfaces in zinc oxide based varistors. Journal of the European Ceramic Society 2000, 20, 2759–2765. [Google Scholar] [CrossRef]
- Elfwing, M.; Olsson, E. Electron holography study of active interfaces in zinc oxide varistor materials. Journal of Applied Physics 2002, 92, 5272–5280. [Google Scholar] [CrossRef]
- Cho, K.G.; Kim, H.S.; Jang, S.S.; Kyung, H.; Kang, M.S.; Lee, K.H.; Yoo, W.C. Optimizing electrochemically active surfaces of carbonaceous electrodes for ionogel based supercapacitors. Advanced Functional Materials 2020, 30, 2002053. [Google Scholar] [CrossRef]
- Zhukov, V.; Winkler, A.; Rendulic, K. The energetics of coadsorbate interaction on catalytically active surfaces. In Proc. ÖPG 93, Österreichischen Physikalischen Gesellschaft, Graz, Austria (20 Sep 1993 – 24 Sep 1993).
- Roginskii, S.Z. Isotopic methods for studying the heterogeneity of active surfaces and interactions in the adsorption layer. Zhurnal Fizicheskoi Khimii 1958, 32, 737–745. [Google Scholar]
- Keier, N.P.; Roginskii, S.Z. Investigation of the nonhomogeneity of active surfaces by the differential isotopic method. I. Active surfaces of metallic nickel and of zinc oxide. Izvest. Akad. Nauk SSSR 1950, (1), 51. [Google Scholar]
- Charcosset, H.; Barthome. D.; Nicolova, R. ; Revillon, A. ; Tournaya. L.; Trambouz. Y. Method for determining active surfaces of some catalytic systems by chemisorption. Bulletin De La Societe Chimique De France 1967, 12, 4555. [Google Scholar]
- Flosdorf, E.W.; Kistiakowsky, G.B. Heats of adsorption on catalytically active surfaces. J. Phys. Chem. 2002, 34, 1907–1918. [Google Scholar] [CrossRef]
- Chapaeva, A.; Chokaev, K.K.; Loginov, A. The formation of the active surfaces of modified lanthanide catalysts. I, Paramagnetic centres of chromium-containing yttrium and scandium oxides. Russian Journal of Physical Chemistry 1990, 64, 1034–1036. [Google Scholar]
- Niklewsk, J.B.; Sis, L.; Wirtz, G. Characterization of catalitically active surfaces with scanning electron microscope. American Ceramic Society Bulletin 1971, 50, 381. [Google Scholar]
- Iwasawa, Y.; Shido, T.; Fukui, K. Molecular design and characterization of active surfaces for molecular-level understanding and development of catalysis. Abstracts of Papers of the American Chemical Society 2001, 221, U310. [Google Scholar]
- Tada, M.; Iwasawa, Y. Chemical design and in situ characterization of active surfaces for selective catalysis. Annu. Rev. Mater. Res. 2005, 35, 397–426. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Palivan, C.G.; Gademann, K.; Meier, W.; Calame, M.; Mikhalevich, V.; Zhang, X.; Piel, E.; Szponarski, M.; Wiesler, A.; Lanzilotto, A.; Constable, E.C.; Fanget, A.; Stoop, S.L. “Active surfaces” as possible functional systems in detection and chemical (bio) reactivity. CHIMIA International Journal for Chemistry 2016, 70, 402–412, [https://pubmed.ncbi.nlm.nih.gov/27363368/]. [Google Scholar] [CrossRef]
- Lewis, D.R. Electrical condition of catalytically active surfaces. Thesis, University of Manchester, Manchester, 1926.
- Bowden, F.P.; Rideal, E.K. On the electrolytic behaviour of thin films. Part II. The areas of catalytically active surfaces. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 1928, 120, 80–89. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.V.; Zhang, L.; Tang, Z.; Wan, H. Active surfaces for CO oxidation on palladium in the hyperactive state. Langmuir 2010, 26, 18113–18118, [https://pubmed.ncbi.nlm.nih.gov/21053982/]. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.; Wan, H. Kinetics and active surfaces for CO oxidation on Pt-group metals under oxygen rich conditions. Topics in Catalysis 2013, 56, 1299–1313. [Google Scholar] [CrossRef]
- Kondoh, H.; Toyoshima, R.; Monya, Y.; Yoshida, M.; Mase, K.; Amemiya, K.; Mun, B.S. In situ analysis of catalytically active Pd surfaces for CO oxidation with near ambient pressure XPS. Catalysis Today 2016, 260, 14–20. [Google Scholar] [CrossRef]
- Ertl, G.; Norton, P.R.; Rüstig, J. Kinetic oscillations in the platinum-catalyzed oxidation of CO. Physical Review Letters 1982, 49, 177. [Google Scholar] [CrossRef]
- Imbihl, R.; Cox, M.P.; Ertl, G.; Müller, H.; Brenig, W. Kinetic oscillations in the catalytic CO oxidation on Pt (100): Theory. J. Chem. Phys. 1985, 83, 1578–1587. [Google Scholar] [CrossRef]
- Eiswirth, M.; Ertl, G. Kinetic oscillations in the catalytic CO oxidation on a Pt (110) surface. Surface Science 1986, 177, 90–100. [Google Scholar] [CrossRef]
- Imbihl, R.; Cox, M.P.; Ertl, G. Kinetic oscillations in the catalytic CO oxidation on Pt (100): Experiments. J. Chem. Phys. 1986, 84, 3519–3534. [Google Scholar] [CrossRef]
- Lyons, M.E. The mechanism of mediated electron transfer at redox active surfaces. Electroanalysis 2015, 27, 992–1009. [Google Scholar] [CrossRef]
- Lan, Y.M.; Cheng, K.J.; Luk, Y.Y. Development of redox-active surfaces and micelles for biocompatible systems: Caging ferrocene in cyclic oligosaccharides. In Abstracts of Papers of the American Chemical Society 2005, 230, U1110–U1111. [Google Scholar]
- Yzambart, G.; Fabre, B.; Camerel, F.; Roisnel, T.; Lorcy, D. Controlled grafting of tetrathiafulvalene (TTF) containing diacetylenic units on hydrogen-terminated silicon surfaces: from redox-active TTF monolayer to polymer films. J. Phys. Chem. C 2012, 116, 12093–12102. [Google Scholar] [CrossRef]
- Joy, S.; Pal, P.; Mondal, T.K.; Talapatra, G.B.; Goswami, S. Synthesis of amphiphilic azo-anion-radical complexes of chromium(III) and the development of ultrathin redox-active surfaces by the Langmuir–Schaefer technique. Chemistry–A European Journal 2012, 18, 1761–1771, [https://pubmed.ncbi.nlm.nih.gov/22237915/]. [Google Scholar] [CrossRef] [PubMed]
- Fellermann, H. Micelles as containers for protocells. Beiträge des Instituts für Umweltsystemforschung der Universität Osnabrück, 2005; 33, 1-83. [Google Scholar]
- Chang, G.G.; Huang, T.M.; Hung, H.C. Reverse micelles as life-mimicking systems. Proceedings of the National Science Council, Republic of China. Part B, Life Sciences 2000, 24, 89–100. [Google Scholar]
- Ciucci, F.; Chueh, W.C.; Goodwin, D.G.; Haile, S.M. Surface reaction and transport in mixed conductors with electrochemically-active surfaces: a 2D numerical study of ceria. Physical Chemistry Chemical Physics 2011, 13, 2121–2135. [Google Scholar] [CrossRef]
- Suwono, A.; Daguenet, M.; Bodiot, D. Theoretical study of the diffusion or conduction fluxes on a finite number of active surfaces in interaction, one with another, separated by inert zones, in a Newtonian or non-Newtonian viscous fluid in laminar or turbulent flow. International Journal of Heat and Mass Transfer 1976, 19, 239–244. [Google Scholar] [CrossRef]
- Beringuier, H.; Suwono, A.; Delmas, A.; Daguenet, M.; Spinner, B.; Bodiot, D. Experimental study of diffusion flows on a finite number of interacting active surfaces divided each other by inert zones into a newtonian or not viscous-fluid in a laminar or turbulent-flow. Journal de Chimie Physique et de Physico-Chimie Biologique 1976, 73, 868–871. [Google Scholar] [CrossRef]
- Suwono, A.; Daguenet, M. Theoretical study of diffusion or conduction interaction between active surfaces in laminar-flow of a fluid with small schmidt or prandtl numbers. Journal de Chimie Physique et de Physico-Chimie Biologique 1977, 74, 681–684. [Google Scholar] [CrossRef]
- Vurdelja, A. A study on the selective transport in the emulsions containing droplets with active surfaces [Badania transportu selektywnego w środowisku emulsji z czynnymi powierzchniami kropel]. PhD Thesis, Warsaw University of Technology, Faculty of Chemical and Process Engineering, Department of Process Kinetics and Thermodynamics, Warsaw, 2017. [Google Scholar]
- Amelin, A.G.; Kabanov, A.N.; Shchukin, E.R.; Shulimanova, Z.L. Features of the motion of aerosol particles near catalytically active surfaces. Kinet. Catal. 1985, 26, 93–101. [Google Scholar]
- Parisi, J.; Peinke, J.; Röhricht, B.; Rau, U.; Klein, M.; Rössler, O.E. Comparison between a generic reaction-diffusion model and a synergetic semiconductor system. Zeitschrift für Naturforschung A 1987, 42, 655–656. [Google Scholar] [CrossRef]
- Merz, W. Strong solutions for reaction-drift-diffusion problems in semiconductor technology. Journal of Applied Mathematics and Mechanics 2001, 81, 623–635. [Google Scholar] [CrossRef]
- Justin, M.; Betchewe, G.; Doka, S.Y.; Crepin, K.T. Exact solutions of a semiconductor nonlinear reaction diffusion equation through factorization method. Applied Mathematics and Computation 2012, 219, 2917–2922. [Google Scholar] [CrossRef]
- Justin, M.; Marcel, G.; Betchewe, G.; Doka, S.Y.; Crepin, K.T. New exact solutions for a semiconductor nonlinear reaction-diffusion equation: the combination of the factorization method to the projective Riccati equation method. Electronic Journal of Mathematical Analysis and Applications 2017, 5, 271–288. [Google Scholar]
- Gardner, J.W. A non-linear diffusion-reaction model of electrical conduction in semiconductor gas sensors. Sensors and Actuators B: Chemical 1990, 1, 166–170. [Google Scholar] [CrossRef]
- Lavine, I.S.; Levinson, J.A.; Glogovsky, K.G. Modeling and simulation of hydrogen diffusion and reaction in semiconductor photonic materials. In Abstracts of Papers of the American Chemical Society 2014, 247. [Google Scholar]
- Peirce, A.P. Mathematical analysis of chemical systems: The effect of defect structures on chemically active surfaces; Optimal control of quantum molecular systems. PhD Thesis, Princeton University, Princeton, New Jersey, 1987. [Google Scholar]
- Glitzky, A. An electronic model for solar cells including active interfaces and energy resolved defect densities. SIAM Journal on Mathematical Analysis 2012, 44, 3874–3900. [Google Scholar] [CrossRef]
- Masuduzzaman, M.; Weir, B.; Alam, M.A. Probing bulk defect energy bands using generalized charge pumping method. Journal of Applied Physics 2012, 111, 074501. [Google Scholar] [CrossRef]
- Kumar, N.T.; Pinto, M.A.D.C.; Shmavonyan, G. Reaction–diffusion cellular automata framework-based understanding of radiation-induced effects from alpha-particles on the performances of microprocessors/FPGAs/other electronic devices using higher order logic (HOL) System and CAVA library in the R&D of semiconductor industry. International Journal of Applied Research on Information Technology and Computing 2018, 9, 39–49. [Google Scholar] [CrossRef]
- Mietke, A.; Jülicher, F.; Sbalzarini, I.F. Self-organized shape dynamics of active surfaces. Proceedings of the National Academy of Sciences 2019, 116, 29–34, [https://pubmed.ncbi.nlm.nih.gov/30567977/]. [Google Scholar] [CrossRef]
- Alonso, S.; Chen, H.Y.; Bär, M.; Mikhailov, A.S. Self-organization processes at active interfaces. European Physical Journal Special Topics 2010, 191, 131–145. [Google Scholar] [CrossRef]
- Cagnetta, F.; Evans, M.R.; Marenduzzo, D. Kinetic roughening in active interfaces. EPJ Web of Conferences 2020, 230, 00001. [Google Scholar] [CrossRef]
- Rubino, M. Developing active surfaces through the implementation of nanotechnology. Abstracts of Papers of the American Chemical Society 2017, 254. [Google Scholar]
- Neretina, S.; Hughes, R. Nanostructure synthesis at the liquid-substrate interface: A new strategy for obtaining plasmonic and chemically active surfaces. Abstracts of Papers of the American Chemical Society 2017, 254. [Google Scholar]
- Hoffmann, L.; Giomi, L. Active surfaces and defect-mediated morphogenesis. Bulletin of the American Physical Society 2021, 66, C05.00001. [Google Scholar]
- Buten, C.; Kortekaas, L.; Ravoo, B.J. Design of active interfaces using responsive molecular components. Advanced Materials 2020, 32, 1904957, [https://pubmed.ncbi.nlm.nih.gov/31573115/]. [Google Scholar] [CrossRef]
- Rossiter, J.; Yap, B.; Conn, A. Biomimetic chromatophores for camouflage and soft active surfaces. Bioinspiration & Biomimetics 2012, 7, 036009, [https://pubmed.ncbi.nlm.nih.gov/22549047/]. [Google Scholar] [CrossRef]
- Krysiński, P.; Blanchard, G.J. Synthesis and characterization of amphiphilic biomimetic assemblies at electrochemically active surfaces. Langmuir 2003, 19, 3875–3882. [Google Scholar] [CrossRef]
- Garni, M.; Wehr, R.; Avsar, S.Y.; John, C.; Palivan, C.; Meier, W. Polymer membranes as templates for bio-applications ranging from artificial cells to active surfaces. European Polymer Journal 2019, 112, 346–364. [Google Scholar] [CrossRef]
- Komissarov, G.G. A new concept of photosynthesis. In: Process Advancement in Chemistry and Chemical Engineering Research. CRC, Waretown, 2016. p. 303-327.
- Komissarov, G.G.; Lobanov, A.V. Photoinduced processes of hydrogen peroxide formation and decomposition and their role in photosynthesis and biosphere origin. Geochemistry International 2014, 52, 1239–1251. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Komissarov, G.G. Hydrogen peroxide in artificial photosynthesizing systems. Biophysics 2014, 59, 169–182. [Google Scholar] [CrossRef]
- Komissarov, G.G.; Lobanov, A.V.; Nevrova, O.V.; Popov, I.A.; Kononikhin, A.S.; Pekov, S.I.; Nikolaev, E.N. New step towards artificial photosynthesis: photogeneration of organic compounds in the inorganic carbon-hydrogen peroxide-phthalocyanine system. Doklady Physical Chemistry 2013, 453, 275–278. [Google Scholar] [CrossRef]
- Sammaknejad, N.; Zhao, Y.; Huang, B. A review of the expectation maximization algorithm in data-driven process identification. Journal of Process Control 2019, 73, 123–136. [Google Scholar] [CrossRef]
- Czop, P.; Kost, G.; Sławik, D.; Wszołek, G. Formulation and identification of first-principle data-driven models. Journal of Achievements in Materials and Manufacturing Engineering 2011, 44, 179–186. [Google Scholar]
- Chang, H.; Zhang, D. Identification of physical processes via combined data-driven and data-assimilation methods. Journal of Computational Physics 2019, 393, 337–350. [Google Scholar] [CrossRef]
- Brewick, P.T.; Masri, S.F. An evaluation of data-driven identification strategies for complex nonlinear dynamic systems. Nonlinear Dynamics 2016, 85, 1297–1318. [Google Scholar] [CrossRef]
- Meidani, K.; Farimani, A.B. Data-driven identification of 2D partial differential equations using extracted physical features. Computer Methods in Applied Mechanics and Engineering 2021, 381, 113831. [Google Scholar] [CrossRef]
- Rudy, S.; Alla, A.; Brunton, S.L.; Kutz, J.N. Data-driven identification of parametric partial differential equations. SIAM Journal on Applied Dynamical Systems 2019, 18, 643–660. [Google Scholar] [CrossRef]
- Laisk, A.; Eichelmann, H. Towards understanding oscillations: A mathematical model of the biochemistry of photosynthesis. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 1989, 323, 369–384. [Google Scholar] [CrossRef]
- Nedbal, L.; Červený, J.; Rascher, U.; Schmidt, H. E-photosynthesis: A comprehensive modeling approach to understand chlorophyll fluorescence transients and other complex dynamic features of photosynthesis in fluctuating light. Photosynthesis Research 2007, 93, 223–234, [https://pubmed.ncbi.nlm.nih.gov/17492490/]. [Google Scholar] [CrossRef]
- Gebhardt, R.S.; Du, P.; Wodo, O.; Ganapathysubramanian, B. A data-driven identification of morphological features influencing the fill factor and efficiency of organic photovoltaic devices. Computational Materials Science 2017, 129, 220–225. [Google Scholar] [CrossRef]
- Ritzberger, D.; Jakubek, S. Nonlinear data-driven identification of polymer electrolyte membrane fuel cells for diagnostic purposes: A Volterra series approach. Journal of Power Sources 2017, 361, 144–152. [Google Scholar] [CrossRef]
- Cope, F.W. Cooperative interactions in nerve membrane potential and in photosynthesis, evidenced by non-linear Arrhenius plots and critical exponents. Physiol. Chern. Phys. 1977, 9, 247-258. [https://pubmed.ncbi.nlm.nih.gov/594192/].
- Cope, F.W. Sigmoid biological time curves for muscle, nerve, growth, firefly, and infrared phosphorescence of green leaves, melanin, and cytochrome c. Physiol. Chern. Phys. 1977, 9, 443–459. [Google Scholar]
- Cope, F.W. Critical exponent analysis of activation energies of nonlinear Arrhenius plots as a test for cooperative interactions in amorphous semiconductors and in biological systems. Physiol. Chern. Phys. 1977, 9, 329–335. [Google Scholar]
- Cagnetta, F. Active interfaces, a universal approach. PhD Thesis, The University of Edinburgh, Edinburgh, 2020. [Google Scholar]
- Hermann, S.; Schmidt, M. Active interface polarization as a state function. Physical Review Research 2020, 2, 022003. [Google Scholar] [CrossRef]
- Hannezo, E. A toy model for active interfaces. Physics 2018, 11, 61. [Google Scholar] [CrossRef]
- Al Hammal, O.; De Los Santos, F.; Munoz, M.A. A non-order parameter Langevin equation for a bounded Kardar–Parisi–Zhang universality class. Journal of Statistical Mechanics: Theory and Experiment, 2005; 10, P10013. [Google Scholar] [CrossRef]
- Sasamoto, T.; Spohn, H. One-dimensional Kardar-Parisi-Zhang equation: an exact solution and its universality. Phys. Rev. Lett. 2010, 104, 230602, [https://pubmed.ncbi.nlm.nih.gov/20867222/]. [Google Scholar] [CrossRef]
- Corwin, I. The Kardar–Parisi–Zhang equation and universality class. Random Matrices: Theory and Applications 2012, 1, 1130001. [Google Scholar] [CrossRef]
- Sasamoto, T. The 1D Kardar–Parisi–Zhang equation: height distribution and universality. Progress of Theoretical and Experimental Physics 2016, 2, 022A01. [Google Scholar] [CrossRef]
- Mukherjee, S. Conserved Kardar-Parisi-Zhang equation: Role of quenched disorder in determining universality. Phy.l Rev. E 2021, 103, 042102. [Google Scholar] [CrossRef] [PubMed]
- Nattermann, T.; Tang, L.H. Kinetic surface roughening. I. The Kardar-Parisi-Zhang equation in the weak-coupling regime. Phys. Rev. A 1992, 45, 7156. [Google Scholar] [CrossRef] [PubMed]
- Fogedby, H.C. Localized growth modes, dynamic textures, and upper critical dimension for the Kardar-Parisi-Zhang Equation in the weak-noise limit. Phys. Rev. Lett. 2005, 94, 195702. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Alava, M.; Kertész, J. Self-organized criticality in the Kardar-Parisi-Zhang equation. Europhysics Letters (EPL) 2002, 57, 665–671. [Google Scholar] [CrossRef]
- Fogedby, H.C. Patterns in the Kardar-Parisi-Zhang equation. Pramana 2008, 71, 253–262. [Google Scholar] [CrossRef]
- Katzav, E. Growing surfaces with anomalous diffusion: Results for the fractal Kardar-Parisi-Zhang equation. Phys. Rev. E 2003, 68, 031607. [Google Scholar] [CrossRef]
- Le Doussal, P.; Thiery, T. Diffusion in time-dependent random media and the Kardar-Parisi-Zhang equation. Phys. Rev. E 2017, 96, 010102. [Google Scholar] [CrossRef]
- Antonov, N.V.; Gulitskiy, N.M.; Kakin, P.I.; Kostenko, M.M. Effects of turbulent environment on the surface roughening: The Kardar-Parisi-Zhang model coupled to the stochastic Navier–Stokes equation. Physica Scripta 2020, 95, 084009. [Google Scholar] [CrossRef]
- Sayfidinov, O.; Bognár, G.V. Numerical solutions of the Kardar-Parisi-Zhang interface growing equation with different noise terms. In Vehicle and Automotive Engineering 2020, 3, 302–311. [Google Scholar] [CrossRef]
- Kechagia, P.; Yortsos, Y.C.; Lichtner, P. Nonlocal Kardar-Parisi-Zhang equation to model interface growth. Phys. Rev. E 2001, 64, 016315, [https://pubmed.ncbi.nlm.nih.gov/11461399/]. [Google Scholar] [CrossRef]
- Hartmann, A.K.; Krajenbrink, A.; Le Doussal, P. Probing large deviations of the Kardar-Parisi-Zhang equation at short times with an importance sampling of directed polymers in random media. Phys. Rev. E 2020, 101, 012134. [Google Scholar] [CrossRef]
- Santalla, S.N.; Rodríguez-Laguna, J.; Cuerno, R. Circular Kardar-Parisi-Zhang equation as an inflating, self-avoiding ring polymer. Phys. Re. E 2014, 89, 010401, [https://pubmed.ncbi.nlm.nih.gov/24580156/]. [Google Scholar] [CrossRef]
- Balibar, S.; Bouchaud, J.P. Kardar-Parisi-Zhang equation and the dynamic roughening of crystal surfaces. Phys. Rev. Lett. 1992, 69, 862. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kahng, B. Exact derivation of the Kardar-Parisi-Zhang equation for the restricted solid-on-solid model. Phys. Rev. E 1995, 51, 796–798, [https://pubmed.ncbi.nlm.nih.gov/9962709/]. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.A.; Sano, M. Evidence for geometry-dependent universal fluctuations of the Kardar-Parisi-Zhang interfaces in liquid-crystal turbulence. J. Stat. Phys. 2012, 147, 853–890. [Google Scholar] [CrossRef]
- Golubović, L.; Wang, Z.G. Kardar-Parisi-Zhang model and anomalous elasticity of two-and three-dimensional smectic-A liquid crystals. Phys. Rev. 1994, 49, 2567–2578, [https://pubmed.ncbi.nlm.nih.gov/9961517/]. [Google Scholar] [CrossRef]
- Golubović, L.; Wang, Z.G. Erratum: Kardar-Parisi-Zhang model and anomalous elasticity of two-and three-dimensional smectic-A liquid crystals [Phys. Rev. E 49, 2567 (1994)]. Phys. Rev. E 1994, 50, 4265, [https://pubmed.ncbi.nlm.nih.gov/9962490/]. [Google Scholar] [CrossRef]
- Schilardi, P.L.; Azzaroni, O.; Salvarezza, R.C.; Arvia, A.J. Validity of the Kardar-Parisi-Zhang equation in the asymptotic limit of metal electrodeposition. Physical Review-Section B-Condensed Matter 1999, 59, 4638–4641. [Google Scholar] [CrossRef]
- Lütt, M.; Schlomka, J.P.; Tolan, M.; Stettner, J.; Seeck, O.H.; Press, W. Kardar-Parisi-Zhang growth of amorphous silicon on Si/SiO2. Phys. Rev. B 1997, 56, 4085–4091. [Google Scholar] [CrossRef]
- Barna, I.F.; Bognár, G.; Mátyás, L.; Guedda, M.; Hriczó, K. Travelling-wave solutions of the Kardar-Parisi-Zhang interface growing equation with different kind of noise terms. In AIP Conference Proceedings 2020, 2293, 280005. [Google Scholar] [CrossRef]
- Lauter, R.; Mitra, A.; Marquardt, F. From Kardar-Parisi-Zhang scaling to explosive desynchronization in arrays of limit-cycle oscillators. Phys. Rev. E 2017, 96, 012220. [Google Scholar] [CrossRef] [PubMed]
- Lassig, M.; Kinzelbach, H. Phase transition of the Kardar-Parisi-Zhang equation in four substrate dimensions-Reply. Phys. Rev. Lett. 1998, 80, 889–889. [Google Scholar]
- Rieß, W. In situ measurements of respiration and mineralisation processes. Interaction between fauna and geochemical fluxes at active interfaces. PhD Thesis, University of Bremen, Bremen, Germany, 1999. [Google Scholar]
- Melkikh, A.V.; Sutormina, M. Protocells and LUCA: Transport of substances from first physicochemical principles. Progress in Biophysics and Molecular Biology 2019, 145, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Gradov, O.V.; Gradova, M.A. "MS-patch-clamp" or the possibility of mass spectrometry hybridization with patch-clamp setups for single cell metabolomics and channelomics. Advances in Biochemistry 2015, 3, 66–71. [Google Scholar] [CrossRef]
- Zhang, L.; Vertes, A. Einzelzell-massenspektrometrie zur untersuchung zellulärer heterogenität. Angewandte Chemie, 2018, 130, 4554–4566. [Google Scholar] [CrossRef]
- Zhang, L.; Vertes, A. Single-cell mass spectrometry approaches to explore cellular heterogeneity. Angewandte Chemie International Edition 2018, 57, 4466–4477, [https://pubmed.ncbi.nlm.nih.gov/29218763/]. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B.; Freund, M.S.; Hammond, P.T. Catalytic, conductive bipolar membrane interfaces through layer-by-layer deposition for the design of membrane-integrated artificial photosynthesis systems. Chem. Sus. Chem. 2017, 10, 4599–4609. [Google Scholar] [CrossRef]
- Hartman, H. Speculations on the origin and evolution of photosynthesis and the membrane. Origins of Life 1986, 16, 384. [Google Scholar] [CrossRef]
- Nakamura, H. Origin of the proto-cell membrane - great importance of phospholipid bilayer. In Exobiology: Matter, Energy, and Information in the Origin and Evolution of Life in the Universe (Proceedings of the Fifth Trieste Conference on Chemical Evolution: An Abdus Salam Memorial Trieste, Italy, 22–26 September 1997); Chela-Flores, J., Raulin, F., Eds.; Springer, Dordrecht, 1998; pp. 191-194. [CrossRef]
- Kundu, N.; Mondal, D.; Sarkar, N. Dynamics of the vesicles composed of fatty acids and other amphiphile mixtures: unveiling the role of fatty acids as a model protocell membrane. Biophysical Reviews 2020, 12, 1117–1131, https://pubmed.ncbi.nlm.nih.gov/32926295/. [Google Scholar] [CrossRef]
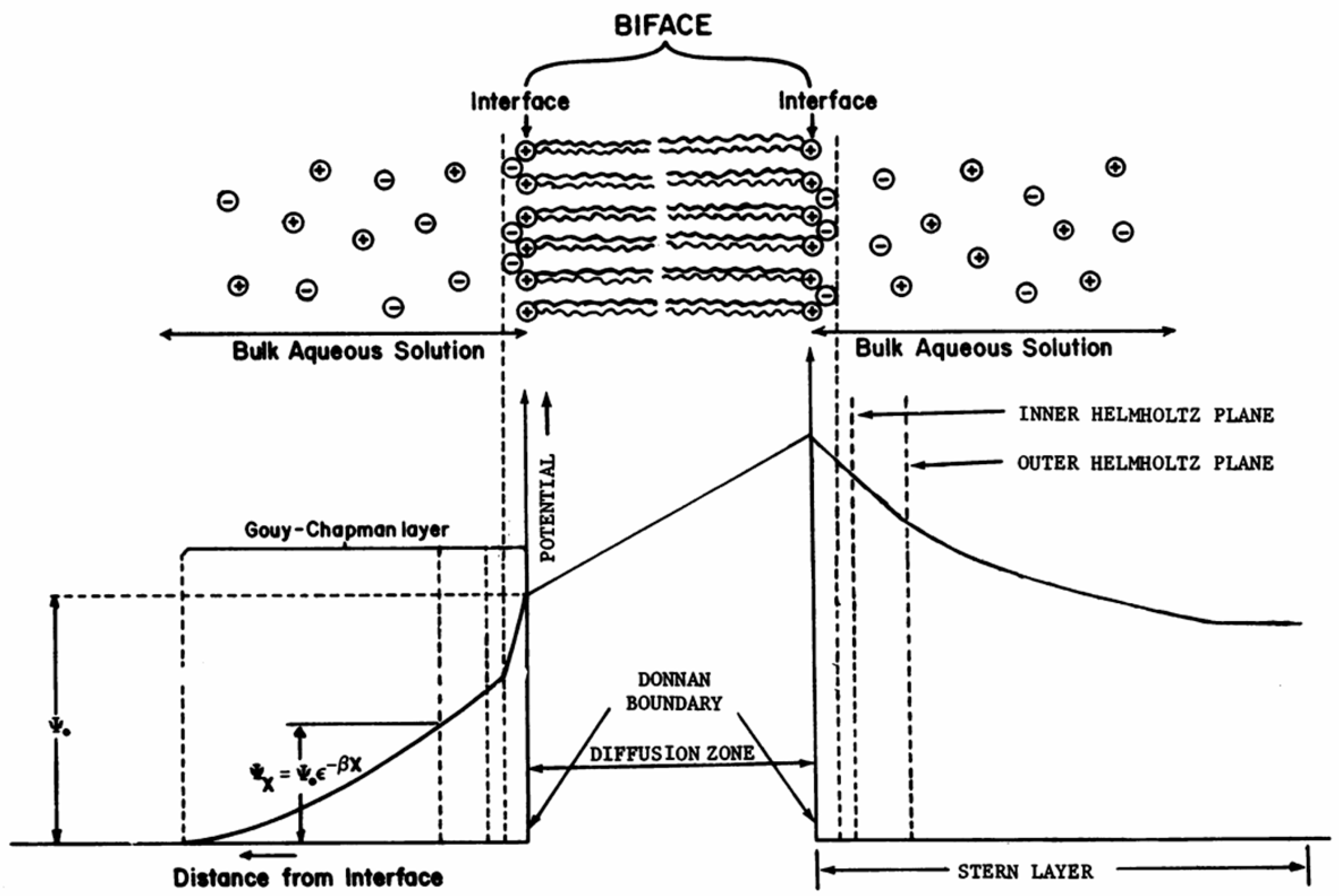
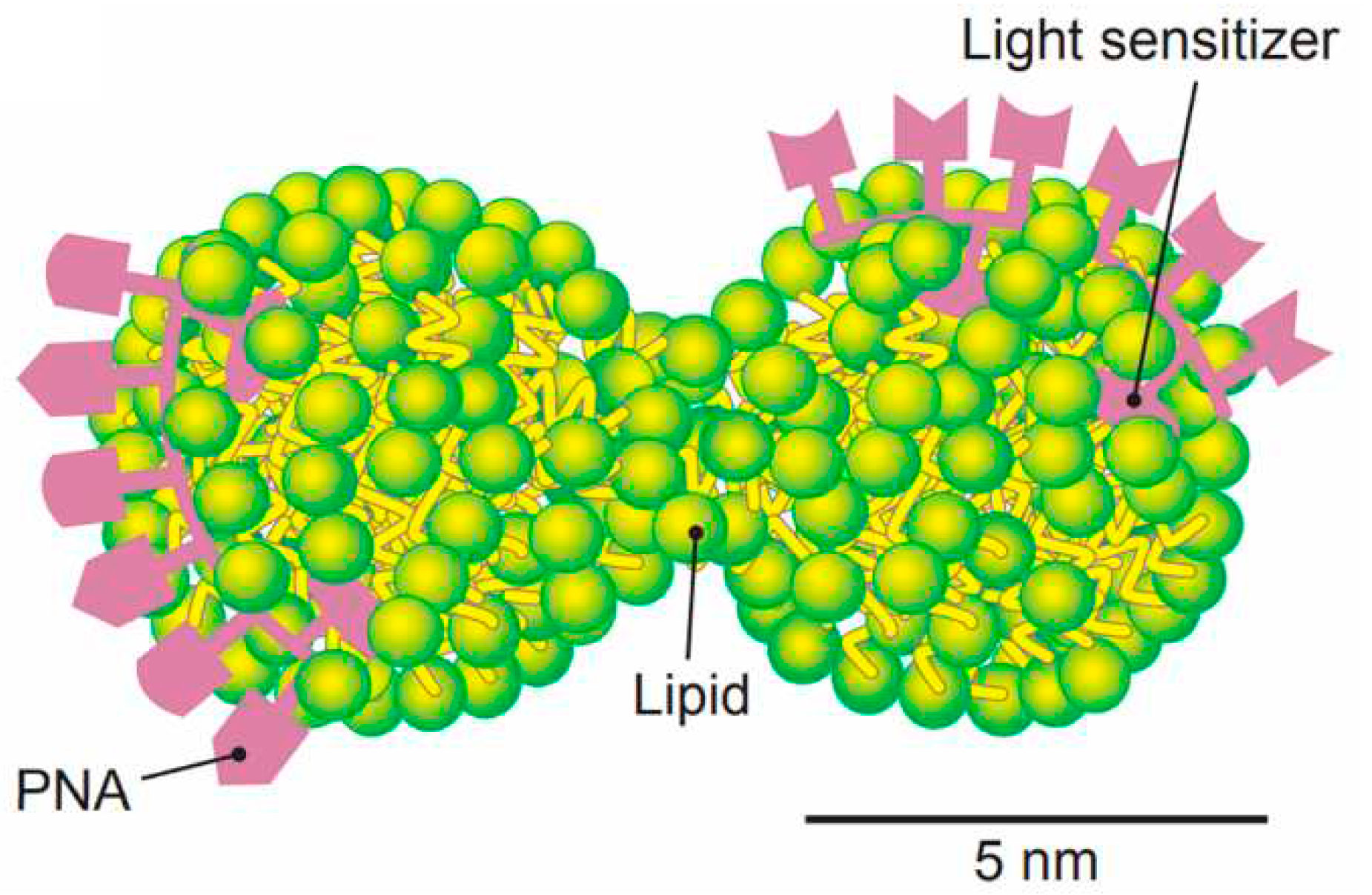
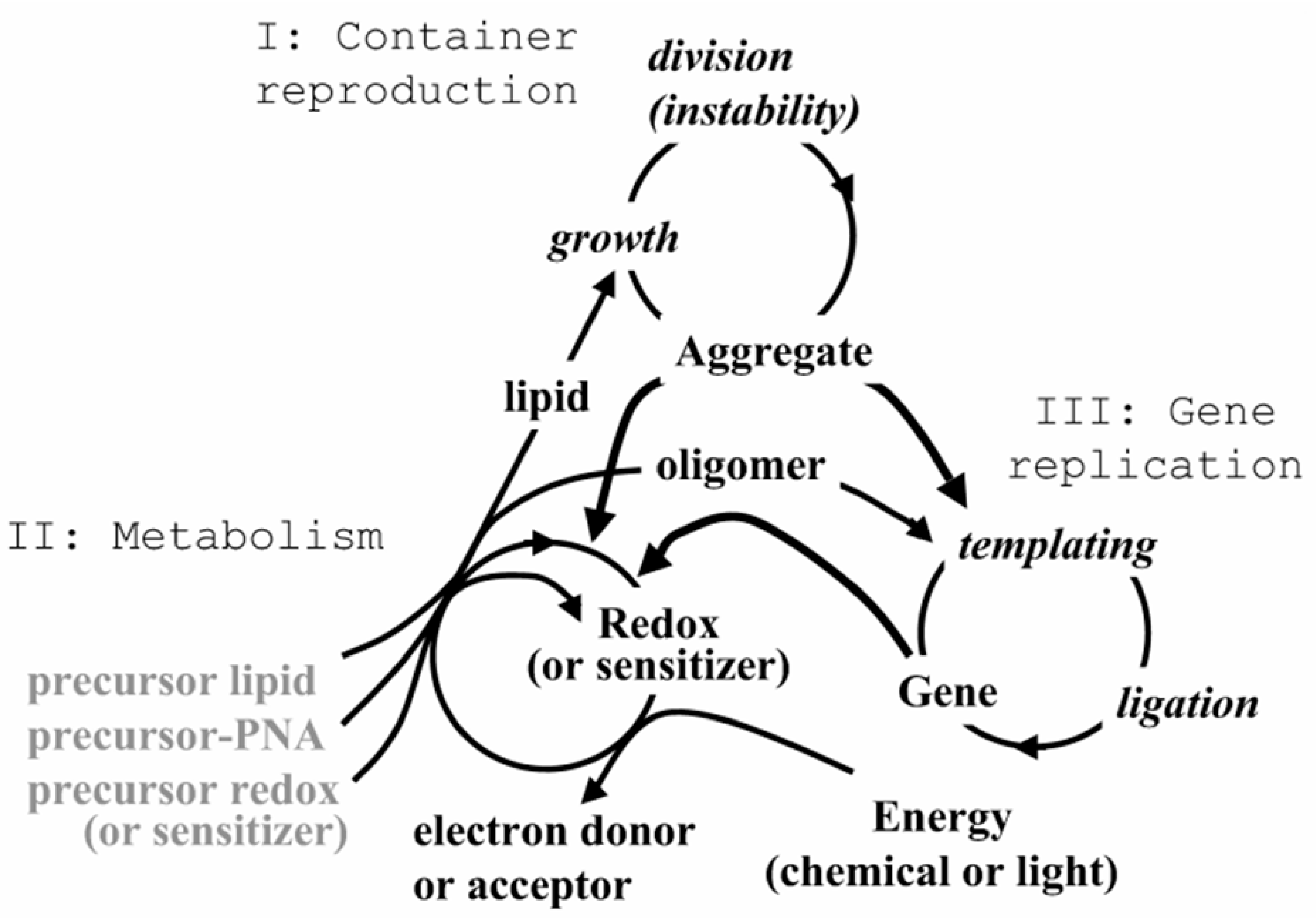
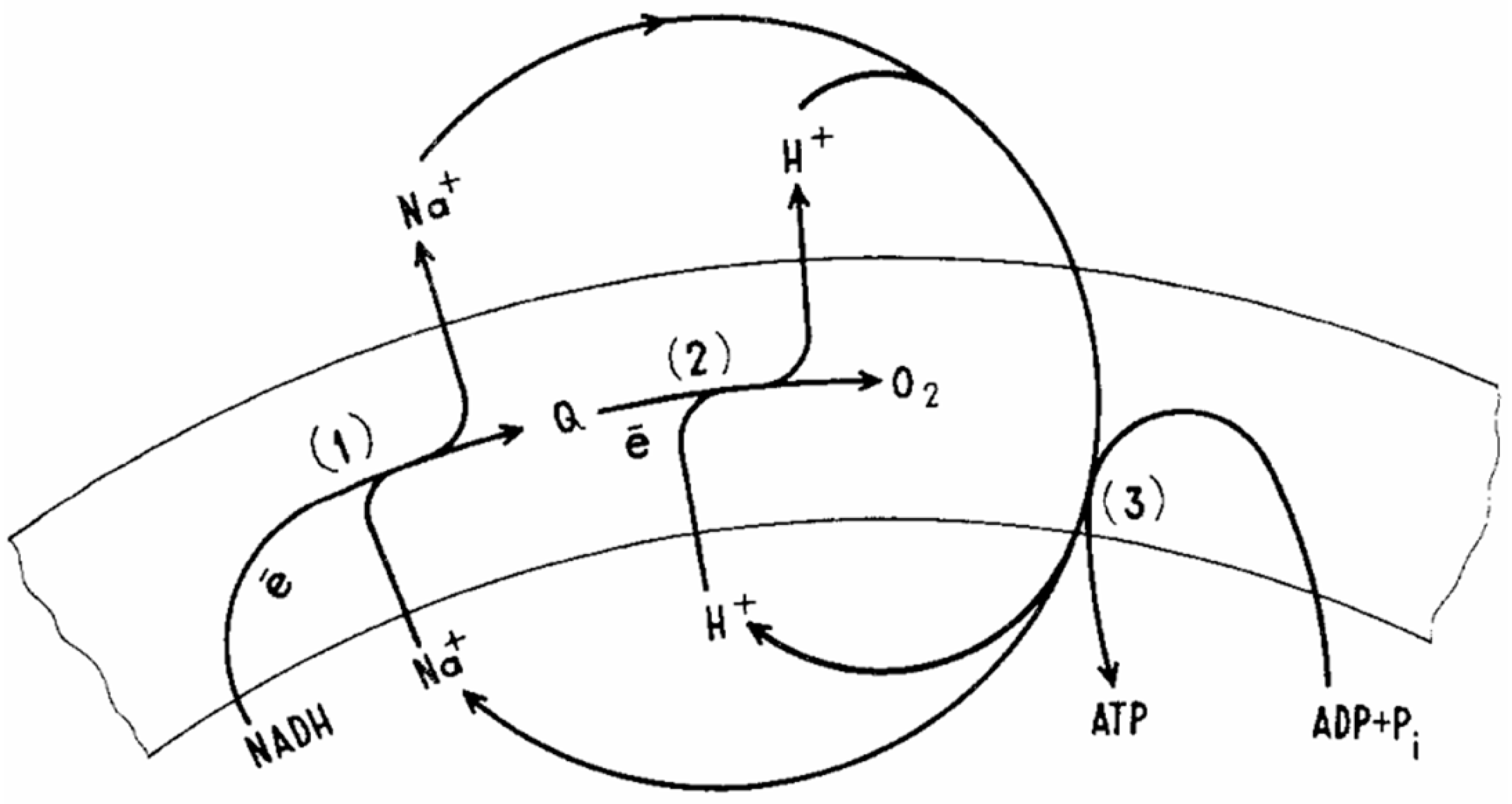
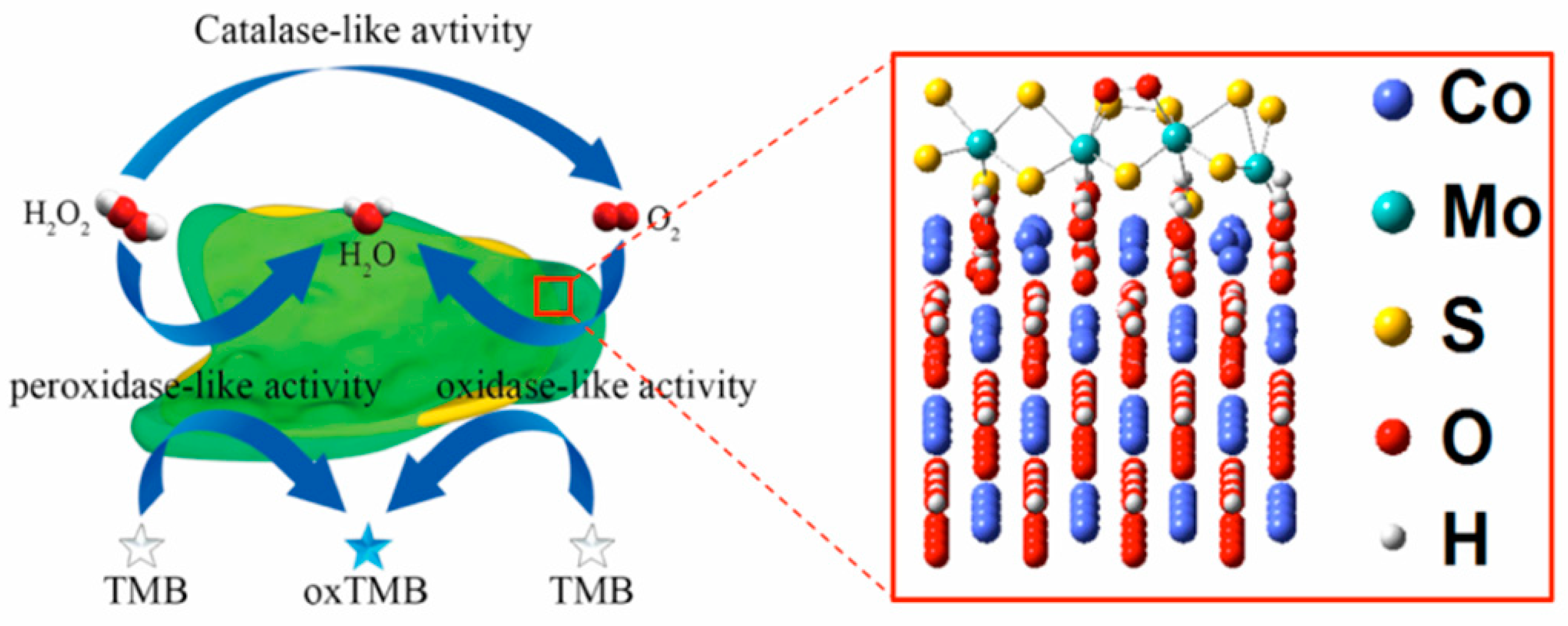
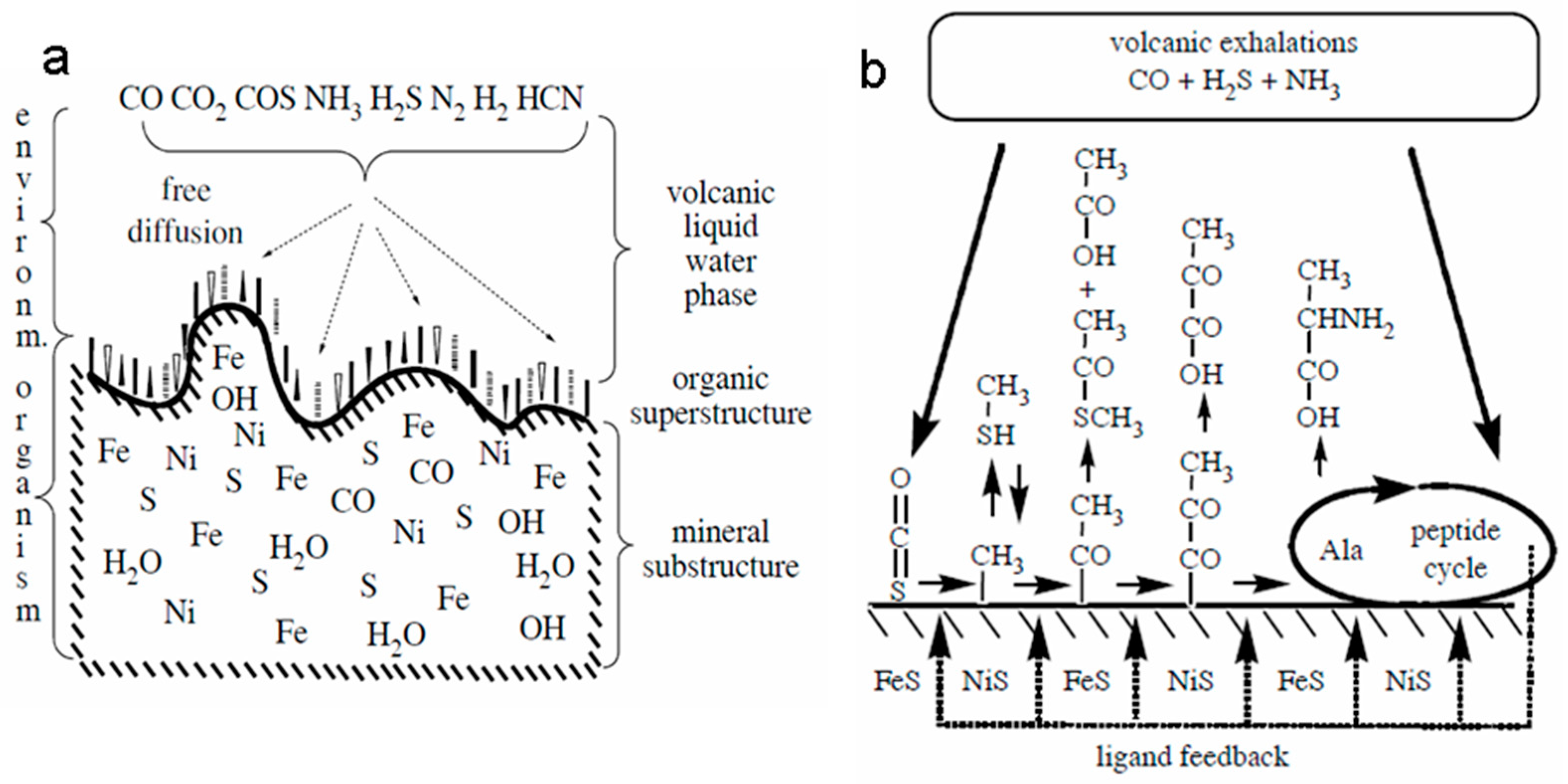
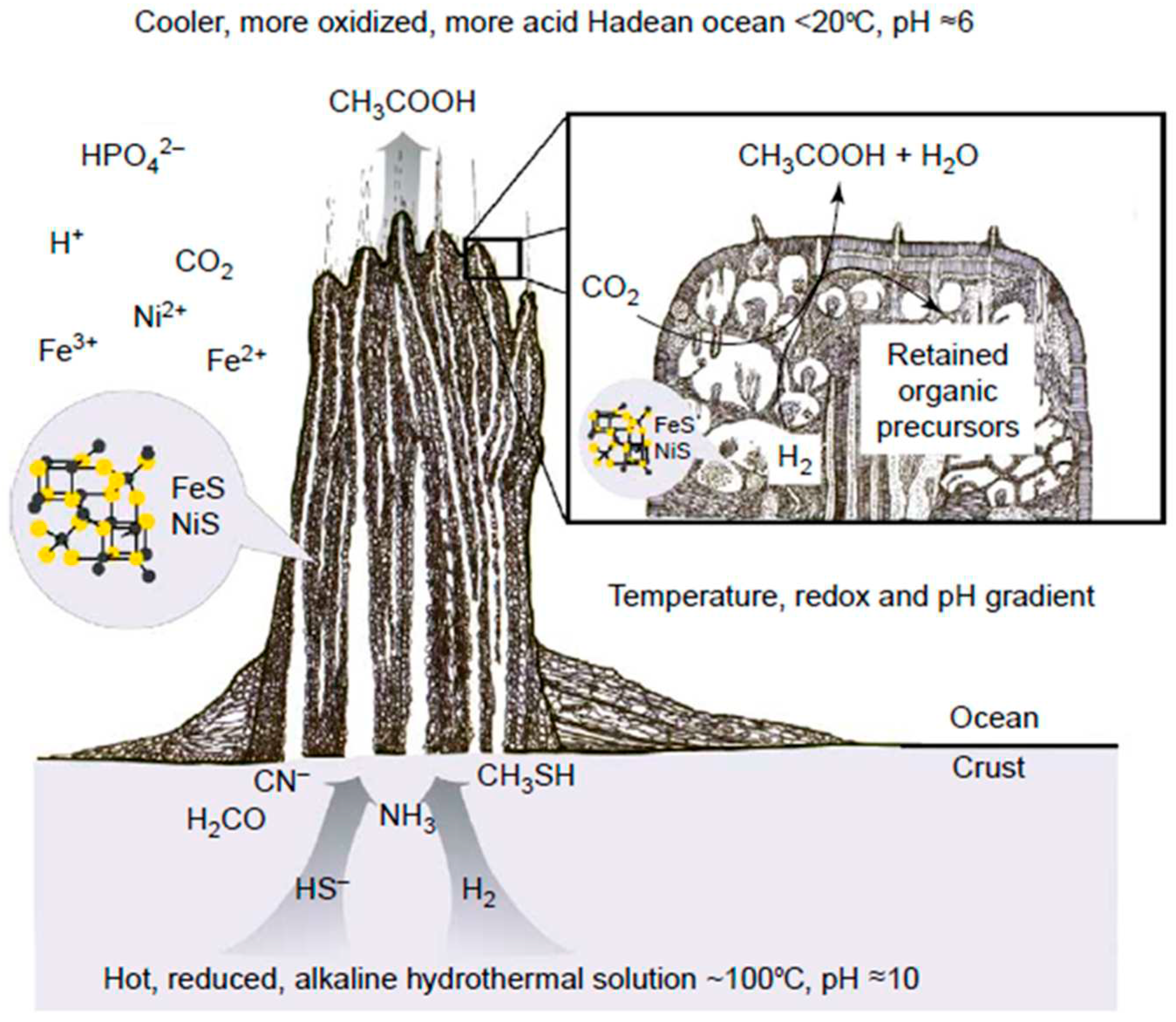
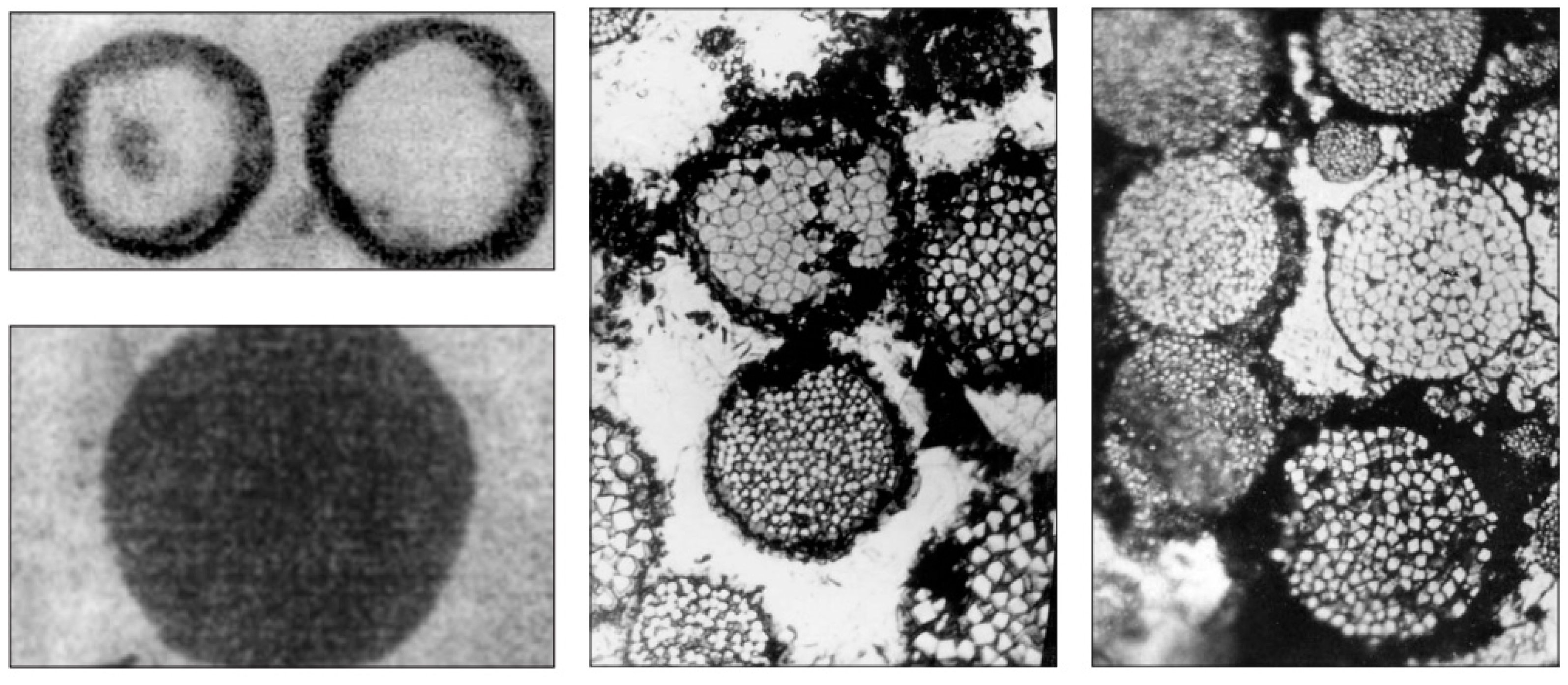
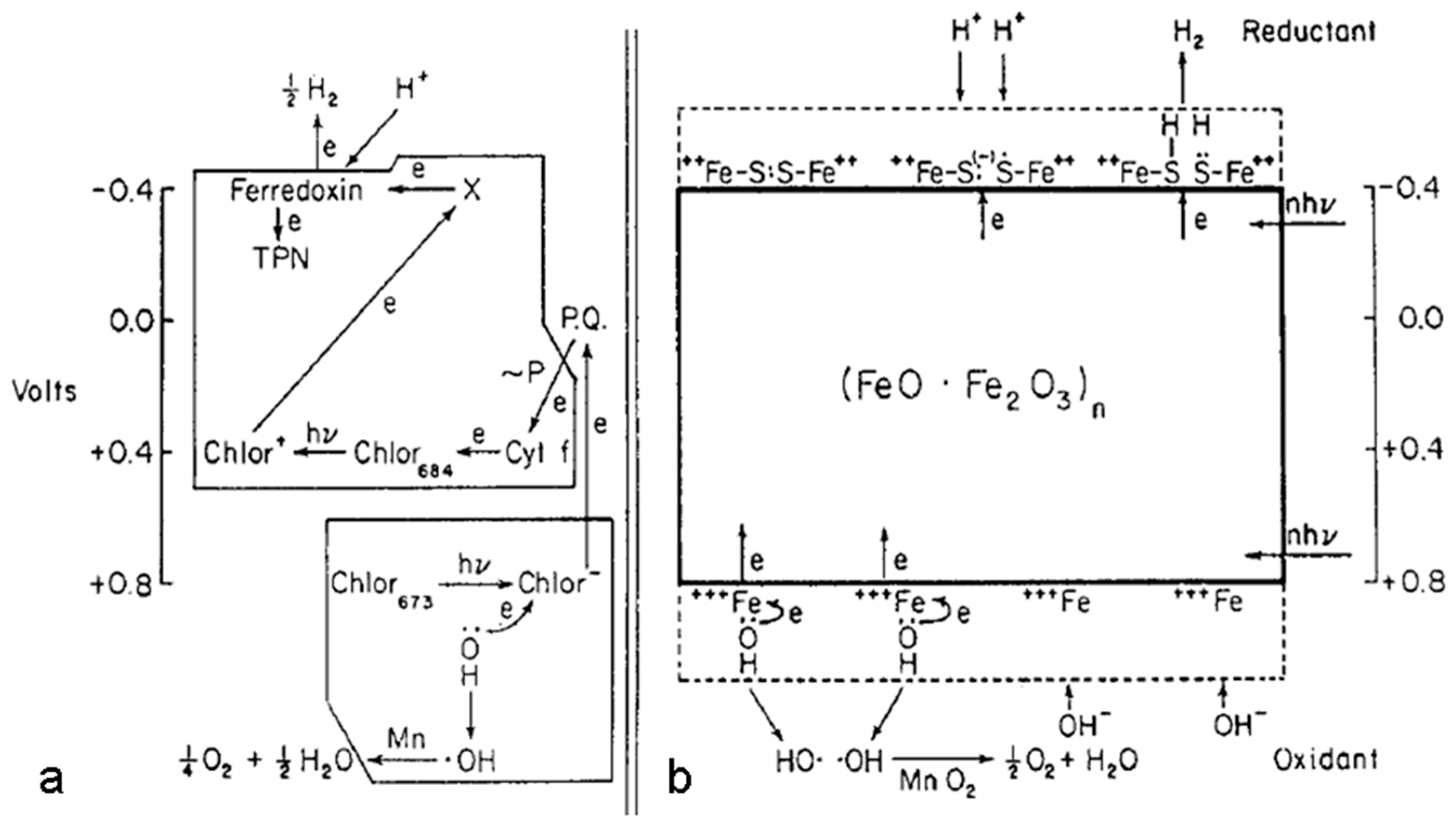
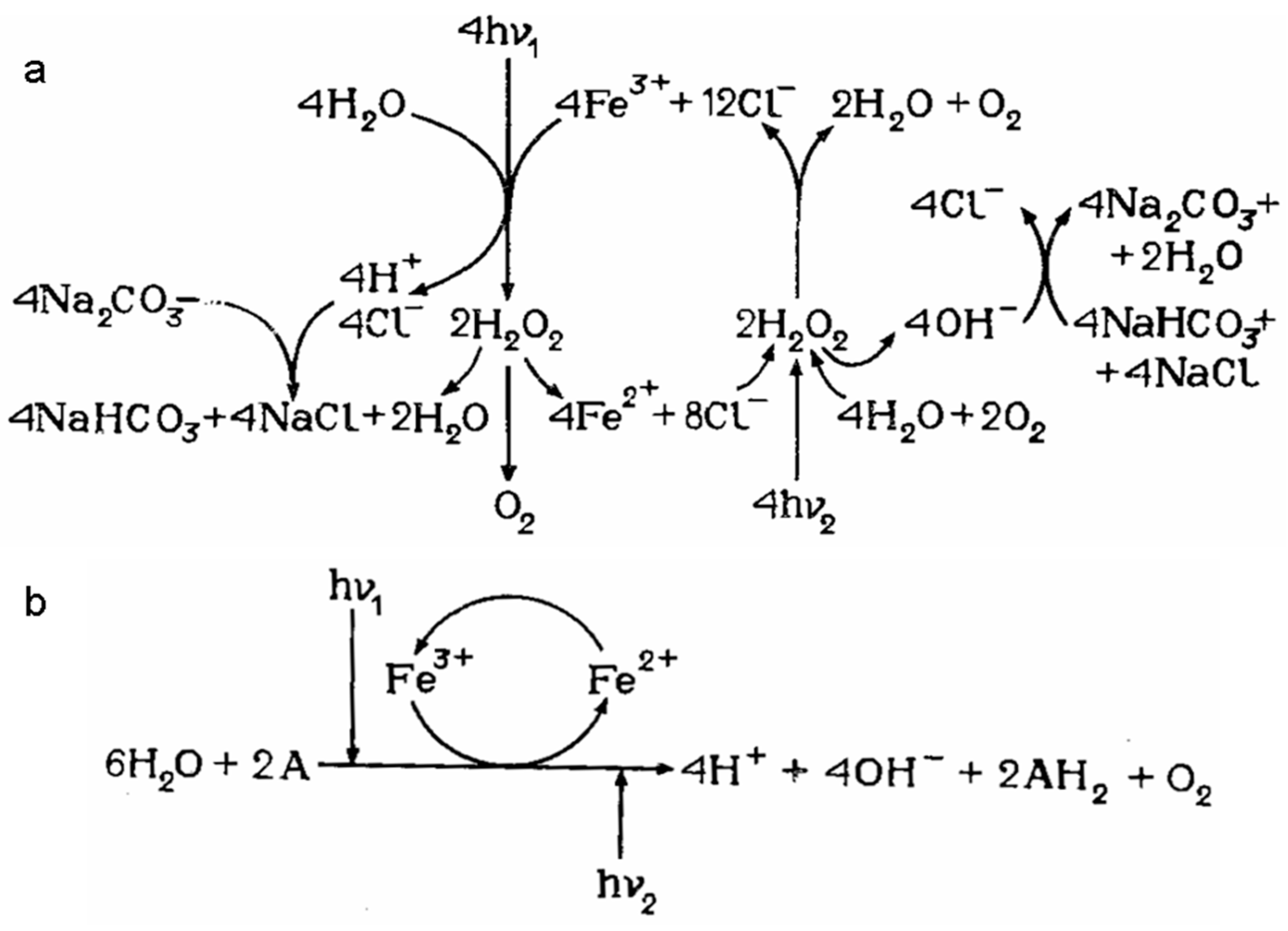
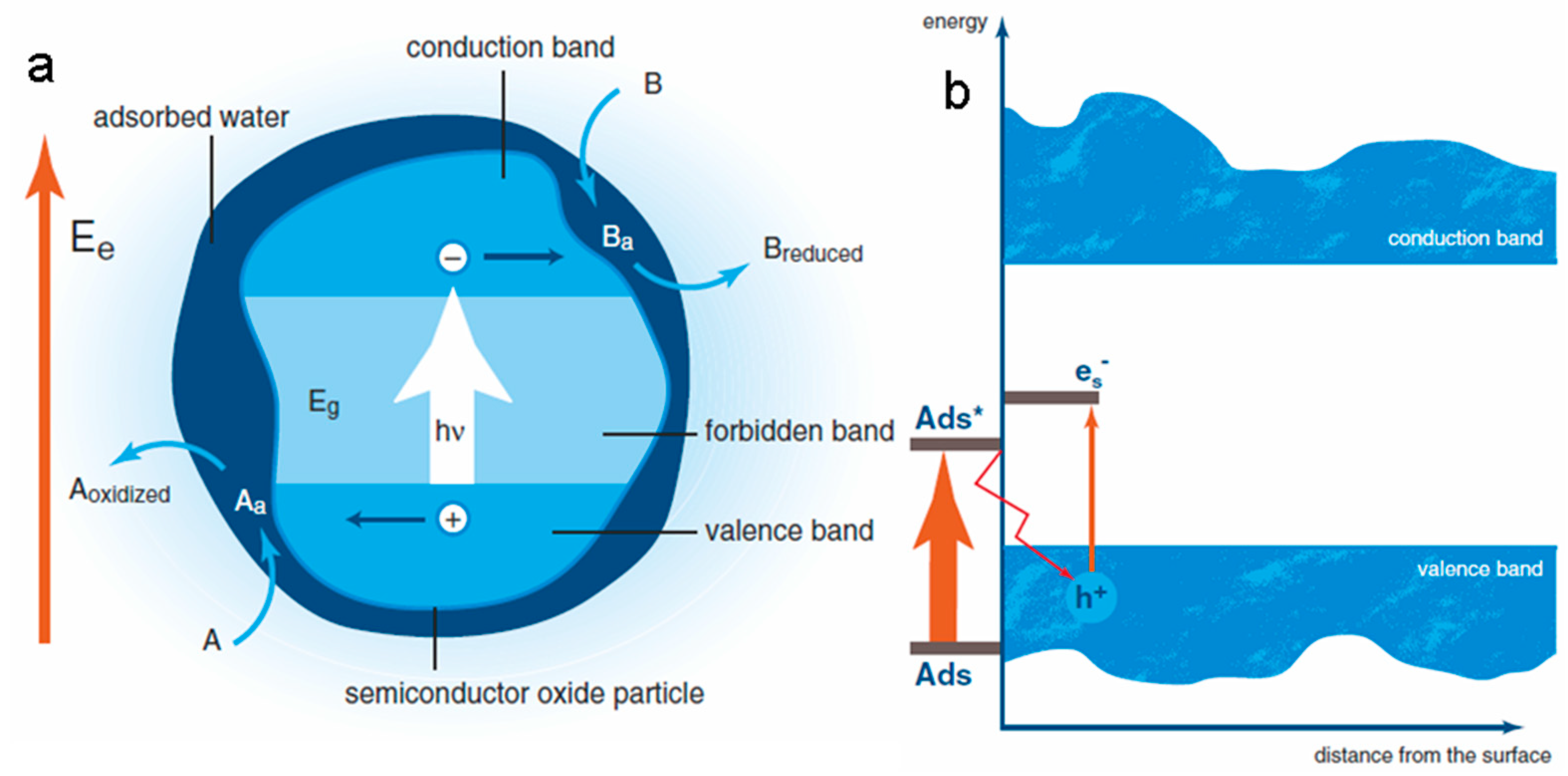
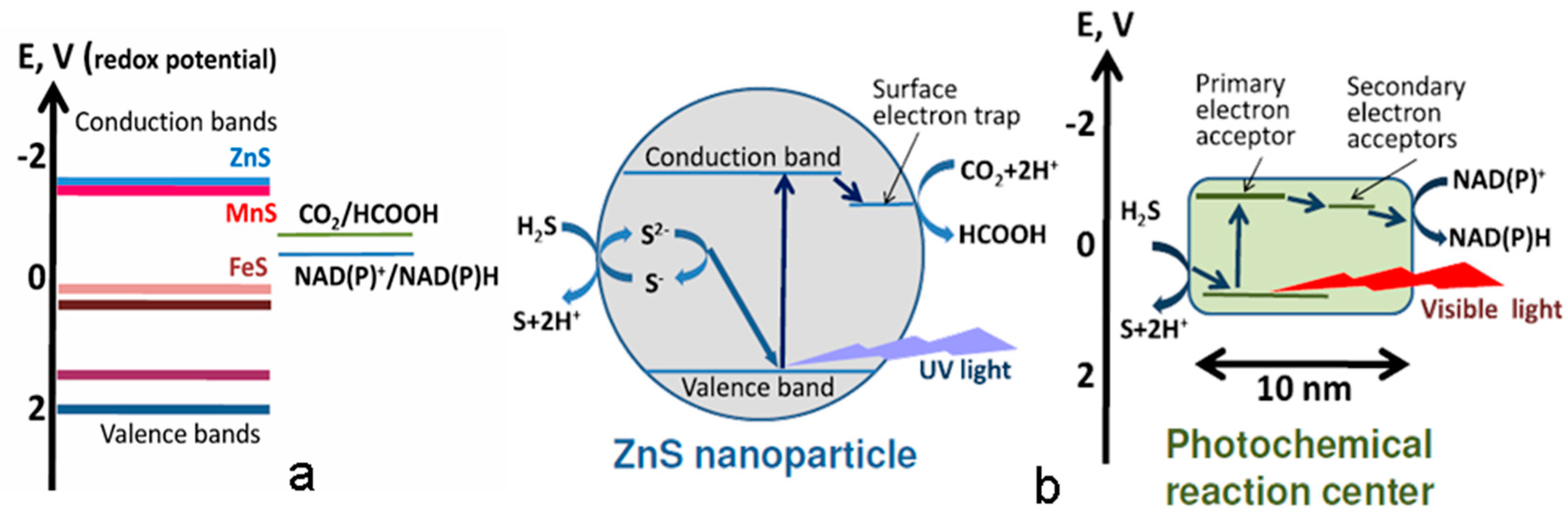
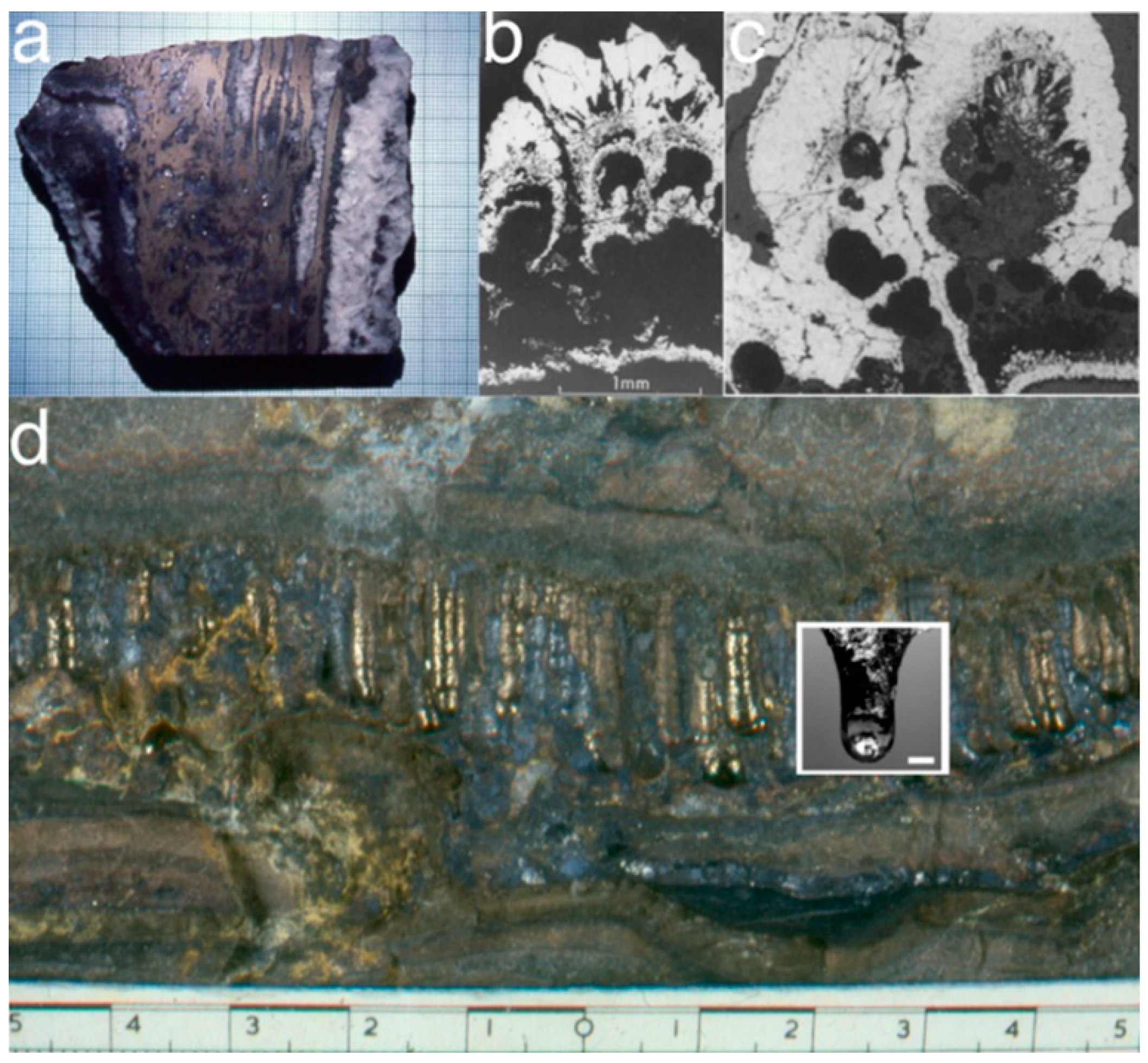
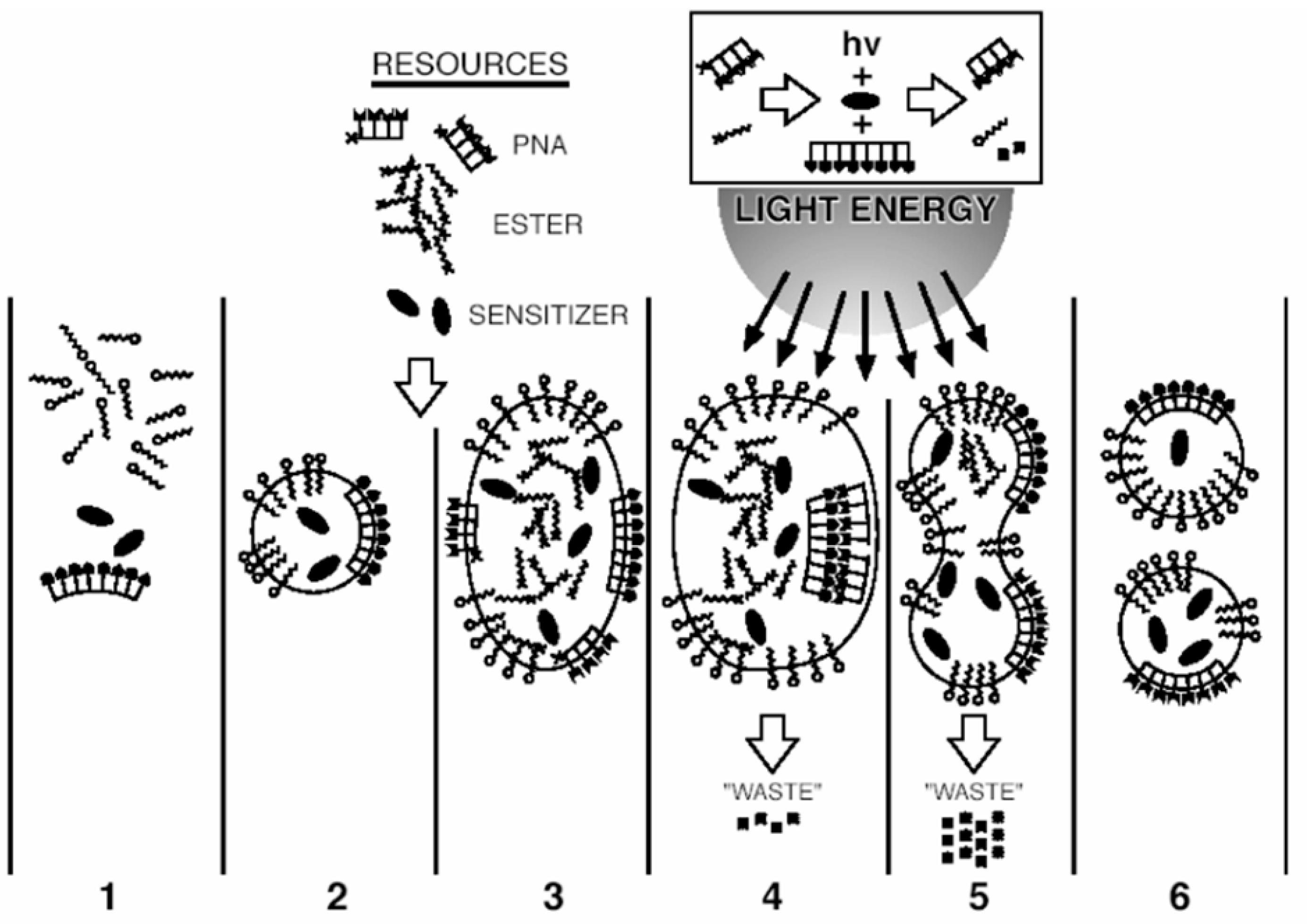
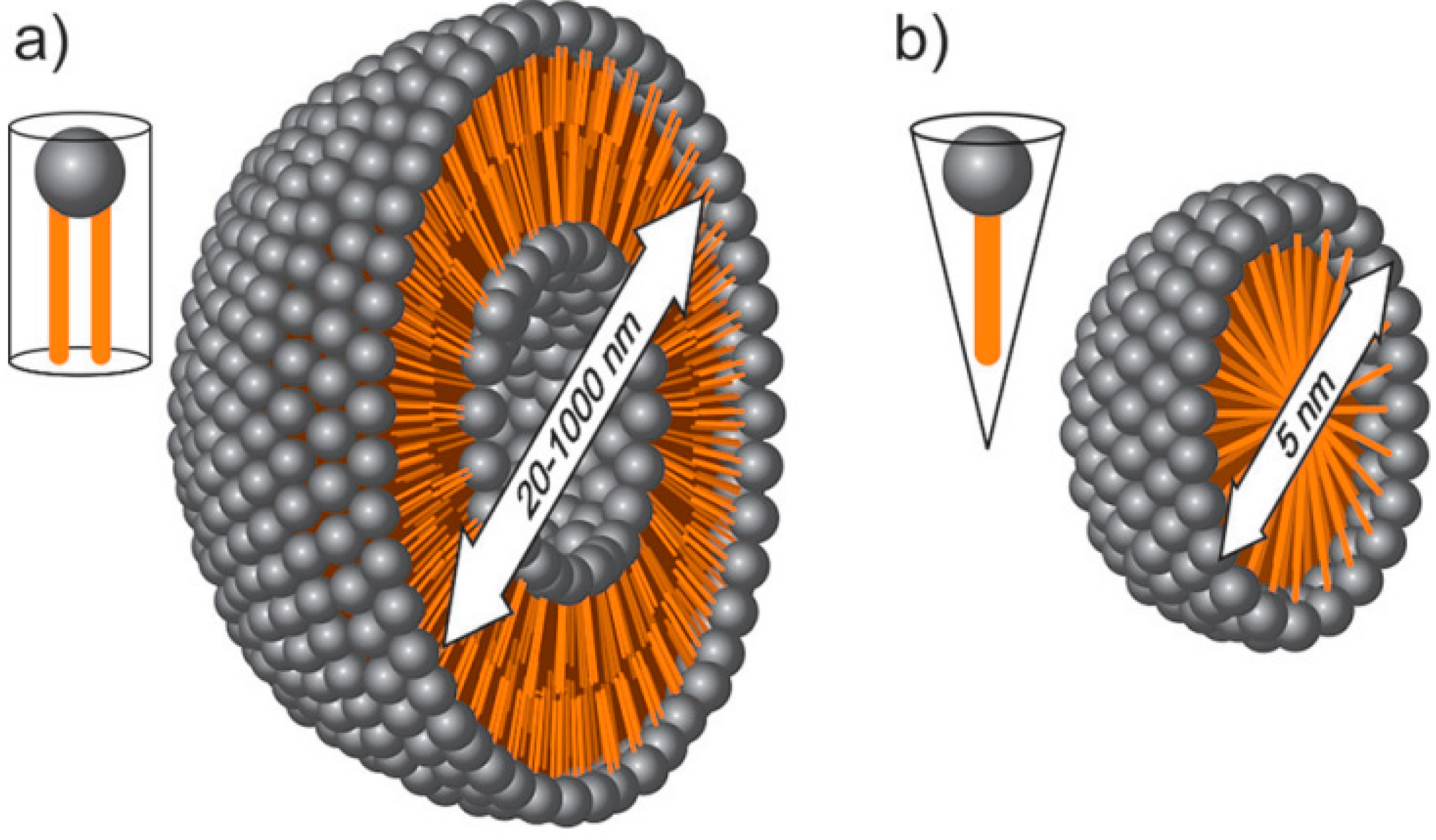
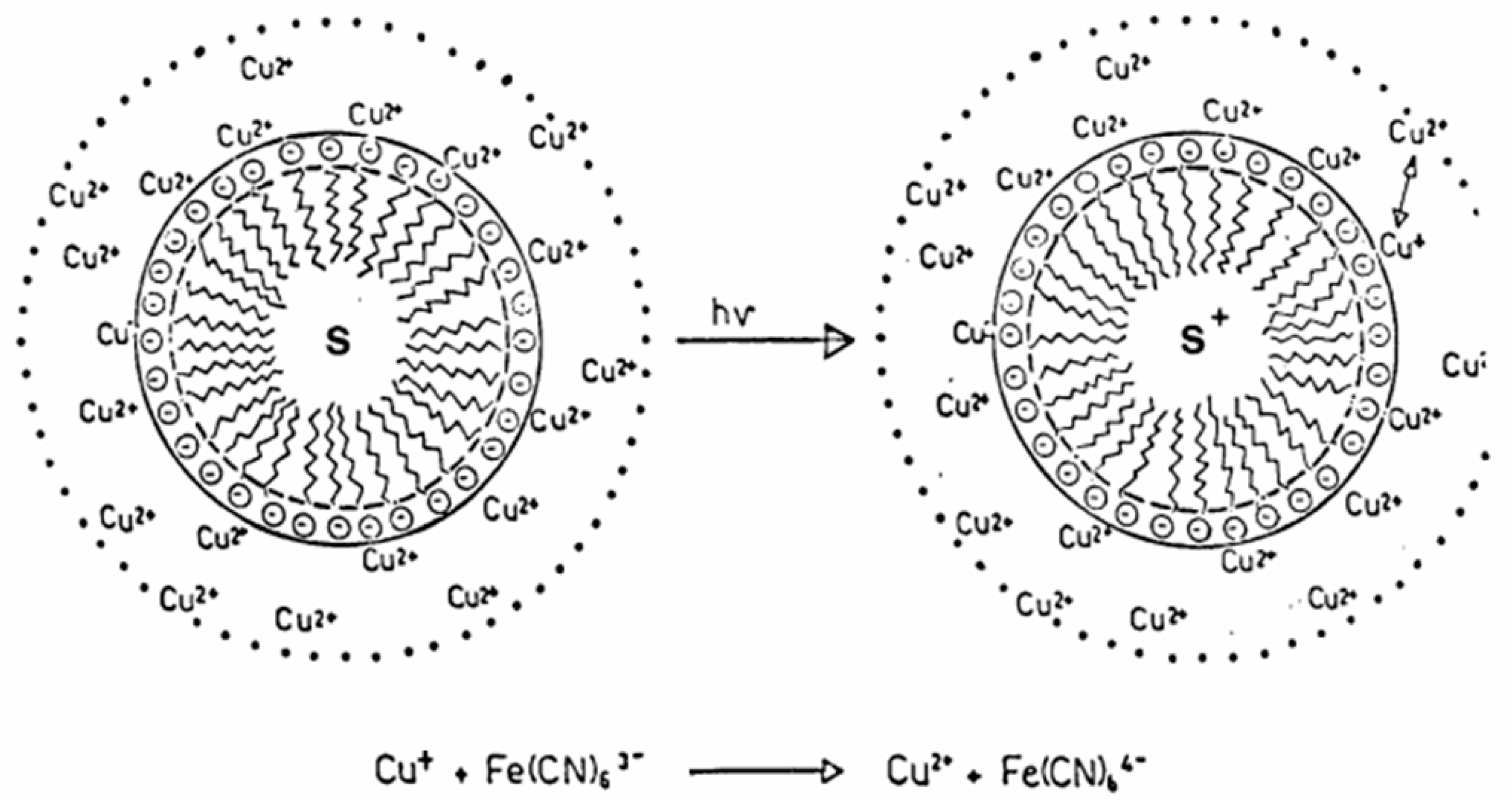
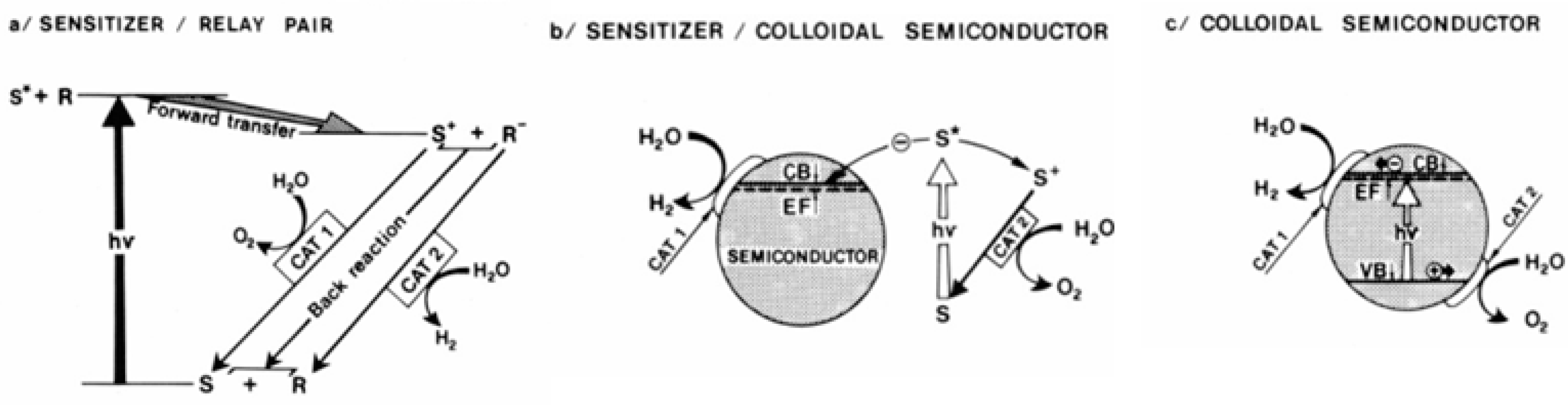
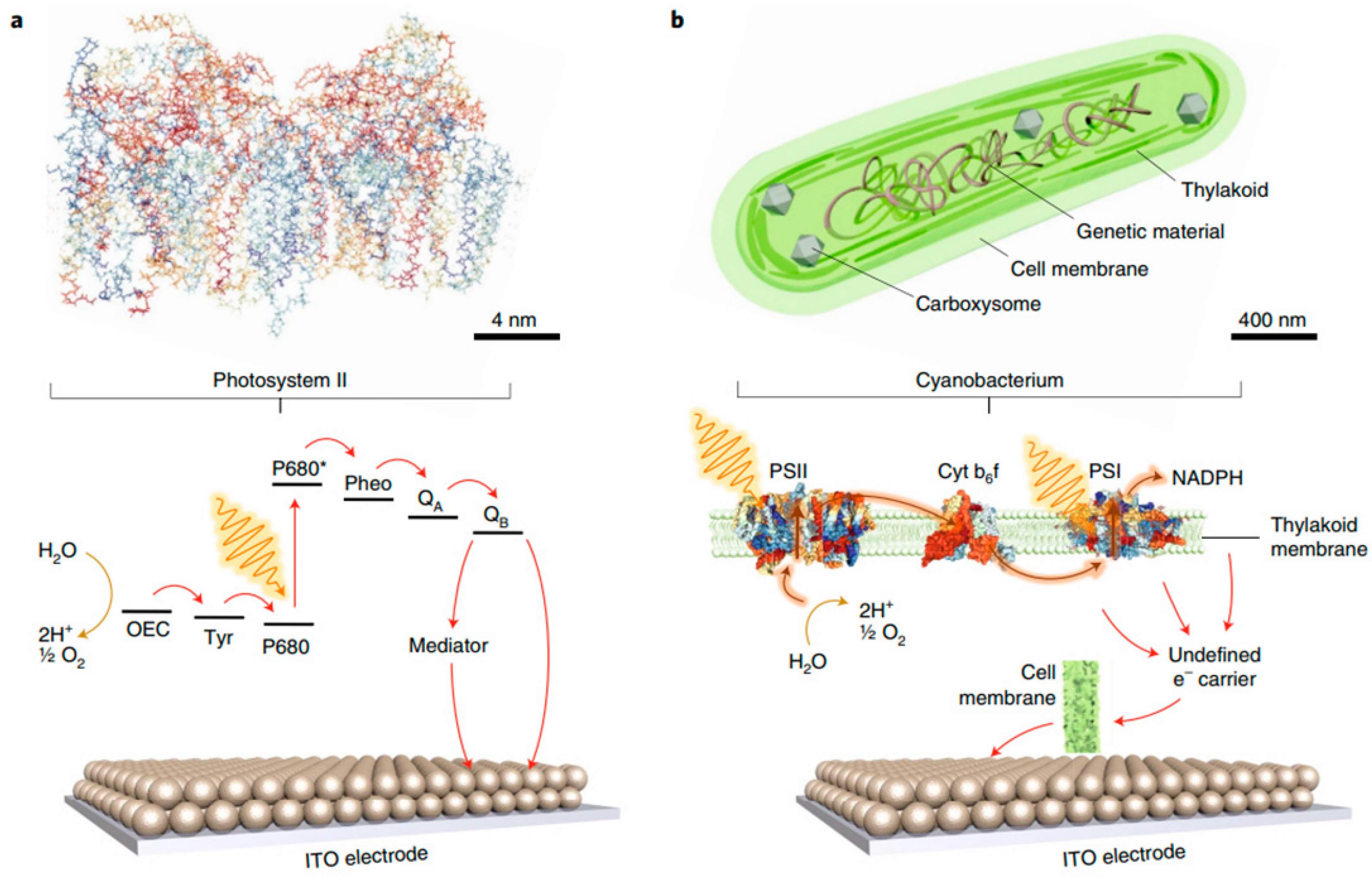
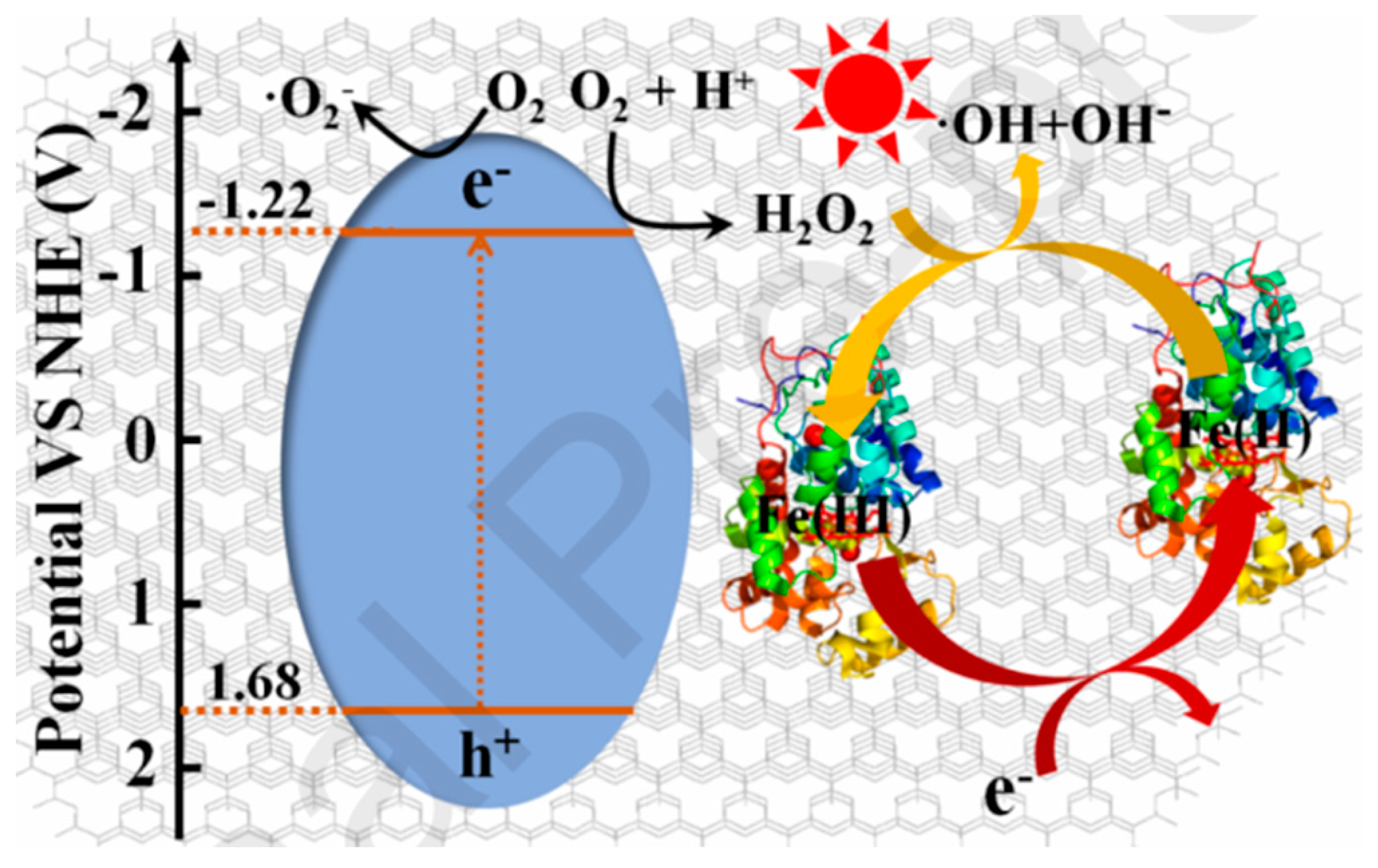
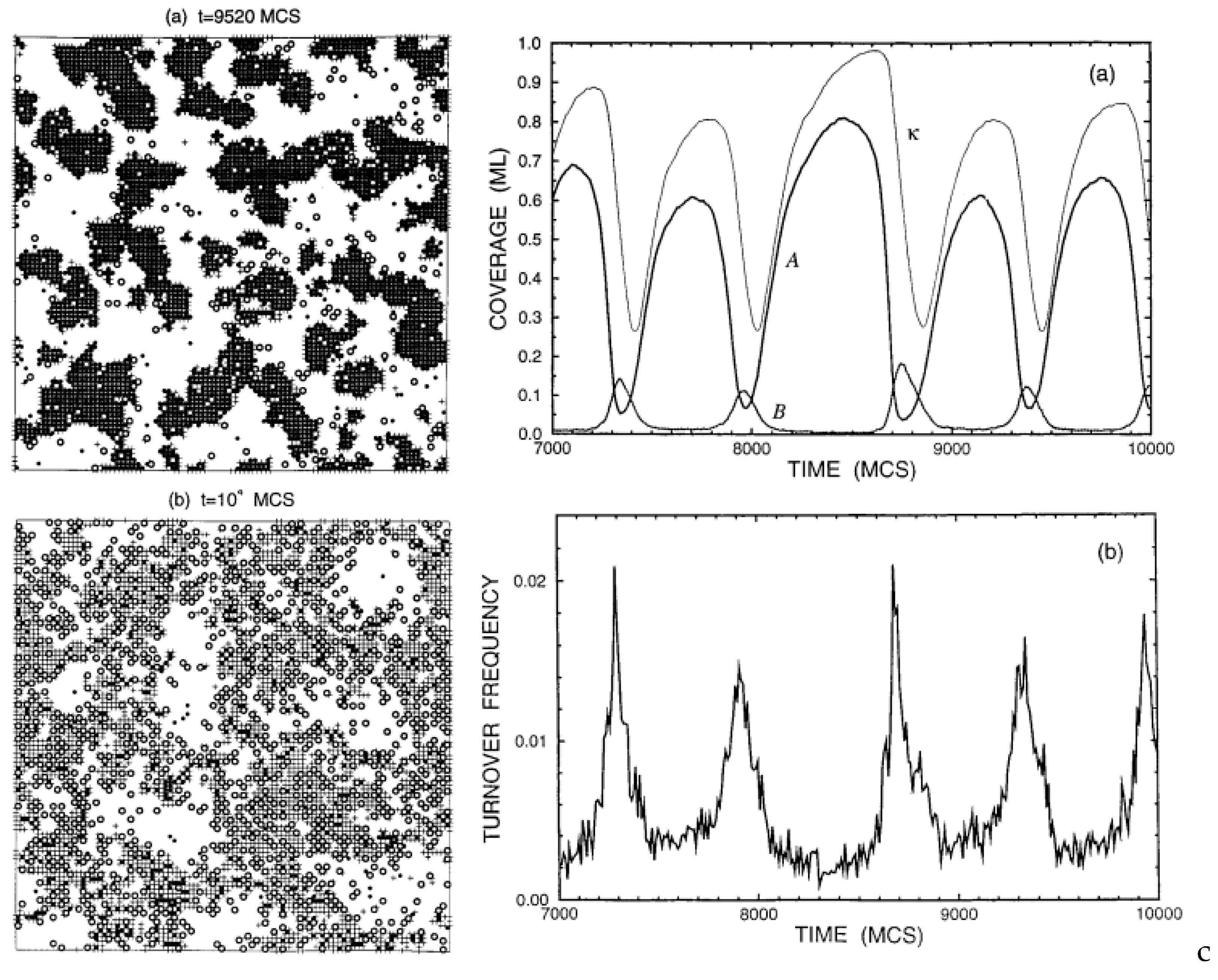
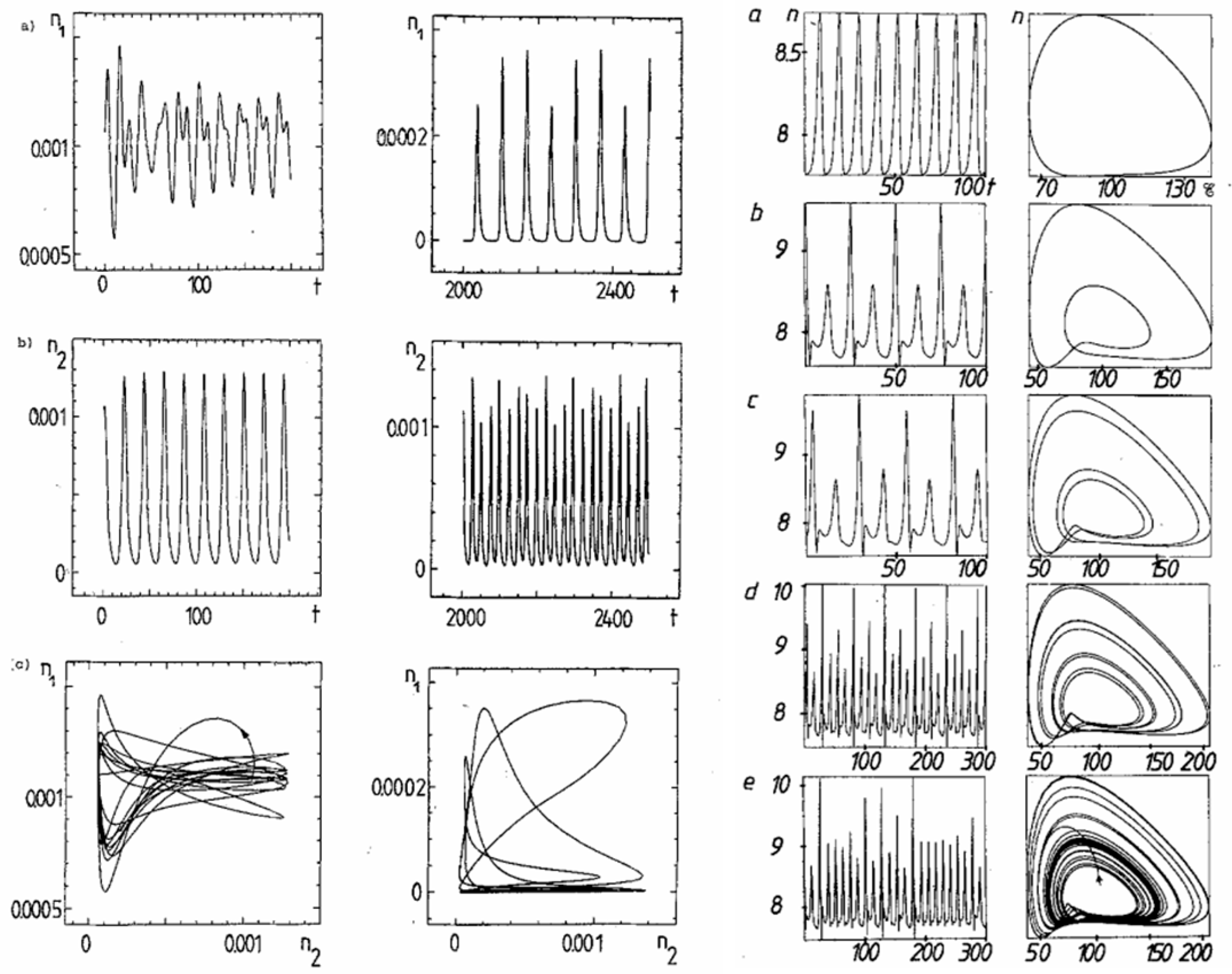
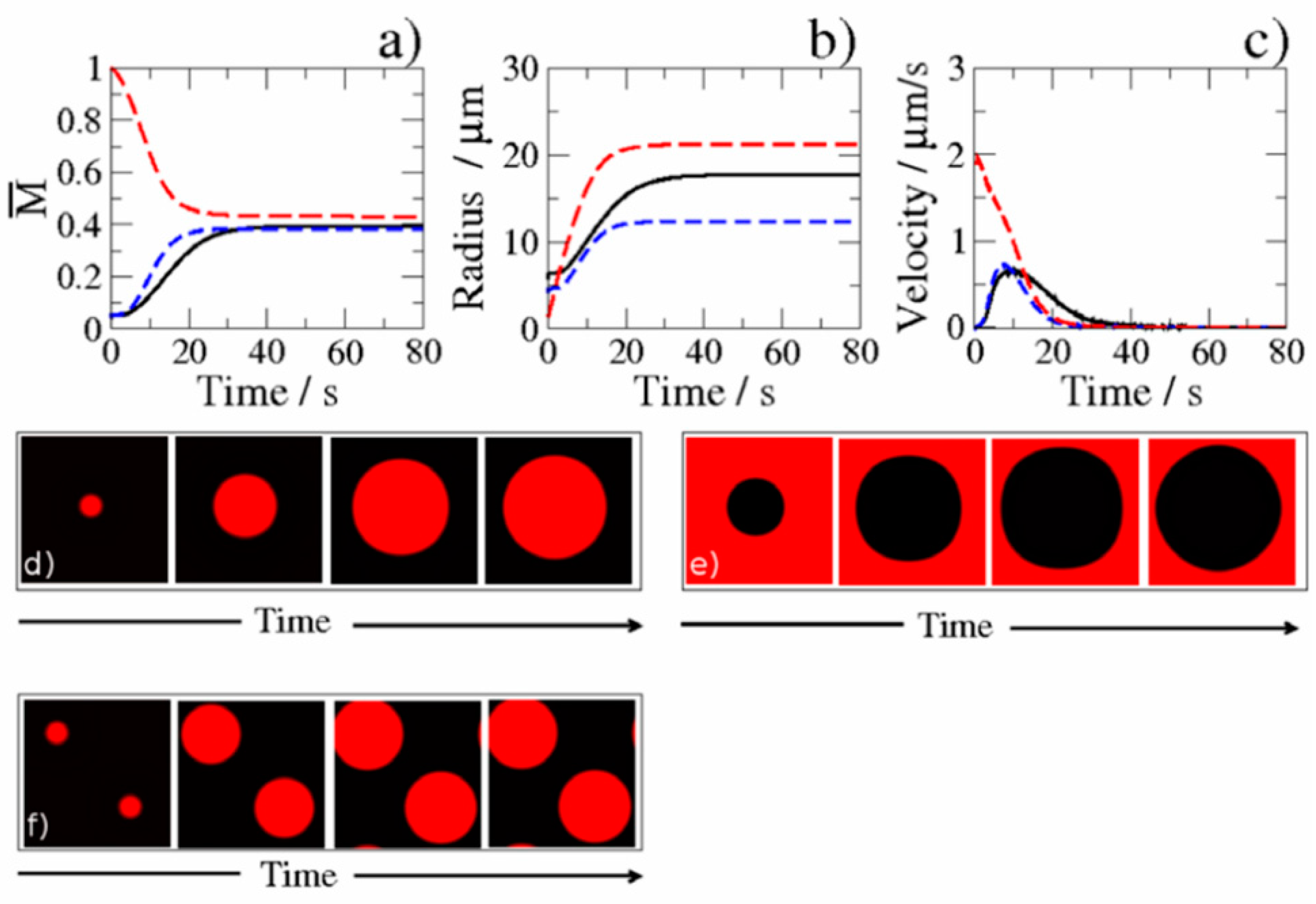
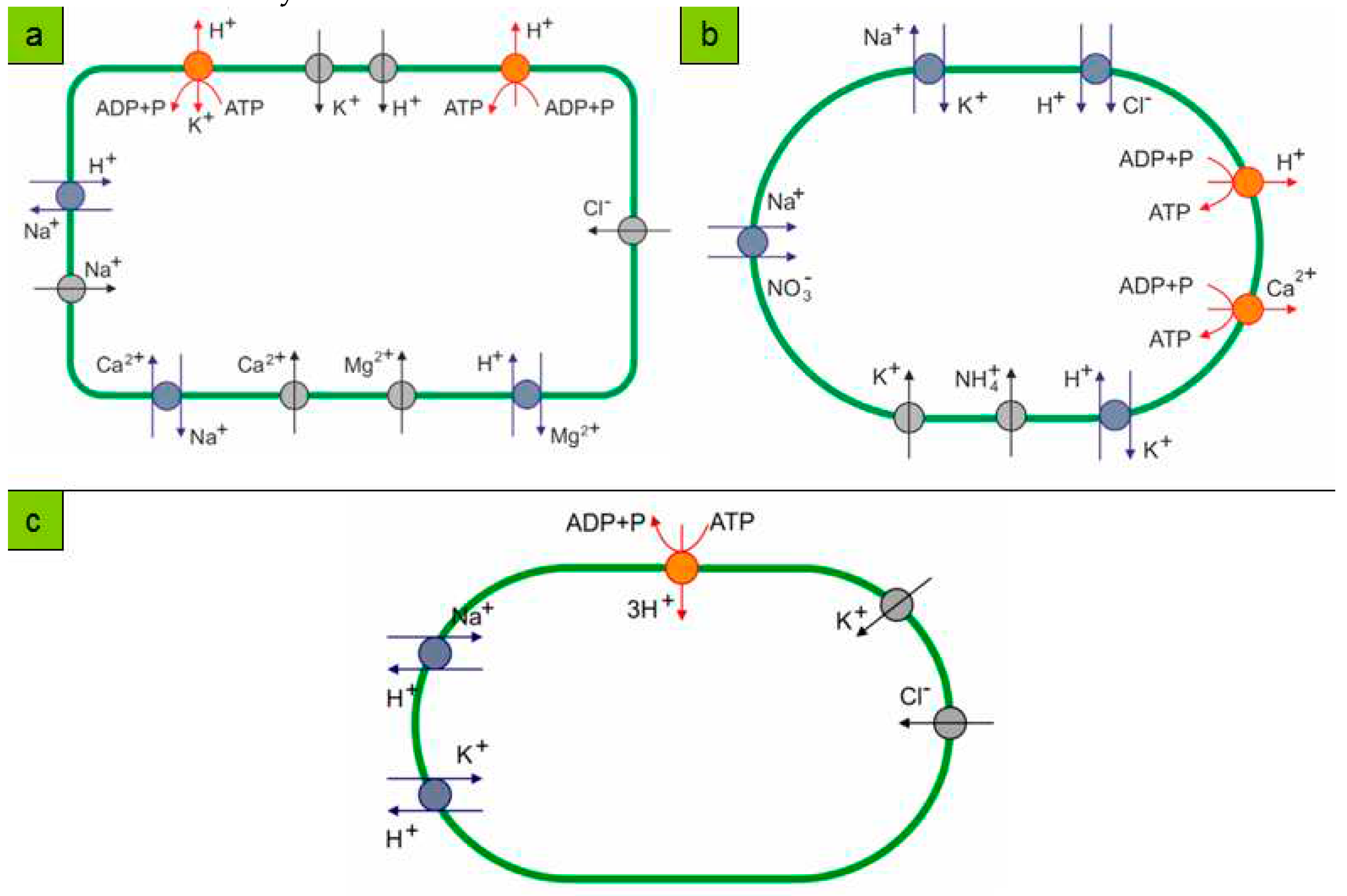
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




