Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
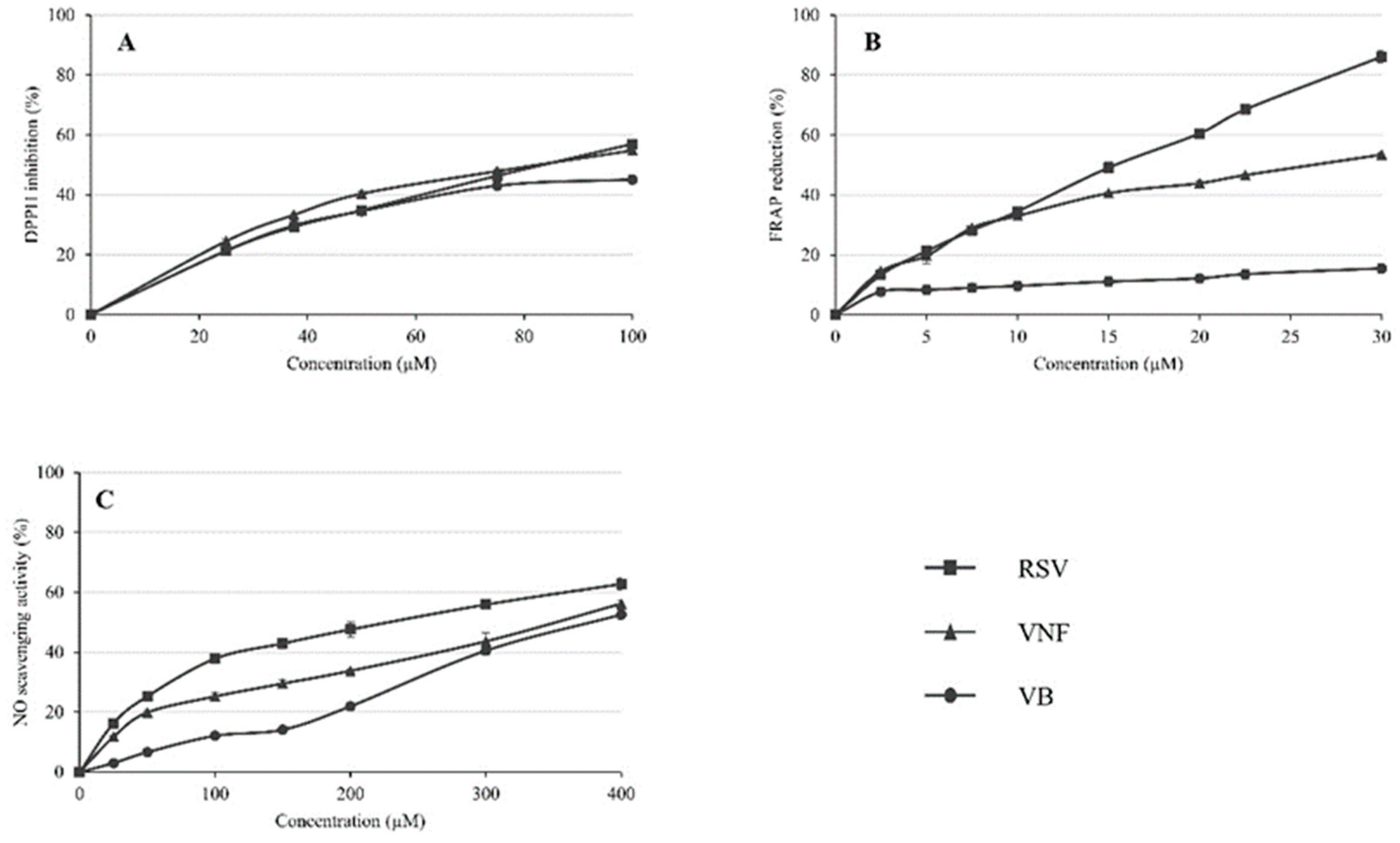
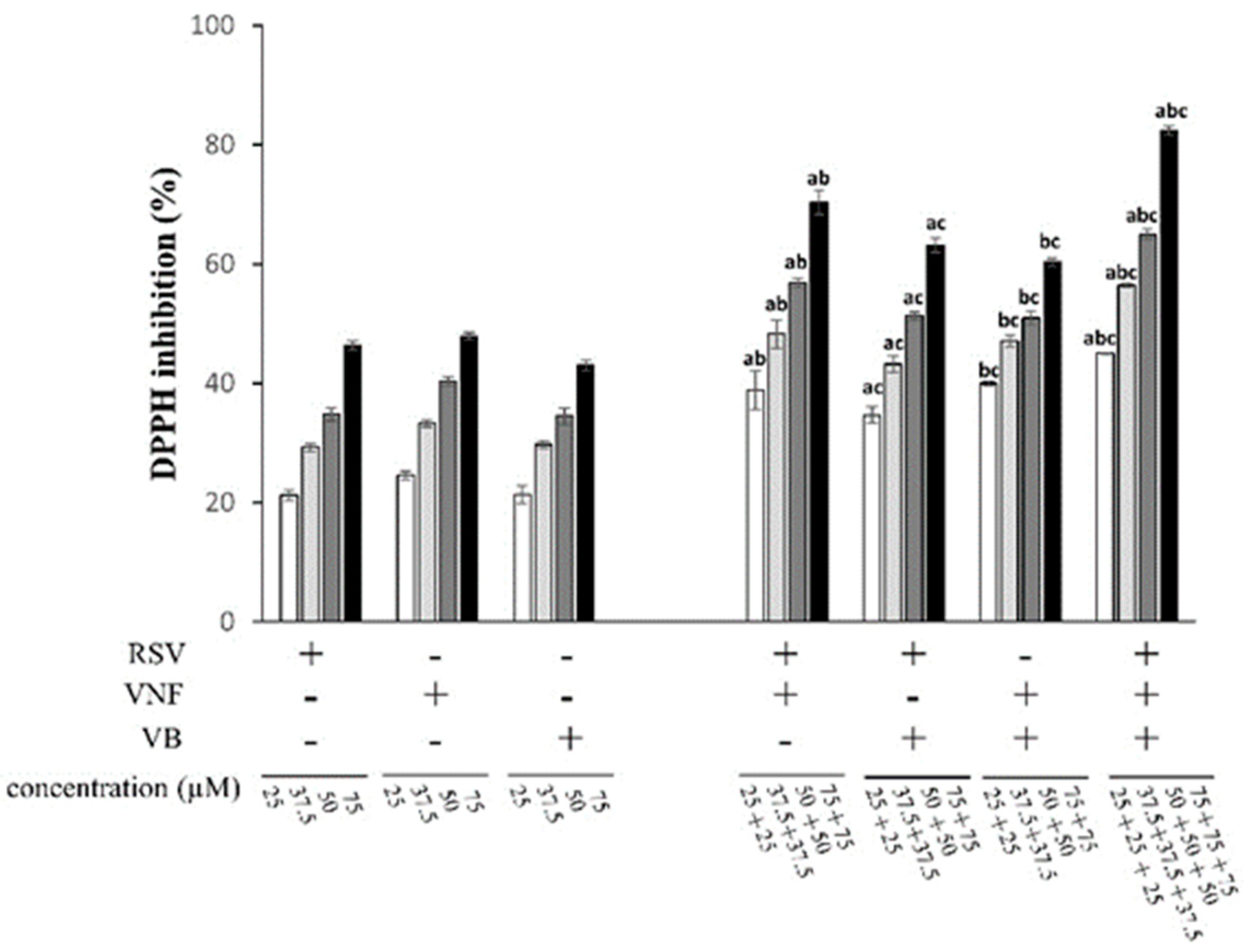
2.1. Determination of antioxidant activities by the DPPH scavenging assay
| DPPH | FRAP | NO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | CI | Interaction | IC50 (µM) | CI | Interaction | IC50 (µM) | CI | Interaction | |
| RSV | 83.69 | 15.38 | 229 | data | data 1 | ||||
| VNF | 82.61 | 26.19 | 350.84 | ||||||
| VB | 139.67 | ND | 378.84 | ||||||
| RSV+VNF | 79.90 | 0.93 | Additive | 14.72 | 0.78 | Synergy | 285.12 | 1.04 | Additive |
| RSV+VB | 95.81 | 0.93 | Additive | ND | 1.68 | Antagonism | 271.51 | 0.96 | Additive |
| VNF+VB | 93.63 | 0.90 | Additive | ND | 1.18 | Antagonism | ND | 1.11 | Antagonism |
| RSV+VNF+VB | 91.24 | 0.97 | Additive | 25.53 | 0.83 | Synergy | 307.76 | 0.85 | Synergy |
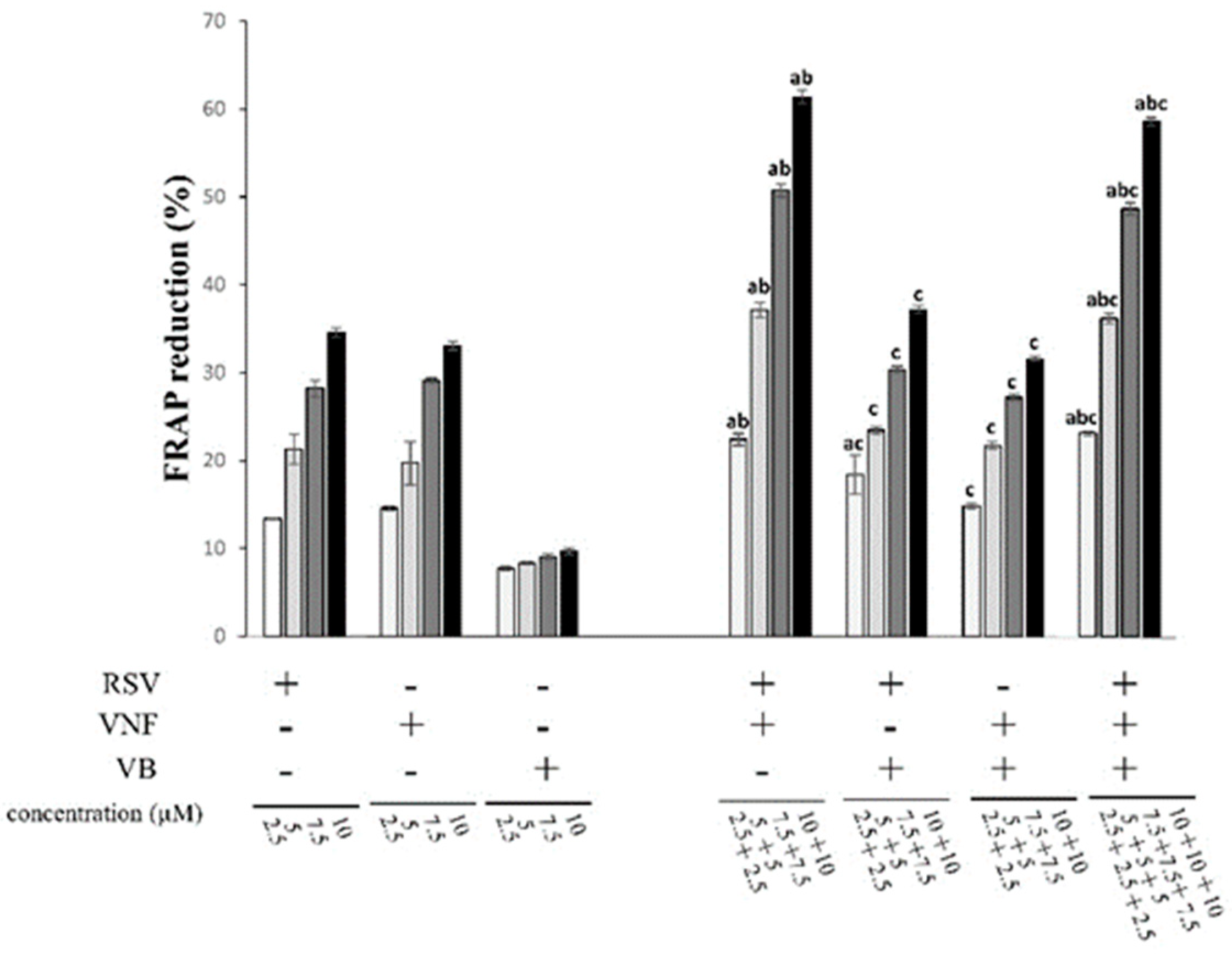
2.2. Determination of antioxidant activities by the FRAP assay
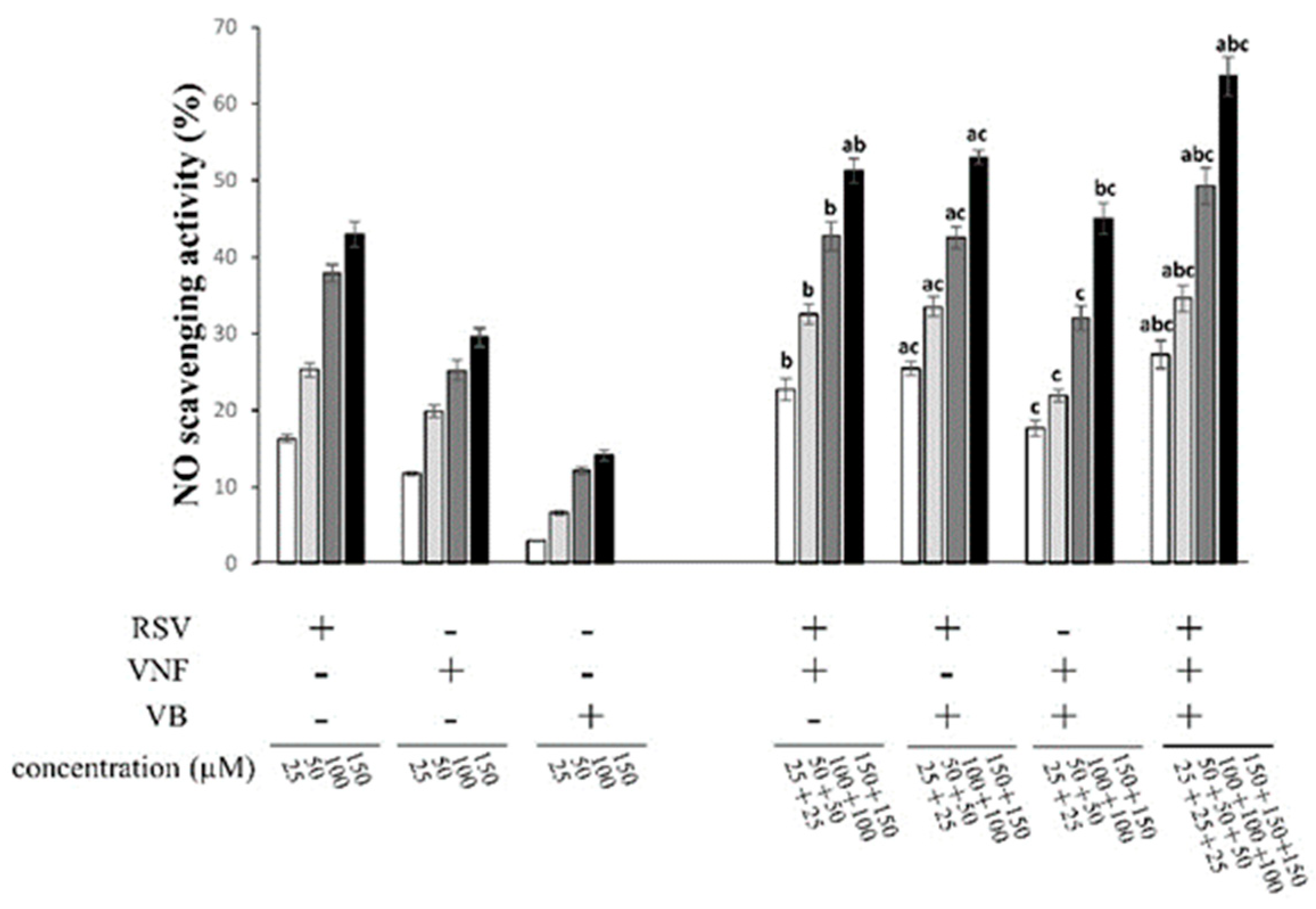
2.3. Determination of antioxidant activity by NO scavenging assay
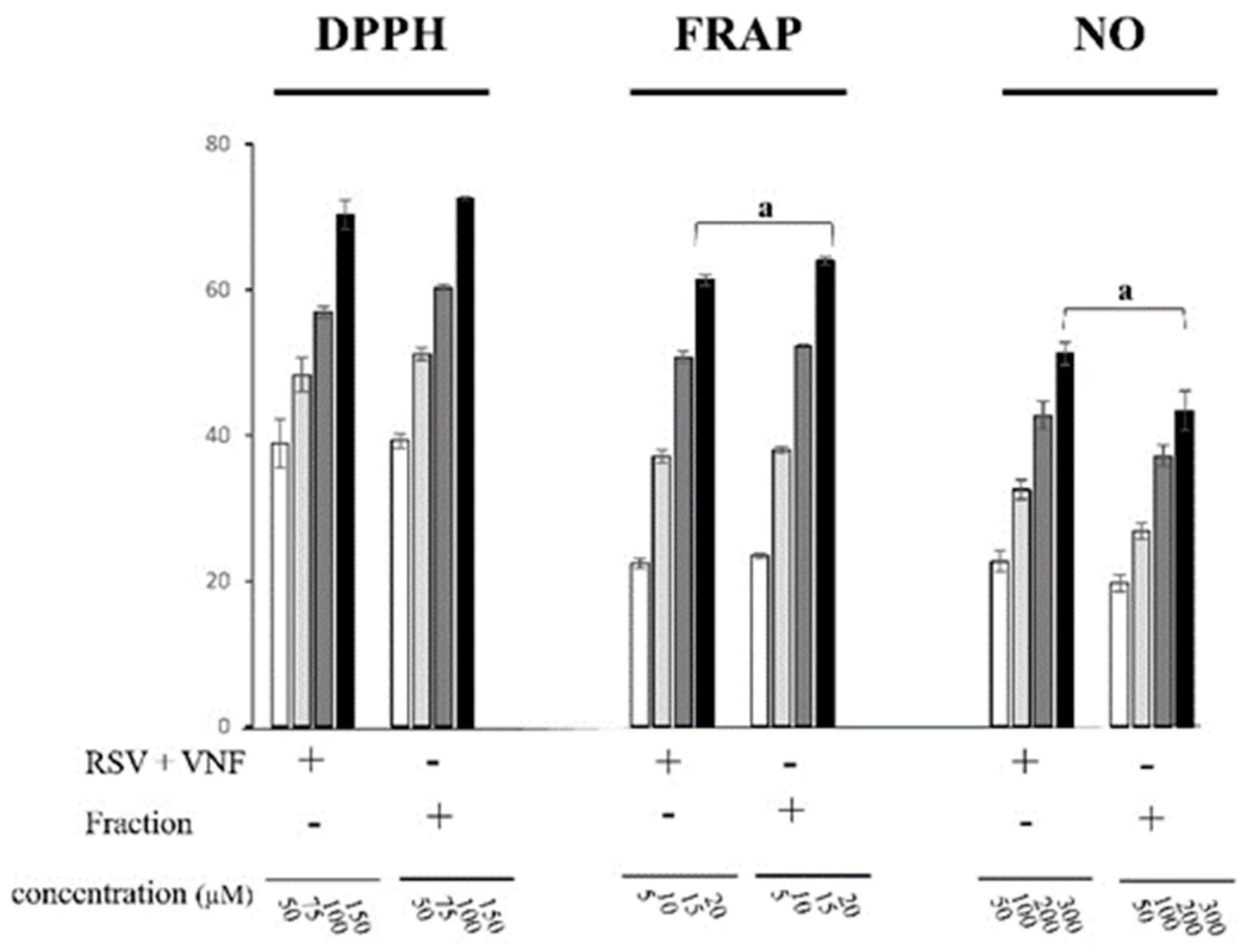
2.4. Comparison of the antioxidant activity between the combination RSV+VNF and the fraction
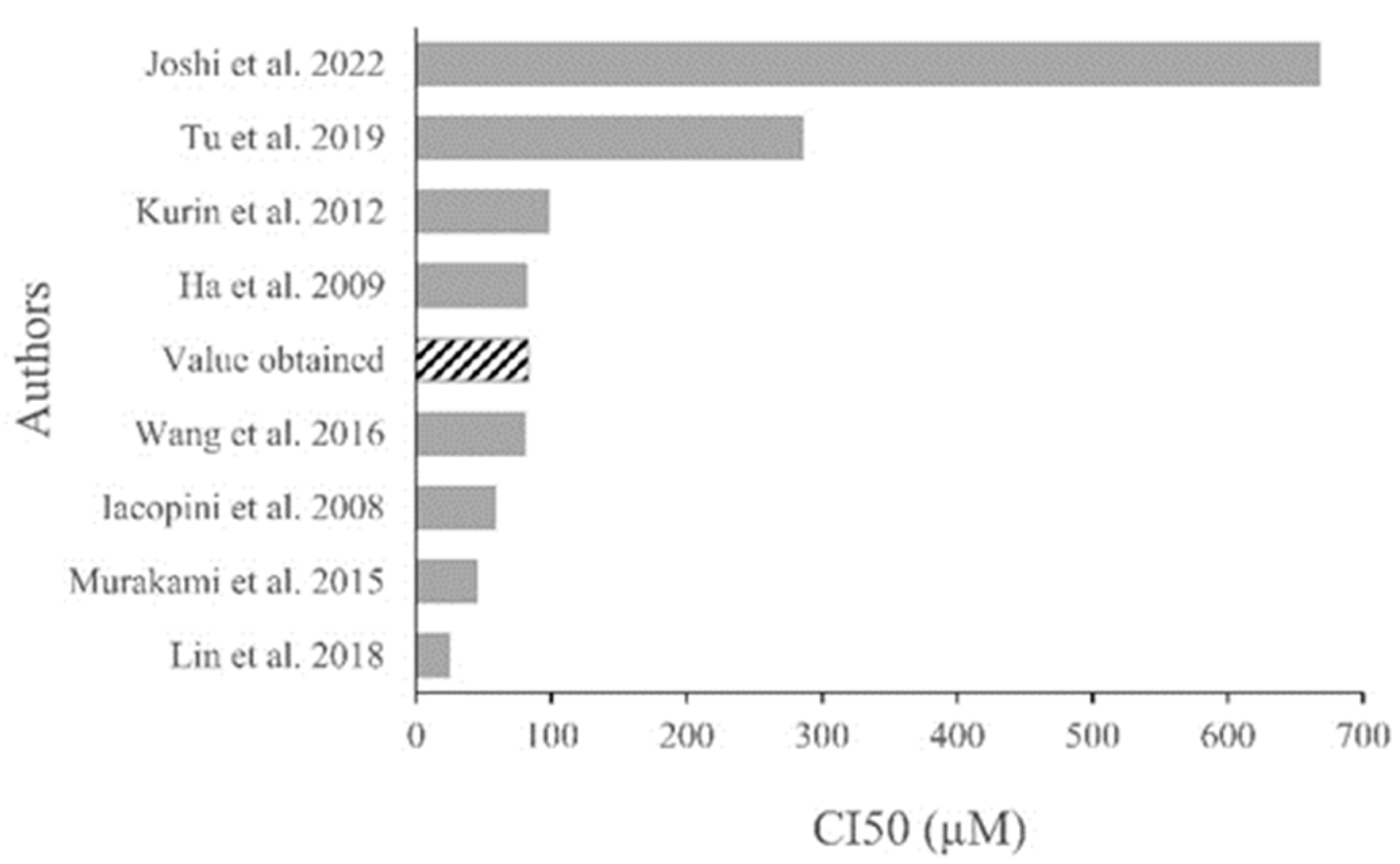
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. DPPH Scavenging Assay
4.3. FRAP assay
4.4. NO Scavenging Assay
4.5. Statistical analysis and determination of the interactions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Medicine and Cellular Longevity 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am J Biomed Sci 2013, 5, 126–139. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural Stilbenes: An Overview. Nat Prod Rep 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about in vitro Evaluation of Antioxidant Properties. Anal Chim Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Guilland, J.-C. Les interactions entre les vitamines A, D, E et K: synergie et/ou compétition. OCL 2011, 18, 59–67. [Google Scholar] [CrossRef]
- Aftab, N.; Vieira, A. Antioxidant Activities of Curcumin and Combinations of This Curcuminoid with Other Phytochemicals. Phytother Res 2010, 24, 500–502. [Google Scholar] [CrossRef]
- Ha, D.T.; Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and Lipoxygenase Inhibitory Activity of Oligostilbenes from the Leaf and Stem of Vitis Amurensis. Journal of Ethnopharmacology 2009, 125, 304–309. [Google Scholar] [CrossRef]
- Liu, W.-B.; Hu, L.; Hu, Q.; Chen, N.-N.; Yang, Q.-S.; Wang, F.-F. New Resveratrol Oligomer Derivatives from the Roots of Rheum Lhasaense. Molecules 2013, 18, 7093–7102. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.-M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol Rev 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Nassra, M.; Krisa, S.; Papastamoulis, Y.; Kapche, G.D.; Bisson, J.; André, C.; Konsman, J.-P.; Schmitter, J.-M.; Mérillon, J.-M.; Waffo-Téguo, P. Inhibitory Activity of Plant Stilbenoids against Nitric Oxide Production by Lipopolysaccharide-Activated Microglia. Planta Med. 2013, 79, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Chen, B.; Wei, G.; Chen, D. E-Configuration Improves Antioxidant and Cytoprotective Capacities of Resveratrols. Molecules 2018, 23, 1790. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Deepa, P.R.; Sharma, P.K. Effect of Different Proportions of Phenolics on Antioxidant Potential: Pointers for Bioactive Synergy/Antagonism in Foods and Nutraceuticals. Proc Natl Acad Sci India Sect B Biol Sci 2022, 92, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Chen, Y.; Zou, J.; Li, X. Biotransformation of Resveratrol: New Prenylated Trans-Resveratrol Synthesized by Aspergillus sp. SCSIOW2. Molecules 2016, 21, 883. [Google Scholar] [CrossRef] [PubMed]
- Agbadua, O.G.; Kúsz, N.; Berkecz, R.; Gáti, T.; Tóth, G.; Hunyadi, A. Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants 2022, 11, 1832. [Google Scholar] [CrossRef]
- Skroza, D.; Generalić Mekinić, I.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the Potential Synergistic Effect of Resveratrol with Other Phenolic Compounds: A Case of Binary Phenolic Mixtures. Journal of Food Composition and Analysis 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Chan, M.M.Y.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between Ethanol and Grape Polyphenols, Quercetin, and Resveratrol, in the Inhibition of the Inducible Nitric Oxide Synthase Pathway. Biochemical Pharmacology 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J Agric Food Chem 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, Epicatechin, Quercetin, Rutin and Resveratrol in Red Grape: Content, in Vitro Antioxidant Activity and Interactions. Journal of Food Composition and Analysis 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Abraham, S.K.; Eckhardt, A.; Oli, R.G.; Stopper, H. Analysis of in Vitro Chemoprevention of Genotoxic Damage by Phytochemicals, as Single Agents or as Combinations. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2012, 744, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant Activity of Phenolic Compounds in 2,2′-Azobis (2-Amidinopropane) Dihydrochloride (AAPH)-Induced Oxidation: Synergistic and Antagonistic Effects. J Amer Oil Chem Soc 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





