1. Introduction
Mangrove forests play a crucial role in providing valuable ecosystem services, contributing to a staggering annual value of at least US $1.6 billion (Polidoro, Carpenter et al. 2010). These services encompass a wide range of benefits that support coastal livelihoods on a global scale (Dahdouh-Guebas, Jayatissa et al. 2005, Duke, Meynecke et al. 2007, Ellison 2008, Abrantes, Johnston et al. 2015). However, mangrove forests are disappearing worldwide by 1 to 2% per year (Duke, Meynecke et al. 2007). Clearing for coastal development, expansion of aquaculture, logging for timber, and fuel production are among the primary drivers behind this concerning trend (Daru, Yessoufou et al. 2013, Kirui, Kairo et al. 2013, Yessoufou and Stoffberg 2016). It has been shown that over 40% of the assessed vertebrate species endemic to mangrove forests are currently facing global threats to their survival (Luther and Greenberg 2009). As a result, urgent attention is needed to monitor the state of mangrove forests and get a better understanding of mangrove forest dynamics in response to disturbances.
According to the critical slowing down theory, the response of an ecosystem, such as the mangrove forest ecosystem, to disturbance is not always a gradual process; it can lead to sudden and irreversible changes (Scheffer 1990, Scheffer 2001, Scheffer, Carpenter et al. 2001). In fact, even gradual changes in the environment may not result in a corresponding gradual response from the ecosystem. Instead, they can trigger sudden, unpredictable, and irreversible shifts known as regime shifts (Capon, Lynch et al. 2015). Apart from the growing evidence of critical changes occurrences in different ecosystems around the world (Barbier, Koch et al. 2008, Guttal and Jayaprakash 2008, Lenton 2011, Verbesselt, Umlauf et al. 2016, Alibakhshi, Groen et al. 2017), the need to enhance the understanding of ecosystem states and generate early warning signals of critical transition in ecosystems is highlighted.
Abrupt changes in the state of an ecosystem can occur when ecosystems are unable or slow to cope with the effects of disturbances, which is a sign of low resilience (Carpenter and Brock 2006, Carpenter, Brock et al. 2008, Scheffer, Bascompte et al. 2009, Carpenter and Brock 2011, Carpenter, Cole et al. 2011). Disturbances can push the state of an ecosystem to a state that is near a critical threshold. Once a critical threshold is reached, even a small disturbance can trigger a significant transition to a new state, where it is challenging and sometimes impossible to return to the previous state (Scheffer, Carpenter et al. 2001, Carpenter and Brock 2006, Carpenter, Brock et al. 2008, Scheffer, Bascompte et al. 2009, Carpenter and Brock 2011, Carpenter, Cole et al. 2011). This knowledge is crucial for assessing the current state of an ecosystem and determining whether it is approaching a critical transition. Understanding the current state of an ecosystem is particularly crucial in mangrove forests, as it can inform conservation efforts can safeguard abundant resources, especially for locals (Martínez, Intralawan et al. 2007). Detecting the state of an ecosystem can prevent irreversible changes and facilitate early interventions (Lenton, Held et al. 2008, Hirota, Holmgren et al. 2011, Alibakhshi 2023).
Various statistical analyses have been developed to quantify the state of an ecosystem (Carpenter and Brock 2006, Dakos, Carpenter et al. 2012, Kéfi, Guttal et al. 2014). For example, an increasing trend in autocorrelation and standard deviation of a ‘state variable’ over time (Dakos, Van Nes et al. 2012) can serve as early warning signals (Dakos, Carpenter et al. 2012, Dakos, Van Nes et al. 2012, Lenton, Livina et al. 2012, Dakos, Carpenter et al. 2015). A ‘state variable’ is an indicator that can present the state of an ecosystem and measure the proximity of the ecosystem to critical conditions. Increased skewness can also be used to measure early warning signals of critical transition, as disturbances can lead to alterations in the asymmetrical distribution of the time series of a state variable before the state change occurrences (Guttal and Jayaprakash 2008, Dakos, Carpenter et al. 2012).
Despite the availability of various methods for assessing early warning signals, the anticipation of critical transitions in ecosystems poses significant challenges. Hence, the application of the methods to assess the state of ecosystems and identify early warning signals of reduced resilience is still limited. This limitation stems from the complexity of ecosystem processes, compounded by the requirement for high-frequency time series data of ecosystem state variables (Dakos, Carpenter et al. 2015). To address this problem, this study uses the high spatio-temporal resolution of satellite images. Satellite images offer high-frequency time series of data, comprehensive coverage, and multispectral capabilities, making them a powerful tool for the aim of this study.
This study aims to investigate the potential of time series analysis of remote sensing data in detecting regime shifts in mangrove forest ecosystems and establishing an early warning system. The application of the critical slowing down theory in monitoring mangrove forests has been rarely tested (Wang, Zhang et al. 2023), especially in the Middle East. This is the first study that explores the potential of remote sensing to explore early warning signals of impending critical transition in mangrove forests in the Middle East. To achieve the aim of this study, it is crucial to select the right ecosystem state variables. A prior research study has demonstrated that utilizing remotely sensed indicators capable of simultaneously capturing variations in both water and vegetation can provide a more comprehensive understanding of ecosystem states compared to relying solely on either vegetation-based or water-based indices (Alibakhshi, Groen et al. 2017). Hence, in this study, we used a vegetation-based indicator, namely the Normalized Difference Vegetation Index (NDVI), a water-based indicator, namely the Modified Normalized Difference Water Index (MNDW), and a vegetation-water-based indicator, namely Modified Vegetation Water Ratio (MVWR). Mangrove forest ecosystems in Qeshm Island and Gabrik are chosen as study sites, where Qeshm Island is selected as a representative of an unhealthy ecosystem, while Gabrik serves as the reference site for comparison. To assess the land-use and land-cover change in the study sites, Landsat data has been used.
2. Material and methods
2.1. Study sites
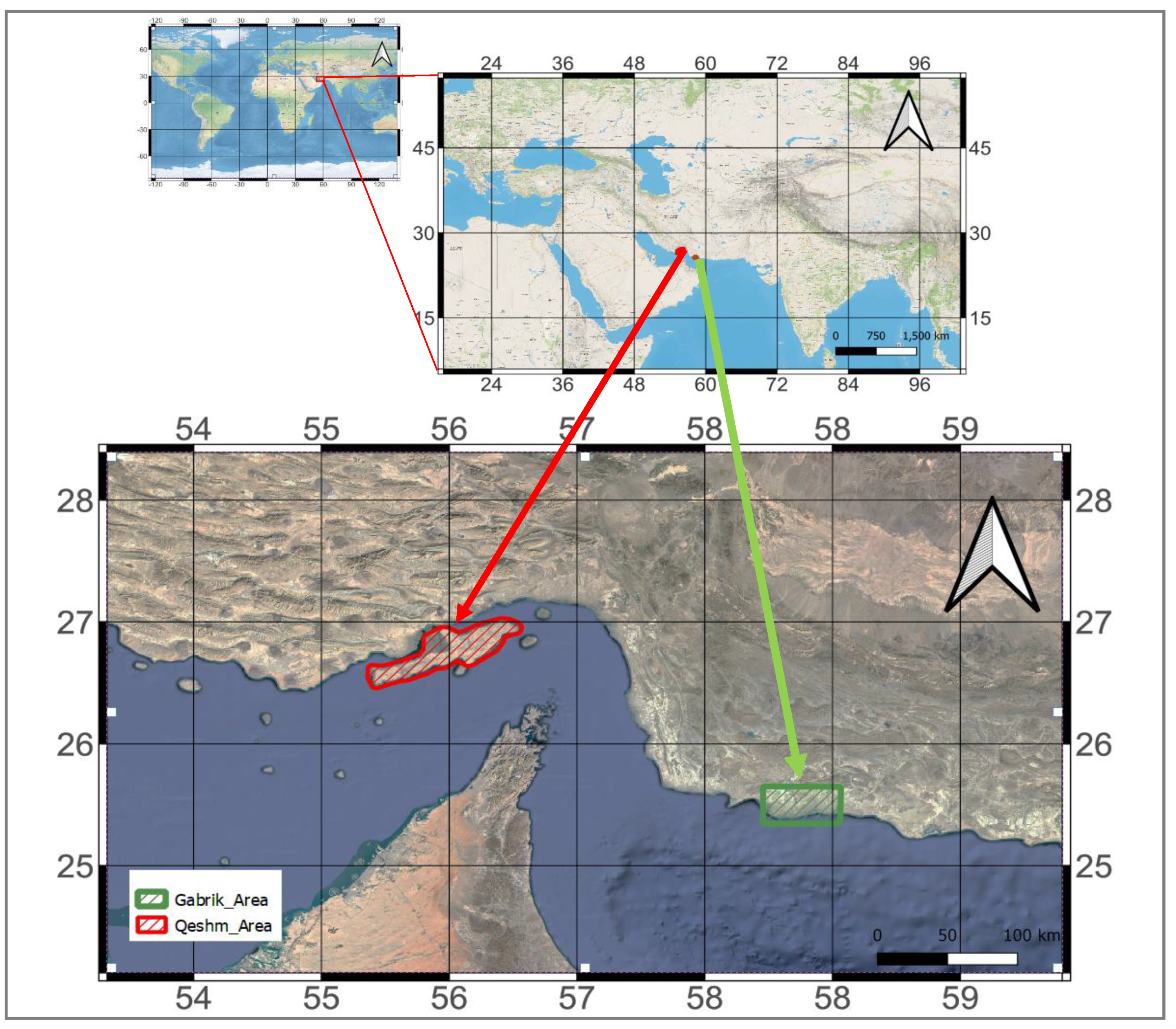
This study focuses on Qeshm Island (Mazraeh and Pazhouhanfar 2018, Kourosh Niya, Huang et al. 2019), located in the southern region of Iran, between the Persian Gulf and the Oman Sea. Qeshm Island, with a total area of 1667 km2 has undergone substantial land-use and land-cover change (Kourosh Niya, Huang et al. 2019). Another study site, Gabrik, with a total area of 2496 km2, has been selected as a reference case study, representing a healthy ecosystem. Both study sites are characterized by the presence of mangrove forest ecosystems (Zahed, Rouhani et al. 2010, Naderloo, Türkay et al. 2013).
The geographical coordinates of Qeshm Island range from 55° 15’ 38” E to 56° 16’ 52” E and 26° 32’ 20” N to 27° 00’ 00” N. The Gabrik is located southeast of Qeshm Island with geographical coordinates ranging from 55° 15’ 38” E to 56° 16’ 52” E and 26° 32’ 20” N to 27° N00’ 00” N (
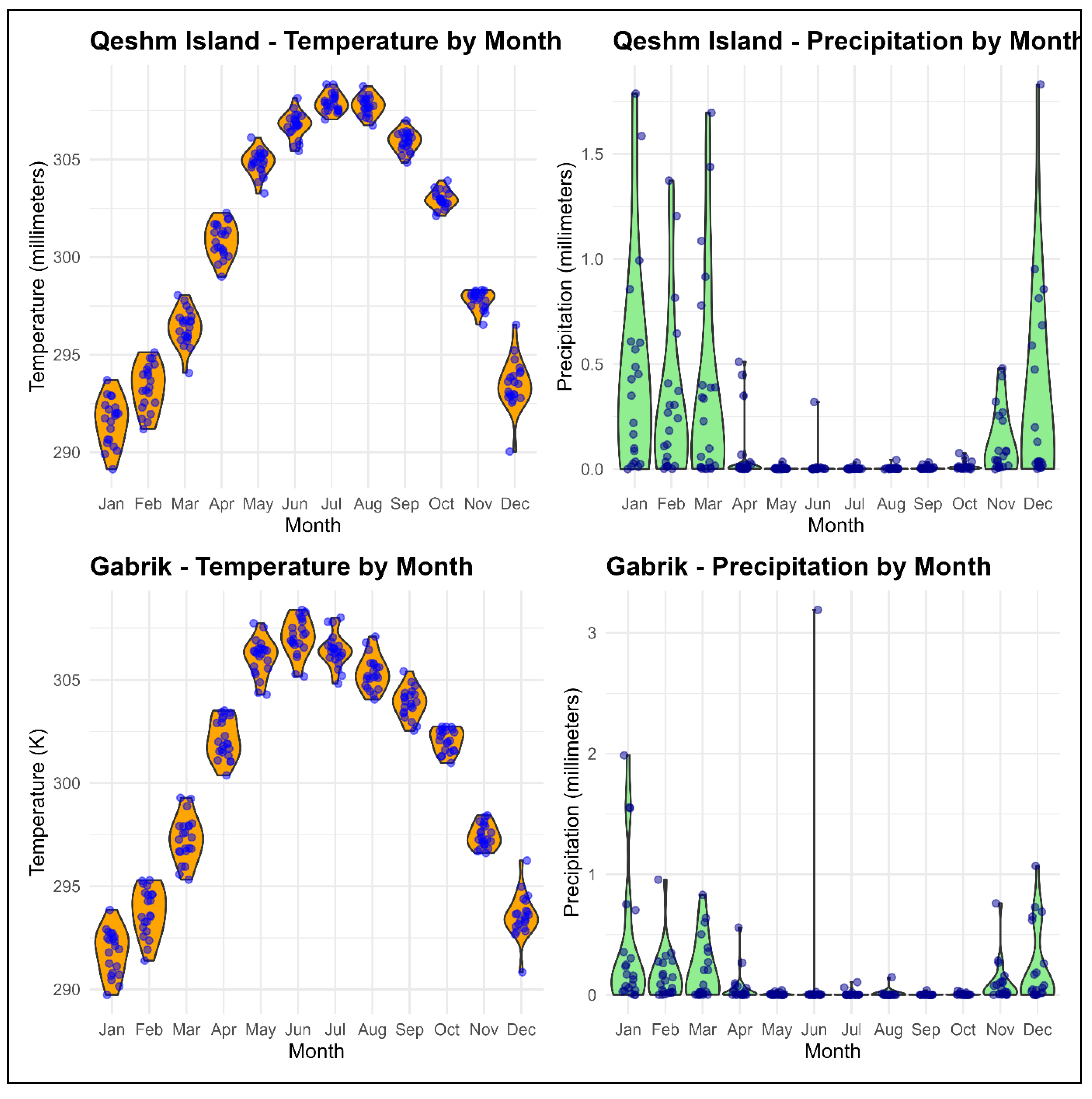
Figure 1). Based on ECMWF Reanalysis version 5 (ERA-5) data (Muñoz Sabater 2019), the mean temperature in Qeshm Island is 301 Kelvin (28 degrees Celsius), and the mean precipitation is 36 mm from February 18, 2000 to July 31, 2021. In Gabrik, the mean temperature is 301 Kelvin (28 degrees Celsius), with an average precipitation of 28 mm from February 18, 2000 to July 31, 2021. These show the similarity of climatic conditions and local weather patterns of Qeshm Island and Gabrik study sites (
Figure 2). According to the population dataset provided by WorldPop (Sorichetta, Hornby et al. 2015), the estimated population residing in the region of Gabrik is reported to be remarkably low, with a mere 74 individuals. On the other hand, a significantly higher population count of 957 individuals are living in Qeshm Island.
2.2. Materials and methods
2.2.1. State variables
The Normalized Difference Vegetation Index has been obtained from the latest version of the Moderate Resolution Imaging Spectroradiometer (MODIS) product, specifically MOD09A1 (version 006). The 006 version of MOD09A1 has undergone numerous enhancements in the algorithm (Didan, 2015). The data utilized in this study was acquired from the Google Earth Engine platform (Gorelick et al., 2017). The dataset utilized herein possesses a spatial resolution of 500 meters with a temporal resolution of 16 days, enabling comprehensive analysis from February 18, 2000, to July 31, 2021. The MOD09A1 product is derived from atmospherically corrected bi-directional surface reflectance. The calculation of the Normalized Difference Vegetation Index (NDVI) involves utilizing the Red and Near-Infrared (NIR) bands, particularly within the wavelengths of 0.620 μm to 0.670 μm and 0.841 μm to 0.876 μm, respectively (Eq. 1). The NDVI (dimensionless) values range from -1 to +1, where a value of -1 indicates bare land, while a value of +1 indicates dense forest.
The Modified Normalized Difference Water Index (MNDWI) is calculated using MOD09A1 (version 006), which is described earlier. Among the various remotely sensed water indices (Mozumder, Tripathi et al. 2014, Rokni, Ahmad et al. 2014, Li, Chen et al. 2015), MNDWI has been acknowledged as the most accurate indicator for extracting water area variations (Ji, Zhang et al. 2009, Chen, Huang et al. 2013). MNDWI, similar to NDVI, is a dimensionless index that ranges from -1 to 1. When MNDWI values are below 0, it indicates low water content, which includes soil and vegetation. On the other hand, MNDWI values above 0 indicate high water content and varying water levels. The calculation of the MNDWI involves utilizing the Green and shortwave infrared (SWIR) bands, specifically within the wavelengths of 0.55 μm to 0.57 μm and 1.23 μm to 1.25 μm, respectively (Eq. 2).
Additionally, the Modified Vegetation Water Ratio (MVWR) was used to measure early warning signs in aquatic ecosystems (Alibakhshi, Groen et al. 2017) (Eq. 3). The MVWR is sensitive to changes in vegetation water content, which are the main component of mangrove forests. It effectively captures variations in water availability and reflects hydrological dynamics, such as seasonal fluctuations and long-term shifts. Moreover, the MVWR is shown as a reliable indicator to assess vegetation health and stress levels (Tehrani and Janalipour 2021), which can make it a powerful indicator for the aim of this study. The calculation of the MNDWI involves:
2.2.2. Map of mangrove forest ecosystems and land-use and land-cover change
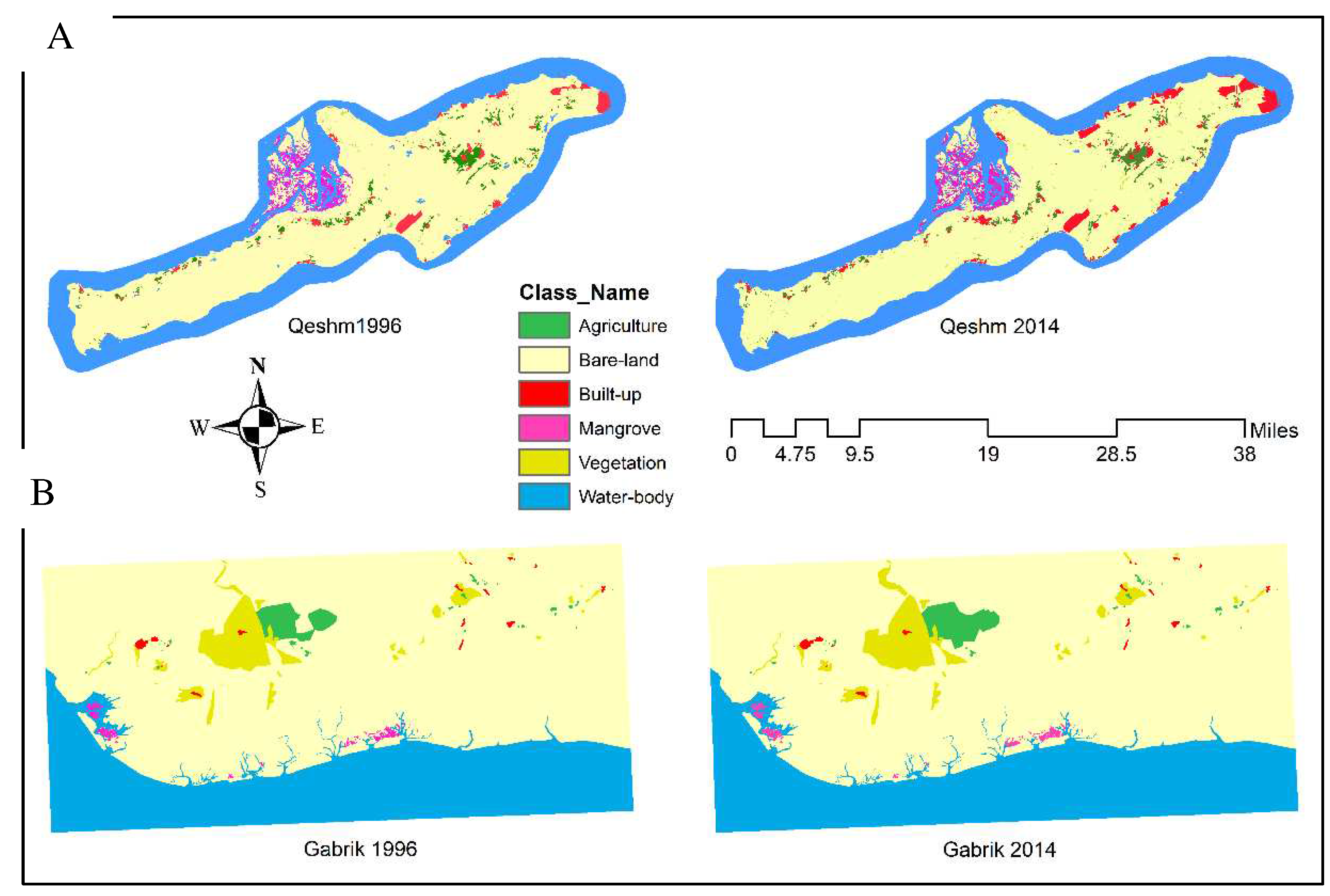
The land-use and land-cover change maps of study sites were calculated with a spatial resolution of 30 meters (Kourosh Niya, Huang et al. 2019), by using Landsat satellite imagery for the years 1996, 2002, 2008, and 2014. The maps provide a classification map of study sites, including six distinct land use classes: agriculture, bare-land, built-up, dense vegetation, mangrove forests, and waterbody.
In addition, the Global mangrove forest Distribution dataset was employed for the year 2000 which provides a map of the world’s mangrove forest ecosystems as of the year 2000 with a spatial resolution of approximately 30 meters (Giri, Ochieng et al. 2011, Giri, Ochieng et al. 2013). The data compilation involved analyzing over 1,000 Landsat Thematic Mapper ™ scenes using a hybrid approach of classification techniques.
2.2.3. Exploring early warning signals of critical transition
First, the extent of mangrove forests in each study site was delineated using the Global mangrove forest Distribution maps (
Section 2.2.2). After that, 100 points were randomly selected in each study site. From each point, the time series of the state variables (
Section 2.2.1) was extracted using a mean function for the period from February 18, 2000, to July 31, 2021. Subsequently, autocorrelation, skewness, and standard deviation were applied to detect early warning signals of critical transition (Dakos, Carpenter et al. 2012). Autocorrelation refers to the degree of correlation between the values of the same variables in time. For autocorrelation calculation, the first step is to define a lag operator, which is represented by (
t) and is a time display. The autocorrelation function (ACF) can be calculated using equation (4):
Standard deviation (SD) measures the degree of variability or distribution for a set of data relative to the mean of the same data. SD is obtained from the variance as shown in equation (5):
Skewness is the third statistical index used in this study, which is calculated using equation (6):
Prior to applying metric-based models, the data needs to undergo detrending and smoothing procedures to mitigate the impact of nonstationary conditions on the leading indicators (Dakos, Carpenter et al. 2012). Several detrending approaches are commonly employed, such as Gaussian, Linear, Loess filters, and first-differencing (Lenton, Livina et al. 2012). These methods detrend the data within a rolling window. In this study, a sensitivity analysis was conducted using Kendall's τ, a nonparametric statistic measuring the association between indicators and time, to identify the optimal size of the rolling window and bandwidth for the Gaussian filter (Bevan and Kendall 1971). Kendall's τ ranges from -1 to +1, where higher values indicate stronger trends, aiming to identify the detrending settings that best capture trends in the leading indicators. To achieve this, the leading indicators for various rolling window sizes (ranging from 25% to 75% of the time series length) and bandwidths (ranging from 25% to 75% of the time series length for the Gaussian filter) with increments of 10%, was calculated using Gaussian, Loess, Linear filters, and first-differencing approaches.
To ensure that the observed trends in the leading indicators were not due to random chance, 1000 surrogate datasets were generated. These datasets were created by fitting the best linear autoregressive moving average model (ARMA) based on AIC to the residuals obtained after detrending the data. Each surrogate dataset had the same length as the residual time series. Following previous research, the trend estimations from the original data with those from the surrogate data, had similar correlation structures and probability distributions. Kendall's τ was employed to estimate trends in autocorrelation, skewness, and standard deviation was compared. The probability of finding a trend by chance was measured by comparing Kendall's τ of the original data with the number of cases in which the statistic was equal to or smaller than the estimates of the simulated records, denoted as P(τ∗≤τ) (Dakos, Carpenter et al. 2012).
In this study, we only used a large variety of freely available remotely sensed data. All statistical analyses and visualizations were performed in R statistical software (Pinheiro, Bates et al. 2000) QGIS (Qgis 2016), and Google Earth Engine.
3. Results
The results in this section include analyses of land-use and land-cover change in Qeshm Island and Gabrik, highlighting changes in agriculture, bare land, built-up areas, mangroves, vegetation, and water bodies classes (Section 4.1). Time series of state variables, including NDVI, MNDWI, and MVWR are represented, which show temporal dynamics and seasonal variations of mangrove forests in study sites (Section 4.2). The early warning signals and the results of sensitivity analyses have been presented (Section 4.3).
3.1. Land-use and land-cover change
Qeshm Island experienced various changes in land-use and land-cover change during the study period (
Table 1). The area dedicated to agriculture witnessed a marginal increase of 338 units (0.65% change). However, there was a significant decrease in bare land, with a change of -57,360 units (-3.65% change). On the other hand, built-up areas expanded dramatically, showing an increase of 35,759 units (64.40% change). Mangroves and vegetation also exhibited positive growth, with changes of 6,049 units (9.07% change) and 6,880 units (70.30% change), respectively. Water bodies slightly expanded, with a change of 8,336 units (0.82% change).
In Gabrik agriculture experienced substantial growth (
Table 1), with a change of 4,708 units (15.22% change). Bare land decreased, albeit to a lesser extent, with a change of -8,629 units (-0.52% change). The built-up areas expanded modestly, with a change of 215 units (4.04% change). Mangroves and vegetation also showed positive growth, with changes of 790 units (9.47% change) and 2,628 units (3.22% change), respectively. Water bodies remained relatively stable, with a minimal change of 288 units (0.04% change).
3.2. Time series of state variables
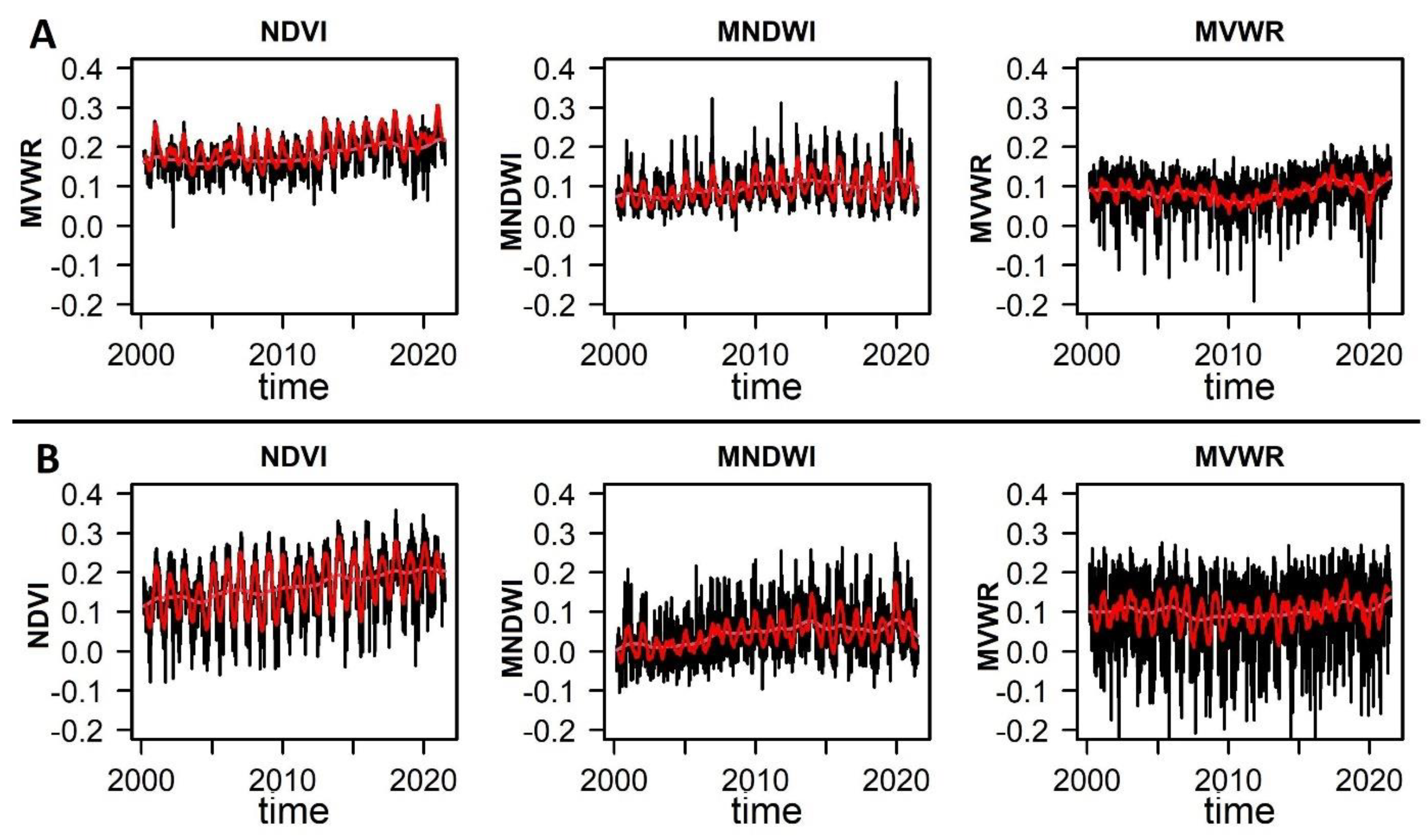
The extracted data for the NDVI, MNDVI, and MVWR indices reveal the temporal dynamics and seasonal variations in the study sites (
Figure 4). Both study sites showed NDVI values, ranging from 0.01 to 0.36. Similarly, MNDWI values, ranging from -0.01 to 0.36, and MVWR values, ranging from -0.242 to 0.263. More specifically, Qeshm Island has a slightly higher average NDVI value of 0.20, indicating a relatively denser green vegetation cover compared to Gabrik, which has an average NDVI of 0.17. This suggests that Qeshm Island may have higher overall vegetation density. In terms of water presence, Gabrik exhibits a lower average MNDWI value of 0.04, suggesting a relatively lower presence of water bodies compared to Qeshm Island, which has an average MNDWI of 0.10. This indicates that Qeshm Island may have more abundant water bodies within its study area. Regarding vegetation water content, Gabrik and Qeshm Island have similar average MVWR values of 0.10 and 0.09, respectively.
3.3. Early warning signals of a critical transition
In Qeshm Island, where forests have been disturbed, NDVI and MNDWI exhibited significant positive autocorrelation (0.505, 0.864) and substantial standard deviation (0.891, 0.903). Notably, MVWR, shows negative autocorrelation (-0.100) and lower standard deviation (-0.074), suggesting less sensitivity compared to NDVI and MNDWI. The skewness values of NDVI (0.288) indicate a more pronounced increasing trend compared to MNDWI (-0.584) and MVWR (-0.212). In Gabrik, the reference study site, NDVI exhibited negative autocorrelation (-0.192) and lower standard deviation (-0.186) than MNDWI and MVWR. The MNDWI demonstrated an uptick in both autocorrelation (0.789), standard deviation (0.891), and skewness (-0.576), while MVWR exhibited corresponding declines of -0.722, -0.118, and -0.335 respectively.
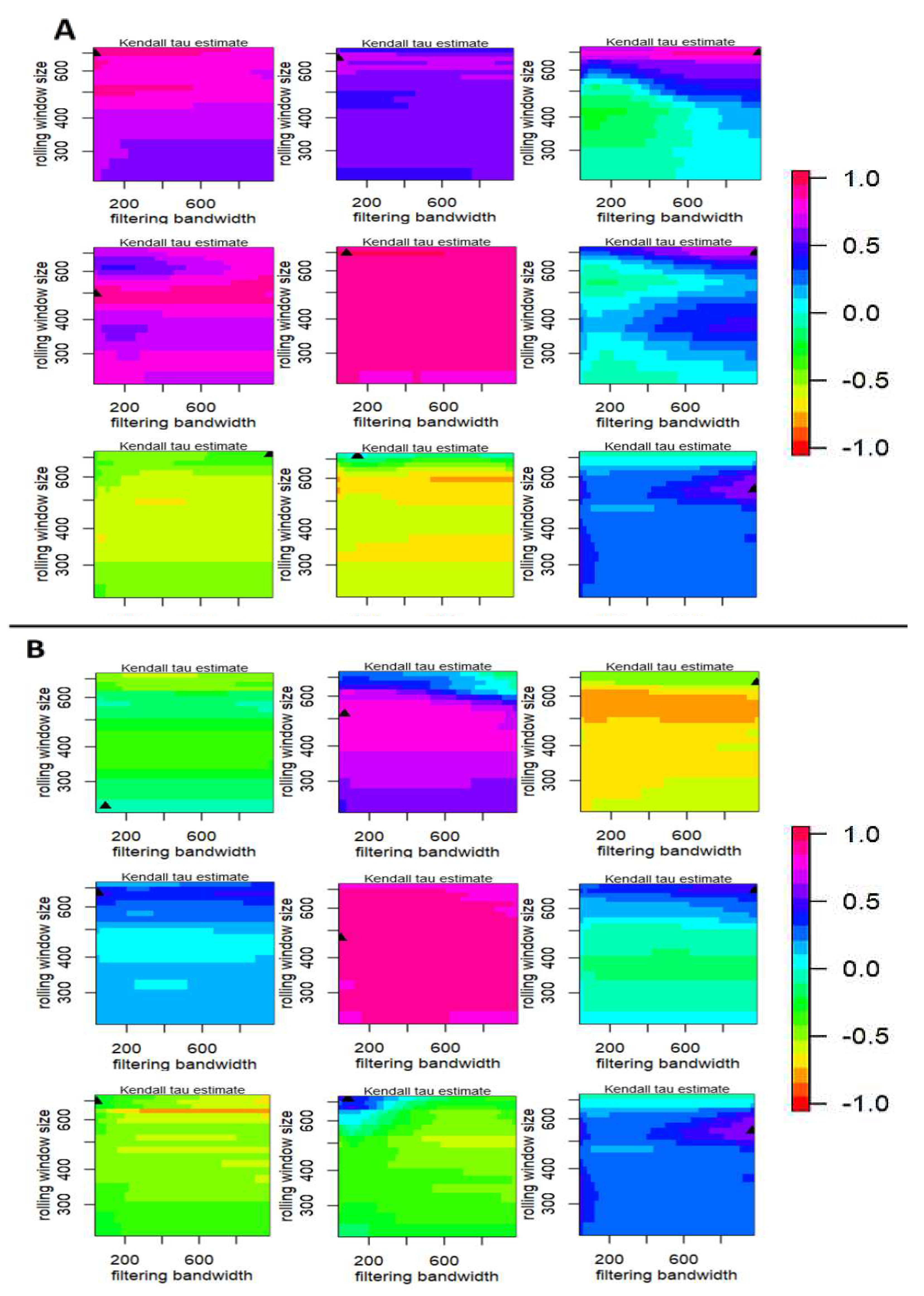
Contour plots (
Figure 6) illustrate the impact of varying the width of the rolling window and applying Gaussian filtering on the discerned trend in the statistical metrics, as quantified by Kendall's τ. Additionally,
Figure 1A and
Figure 2A depict histograms representing the frequency distribution of the trend statistic of the marked upside triangles in
Figure 6. The results of the sensitivity analysis show NDVI, MNDWI, and MVWR consistently presented less variation in color gradients in the plot, indicating a consistently high Kendall’s τ with different setting to perform the analysis. This consistently high ranking suggests the resilience of NDVI, MNDWI, and MVWR to variations in rolling window widths and Gaussian filtering bandwidths.
4. Discussion
This study illustrates the potential of remote sensing coupled with critical slowing down theory to explore the resilience of the mangrove forest ecosystem, which contributes to the state-of-the-art knowledge on monitoring of mangroves. While Qeshm Island and Gabrik share similarities in climate, geography, and annual rainfall (Fig. 1 and Fig. 2), the analysis of remote sensing indices, including NDVI, MNDWI, and MVWR, revealed significant differences between the two study areas (Fig. 4). The time series of NDVI in Qeshm Island showed increases in autocorrelation, standard deviation, and skewness, unlike Gabrik (Fig. 5), signaling the reduced resilience within the mangroves. Importantly, the increases in the statistical metric do not necessarily predict a state shift in the ecosystem's state but rather suggest a potential decrease in resilience (Dakos, Carpenter et al. 2015). Although the signals cannot directly warn of the upcoming transition in the mangroves’ state, the projection models anticipated the loss of up to half of the current mangrove areas (i.e., occurrence of tree less state) in Iran by the end of the 21st century due to the impending threats posed by disturbances (Mafi-Gholami, Zenner et al. 2020). In addition, a comparison of three hybrid models to simulate land use change suggested a decline in the total area of mangrove forests (Kourosh Niya, Huang et al. 2020).
The mangroves in Qeshm Island are significantly influenced by several stressors, such as pollution and eutrophication, biological stressors, extreme events, and land-use and-cover change. More specifically, eutrophication and pollution from nutrient-rich wastewater effluent, have been identified as one of the main factors influencing mangrove health in the region (Akbarzadeh-Chomachaei, Koohkan et al. 2023). Heavy metal pollution in coastal sediments, as evidenced in Qeshm Island raises concerns regarding mangrove ecosystem integrity (Akbarzadeh-Chomachaei, Koohkan et al. 2023). Petroleum pollution in sediments from Qeshm Island compounds these environmental challenges (Ebrahimi-Sirizi and Riyahi-Bakhtiyari 2013, Hamzeh, Koochaknejad et al. 2021). Along with such anthropogenic disturbances, biological stressors also play a significant role in disturbing mangrove forests. The proliferation of harmful algal blooms, a consequence of eutrophication, has emerged as a significant biological stressor impacting mangrove ecosystems (Mirza Esmaeili, Mortazavi et al. 2021). Additionally, the presence of a fungal pathogen and the documented damage on mangroves highlights the multifaceted biological threats faced by these mangroves (Goudarzi and Moslehi 2020, Moslehi, Bernier et al. 2023). In addition to all these stressors, we showed that the expansion of built-up areas was significantly increased in Qeshm Island than in Gabrik (
Table 1). Gabrik site, which is known as the Ramsar International Wetland site is considered a protected area. Gabrik site, opposite Qeshm Island, is shown to be less affected by pollution and heavy metals (Zarezadeh and Rezaee 2016) and land-use land-cover-change (
Table 1).
Although the adverse impacts of the abovementioned disturbances (i.e., pollution and eutrophication, biological stressors, climate change, extreme events, and land-use land-cover change) in the Qeshm Island are well documented, the area of mangroves in both sites exhibits an increase (
Table 1). This observed expansion could be attributed to mangrove adaptation through landward migration and expansion (Etemadi, Smoak et al. 2018) as well as reforestation projects in the regions (Mahmoudi, Mafi-Gholami et al. 2022, Farshid, Moradi Balef et al. 2023). Not only has the area of mangroves increased during the past decade, but also the NDVI did not show declines (Fig. 4). NDVI is sensitive to greenness and vegetation health (Alatorre, Sánchez-Carrillo et al. 2016, Li, Jia et al. 2019, Alibakhshi 2020, Cabello, Germentil et al. 2021) and is widely utilized and validated in various studies. NDVI proves effective in assessing vegetation dynamics by capturing the reflectance of near-infrared (NIR) radiation and absorption of red light, indicative of healthy vegetation (Verbesselt, Hyndman et al. 2010, Verbesselt, Hyndman et al. 2010, Ruan, Yan et al. 2022, Tran, Reef et al. 2022). In fact, given previous reports of reduced resilience in the mangroves, one might have anticipated a decline in the NDVI time series. However, the absence of such a decline (Fig. 4) does not necessarily indicate the health of the mangrove forest in the region. The elevation in eutrophication along with reported instances of algal blooming (Hamzeh, Koochaknejad et al. 2021, Mirza Esmaeili, Mortazavi et al. 2021) can increase the NDVI (Rada, Nikitina et al. 2023). Hence, the pattern of NDVI trend (Fig. 4) in the mangroves is most likely due to an increase of the chlorophylls in the water following the increase of eutrophic level and algal blooming occurrences, not an increase in the tree covers or improvements in the mangrove forests health.
Although the trend analyses of NDVI cannot reflect the dynamics of mangroves in the study sites, the results of statistical metrics of NDVI, i.e., increase in standard deviation, autocorrelation, and skewness provided valuable knowledge on the state of the mangroves (
Table 2). The increases in the autocorrelation, standard deviation, and skewness (
Table 2) of NDVI in Qeshm Island may suggest mangrove forests are slow in recovering themself which is in line with previous studies (Ghanbarzad Dashti, Farzingohar et al. 2021). The negative trend of change in autocorrelation, standard deviation, and skewness observed in the MVWR index in Qeshm Island suggest unreliable signals and interpretations. Similarly, the high autocorrelation and standard deviation values observed in the MNDWI index in the reference site show limitations in the application of water indicators in exploring the state of the mangroves. These findings might be attributed to the complexity of mangrove forest structures and also the adaptability of mangroves, particularly in response to variations in water areas (as indicated by MNDWI in this study) through landward migration. (Etemadi, Smoak et al. 2018). Concerning the modest outcomes of skewness, signifying a decline in all cases except for NDVI in Qeshm Island (
Table 1), it is noteworthy that skewness excels when there is an escalation in the asymmetry of fluctuations near a tipping point. It has shown that, while autocorrelation consistently rises before a transition, the standard deviation may be underestimated as the fluctuations in an ecosystem increasingly lean towards low frequencies near a transition (Dakos, Van Nes et al. 2012, Alibakhshi, Groen et al. 2017).
In selecting the satellite data to perform the analyses, we strategically selected MODIS due to its advantages in terms of both temporal frequency and historical data availability. MODIS offers a longer archive of data along with high temporal resolution compared to other widely used satellite images such as Landsat and Sentinel, enabling us to capture dynamic changes in environmental conditions.
The findings of the sensitivity analysis (Fig. 6) illustrated the robustness of NDVI, MNDWI, and MVWR as early warning signal generators in mangrove forests. More specifically, the consistently high Kendall’s τ observed in NDVI, even under varying conditions of rolling window widths and Gaussian filtering bandwidths may suggest its stability and reliability in presenting the state of the ecosystem. This robustness may be attributed to NDVI's sensitivity to vegetation changes, making it less prone to fluctuations introduced by the applied methodologies.
Despite the significance of the results presented in this study, it is essential to acknowledge certain limitations. Firstly, the analysis solely relies on remote sensing data, which may have limitations in accurately capturing certain ecological processes and dynamics at a finer spatial scale. Additionally, the study focused on a specific region (Qeshm Island and Gabrik), limiting the generalizability of the results to other mangrove forest ecosystems. However, it should be noted that, due to the current political situation in some countries such as Iran, accessing field data is difficult, and thus the common understanding of ecosystem dynamics is strongly based on satellite data.
The results of this study have critical implications for biodiversity, ecosystem resilience, and socio-economic factors. Mangrove forest ecosystems are crucial for biodiversity conservation, harbouring a diverse range of plant and animal species and providing vital habitats and breeding grounds for numerous marine and avian organisms, contributing to the overall ecological resilience of coastal ecosystems (Nagelkerken, Van der Velde et al. 2000, Lugendo, Nagelkerken et al. 2007, Kathiresan 2012). Early warning signals offer opportunities for proactive conservation to maintain biodiversity and ecosystem stability. By providing a robust approach that can effectively evaluate the state of the mangrove forests, combined with a thorough conservation and management system, it is possible to effectively monitor and enhance the state of mangrove forests and safeguard the well-being of the communities dependent on these forests.
5. Conclusion
This study contributes to the state-of-the-art knowledge on the monitoring of mangroves and provides new information on the early warning signals of reduced resilience in mangrove forests. By utilizing remote sensing analysis and time series analysis, there is potential to enhance the global capacity for detecting regime shifts in mangrove ecosystems The utilization of remote sensing indices, particularly NDVI, emerges as a robust indicator of ecosystem dynamics. NDVI outperforms MVWR and MNDWI in generating early warning signals of critical transition. Furthermore, the study highlights significant differences between the mangrove forests of Qeshm Island and Gabrik, with Qeshm Island exhibiting more pronounced fluctuations and variability in ecosystem dynamics. The practical implications of this study involve informing policy frameworks and international initiatives for the conservation and sustainable management of mangrove forest ecosystems. To promote the conservation of mangrove forest ecosystems, several policy recommendations can be made. Strict regulations and land use planning should control urban expansion, particularly in sensitive coastal regions like Qeshm Island. Buffer zones and protected areas must be established. Sustainable agricultural practices and reduced use of harmful chemicals are essential. Raising public awareness through education programs is crucial, along with fostering collaboration between governmental organizations, research institutions, and local communities to develop integrated management plans. The findings highlight the need for region-specific conservation approaches and highlight the value and vulnerability of these ecosystems.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgments
This study is inspired by a previous study conducted by Sara Alibakhshi (Alibakhshi, Groen et al. 2017). All the analyses presented in this study have been carried out by Sara Alibakhshi, who is the lead author. However, it is important to note that the land-use and land cover change data utilized in this study were obtained from Kourosh Niya (Kourosh Niya, Huang et al. 2019). We extend our sincere gratitude to Kourosh Niya for generously providing the data and background knowledge about study sites. Additionally, we would like to express our appreciation to Prof. Jinliang Huang and Xiamen University for his support, assistance, and contributions to this study. This research was supported by the Finnish Research Impact Foundation.
References
- Abrantes, K.G.; Johnston, R.; Connolly, R.M.; Sheaves, M. Importance of Mangrove Carbon for Aquatic Food Webs in Wet–Dry Tropical Estuaries. Estuaries Coasts 2014, 38, 383–399. [Google Scholar] [CrossRef]
- Akbarzadeh-Chomachaei, G.; Koohkan, H.; Dehghani, R.; Mortazavi, M.; Gozari, M. Comparison of heavy metals pollution in coastal sediments of Bandar Abbas, Qeshm Island and Hormuz-Lark, Persian Gulf. Int. J. Environ. Sci. Technol. 2023, 20, 10861–10876. [Google Scholar] [CrossRef]
- Alatorre, L.C.; Sánchez-Carrillo, S.; Miramontes-Beltrán, S.; Medina, R.J.; Torres-Olave, M.E.; Bravo, L.C.; Wiebe, L.C.; Granados, A.; Adams, D.K.; Sánchez, E.; et al. Temporal changes of NDVI for qualitative environmental assessment of mangroves: Shrimp farming impact on the health decline of the arid mangroves in the Gulf of California (1990–2010). J. Arid. Environ. 2016, 125, 98–109. [Google Scholar] [CrossRef]
- Alibakhshi, S. Remotely sensed monitoring of land surface albedo and ecosystem dynamics. Department of Built Environment, Aalto University. 2020. [Google Scholar]
- Alibakhshi, S. A robust approach and analytical tool for identifying early warning signals of forest mortality. Ecol. Indic. 2023, 155. [Google Scholar] [CrossRef]
- Alibakhshi, S.; Groen, T.A.; Rautiainen, M.; Naimi, B. Remotely-Sensed Early Warning Signals of a Critical Transition in a Wetland Ecosystem. Remote. Sens. 2017, 9, 352. [Google Scholar] [CrossRef]
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal Ecosystem-Based Management with Nonlinear Ecological Functions and Values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Bevan, J.M.; Kendall, M.G. Rank Correlation Methods. The Statistician 1971, 20, 74–74. [Google Scholar] [CrossRef]
- Cabello, K.; Germentil, M.; Blanco, A.; Macatulad, E.; Salmo, S., III. Post-Disaster Assessment of Mangrove Forest Recovery in Lawaan-Balangiga, Eastern Samar Using Ndvi Time Series Analysis. ISPRS Annals of the Photogrammetry, Remote Sensing and Spatial Information Sciences 2021, 3, 243–250. [Google Scholar] [CrossRef]
- Capon, S.J.; Lynch, A.J.J.; Bond, N.; Chessman, B.C.; Davis, J.; Davidson, N.; Finlayson, M.; Gell, P.A.; Hohnberg, D.; Humphrey, C.; et al. Regime shifts, thresholds and multiple stable states in freshwater ecosystems; a critical appraisal of the evidence. Sci. Total. Environ. 2015, 534, 122–130. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Brock, W.A. Rising variance: a leading indicator of ecological transition. Ecol. Lett. 2006, 9, 311–318. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Brock, W.A. Early warnings of unknown nonlinear shifts: a nonparametric approach. Ecology 2011, 92, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Brock, W.A.; Cole, J.J.; Kitchell, J.F.; Pace, M.L. Leading indicators of trophic cascades. Ecol. Lett. 2007, 11, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Batt, R.; Brock, W.A.; Cline, T.; Coloso, J.; Hodgson, J.R.; Kitchell, J.F.; Seekell, D.A.; et al. Early Warnings of Regime Shifts: A Whole-Ecosystem Experiment. Science 2011, 332, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, C.; Ticehurst, C.; Merrin, L.; Thew, P. An Evaluation of MODIS Daily and 8-day Composite Products for Floodplain and Wetland Inundation Mapping. Wetlands 2013, 33, 823–835. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; Di Nitto, D.; Bosire, J.O.; Seen, D.L.; Koedam, N. How effective were mangroves as a defence against the recent tsunami? Current Biology 2005, 15, R443–R447. [Google Scholar] [CrossRef] [PubMed]
- Dakos, V.; Carpenter, S.R.; Brock, W.A.; Ellison, A.M.; Guttal, V.; Ives, A.R.; Kéfi, S.; Livina, V.; Seekell, D.A.; van Nes, E.H.; et al. Methods for Detecting Early Warnings of Critical Transitions in Time Series Illustrated Using Simulated Ecological Data. PLoS ONE 2012, 7, e41010. [Google Scholar] [CrossRef]
- Dakos, V.; Carpenter, S.R.; van Nes, E.H.; Scheffer, M. Resilience indicators: Prospects and limitations for early warnings of regime shifts. Philosophical Transactions of the Royal Society B: Biological Sciences 2015, 370, 1–10. [Google Scholar] [CrossRef]
- Dakos, V.; van Nes, E.H.; D'Odorico, P.; Scheffer, M. Robustness of variance and autocorrelation as indicators of critical slowing down. Ecology 2012, 93, 264–271. [Google Scholar] [CrossRef]
- Daru, B.H.; Yessoufou, K.; Mankga, L.T.; Davies, T.J. A Global Trend towards the Loss of Evolutionarily Unique Species in Mangrove Ecosystems. PLoS ONE 2013, 8, e66686. [Google Scholar] [CrossRef]
- Duke, N. C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef]
- Ebrahimi-Sirizi, Z.; Riyahi-Bakhtiyari, A. Petroleum pollution in mangrove forests sediments from Qeshm Island and Khamir Port—Persian Gulf, Iran. Environ. Monit. Assess. 2012, 185, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M. Managing mangroves with benthic biodiversity in mind: Moving beyond roving banditry. J. Sea Res. 2008, 59, 2–15. [Google Scholar] [CrossRef]
- Etemadi, H.; Smoak, J.M.; Sanders, C.J. Forest migration and carbon sources to Iranian mangrove soils. J. Arid. Environ. 2018, 157, 57–65. [Google Scholar] [CrossRef]
- Farshid, Z.; Balef, R.M.; Zendehboudi, T.; Dehghan, N.; Mohajer, F.; Kalbi, S.; Hashemi, A.; Afshar, A.; Bafghi, T.H.; Baneshi, H.; et al. Reforestation of grey mangroves (Avicennia marina) along the northern coasts of the Persian Gulf. Wetl. Ecol. Manag. 2022, 31, 115–128. [Google Scholar] [CrossRef]
- Ghanbarzad Dashti, S.; Farzingohar, M.; Souri, A. Temperature and Salinity Effects in Sensitive Area of Qeshm Island: Mangrove Forests. International Journal of Coastal, Offshore And Environmental Engineering 2021, 6, 13–18. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Global mangrove forests distribution, 2000. NASA Socioeconomic Data and Applications Center (SEDAC), Palisades. 2013. doi 10: H4J67DW68.
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Goudarzi, A.; Moslehi, M. Distribution of a devastating fungal pathogen in mangrove forests of southern Iran. Crop. Prot. 2019, 128, 104987. [Google Scholar] [CrossRef]
- Guttal, V.; Jayaprakash, C. Changing skewness: an early warning signal of regime shifts in ecosystems. Ecol. Lett. 2008, 11, 450–460. [Google Scholar] [CrossRef]
- Hamzeh, M.A.; Koochaknejad, E.; Hamzei, S. Historical eutrophication and pollution records off Bandar Abbas coast (North of Strait of Hormuz) using benthic foraminiferal ecology and geochemistry of trace elements from a sediment core. Reg. Stud. Mar. Sci. 2021, 47, 101929. [Google Scholar] [CrossRef]
- Hirota, M.; Holmgren, M.; Van Nes, E.H.; Scheffer, M. Global Resilience of Tropical Forest and Savanna to Critical Transitions. Science 2011, 334, 232–235. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Wylie, B. Analysis of Dynamic Thresholds for the Normalized Difference Water Index. Photogramm. Eng. Remote Sens. 2009, 75, 1307–1317. [Google Scholar] [CrossRef]
- Kathiresan, K. Importance of mangrove ecosystem. International Journal of Marine Science 2012, 2. [Google Scholar] [CrossRef]
- Kéfi, S.; Guttal, V.; Brock, W.A.; Carpenter, S.R.; Ellison, A.M.; Livina, V.N.; Seekell, D.A.; Scheffer, M.; van Nes, E.H.; Dakos, V. Early Warning Signals of Ecological Transitions: Methods for Spatial Patterns. PLOS ONE 2014, 9, e92097. [Google Scholar] [CrossRef] [PubMed]
- Kirui, K.; Kairo, J.; Bosire, J.; Viergever, K.; Rudra, S.; Huxham, M.; Briers, R. Mapping of mangrove forest land cover change along the Kenya coastline using Landsat imagery. Ocean Coast. Manag. 2013, 83, 19–24. [Google Scholar] [CrossRef]
- Niya, A.K.; Huang, J.; Karimi, H.; Keshtkar, H.; Naimi, B. Use of Intensity Analysis to Characterize Land Use/Cover Change in the Biggest Island of Persian Gulf, Qeshm Island, Iran. Sustainability 2019, 11, 4396. [Google Scholar] [CrossRef]
- Niya, A.K.; Huang, J.; Kazemzadeh-Zow, A.; Karimi, H.; Keshtkar, H.; Naimi, B. Comparison of three hybrid models to simulate land use changes: a case study in Qeshm Island, Iran. Environ. Monit. Assess. 2020, 192, 1–19. [Google Scholar] [CrossRef]
- Lenton, T.M. Early warning of climate tipping points. Nat. Clim. Chang. 2011, 1, 201–209. [Google Scholar] [CrossRef]
- Lenton, T. M.; Held, H.; Kriegler, E.; Hall, J.W.; Lucht, W.; Rahmstorf, S.; Schellnhuber, H.J. Tipping elements in the Earth's climate system. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Lenton, T.M.; Livina, V.N.; Dakos, V.; van Nes, E.H.; Scheffer, M. Early warning of climate tipping points from critical slowing down: comparing methods to improve robustness. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2012, 370, 1185–1204. [Google Scholar] [CrossRef]
- Li, H.; Jia, M.; Zhang, R.; Ren, Y.; Wen, X. Incorporating the Plant Phenological Trajectory into Mangrove Species Mapping with Dense Time Series Sentinel-2 Imagery and the Google Earth Engine Platform. Remote. Sens. 2019, 11, 2479. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Cai, D.; Li, R. Determination of an appropriate ecological hydrograph for a rare fish species using an improved fish habitat suitability model introducing landscape ecology index. Ecol. Model. 2015, 311, 31–38. [Google Scholar] [CrossRef]
- Lugendo, B. R.; Nagelkerken, I.; Kruitwagen, G.; Van Der Velde, G.; Mgaya, Y.D. Relative importance of mangroves as feeding habitats for fishes: a comparison between mangrove habitats with different settings. Bulletin of Marine Science 2007, 80, 497–512. [Google Scholar]
- Luther, D.A.; Greenberg, R. Mangroves: A Global Perspective on the Evolution and Conservation of Their Terrestrial Vertebrates. BioScience 2009, 59, 602–612. [Google Scholar] [CrossRef]
- Mafi-Gholami, D.; Zenner, E.K.; Jaafari, A.; Bui, D.T. Spatially explicit predictions of changes in the extent of mangroves of Iran at the end of the 21st century. Estuarine, Coast. Shelf Sci. 2020, 237, 106644. [Google Scholar] [CrossRef]
- Mahmoudi, B.; Mafi-Gholami, D.; Ng, E. Evaluation of mangrove rehabilitation and afforestation in the southern coasts of Iran. Estuarine, Coast. Shelf Sci. 2022, 277. [Google Scholar] [CrossRef]
- Martínez, M. L.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecological economics 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Mazraeh, H.M.; Pazhouhanfar, M. Effects of vernacular architecture structure on urban sustainability case study: Qeshm Island, Iran. Front. Arch. Res. 2018, 7, 11–24. [Google Scholar] [CrossRef]
- Esmaeili, F.M.; Mortazavi, M.S.; Banadaki, A.D.; Saraji, F.; Nozar, S.L.M. Algal blooms historical outbreaks in the northern coastal waters of the Persian Gulf and Oman Sea (1980–2015). Environ. Monit. Assess. 2021, 193, 1–12. [Google Scholar] [CrossRef]
- Moslehi, M.; Bernier, L.; Zakeri, O.; Ahmadi, A. First report of Streblote solitaria (Lepidoptera: Lasiocampidae) damage on Avicennia marina trees in southern mangroves of Iran. Acta Ecol. Sin. 2023. [Google Scholar] [CrossRef]
- Mozumder, C.; Tripathi, N.K.; Tipdecho, T. Ecosystem evaluation (1989–2012) of Ramsar wetland Deepor Beel using satellite-derived indices. Environ. Monit. Assess. 2014, 186, 7909–7927. [Google Scholar] [CrossRef]
- Muñoz Sabater, J. ERA5-land monthly averaged data from 1981 to present, Copernicus Climate Change Service (C3S) Climate Data Store (CDS). Earth Syst. Sci. Data 2019, 55, 5679–5695. [Google Scholar]
- Naderloo, R.; Türkay, M.; Sari, A. Intertidal habitats and decapod (Crustacea) diversity of Qeshm Island, a biodiversity hotspot within the Persian Gulf. Marine biodiversity 2013, 43, 445–462. [Google Scholar] [CrossRef]
- Nagelkerken, I.; van der Velde, G.; Gorissen, M.; Meijer, G.; Hof, T.V.; Hartog, C.D. Importance of Mangroves, Seagrass Beds and the Shallow Coral Reef as a Nursery for Important Coral Reef Fishes, Using a Visual Census Technique. Estuarine, Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. The R Development Core Team, 2013. NLME: linear and nonlinear mixed effects models: 2000, 1-3.
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Qgis, S. QGIS Geographic Information System v.2.16.3. Open Source Geospatial Foundation. 2016. URL: http://qgis.osgeo.org.
- Rada, A.; Nikitina, O.; Syrova, M. Estimation of the level of eutrophication of coastal waters of the Baltic Sea on the basis of Earth remote sensing data. E3S Web Conf. 2023, 411, 02001. [Google Scholar] [CrossRef]
- Rokni, K.; Ahmad, A.; Selamat, A.; Hazini, S. Water Feature Extraction and Change Detection Using Multitemporal Landsat Imagery. Remote Sens. 2014, 6, 4173–4189. [Google Scholar] [CrossRef]
- Ruan, L.; Yan, M.; Zhang, L.; Fan, X.; Yang, H. Spatial-temporal NDVI pattern of global mangroves: A growing trend during 2000–2018. Sci. Total. Environ. 2022, 844, 157075. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M. Multiplicity of stable states in freshwater systems. Hydrobiologia 1990, 200-201, 475–486. [Google Scholar] [CrossRef]
- Scheffer, M. Alternative Attractors of Shallow Lakes. Sci. World J. 2001, 1, 254–263. [Google Scholar] [CrossRef]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Sorichetta, A.; Hornby, G.M.; Stevens, F.R.; Gaughan, A.E.; Linard, C.; Tatem, A.J. High-resolution gridded population datasets for Latin America and the Caribbean in 2010, 2015, and 2020. Sci. Data 2015, 2, 150045. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, N.A.; Janalipour, M. Predicting ecosystem shift in a Salt Lake by using remote sensing indicators and spatial statistics methods (case study: Lake Urmia basin). Environ. Eng. Res. 2020, 26, 200225. [Google Scholar] [CrossRef]
- Tran, T.V.; Reef, R.; Zhu, X. A Review of Spectral Indices for Mangrove Remote Sensing. Remote. Sens. 2022, 14, 4868. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting trend and seasonal changes in satellite image time series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Zeileis, A.; Culvenor, D. Phenological change detection while accounting for abrupt and gradual trends in satellite image time series. Remote Sens. Environ. 2010, 114, 2970–2980. [Google Scholar] [CrossRef]
- Verbesselt, J.; Umlauf, N.; Hirota, M.; Holmgren, M.; Van Nes, E.H.; Herold, M.; Zeileis, A.; Scheffer, M. Remotely sensed resilience of tropical forests. Nat. Clim. Chang. 2016, 6, 1028–1031. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Cao, Y.; Wang, R.; Kattel, G.; He, D.; You, W. Pattern changes and early risk warning of Spartina alterniflora invasion: a study of mangrove-dominated wetlands in northeastern Fujian, China. J. For. Res. 2023, 34, 1447–1462. [Google Scholar] [CrossRef]
- Yessoufou, K.; Stoffberg, G. Biogeography, threats and phylogenetic structure of mangrove forest globally and in South Africa: A review. South Afr. J. Bot. 2016, 107, 114–120. [Google Scholar] [CrossRef]
- Zahed, M.A.; Rouhani, F.; Mohajeri, S.; Bateni, F.; Mohajeri, L. An overview of Iranian mangrove ecosystems, northern part of the Persian Gulf and Oman Sea. Acta Ecol. Sin. 2010, 30, 240–244. [Google Scholar] [CrossRef]
- Zarezadeh, R.; Rezaee, P. Study on accumulation of heavy metals in Mangrove sediments, Gabrik Creek (Jask). Journal of Natural environment 2016, 69, 61–78. [Google Scholar]
Figure 1.
Location of the study sites Qeshm Island and Gabrik.
Figure 1.
Location of the study sites Qeshm Island and Gabrik.
Figure 2.
Monthly temperature in Kelvin (°K) and precipitation millimetres (mm) for Qeshm Island and Gabrik from February 18, 2000, to July 31, 2021.
Figure 2.
Monthly temperature in Kelvin (°K) and precipitation millimetres (mm) for Qeshm Island and Gabrik from February 18, 2000, to July 31, 2021.
Figure 3.
Land-use maps of Qeshm Island (A) and Gabrik (B) were extracted from Landsat images from 1996 to 2014.
Figure 3.
Land-use maps of Qeshm Island (A) and Gabrik (B) were extracted from Landsat images from 1996 to 2014.
Figure 4.
The time series of three remotely sensed indices. The first column represents the time series of the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, at 500-m spatial resolution in Qeshm Island (A) and Gabrik (B). The red line illustrates the trend obtained using a moving average with a window size of 20-time steps.
Figure 4.
The time series of three remotely sensed indices. The first column represents the time series of the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, at 500-m spatial resolution in Qeshm Island (A) and Gabrik (B). The red line illustrates the trend obtained using a moving average with a window size of 20-time steps.
Figure 5.
Early warning signals analysis using time series of remotely sensed indices. The first column represents the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, at 500-m spatial resolution in Qeshm Island (A), and Gabrik (B). The red line illustrates the rolling window size of 50 percent which was used in the analysis. The acf(1) refers to autocorrelation at lag one, SD refers to standard deviation. Please note that the Y-axes have different ranges of values.
Figure 5.
Early warning signals analysis using time series of remotely sensed indices. The first column represents the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, at 500-m spatial resolution in Qeshm Island (A), and Gabrik (B). The red line illustrates the rolling window size of 50 percent which was used in the analysis. The acf(1) refers to autocorrelation at lag one, SD refers to standard deviation. Please note that the Y-axes have different ranges of values.
Figure 6.
Sensitivity analysis for rolling window metrics for time series of remotely sensed indices. The first column represents the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, in Qeshm Island (Panel A), and Gabrik (Panel B). In each panel, the first row refers to autocorrelation (acf(1)), the first row refers to standard deviation, and the third row refers to skewness.
Figure 6.
Sensitivity analysis for rolling window metrics for time series of remotely sensed indices. The first column represents the Normalized Difference Vegetation Index (NDVI), the second column represents the Modified Normalized Water Index (MNDWI), and the third column represents the Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021, in Qeshm Island (Panel A), and Gabrik (Panel B). In each panel, the first row refers to autocorrelation (acf(1)), the first row refers to standard deviation, and the third row refers to skewness.
Table 1.
Land-use and land-cover change in Qeshm Island and Gabrik between 1996 and 2014, obtained from Landsat data at 30-m spatial resolution.
Table 1.
Land-use and land-cover change in Qeshm Island and Gabrik between 1996 and 2014, obtained from Landsat data at 30-m spatial resolution.
| Study sites |
Class |
1996 (number of pixels) |
2014 (number of pixels) |
Change (number of pixels) |
Percentage (%) |
| Qeshm |
Agriculture |
51615 |
51953 |
338 |
0.65% |
| Bare-land |
1571765 |
1514405 |
-57360 |
-3.65% |
| Built-up |
55525 |
91284 |
35759 |
64.40% |
| Mangrove |
66672 |
73552 |
6049 |
9.07% |
| Vegetation |
9787 |
15836 |
6880 |
70.30% |
| Water-body |
1012778 |
1021114 |
8336 |
0.82% |
| Gabrik |
Agriculture |
30929 |
35637 |
4708 |
15.22% |
| |
Bare-land |
1674507 |
1665878 |
-8629 |
-0.52% |
| |
Built-up |
5317 |
5532 |
215 |
4.04% |
| |
Mangrove |
8342 |
9132 |
790 |
9.47% |
| |
Vegetation |
81579 |
84207 |
2628 |
3.22% |
| |
Water-body |
657401 |
657689 |
288 |
0.04% |
Table 2.
Kendall’s τ of autocorrelation, standard deviation, and skewness trend change in Qeshm Island and Gabrik (significant at P < 0.1) of Normalized Difference Vegetation Index (NDVI), Modified Normalized Water Index (MNDWI), and Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021.
Table 2.
Kendall’s τ of autocorrelation, standard deviation, and skewness trend change in Qeshm Island and Gabrik (significant at P < 0.1) of Normalized Difference Vegetation Index (NDVI), Modified Normalized Water Index (MNDWI), and Modified Vegetation Water Ratio (MVWR) from February 18, 2000, to July 31, 2021.
| Statistical Measures |
Qeshm |
Gabrik |
| NDVI |
MNDWI |
MVWR |
NDVI |
MNDWI |
MVWR |
| Autocorrelation |
0.505 |
0.864 |
-0.100 |
-0.192 |
0.789 |
-0.722 |
| Standard Deviation |
0.891 |
0.903 |
-0.074 |
-0.186 |
0.891 |
-0.118 |
| Skewness |
0.288 |
-0.584 |
-0.212 |
0.431 |
-0.576 |
-0.335 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).