Introduction: Genetic Content of the Cell
Cells are made up of water, that accounts for 70% of the total mass of the cell. They also contain inorganic ions and organic ions that contain ions. Cells are the fundamental elements of life, this is because they are given in discrete and well recognized grouping. This ease of recognition is due to the fact that all cells have structures that are surrounded by a cell membrane (also known as the plasma membrane). This membrane contain molecules referred to as phospholipids, which prevent hydrophilic substances from entering or escaping the cell. The membrane also contains proteins, that determine what substances are fit or not fit to enter the cell, fasten cells to each other, such that the function as one, serve as signal transmitters from the cell to the environment, back to the cell, and identify the cell as part of the same organism or different. Carbohydrates are found in lesser quantities and they are seen either attached to the proteins or the lipid. We can go on and on about the parts of a cell, but what we are concerned with as regards the cell is the genetic blueprint of the cell, that which makes the cell what it is. And this is to be found in the cytoplasm and nucleus. What is being said here is that the cytoplasm of prokaryotes, which includes bacteria, contain DNA. However in eukaryotes (plants and animals), DNA is found in the nucleus of the cell. But DNA is also found in the other sites away from the nucleus, which includes the mitochondria (mtDNA) - which synthesizes energy (ATP), useful for the cell, and the chloroplasts (cpDNA) for plants. The genetic materials found in the DNA plays a basic role in determining the structure and nature of cell substances and they are capable of propagation and variation.
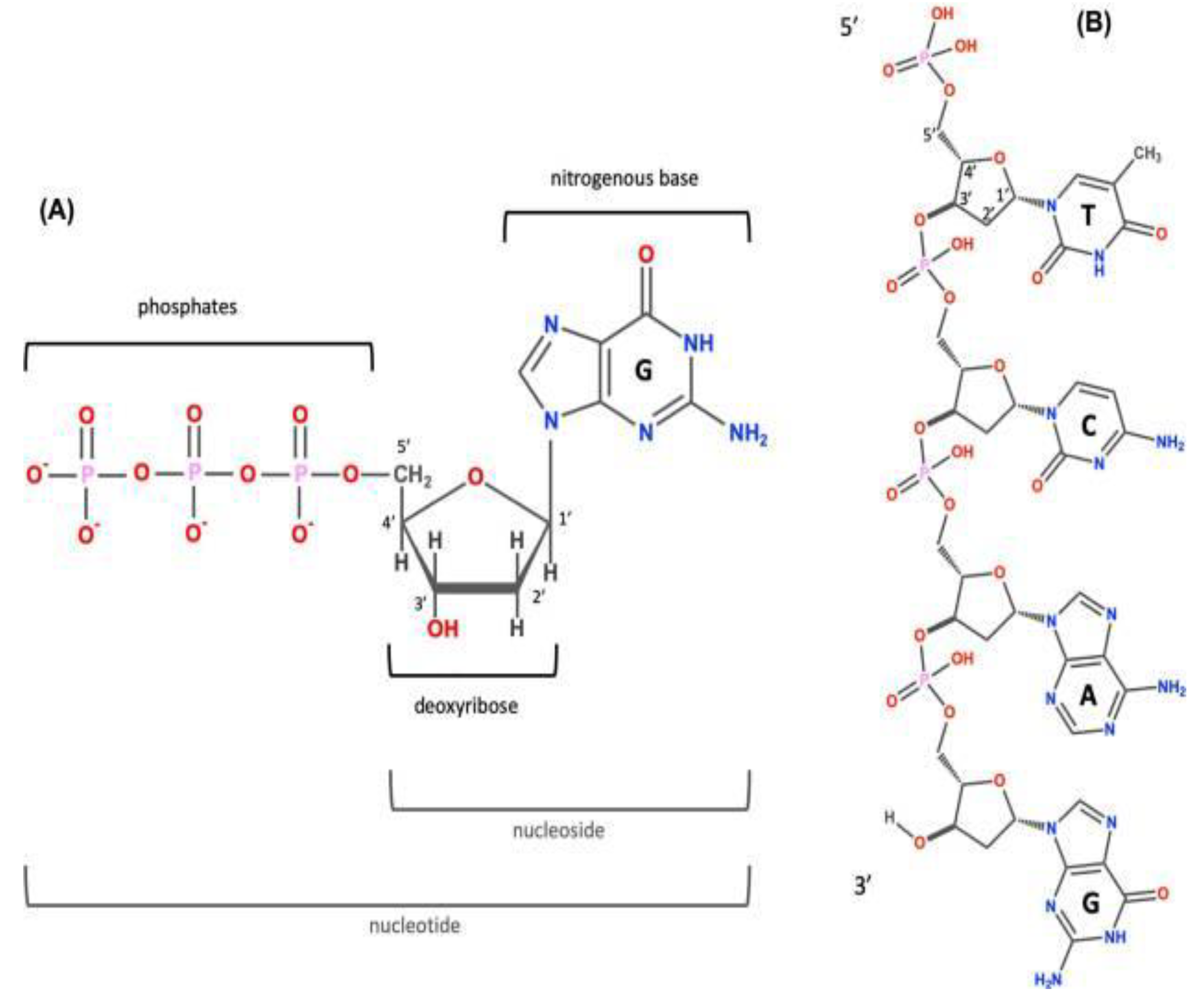
DNA (deoxyribonucleic acid) is the hereditary/genetic material in animals (but our focus at this initial point here is the human person). Nearly all the cells in the body of an individual has the same DNA. That is to say that the for those cells that have a nucleus, in the human body, the DNA code is the same. DNA information is stored in codes, that are comprised of four nitrogenous (chemical) bases which are, adenine (A), guanine (G), cytosine (C), and thymine (T). These chemical bases pair up with each other, A with T and C with G, to form units or hydrogen bonds called base pairs (1). Every base has its own phosphate and sugar molecule attached to it. Thus giving it the name nucleotide. Nucleotides are arranged in two long strands that form a spiral called a double helix structure. This structure, along with the chemical stability molecules, makes DNA the ideal genetic material, for the copying and transmission of the genetic code to the next generation (2). Nucleotides are units whose macromolecules are referred to as nucleic acids. Nucleic acids come in two varieties: DNA and RNA (ribonucleic acid). Thus, it is evident that DNA and RNA are polymers of nucleotides.
The human DNA has four bases as already noted, but these can be segmented into two bases namely, the double-ring purine bases: adenine and guanine; and the single-ring pyrimidine bases: cytosine and thymine. The carbon in the deoxyribose ring are numbers called 1′ to 5′. The phosphate group is acidic, and this is the reason why it is called a nucleic acid. After the synthesis, one phosphate group remains, it is this that is joined to 5′, as seen in
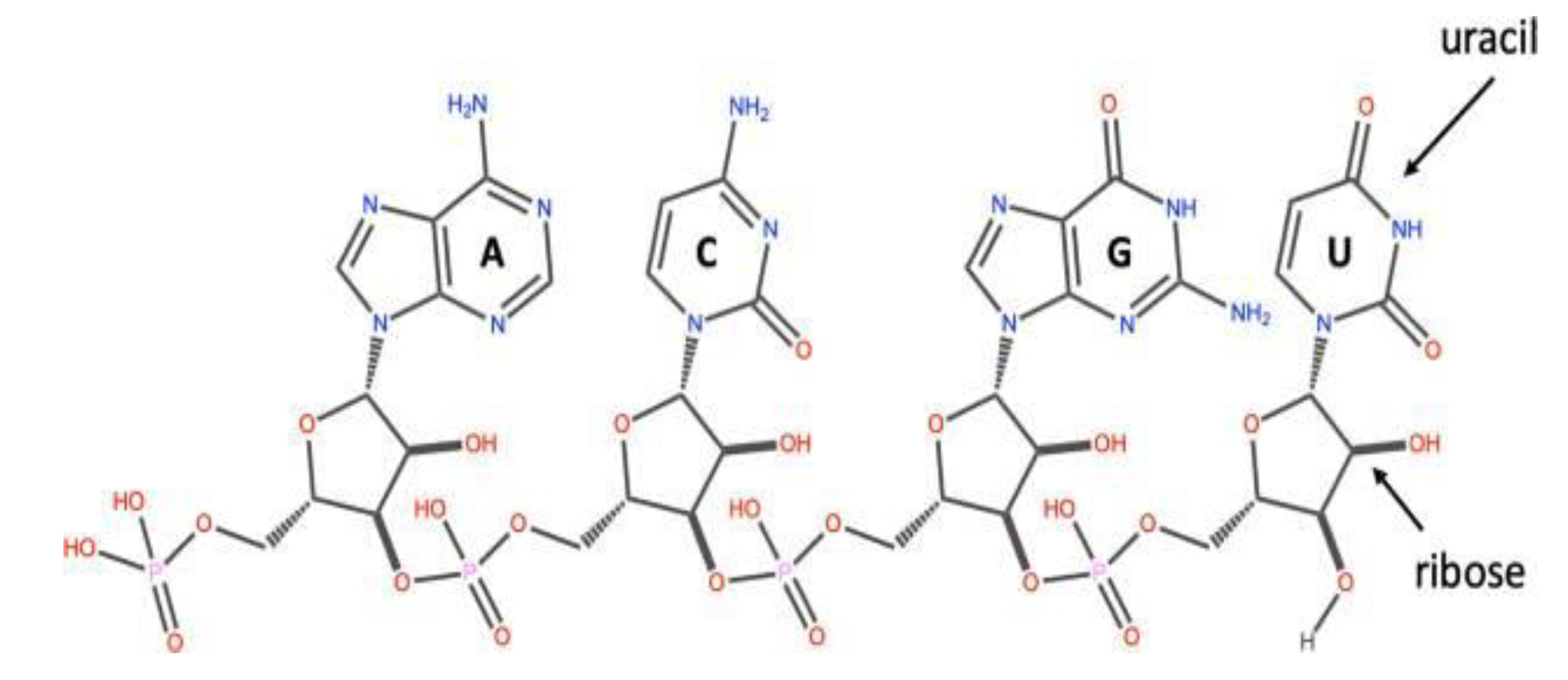
Figure 1. The RNA molecules in the cell are similar to the DNA. They also have four chemical bases: guanine, cytosine, adenine and uracil. They are easily broken down than the DNA and they (although not all) mostly do not form stable secondary compounds and the third tier carbon of the ribose is linked to the fifth tier of another by means of a phosphodiester bond, as seen in
Figure 2 (2).
What should be noted here is that every thing that is needed for biological life to thrive is contained in the nucleic acids of the cell. it is necessary to recall that the cell is bathed in water, 70%. This will also indicate that these nucleic acids are bathed in water. Water is therefore fundamental to continuity to life. However, can it be said that water is the foundation to the origin story?
Water as the Foundation?
The early Greek philosophers, the Milesians, pondered on the fundamental origin of all things. And of the them all, it was Thales of Miletus (ca 624-527BC) that noted water to be the origin and source of all that there is. There is no recorded evidence of Thales ever putting down in writing his thoughts, but we know from philosophers like Aristotle that he, Thales, endorsed water as the foundation of all that there is. Aristotle reported saying: “‘Thales says that it [the nature of things] is water’” (3). That the nature of all things is water indicates that water, according to Thales, was the primary principle from which all other things get their origin. According to the hypothesis of Thales, it could be deciphered that he reason that water had the propensity to becoming a lot of things, and that it takes on different states, that defined nature was also what necessitated his reason for choosing water. Plato in his Timaeus 49B-C, makes known a process that is rotational in nature, one that involves water taking on differing states as seen in evaporation and its attendant consequences. It can be deduced that he had the thinking of Thales in mind. Aristotle noted that Thales had arrived at this based on observation. According to him, “Thales may have observed, ‘that the nurture of all creatures is moist, and that warmth itself is generated from moisture and lives by it; and that from which all things come to be is their first principle’” (4). Then, in the lines 983 b26-27, Aristotle becomes more definite as he says that, “Besides this, another reason for the supposition would be that the semina of all things have a moist nature...” (5)
In ancient China, G. Shuidi (ca 475-221BC) considered water to the be the source of all that there is, based on his observation of blood. He noted that since blood and vessels are what ensures that food is transported to every part of the body, then this stands as the foundation of all life. That is to say every life is immersed in a kind of blood and vessel composition, allowing for the origin and continuity of life (6) (Xie, H.; Zhu, Y. Translation of Guan zi; People’s Publishing House: Guizhou, China, 1996, 1080). Y. Quan (ca 280), developed his own theory the moist sandy part of the water eventually becomes ground after desiccation, and the evaporated water becomes CHI, and CHI is the fundamental elements that comprise our world (7). In India, the “Rig Veda” (ca 1500 BC–900 BC) notes in that everything is chaos, everything is water (8).
One might allude that the above were merely conjectures arrived at by simple minded observation from these philosophers. Yet it would be wise not to dismiss this with a wave of the hand. This is because science is beginning to realize that the best answers to questions are those that are simple and fundamental. Water bears chemical properties that enables it foster life in all ramifications - as a solvent, a conductor of electricity, an integral element in metabolism; it also plays host to other acts such as catalyst, reactant, product, messenger, controller, chaperone etc. Whatever pertains to life, depends on the dissolvable qualities of all that there is. What is being alluded here is captured by E. Brini et al. (9), who note that
Life depends on the solubilities of gases in water. Humanity depends on sea life for food, and they require conditions under which oxygen (O2) has sufficient solubility in water. Marine plants require carbon dioxide (CO2), which must be dissolved in water, in order for photosynthesis to produce carbohydrates, which releases oxygen. Gas solubilities in water depend on temperature, pressure, and salinity.
On a lesser yet not unimportant note, we can assert that electricity itself dissolves in water which makes it easy for current to pass through water effortlessly and for it to become better enhanced. This comment will be better understood as the work progresses, particularly when we deal with the analysis of water.
A further Analysis on Water
As a solvent, Water provides protons H+ and hydroxyls OH- for varied reactions. It is also a necessary tool for hydrolysis. When we speak of water as the major element in hydrolysis, we seem to hit a wall as regards the origin of life. This is because we speak of a progression as regards life in its origin, that is to say a build-up, an increase, evolution wise. Hydrolysis speaks of a break down, a decrease from major to minor, a backward step. Nonetheless, this is an elemental function of water, which is the dissociation of bigger molecules into smaller ones, when water is added. Hydrolysis is even involved in metabolism, as it helps in breaking down proteins, fats and complex carbohydrates in food.
The general formula for a hydrolysis reaction is
We can further compute it in nature as
It is the reverse of a condensation reaction and water is consumed in the process, as it breaks the larger molecule into smaller ones, that comprised the former. The protons and hydoxyl ions do catalyse hydrolysis reactions, making them highly pH-dependent processes (10). Water molecules “fastly” cleave ester and amide bonds thereby hydrolyzing nucleic acids and proteins. In “hydrolysis OH- replaces another moiety in the molecule (e.g., phosphate, amino or thiol group) by nucleophilic substitution” (11). We have already noted that water is also a very integral aspect of metabolism. For instance, the metabolism of the E. coli, witnesses to this hydrolytic action of water. In such, the most common reactant is H+, followed by water, which participates as a substrate or product in over 500 reactions (12).
The essentially and “rudimentarily” speak of the characteristics, chemical and physical, of water, we also need to note that water itself is composed of two atoms of hydrogen and one atom of oxygen. Structurally speaking, the Hydrogen and Oxygen molecules makes water to be a very important asset as regards chemical reactions.
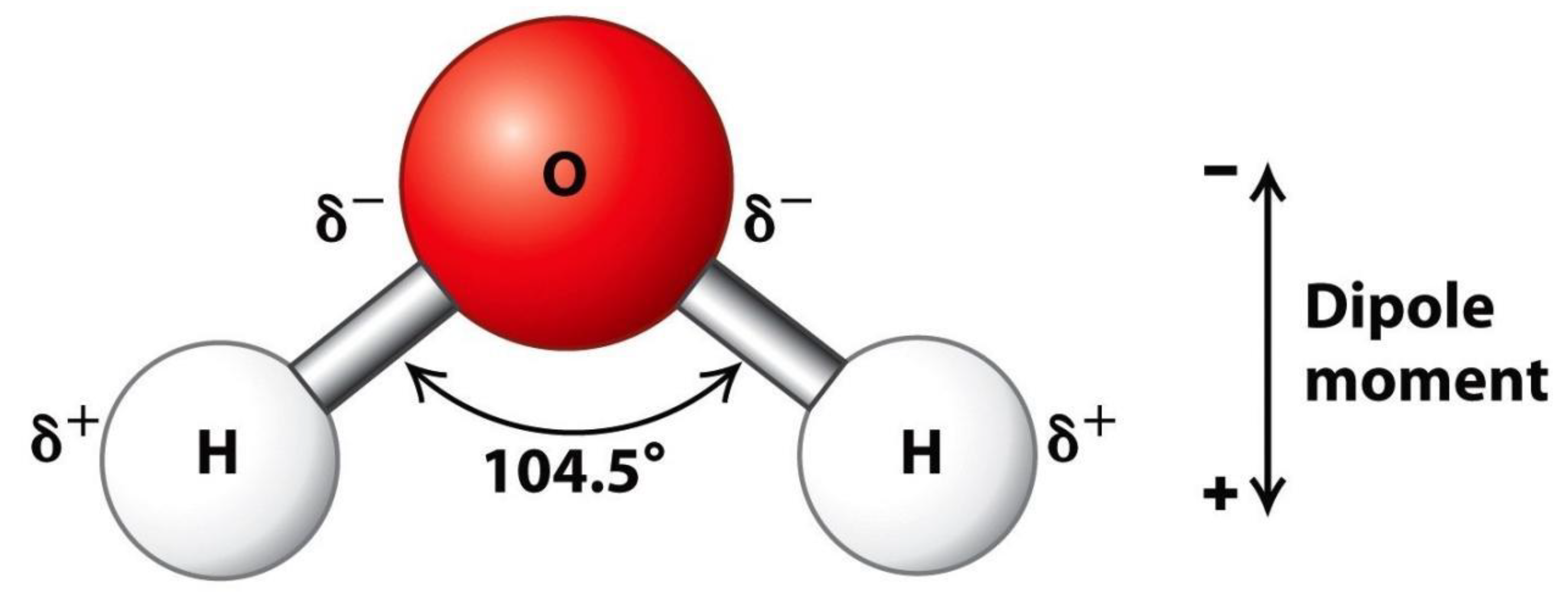
Structural Formula Of Water: We have already noted that a water molecule has two hydrogen and one oxygen atom. The atoms of hydrogen are bonded to that of the oxygen in a strange fashion, thus making the structure of the water molecule bent. this is because Water has four electron pairs and the
coordination geometry of oxygen is based upon a tetrahedral arrangement of electron pairs. Since there are only two bonded groups, there are two lone pairs. Since the lone pairs are not 'seen', the
shape of water is bent. The two lone pairs compress the H-O-H bond angle below the ideal tetrahedral angle to 104.5° (as visible in
Figure 3).
It should be noted that all of the electron pairs—shared and unshared—repel each other. Due to the fact that oxygen is more electronegative than hydrogen, the oxygen side of the molecule drags the electrons, keeping them away from the hydrogen side. This gives the oxygen end of the water molecule a partial negative charge, while the hydrogen end has a partial positive charge. This is the reason Water is classified as a polar molecule because of its polar covalent bonds and its bent shape. Polar molecules have the propensity of attracting each other by means of the law of attraction in dipole to dipole forces. This ensures that the positive end of one water molecule attracts the negative end of another molecule. This also works in the case of every polar substance. Therefore in the water element, there are a lot of hydrogen bonds that is formed as a result of the high electronegativity of the oxygen. As a dipole to dipole bond, hydrogen bonds are very strong. It was earlier asserted that electricity dissolves in water, and it is apparently such that the positively charged ions in the current is drawn to the negative side of the ions in the water molecules and the negatively charged ions are drawn to the positive side of the water ionic charge. This enables the current to easily wade through water (we ought to note that pure water is not a good conductor, but water is hardly ever in its 100% pure state, this enhances its electric conductivity).
Prying Into The Fundamentals
The work so far has bothered on the fundamental elements of living materials. We started off from the cell, but realizing that even in the cell, the nucleus and its attendant nucleic acids are those that bear the distinguishing marks of the cell, we had to dig deeper. It brought us to the DNA (RNA) fundamental composition. We also went a little further, recollecting that DNA (RNA) themselves are bathed in water, which takes up a huge space in the cell. To this, water in its fundamentals needed to be analyzed. However, the mission is about the origin of life. Therefore how does this all come to bear?
Before we delve further into this, there is a need for us to assert here that nucleic acids are polar molecules, this is because the sugar-phosphate backbone of the DNA is hydrophilic, but the interior of the DNA is hydrophobic. Therefore making the DNA molecule polar.
Fundamentally speaking, all living organisms in the world are built from six elements namely: Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus and Sulfur (CHNOPS). According to G. Mandala and M. Ubbu (13), recounting the thoughts provided for by M. Pasek:
...Carbon easily enters bonds with other atoms in carbon. This means that it creates large chains that act as fine bones for other atoms to which they are bound,...carbon atoms are essential elements for building large molecules... nitrogen, hydrogen and oxygen...show the effects of acid-base, which allows them to combine with carbon to form amino acids, fats, lipids and nucleobases in which DNA and RNA are formed. Sulfur provides electron shuffles,...with their high levels of electrons, sulfides and sulfates help stimulate reactions. Some organisms use selenium instead of sulfur in their enzymes, but not many. Phosphorus, which is commonly found in the phosphate molecule, is essential for the body's metabolism, since polyphosphate molecules such as ATP (adenosinetriphosphate) are able to store large amounts of energy in their chemical bonds. Breaking the bond releases its power; do this often, say, a group of muscle cells, and you can move your arm.
Once these DNA is formed by these fundamentals (as I refer to these elements), then life builds from there and is replicated. However we arrive at a burning question, which is: from whence do these ingredients of life arise?
Let us trace it back to T > 0. I am firmly in support of the latter because whatever happened at T = 0 and T < 0 (if there is anything like this) is not privy to any mind or intelligence. After the Big Bang at T > 0, there was a release of mass (energy) at very high temperature. The major conjecture here is that two primordial gases were born, first hydrogen, which is the simplest of all elements with one proton, no neutron and one electron (even though there are isotopes of hydrogen called deuterium - one proton and one neutron; and tritium - one proton and two neutrons). It was the fusion of hydrogen atoms with each other at high temperatures that yielded helium (and this is what we also see in Stars - the fusion of hydrogen to become helium at high temperatures and an abundant release of energy). According to the report given by NASA (14),
Small stars fuse hydrogen into helium, and then fuse helium into carbon and nitrogen. Carbon is a basic building block of life and nitrogen is a part of all proteins – essential to life
Large stars make heavy elements as well as light elements through the process of fusion in their cores. For example, large stars create the calcium in your bones and the oxygen you breathe, the silicon in the soil, and the sulfur that’s in your hair.
The explosive power of supernovae creates and disperses a wide range of elements. The gold used in jewelry and the titanium used in light-weight eyeglass frames were formed in supernovae. Supernovae also provide the iron in your blood.
The nuclei of the elements formed in the big bang, stars, and supernovae rain down on us from space in the form of cosmic rays. Lithium, used in watch batteries, comes partly from cosmic rays.
This fusion that is taking place is nuclear fusion. This is a process in which two light atoms combine together at high temperatures becoming a single heavier atom and the process gives off as residue high amounts of energy. It is noted that this takes place at high temperatures, at levels when mass is in its plasma stage. With all that has been gathered so far, we note that
...all living organisms store and transmit hereditary information using two kinds of molecules: DNA and RNA. Each of these molecules is in turn composed of four kinds of subunits known as nucleotides. The sequences of nucleotides in particular lengths of DNA or RNA, known as genes, direct the construction of molecules known as proteins, which in turn catalyze biochemical reactions, provide structural components for organisms, and perform many of the other functions on which life depends. Proteins consist of chains of subunits known as amino acids. The sequence of nucleotides in DNA and RNA therefore determines the sequence of amino acids in proteins; this is a central mechanism in all of biology. Experiments conducted under conditions intended to resemble those present on primitive Earth have resulted in the production of some of the chemical components of proteins, DNA, and RNA. Some of these molecules also have been detected in meteorites from outer space and in interstellar space by astronomers using radio-telescopes. Scientists have concluded that the "building blocks of life" could have been available early in Earth's history. An important new research avenue has opened with the discovery that certain molecules made of RNA, called ribozymes, can act as catalysts in modem cells. It previously had been thought that only proteins could serve as the catalysts required to carry out specific biochemical functions. Thus, in the early prebiotic world, RNA molecules could have been "autocatalytic"—that is, they could have replicated themselves well before there were any protein catalysts (called enzymes) (15).
Did Life really Begin from the Stars?
It is already projected as one of the solution to the origin of life conundrum, that is to say that earthly life came from the stars. However favorable this is with respect to the above anaylses already offered, I would not fully subscribe to life originating from the stars. This is because what came from the stars or from the universe is not what we presently have now as biological life. Interactions have taken place over time, this is what we have always known as evolution.
Darwin spoke of “descent with modification”, what we now come to refer to as evolution. however in his own elaboration of how this works, he highlighted what he referred to as Natural Selection, in which individuals in a population inherits traits that makes them survive and extend their species regardless of the harshness of the environment (scarcity of resources, predators and the likes). Individuals with better traits will be better suited for adaptation, survival and transmission. Such Darwinian thought is today acceptable for understanding how individual species have become modified over the years. Nonetheless, I propose Biological Interaction (Biological Communication) as the process on which evolution is founded. What do I mean?
Let us take cue from the example given on the Khan Academy website (16) about natural selection:
...a group of mice with heritable variation in fur color (black vs. tan) has just moved into a new area where the rocks are black. This environment features hawks, which like to eat mice and can see the tan ones more easily than the black ones against the black rock. Because the hawks can see and catch the tan mice more easily, a relatively large fraction of the tan mice are eaten, while a much smaller fraction of the black mice are eaten. If we look at the ratio of black mice to tan mice in the surviving ("not-eaten") group, it will be higher than in the starting population. Fur color is a heritable trait (one that can be passed from parent to child). So, the increased fraction of black mice in the surviving group means an increased fraction of black baby mice in the next generation. After several generations of selection, the population might be made up almost entirely of black mice.
Can it be said that there is an interaction between the hawk and the mice? Indeed there is. The interaction might be not be conventional sort that we are used to, but it is nonetheless an interaction, a communication between the hawk and the mice. This interaction is what prevents the hawk from “decay to equilibrium”. What does preventing or avoiding decay to equilibrium by a living organism mean?
According to Schrodinger, “The obvious answer is: By eating, drinking, breathing and (in the case of plants) assimilating. The technical term is metabolism.” (17). The Greek word for metabolism is metaballein that which stands for exchange or change. The exchange is between the organism and its immediate or proximate environment. By eating, drinking, breathing and the likes, there is an exchange between the organism and the environment. The decay to equilibrium according to Schrodinger occurs when the organism feeds on negentropy. The eating, drinking, breathing etc., that the organism does is simply just the process by which it feeds on negentropy thereby reducing the speed at which the organism attains to thermodynamical equilibrium (death) (17).
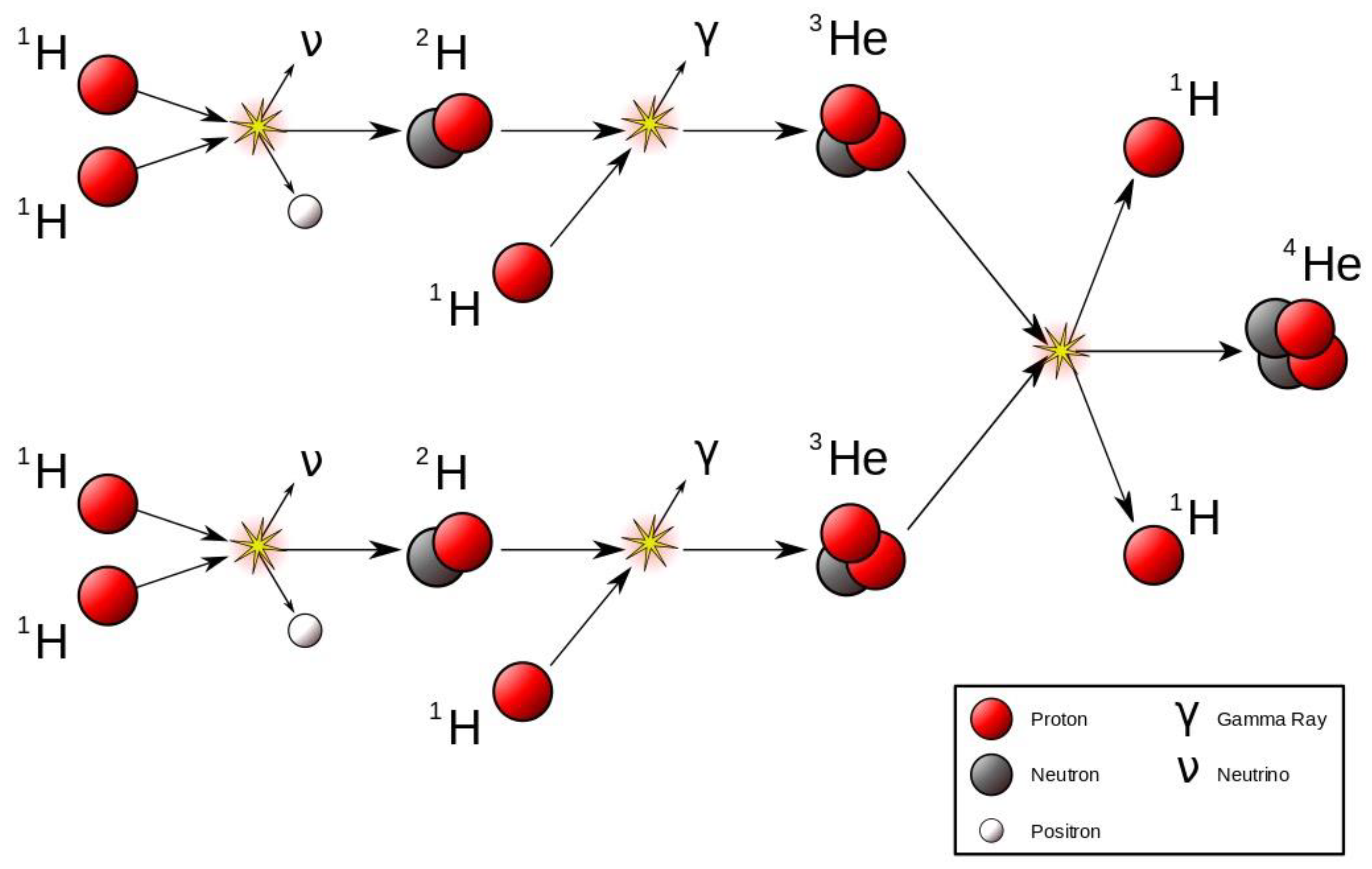
The mice comprises the environment (proximate or remote) of the hawk as much as the former comprises the environment of the latter. Thus when the hawk goes on again and again eating these mice, randomly choosing one over another, it does not change the notion of a biological interaction. Even if we take this out, reaching out to the stars, the fusion between the hydrogen that leads to the element of helium, is yet an example of interaction. This takes place in three stages namely:
the proton in one hydrogen combines with the proton of another hydrogen, becoming a neutron to form a nucleus of deuterium, which is a heavy isotope of hydrogen.
the deuterium nucleus combines with another hydrogen proton to form the light helium isotope called helium-3.
Eventually, two helium-3 nuclei combine to form helium-4, releasing two protons.
These three steps can be well elaborated in
Figure 5.
Biological interaction is the process by which living organism stands in reference to their individual environment in order to avoid decay to thermodynamical equilibrium, a process that involves the living organism feeding on negentropy.
From the Stars to the Earth: Biological Interaction
All what has been said so far is that life we have it now, could not have originated from the stars, nonetheless it traces itself fundamentally to the action of the stars, which when we extrapolate, takes us back to the big bang. Life as we have it now has firmly related to a process referred to as biological interaction. Water the sustains and supports life comes from the interaction between the hydrogen atoms, that is so heavily abundant in space, and the oxygen atoms again that is again found in space although in far lesser quantities than that of hydrogen. The continued interaction between these two atoms necessitated the origin of water. These two atoms with their mass numbers, their electron affinity, electronegativity, have provided the necessary ambience for every form of biological life - unicellular to multicellular - to thrive in water. The many variations that is witnessed today on the earth owes its existence to the ever constant interaction that goes on in life, again from the unicellular to the multicellular.
We can assert that this interaction started immediately after the big bang, and it continues in the universe. A good example is in the explosion of stars, which happens with fusion (and we have already denoted how interaction takes place as regards fusion). The phenomena of molten magma solidifying to become hills, mountains, rocks or soil, again is tied to its interaction with the cooling effect of the upper atmosphere. In whatever situation, what is alluded here is that life thrives on interaction.
Interactions is presently ongoing in the earth’s environment and outside the earth in time. Therefore, whatever other variations may occur on the earth as pertaining to her inhabitants is already in motion, with the constant interaction that is taking place.
Other Bibliography
Aristotle, Analytica Posteriora; De Caelo; De Anima; De Generatione Animalium; Historia Animalium; Metaphysics; Politics; Historia Animalium.
Cicero, De Republica; De Natura Deorum
Diogenes Laertius, Lives of Eminent Philosophers
Pliny (the Elder), Naturalis Historia
Pliny (the Younger), Epistulae
Pseudo-Plutarch, Epitome
Seneca, Quaestiones Naturales
Theophrastus, ap. Simplicius, in Physics
Rahman A.; Stillinger F. H. Molecular Dynamics Study of Liquid Water in J. Chem. Phys. 1971, 55, 3336–3359. 10.1063/1.1676585.
Ben-Naim A.; Stillinger F. H.. Aspects of the Statistical-Mechanical Theory of Water. In Structure and Transport Processes in Water and Aqueous Solutions; Horne R. A., Ed.; Wiley-Interscience: New York, 1972.
Stillinger F. H.; Rahman A. Improved Simulation of Liquid Water by Molecular Dynamics in J. Chem. Phys. 1974, 60, 1545–1557. 10.1063/1.1681229.
Maury C.P. Origin of life. Primordial genetics: Information transfer in a pre-RNA world based on self-replicating beta-sheet amyloid conformers in J. Theor. Biol. 2015;382:292–297. doi: 10.1016/j.jtbi.2015.07.008.
Carter C.W. What RNA World? Why a Peptide/RNA Partnership Merits Renewed Experimental Attention in Life. 2015; 5:294–320. doi: 10.3390/life5010294.
Wächtershäuser G. The place of RNA in the origin and early evolution of the genetic machinery in Life. 2014;4:1050–1091. doi: 10.3390/life4041050.
Luisi P.L. A new start from ground zero? in Orig. Life Evol. Biosph. 2014;44:303–306. doi: 10.1007/s11084-014-9386-1.
Benner S.A. Paradoxes in the origin of life in Orig. Life Evol. Biosph. 2014;44:339–343. doi: 10.1007/s11084-014-9379-0.
Kvenvolden K.A., Peterson E., Pollock G.E. Optical Configulation of Amino-Acids in Pre-Cambrian Fig Tree Chert in Nature. 1969; 221:141–143. doi: 10.1038/221141a0.
Nagy B., Engel M.H., Zumberge J.E., Ogiono H., Chang S.Y. Amino acids and hydrocarbons ~3,800-Myr old in the Isua Rocks, southwestern Greenland in Nature. 1981;289:53–56. doi: 10.1038/289053a0.
Van Zuilen M.A., Lepland A., Arrhenius G. Reassessing the evidence for the earliest traces of life in Nature. 2002;418:627–630. doi: 10.1038/nature00934.
Botta O., Glavin D.P., Kminek G., Bada J.L. Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites in Orig. Life Evol. Biosph. 2002;32:143–163. doi: 10.1023/A:1016019425995.
Stillinger F. H. Theory and Molecular Models for Water in Adv. Chem. Phys. 1975, 31, 1–101. 10.1002/9780470143834.ch1.
Stillinger F. H.; Weber T. A. Inherent Structure in Water in J. Phys. Chem. 1983, 87, 2833–2840. 10.1021/j100238a027.
Bloomfield VA, Crothers DM, Tinoco I Jr., Interaction of nucleic acids and water and ions. Nucleic acids: structures, properties and functions, Chap.11. University Science Books, Sausalito, 475–534, 2000.
Bonner G, Klibanov AM. Structural stability of DNA in nonaqueous solvents in Biotechnol Bioeng. 2000;68:339–344. doi: 10.1002/(SICI)1097-0290(20000505)68:3<339::AID-BIT12>3.0.CO;2-O.
Doolittle WF, Bapteste E. Pattern pluralism and the Tree of Life hypothesis in Proc Natl Acad Sci USA. 2007; 104:2043–2049.
Duche D, Baty D, Chartier M, Letellier L. Unfolding of colicin A during its translocation through the Escherichia coli envelope as demonstrated by disulfide bond engineering in J Biol Chem. 1994; 269: 24820–24825.
Edgell DR, Doolittle WF. Archaea and the origin(s) of DNA replication proteins in Cell. 1997; 89:995–998.
Edwards RA, Rohwer F. Viral metagenomics in Nat Rev Microbiol. 2005; 3:504–510.
Ellis RJ. Macromolecular crowding: obvious but underappreciated in Trends Biochem Sci. 2001; 26:597–604.
Embley TM. Multiple secondary origins of the anaerobic lifestyle in eukaryotes in Philos Trans R Soc Lond B Biol Sci. 2006; 361:1055–1067.
Darwin C., On the Origin of Species: 150th Anniversary Edition, Signet, 2003.
Bourdeau V, Ferbeyre G, Pageau M, Paquin B, Cedergren R. The distribution of RNA motifs in natural sequences in Nucleic Acids Res. 27:4457–4467, 1999. doi: 10.1093/nar/27.22.4457.
Elton D. C.; Fernández-Serra M.-V. Polar Nanoregions in Water: A Study of the Dielectric Properties of TIP4P/2005, TIP4P/2005f and TTM3F in J. Chem. Phys. 140, 124504, 2014. 10.1063/1.4869110.
Hasegawa T.; Tanimura Y. A Polarizable Water Model for Intramolecular and Intermolecular Vibrational Spectroscopies in J. Phys. Chem. B, 115, 5545–5553, 2011. 10.1021/jp111308f.
Cuervo A, Dans PD, Carrascosa JL, Orozco M, Gomila G, Fumagalli L. Direct measurement of the dielectric polarization properties of DNA in Proc Natl Acad Sci U S A.111:E3624–E3630, 2014. doi: 10.1073/pnas.1405702111.
Cui S, Yu J, Kuhner F, Schulten K, Gaub HE. Double-stranded DNA dissociates into single strands when dragged into a poor solvent in J Am Chem Soc. 129:14710–14716, 2007. doi: 10.1021/ja074776c.
Pamuk B.; Soler J. M.; Ramírez R.; Herrero C. P.; Stephens P. W.; Allen P. B.; Fernández-Serra M.-V. Anomalous Nuclear Quantum Effects in Ice in Phys. Rev. Lett. 108, 193003. 2012. 10.1103/PhysRevLett.108.193003.
Mahoney M. W.; Jorgensen W. L. Quantum, Intramolecular Flexibility, and Polarizability Effects on the Reproduction of the Density Anomaly of Liquid Water by Simple Potential Functions in J. Chem. Phys., 115, 10758–10768, 2001. 10.1063/1.1418243.
Kimoto M, Hikida Y, Hirao I. Site-specific functional labeling of nucleic acids by in vitro replication and transcription using unnatural base pair systems in Israel Journal of Chemistry 53:450–468, 2013.
Li Z, Lavergne T, Malyshev DA, Zimmermann J, Adhikary R, Dhami K, Ordoukhanian P, Sun Z, Xiang J, Romesberg FE. Site-specifically arraying small molecules or proteins on DNA using an expanded genetic alphabet in Chemistry 19:14205–14209, 2013.
Yu H.; van Gunsteren W. F. Charge-On-Spring Polarizable Water Models Revisited: From Water Clusters to Liquid Water to Ice in J. Chem. Phys. 121, 9549–9564, 2004,. 10.1063/1.1805516.
Xu H.; Stern H. A.; Berne B. J. Can Water Polarizability Be Ignored in Hydrogen Bond Kinetics? in J. Phys. Chem. B 2002, 106, 2054–2060. 10.1021/jp013426o.
Car R.; Parrinello M. Unified Approach for Molecular Dynamics and Density-Functional Theory in Phys. Rev. Lett. 55, 2471–2474, 1985. 10.1103/PhysRevLett.55.2471.
Laasonen K.; Sprik M.; Parrinello M.; Car R. ”Ab Initio” Liquid Water in J. Chem. Phys. 99, 9080–9089, 1993. 10.1063/1.465574.
Crick F.H.C., Barnett L., Brenner S. and Watts-Tobin R.J. General nature of the genetic code for proteins in Nature 192, 1227–1232, 1961. 10.1038/1921227a0
Franklin R.E. and Gosling R.G. Molecular configuration in sodium thymonucleate in Nature 171, 740–741, 1953. 10.1038/171740a0
Meselson M. and Stahl F.W. The replication of DNA in Escherichia coli in Proc. Natl. Acad. Sci. U.S.A. 44, 671–682, 1958. 10.1073/pnas.44.7.671
Watson J. and Crick F. Molecular structure of nucleic acid. A structure for deoxyribose nucleic acid in Nature 171, 737–738, 1953. 10.1038/171737a0
Dahm R. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research in Hum. Genet. 122, 565–581, 2008. 10.1007/s00439-007-0433-0
McCarty M. Discovering genes are made of DNA in Nature 421, 406, 2003. 10.1038/nature01398
Dalrymple, G. Brent The Age of the Earth, Stanford University Press: Stanford, CA. 1991.
Longair, Malcolm S. Our Evolving Universe, Cambridge University Press: New York, 1996.
Silk, Joseph A Short History of the Universe, Scientific American Library: New York, 1994.
Weinberg, Steven The First Three Minutes: A Modern View of the Origin on the Universe, Basic Books: New York, 1993.
Dawkins, Richard Climbing Mount Improbable, W.W. Norton: New York and London, 1996.
Fortey, Richard Life: A Natural History of the First Four Billion Years of Life on Earth, Alfred P. Knopf: New York, 1998.
Gould, Stephen J. The Panda’s Thumb, W.W. Norton: New York, 1992
Weiner, Jonathan The Beak of the Finch: A Story of Evolution in Our Time, Alfred P. Knopf: New York, 1994.
Whitfield, Philip From So Simple a Beginning, Macmillan: New York, 1993.
References
- Hoshika S, et al., Hachimoji DNA and RNA: A genetic system with eight building blocks in SCIENCE Vol 363, Issue 6429, Feb 2019, 884-887. [CrossRef]
- Minchin S - Lodge, J. Understanding biochemistry: structure and function of nucleic acids in Essays Biochem. 16;63(4), Oct 2019, 433-456. [CrossRef]
- Aristotle, Metaph. 983 b20.
- Aristotle Metaph. 983 b23-25.
- Aristotle, Metaph. 983 b26-27.
- Xie, H.; Zhu, Y. Translation of Guan zi; People’s Publishing House: Guizhou, China, 1996; Volume 1080. [Google Scholar]
- Xiao, F. On Yang Quan. J. Wuhan Univ. Humanit. Ed. 1980, 4, 5–12.
- Huang, X. History of Indian Philosophy; The Commercial Press: Beijing, China, 1989; pp. 41–42. [Google Scholar]
- Brini E, Fennell CJ, Fernandez-Serra M, Hribar-Lee B, Lukšič M, Dill KA. How Water's Properties Are Encoded in Its Molecular Structure and Energies in Chem Rev. 2017 Oct 11;117(19):12385-12414. 1: Oct 11;117(19). [CrossRef]
- Speight, J.G. , Hydrolysis in Reaction Mechanisms in Environmental Engineering, CD&W Inc., Laramie-Wyoming, USA, 203-229.
- Vieiria A.N. et al., The Ambivalent role of Water at the Origins of Life in FEBS Letters, 594(17), 2020, 2717-2733. [CrossRef]
- Sousa, F.L. , Hordijk W., Steel M and Martin W.F., Auticatalytic sets in E. coli Metabolism in J. Syst Chem 6, 4. [CrossRef]
- Mandala G. - Ubbu M., The Elements of Life: Highlight are Involved in All Aspects of Life in JETIR May 2019, Volume 6, Issue 5, 1-7, (ISSN-2349-5162). 20 May.
- NASA, https://www.nasa.gov/pdf/190389main_Cosmic_Elements_Poster_Back.pdf.
- National Academy of Sciences (US). Science and Creationism: A View from the National Academy of Sciences: Second Edition. Washington (DC): National Academies Press (US); 1999. The Origin of the Universe, Earth, and Life. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230211/.
- Khan Academy, https://www.khanacademy.org/science/ap-biology/natural-selection/natural-selection-ap/a/darwin-evolution-natural-selection, (retrieved 12-12-22).
- Schrodinger, E. , What is life? The Physical Aspect of The Living Cell and Mind and Matter, Cambridge University Press, Cambridge, 1977, 74.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).