Submitted:
12 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
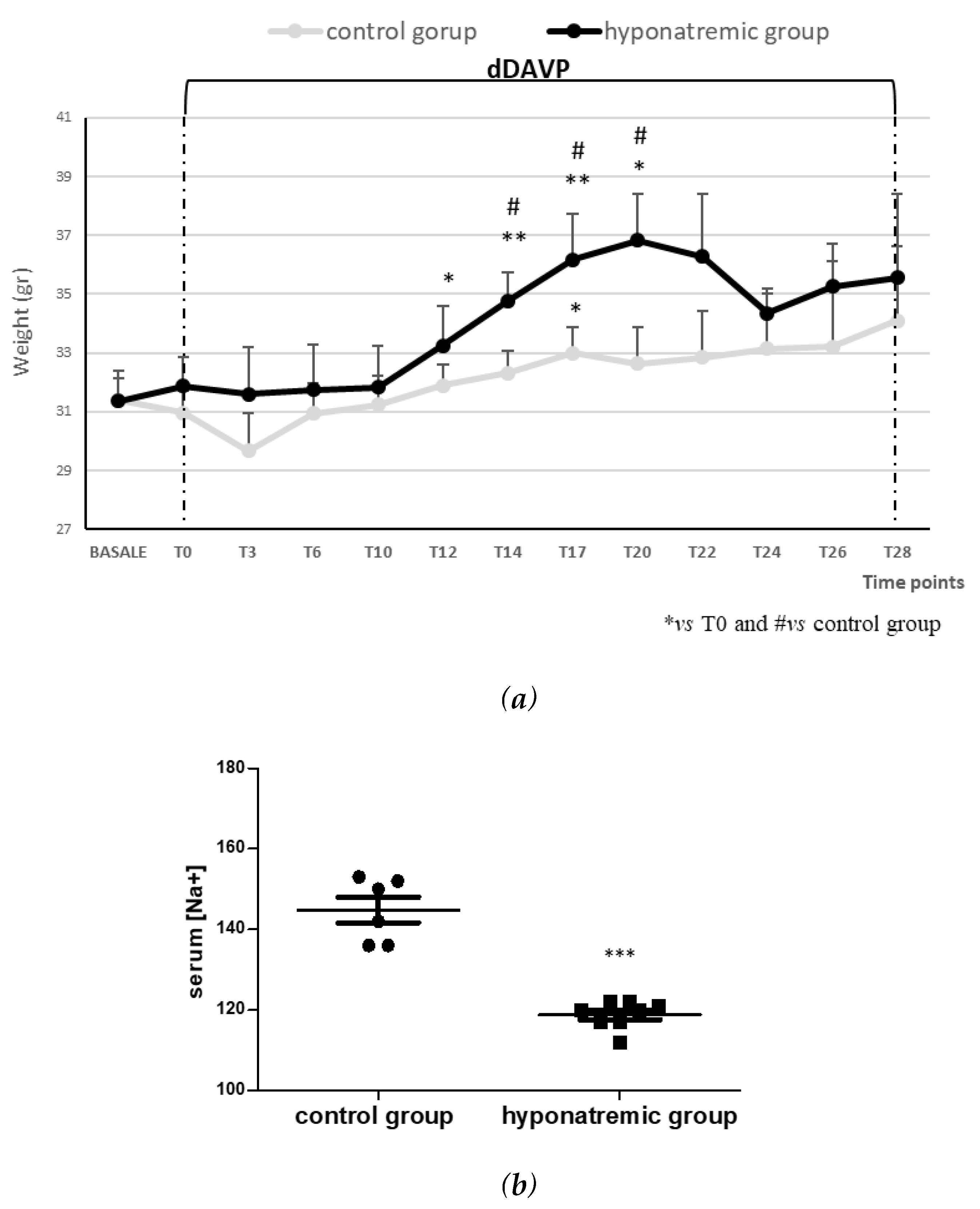
Induction of hyponatremia in nude mice
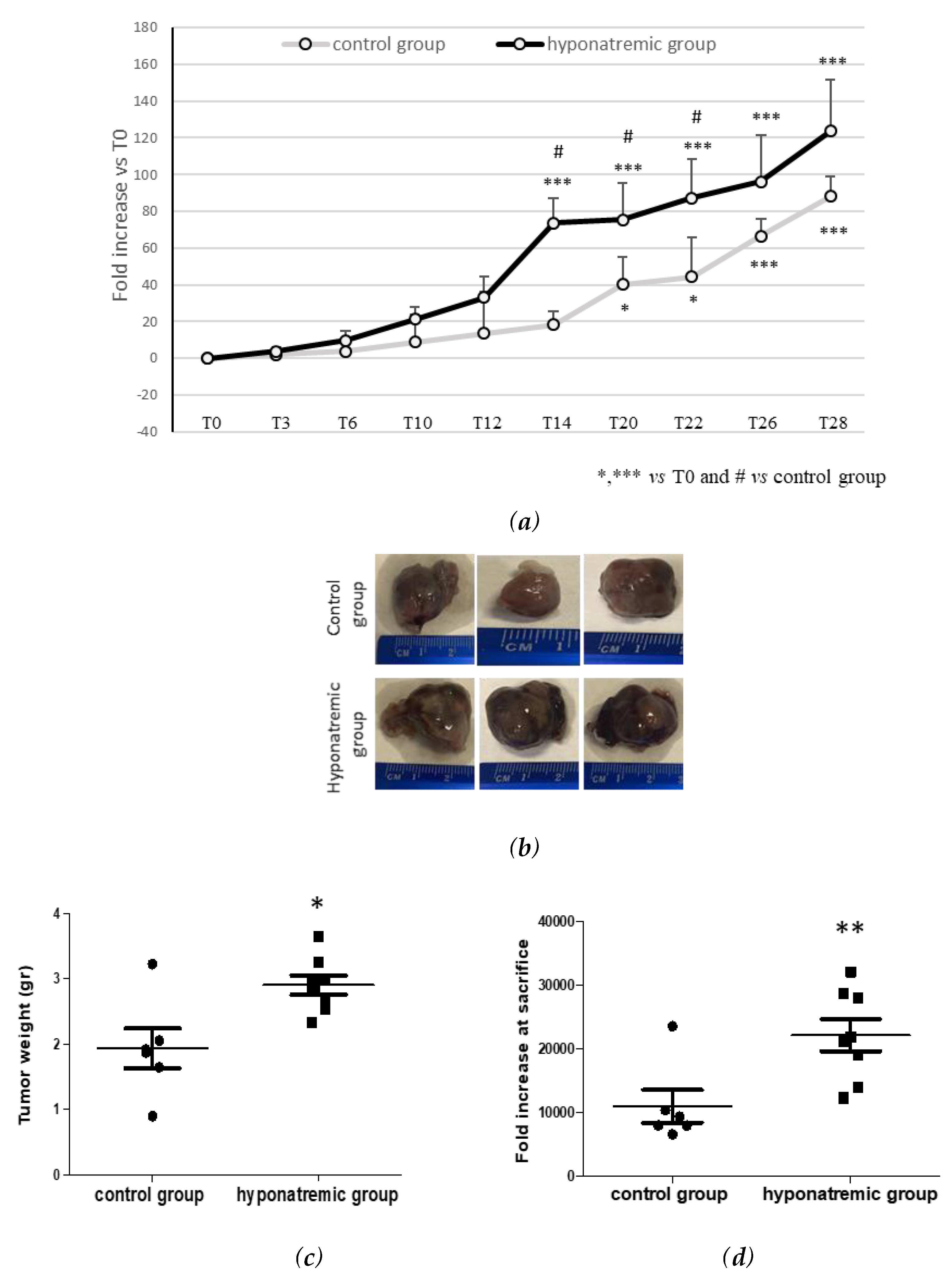
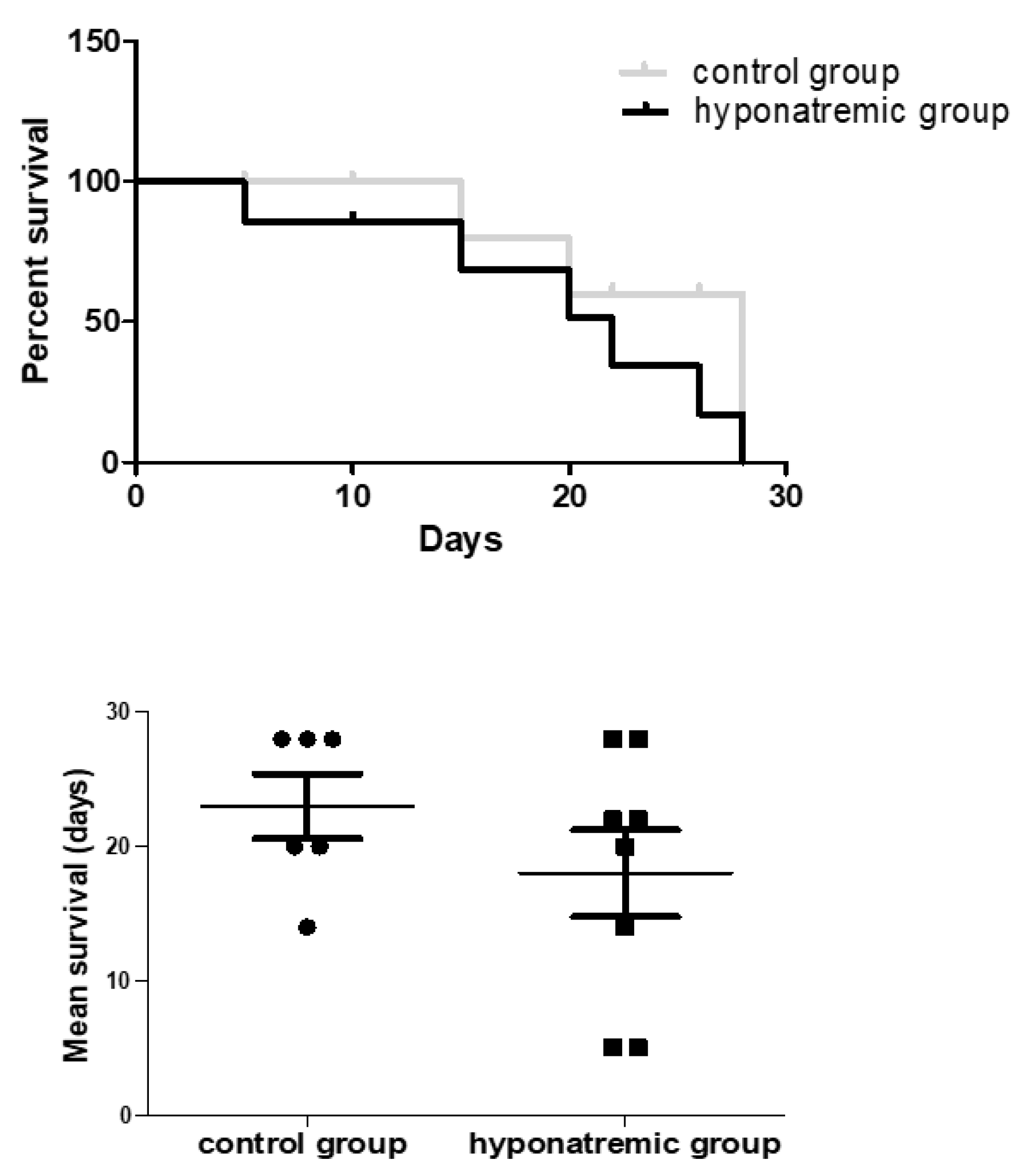
Analysis of tumor masses and survival
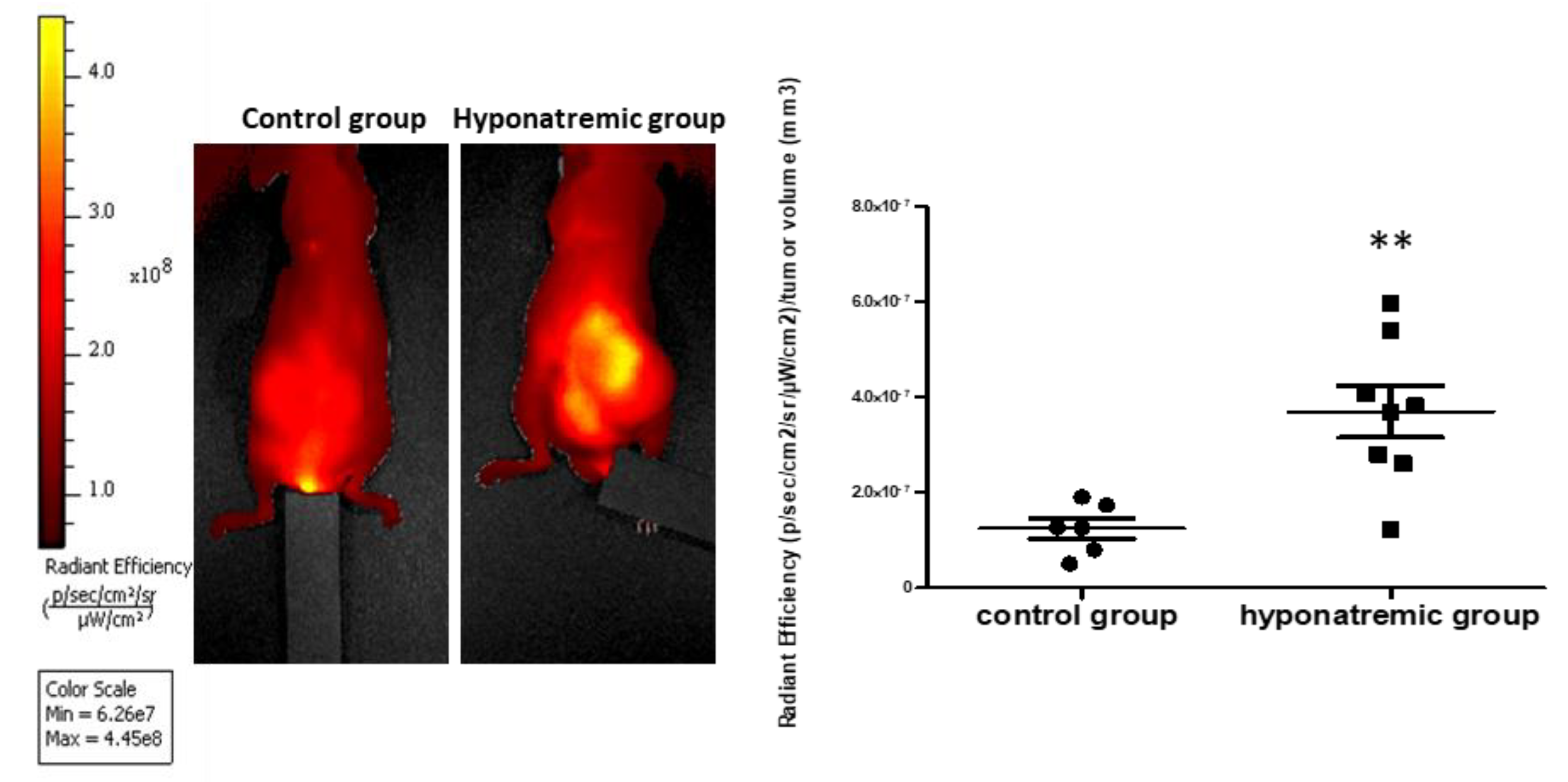
Analysis of tumor progression with IVIS Lumina 5 System
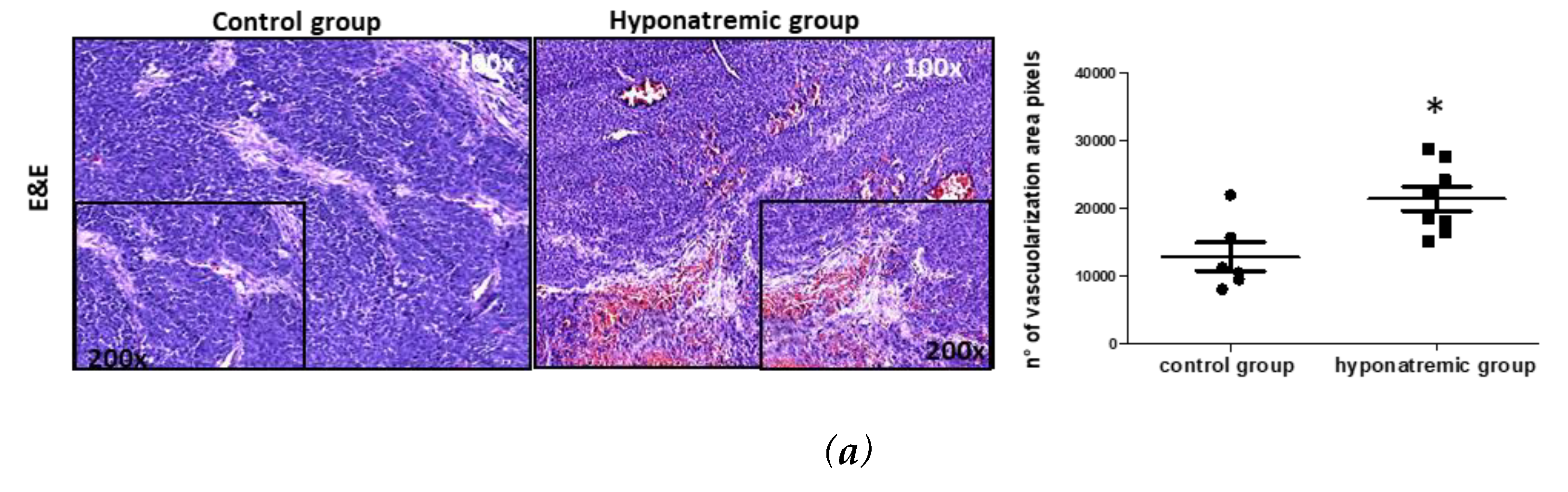
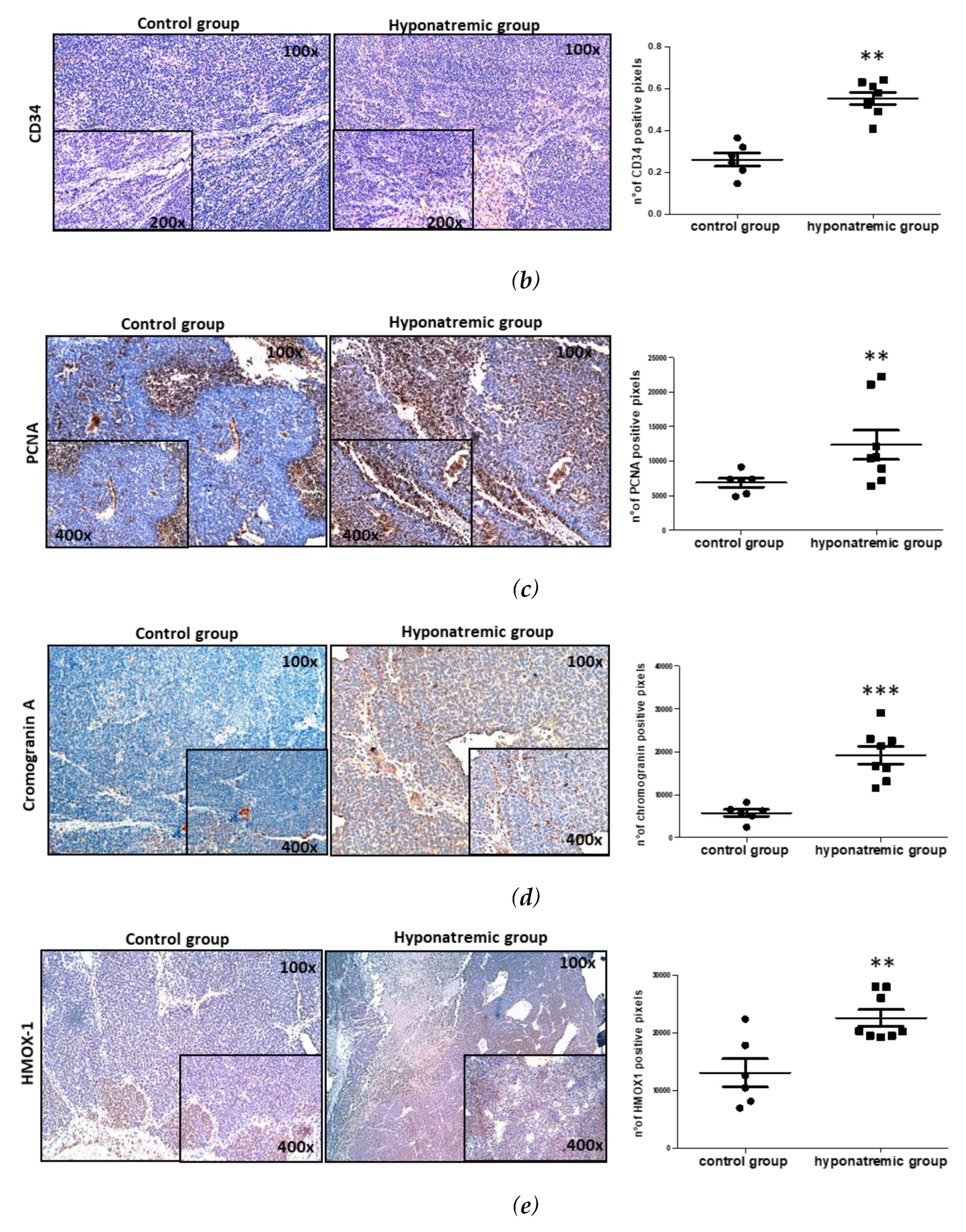
Histological and immunohistochemical analysis
Analysis of MMPs activity in vivo with IVIS Lumina S5
3. Discussion
4. Materials and Methods
Chemicals and reagents
Cell cultures and cell transfection
A murine xenograft model of neuroblastoma
In vivo imaging: IVIS Lumina S5 Imaging System
Serum [Na+] analysis
Tissues preparation and morphological characterization
Immunohistochemical analysis
Statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Adrogué, H.J.; Madias, N.E. Hyponatremia. N. Engl. J. Med. 2000, 342, 1581–1589. [Google Scholar] [CrossRef]
- Rondon-Berrios, H.; Agaba, E.I.; Tzamaloukas, A.H. Hyponatremia: Pathophysiology, Classification, Manifestations and Management. Int. Urol. Nephrol. 2014, 46, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.M.; Shah, P.; Lei, X.; Lahoti, A.; Salahudeen, A.K. Hyponatremia in Hospitalized Cancer Patients and Its Impact on Clinical Outcomes. Am. J. kidney Dis. Off. J. Natl. Kidney Found. 2012, 59, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Rinaldi, S.; Caramanti, M.; Grohè, C.; Santoni, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; Cascinu, S. Hyponatremia in Cancer Patients: Time for a New Approach. Crit. Rev. Oncol. Hematol. 2016, 102, 15–25. [Google Scholar] [CrossRef]
- Castillo, J.J.; Glezerman, I.G.; Boklage, S.H.; Chiodo, J. 3rd; Tidwell, B.A.; Lamerato, L.E.; Schulman, K.L. The Occurrence of Hyponatremia and Its Importance as a Prognostic Factor in a Cross-Section of Cancer Patients. BMC Cancer 2016, 16, 564. [Google Scholar] [CrossRef]
- Sbardella, E.; Isidori, A.M.; Arnaldi, G.; Arosio, M.; Barone, C.; Benso, A.; Berardi, R.; Capasso, G.; Caprio, M.; Ceccato, F.; et al. Approach to Hyponatremia According to the Clinical Setting: Consensus Statement from the Italian Society of Endocrinology (SIE), Italian Society of Nephrology (SIN), and Italian Association of Medical Oncology (AIOM). J. Endocrinol. Invest. 2018, 41, 3–19. [Google Scholar] [CrossRef]
- Grohé, C. Hyponatremia in Oncology Patients. Front. Horm. Res. 2019, 52, 161–166. [Google Scholar] [CrossRef]
- Gandhi, L.; Johnson, B.E. Paraneoplastic Syndromes Associated with Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2006, 4, 631–638. [Google Scholar] [CrossRef]
- Rawson, N.S.; Peto, J. An Overview of Prognostic Factors in Small Cell Lung Cancer. A Report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br. J. Cancer 1990, 61, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Wang, Z.H.; Zhou, H.W.; Liu, M.; Gu, Y.J.; Sun, J.Z. Clinical Outcome of 30 Patients with Bone Marrow Metastases. J. Cancer Res. Ther. 2018, 14, S512–S515. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bae, E.H.; Ma, S.K.; Kweon, S.S.; Kim, S.W. Prognostic Impact of Hyponatraemia in Patients with Colorectal Cancer. Color. Dis. Off. J. Assoc. Coloproctology Gt. Britain Irel. 2015, 17, 409–416. [Google Scholar] [CrossRef]
- Ginès, P.; Guevara, M. Hyponatremia in Cirrhosis: Pathogenesis, Clinical Significance, and Management. Hepatology 2008, 48, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Cescon, M.; Cucchetti, A.; Grazi, G.L.; Ferrero, A.; Viganò, L.; Ercolani, G.; Zanello, M.; Ravaioli, M.; Capussotti, L.; Pinna, A.D. Indication of the Extent of Hepatectomy for Hepatocellular Carcinoma on Cirrhosis by a Simple Algorithm Based on Preoperative Variables. Arch. Surg. 2009, 144, 57–63, discussion 63. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, N.S.; Brown, J.E.; Brown, S.R.; Rafiq, R.; Morgan, R.; Patel, P.M.; O’Donnell, D.; Harnden, P.; Rogers, M.; Cocks, K.; et al. Prognostic Factors in Renal Cell Carcinoma: Association of Preoperative Sodium Concentration with Survival. Clin. cancer Res. an Off. J. Am. Assoc. Cancer Res. 2008, 14, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Schutz, F.A.B.; Xie, W.; Donskov, F.; Sircar, M.; McDermott, D.F.; Rini, B.I.; Agarwal, N.; Pal, S.K.; Srinivas, S.; Kollmannsberger, C.; et al. The Impact of Low Serum Sodium on Treatment Outcome of Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Cancer Database Consortium. Eur. Urol. 2014, 65, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeinah, G.F.; Al-Kindi, S.G.; Hassan, A.A.; Allam, A. Hyponatraemia in Cancer: Association with Type of Cancer and Mortality. Eur. J. Cancer Care (Engl). 2015, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Rinaldi, S.; Belfiori, G.; Partelli, S.; Crippa, S.; Torniai, M.; Falconi, M. The Role of Hyponatraemia Before Surgery in Patients With Radical Resected Pancreatic Cancer. Clin. Med. Insights. Oncol. 2020, 14, 1179554920936605. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Li, C.-C.; Wen, S.-C.; Singla, N.; Woldu, S.L.; Robyak, H.; Huang, C.-N.; Ke, H.-L.; Li, W.-M.; Lee, H.-Y.; et al. Validation of Hyponatremia as a Prognostic Predictor in Multiregional Upper Tract Urothelial Carcinoma. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Guo, J.-Y.; Gong, T.-T.; Yang, Z.; Liu, Y.; Wang, L.; Wang, Y.-N.; Wu, Q.-J. Prognostic Value of Preoperative Hyponatremia in Patients with Epithelial Ovarian Cancer. J. Cancer 2019, 10, 836–842. [Google Scholar] [CrossRef]
- Berardi, R.; Mocchegiani, F.; Rinaldi, S.; Fiordoliva, I.; Rovinelli, F.; Caramanti, M.; Federici, A.; Burattini, M.; Morgese, F.; Torniai, M.; et al. Hyponatremia Is a Predictor of Clinical Outcome for Resected Biliary Tract Cancers: A Retrospective Single-Center Study. Oncol. Ther. 2020, 8, 115–124. [Google Scholar] [CrossRef]
- Dhaliwal, H.S.; Rohatiner, A.Z.; Gregory, W.; Richards, M.A.; Johnson, P.W.; Whelan, J.S.; Gallagher, C.J.; Matthews, J.; Ganesan, T.S.; Barnett, M.J. Combination Chemotherapy for Intermediate and High Grade Non-Hodgkin’s Lymphoma. Br. J. Cancer 1993, 68, 767–774. [Google Scholar] [CrossRef]
- Hansen, O.; Sørensen, P.; Hansen, K.H. The Occurrence of Hyponatremia in SCLC and the Influence on Prognosis: A Retrospective Study of 453 Patients Treated in a Single Institution in a 10-Year Period. Lung Cancer 2010, 68, 111–114. [Google Scholar] [CrossRef]
- Berardi, R.; Santoni, M.; Newsom-Davis, T.; Caramanti, M.; Rinaldi, S.; Tiberi, M.; Morgese, F.; Torniai, M.; Pistelli, M.; Onofri, A.; et al. Hyponatremia Normalization as an Independent Prognostic Factor in Patients with Advanced Non-Small Cell Lung Cancer Treated with First-Line Therapy. Oncotarget 2017, 8, 23871–23879. [Google Scholar] [CrossRef]
- Balachandran, K.; Okines, A.; Gunapala, R.; Morganstein, D.; Popat, S. Resolution of Severe Hyponatraemia Is Associated with Improved Survival in Patients with Cancer. BMC Cancer 2015, 15, 163. [Google Scholar] [CrossRef]
- Benvenuti, S.; Deledda, C.; Luciani, P.; Modi, G.; Bossio, A.; Giuliani, C.; Fibbi, B.; Peri, A. Low Extracellular Sodium Causes Neuronal Distress Independently of Reduced Osmolality in an Experimental Model of Chronic Hyponatremia. Neuromolecular Med. 2013, 15, 493–503. [Google Scholar] [CrossRef]
- Fibbi, B.; Marroncini, G.; Naldi, L.; Anceschi, C.; Errico, A.; Norello, D.; Peri, A. Hyponatremia and Cancer: From Bedside to Benchside. Cancers (Basel). 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Fibbi, B.; Errico, A.; Grappone, C.; Maggi, M.; Peri, A. Effects of Low Extracellular Sodium on Proliferation and Invasive Activity of Cancer Cells in Vitro. Endocrine 2020, 67, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Anceschi, C.; Naldi, L.; Fibbi, B.; Baldanzi, F.; Martinelli, S.; Polvani, S.; Maggi, M.; Peri, A. Low Sodium and Tolvaptan Have Opposite Effects in Human Small Cell Lung Cancer Cells. Mol. Cell. Endocrinol. 2021, 537, 111419. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Anceschi, C.; Naldi, L.; Fibbi, B.; Brogi, M.; Lanzilao, L.; Fanelli, A.; Maggi, M.; Peri, A. Hyponatremia-Related Liver Steatofibrosis and Impaired Spermatogenesis: Evidence from a Mouse Model of the Syndrome of Inappropriate Antidiuresis. J. Endocrinol. Invest. 2023, 46, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Giuliani, C.; Parenti, G.; Norello, D.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Moderate Hyponatremia Is Associated with Increased Risk of Mortality: Evidence from a Meta-Analysis. PLoS One 2013, 8, e80451. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Civin, C.I.; Strauss, L.C.; Brovall, C.; Fackler, M.J.; Schwartz, J.F.; Shaper, J.H. Antigenic Analysis of Hematopoiesis. III. A Hematopoietic Progenitor Cell Surface Antigen Defined by a Monoclonal Antibody Raised against KG-1a Cells. J. Immunol. 1984, 133, 157–165. [Google Scholar] [CrossRef]
- Fina, L.; Molgaard, H. V; Robertson, D.; Bradley, N.J.; Monaghan, P.; Delia, D.; Sutherland, D.R.; Baker, M.A.; Greaves, M.F. Expression of the CD34 Gene in Vascular Endothelial Cells. Blood 1990, 75, 2417–2426. [Google Scholar] [CrossRef]
- Kapoor, S.; Shenoy, S.P.; Bose, B. CD34 Cells in Somatic, Regenerative and Cancer Stem Cells: Developmental Biology, Cell Therapy, and Omics Big Data Perspective. J. Cell. Biochem. 2020, 121, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Somasundaram, D.B.; Herman, T.S.; Aravindan, S. Significance of Hematopoietic Surface Antigen CD34 in Neuroblastoma Prognosis and the Genetic Landscape of CD34-Expressing Neuroblastoma CSCs. Cell Biol. Toxicol. 2021, 37, 461–478. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Beach, D. Proliferating Cell Nuclear Antigen and P21 Are Components of Multiple Cell Cycle Kinase Complexes. Mol. Biol. Cell 1993, 4, 897–906. [Google Scholar] [CrossRef]
- Kumar, A.; Kurmi, B. Das; Singh, A.; Singh, D. Potential Role of Resveratrol and Its Nano-Formulation as Anti-Cancer Agent. Explor. Target. anti-tumor Ther. 2022, 3, 643–658. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A Comprehensive and Systematic Review on Potential Anticancer Activities of Eugenol: From Pre-Clinical Evidence to Molecular Mechanisms of Action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wang, W.; Tang, X.; Goh, R.M.W.-J.; Thuya, W.L.; Ho, P.C.L.; Chen, L.; Wang, L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers (Basel). 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R.J. The Extended Granin Family: Structure, Function, and Biomedical Implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Gustafsson, B.I.; Moss, S.F.; Pavel, M.; Tsolakis, A. V; Kidd, M. Chromogranin A--Biological Function and Clinical Utility in Neuro Endocrine Tumor Disease. Ann. Surg. Oncol. 2010, 17, 2427–2443. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, R.J.; Seeger, R.C.; Yu, A.L.; O’Connor, D.T. Chromogranin A in Children with Neuroblastoma. Serum Concentration Parallels Disease Stage and Predicts Survival. J. Clin. Invest. 1990, 85, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Macri, L.; Faulkner, L.B.; Tintori, V.; Paoli, A.; Garaventa, A.; Bussolati, G. Detection Procedures for Neuroblastoma Cells Metastatic to Blood and Bone Marrow: Blinded Comparison of Chromogranin A Heminested Reverse Transcription Polymerase Chain Reaction to Tyrosine Hydroxylase Nested Reverse Transcription Polymerase Chain Reacti. Diagnostic Mol. Pathol. Am. J. Surg. Pathol. part B 2002, 11, 98–106. [Google Scholar] [CrossRef]
- Zhang, D.; Babayan, L.; Ho, H.; Heaney, A.P. Chromogranin A Regulates Neuroblastoma Proliferation and Phenotype. Biol. Open 2019, 8. [Google Scholar] [CrossRef]
- Fibbi, B.; Marroncini, G.; Anceschi, C.; Naldi, L.; Peri, A. Hyponatremia and Oxidative Stress. Antioxidants 2021, 10, 11–14. [Google Scholar] [CrossRef]
- Hemmati, M.; Yousefi, B.; Bahar, A.; Eslami, M. Importance of Heme Oxygenase-1 in Gastrointestinal Cancers: Functions, Inductions, Regulations, and Signaling. J. Gastrointest. Cancer 2021. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Q.; Bao, L.; Li, M.; Chang, K.; Yi, X. Cytoprotective Role of Heme Oxygenase-1 in Cancer Chemoresistance: Focus on Antioxidant, Antiapoptotic, and Pro-Autophagy Properties. Antioxidants (Basel, Switzerland) 2023, 12. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Ahmad, I.M.; Rachagani, S.; Banerjee, K.; Thompson, C.M.; Maurer, H.C.; Olive, K.P.; Bailey, K.L.; Britigan, B.E.; Kumar, S. Enhancing Responsiveness of Pancreatic Cancer Cells to Gemcitabine Treatment under Hypoxia by Heme Oxygenase-1 Inhibition. Transl. Res. 2019, 207, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xuan, Z.; Liu, X.; Zheng, M.; Yang, C.; Wang, H. Immunomodulatory Role of Metalloproteinase ADAM17 in Tumor Development. Front. Immunol. 2022, 13, 1059376. [Google Scholar] [CrossRef] [PubMed]
- Holland-Bill, L.; Christiansen, C.F.; Farkas, D.K.; Donskov, F.; Jørgensen, J.O.L.; Sørensen, H.T. Diagnosis of Hyponatremia and Increased Risk of a Subsequent Cancer Diagnosis: Results from a Nationwide Population-Based Cohort Study. Acta Oncol. 2018, 57, 522–527. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




