Submitted:
13 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Assess the severity of melasma,
- Evaluate the impact of melasma on the QoL of affected patients,
- Identify predictors of QoL through stepwise regression analysis.
2. Materials and Methods
2.1. Study design and setting
2.2. Study population
2.3. Sample size and sampling techniques
2.4. Data collection
2.6. Statistical analysis
3. Results
3.1. Study Respondents Characteristics
3.2. Descriptive Analysis of the Dependent and Predictor Variables
3.3. Severity of Melasma Index (MASI)
3.4. QoL (MELASQoL)
3.5. Stepwise Regression Analysis
| = | 51.730 | + | 0.315 Masi | - | 4.686 Cheeks | - | 4.148 Education | - | 2.519 Menopausal | ||
| se | = | (5.249) | (0.120) | (1.376) | (2.044) | (1.243) | r2 = 0.145 | ||||
|
t |
= |
(9.856) |
(2.628) |
(-3.405) |
(-2.029) |
(-2.027) |
F (4, 145) = 6.153 |
||||
| p < 0.001 | |||||||||||
| p | = | (< 0.001) | (.010) | (< 0.001) | (.044) | (.045) |
4. Discussion
- Cost: Some women do not use sun protection cream because they cannot afford it or believe it is too costly.
- Skin reactions: Some women experience skin reactions, irritation, or sensitivity to sun protection cream, which discourages them from using it.
- Appearance: Some women avoid using sun protection cream because they believe it makes their face look white or pale and “creates” pimples.
- No perceived need: Some women do not see the necessity of using sun protection cream, particularly if they spend most of their time indoors or do not spend much time in direct sunlight.
- Use of alternative products: A few women report using moisturizers or other products that have SPF as an alternative to dedicated sun protection cream.
- Efficacy concerns: A small number of women believe that sun protection cream is ineffective or does not work as advertised.
- Other reasons: Some women simply responded with “N/A” or “none”, indicating that they have no particular reason for not using sun protection cream, while others did not provide a reason at all.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dréno B, Araviiskaia E, Berardesca E, Gontijo G, Sanchez Viera M, Xiang L, et al. Microbiome in healthy skin, update for dermatologists. Journal of the European Academy of Dermatology and Venereology. 2016;30(12):2038-47. [CrossRef]
- Bodeker, G.; Ryan, T.J.; Volk, A.; Harris, J.; Burford, G. Integrative Skin Care: Dermatology and Traditional and Complementary Medicine. J. Altern. Complement. Med. 2017, 23, 479–486. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Skin Depigmenting Agents in Anti-Aging Cosmetics: A Medicinal Perspective on Emerging Ingredients. Appl. Sci. 2022, 12, 775. [Google Scholar] [CrossRef]
- Siddiqui S, Qayyum M. Correlation between quality of life and clinical severity of melasma in Pakistani women: Maryam Qayyum, Saadiya Siddiqui, Mohsina, Mahmoona Ilyas, Atif Shahzad, Nadia Ali Zafar. Journal of Pakistan Association of Dermatologists. 2022;32(4):683-9.
- Amatya, B.; Jha, A.K.; Shrestha, S. Frequency of different types of facial melanoses referring to the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital in 2019, and assessment of their effect on health-related quality of life. BMC Dermatol. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zeng, X.; Ying, J.; Cai, Y.; Qiu, Y.; Xiang, W. Evaluating the quality of life among melasma patients using the MELASQoL scale: A systematic review and meta-analysis. PLOS ONE 2022, 17, e0262833. [Google Scholar] [CrossRef] [PubMed]
- Jusuf NK, Putra IB, Mahdalena M. Is there a correlation between severity of melasma and quality of life? Open access Macedonian journal of medical sciences. 2019;7(16):2615.
- Mpofana, N.; Abrahamse, H. The Management of Melasma on Skin Types V and VI Using Light Emitting Diode Treatment. Photomed. Laser Surg. 2018, 36, 522–529. [Google Scholar] [CrossRef]
- Majid I, Aleem S. Melasma: Update on Epidemiology, Clinical Presentation, Assessment, and Scoring. Journal of Skin and Stem Cell. 2021;8(4).
- Walker, S.; Shah, M.; Hubbard, V.; Pradhan, H.; Ghimire, M. Skin disease is common in rural Nepal: results of a point prevalence study. Br. J. Dermatol. 2007, 158, 334–338. [Google Scholar] [CrossRef] [PubMed]
- El-Essawi, D.; Musial, J.L.; Hammad, A.; Lim, H.W. A survey of skin disease and skin-related issues in Arab Americans. J. Am. Acad. Dermatol. 2007, 56, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Parthasaradhi, A.; Al Gufai, A.F.; H, A.; M, A.; A, A.; A, S.; M, A.; A, A.; N, M.; H, A.S. The pattern of skin diseases in Hail Region, Saudi Arabia. Ann. Saudi Med. 1998, 18, 558–561. [Google Scholar] [CrossRef]
- Tendero, M.P.A.; Romero, I.B.; Rincón, J.M.R.; Payá, J.S.; Costa, A.L.; Crespo, M.P.; Salvador, J.F.S. Dermatoses in Latin American immigrants seen in a tertiary hospital. Eur. J. Dermatol. 2009, 19, 157–162. [Google Scholar] [CrossRef]
- Werlinger, K.D.; Guevara, I.L.; González, C.M.; Rincón, E.T.; Caetano, R.; Haley, R.W.; Pandya, A.G. Prevalence of Self-diagnosed Melasma Among Premenopausal Latino Women in Dallas and Fort Worth, Tex. Arch. Dermatol. 2007, 143, 423–431. [Google Scholar] [CrossRef]
- Hiletework, M. Skin diseases seen in Kazanchis health center. . 1998, 36, 245–54. [Google Scholar] [PubMed]
- Dlova, N.C.; Akintilo, L.O.; Taylor, S.C. Prevalence of pigmentary disorders: A cross-sectional study in public hospitals in Durban, South Africa. Int. J. Women’s Dermatol. 2019, 5, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Sangha, A.M. Dermatological Conditions in SKIN OF COLOR-Melasma: Topical and Systemic Management. . 2022, 15, S17–S19. [Google Scholar] [PubMed]
- Tamega AdA, Miot H, Moço N, Silva M, Marques M, Miot L. Gene and protein expression of oestrogen-β and progesterone receptors in facial melasma and adjacent healthy skin in women. International Journal of Cosmetic Science. 2015;37(2):222-8.
- E Grimes, P.; Yamada, N.; Bhawan, J. Light Microscopic, Immunohistochemical, and Ultrastructural Alterations in Patients with Melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Maranzatto CFP, Miot HA, Miot LDB, Meneguin S. Psychometrican analysis and dimensional structure of the Brazilian version of melasma quality of life scale (MELASQoL-BP). Anais brasileiros de dermatologia. 2016; 91: 422-8.
- Kim, E.H.; Kim, Y.C.; Lee, E.-S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Torres-Álvarez, B.; Mesa-Garza, I.G.; Castanedo-Cázares, J.P.M.; Fuentes-Ahumada, C.B.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and Immunohistochemical Study in Melasma: Evidence of Damage in the Basal Membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef]
- Mpofana, N.; Chibi, B.; Visser, T.; Paulse, M.; Finlayson, A.J.; Ghuman, S.; Gqaleni, N.; Hussein, A.A.; Dlova, N.C. Treatment of Melasma on Darker Skin Types: A Scoping Review. Cosmetics 2023, 10, 25. [Google Scholar] [CrossRef]
- Jobanputra, R.; Bachmann, M.; MBChB, D.R.J.; MBChB, M.M.B. The effect of skin diseases on quality of life in patients from different social and ethnic groups in Cape Town, South Africa. Int. J. Dermatol. 2000, 39, 826–831. [Google Scholar] [CrossRef]
- Ali R, Aman S, Nadeem M, Kazmi AH. Quality of life in patients of melasma. Journal of Pakistan Association of Dermatologists. 2013;23(2):143-8.
- Cestari, T.; Hexsel, D.; Viegas, M.; Azulay, L.; Hassun, K.; Almeida, A.; Rêgo, V.; Mendes, A.; Filho, J.; Junqueira, H. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: the MelasQoL-BP study and improvement of QoL of melasma patients after triple combination therapy. Br. J. Dermatol. 2006, 156, 13–20. [Google Scholar] [CrossRef]
- Fleck M, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, et al. Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL-bref. Revista de saude publica. 2000; 34: 178-83.
- Dogramaci AC, Havlucu DY, Inandi T, Balkrishnan R. Validation of a melasma quality of life questionnaire for the Turkish language: the MelasQoL-TR study. Journal of dermatological treatment. 2009;20(2):95-9.
- Balkrishnan, R.; Mcmichael, A.; Camacho, F.; Saltzberg, F.; Housman, T.; Grummer, S.; Feldman, S.; Chren, M.-M. Development and validation of a health-related quality of life instrument for women with melasma. Br. J. Dermatol. 2003, 149, 572–577. [Google Scholar] [CrossRef]
- Dominguez, A.R.; Balkrishnan, R.; Ellzey, A.R.; Pandya, A.G. Melasma in Latina patients: Cross-cultural adaptation and validation of a quality-of-life questionnaire in Spanish language. J. Am. Acad. Dermatol. 2006, 55, 59–66. [Google Scholar] [CrossRef]
- Sarkar, R.; Garg, S.; Dominguez, A.; Balkrishnan, R.; Jain, R.; Pandya, A. Development and validation of a Hindi language health-related quality of life questionnaire for melasma in Indian patients. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 16–22. [Google Scholar] [CrossRef]
- Freitag, F.; Cestari, T.; Leopoldo, L.; Paludo, P.; Boza, J. Effect of melasma on quality of life in a sample of women living in southern Brazil. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Schmitt, A.; Boussetta, S.; Rahhali, N.; Taieb, C. Melasma: Measure of the Impact on Quality of Life Using the French Version of MELASQOL after Cross-cultural Adaptation. Acta Dermato-Venereologica 2010, 90, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? Journal of Investigative Dermatology. 2005;125(4):659-64.
- Thayer, JD. Stepwise Regression as an Exploratory Data Analysis Procedure. 2002.
- Falk R, Miller N. A primer for soft modeling University of Akron Press. Akron, Ohio. 1992.
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences: Routledge; 2013.
- Chin, WW. Commentary: Issues and opinion on structural equation modeling. JSTOR; 1998. p. vii-xvi.
- Ikino, J.K.; Nunes, D.H.; Da Silva, V.P.M.; Fröde, T.S.; Sens, M.M. Melasma and assessment of the quality of life in Brazilian women. An. Bras. de Dermatol. 2015, 90, 196–200. [Google Scholar] [CrossRef] [PubMed]
- D’Elia MPB, Brandão MC, de Andrade Ramos BR, da Silva MG, Miot LDB, Dos Santos SEB, et al. African ancestry is associated with facial melasma in women: a cross-sectional study. BMC medical genetics. 2017;18: 1-7.
- Guinot, C.; Cheffai, S.; Latreille, J.; Dhaoui, M.; Youssef, S.; Jaber, K.; Nageotte, O.; Doss, N. Aggravating factors for melasma: a prospective study in 197 Tunisian patients. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Oh, B.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. The relation between the amount of sunscreen applied and the sun protection factor in Asian skin. J. Am. Acad. Dermatol. 2010, 62, 218–222. [Google Scholar] [CrossRef]
- Teramura, T.; Mizuno, M.; Asano, H.; Naito, N.; Arakane, K.; Miyachi, Y. Relationship between sun-protection factor and application thickness in high-performance sunscreen: double application of sunscreen is recommended. Clin. Exp. Dermatol. 2012, 37, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Diffey, BL. When should sunscreen be reapplied? Journal of the American Academy of Dermatology. 2001;45(6):882-5.
- Heerfordt, I.M.; Torsnes, L.R.; Philipsen, P.A.; Wulf, H.C. Photoprotection by sunscreen depends on time spent on application. Photodermatol. Photoimmunol. Photomed. 2017, 34, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, A.; Wulf, H. The relation between sun protection factor and amount of suncreen applied in vivo. Br. J. Dermatol. 2007, 156, 716–719. [Google Scholar] [CrossRef]
- Petersen B, Wulf HC. Application of sunscreen− theory and reality. Photodermatology, photoimmunology & photomedicine. 2014 Apr;30(2-3):96-101.
- Seetan K, Shatanawi M, Ali A, Khamees Aa, Alsheikh A, Alawneh A, et al. Disease characteristics, determinants, and perception of use of sunscreen and sun-protective behaviors among patients of color with melasma: A cross-sectional study. Photodermatology, Photoimmunology & Photomedicine. 2022;38(5):495-500.
- Rigel, D.S.; Taylor, S.C.; Lim, H.W.; Alexis, A.F.; Armstrong, A.W.; Fuxench, Z.C.C.; Draelos, Z.D.; Hamzavi, I.H. Photoprotection for skin of all color: Consensus and clinical guidance from an expert panel. J. Am. Acad. Dermatol. 2021, 86, S1–S8. [Google Scholar] [CrossRef]
- Albrecht S, Jung S, Müller R, Lademann J, Zuberbier T, Zastrow L, et al. Skin type differences in solar-simulated radiation-induced oxidative stress. British Journal of Dermatology. 2019;180(3):597-603.
- Mpofana N, Ramhurry C. An Investigation into the effectiveness of light emitting diodes on treating melasma on skin type VI. American Journal of Dermatology and Venereology. 2014.
- Lucas, R.M.; Norval, M.; Wright, C.Y. Solar ultraviolet radiation in Africa: a systematic review and critical evaluation of the health risks and use of photoprotection. Photochem. Photobiol. Sci. 2015, 15, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.Y.; Norval, M.; Summers, B.; Davids, L.M.; Coetzee, G.; Oriowo, M. Solar ultraviolet radiation exposure and human health in South Africa: finding a balance. South Afr. Med J. 2012, 102, 665. [Google Scholar] [CrossRef] [PubMed]
- Sarkar R, Ailawadi P, Garg S. Melasma in men: A review of clinical, etiological, and management issues. The Journal of clinical and aesthetic dermatology. 2018;11(2):53.
- Handel, A.C.; Miot, L.D.B.; Miot, H.A. Melasma: a clinical and epidemiological review. An. Bras. de Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Vázquez M, Maldonado H, Benmamán C, Sanchez JL. Melasma in men: a clinical and histologic study. International journal of dermatology. 1988;27(1):25-7.
- Vachiramon V, Suchonwanit P, Thadanipon K. Melasma in men. Journal of Cosmetic Dermatology. 2012;11(2):151-7.
- Dlova, N.C.; Nevondo, F.T.; Mwangi, E.M.; Summers, B.; Tsoka-Gwegweni, J.; Martincigh, B.S.; Mulholland, D.A. Chemical analysis and in vitro UV-protection characteristics of clays traditionally used for sun protection in South Africa. Photodermatol. Photoimmunol. Photomed. 2013, 29, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.-H.; Seo, K.-I.; Park, J.-Y.; Lim, J.-G.; Eun, H.-C.; Park, K.-C. A Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin C Iontophoresis in Melasma. Dermatology 2003, 206, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. The Therapeutic Use of Antioxidants for Melasma. J. Drugs Dermatol. 2020, 19, 788–792. [Google Scholar] [CrossRef]
- Ismail ESA, Patsatsi A, Abd el-Maged WM, Nada EEDAeA. Efficacy of microneedling with topical vitamin C in the treatment of melasma. Journal of cosmetic dermatology. 2019;18(5):1342-7.
- Wolf, G.; Cartwright, B. Rules for coding dummy variables in multiple regression. Psychol. Bull. 1974, 81, 173–179. [Google Scholar] [CrossRef]
- Cohen, A. Dummy Variables in Stepwise Regression. Am. Stat. 1991, 45, 226. [Google Scholar] [CrossRef]
- Hardy, MA. Regression with dummy variables: Sage; 1993.
- Mansfield, E.R.; Helms, B.P. Detecting Multicollinearity. Am. Stat. 1982, 36, 158. [Google Scholar] [CrossRef]
- Alin, A. Multicollinearity. Wiley interdisciplinary reviews: computational statistics. 2010;2(3):370-4.
- Farrar, D.E.; Glauber, R.R. Multicollinearity in Regression Analysis: The Problem Revisited. Rev. Econ. Stat. 1967, 49, 92. [Google Scholar] [CrossRef]
- KrupaShankar, D.S.R.; Somani, V.K.; Kohli, M.; Sharad, J.; Ganjoo, A.; Kandhari, S.; Mysore, V.R.; Aurangabadkar, S.; Malakar, S.; Vedamurthy, M.; et al. A Cross-Sectional, Multicentric Clinico-Epidemiological Study of Melasma in India. Dermatol. Ther. 2014, 4, 71–81. [Google Scholar] [CrossRef]
- Anderson, L.; Rodrigues, M. Quality of life in a cohort of melasma patients in Australia. Australas. J. Dermatol. 2018, 60, 160–162. [Google Scholar] [CrossRef]
- Kim HY, Park GH, Park EJ, Kwon IH, Kim KH, Kim KJ. Usefulness of melasma quality of life scale (MELASQOL) when evaluating the quality of life in Korean melasma patients. Korean Journal of Dermatology. 2013:422-8.
- Pollo, C.F.; Miot, L.D.; Meneguin, S.; Miot, H.A. Factors associated with quality of life in facial melasma: a cross-sectional study. Int. J. Cosmet. Sci. 2018, 40, 313–316. [Google Scholar] [CrossRef]
- Kothari, P.; Sharma, Y.K.; Patvekar, M.; Gupta, A. Correlating impairment of quality of life and severity of melasma: A cross-sectional study of 141 patients. Indian J. Dermatol. 2018, 63, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Harumi, O.; Goh, C.L. The Effect of Melasma on the Quality of Life in a Sample of Women Living in Singapore. . 2016, 9, 21–4. [Google Scholar] [PubMed]
- Kwon, S.; Na, J.; Choi, J.; Park, K. Melasma: Updates and perspectives. Exp. Dermatol. 2018, 28, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Micek, I.; Pawlaczyk, M.; Kroma, A.; Seraszek-Jaros, A.; Urbańska, M.; Gornowicz-Porowska, J. Treatment of melasma with a low-fluence 1064 nm Q-switched Nd:YAG laser: Laser toning in Caucasian women. Lasers Surg. Med. 2021, 54, 366–373. [Google Scholar] [CrossRef]
- Dorgham, N.A.; Hegazy, R.A.; Sharobim, A.K.; Dorgham, D.A. Efficacy and tolerability of chemical peeling as a single agent for melasma in dark-skinned patients: A systematic review and meta-analysis of comparative trials. J. Cosmet. Dermatol. 2020, 19, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Myers RH, Myers RH. Classical and modern regression with applications: Duxbury press Belmont, CA; 1990.
- Menard, S. Applied logistic regression analysis: Sage; 2002.
- Allison, PD. Logistic regression using SAS: Theory and application: SAS institute; 2012.
- Rathi, S.K.; Achar, A. Melasma: A clinico-epidemiological study of 312 cases. Indian J. Dermatol. 2011, 56, 380–2. [Google Scholar] [CrossRef]
- Moin, A.; Jabery, Z.; Fallah, N. Prevalence and awareness of melasma during pregnancy. Int. J. Dermatol. 2006, 45, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Tamega AdA, Miot L, Bonfietti C, Gige T, Marques MEA, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. Journal of the European Academy of Dermatology and Venereology. 2013;27(2):151-6.
- Holme, S.; Beattie, P.; Fleming, C. Cosmetic camouflage advice improves quality of life. Br. J. Dermatol. 2002, 147, 946–949. [Google Scholar] [CrossRef]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: an Up-to-Date Comprehensive Review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part II. Journal of the American Academy of Dermatology. 2011;65(4):699-714.
- Lynch, J.W.; Smith, G.D.; A Kaplan, G.; House, J.S. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. BMJ 2000, 320, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Renzi, C.; Abeni, D.; Picardi, A.; Agostini, E.; Melchi, C.; Pasquini, P.; Puddu, P.; Braga, M. Factors associated with patient satisfaction with care among dermatological outpatients. Br. J. Dermatol. 2001, 145, 617–623. [Google Scholar] [CrossRef]
- Simpson, ER. Sources of estrogen and their importance. The Journal of steroid biochemistry and molecular biology. 2003;86(3-5):225-30.
- Koothirezhi R, Ranganathan S. Postmenopausal syndrome. 2020.
- Cario, M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef]
- Pandya AG, Hynan LS, Bhore R, Riley FC, Guevara IL, Grimes P, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. Journal of the American Academy of Dermatology. 2011;64(1):78-83. e2.
- Abou-Taleb, D.A.; Ibrahim, A.K.; Youssef, E.M.; Moubasher, A.E. Reliability, Validity, and Sensitivity to Change Overtime of the Modified Melasma Area and Severity Index Score. Dermatol. Surg. 2017, 43, 210–217. [Google Scholar] [CrossRef]
- Thng TGS, Chuah SY. The Scoring Aid: MASI and Modified MASI. Melasma and Vitiligo in Brown Skin. 2017:63-70. [CrossRef]
- Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. Journal of the American Academy of Dermatology. 2006;54(5):S272-S81. [CrossRef]
| On a Likert scale of 1 (not bothered at all) to 7 (bothered all the time), the subject rates how s/he feels about themselves: |
|
| Score | Darkness (D) | Homogeneity (H) | Area (A) |
|---|---|---|---|
| 0 | Absent | Minimal | No involvement |
| 1 | Slight | Slight | < 10% |
| 2 | Mild | Mild | 10-29% |
| 3 | Marked | Marked | 30-49% |
| 4 | Maximum | Maximum | 50-69% |
| 5 | 70-89% | ||
| 6 | 90-100% |
| Variables | Notes | Mean | Std. Deviation |
|---|---|---|---|
| MELASQoL | Scale from 7 to 70 | 56.29 | 7.35 |
| Children | Number of children | 2.10 | 1.13 |
| Skin care regime | Total number of skin products used | 2.43 | 2.11 |
| Age | Years | 47.30 | 10.21 |
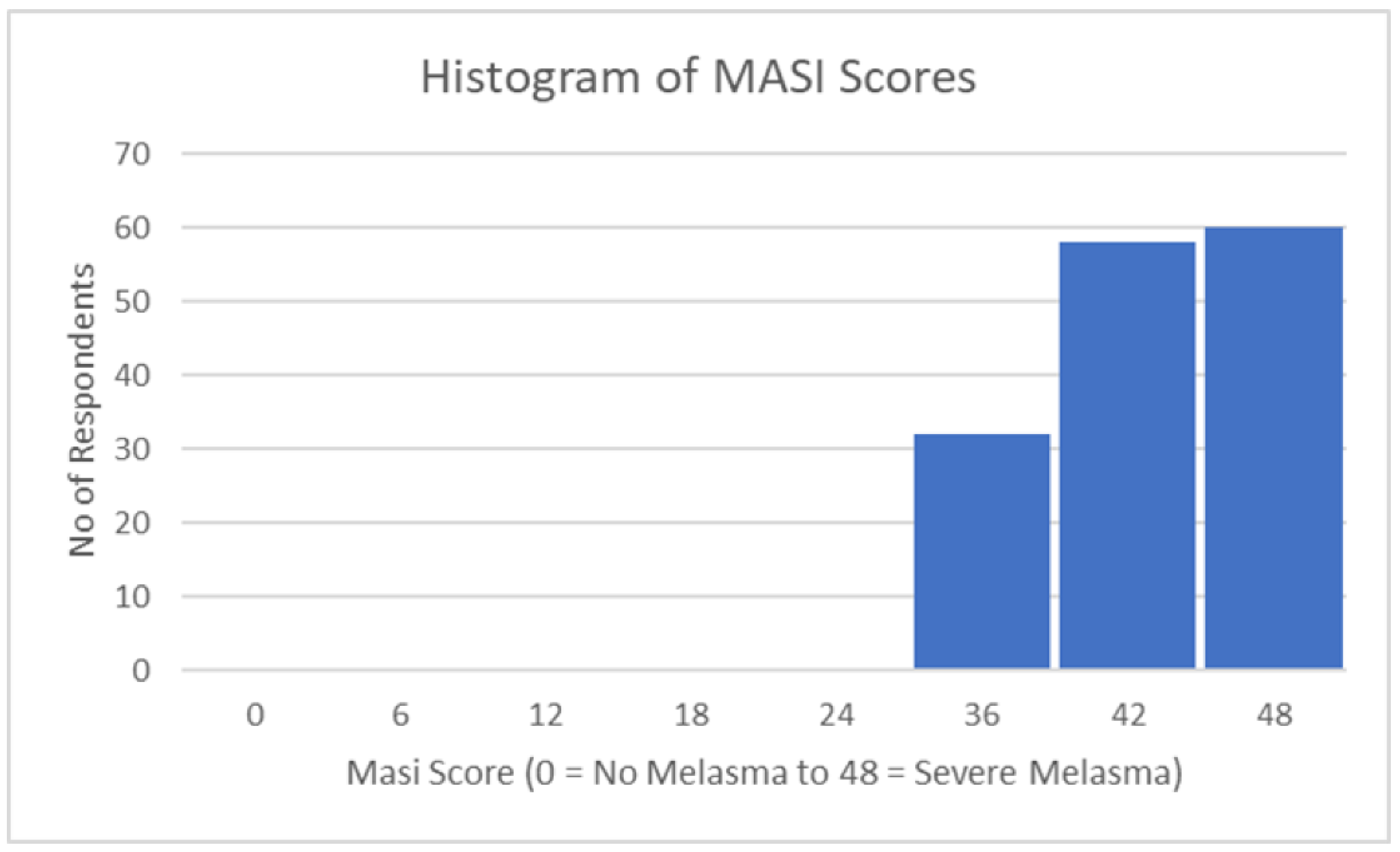
| MASI | MASI grading scale | 40.62 | 4.87 |
| Sun exposure | Times a day | 0.95 | 0.42 |
| SPF | Range from 4 to 100 | 45.09 | 27.64 |
| Sun exposure | Minutes per day | 113.00 | 47.33 |
| Duration of melasma | Years | 6.38 | 4.78 |
| Treatment duration | Months | 10.54 | 13.56 |
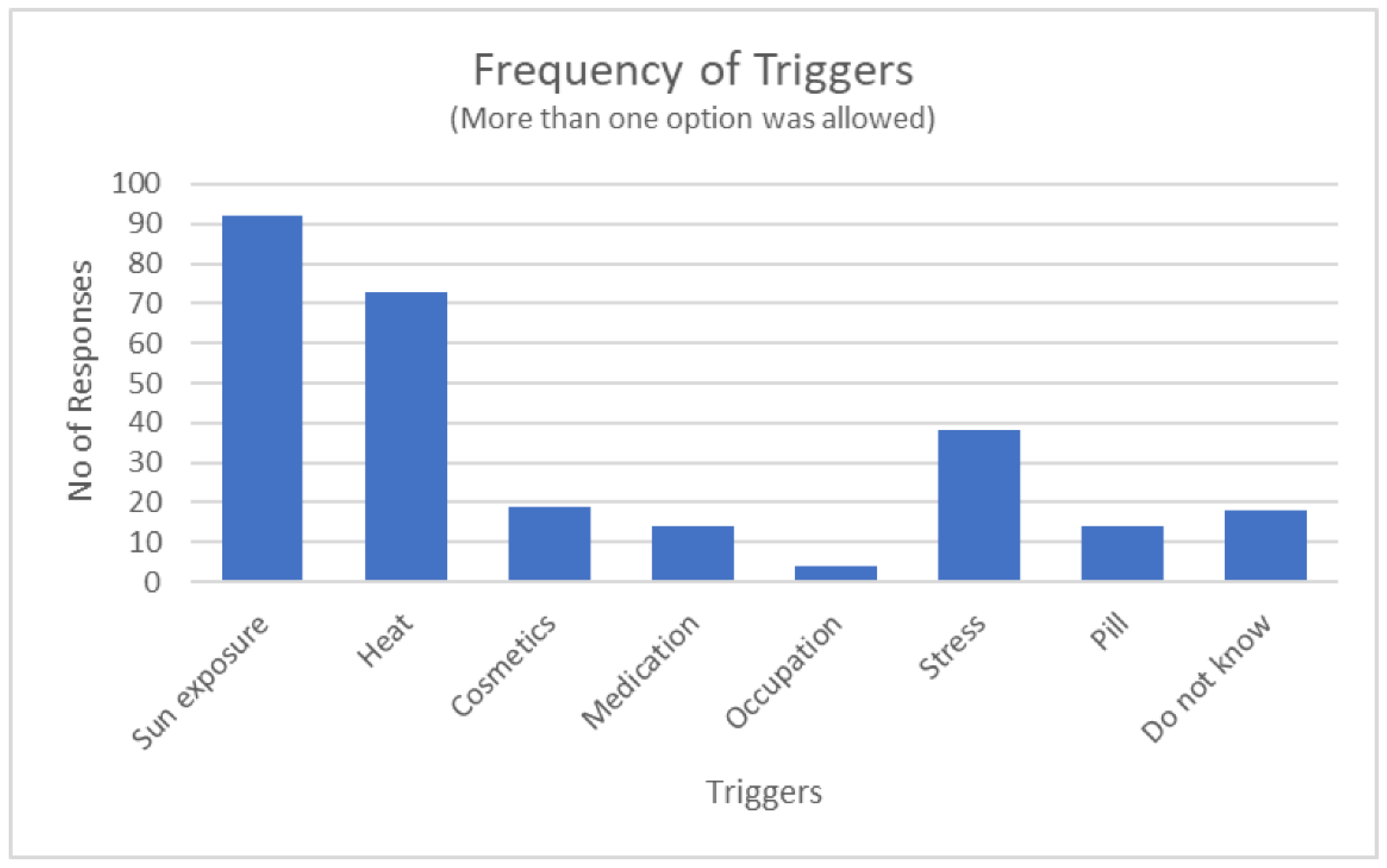
| Triggers | Total number of triggers | 1.81 | 0.72 |
| Sport participation | Number of days per week | 1.01 | 2.22 |
| Dummy Variables |
Frequency of “0” |
Percentage (%) |
Frequency of “1” |
Percentage (%) |
|
|---|---|---|---|---|---|
| Gender | 7 | 5% | 143 | 95% | |
| Education | 13 | 9% | 137 | 91% | |
| Use of sun protection | 16 | 11% | 134 | 89% | |
| Previously consulted with the Doctor | 20 | 13% | 130 | 87% | |
| Familiarity with the word melasma | 16 | 11% | 134 | 89% | |
| Suffers from melasma | 9 | 6% | 141 | 94% | |
| Forehead | 88 | 59% | 62 | 41% | |
| Cheeks | 34 | 23% | 116 | 77% | |
| Jawline | 116 | 77% | 34 | 23% | |
| Nose | 134 | 89% | 16 | 11% | |
| Sides of the face | 111 | 74% | 39 | 26% | |
| Current melasma treatment | 18 | 12% | 132 | 88% | |
| Use of plants as an alternative treatment | 91 | 61% | 59 | 39% | |
| Family history | 91 | 61% | 59 | 39% | |
| Participation in outdoor sport | 117 | 78% | 33 | 22% | |
| Post-menopausal | 101 | 67% | 49 | 33% | |
| HRT | 140 | 93% | 10 | 7% | |
| MASI Statistics | |
|---|---|
| Mean | 40.62 |
| Standard Error | 0.40 |
| Median | 40.00 |
| Mode | 40.00 |
| Standard Deviation | 4.87 |
| Sample Variance | 23.73 |
| Kurtosis | -1.03 |
| Skewness | -0.18 |
| Range | 17.00 |
| Minimum | 31.00 |
| Maximum | 48.00 |
| Sample size | 150 |
| Confidence Level (95,0%) | 0,786 |
| MELASQoL | Likert Scalea | Descriptives | Frequency Distribution |
||||||||
| Questions on… | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Count | Mean | Stdev | |
| Appearance | 0 | 0 | 0 | 3 | 38 | 77 | 32 | 150 | 5,920 | 0,735 |  |
| Frustration | 0 | 0 | 0 | 4 | 37 | 77 | 32 | 150 | 5,913 | 0,748 | |
| Embarrassment | 0 | 0 | 2 | 7 | 40 | 70 | 31 | 150 | 5,807 | 0,862 | |
| Depressed | 0 | 0 | 1 | 5 | 44 | 79 | 21 | 150 | 5,760 | 0,754 | |
| Others | 0 | 0 | 0 | 7 | 39 | 82 | 22 | 150 | 5,793 | 0,742 | |
| Desire | 1 | 0 | 1 | 13 | 40 | 71 | 24 | 150 | 5,667 | 0,943 | |
| Affection | 1 | 0 | 1 | 13 | 44 | 66 | 25 | 150 | 5,647 | 0,953 | |
| Unattractive | 1 | 0 | 2 | 10 | 39 | 74 | 24 | 150 | 5,693 | 0,938 | |
| Unproductive | 1 | 1 | 1 | 21 | 37 | 70 | 19 | 150 | 5,520 | 1,018 | |
| Freedom | 1 | 0 | 1 | 11 | 41 | 76 | 20 | 150 | 5,660 | 0,901 | |
| a7-point Likert Scale ranging from 1 = “Not bothered at all” to 7 = “Constantly bothered”. | |||||||||||
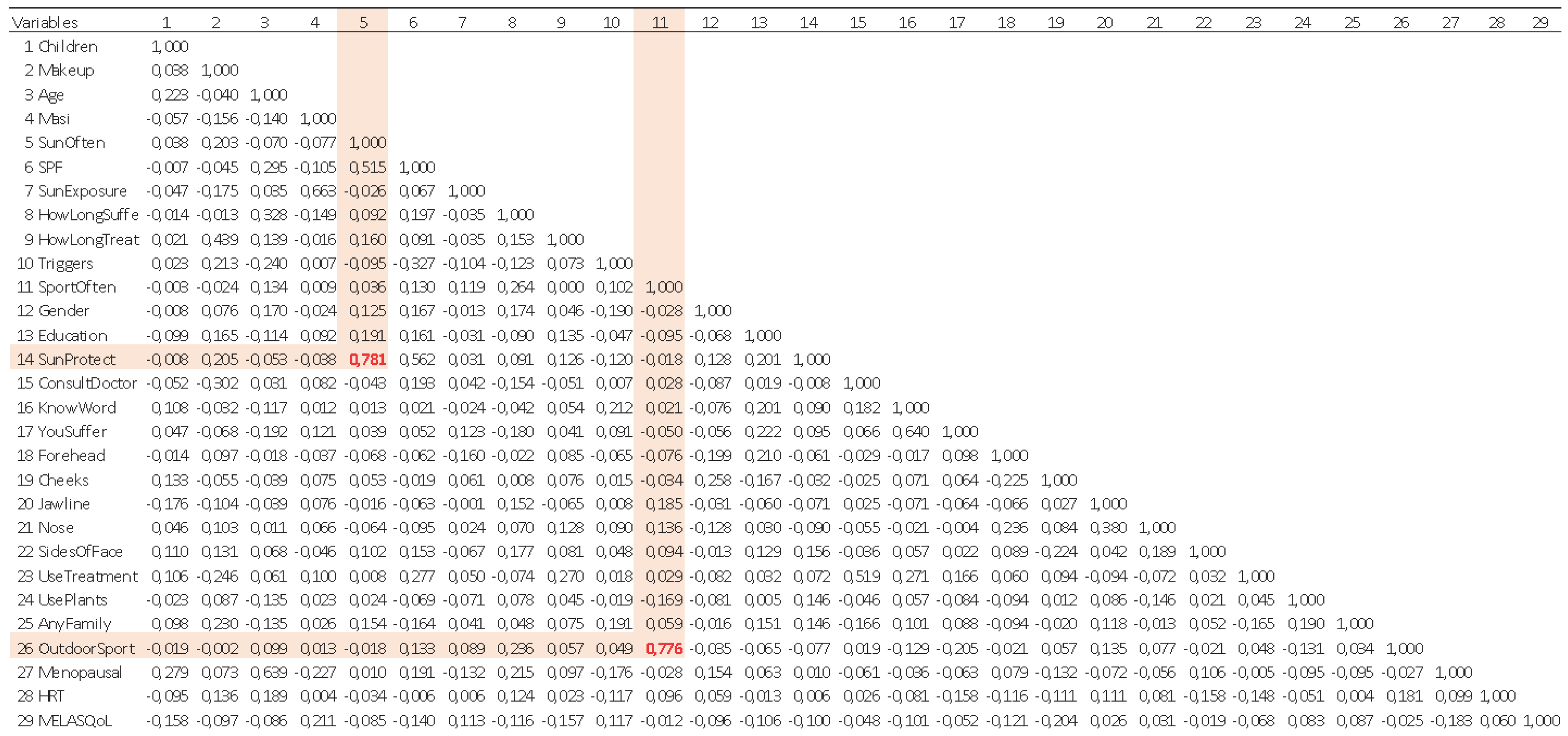
| Model | R | R2 | Adjusted R2 | Std. Error | Durbin-Watson |
|---|---|---|---|---|---|
| 1 | ,211a | 0,044 | 0,038 | 7,210 | |
| 2 | ,305b | 0,093 | 0,081 | 7,047 | |
| 3 | ,348c | 0,121 | 0,103 | 6,962 | |
| 4 | ,381d | 0,145 | 0,122 | 6,889 | 1,951 |
| a Predictors: (Constant), MASI | |||||
| b Predictors: (Constant), MASI, Cheeks | |||||
| c Predictors: (Constant), MASI, Cheeks, Education | |||||
| d Predictors: (Constant), MASI, Cheeks, Education, menopausal | |||||
| e Dependent Variable: MELASQoL | |||||
 |
| Modela | Sum of Squares | Df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| 1 | Regression | 357.63 | 1 | 357.630 | 6.880 | .010b |
| Residual | 7692.768 | 148 | 51.978 | |||
| Total | 8050.398 | 149 | ||||
| 2 | Regression | 749.586 | 2 | 374.793 | 7.546 | <.001c |
| Residual | 7300.812 | 147 | 49.665 | |||
| Total | 8050.398 | 149 | ||||
| 3 | Regression | 973.157 | 3 | 324.386 | 6.692 | <.001d |
| Residual | 7077.241 | 146 | 48.474 | |||
| Total | 8050.398 | 149 | ||||
| 4 | Regression | 1168.115 | 4 | 292.029 | 6.153 | <.001e |
| Residual | 6882.283 | 145 | 47.464 | |||
| Total | 8050.398 | 149 | ||||
| a Dependent Variable: MELASQoL | ||||||
| b Predictors: (Constant), MASI | ||||||
| c Predictors: (Constant), MASI, Cheeks | ||||||
| d Predictors: (Constant), MASI, Cheeks, Education | ||||||
| e Predictors: (Constant), MASI, Cheeks, Education, menopausal | ||||||
| Model | Unstandardized Coefficients |
Std. Error |
Standardized Coefficients |
t-values | Significance | Collinearity Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | Beta | Tolerance | VIF | |||||||
| 1 | (Constant) | 43,376 | 4,960 | 8,745 | <,001 | |||||
| MASI | 0,318 | 0,121 | 0,211 | 2,623 | 0,010 | 1,000 | 1,000 | |||
| 2 | (Constant) | 45,356 | 4,900 | 9,257 | <,001 | |||||
| MASI | 0,343 | 0,119 | 0,227 | 2,886 | 0,004 | 0,994 | 1,006 | |||
| Cheeks | -3,872 | 1,378 | -0,221 | -2,809 | 0,006 | 0,994 | 1,006 | |||
| 3 | (Constant) | 48,710 | 5,086 | 9,577 | <,001 | |||||
| MASI | 0,370 | 0,118 | 0,245 | 3,132 | 0,002 | 0,983 | 1,017 | |||
| Cheeks | -4,391 | 1,383 | -0,251 | -3,175 | 0,002 | 0,964 | 1,037 | |||
| Education | -4,426 | 2,061 | -0,170 | -2,148 | 0,033 | 0,961 | 1,040 | |||
| 4 | (Constant) | 51,730 | 5,249 | 9,856 | <,001 | |||||
| MASI | 0,315 | 0,120 | 0,209 | 2,628 | 0,010 | 0,933 | 1,071 | |||
| Cheeks | -4,686 | 1,376 | -0,268 | -3,405 | <,001 | 0,953 | 1,049 | |||
| Education | -4,148 | 2,044 | -0,159 | -2,029 | 0,044 | 0,957 | 1,045 | |||
| Menopausal | -2,519 | 1,243 | -0,161 | -2,027 | 0,045 | 0,931 | 1,074 | |||
| a Dependent Variable: MELASQoL | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





