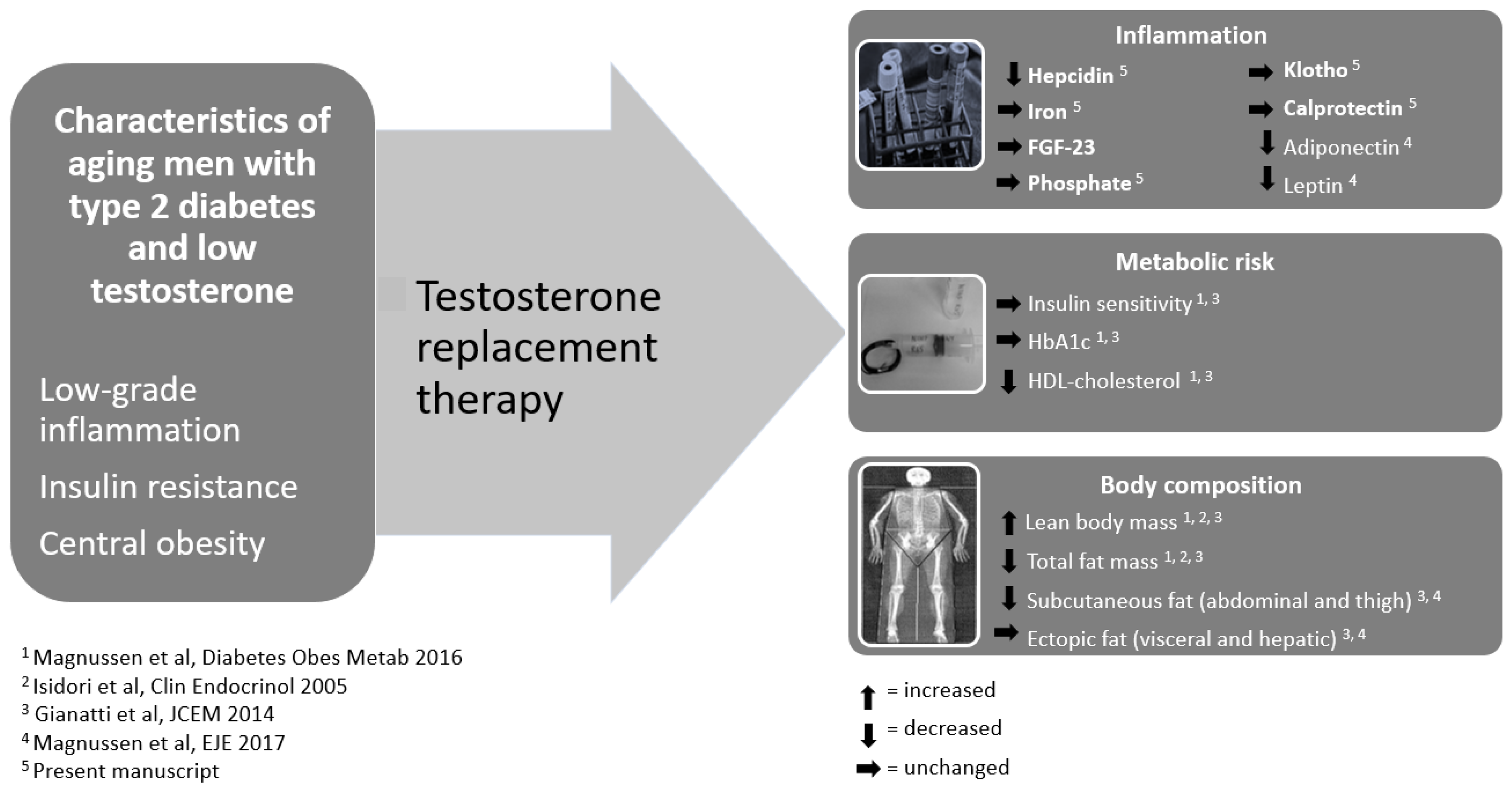
1. Introduction
Type 2 diabetes mellitus (T2D) is associated with high risk of cardiovascular disease (CVD) [
1]. Chronic low-grade inflammation is a hallmark of T2D and inflammation contributes to the development of CVD [
2]. Inflammation [
3], central obesity [
4], and T2D are associated with lower testosterone levels and 58% of men with T2D have hypogonadism [
5,
6]. High endogenous testosterone in men is associated with lower risk of CVD [
7], but the effect of testosterone replacement therapy (TRT) on CVD, in patients at risk, is debated. TRT increases lean body mass and reduces fat mass [
8,
9,
10] including abdominal subcutaneuos fat [
11,
12], whereas no change is seen in insulin resistance, visceral fat, or hepatic fat [
10,
12,
13]. HDL cholesterol and adiponectin levels decrease during TRT compared to placebo [
10], which could suggest increased cardiovascular risk. A large randomized controlled trial (RCT) observed increased number of cardiovascular events during TRT compared to placebo in old men with mobility limitations [
14]. Therefore, since 2015, a warning regarding risk of CVD was applied to testosterone products from the U.S. Food and Drug Administration advisory committee [
15]. However, a recent meta-analysis reported no evidence of increased CVD risk during TRT [
16] and additional data are needed to ensure the safety of TRT also in T2D. Others and we previously reported decreased leptin and leptin/adiponectin ratio in men with T2D during TRT [
12,
17,
18,
19]. Decreased leptin may be of benefit regarding risk of CVD [
12], whereas decreased adiponectin levels might add to CVD risk [
12,
18].
Hepcidin is a peptide hormone produced in the liver [
20]. Hepcidin is responsible for iron homeostasis and high hepcidin decreases iron levels, but hepcidin also increase as an acute phase reactant [
21]. High hepcidin levels may predispose to atherosclerotic lesions and CVD by enhancing iron retention in vascular plaque macrophages thus promoting foam cell formation and plaque instability [
22]. Higher hepcidin levels were seen in 166 men with T2D on metformin mono-therapy compared to 146 healthy controls in an observation study [
23]. Three previous placebo-controlled RCT studies showed decreased hepcidin after 1─3 months TRT [
17,
24,
25]; however, one of the RCT’s reported unchanged hepcidin after 6 months of TRT compared to placebo [
25] and another study only reported results of hepcidin after 3 months of TRT despite a study duration of 12 months [
24].
Activation of the inflammatory pathways fibroblast growth factor 23 (FGF23)-phosphate-klotho [
26,
27], and calprotectin [
28] could decrease circulating levels of iron thus stimulating hepcidin production. We previously reported that phosphate and calprotectin decreased during TRT in aging men with low bioavailable testosterone levels and without T2D [
29]. The effect of TRT on the FGF23-phosphate-klotho or calprotectin pathways has not been investigated in T2D.
In the current study, we investigated changes in the inflammatory cardiovascular risk markers hepcidin-iron, FGF23-phosphate-klotho, and calprotectin pathways during 24 weeks of TRT in men with T2D; secondarily, we investigated if possible changes in inflammatory markers during TRT could be a direct or indirect effect.
2. Materials and Methods
This 24-week, randomized, double-blinded, placebo-controlled trial was conducted at Odense University Hospital (Denmark) from April 2012 to November 2013. Men, aged 50–70 years with T2D on metformin monotherapy for minimum three months and bioavailable testosterone levels <7.3 nmol/L, were included. The study was approved by the local Ethics Committee (identifier: S-20120002) and the Danish Health and Medicines Authority (identifier: 2011-002102-73). The trial was declared in ClinicalTrials.gov (identifier: NCT01560546) and all patients gave written informed consent at the screening visit. The study population is reported in detail elsewhere [
10].
This data represent a secondary analysis of our study that evaluated the effect of TRT on lean body mass in men with T2D and low bioavailable testosterone [
10].
Study design
Patients were randomly assigned to 5 g gel daily containing 50 mg testosterone, Testim
® (TRT, n=22) or placebo (n=21). Patients were increased to 10 g gel daily if bioavailable testosterone levels were <7.3 nmol/L after three weeks treatment [
10]. The patients were examined before and after 24 weeks of TRT. The sample size of the study was determined by the anticipated effect of TRT on total lean body mass [
9] with an assumption of type 1 error (α)=0.05, type 2 error (β)=0.1, SD=1.3 kg, along with a 25% dropout rate, resulting in 20 patients in each group. The patients had fasting blood samples performed. In the present study, the primary outcome measures included changes in hepcidin-iron, FGF23, phosphate, klotho, and calprotectin. Two non-testosterone-related serious adverse events occurred in the study. Safety monitoring were handled externally to ensure continued blinding.
Biochemical variables
Bioactive hepcidin 25 (EIA-5782) FGF23 (EIA-6060), soluble alpha-klotho (EIA-5605), and calprotectin (EIA-5111) were measured using enzyme-linked immunosorbent assays (ELISA) according to the manufacturer instructions. All kits were from DRG Instruments GmbH, Marburg, Germany. Hepcidin and FGF23 showed intra-assay CVs below 8% on kit-controls and for hepcidin a CV% of 9.1 on serum pool control material. Klotho showed a CV% of 10.4 on serum pool control, no kit controls were included with the kit. Calprotectin had a CV between 18.7% and 21.7% on kit-controls and 7.1% on serum-pool control material.
Phosphate and iron were analyzed on Cobas8000 (Cobas®, Roche, Basel, Switzerland) according to routine procedures, accredited under ISO 15189, using photometric assays (PHOS2 ver.2 #05171377 190 and IRON Gen.2 #05169291 190). The procedures included internal and external quality controls.
Lean body mass and total fat mass evaluated by Dual-energy X-ray absorptiometry (DXA)
Results for lean body mass and total fat mass were obtained by DXA scans using a Hologic Discovery device (Waltham, MA) as described in detail elsewhere [
12].
Regional body fat mass evaluated by Magnetic resonance imaging (MRI)
MRI was performed with a 3.0-T high-field MR Unit (Phillips Achieva, Phillips Healthcare). Three abdominal slices and one femoral slice were achieved using an axial, T1-weighted gradient-echo sequence. In-house developed software using MATLAB (MathWorks, Natick, Massachusetts, United States) was applied for automatic segmentation of the images, yielding subcutaneous abdominal adipose tissue (SAT, %fat of total abdominal volume), visceral adipose tissue (VAT, %fat of total abdominal volume) and thigh subcutaneous fat area (TFA, %fat of total thigh volume) as described in detail elsewhere [
12].
Hepatic fat content evaluated by Magnetic resonance spectroscopy (MRS)
Single-voxel liver 1H MRS was performed to measure the hepatic fat content. MRS measurements were performed using a Philips Achieva 3.0-T MR scanner (Philips Healthcare). The MRS data were acquired using a SENSE XL torso coil with 16 channels, following shimming, with volumes of interest (30 × 30 × 30 mm3) manually placed within the right lobe of the liver (segment six or seven). The point-resolved spectroscopy (PRESS) technique was performed without water suppression (repetition time ms/echo time ms, 2000/35). We collected spectra during a single breath hold (17.5 s). The water peak and the major fat peak of methylene, located at 4.7 and 1.3 ppm respectively, were automatically fitted by using a spectroscopic analysis package included in the Philips workstation. Area ratios (hepatic fat/water ratio) were calculated for each patient. An experienced MR spectroscopist, who was blinded to the treatment allocation, reviewed automated spectral results.
Whole-body insulin sensitivity estimated by euglycemic–hyperinsulinemic clamp
After an overnight fast, a 2-h basal tracer equilibration period was followed by a 4-h period with insulin infusion at a rate of 40 U/m2/min. A [3-3H]-glucose infusion was used throughout the 6-h study, and [3-3H]-glucose was added to the glucose infusates to maintain plasma-specific activity constant at baseline levels during the 4-h clamp period. By varying the infusion of 20% glucose based on bedside plasma glucose measurements every 10–20 min, plasma-glucose was kept constant at approximately 5.5 mmol/L. Steele’s non-steady-state formulas were used to calculate the rates of total glucose appearance and glucose disposal (Rd). Insulin-stimulated Rd was taken as an estimate of whole-body insulin sensitivity.
Statistical methods
We performed per-protocol analyses. Descriptive statistics were performed providing results expressed as arithmetic mean ±standard deviation, geometric mean (95% CI), or median (interquartile range) as appropriate for each group (TRT or placebo) at baseline and after 24 weeks of treatment. Differences in baseline values were analyzed using unpaired t-test on normally distributed data. Wilcoxon rank-sum tests were conducted at baseline if data could not be transformed to normally distributed data using natural logarithm. Outcome measurements were assessed by multiple linear regression analyses controlled for baseline values on normally distributed data for the placebo-controlled mean effect of intervention between groups (β). The models were checked with residual plots and Box–Cox analysis. Absolute changes during 24 weeks from baseline are given as delta values (∆). For nonparametric data, outcome measurements were assessed by delta values using the Wilcoxon-Mann-Whitney test for comparison of treatment groups.
The tests were done two-sided and results of P<0.05 were considered statistically significant. Pearson’s bivariate correlation test or non-parametric Spearman rank correlation were performed as appropriate for analysis of correlations between the measured parameters (hepcidin-iron, FGF23, phosphate, klotho, and calprotectin) as well as correlations with previously obtained results for lean body mass, total fat mass, insulin sensitivity during clamp, hepatic fat, SAT, VAT, TFA, HDL-cholesterol, adiponectin, leptin, bioavailable testosterone levels, hemoglobin, and hematocrit [
10]. Statistical analyses were performed with STATA, version 16.
3. Results
At baseline, TRT and placebo groups were comparable regarding all study outcomes (
Table 1).
During TRT, hepcidin levels decreased compared to placebo after 24 weeks of treatment (
Table 1). Iron, FGF23, phosphate, klotho, and calprotectin were unchanged during 24 weeks of TRT compared to placebo (
Table 1).
Lean body mass, hemoglobin, and hematocrit increased, whereas total fat mass, HDL-cholesterol, adiponectin, and leptin decreased in the TRT group (
Table 1). Furthermore, insulin sensitivity and ectopic fat, viscerally and in the liver, were unchanged during TRT (
Table 1,
Figure 1).
3.1. Correlations
In the TRT-group, no significant correlations were found between delta values of hepcidin and iron, FGF23-phosphate-klotho, calprotectin, hemoglobin, hematocrit, HDL-cholesterol, adiponectin, leptin, insulin sensitivity, or body composition.
4. Discussion
The main finding of the present study was a significant reduction of hepcidin following 24 weeks of TRT compared to placebo in men with T2D and low bioavailable testosterone levels. The decrease in hepcidin was not related to changes in circulating iron levels or body composition, which suggested a direct effect of TRT.
Our finding of reduced hepcidin during TRT for 24 weeks added significant knowledge to previous RCTs on the topic in aging men with T2D [
17], mobility problems [
25], and anemia [
24]. Hepcidin levels decreased during 1–3 months TRT in all three studies [
17,
24,
25], but was unchanged compared to placebo after 6 months in the study by Bachman et al. [
25] and not reported in the study by Artz et al. [
24]. Interestingly, the included study populations differed significantly in previous studies [
17,
24,
25]. Our present cohort included men (aged 50─70 years) with T2D treated with continuous, stable metformin monotherapy throughout the study, which implied well-regulated glycemic control, and a duration of T2D of app. 3.5 years before study inclusion. Consistent to our study, Artz et al. [
24] measured hepcidin by a competitive assay and baseline hepcidin was comparable to ours (each app. 20 ng/mL) [
24]. However, Artz et al. [
24] included aging men with anemia, divided the study cohort according to type of anemia, and found that TRT suppressed hepcidin levels in men with unexplained anemia, whereas hepcidin levels were unchanged in men with iron deficiency [
24]. The authors concluded that TRT stimulated erythropoiesis associated with increased iron mobilization, but this effect was attenuated by iron deficiency [
24]. Anemia is an important driver of hepcidin [
30], but notably, all men in our study had normal hemoglobin and iron levels by study inclusion. Iron as well as FGF23-phosphate-klotho pathway and calprotectin were unchanged during TRT, and delta hepcidin was not associated with changes in iron in the present study. These findings supported that decreased hepcidin during TRT was not mediated by increased iron mobilization opposite the conclusion by Artz et al. [
24] at least after 24 weeks of TRT.
In contrast to our study, Dhindsa et al. [
17] included men with T2D of longer duration (app. 10 years) using varying antidiabetic treatment with the allowance to change anti-hyperglycemic medications during the trial including insulin (50%) and supra-physiological testosterone dose was applied [
17]. The study by Bachman et al. [
25] included older men (>65 years) with mobility limitation and high burden of chronic diseases including 50% with heart disease, and the study was terminated due to increased CVD events in the TRT group [
25]. Hepcidin production is stimulated by inflammation [
20] and hepcidin levels are associated with obesity and duration and severity of T2D [
23,
31]. In accordance, our level of hepcidin (20 ng/mL) was considerably lower compared to hepcidin levels in the study by Dhindsa et al. [
17] and Bachman et al. [
25], i.e., 200 ng/mL [
17] and 100 ng/mL [
25], respectively, which could suggest more inflammation [
17,
25]. Furthermore, Bachman et al. [
25] and Dhindsa et al. [
17] used conventional ELISA [
17,
25] and technical differences in the methods applied could affect study results [
32].
Insulin sensitivity, evaluated by euglycemic clamp [
10], was unchanged in the present study, and changes in hepcidin was not related to changes in body composition. These findings further suggested a direct effect of TRT on hepcidin. Our previous observation of reduced phosphate and calprotectin in aging men without T2D during TRT compared to placebo [
29] was not observed in men with T2D.
Strengths and limitations apply in the present study. Our study was strengthened by the inclusion of patients based on biochemical hypogonadism, testosterone evaluation by the gold standard method i.e., liquid chromatography tandem mass spectrometry, per-protocol-analyses, a low dropout rate, and no dropouts were due to adverse effects of the testosterone gel. The original study was primarily designed to evaluate the effects of TRT on lean body mass. We acknowledge that the present study could be underpowered to identify significant alterations in some of the inflammatory markers.
In conclusion, TRT decreased hepcidin levels in older men with T2D and low bioavailable testosterone levels. Our data supported a direct anti-inflammatory effect of TRT after 24 weeks as changes in hepcidin were not associated with changes in iron, lean body mass or fat deposits.
Author Contributions
MSA, DG, and LVM conceived and designed the study protocol. LHJ was responsible for hormone analyses. LVM was responsible for data collection. LVM and LHJ contributed equally to data analyses and writing. MSA and DG contributed to interpretation of data and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Novo Nordic Foundation, the Danish Diabetes Academy (through a grant from the Novo Nordic Foundation), Odense University Hospital, Institute of Clinical Research at the University of Southern Denmark, the Region of Southern Denmark, Consultant Council Scholarship Committee of Odense University Hospital, and the Christenson-Ceson Family Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee (identifier: S-20120002) and the Danish Health and Medicines Authority (identifier: 2011-002102-73). The trial was declared in ClinicalTrials.gov (identifier: NCT01560546),.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank all the participating patients in the study.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011;123(4):e18-e209. [CrossRef]
- Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity 2022;55(1):31-55. [CrossRef]
- Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes care 2006;29(10):2289-94. [CrossRef]
- Nielsen TL, Hagen C, Wraae K, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. The Journal of clinical endocrinology and metabolism 2007;92(7):2696-705. [CrossRef]
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. The Journal of clinical endocrinology and metabolism 2007;92(11):4241-7. [CrossRef]
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes care 2010;33(6):1186-92. [CrossRef]
- Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. Journal of the American College of Cardiology 2011;58(16):1674-81. [CrossRef]
- Frederiksen L, Hojlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordrecht, Netherlands) 2012;34(1):145-56. [CrossRef]
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical endocrinology 2005;63(3):280-93. [CrossRef]
- Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund K, Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes Metab 2016;18(10):980-9. [CrossRef]
- Frederiksen L, Hojlund K, Hougaard DM, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. European journal of endocrinology / European Federation of Endocrine Societies 2012;166(3):469-76. [CrossRef]
- Magnussen LV, Andersen PE, Diaz A, et al. MR spectroscopy of hepatic fat and adiponectin and leptin levels during testosterone therapy in type 2 diabetes: a randomized, double-blinded, placebo-controlled trial. European Journal of Endocrinology 2017;177(2):157-68. [CrossRef]
- Gianatti EJ, Dupuis P, Hoermann R, et al. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes care 2014;37(8):2098-107. [CrossRef]
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. The New England journal of medicine 2010;363(2):109-22. [CrossRef]
- Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “Age-Related Hypogonadism” — FDA Concerns. New England Journal of Medicine 2015;373(8):689-91. [CrossRef]
- Hudson J, Cruickshank M, Quinton R, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev 2022;3(6):e381-e93. [CrossRef]
- Dhindsa S, Ghanim H, Batra M, et al. Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and type 2 diabetes. Clinical endocrinology 2016;85(5):772-80. [CrossRef]
- Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. European journal of endocrinology / European Federation of Endocrine Societies 2007;156(5):595-602. [CrossRef]
- Gianatti EJ, Dupuis P, Hoermann R, Zajac JD, Grossmann M. Effect of testosterone treatment on constitutional and sexual symptoms in men with type 2 diabetes in a randomized, placebo-controlled clinical trial. The Journal of clinical endocrinology and metabolism 2014;99(10):3821-8. [CrossRef]
- Sangkhae V, Nemeth E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv Nutr 2017;8(1):126-36. [CrossRef]
- Chambers K AM, Sharma S. Physiology, Hepcidin. 2022 Jan.
- Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta 2009;1790(7):718-23. [CrossRef]
- Andrews M, Soto N, Arredondo-Olguín M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition (Burbank, Los Angeles County, Calif.) 2015;31(1):51-7. [CrossRef]
- Artz AS, Stephens-Shields AJ, Bhasin S, Ellenberg SS, Cohen HJ, Snyder PJ. Markers of Iron Flux during Testosterone-Mediated Erythropoiesis in Older Men with Unexplained or Iron-Deficiency Anemia. The Journal of clinical endocrinology and metabolism 2020;105(11):3396-403. [CrossRef]
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. The journals of gerontology. Series A, Biological sciences and medical sciences 2014;69(6):725-35. [CrossRef]
- Yeung SMH, Binnenmars SH, Gant CM, et al. Fibroblast Growth Factor 23 and Mortality in Patients With Type 2 Diabetes and Normal or Mildly Impaired Kidney Function. Diabetes care 2019;42(11):2151-53. [CrossRef]
- Nie F, Wu D, Du H, et al. Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J Diabetes Complications 2017;31(3):594-98. [CrossRef]
- Pedersen L, Nybo M, Poulsen MK, Henriksen JE, Dahl J, Rasmussen LM. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC cardiovascular disorders 2014;14:196. [CrossRef]
- Pedersen L, Christensen LL, Pedersen SM, Andersen M. Reduction of calprotectin and phosphate during testosterone therapy in aging men: a randomized controlled trial. J Endocrinol Invest 2017;40(5):529-38. [CrossRef]
- Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and Anemia: A Tight Relationship. Front Physiol 2019;10:1294. [CrossRef]
- Aregbesola A. Serum hepcidin concentrations and type 2 diabetes. World Journal of Diabetes 2015;6(7):978. [CrossRef]
- Zaritsky J, Young B, Wang HJ, et al. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol 2009;4(6):1051-6. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).