Submitted:
13 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
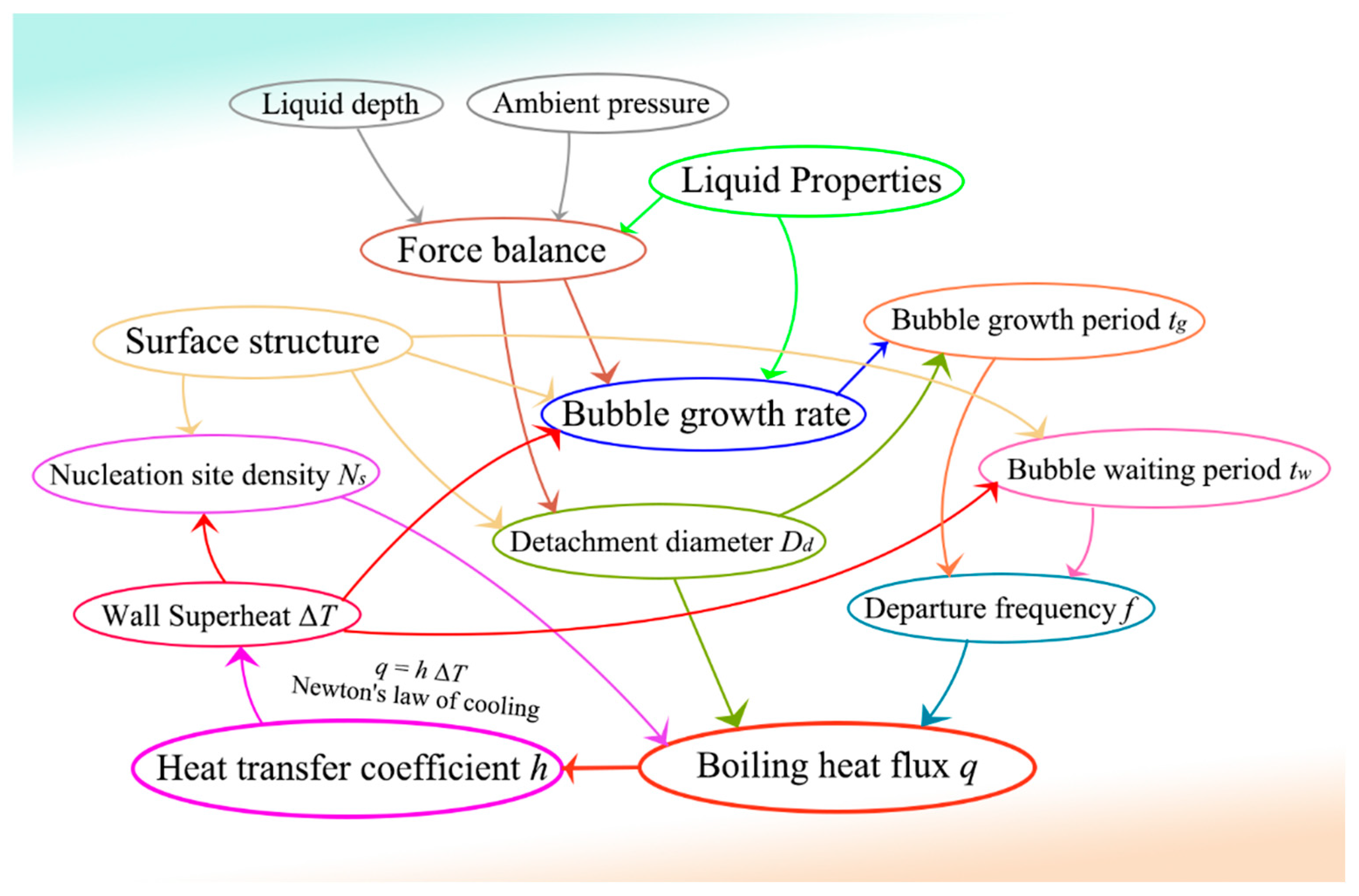
1. Introduction
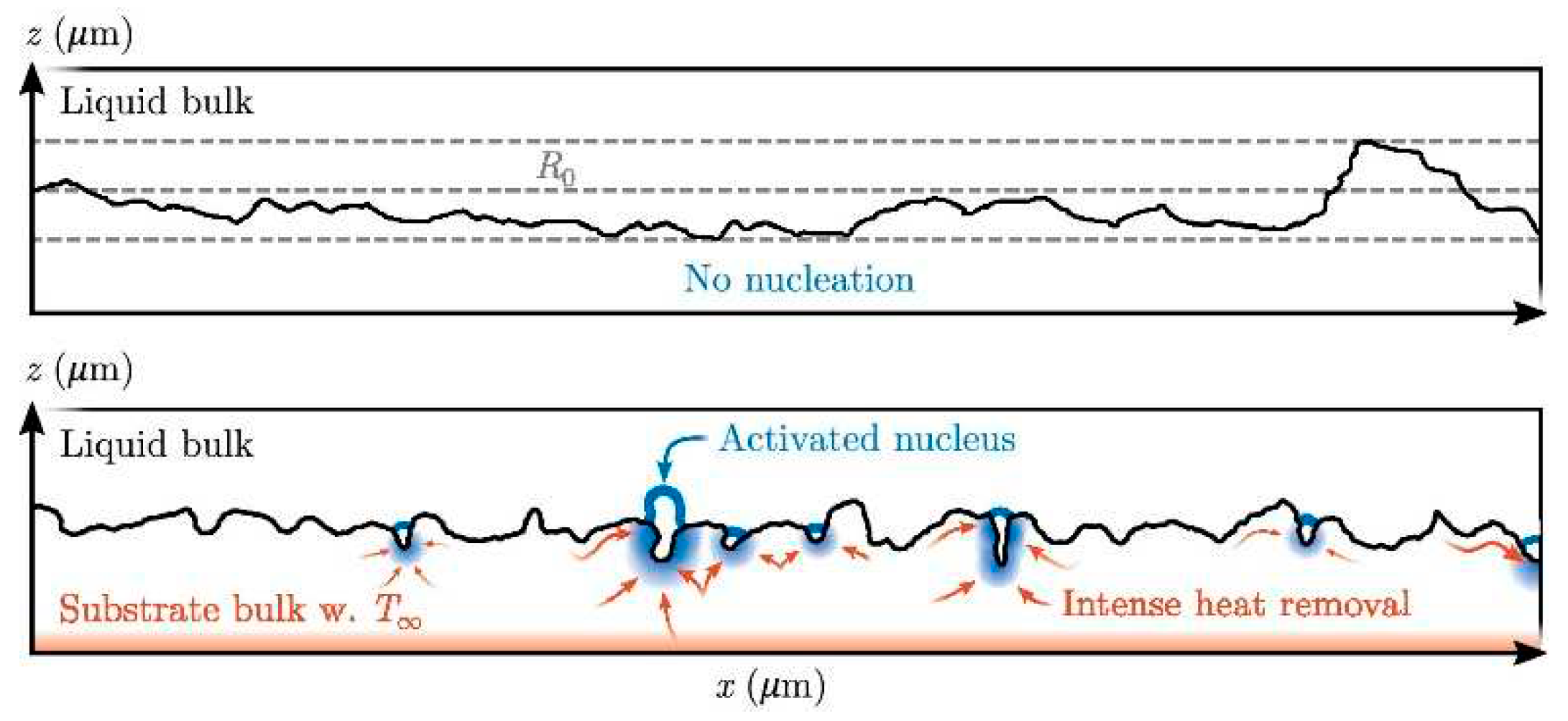
2. Bubble nucleation
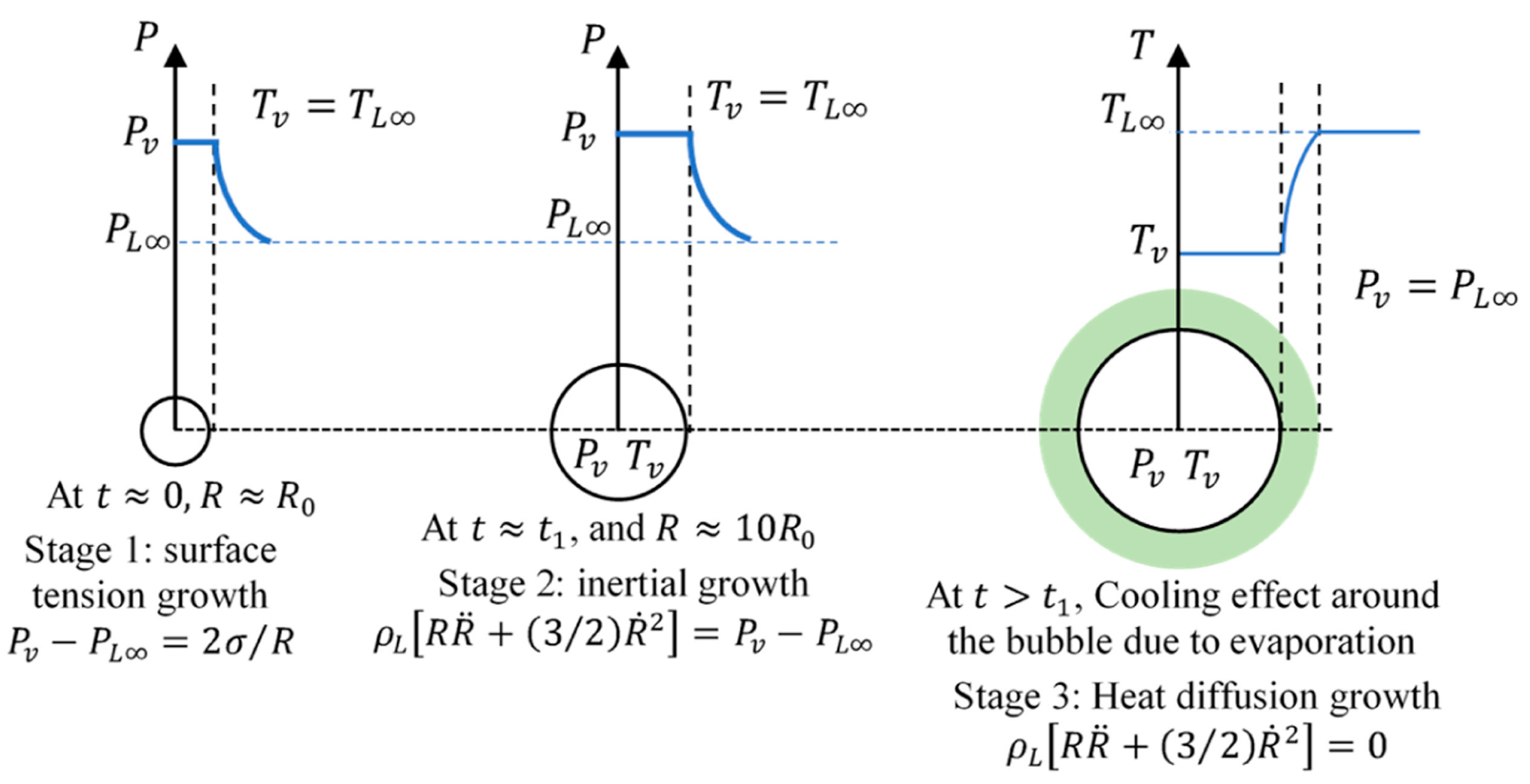
2.1. Homogeneous nucleation
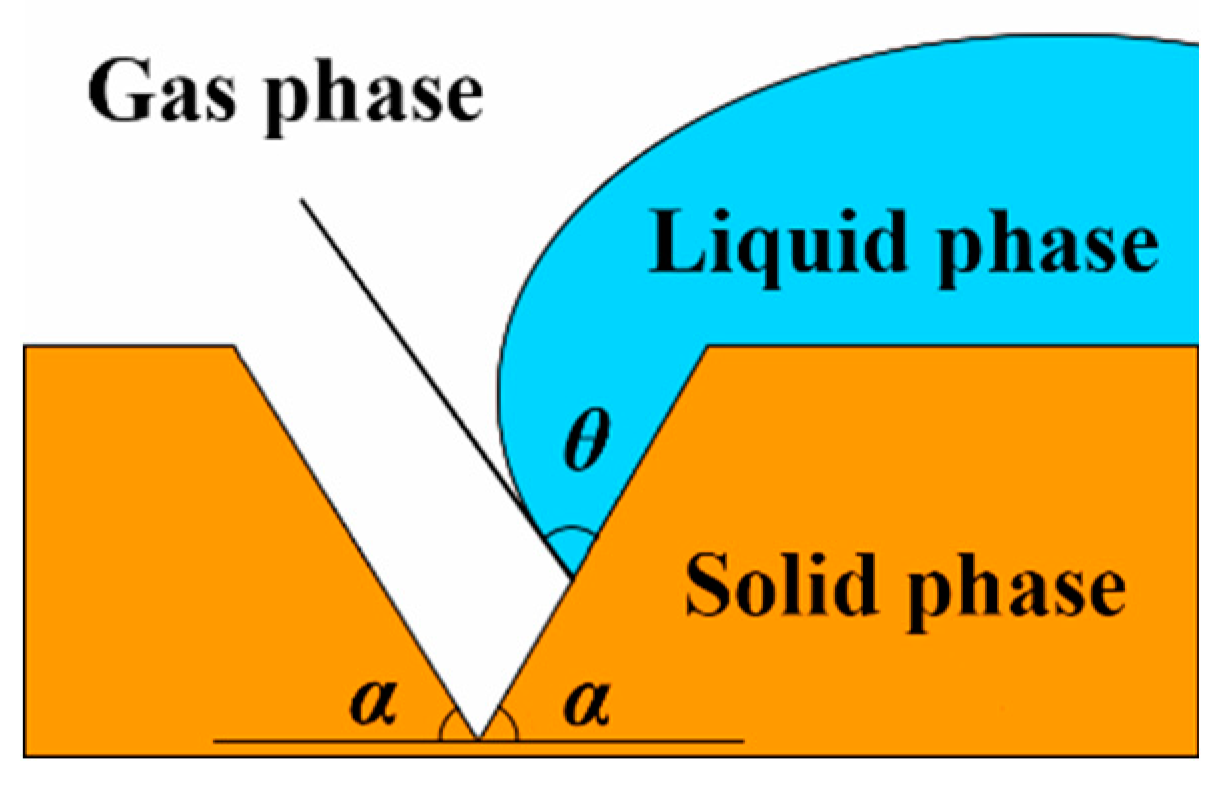
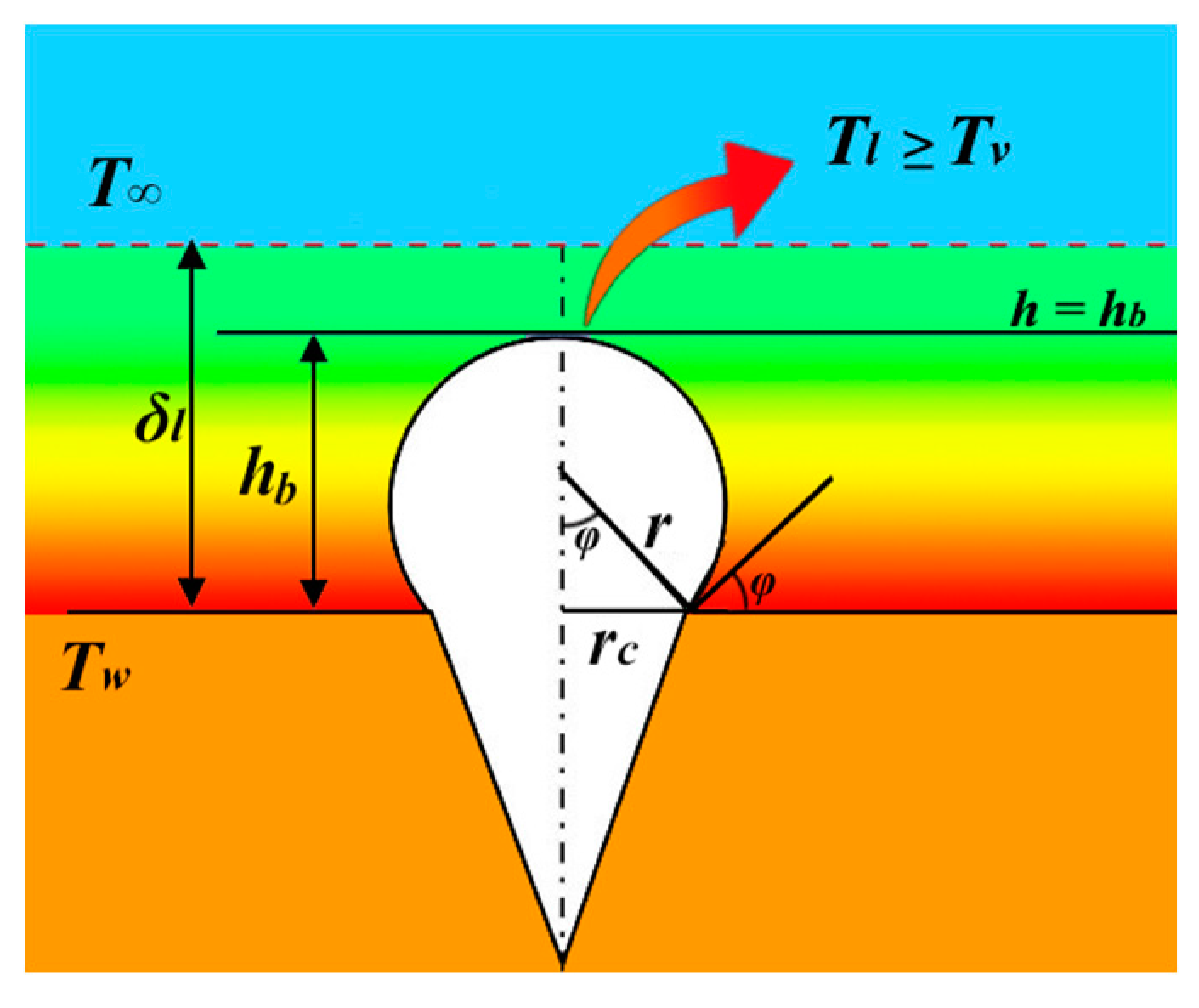
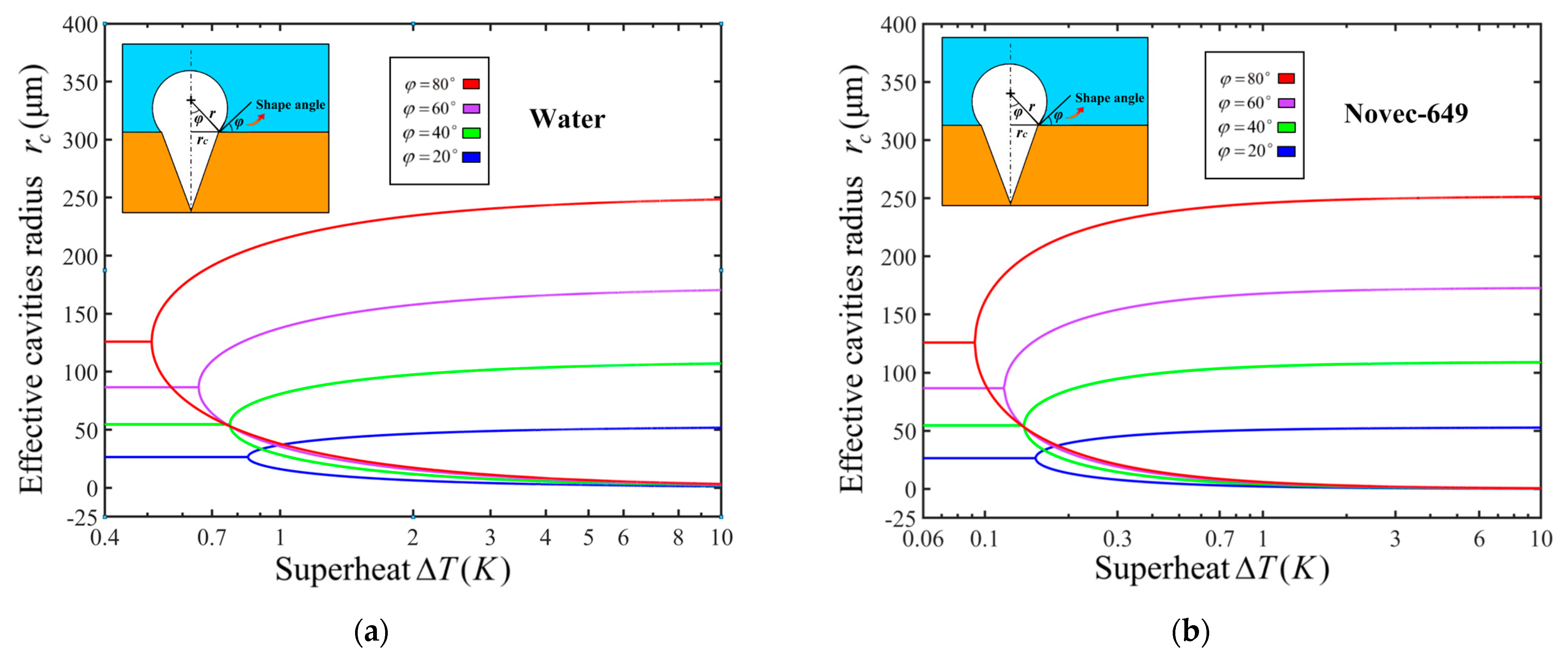
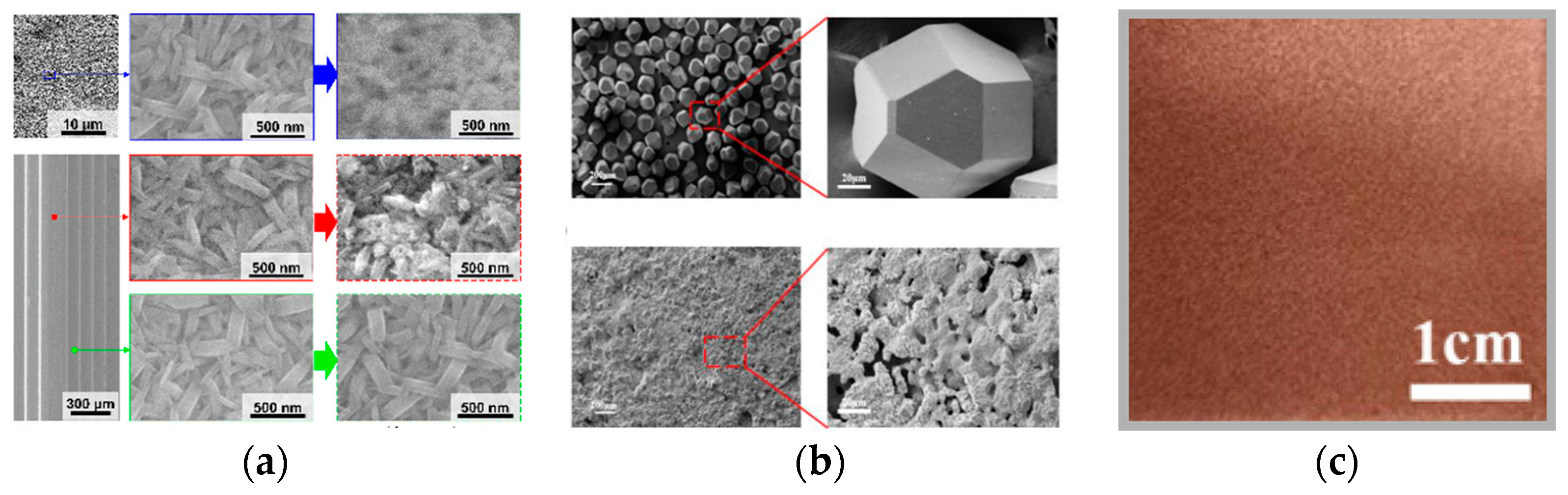
2.2. Heterogeneous nucleation
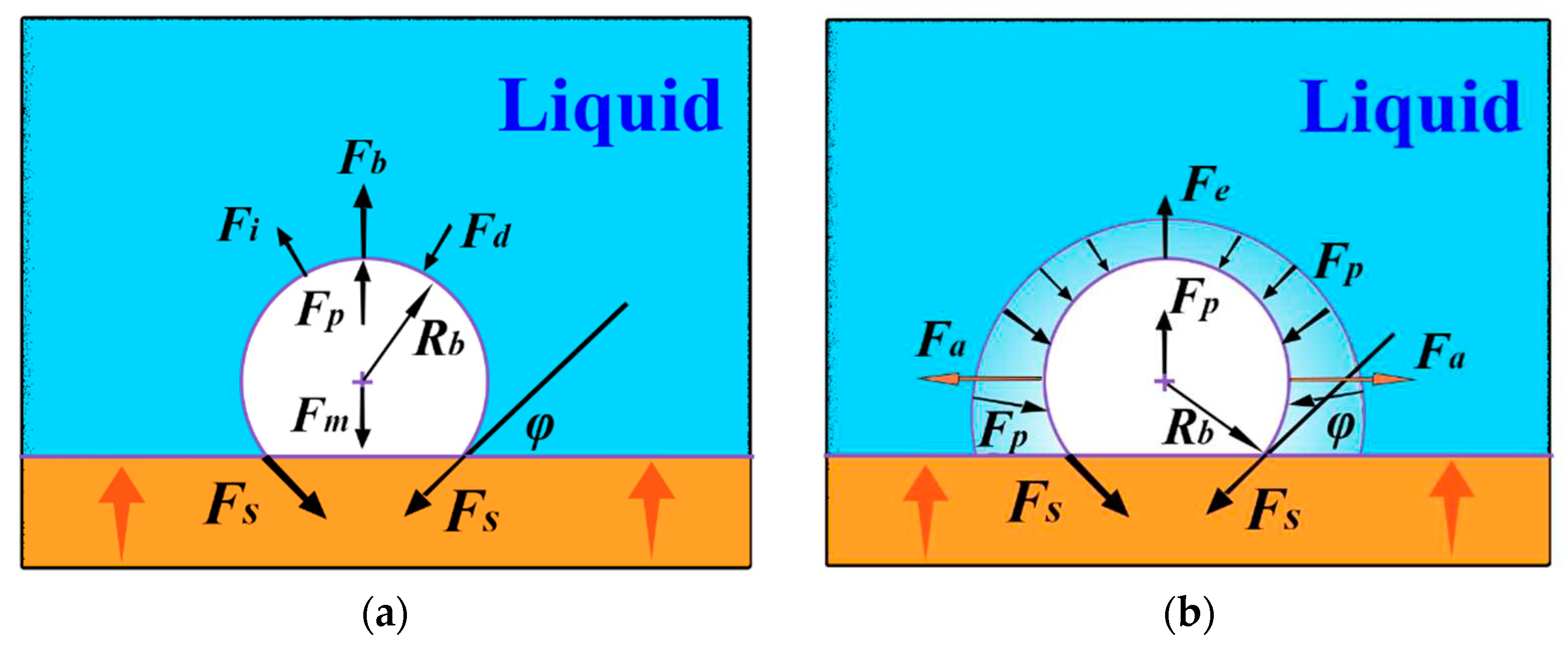
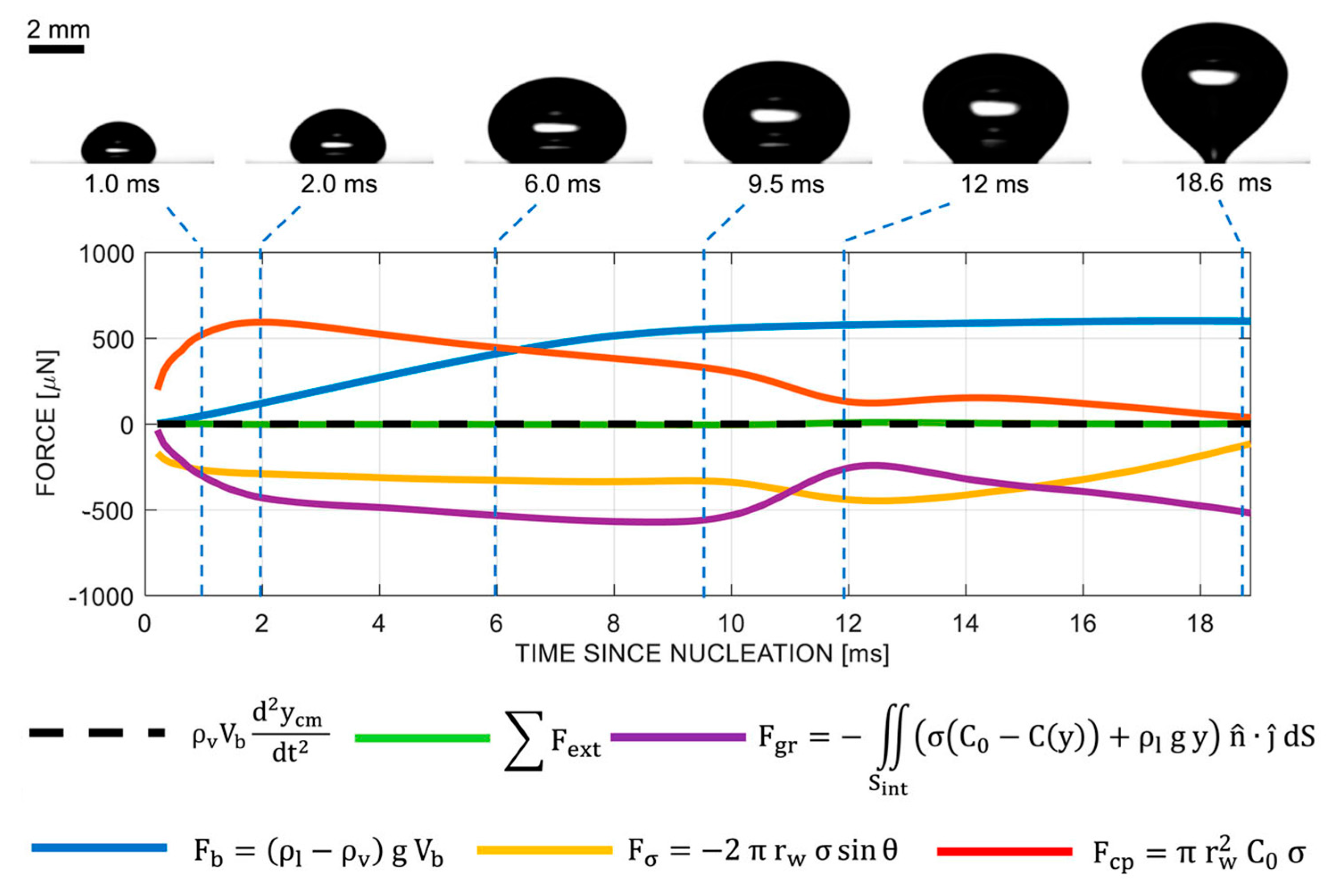
3. Mechanical analysis of the bubble
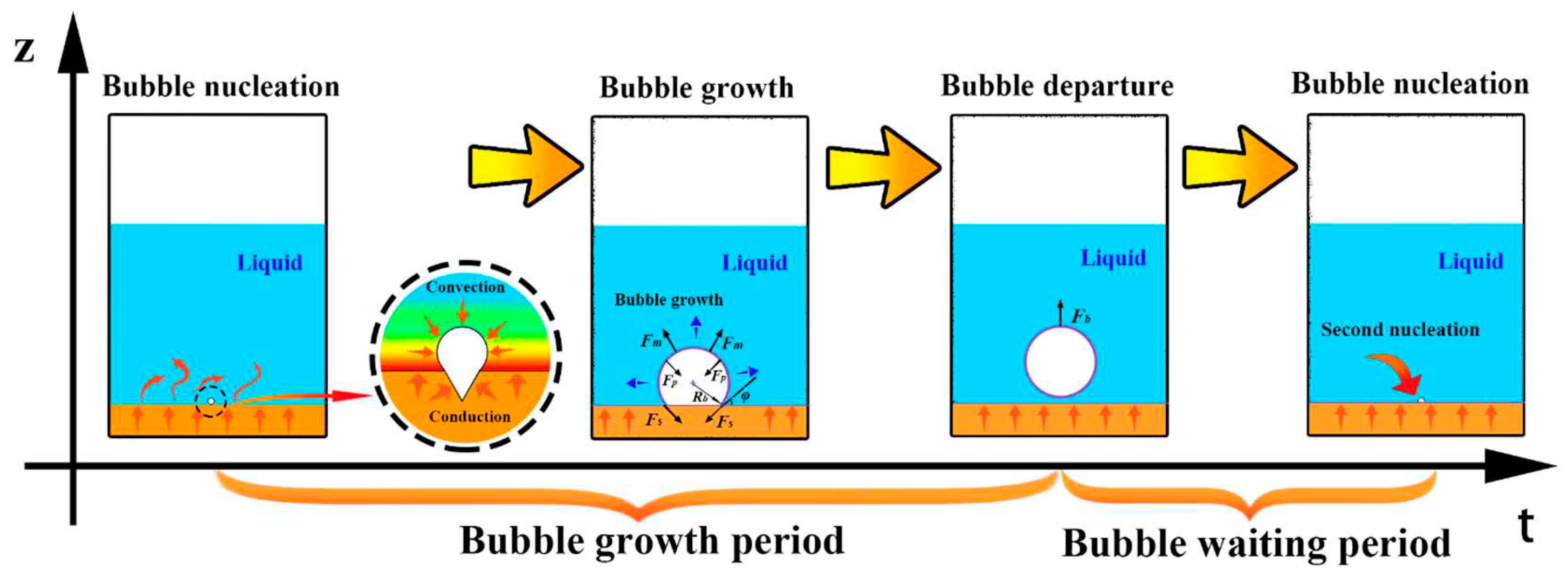
4. Bubble growth and detachment model
4.1. Bubble growth in uniform boiling
4.2. Bubble growth in non-uniform boiling
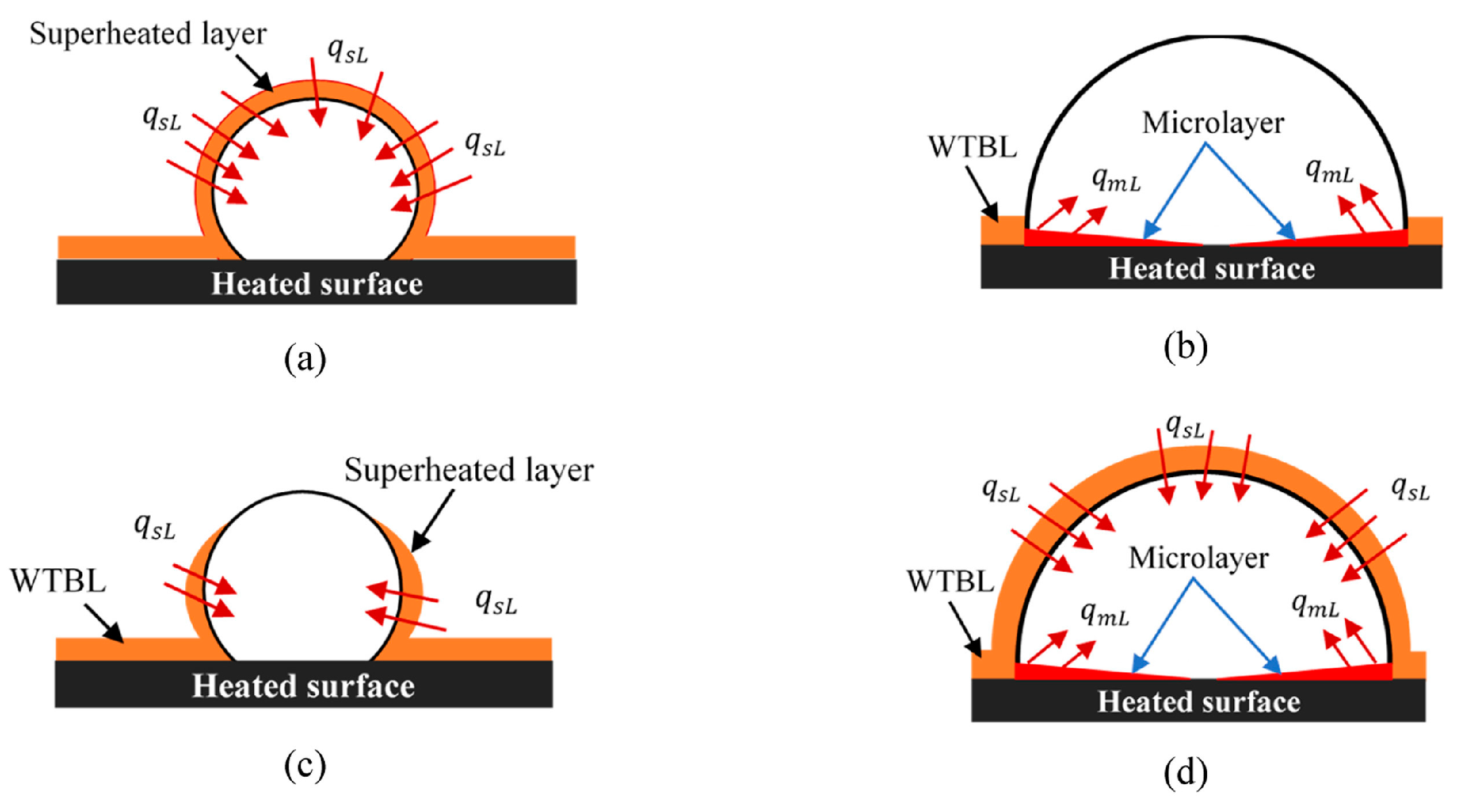
4.2.1. Thermal boundary layer bubble growth modeling
4.2.2. Empirical modeling of bubble growth
4.3. Bubble detachment diameter
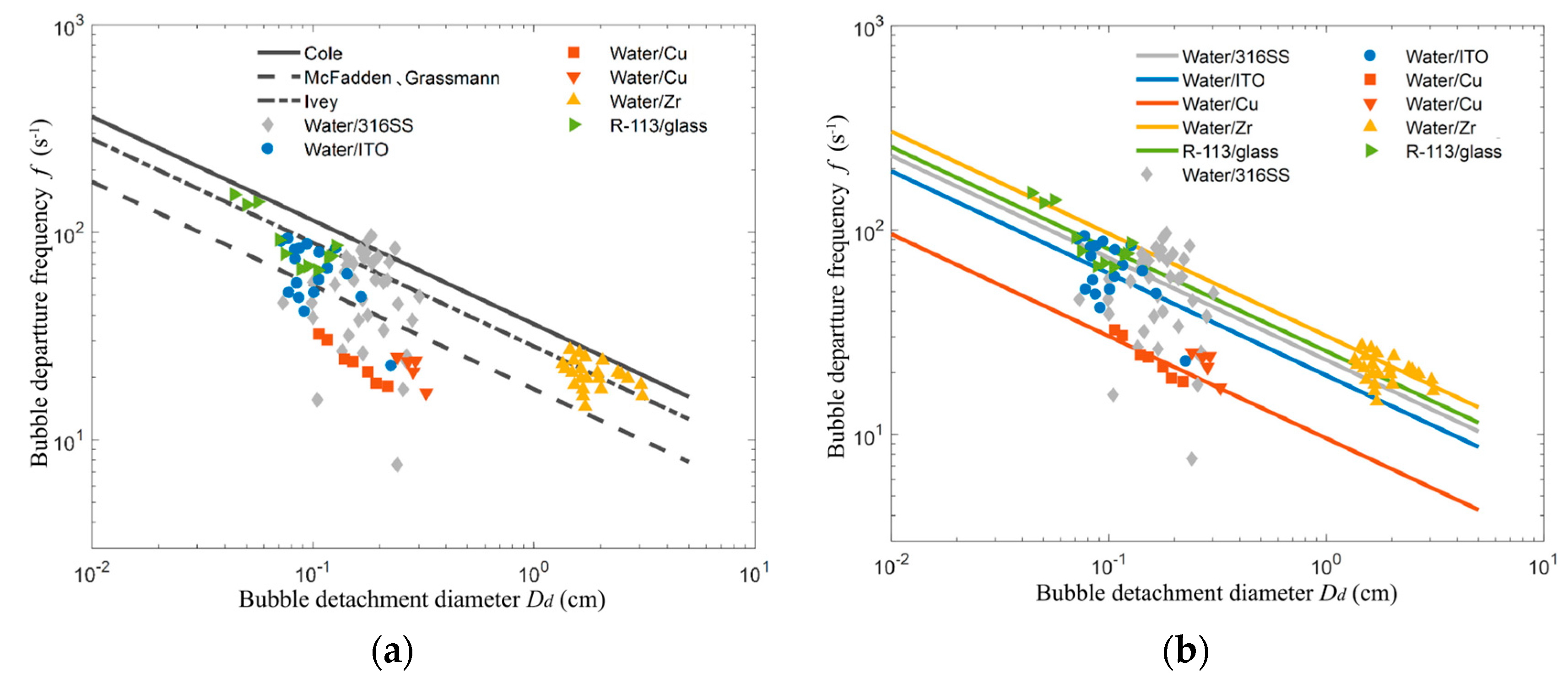
5. Bubble departure frequency
5.1. Bubble growth period tg
5.2. Bubble waiting period tw
5.3. Bubble departure frequency
6. Boiling heat transfer coefficient h and heat flux q
6.1. Boiling heat transfer coefficient h
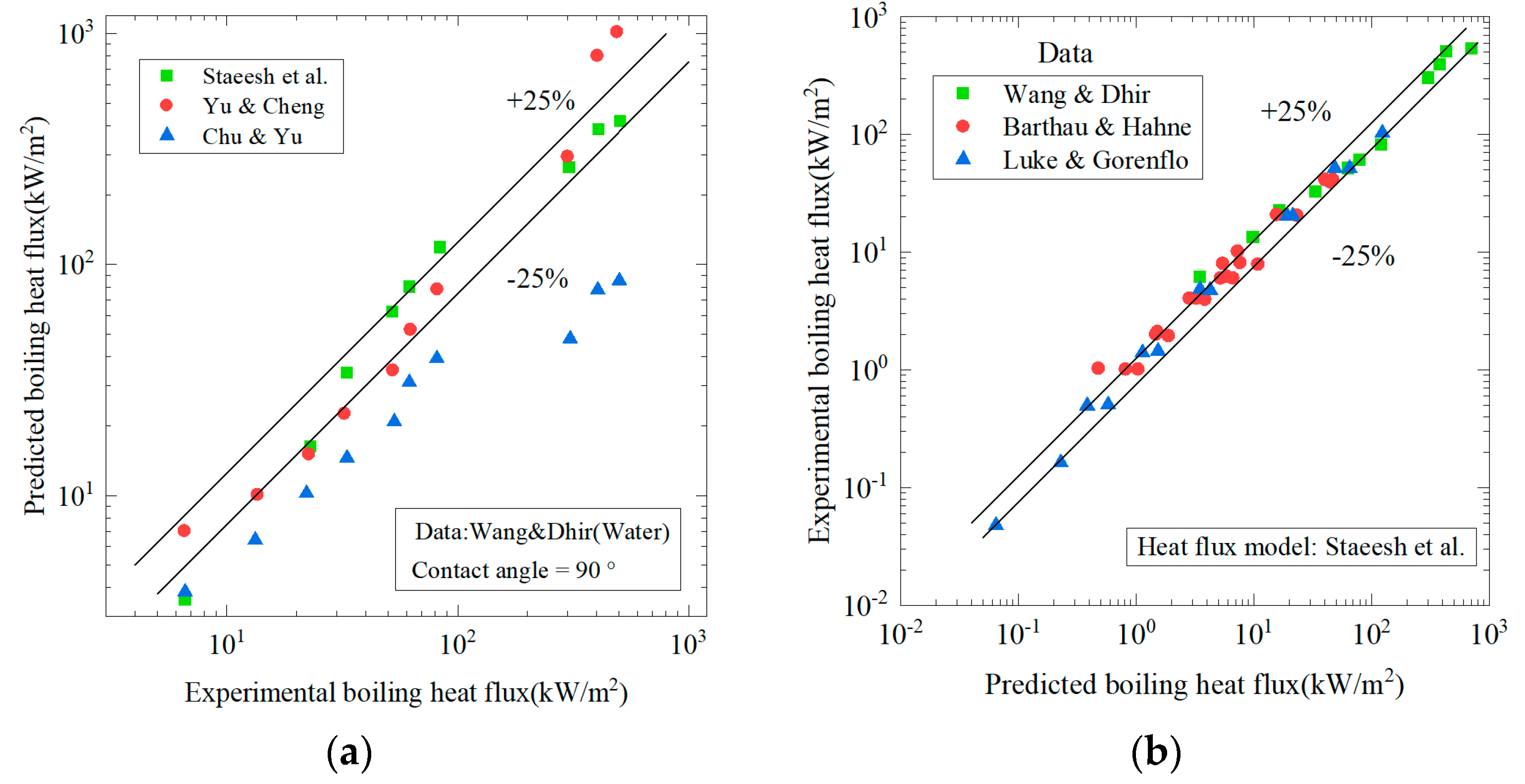
6.2. Heat flux q
7. Conclusions
Acknowledgments
Abbreviations
| \Nomenclature | |||
| Abbreviations | Greek symbols | ||
| Ar | Archimedes number | thermal diffusivity(m2 s-1) | |
| Ca | Capillary number() | density(kg m-3) | |
| CL | lift-off coefficient | difference | |
| Cp | specific heat at constant pressure(J kg-1K-1) | dynamic viscosity (kg m-1s-1) | |
| Dd | bubble departure diameter (m) | kinematic viscosity (m2s-1) | |
| f | bubble departure frequency (s-1) | thermal boundary layer thickness (μm) | |
| Fb | buoyancy force(N) | surface tension (N m-1) | |
| Fdu | unsteady drag force(N) | contact angle (°) | |
| Fm | excess vapor pressure (N) | shape angle (°) | |
| Fp | liquid static pressure(N) | ||
| Fs | surface tension(N) | Superscripts | |
| g | gravitational acceleration(m s-2) | + | non-dimensional |
| h | heat transfer coefficient(W m-2 K-1) | ||
| H | height of liquid(m) | Subscripts | |
| hlv | latent heat of vaporization (J kg-1) | c | cavity |
| Ja | Jakob number() | d | bubble departure |
| k | thermal conductivity (W m-1 K-1) | l | liquid phase |
| Nu | Nusselt number | max | maximum |
| Ns | active nucleation site density (m-2) | me | microlayer evaporation |
| p | pressure (bar) | min | minimum |
| Pr | Prandtl number | nc | natural convection |
| q | heat flux (W m-2) | r | reduced property |
| Ra | surface roughness (μm) | sat | saturation condition |
| Rb | bubble radius (m) | tc | transient conduction |
| Rc | cavity radius (m) | s | heating surface |
| S | dimensionless surface parameter | v | vapor phase |
| T | temperature (K) | w | wall |
| t | time (s) | bulk | |
| tw | bubble waiting period (s) | ||
| tg | bubble growth period (s) | ||
| v | specific volume(m3 kg-1) | ||
References
- Wu, X.; Huang, J.; Zhuang, Y.; Liu, Y.; Yang, J.; Ouyang, H.; Han, X. Prediction Models of Saturated Vapor Pressure, Saturated Density, Surface Tension, Viscosity and Thermal Conductivity of Electronic Fluoride Liquids in Two-Phase Liquid Immersion Cooling Systems: A Comprehensive Review. Appl. Sci. 2023, 13, 4200. [Google Scholar] [CrossRef]
- Wu, X.; Li, C.; Yang, J.; Liu, Y.; Han, X. Theoretical and experimental research on flow boiling heat transfer in microchannels for IGBT modules. Int. J. Heat Mass Transf. 2023, 205. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, M.; Jiang, Y.; Liu, J. Two-stage multichannel liquid–metal cooling system for thermal management of high-heat-flux-density chip array. Energy Convers. Manag. 2022, 259, 115591. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, Y.; Zhang, S.; Yuan, W.; Tang, H. A review on fabrication and pool boiling enhancement of three-dimensional complex structures. Renew. Sustain. Energy Rev. 2022, 162. [Google Scholar] [CrossRef]
- Chu, H.; Yu, X.; Jiang, H.; Wang, D.; Xu, N. Progress in enhanced pool boiling heat transfer on macro- and micro-structured surfaces. Int. J. Heat Mass Transf. 2023, 200. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Chakraborty, A.; Lee, S.-E.; Kim, G.; Yu, C. Recent Advances in Two-Phase Immersion Cooling with Surface Modifications for Thermal Management. Energies 2022, 15, 1214. [Google Scholar] [CrossRef]
- Pham, M.; Bois, G.; Francois, F.; Baglietto, E. Assessment of State-of-the-art multiphase CFD modeling for subcooled flow boiling in reactor applications. Nucl. Eng. Des. 2023, 411. [Google Scholar] [CrossRef]
- Kuczyński, W.; Charun, H.; Piątkowski, P.; Bałasz, B.; Chliszcz, K. A regressive model for dynamic impulsive instabilities during the condensation of R134a, R1234ze(E) and R1234yf refrigerants. Int. J. Heat Mass Transf. 2021, 169, 120963. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, K.; Yan, Y.; Ouyang, H.; Han, X. Investigation on the influence of different heat transmission distances on thermo-hydrodynamic characteristics of pulsating heat pipes. Appl. Therm. Eng. 2023, 234. [Google Scholar] [CrossRef]
- Wei, R.; Hu, C.; Yang, J.; Wu, F.; Hu, J.; Li, F.; Li, C. Pressure-drop characteristics of CO2 boiling flow in the regenerative-cooling channel of an Mg/CO2 powder rocket engine for Mars missions. Acta Astronaut. 2022, 199, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wen, X.; Cai, W.; Jiang, Y.; Wen, C.; Wang, Y.; Hu, L.; Yu, H.; Zhu, H.; et al. Experimental studies on two-phase immersion liquid cooling for Li-ion battery thermal management. J. Energy Storage 2023, 72. [Google Scholar] [CrossRef]
- Narumanchi, S.; Troshko, A.; Bharathan, D.; Hassani, V. Numerical simulations of nucleate boiling in impinging jets: Applications in power electronics cooling. Int. J. Heat Mass Transf. 2008, 51, 1–12. [Google Scholar] [CrossRef]
- Birbarah, P.; Gebrael, T.; Foulkes, T.; Stillwell, A.; Moore, A.; Pilawa-Podgurski, R.; Miljkovic, N. Water immersion cooling of high power density electronics. Int. J. Heat Mass Transf. 2019, 147, 118918. [Google Scholar] [CrossRef]
- Fan, S.; Duan, F. A review of two-phase submerged boiling in thermal management of electronic cooling. Int. J. Heat Mass Transf. 2020, 150, 119324. [Google Scholar] [CrossRef]
- Mahmoud, M.; Karayiannis, T. Pool boiling review: Part I – Fundamentals of boiling and relation to surface design. Therm. Sci. Eng. Prog. 2021, 25, 101024. [Google Scholar] [CrossRef]
- Ghazivini, M.; Hafez, M.; Ratanpara, A.; Kim, M. A review on correlations of bubble growth mechanisms and bubble dynamics parameters in nucleate boiling. J. Therm. Anal. Calorim. 2021, 147, 6035–6071. [Google Scholar] [CrossRef]
- Hasan, M.N.; Monde, M.; Mitsutake, Y. Lower limit of homogeneous nucleation boiling explosion for water. Int. J. Heat Mass Transf. 2011, 54, 3226–3233. [Google Scholar] [CrossRef]
- Nimkar, N.D.; Bhavnani, S.H.; Jaeger, R.C. Effect of nucleation site spacing on the pool boiling characteristics of a structured surface. Int. J. Heat Mass Transf. 2006, 49, 2829–2839. [Google Scholar] [CrossRef]
- Salla, J.; Demichela, M.; Casal, J. BLEVE: A new approach to the superheat limit temperature. J. Loss Prev. Process. Ind. 2006, 19, 690–700. [Google Scholar] [CrossRef]
- Zhao, Z.; Glod, S.; Poulikakos, D. Pressure and power generation during explosive vaporization on a thin-film microheater. Int. J. Heat Mass Transf. 2000, 43, 281–296. [Google Scholar] [CrossRef]
- Li, J.; Peterson, G.; Cheng, P. Mechanical nonequilibrium considerations in homogeneous bubble nucleation for unsteady-state boiling. Int. J. Heat Mass Transf. 2005, 48, 3081–3096. [Google Scholar] [CrossRef]
- Islam, A.; Monde, M.; Woodfield, P.L.; Mitsutake, Y. Jet impingement quenching phenomena for hot surfaces well above the limiting temperature for solid–liquid contact. Int. J. Heat Mass Transf. 2008, 51, 1226–1237. [Google Scholar] [CrossRef]
- Petersen, K.; Rahbarimanesh, S.; Brinkerhoff, J. Progress in physical modelling and numerical simulation of phase transitions in cryogenic pool boiling and cavitation. Appl. Math. Model. 2023, 116, 327–349. [Google Scholar] [CrossRef]
- Bankoff, S.G. Entrapment of gas in the spreading of a liquid over a rough surface. AIChE J. 1958, 4, 24–26. [Google Scholar] [CrossRef]
- Cornwell, K. On boiling incipience due to contact angle hysteresis. Int. J. Heat Mass Transf. 1982, 25, 205–211. [Google Scholar] [CrossRef]
- Hsu, Y. On the Size Range of Active Nucleation Sites on a Heating Surface. Journal of Heat Transfer 1962, 207–213. [Google Scholar] [CrossRef]
- Qi, Y.; Klausner, J.F. Heterogeneous nucleation with artificial cavities. Journal of Heat Transfer 2005, 127, 1189–1196. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Q.; Yang, R. Theoretical analysis of bubble nucleation in liquid film boiling. Int. J. Heat Mass Transf. 2022, 192. [Google Scholar] [CrossRef]
- Iyer, S.; Kumar, A.; Coventry, J.; Lipiński, W. Modelling of bubble growth and detachment in nucleate pool boiling. Int. J. Therm. Sci. 2023, 185. [Google Scholar] [CrossRef]
- Kaniowski, R.; Pastuszko, R. Pool boiling experiment with Novec-649 in microchannels for heat flux prediction. Exp. Therm. Fluid Sci. 2023, 141. [Google Scholar] [CrossRef]
- Nam, Y.; Aktinol, E.; Dhir, V.K.; Ju, Y.S. Single bubble dynamics on a superhydrophilic surface with artificial nucleation sites. Int. J. Heat Mass Transf. 2011, 54, 1572–1577. [Google Scholar] [CrossRef]
- Hiroto Sakashita, T.K. Method for predicting boiling curves of saturated nucleate boiling. International Journal of Heat and Mass Transfer 2001, 44, 673–682. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Graham, R.W. .An analytical and experimental study of the thermal boundary layer & ebullition cycle in nucleate boiling[J], 1961.
- Xiao, B.; Jiang, G.; Zheng, D.; Chen, L.; Liu, B. Calculation of Active Nucleation Site Density in Boiling Systems. Res. J. Appl. Sci. Eng. Technol. ` 2013, 6, 587–592. [Google Scholar] [CrossRef]
- Wang, C.H.; Dhir, V.K. Effect of Surface Wettability on Active Nucleation Site Density During Pool Boiling of Water on a Vertical Surface. ASME Journal of Heat and Mass Transfer 1993, 115, 659–669. [Google Scholar] [CrossRef]
- Suszko, A.; El-Genk, M.S. Saturation boiling of PF-5060 on rough Cu surfaces: Bubbles transient growth, departure diameter and detachment frequency. Int. J. Heat Mass Transf. 2015, 91, 363–373. [Google Scholar] [CrossRef]
- Bourdon, B.; Di Marco, P.; Rioboo, R.; Marengo, M.; De Coninck, J. Enhancing the onset of pool boiling by wettability modification on nanometrically smooth surfaces. Int. Commun. Heat Mass Transf. 2013, 45, 11–15. [Google Scholar] [CrossRef]
- Jo, H.; Ahn, H.S.; Kang, S.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Mahmoud, M.; Karayiannis, T. Pool boiling review: Part II – Heat transfer enhancement. Therm. Sci. Eng. Prog. 2021, 25, 101023. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lee, M.-R.; Wu, C.-H.; Chen, P.-H. Effect of interlaced wettability on horizontal copper cylinders in nucleate pool boiling. Appl. Therm. Eng. 2017, 112, 1187–1194. [Google Scholar] [CrossRef]
- Jo, H.; Kaviany, M.; Kim, S.H.; Kim, M.H. Heterogeneous bubble nucleation on ideally-smooth horizontal heated surface. Int. J. Heat Mass Transf. 2014, 71, 149–157. [Google Scholar] [CrossRef]
- Shi, J.; Feng, D.; Chen, Z.; Ma, Q. Numerical study of a hybrid thermal lattice Boltzmann method for pool boiling heat transfer on a modeled hydrophilic metal foam surface. Appl. Therm. Eng. 2023, 229. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Jiang, H.; Liu, Y.; Li, X.; Shan, D.; Guo, B. Achieving robust and enhanced pool boiling heat transfer using micro–nano multiscale structures. Appl. Therm. Eng. 2023, 227. [Google Scholar] [CrossRef]
- Lv, Z.; An, Y.; Huang, C. Enhanced pool boiling heat transfer by adding metalized diamond in copper porous materials. Appl. Therm. Eng. 2023, 226. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Ni, L.; Jiang, J.; Yu, Y.; Pan, Y.; Zhu, Z. Fabrication of tungstate metal foams as efficient catalysts for dimethyl sulfoxide oxidation in a microreactor. J. Ind. Eng. Chem. 2024, 129, 445–455. [Google Scholar] [CrossRef]
- Klausner, J.; Mei, R.; Bernhard, D.; Zeng, L. Vapor bubble departure in forced convection boiling. Int. J. Heat Mass Transf. 1993, 36, 651–662. [Google Scholar] [CrossRef]
- Van Helden WG, J.; Van Der Geld CW, M.; Boot PG, M. Forces on bubbles growing and detaching in flow along a vertical wall. International Journal of Heat and Mass Transfer 1995, 38, 2075–2088. [Google Scholar] [CrossRef]
- Mei, R.; Klausner, J.F. Unsteady force on a spherical bubble at finite Reynolds number with small fluctuations in the free-stream velocity. Phys. Fluids A: Fluid Dyn. 1992, 4, 63–70. [Google Scholar] [CrossRef]
- Auton, T.R. The lift force on a spherical body in a rotational flow. J. Fluid Mech. 1987, 183, 199–218. [Google Scholar] [CrossRef]
- Thorncroft, G.E.; Klausner, J.F. BUBBLE FORCES AND DETACHMENT MODELS. Multiph. Sci. Technol. 2001, 13, 42. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Osada, H.; Inagaki, M.; Horinouchi, N. Dynamic modeling on bubble growth, detachment and heat transfer for hybrid-scheme computations of nucleate boiling. Int. J. Heat Mass Transf. 2013, 56, 640–652. [Google Scholar] [CrossRef]
- Xueli Wang a B Z W B, Jinjia Wei C a, Bengt Sundén. Correlations for prediction of the bubble departure radius on smooth flat surface during nucleate pool boiling. International Journal of Heat and Mass Transfer 2019, 132, 699–714. [Google Scholar] [CrossRef]
- Bhati, J.; Paruya, S. Numerical simulation of bubble dynamics in pool boiling at heated surface. Int. J. Heat Mass Transf. 2020, 152. [Google Scholar] [CrossRef]
- Paruya, S.; Bhati, J.; Akhtar, F. Numerical model of bubble shape and departure in nucleate pool boiling. Int. J. Heat Mass Transf. 2021, 180, 121756. [Google Scholar] [CrossRef]
- Iyer, S.; Kumar, A.; Coventry, J.; Lipiński, W. Mechanistic modelling of bubble growth in sodium pool boiling. Appl. Math. Model. 2023, 117, 336–358. [Google Scholar] [CrossRef]
- Beer, H.; Best, R.; Hahne, H.; et al. Nucleate Boiling: Bubble Growth and Dynamics. Heat Transfer in Boiling 1977, 21–52. [Google Scholar]
- Bucci, M.; Buongiorno, J.; Bucci, M. The not-so-subtle flaws of the force balance approach to predict the departure of bubbles in boiling heat transfer. Physics of Fluids 2021, 33. [Google Scholar] [CrossRef]
- Lima, S.F. Using surface integrals for checking Archimedes’ law of buoyancy. European Journal of Physics 2012, 33, 101–113. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Fei, G.; Zhou, T.; Zhao, C. Effects of surface wettability on bubble departure and critical heat flux: A parametric study based on 3-D dynamic force analysis model. Int. J. Therm. Sci. 2023, 186. [Google Scholar] [CrossRef]
- Kopchikov, I.A.; Voronin, G.I.; Kolach, T.A.; et al. Liquid boiling in a thin film. International Journal of Heat and Mass Transfer 1969, 12, 791–796. [Google Scholar] [CrossRef]
- Labuntsov, D. Study of the growth of bubbles during boiling of saturated water within a wide range of pressures by means of high-speed moving pictures. Teplofizika Vysokikh Temperatur 1964, 2, 446–453. [Google Scholar]
- Akiyama, M.; Tachibana, H.; Ogawa, N. Effects of system pressure on bubble growth rate. Trans, JSME 1969, 35, e126. [Google Scholar]
- Miglani, A.; Joo, D.; Basu, S.; Kumar, R. Nucleation dynamics and pool boiling characteristics of high pressure refrigerant using thermochromic liquid crystals. Int. J. Heat Mass Transf. 2013, 60, 188–200. [Google Scholar] [CrossRef]
- Gao, W.; Qi, J.; Yang, X.; Zhang, J.; Wu, D. Experimental investigation on bubble departure diameter in pool boiling under sub-atmospheric pressure. Int. J. Heat Mass Transf. 2019, 134, 933–947. [Google Scholar] [CrossRef]
- Mahmoud, M.; Karayiannis, T. Bubble growth on a smooth metallic surface at atmospheric and sub-atmospheric pressure. Int. J. Heat Mass Transf. 2023, 209. [Google Scholar] [CrossRef]
- Mahmoud, M.; Karayiannis, T. Bubble growth models in saturated pool boiling of water on a smooth metallic surface: Assessment and a new recommendation. Int. J. Heat Mass Transf. 2023, 208. [Google Scholar] [CrossRef]
- Sernas, V.; Hooper, F.C. The initial vapor bubble growth on a heated wall during nucleate boiling. International Journal of Heat and Mass Transfer 1969, 12, 1627–1630. [Google Scholar] [CrossRef]
- Forster, H.K.; Zuber, N. Growth of a Vapor Bubble in a Superheated Liquid. J. Appl. Phys. 1954, 25, 474–478. [Google Scholar] [CrossRef]
- Plesset, M.S.; Zwick, S.A. The Growth of Vapor Bubbles in Superheated Liquids. J. Appl. Phys. 1954, 25, 493–500. [Google Scholar] [CrossRef]
- Fritz, W.; Ende, W. Über den verdampfungsvorgang nach kinematographischen aufnahmen an dampfblasen. Physikalische Zeitschrift Der Sowjetunion 1936, 37, 391–401. [Google Scholar]
- Plesset, M.S.; Zwick, S.A. The Growth of Vapor Bubbles in Superheated Liquids. J. Appl. Phys. 1954, 25, 493–500. [Google Scholar] [CrossRef]
- Forster, H.K.; Zuber, N. Growth of a Vapor Bubble in a Superheated Liquid. J. Appl. Phys. 1954, 25, 474–478. [Google Scholar] [CrossRef]
- Scriven, L. On the dynamics of phase growth. Chem. Eng. Sci. 1959, 10, 1–13. [Google Scholar] [CrossRef]
- Avdeev, A.A.; Zudin, Y.B. Inertial-thermal governed vapor bubble growth in highly superheated liquid. Heat Mass Transf. 2005, 41, 855–863. [Google Scholar] [CrossRef]
- Mikic, B.B.; Rohsenow, W.M.; Griffith, P. On bubble growth rates. International Journal of Heat and Mass Transfer 1970, 13, 657–666. [Google Scholar] [CrossRef]
- Strenge, P.H.; Orell, A.; Westwater, J.W. Microscopic study of bubble growth during nucleate boiling. AIChE Journal 1961, 7, 578–583. [Google Scholar] [CrossRef]
- Zuber, N. The dynamics of vapor bubbles in nonuniform temperature fields. Int. J. Heat Mass Transf. 1961, 2, 83–98. [Google Scholar] [CrossRef]
- Van Stralen, S. The mechanism of nucleate boiling in pure liquids and in binary mixtures—part I. Int. J. Heat Mass Transf. 1966, 9, 995–1020. [Google Scholar] [CrossRef]
- Cole, R.; Shulman, H.L. Bubble growth rates at high Jakob numbers. International Journal of Heat and Mass Transfer 1966, 9, 1377–1390. [Google Scholar] [CrossRef]
- Du, J.; Zhao, C.; Bo, H. A modified model for bubble growth rate and bubble departure diameter in nucleate pool boiling covering a wide range of pressures. Appl. Therm. Eng. 2018, 145, 407–415. [Google Scholar] [CrossRef]
- Benjamin, R.; Balakrishnan, A. Nucleate pool boiling heat transfer of pure liquids at low to moderate heat fluxes. Int. J. Heat Mass Transf. 1996, 39, 2495–2504. [Google Scholar] [CrossRef]
- Alavi Fazel, S.A.; Shafaee, S.B. Bubble dynamics for nucleate pool boiling of electrolyte solutions[J], 2010.
- Phan, H.T.; Caney, N.; Marty, P.; Colasson, S.; Gavillet, J. A model to predict the effect of contact angle on the bubble departure diameter during heterogeneous boiling. Int. Commun. Heat Mass Transf. 2010, 37, 964–969. [Google Scholar] [CrossRef]
- Nam, Y.; Aktinol, E.; Dhir, V.K.; Ju, Y.S. Single bubble dynamics on a superhydrophilic surface with artificial nucleation sites. Int. J. Heat Mass Transf. 2011, 54, 1572–1577. [Google Scholar] [CrossRef]
- Friz, W. Maximum volume of vapor bubbles. Phys. Z. 1935, 36, 379–354. [Google Scholar]
- Bovard, S.; Asadinia, H.; Hosseini, G.; Fazel, S.A.A. Investigation and experimental analysis of the bubble departure diameter in pure liquids on horizontal cylindrical heater. Heat Mass Transf. 2016, 53, 1199–1210. [Google Scholar] [CrossRef]
- Kumar, N.; Ghosh, P.; Shukla, P. Development of an approximate model for the prediction of bubble departure diameter in pool boiling of water. Int. Commun. Heat Mass Transf. 2021, 127, 105531. [Google Scholar] [CrossRef]
- Cole, R. Bubble frequencies and departure volumes at subatmospheric pressures. AIChE J. 1967, 13, 779–783. [Google Scholar] [CrossRef]
- Dong, L.; Quan, X.; Cheng, P. An experimental investigation of enhanced pool boiling heat transfer from surfaces with micro/nano-structures. Int. J. Heat Mass Transf. 2014, 71, 189–196. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, R. Ultrahigh Flux Thin Film Boiling Heat Transfer Through Nanoporous Membranes. Nano Lett. 2018, 18, 3096–3103. [Google Scholar] [CrossRef]
- Lim, D.Y.; Bang, I.C. Controlled bubble departure diameter on biphilic surfaces for enhanced pool boiling heat transfer performance. International Journal of Heat and Mass Transfer 2020, 150, 119360. [Google Scholar] [CrossRef]
- Marie, A.; Cioulachtjian, S.; Lips, S.; Sartre, V. Thermal interactions between nucleation sites and the solid wall during pool boiling of a pure fluid: A review. Int. J. Therm. Sci. 2022, 174, 107388. [Google Scholar] [CrossRef]
- Hatton, A.P.; Hall, I.S. Photographic study of boiling on prepared surfaces. Proc. 3rd int. heat transfer conf. Aiche Journal 1966, 4, 24–37. [Google Scholar]
- Lee, H.C.; Oh, B.D.; Bae, S.W.; et al. Single bubble growth in saturated pool boiling on a constant wall temperature surface. International Journal of Multiphase Flow 2003, 29, 1857–1874. [Google Scholar] [CrossRef]
- Chi-Yeh H, Griffith, Peter The mechanism of heat transfer in nucleate pool boiling. International Journal of Heat and Mass Transfer 1965, 8, 887–904. [CrossRef]
- Hsu, Y.Y.; Graham, R.W. Transport Processes in Boiling and Two-Phase Systems[J], 1986.
- Van Stralen S J D, Sohal M S, Cole R; et al. Bubble growth rates in pure and binary systems: Combined effect of relaxation and evaporation microlayers. International Journal of Heat and Mass Transfer 1975, 18, 453–467.
- Jakob, M.; Fritz, W. Versuche über den Verdampfungsvorgang. Forschung auf dem Gebiet des Ingenieurwesens A 1931, 2, 435–447. [Google Scholar] [CrossRef]
- Jakob, M. Heat Transfer, Chapter 29, 1: New York: Wiley and Sons, 1949.
- Sakashita, H.; Ono, A. Boiling behaviors and critical heat flux on a horizontal plate in saturated pool boiling of water at high pressures. Int. J. Heat Mass Transf. 2009, 52, 744–750. [Google Scholar] [CrossRef]
- Hamzekhani, S.; Falahieh, M.M.; Kamalizadeh, M.R.; Nazari, Z. Experimental study on bubble departure frequency for pool boiling of water/NaCl solutions. Heat Mass Transf. 2015, 51, 1313–1320. [Google Scholar] [CrossRef]
- Cole, R. A photographic study of pool boiling in the region of the critical heat flux. AIChE J. 1960, 6, 533–538. [Google Scholar] [CrossRef]
- McFadden, P.; Grassmann, P. The relation between bubble frequency and diameter during nucleate pool boiling. Int. J. Heat Mass Transf. 1962, 5, 169–173. [Google Scholar] [CrossRef]
- Ivey, H.J. Relationships between bubble frequency, departure diameter and rise velocity in nucleate boiling. International Journal of Heat and Mass Transfer 1967, 10, 1023–1040. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, S.; Lu, Z.; Cheng, P.; Wang, E.N. A unified relationship between bubble departure frequency and diameter during saturated nucleate pool boiling. Int. J. Heat Mass Transf. 2020, 165, 120640. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.H. On the departure behaviors of bubble at nucleate pool boiling. International Journal of Multiphase Flow 2006, 32, 1269–1286. [Google Scholar] [CrossRef]
- Goel, P.; Nayak, A.K.; Kulkarni, P.P.; Joshi, J.B. Experimental study on bubble departure characteristics in subcooled nucleate pool boiling. Int. J. Multiph. Flow 2017, 89, 163–176. [Google Scholar] [CrossRef]
- Gerardi C, Buongiorno J, Hu L-W; et al. Measurement of nucleation site density, bubble departure diameter and frequency in pool boiling of water using high-speed infrared and optical cameras[J], 2009.
- Han, C.-Y. The mechanism of heat transfer in nucleate pool boiling[D]. Massachusetts Institute of Technology, 1962.
- Chang, Y.; Ferng, Y. Experimental investigation on bubble dynamics and boiling heat transfer for saturated pool boiling and comparison data with previous works. Appl. Therm. Eng. 2019, 154, 284–293. [Google Scholar] [CrossRef]
- Sapozhnikov, S.Z.; Mityakov, V.Y.; Mityakov, A.V. Heatmetry: The science and practice of heat flux measurement[M]. Springer Nature, 2020.
- McHale, J.P.; Garimella, S.V. Nucleate boiling from smooth and rough surfaces – Part 1: Fabrication and characterization of an optically transparent heater–sensor substrate with controlled surface roughness. Exp. Therm. Fluid Sci. 2012, 44, 456–467. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Z.; Sundén, B. Heat transfer prediction and critical heat flux mechanism for pool boiling of NOVEC-649 on microporous copper surfaces. Int. J. Heat Mass Transf. 2019, 141, 818–834. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kumar, B.; Kumar, N.; Raj, R. Simultaneous enhancement of critical heat flux and heat transfer coefficient via in-situ deposition of ionic liquids during pool boiling. Int. J. Heat Mass Transf. 2023, 208. [Google Scholar] [CrossRef]
- Yu, X.; Xu, N.; Yu, S.; Han, Y.; Chu, H. Effect of orthogonal channel structure on the heat transfer in pool boiling and its heat flux prediction model. Int. J. Therm. Sci. 2023, 187. [Google Scholar] [CrossRef]
- Konopko, L.A.; Nikolaeva, A.A.; Huber, T.E.; Kobylianskaya, A.K. Miniaturized Heat-Flux Sensor Based on a Glass-Insulated Bi–Sn Microwire. Semiconductors 2019, 53, 662–666. [Google Scholar] [CrossRef]
- Rassoulinejad-Mousavi, S.M.; Al-Hindawi, F.; Soori, T.; Rokoni, A.; Yoon, H.; Hu, H.; Wu, T.; Sun, Y. Deep learning strategies for critical heat flux detection in pool boiling. Appl. Therm. Eng. 2021, 190, 116849. [Google Scholar] [CrossRef]
- Suh, Y.; Bostanabad, R.; Won, Y. Deep learning predicts boiling heat transfer. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Barathula, S.; Chaitanya, S.; Srinivasan, K. Evaluation of machine learning models in the classification of pool boiling regimes up to critical heat flux based on boiling acoustics. Int. J. Heat Mass Transf. 2023, 201. [Google Scholar] [CrossRef]
- Dunlap, C.; Pandey, H.; Weems, E.; Hu, H. Nonintrusive heat flux quantification using acoustic emissions during pool boiling. Appl. Therm. Eng. 2023, 228. [Google Scholar] [CrossRef]
- Hobold, G.M.; da Silva, A.K. Visualization-based nucleate boiling heat flux quantification using machine learning. Int. J. Heat Mass Transf. 2019, 134, 511–520. [Google Scholar] [CrossRef]
- Kim, J. Review of nucleate pool boiling bubble heat transfer mechanisms. Int. J. Multiph. Flow 2009, 35, 1067–1076. [Google Scholar] [CrossRef]
- Mohanty, R.L.; Das, M.K. A critical review on bubble dynamics parameters influencing boiling heat transfer. Renewable and Sustainable Energy Reviews 2017, 78, 466–494. [Google Scholar] [CrossRef]
- Jung, D.; Kim, Y.; Ko, Y.; Song, K. Nucleate boiling heat transfer coefficients of pure halogenated refrigerants. Int. J. Refrig. 2003, 26, 240–248. [Google Scholar] [CrossRef]
- Jung, D.; Lee, H.; Bae, D.; Oho, S. Nucleate boiling heat transfer coefficients of flammable refrigerants. Int. J. Refrig. 2004, 27, 409–414. [Google Scholar] [CrossRef]
- Rao, G.V.; Balakrishnan, A. Heat transfer in nucleate pool boiling of multicomponent mixtures. Experimental Thermal and Fluid Science 2004, 29, 87–103. [Google Scholar]
- Judd, R.L.; Hwang, K.S. A Comprehensive Model for Nucleate Pool Boiling Heat Transfer Including Microlayer Evaporation. Journal of Heat Transfer 1976, 98, 623–629. [Google Scholar] [CrossRef]
- Fazel, S.A.; Mahboobpour, M. Pool boiling heat transfer in monoethyleneglycol aqueous solutions. Exp. Therm. Fluid Sci. 2013, 48, 177–183. [Google Scholar] [CrossRef]
- Mikic, B.B.; Rohsenow, W.M. A New Correlation of Pool-Boiling Data Including the Effect of Heating Surface Characteristics. J. Heat Transf. 1969, 91, 245–250. [Google Scholar] [CrossRef]
- Han, C.; Griffith, P. The Mechanism of Heat Transfer in Nucleate Pool Boiling Part L. International Journal of Heat and Mass Transfer 1965, 8, 887–903. [Google Scholar]
- Paul, D.D.; Abdel-Khalik, S.I. A statistical analysis of saturated nucleate boiling along a heated wire. International Journal of Heat and Mass Transfer 1983, 26, 509–519. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, P. A Fractal Model for Nucleate Pool Boiling Heat Transfer. J. Heat Transf. 2002, 124, 1117–1124. [Google Scholar] [CrossRef]
- Wang, C.H.; Dhir, V.K. Effect of Surface Wettability on Active Nucleation Site Density During Pool Boiling of Water on a Vertical Surface. Journal of Heat Transfer 1993, 115, 659–669. [Google Scholar] [CrossRef]
- Chu, H.; Yu, B. A new comprehensive model for nucleate pool boiling heat transfer of pure liquid at low to high heat fluxes including CHF. Int. J. Heat Mass Transf. 2009, 52, 4203–4210. [Google Scholar] [CrossRef]
- Sateesh, G.; Das, S.K.; Balakrishnan, A. Analysis of pool boiling heat transfer: effect of bubbles sliding on the heating surface. Int. J. Heat Mass Transf. 2005, 48, 1543–1553. [Google Scholar] [CrossRef]
- Barthau, G.; Hahne, E. Nucleation site density and heat transfer in nucleate pool boiling of refrigerant R 134a in a wide pressure range[C]. 3rd European thermal sciences conference (Heidelberg, 10-13 September 2000), 2000: 731-736.
- Luke, A.; Gorenflo, D. Heat transfer and size distribution of active nucleation sites in boiling propane outside a tube. Int. J. Therm. Sci. 2000, 39, 919–930. [Google Scholar] [CrossRef]
- Zueter, A.F.; Tareen, M.S.; Newman, G.; Sasmito, A.P. Dynamic CFD modeling coupled with heterogeneous boiling for deep two phase closed thermosyphons in artificial ground freezing. Int. J. Heat Mass Transf. 2023, 203. [Google Scholar] [CrossRef]
- Yan, P.; Jin, H.; He, G.; Guo, X.; Ma, L.; Yang, S.; Zhang, R. CFD simulation of hydrodynamics in a high-pressure bubble column using three optimized drag models of bubble swarm. Chem. Eng. Sci. 2019, 199, 137–155. [Google Scholar] [CrossRef]
- Mohammed, H.I.; Giddings, D.; Walker, G.S. CFD simulation of a concentrated salt nanofluid flow boiling in a rectangular tube. Int. J. Heat Mass Transf. 2018, 125, 218–228. [Google Scholar] [CrossRef]
- Kharangate, C.R.; Mudawar, I. Review of computational studies on boiling and condensation. Int. J. Heat Mass Transf. 2017, 108, 1164–1196. [Google Scholar] [CrossRef]

| Ref. | Liquid density (kg/m3 ) |
Vapor density (kg/m3 ) |
Surface tension (mN/m) |
Latent heat (kj/kg) |
Saturation temperature(°C) |
|---|---|---|---|---|---|
| Water [29] | 958.35 | 0.597 | 59 | 2256.5 | 100 |
| Novec-649 [30] | 1513 | 13.42 | 10.8 | 88 | 49 |
| Klausner[46] model | Wang[52] model | Siddharth[55] model | |
|---|---|---|---|
| Fs | |||
| Fb | |||
| Fcp | |||
| Fd | |||
| Fi |
| Types of forces | Equation expressing a relation |
|---|---|
| Fs | |
| FP |
|
| FG | |
| Fe | |
| Fm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).