Preprint
Article
Microorganism Isolates Stimulate Forest Crop Growth and Protection
Altmetrics
Downloads
126
Views
66
Comments
0






















This version is not peer-reviewed
Submitted:
12 October 2023
Posted:
16 October 2023
You are already at the latest version
Alerts
Abstract
The antimicrobial properties of the new strains of microorganisms isolated from natural sources of various ecological niches in the Moscow Region and the Republic of Tatarstan were studied. An antifungal activity of isolates was established in a test culture of toxin-producing microscopic fungi that cause various plant diseases: Fusarium oxysporum, Aspergillus flavus, Penicillium spp. and Candida albicans. Of the 46 studied microorganisms, 4 isolates (Bacillus subtilis, Propionibacterium freudenreichii, Lactobacillus plantarum and Streptomyces spp.) showed an ability to produce biologically active metabolites with a pronounced antimicrobial potential. This is promising in the growth and protection of the Scots pine, pedunculate oak and small-leaved linden forest crops from diseases caused by fungi Lophodermium pinastri Chev. and Microsphaera alphitoides stimulation. Based on selected isolates, a Bacterial product LRV composition has been developed. A Scots pine, pedunculate oak and small-leaved linden seedlings single and double foliar treatment with Bacterial product LRV at a concentration of 10 ml/l led to an increase in the growth of an above-ground part by 31.8, 51.9 and 25.4 %, respectively, and an underground part – by 25.0, 37.2 and 25.7 %, respectively, compared with the control. The weight of seedling at the end of the study exceeded the control variant by an average of 26.0, 44.0 and 78.0 %, respectively. A use of a plant protection Bacterial product LRV did not have a significant effect on a powdery mildew and Schütte disease damage to forest crops. A Biogeosystem Technique methodology has been developed to improve a long-term forest growing.
Keywords:
Subject: Environmental and Earth Sciences - Environmental Science
1. Introduction
At the moment, an ecological and biological function of forests in ensuring land restoration and conservation of biological resources are of a vital importance for the human society well-being. Currently, violations in forest cultivation have a negative impact on both climate and anthropogenic disturbed lands [1,2,3]. A restoration of forest and agricultural lands ensures a sustainable development of the ecosystem. It is an important tool in a land management that increases biodiversity, CO2 sequestration, carbon content in the soil (as part of humus) and prevents an uncontrolled transport of pollutants within the ecosystem [4,5,6,7,8]. A forestry on unproductive lands unsuitable for agriculture, as well as the forest restoration and re-introduction of forest species, are of a paramount importance in the economic and recreational sphere of social development [9,10,11,12].
In Russia, a re-afforestation and a forest improvement are underway. The state program "Development of forestry in the Tatarstan Republic" was launched in 2013. The state program was focused on a restoration of felled and thinned plantings and an improvement of forest zones and forest park facilities. An important purpose was the forest fire detection and extinguishing, forest protection from harmful organisms and adverse factors, forest high-quality use intensification, and forest protection and reproduction. Modern advances in the development of plant protection products have been achieved almost exclusively by the chemical industry. At the moment, chemical plant protection products are the main means in combating phytopathogens [1].
Many authors have shown that a long-term use of agrochemicals is associated with persistent levels of environmental pollution, which causes environmental risks to biological systems. Long-term exposure to synthetic fungicides has led to a decrease in application efficiency due to the development of resistance mechanisms in plant pathogens [2]. This has led growers to increase the use of chemicals, with consequent accumulation of residues in agricultural products and their by-products, which in turn have harmful effects on both human and animal health [3]. These reasons have a strong impact on public perception and market demand, creating a need to move towards the products free of synthetic pesticides in a healthier and more environmentally friendly manner. From this perspective, a promising alternative to pest management is the biological control approach, in which human intervention exploits the natural antagonistic effects of biological agents to mitigate the harmful effects of plant pathogens [4].
The mechanisms of biological control carried out by biological agents are diverse and depend on the specific features of both the pathogen and the antagonist, as well as on their density and a specificity of the interactions that occur between them [5,6]. A successful biological control is usually characterized by an activation of multiple mechanisms and targets synergistically aimed at controlling a pathogen and/or its deleterious effects on biological targets [7,8]. According to technological standards, a biological protective equipment must be non-toxic for humans and animals, and for the environment [9,10,11]. The Moscow region is provided to the western part of the Volga basin. Due to its natural location, the Republic of Tatarstan is located in the zone of the Middle Volga upland. A direct antagonism (eg, hyperparasitism and predation) occurs when there is a very high affinity between a pathogen and its antagonist [12,13]. Existing pathogen suppression mechanisms include a production of volatiles, antibiotics, and other secondary metabolites of the microbial life cycle. A production of volatile organic compounds is of an ever-increasing interest in the scientific community due to the various benefits of their application [14].
An aim of the study was as follows. An extraction of isolates of microorganism strains from natural sources in various ecological niches in the Moscow region and in the Republic of Tatarstan. A synthesis of a biological product, stimulating a Scotch pine, pedunculate oak and small-leaved linden seedlings protection from diseases, caused by fungi Lophodermium pinastri Chev. and Microsphaera alphitoides. A Biogeosystem Technique methodology development to improve a long-term forest growing.
2. Materials and Methods
2.1. Region of Investigations
The research was carried out in the Kaibitsky forestry of the Republic of Tatarstan. A Kaibitsky forestry area belongs to the Volga region. The Volga region is a typical northern forest-steppe, which geographical conditions differ from other parts of the Republic of Tatarstan. The terrain day surface is characterized by the outcrops of deposits of various ages and lithological compositions. A zonal relief-forming process and a corresponding structure of soil and vegetation cover are typical for the region. The Republic of Tatarstan includes eight regions.
The territory of Kaibitsky forestry is characterized by temperate continental climate with warm summers and moderately cold winters. An average annual air temperature varies from +2.7 to +3.10 °C [15]. The warmest month is July, an average temperature is circa +19.0 °C to +19.60 °C. The coldest month is January with average temperature from −13.0 °C to −13.70 °C. The absolute minimum air temperature is −46.50 °C, and the absolute maximum air temperature is +37 °C. An air temperature amplitude fluctuation negatively affects forest biogeocenoses of the Volga region. According to Kolobov N.V. [16], in the period from 1884 to 1963 (80 years) there were 26–27 dry years or 32–35%. Since 1951, the warmest years were 1981, 1975, 1995, 2008 and 2010. According to temperature regime data, 2010 was abnormally warm (State Report on the state of natural resources and environmental protection of the Republic of Tatarstan in 2010, Kazan, 2011) (https://eco.tatarstan.ru/gosdoklad.htm).
An average duration of the warm period with an air temperature above 0°C is circa 200–210 days [15]. The vegetation period is 164–175 days long. A steady transition of an average daily air temperature through 0 °C in spring occurs in the first decade of April, and in autumn at the end of October. The pre-Volga region is the warmest part of the Republic of Tatarstan in terms of the winter period temperature sum.
The Volga region is a relatively humid territory. In winter, an air relative humidity is 80–85 %. An air relative humidity in summer is 60–70 %. An annual amount of precipitation is 410–490 mm. A precipitation during the growing season is 200–240 mm. However, the precipitation is distributed unevenly through the territory. In the elevated parts of the region, adjacent to the Volga River, the fall is higher than 450 mm/year. These indicators reflect an influence of relief elements on a distribution of precipitation. The most amount of precipitation (about 70%) falls during the warm period of the year from April to October.
An oldest geological formation in the Volga region territory are the metamorphosed rocks of the crystalline basement of the Russian platform, which are overlain by deposits of Devonian, Carboniferous and Permian Paleozoic, Jurassic and Cretaceous Mesozoic, Tertiary and Quaternary periods. The main composing rocks in the region are the Upper Permian deposits, which are represented by the Tatar and Kazan tiers. The Kazan tier is characterized by a content of dolomite and limestone of light gray and almost white color. At the base of these strata, the red-colored sandy-clay deposits lay, which are not common in the region. The Kazan tier of limestone and dolomite outcrops are observed in the cliffs of the Volga River right bank. These outcrops are also found in the mouths of deep ravines.
2.2. Experiment Location, Conditions and Layout
In the forest nursery of Kaibitsky Berlibash forestry located on the Berl river (55°19′17″ N 48°06′28″ E), a development of the Scotch pine (Pinus sylvestris), pedunculate oak (Quércus róbur) and small-leaved linden (Tília cordáta) seedlings under an application of biological preparation was studied. The bacterial product has been prepared on a basis of bacterial isolates association (Bacterial product LRV) to provide the seedlings growth stimulation and protection from phytopathogens. The experiment was carried out in triplicate.
A total area under experiment was 200 m2. An individual experimental plot accounting area was 7 m2. The experiment options of a biological preparation application were as follows:
1. Control – seedlings sprayed with water once;
1a. Control – seedlings sprayed with water twice;
2. Experimental group 1 – seedlings treated with the Bacterial product LRV at a dose of 10 ml/l once;
2a. Experimental group 2 – seedlings treated with the Bacterial product LRV at a dose of 10 ml/l twice;
3. Experimental group 3 – seedlings treated with the Bacterial product LRV at a dose of 4 ml/l once;
3a. Experimental group 4 – seedlings treated with the Bacterial product LRV at a dose of 4 ml/l twice.
The accounting experimental plot site area of 3 row seedling strips were allocated by protective 2 row seedling strips. The Figure 1 shows a treatment of seedlings with a tractor sprayer in the first half 2022 growing season in the Kaibitskoye forestry.
A Bacterial product LRV solution spraying dose was 100 ml/m². A foliar treatment of seedlings with Bacterial product LRV was carried out after the plant leaves bloomed. A bacterial product working solution preparation was carried out immediately before the seedlings were processed. The seedling plots treated with 100 ml/m² water served as a control. A seedlings re-treatment was carried out a month after first treatment. At the end of the growing season, the seedlings number was counted in each repetition of experiment.
At the end of September, 50 seedlings were selected from each variant. An aerial part of seedling was separated from an underground part. For each seedling, the length of the root bundle, height, growth per year, and diameter of the root collar were measured. After complete drying in an oven at a temperature 105 °C, the weight of root and aerial parts of seedling was determined. The foliar treatment of seedlings with preparation was carried out after the plant leaves bloomed. A re-treatment of seedlings with Bacterial product LRV was carried out a month after the first one. At the end of growing season, the seedlings number was counted in each repetition of experiment.
Samples for a Bacterial product LRV synthesis taken from various natural sources in the Moscow region and the Republic of Tatarstan served as a material for obtaining microorganism cultures. For selection, the microorganisms were cultivated on various liquid (MPA, Sabouraud broth) and dense (MPA, wort agar, Sabouraud agar, MRS, M9) nutrient media with a subsequent study of their morphological, tinctorial and biological properties [15,16,17,18,19,20].
An antifungal activity of isolates was established in a test culture of toxin-producing microscopic fungi that cause various plant diseases: Fusarium oxysporum, Aspergillus flavus, Penicillium spp. and Candida albicans (Collection of Microorganisms of the All-Russian Research Institute of Phytopathology, Moscow region, Russia). The bacterial and test isolates were cultivated at 37±1 °C and 28±2 °C respectively in a test tube on a slant agar of a following composition (%): glucose – 0.63, enzymatic peptone – 2.1, sodium chloride – 0.65, sodium hydrogen phosphate – 0.35, potassium dihydroorthophosphate – 0.06, microbiological agar – 0.12 [21].
An antagonistic activity of isolates of microorganisms against microscopic fungi was determined using a plate method (double culture method) [22]. To obtain an isolate sample, individual bacterial isolate was cultivated in a Petri dish on a Luria-Bertani (LB) agar medium at 37±1°C for 48 h to the moment of a continuous microorganism mycelium cover formation. The fungal colonies were previously grown in another Petri dish on a Czapek-Dox agar medium at 28±2°C for 7 days till the continuous microscopic fungi cover surface formation. Then, the test microorganism culture mycelium discs 6 mm in diameter were cut out from the microorganism mycelium cover on the LB agar medium surface. These blocks with a test culture were transferred to another Petri dish and placed on a surface of a fungal colony on the Czapek's agar medium. The disks with a mycelium of test isolate were evenly distributed at a distance 3 cm from one another and from an edge of Petri dish with a fungal colony on the Czapek's agar medium. As a control, fungi cultures without texted bacterial strain application were used. An incubation of microorganisms was carried out at 28 °C for 5 days. After a cultivation, the maximum and minimum size of the pathogen growth inhibition zone was measured.
An antifungal activity of lactic acid microorganism isolates was also assessed by a counter cultures method [23]. For this, mycelium disks (diameter 6 mm) were cut out from fungal colonies previously grown on Czapek-Dox agar for 7 days at 28±2 °C and placed in the center of MRS-SA agar plates (without sodium acetate and ammonium citrate). The studied isolates were inoculated with a specified medium, placed at a distance of 2 cm from an edge of the agar plates. As a control, plates with disks of fungi without bacterial strains were used. Microorganisms were incubated at 30 °C for 120 h.
A determination of antifungal properties of the biologically active metabolites of isolated microorganisms in relation to pathogens of various diseases of agricultural plants was carried out by the method described in [25,26,27,28,29].
An identification of isolated microorganisms was carried out on the basis of morphological-cultural and physiological-biochemical properties, guided by the determinants of Bergey and Kaufman [30,31,32]. A primary identification of the strains was carried out by studying their tinctorial properties using the Gram stain method [33]. The morphological features of microorganism cells were studied by obtaining live and fixed stained preparations using a bright field and phase contrast microscopy [33]. A motility of the studied bacteria was determined in a “crushed drop” and a “hanging drop” preparations from the daily cultures with their further microscopy.
An ability of isolates to grow in the agar media was assessed by cultivating microorganisms for two days at a temperature of 10–50°C and a pH of 5.0–8.0 [33]. An enzymatic activity and a carbohydrate fermentation tests were determined using the generally accepted methods [34,35]. The study of a fermentation of sugars by isolates was carried out using the "variegated series" method. The glucose, lactose, galactose, sucrose, maltose, fructose, rhamnose, arabinose and sorbitol were used as carbohydrates. Microorganisms were seeded in a liquid nutrient medium with the appropriate 1% substrate (carbohydrate) and bromcresol purple dye at a concentration of 0.03 mg/ml. Accounting for the results of the test for the ability of microorganisms to ferment carbohydrates was carried out by changing the color of the medium (from purple to yellow).
To study the enzyme activity of the selected isolates, bacterial cultures were grown on agar modified corn-lactose and MRS media, as well as on a synthetic medium containing (g/l) sodium citrate – 1.29; (NH4)2HPO4 – 4.75; K2HPO4 – 9.6; MgSO4•7H2O – 0.18 (рН 7.0±0.2) [36,37,38,39]. Carboxymethylcellulose (CMC), water-soluble starch, and casein at a concentration of 1.0% were used as sources of carbon and nitrogen. Olive oil, tweens 20, 40, 60, and 80 were added to the medium at a concentration of 0.5%. Bacteria were cultivated at 37 ± 1°C until hydrolysis zones appeared around their colonies, which were used to judge the ability of isolates to produce hydrolytic enzymes.
A Bacterial product LRV effect on a Scots pine biennial seedlings infection with Schutte’s disease and pedunculate oak biennial seedlings damage by powdery mildew was studied.
2.3. Sampling and Analyzes
The samples of soils, fermented dairy products, raw cow's milk, farm birds and animals gastrointestinal tract and feces contents , as well as agricultural crops were collected on the territory of the Moscow region and the Republic of Tatarstan (https://legalacts.ru/doc/mr-420220-20-42-metody-kontrolja-biologicheskie-i-mikrobiologicheskie-faktory) (accessed May 29, 2023).
The pH value was measured using a potentiometric pH meter during extraction of a water-soluble salt. The ratio of the inoculate of microorganisms and distilled water was 1:2 (https://elibrary.ru/item.asp?id=42960377) (accessed May 29, 2023).
An antagonistic microorganism activity against microscopic fungi was determined using the Methodological Instructions MU 2.3.2.2789—10. The microbiological studies were carried out in a compliance with the Sanitary rules for a safe operation with the microorganisms of III — IV pathogenicity groups (https://ohranatruda.ru/upload/iblock/846/4293757373.pdf) (accessed May 29, 2023).
2.4. Biogeosystem Technique methodology
A current technology is incapable in the soil structure and soil water regime improvements in Biological product LRV priority functioning and a long-term stable productive forest formation. A restorative forest management Biogeosystem Technique methodology is technical and technological solutions of a new generation, focusing on a soil priming action and a soil priming effect enhancement.
2.5. Data Processing and Statistical Analysis
3. Results
From soil samples, raw cow's milk, fermented milk products, the gastrointestinal tract contents, farm bird and animal feces, as well as crops grown in the Moscow region and in the Republic of Tatarstan, 46 isolates of microorganisms from various taxonomic groups were obtained. The study of the phenotypic characteristics of the isolated strains, namely, cell morphology, shape and color of colonies, acid production, growth pattern, Gram stain and other features, showed a genetic diversity of microorganisms. An active growth of bacteria on selective media was noted from the first day of their cultivation. Among isolated microorganisms, the most belonged to bacteria of genus Bacillus, Lactobacillus, Lactococcus and Streptomyces.
Of the 46 studied microorganisms, 4 strains (Bacillus spp., Propionibacterium spp., Lactobacillus spp., Streptomyces spp.) had a pronounced ability to inhibit growth and development of the various groups of microscopic fungi. A microorganism’s antagonistic activity against microscopic fungi is presented in Table 1.
The selected microorganism strains with an antimicrobial potential were classified corresponding to determinants "Bergey" and "Kaufman" as Bacillus subtilis, Lactobacillus plantarum, Propionibacterium freudenreichii and Streptomyces spp. species (Table 2).
For a more detailed characterization of metabolic features of selected cultures of microorganisms, optimal temperature and pH for growth, as well as an ability to use various substrates for metabolism, were assessed (Table 2). The isolates had a temperature from 9 to 46°C and a pH from 3.0 to 9.0 wide limits of cultivation. Some strains had an acid-forming activity and an ability to grow in media with carbon-containing compounds. The bacteria fermented various carbohydrates such as fructose, maltose, glucose, galactose, mannitol, sorbitol, mannose, sucrose, and many other sugars, indicating their ability to use various organic carbon compounds for their metabolism. The strains had the ability to produce amylase, protease, cellulase and lipase enzymes. The most active producers of hydrolases were B. subtilis and L. plantarum strains.
Thus, out of 46 microorganisms isolated, 4 strains were selected, namely Bacillus subtilis, Propionibacterium freudenreichii, Lactobacillus plantarum and Streptomyces, capable of producing biologically active substances with a pronounced antimicrobial effect. Of the isolated microorganisms with antimicrobial activity, strains of B. subtilis and L. plantarum are of a greatest interest for studying the properties of hydrolases. The results obtained open up a possibility of using the microorganisms, selected by us, and their metabolites as a protective agent or a stimulating additive to agronomic biological preparations.
Table 3 presents some biometric indicators of the seedlings and saplings before treatment with Bacterial product LRV at the nursery of the Kaibitskoye forestry.
The Scots pine, pedunculate oak and small-leaved linden biennial seedlings biometric indicators in the forest nursery of the Kaibitskoe forestry are presented in Table 4.
For pedunculate oak biennial seedlings, maximum growth and development was observed when the Bacterial product LRV was sprayed twice at a dose of 10 ml/l. A seedling weight in this variant exceeds the control variant by 26 %. With a double application of the Bacterial product LRV at a dose of 4 ml/l, weight of a seedling exceeds the control by 24 %. In these variants, the seedlings’ biometric indicators are significantly ahead of those in other variants of the experiment.
A double foliar treatment of the small-leaved linden biennial seedlings with Bacterial product LRV at a dose of 10 ml/l provided an increase in a plant above-ground part growth by 51.9 % and an underground part by 37.2 % compared with the control. A total weight of seedlings at the end of the study exceeds the control variant by an average of 44.0 %.
In Scotch pine biennial seedlings, a greatest increase in biometric parameters was shown in the variant with a double application of the Bacterial product LRV at a dose of 10 ml/l. A double foliar spraying of the Scotch pine with the Bacterial product LRV at the indicated dose led to an increase in a plant above-ground part growth by an average of 25.7 % and an underground part by 25.4 % compared with the control. Accordingly, an increase in an accumulation of seedlings shoots and roots biomass was observed by 3.3 % and 3.1 %, respectively.
An important object of the study was a Bacterial product LRV effect on fungi caused forest species disease.
Figure 2 and Figure 3 show an Bacterial preparation LRV effect on the growth and development of the above-ground part of seedlings of pedunculate oak and small-leaved linden in the forest nursery of the Kaibitskoe forestry.
We studied a Bacterial product LRV effect on a Scots pine biennial seedlings infection with Schutte’s disease (Table 5).
As it can be seen from the Table 5, the Bacterial product LRV did not have a pronounced effect on a spread of Scots pine Schutte’s disease (Figure 4).
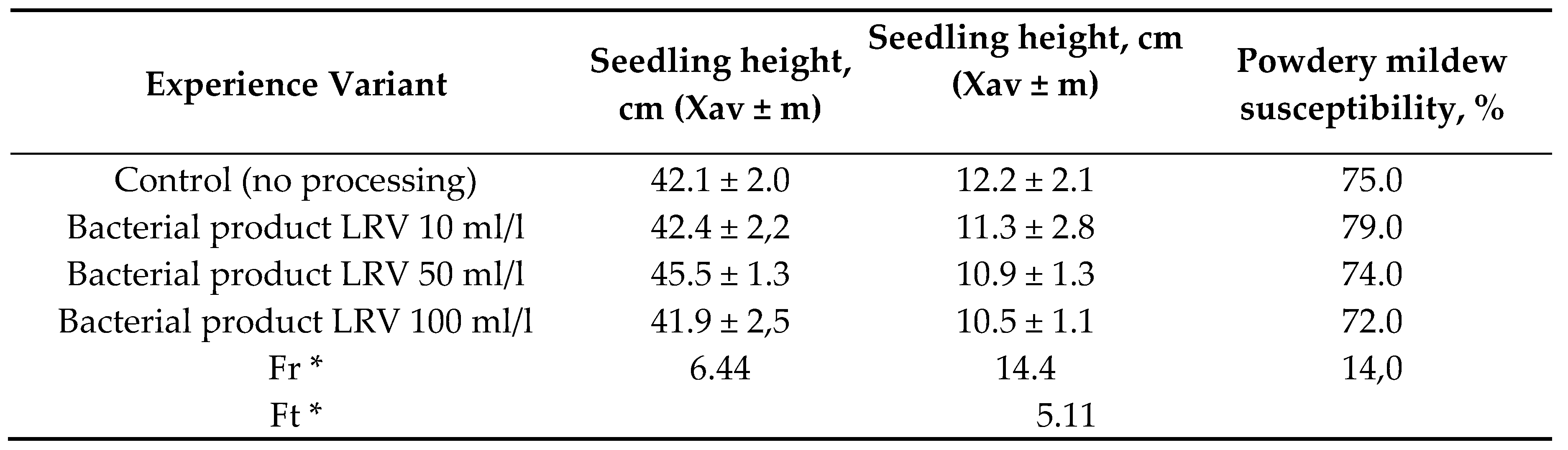
We studied a Bacterial product LRV effect on a powdery mildew of pedunculate oak biennial seedlings in the forest nursery of the Kaibitskoe forestry (Table 6).
As it can be seen from the Table 6, a use of different doses of Bacterial product LRV did not have a significant effect on a powdery mildew spread in pedunculate oak seedlings. This indicator value 72.0–79.0 % was at a level of control option. At the same time, a Bacterial product LRV treatment with doses of 10 ml/l, 50 ml/l and 100 ml/l had a pronounced positive effect on a pedunculate oak and small-leaved linden seedlings of above-ground and underground parts development in conditions of the Kaibitsky forestry. A Bacterial product LRV effect at a dose of 50 ml/l on an above-ground part of pedunculate oak seedling is clearly shown in Figure 5.
The results indicate a positive influence of the Bacterial product LRV on the growth and development of forest crop seedlings.
The results obtained during the study indicate a potential of the Bacterial product LRV use to enhance the growth and development of young seedlings of forest crops and allow recommending it for use in forestry nurseries.
The standard silviculture pre-planting and management fail in providing a soil fine aggregate structure, a multi-level hierarchy and a well-balanced water regime suppressing tree ontogenesis.
4. Discussion
An environmental and economic importance of Scots pine, pedunculate oak and small-leaved linden is high. However, morphological, biological and ecological features of these species determine their susceptibility to diseases caused by fungi Lophodermium pinastri Chev. and Microsphaera alphitoides. Thus, improved measures required for protection and reproduction of Scots pine, pedunculate oak and small-leaved linden [41,42,43]. A crops death important cause in the territory of coniferous-deciduous forests is an unhealthy and low-quality seedlings use for reforestation. A cultivation of planting material in the territories of forest nurseries is associated with a complex of silviculture standard measures, which lead to a decrease in a content of nutrients, lowering a soil agrochemical and biological potential. In consequence, a content and a toxicogenicity of phytopathogenic microorganisms, and a degradation of a stand and the soil increased [44,45,46,47]. In this regard, it is relevant to search for new ways to increase a forest crop seedlings productivity while minimizing an adverse environmental impact. This indicates a need to solve the problem of growing planting material in nursery farms at a new higher biological and technological level.
New environmentally friendly developments, reducing a degree of negative impact of biotic and anthropogenic stress factors on the trees and soil are needed. A number of publications have shown that a highly effective and environmentally friendly natural growth regulators use makes it possible to stimulate the growth and development of plants, increase the standard planting material yield and its resistance to fungal diseases and stress factors. This improves a planting material quality. Of particular interest is a study of substances of bacterial origin that stimulate the tree species seedlings growth and development and protective mechanisms of their influence on plant organism, which include biologically active metabolites of spore-forming and lactic acid microorganisms.
In the present study, we isolated from natural sources of various ecological niches in the Moscow region and the Republic of Tatarstan the new strains of microorganisms as a potential basis for a complex biological product for stimulating the growth and protection of Scots pine, pedunculate oak, and small-leaved linden from diseases caused by fungi Lophodermium pinastri Chev. and Microsphaera alphitoides. It was found that isolates of Bacillus subtilis, Propionibacterium freudenreichii, Lactobacillus plantarum and Streptomyces spp. differed from each other in an ability to produce biologically active metabolites with a pronounced antimicrobial potential against phytopathogenic fungi (Table 1). A number of studies have shown that a capability of antagonist bacteria to inhibit phytopathogenic fungi is a strain-specific trait [55,56,57,58,59].
The isolates, selected in the course of the study for the ability to suppress toxin-producing microscopic fungi, produces such hydrolytic enzymes as amylase, protease, cellulase and lipase. The most active producers of hydrolases were B. subtilis and L. plantarum. Isolates of Propionibacterium freudenreichii and Lactobacillus plantarum, characterized with an acid-forming activity (Table 2). Antifungal properties of Bacillus subtilis strains are associated with their ability to produce enzymatic complexes (chitinase, chitosanase, protease, cellulase, glucanase, lipase), which effectively break down the main components of a cell membrane of fungi, lipopeptides (surfactin, iturin, fengycin), affecting target phytopathogen cells at a membrane level through an interaction with an ergosterol [57,58]. This prevents an adhesion of competitive microorganisms on the parts of a plant organism and induces a plant systemic resistance to pathogens and unfavorable abiotic and anthropogenic factors. In addition, the bacterial strains of these species form fungicidal and fungistatic peptides (they are synthesized by the ribosomal multienzyme mechanism), as well as various siderophores (for example, bacilliboctin), which action can be realized through a competition for Fe in order to reduce a Fe availability for pathogens [60,61,62,63].
The siderophores, produced by Bacillus subtilis, are involved in the suppression of a number of plant diseases [64]. An antifungal potential of bacterial strains of Propionibacterium freudenreichii and Lactobacillus plantarum species is associated with their ability to produce a combination of organic acids (lactic, acetic, propionic, citric, 3-phenyl-lactic, transcinnamic, benzoic and oleamide), fatty acids (methyl esters of 9,12-otadecadienoic and hexadecanoic, 12-hydroxydodecanoic, stearic, lauric and palmitic), bacteriocin-like polypeptides, reuterin, hydrogen peroxide, diketopiperazine and diacetyl [65,66,67,68,69,70,71]. Similar results have been established in the works of many researchers, which show that various Streptomyces spp. produce secondary metabolites with antifungal properties [72,73,74]. An ability of Streptomyces spp. strains to inhibit phytopathogenic fungi is associated with a production of chitinase, betaine, azine, morpholine, pyrazole, fungichromine, actiphenol, ethyl 3-(2-methyl-2-propanyl)-1H-pyrazole-5-carboxylate, 6-amino-5-nitrosopyrimidine-2,4-diol, naphthalene benzaldehyde, carvacrol, and phenol, as well as many other compounds.
A feature of the isolates we selected was an ability to grow in a wide range of a temperature from 9 to 46 °C and a pH from 3.0 to 9.0 (Table 2). This opens up the prospects of the joint use of isolates with the herbicidal and fungicidal agents when the acidity of mixture decreases significantly.
In our study, a Scotch pine, pedunculate oak and small-leaved linden seedlings foliar treatment with a product based on the selected bacterial isolates (Bacterial product LRV) increases the plant growth and aboveground and underground biomass compared to the control. An effect of Bacterial product LRV on the forest crops is dose-dependent (Table 4). According to publications [54,57,58], bacteria are able to enhance growth and development of a host plant, producing biologically active metabolites and inhibiting phytopathogenic microorganisms. Bacillus spp. strains exert a growth-stimulating activity and increase a seed germination energy. These strains also provide a plant resistance to stress-factors such as frost, drought, high temperatures, and fungal and bacterial diseases.
Natural substances formed by bacilli have a number of significant advantages: a fairly wide range of biological activity, a positive general effect on a plant organism metabolism. The natural substances have a low toxicity and are of a high safety for the humans and environment. It is important that these kind substances show their activity in an extremely low concentration. This makes their use environmentally and economically beneficial. Some researchers noted [73,75] that streptomycetes and their metabolites are able to provide a stimulating effect on the growth and development of plants. This normalizes a plant cell physiology and biochemistry and increase a leaf surface index, the photosynthesis and respiration intensity, regulating a transpiration coefficient and a plant water consumption and reducing the trace elements digestible form deficiency. These factors generally affect the productivity and quality of grown products.
In our study, the Biological product LRV at various doses (Table 5 and Table 6) did not significantly affect the powdery mildew and Schutte's disease in forest plantations of Scotch pine, pedunculate oak and small-leaved linden. An observed increase in the growth and biomass of the aboveground and underground parts of forest cultures seedlings is associated with an ability of isolates selected by us to produce biologically active compounds that can function as plant growth regulators. An inhibitory effect on other phytopathogenic fungi, different from those presented in this work, capable of to infect and cause diseases of Scotch pine, pedunculate oak and small-leaved linden, is highly probable.
The data on the Propionibacterium freudenreichii and Lactobacillus plantarum strains and consortiums based on their ability to increase the forest crop seedlings growth and development are not available. In recent years, an interest has increased in research on lactic acid microorganisms as another class of plant growth promoters and potentially beneficial bacteria against phytopathogens. There are data on an increase in an agricultural plants stress resistance under an influence of exogenous treatment with metabolites of lactic acid microorganisms [76] and a successful use of this kind preparations in medicine, veterinary medicine, and viable bacterial cells in crop production [27,65,71,77]. This indicates a possibility of a wide use of lactobacilli and their metabolites in agriculture.
The data on a Bacillus subtilis, Propionibacterium freudenreichii, Lactobacillus plantarum and Streptomyces spp. strains combined use as a part of a consortium to increase the productivity of Scots pine, pedunculate oak and small-leaved linden opens a prospect of these valuable tree species restoration. The biologically active preparations that have both growth-stimulating and immuno-inducing effects are of a special interest now.
The results obtained during the study indicate a potential of a Bacterial product LRV use to enhance the growth and development of seedlings of forest crops and allow recommending it for use in forestry nurseries. Further study of the bacterial product obtained in our research should reveal its ability to increase an adaptive potential of forest crops at the early ontogenesis stages.
A further Bacterial product LRV promotion is possible using a BGT* methodology. The BGT* is a new theoretical, technical and technological basis of a long-term soil improvement via intra-soil milling processing [78,79] instead of a standard plowing, a drought mitigation and a fresh water saving via intra-soil pulse continuously-discrete humidification [80,81,82] instead of a standard frontal irrigation, and an intra-soil disperse waste ameliorative and nutritional recycling [83,84,85] instead of standard waste landfills.
5. Conclusions
New strains of microorganisms as a potential basis for a complex biological product development for stimulating the seedlings growth and protection were isolated. The isolates selected have an ability to produce biologically active metabolites with a pronounced antimicrobial potential against phytopathogenic fungi. Biological product LRV based on selected bacterial isolates was developed. A foliar treatment of the Scots pine, pedunculate oak, and small-leaved linden seedlings with Biological product LRV increased growth and biomass of the aboveground and underground parts of seedlings compared to untreated plants. The Biological product LRV provided protection of plants from diseases caused by fungi Lophodermium pinastri Chev. and Microsphaera alphitoides. A Biogeosystem Technique methodology has been developed to improve a long-term forest growing.
Author Contributions
Conceptualization, supervision, L.R.V., R.S.M., and V.I.E; Data curation, investigation, methodology, project administration, resources, visualization, L.R.V., R.S.M., S.N.S, V.I.E, and A.I.Y.; Formal analysis, visualization, writing—original draft, S.N.S., S.V.K., R.S.M., S.S.M., and A.I.Y.; Investigation, supervision, V.V.S., R.K.S., T.M.M and I.V.Z.; Funding acquisition, supervision, V.V.S., R.S.M., R.K.S. and V.D.R.; Methodology, validation, V.V.S., R.S.M., R.K.S. and T.M.M.; Validation, software, L.L.S., V.D.R., C.S.S. and A.R.; Writing—review and editing, V.D.R., I.V. Z., T.M.M and A.R.; Validation, formal analysis V.D.R., I.V.Z. and A.R.; Software, writing—review and editing, A.R., V.P.K., S.S.M., M.G.B.; Visualization, V.I.Ch., I.V.Z., and M.G.B.; Writing—original draft, S.V.K., V.P.K., and V.I.Ch.; Writing—review and editing, data curation L.L.S., V.P.K. M.A.S., and V.I.Ch. All authors have read and agreed to the published version of the manuscript.
Funding
The work on assessing the antimicrobial properties of microorganisms isolated from various ecological niches and obtaining compositions based on them was supported by the Russian Science Foundation under grant № 23-26-00161.
Data Availability Statement
The data presented in this study are available in the figures and tables provided in the manuscript are available on request.
Acknowledgments
The authors express their deep gratitude to Sh.Z. Validov (Research Laboratory “Microbial Biotechnologies”, IPM&B, Kazan (Volga) Federal University), T.V. Bagaeva (Department of Biochemistry, Biotechnology, and Pharmacology, IPM&B, Kazan (Volga) Federal University) for their help and helpful advice in analytical experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, P.-H.; Chen, R.-Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology. 2018, 46, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Kurtzman, C.P.; Buyer, J.S. Yeasts associated with nectarines and their potential for biological control of brown rot. Yeast. 2010, 27, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Valiullin, L.R.; Titova, V.Y.; Skvortsov, E.V.; Muhammadiev, R.S.; Validov, S.Z.; Rud, V.Y.; Davydov, V.V.; Glinushkin, A.P. Search for antagonists to protect plant raw materials from pathogens. IOP Conf. Ser.: Earth Environ. Sci. 2021, 663, 012005. [Google Scholar] [CrossRef]
- Miftakhov, A.K.; Diabankana, R.G.C.; Frolov, M.; Validov, S.Z.; Afordoanyi, D.M. Persistence as a Constituent of a Biocontrol Mechanism (Competition for Nutrients and Niches) in Pseudomonas putida PCL1760. Microorganisms. 2023, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Valiullin, L.R.; Mukhammadiev, R.S.; Solovyova, A.S.; Skvortsov, E.V.; Valiullina, D.A.; Kasanova, N.R. Reducing the risks of environmental pollution by agents of biological origin. J. Environ. Manag. Tour. 2020, 3, 555–562. [Google Scholar] [CrossRef]
- Afordoanyi, D.M.; Diabankana, R.G.C.; Akosah, Y.A.; Validov, S.Z. Are formae speciales pathogens really host specific? A broadened host specificity in Fusarium oxysporum f.sp. radicis-cucumerinum. Braz J Microbiol. 2022, 53, 1745–1759. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Kromann, P.; Lojan, P.; Rojas, M.; Franco, J.; Suarez, J.P.; Prestwich, B.D. Identification of mVOCs from andean Rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microb. Ecol. 2014, 268, 285–292. [Google Scholar] [CrossRef]
- Karmanov, A.P.; Kanarsky, A.V.; Kocheva, L.S.; Semenov, E.I.; Belyy, V.A. In vitro study of adsorption efficiency of natural lignins towards aflatoxin B2. React. Funct. Polym. 2021, 167, 105033. [Google Scholar] [CrossRef]
- Wisniewski, M.; Biles, C.; Droby, S.; McLaughlin, R.; Wilson, C.; Chalutz, E. Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. I. Characterization of attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol. 1991, 39, 245–258. [Google Scholar] [CrossRef]
- Cheremisin, A.V.; Valiullin, L.R.; Moroz, A.V. On the need to apply a comprehensive assessment to determine the state of the ecosystem of soil and vegetation cover of urban areas. 2021, 1942, 012093. [CrossRef]
- Kamilova, F.; Validov, S.; Lugtenberg, B. Biological control of tomato foot and root rot caused by Fusarium oxysporum f.sp. radicis-lycopersici by Pseudomonas bacteria. Acta Horticulturae. 2009, 808, 317–320. [Google Scholar] [CrossRef]
- Yermolaev, O.P.; Medvedeva, R.A.; Ivanov, M.А. Modern gully erosion in forest and forest-steppe landscapes of the east of the Russian Plain. Geomorfologiya. 2021, 52, 28–41. [Google Scholar] [CrossRef]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: the evolution of culture media in clinical microbiology. New Microbes New Infect. 2019, 34, 100622. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Mizuno, M.; Yamauchi, M.; Takemura, A.J.; Medrano Romero, V.; Morikawa, K. Adjacent-possible ecological niche: growth of Lactobacillus species co-cultured with Escherichia coli in a synthetic minimal medium. Sci Rep. 2017, 7, 12880. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, F. Characterization of Lactobacillus, Bacillus and Saccharomyces isolated from Iranian traditional dairy products for potential sources of starter cultures. AIMS Microbiol. 2017, 12, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Swearingen, P.; O'sullivan, D.; Warthesen, J. Isolation, characterization, and influence of native, nonstarter lactic acid bacteria on Cheddar cheese quality. J Dairy Sci. 2001, 84, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Dehnad, A.; Hanifian, S.; Khani, S. Isolation and Molecular Identification of Streptomyces spp. with Antibacterial Activity from Northwest of Iran. Bioimpacts. 2013, 3, 129–134. [Google Scholar] [CrossRef]
- Freitas, D.B.; Reis, M.P.; Lima-Bittencourt, C.I.; Costa, P.S.; Assis, P.S.; Chartone-Souza, E.; Nascimento, A.M.A. A. Genotypic and phenotypic diversity of Bacillus spp. isolated from steel plant waste. BMC Res. Notes 2008, 1, 92. [Google Scholar] [CrossRef]
- Begunova, A.V.; Rozhkova, I.V.; Zvereva, E.A.; Glazunova, O.A.; Fedorova, T.V. Lactic and propionic acid bacteria: the formation of a community for the production of functional products with bifidogenic and hypotensitive properties. Appl. Biochem. Microbiol. 2019, 55, 660–669. [Google Scholar] [CrossRef]
- Mardanova, А.М.; Hadieva, G.F.; Lutfullin, M.T.; Khilyas, I.V.; Minnullina, L.F.; Gilyazeva, A.G.; Bogomolnaya, L.M.; Sharipova, M.R. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agricultural Sciences. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Fhoula, I.; Najjari, A.; Turki, Y.; Jaballah, S.; Boudabous, A.; Ouzari, H. Diversity and antimicrobial properties of lactic acid bacteria isolated from rhizosphere of olive trees and desert truffles of Tunisia. Biomed Res Int. 2013, 2013, 405708. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501, Lactobacillus paracasei IMC 502 and SYNBIO against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkova, E.P.; Danilova, I.V.; Shamraichuk, I.L.; Kurakov, A.V.; Netrusov, A.I. Antifungal activity of Propionibacterium freudenreichii and several Lactobacillus species. Mikologiya I Fitopatologiya. 2018, 52, 144–149. [Google Scholar]
- Mukhammadiev, R.S.; Mukhammadieva, A.S.; Skvortsov, E.V.; Valiullin, L.R.; Glinushkin, A.P. Antagonistic properties and biocompatibility as important principles for development of effective and biosafety probiotic drugs. IOP Conf. Ser.: Earth Environ. Sci. 2021, 663, 012008. [Google Scholar] [CrossRef]
- Khadieva, G.F.; Lutfullin, M.T.; Mochalova, N.K.; Sharipova, M.R.; Mardanova, A.M.; Lenina, O.A. New Bacillus subtilis strains as promising probiotics. Microbiology. 2018, 87, 463–471. [Google Scholar] [CrossRef]
- Diabankana, R.G.C.; Afordoanyi, D.M.; Safin, R.I.; Nizamov, R.M.; Karimova, L.Z.; Validov, S.Z. Antifungal Properties, Abiotic Stress Resistance, and Biocontrol Ability of Bacillus mojavensis PS17. Curr Microbiol. 2021, 78, 3124–3132. [Google Scholar] [CrossRef]
- Hola, J. Bergey's Bacteria Key: in 2 vols. M.: Mir, 1997.
- Bergey’s Manual of Systematic Bacteriology, Lippincott Williams & Wilkins, 1994, 9th edition, vol. 1. Translated under the title Opredelitel’ bakterii Berdzhi, Moscow: Mir, 1997.
- Khomyakova, D.V.; Botvinko, I.V.; Netrusov, A.I. Isolation of Hydrocarbon-Oxidizing Psychroactive Bacteria from Oil-Polluted Soils. Applied Biochemistry and Microbiology. 2003, 39, 581–584. [Google Scholar] [CrossRef]
- Netrusov, A.I. Workshop on microbiology. M.: Academy, 2005, 608 p.
- Rodríguez, J.; Vázquez, L.; Flórez, A.B.; Mayo, B. Phenotype testing, genome analysis, and metabolic interactions of three lactic acid bacteria strains existing as a consortium in a naturally fermented milk. Front Microbiol. 2022, 13, 1000683. [Google Scholar] [CrossRef]
- Kali, A.; Srirangaraj, S.; Charles, P.M. A cost-effective carbohydrate fermentation test for yeast using microtitre plate. Indian J. Med. Microbiol. 2015, 33, 293–295. [Google Scholar] [CrossRef]
- EL-Sayed, A.I.M.; El-Borai, A.M.; Akl, S.H. Identification of Lactobacillus strains from human mother milk and cottage cheese revealed potential probiotic properties with enzymatic activity. Sci. Rep. 2022, 12, 22522. [Google Scholar] [CrossRef]
- Ngouénam, J.R.; Momo Kenfack, C.H.; Foko Kouam, E.M.; Kaktcham, P.M.; Maharjan, R.; Ngoufack, F.Z. Lactic acid production ability of Lactobacillus sp. from four tropical fruits using their by-products as carbon source. Heliyon. 2021, 7, e07079. [Google Scholar] [CrossRef]
- Osadchaya, A.I.; Safronova, L.A.; Poltavsky, A.N.; Ilyash, V.M. Hydrolase activity of Antarctic bacilli. Мікрoбіoлoгія і біoтехнoлoгія. 2009, 4, 33–40. [Google Scholar] [CrossRef]
- Mukhammadiev, R.; Mukhammadiev, R.; Skvortsov, E.; Gerner, A.; Valiullin, L. Chitinase production by Trichoderma viride in submerged state fermentation. IOP Conf. Ser.: Earth Environ. Sci. 2020, 578, 012009. [Google Scholar] [CrossRef]
- Mukhammadiev, R.S.; Skvortsov, E.V.; Valiullin, L.R.; Glinushkin, A.P.; Bagaeva, T.V. Isolation, purification, and characterization of a lectin from the fungus Fusarium solani 4. Appl. Biochem. Microbiol. 2021, 57, 206–211. [Google Scholar] [CrossRef]
- Kavaliauskas, D.; Danusevičius, D.; Baliuckas, V. New Insight into Genetic Structure and Diversity of Scots Pine (Pinus sylvestris L.) Populations in Lithuania Based on Nuclear, Chloroplast and Mitochondrial DNA Markers. Forests. 2022, 13, 1179. [Google Scholar] [CrossRef]
- Jansons, Ā.; Zeltiņš, P.; Donis, J.; Neimane, U. Long-Term Effect of Lophodermium Needle Cast on The Growth of Scots Pine and Implications for Financial Outcomes. Forests. 2020, 11, 718. [Google Scholar] [CrossRef]
- Turczański, K.; Bełka, M.; Spychalski, M.; Kukawka, R.; Prasad, R.; Smiglak, M. Resistance Inducers for the Protection of Pedunculate Oak (Quercus robur L.) Seedlings against Powdery Mildew Erysiphe alphitoides. Plants. 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Rivas, Y.; Aponte, H.; Rivera-Salazar, D.; Matus, F.; Martínez, O.; Encina, C.; Retamal-Salgado, J. Microbial Community and Enzyme Activity of Forest Plantation, Natural Forests, and Agricultural Land in Chilean Coastal Cordillera Soils. Forests. 2023, 14, 938. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Nazarov, A.; Chetverikov, S.; Chetverikova, D.; Tuktarova, I.; Ivanov, R.; Urazgildin, R.; Garankov, I.; Kudoyarova, G. Microbial Preparations Combined with Humic Substances Improve the Quality of Tree Planting Material Needed for Reforestation to Increase Carbon Sequestration. Sustainability. 2023, 15, 7709. [Google Scholar] [CrossRef]
- Sheller, M.A.; Shilkina, E.A.; Ibe, A.A.; Razdorozhnaya, T.Y.; Sukhikh, T.V. Phytopathogenic fungi in forest nurseries of Middle Siberia. Forest. 2020, 13, 507–512. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of salt tolerance and interactive impact of Azotobacter chroococcum and/or Alcaligenes faecalis inoculation on canola (Brassica napus L.) Plants Grown Saline Soil. Plants. 2021, 10, 110. [Google Scholar] [CrossRef]
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculatareinforces flax (Linum usitatissimum) growth by improving physiological activities under saline soil conditions. Bot. Stud. 2022, 63, 15. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Cumming, J.R.; Collart, F.R.; Noirot, P.H.; Larsen, P.E. Pseudomonas fluorescens transportome is linked to strain-specific plant growth promotion in aspen seedlings under nutrient stress. Front. Plant Sci. 2017, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, A.; Bakaeva, M.; Timergalin, M.; Chetverikova, D.; Kendjieva, A.; Rameev, T.; Hkudaygulov, G.; Nazarov, A.; Kudoyarova, G.; Chetverikov, S. Effects of Humic Substances on the Growth of Pseudomonas plecoglossicida 2,4-D and Wheat Plants Inoculated with This Strain. Microorganisms. 2022, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Rashwan, E.; Hafez, E.; Omara, A.E.-D.; Mohamed, H.H.; Alshaal, T. Potassium Humate and Plant Growth-Promoting Microbes Jointly Mitigate Water Deficit Stress in Soybean Cultivated in Salt-Affected Soil. Plants. 2022, 11, 3016. [Google Scholar] [CrossRef]
- Ranjbariyan, A.; Shams-Ghahfarokhi, M.; Kalantari, S.; Razzaghi-Abyaneh, M. Molecular Identification Of Antagonistic Bacteria From Tehran Soils And Evaluation Of Their Inhibitory Activities Toward Pathogenic Fungi. Iran J. Microbiol. 2011, 3, 140–146. [Google Scholar]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms. 2022, 10, 1759. [Google Scholar] [CrossRef]
- Mardanova, A.M.; Hadieva, G.F.; Lutfullin, M.T.; Khilyas, I.V.; Minnullina, L.F.; Gilyazeva, A.G.; Bogomolnaya, L.M.; Sharipova, M.R. Bacillus subtilis Strains with Antifungal Activity against the Phytopathogenic Fungi. Agric. Sci. 2016, 8, 1–20. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Moreno-Ramírez, L.; Valdez-Salas, B.; Seleiman, M.F.; El-Hendawy, S.; Aldhuwaib, K.J.; Alotaibi, M.; González-Mendoza, D. New Bacillus subtilis Strains Isolated from Prosopis glandulosa Rhizosphere for Suppressing Fusarium Spp. and Enhancing Growth of Gossypium hirsutum L. Biology. 2023, 12, 73. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; González-Mendoza, D. Antifungical Activity of Autochthonous Bacillus subtilis Isolated from Prosopis juliflora against Phytopathogenic Fungi. Mycobiology. 2017, 45, 385–391. [Google Scholar] [CrossRef]
- Perez, K.J.; Viana, J.d.S.; Lopes, F.C.; Pereira, J.Q.; dos Santos, D.M.; Oliveira, J.S.; Velho, R.V.; Crispim, S.M.; Nicoli, J.R.; Brandelli, A.; et al. Bacillus spp. Isolated from Puba as a Source of Biosurfactants and Antimicrobial Lipopeptides. Front. Microbiol. 2017, 8, 61. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Cyclic Lipopeptide Biosynthetic Genes and Products, and Inhibitory Activity of Plant-Associated Bacillus against Phytopathogenic Bacteria. PLoS ONE. 2015, 10, e0127738. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, T.M.; Asaturova, A.M.; Homyak, A.I.; Tomashevich, N.S. Isolation and characterization of antifungal metabolites of Bacillus subtilis strains bzr 336g and bzr 517 using the modified bioauthography method. Sel'skokhozyaistvennaya Biologiya. 2019, 54, 178–185. [Google Scholar] [CrossRef]
- Rizzi, A.; Roy, S.; Bellenger, J.P.; Beauregard, P.B. Iron Homeostasis in Bacillus subtilis Requires Siderophore Production and Biofilm Formation. Appl. Environ. Microbiol. 2019, 85, e02439-18. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants. 2022, 11, 3065. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkova, E.P.; Danilova, I.V.; Shamraichuk, I.L.; Kurakov, A.V.; Netrusov, A.I. Antifungal activity of Propionibacterium freudenreichii and several Lactobacillus species. Mycology and Phytopathology. 2018, 52, 144–149. [Google Scholar]
- Lind, H.; Sjögren, J.; Gohil, S.; Kenne, L.; Schnürer, J.; Broberg, A. Antifungal compounds from cultures of dairy propionibacteria type strains. FEMS Microbiol. Lett. 2007, 271, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Antone, U.; Ciprovica, I.; Zolovs, M.; Scerbaka, R.; Liepins, J. Propionic Acid Fermentation—Study of Substrates, Strains, and Antimicrobial Properties. Fermentation. 2023, 9, 26. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Salman, M.; Numan, M.; Javed, M.R.; Zubair, M.; Mustafa, G. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol. Biol. Rep. 2020, 47, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hao, X.; Yang, F.; Wang, Y.; Fan, X.; Wang, Y. Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods. 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P.E.J. Antifungal Preservation of Food by Lactic Acid Bacteria. Foods. 2022, 11, 395. [Google Scholar] [CrossRef]
- Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal Attributes of Lactobacillus plantarum MYS6 against Fumonisin Producing Fusarium proliferatum Associated with Poultry Feeds. PLoS One. 2016, 11, e0155122. [Google Scholar] [CrossRef]
- Sholkamy, E.N.; Muthukrishnan, P.; Abdel-Raouf, N.; Nandhini, X.; Ibraheem, I.B.M.; Mostafa, A.A. Antimicrobial and antinematicidal metabolites from Streptomyces cuspidosporus strain SA4 against selected pathogenic bacteria, fungi and nematode. Saudi J. Biol. Sci. 2020, 27, 3208–3220. [Google Scholar] [CrossRef]
- Ghanem, G.A.M.; Gebily, D.A.S.; Ragab, M.M.; Ali, A.M.; Soliman, N.E.K.; El-Moity, T.H.A. Efficacy of antifungal substances of three Streptomyces spp. against different plant pathogenic fungi. Egypt. J. Biol. Pest. Control. 2022, 32, 112. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, B.N.; Verma, S.; Choudhary, P.; Das, S.; Chakdar, H.; Murugan, K.; Goswami, S.K.; Saxena, A.K. Bioactive antifungal metabolites produced by Streptomyces amritsarensis V31 help to control diverse phytopathogenic fungi. Braz. J. Microbiol. 2021, 52, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, Z.F.; Merabet, C.; Tsaki, H. Plant growth promoting actinobacteria, the most promising candidates as bioinoculants? Front. Agron. 2022, 4, 849911. [Google Scholar] [CrossRef]
- Jaffar, N.S.; Jawan, R.; Chong, K.P. The potential of lactic acid bacteria in mediating the control of plant diseases and plant growth stimulation in crop production - A mini review. Front Plant Sci. 2023, 13, 1047945. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, V.P. Device for rotational subsurface loosening. Patent RU No 2376737 C1. Published on 27.12.2009.
- Kalinitchenko, V.P.; Glinushkin, A.P.; Sharshak, V.K.; Ladan, E.P.; Minkina, T.M.; Sushkova, S.N.; Mandzhieva, S.S.; Batukaev, A.A.; Chernenko, V.V.; Ilyina, L.P.; et al. Intra-Soil Milling for Stable Evolution and High Productivity of Kastanozem Soil. Processes 2021, 9, 1302. [Google Scholar] [CrossRef]
- Kalinichenko, V.P. Method of intra-soil pulse discrete irrigation. Patent RU No 2386243 C1. Published on 20.04.2010a.
- Belov, S.V.; Glinushkin, A.P.; Danyleiko, Y.K.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; Izmailov, A.Y. Activated potassium phosphate fertilizer solution for agricultural plants growth stimulation. Frontiers in Physics 2021, 8, 618320. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Glinushkin, A.P.; Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Savchenko, I.V.; Meshalkin, V.P.; Samarin, G.N.; Maleki, A.; Kalinitchenko, V.P. Identification of Organic Matter Dispersions Based on Light Scattering Matrices Focusing on Soil Organic Matter Management. ACS Omega 2020, 5, 33214–33224. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, V.P. Device for application of matter while intra-soil milling. Patent RU No 2387115 C2. Published on 27.04.2010 b.
- Kalinichenko, V.P., Glinushkin, A.P., Sokolov, M.S., Budynkov, N.I., Zinchenko, V.E., Chernenko, V.V., Kozyrev, S.G. Patent RU №2720634 С1. MPC A01G 25/06 (2006.01) A01C 23/02 (2006.01), SPC A01G 25/06 (2020.02) A01C 23/02 (2020.02). The method of introducing a biological product inside the topsoil. Application No. 2019117310/033090 on 06/04/2019. Registered May 12th 2020. Bul. 14. 7 p. : 2 fig. https://new.fips.ru/ofpstorage/Doc/IZPM/RUNWC1/000/000/002/720/634/%D0%98%D0%97-02720634-00001/document.pdf.
- Kalinitchenko, V.P.; Glinushkin, A.P.; Minkina, T.M.; Mandzhieva, S.S.; Sushkova, S.N.; Sukovatov, V.A.; Il’ina, L.P.; Makarenkov, D.A. Chemical soil-biological engineering theoretical foundations, technical means, and technology for environmentally safe intra-soil waste recycling and long-term higher soil productivity. ACS Omega 2020, 5, 17553–17564. [Google Scholar] [CrossRef]
Figure 1.
Treatment of seedlings with a tractor sprayer in the Kaibitskoye forestry in the first half of 2022 growing season.
Figure 1.
Treatment of seedlings with a tractor sprayer in the Kaibitskoye forestry in the first half of 2022 growing season.

Figure 2.
Pedunculate oak sowing in 2021: (a) Control; (b) Double treatment with the Bacterial product LRV in a dose 10 ml/l.
Figure 2.
Pedunculate oak sowing in 2021: (a) Control; (b) Double treatment with the Bacterial product LRV in a dose 10 ml/l.

Figure 3.
Small-leaved linden sowing in 2021: (a) Control; (b) Double treatment with the Bacterial product LRV in a dose 10 ml/l.
Figure 3.
Small-leaved linden sowing in 2021: (a) Control; (b) Double treatment with the Bacterial product LRV in a dose 10 ml/l.

Figure 4.
Scots pine sowing 2020: (a) Control; (b) Bacterial product LRV 50 ml/l.

Figure 5.
Pedunculate oak sowing in 2020: (a) Control; (b) Bacterial product LRV 50 ml/l .

Table 1.
Antagonistic activity of selected microorganisms against microscopic fungi.
| Microorganism | Test culture | ||||
|---|---|---|---|---|---|
| Bacillus spp. | Propionibacterium spp. | Lactobacillus spp. | Streptomyces spp. | Combination | |
| Fusarium oxysporum | + | - | - | - | + |
| Aspergillus flavus | + | + | + | + | + |
| Penicillium spp. | + | + | + | - | + |
| Candida albicans | - | + | - | - | + |
* "-" – absence; "+" – manifestation of antagonistic activity.
Table 2.
Properties of selected microorganisms *.
| Property | Isolate | |||
| Bacillus subtilis |
Propionibacterium freudenreichii |
Lactobacillus plantarum |
Streptomyces spp. | |
| Morphology | ||||
| Cell shape | sticks, in pairs | sticks, in pairs at an angle to each other | sticks, in pairs, can be chains, depending on the composition of the medium | filamentous form |
| Gram stain | + | + | + | + |
| Spore formation | + | - | - | - |
| Mobility | - | - | - | + |
| Cultural | ||||
| The shape of the colonies | round | round | round | rounded convex |
| Colony color | white | white | white | cream color |
| Growth on dense nutrient media | uniform growth | uniform growth over the entire surface of the medium | uniform growth throughout the entire thickness of the medium, near-bottom | uniform growth |
|
Physiological and biochemical: Fermentation of carbohydrates: | ||||
| Glucose | + | + | + | +/- |
| Fructose | + | + | + | + |
| Maltose | + | - | + | - |
| Sucrose | + | - | + | - |
| Galactose | - | + | + | + |
| Lactose | +/- | + | + | +/- |
| Arabinose | +/- | + | + | + |
| Rhamnose | - | + | - | + |
| Sorbitol | + | - | +/- | +/- |
| Optimum growth temperature 0C | 35 to 39 | 30 to 33 | 33 to 35 | 23 to 25 |
| Optimum pH growth | 6.9 to 7.2 | 6.2 to 7.3 | 5.9 to 6.2 | 6.8 to 7.3 |
| Acid-forming activity | - | + | + | - |
| Enzymatic activity: | ||||
| cellulolytic | + | - | + | +/- |
| proteolytic | + | + | + | + |
| amylolytic | - | - | + | - |
| lipolytic | +/- | +/- | - | + |
* "-" – the absence or "+" the presence of a sign.
Table 3.
Indicators of 1–3-year-old sparse seedlings and seedlings of Scots pine, pedunculate oak and small-leaved linden in the nursery of Kaibitskoe forestry *.
Table 3.
Indicators of 1–3-year-old sparse seedlings and seedlings of Scots pine, pedunculate oak and small-leaved linden in the nursery of Kaibitskoe forestry *.
| Wood type and age of seedlings | Height, cm | Diameter, mm | Growth, cm | Root length, cm |
|---|---|---|---|---|
| Scots pine 1-year-old | 5.1 ± 0.1 | 0.82 ± 0.02 | – | 7.0 ± 0.1 |
| Scots pine 2-year-old | 17.7 ± 0.4 | 3.84 ± 0.09 | 13.4 ± 0.2 | 17.5 ± 0.4 |
| Scots pine 3-year-old | 25.9 ± 0.5 | 4.13 ± 0.10 | 14.6 ± 0.3 | 20.1 ± 0.5 |
| Pedunculate oak 1-year-old | 4.3 ± 0.1 | 0.81 ± 0.02 | – | 4.9 ± 0.1 |
| Pedunculate oak 2-year-old | 21.9 ± 0.4 | 3.50 ± 0.08 | 15.5 ± 0.3 | 19.8 ± 0.4 |
| Pedunculate oak 3-year-old | 39.5 ± 0.9 | 6.9 ± 0.16 | 22.8 ± 0.5 | 29.4 ± 0.7 |
| Small-leaved linden 1-year-old | 4.4 ± 0.1 | 0.54 ± 0.01 | - | 6.5 ± 0.1 |
| Small-leaved linden 2-year-old | 12.8 ± 0.3 | 3.60 ± 0.09 | 12.3 ± 0.1 | 11.4 ± 0.2 |
| Small-leaved linden 3 year old | 32.1 ± 0.8 | 5.83 ± 0.14 | 14.7 ± 0.3 | 19.6 ± 0.3 |
Table 4.
Biennial seedlings biometric indicators of in the forest nursery of Kaibitskoye forestry of the Ministry of Forestry of the Republic of Tatarstan.
Table 4.
Biennial seedlings biometric indicators of in the forest nursery of Kaibitskoye forestry of the Ministry of Forestry of the Republic of Tatarstan.
| No. | Experiment option* | Number of seedlings, pcs / m² (Xav) | Seedling root length, cm (Xav ± m**) | Seedling height, cm (Xav ± m) | Average weight of seedling, g; (%) relative to control |
||
|---|---|---|---|---|---|---|---|
| roots (Xav ± m) | above-ground part (Xav ± m) | total seedling weight (% to control) |
|||||
| Pedunculate oak | |||||||
| 1 | 1 | 31 | 40.1 ± 0.5 | 22.7 ± 0.5 | 5.2 ± 0.2 | 3.4 ± 0.2 | 8.6 |
| 1 | 32 | 40.4 ± 0.6 | 30.5 ± 0.4 | 5.3 ± 0.3 | 3.5 ± 0.2 | 8.8 | |
| 2 | 2 | 30 | 44.5 ± 0.2 | 38.0 ± 0.3 | 5.7 ± 0.2 | 3.7 ± 0.3 | 9.4 (109) |
| 2а | 31 | 50.5 ± 1.1 | 40.2 ± 0.4 | 6.9 ± 0.7 | 4.2 ± 0.4 | 11.1 (126) | |
| 3 | 3 | 34 | 47.6 ± 0.4 | 39.4 ± 0.2 | 5.9 ± 0.3 | 3.8 ± 0.2 | 9.7 (112) |
| 3а | 40 | 49.5 ± 0.5 | 40.1 ± 0.4 | 6.5 ± 0.5 | 4.4 ± 0.2 | 10.9 (124) | |
| Fр* | 111.5 | 98.8 | 144.1 | 127.8 | - | ||
| Fт | 147.9 | ||||||
| НСР05 | 0.4 | 0.9 | 0.3 | 0.2 | - | ||
| Small-leaved linden | |||||||
| 4 | 1 * | 21 | 25.2 ± 0.2 | 15.5 ± 0.3 | 3.8 ± 0.2 | 2.5 ± 0.2 | 6.3 |
| 1а * | 20 | 26.1 ± 0.4 | 15.6 ± 0.4 | 3.8 ± 0.4 | 2.8 ± 0.3 | 6.6 | |
| 5 | 2 | 22 | 30.1 ± 0.5 | 21.9 ± 0.2 | 4.9 ± 0.3 | 3.5 ± 0.2 | 8.4 (133) |
| 2а | 22 | 35.8 ± 0.5 | 23.7 ± 0.2 | 5.6 ± 0.8 | 3.9 ± 0.6 | 9.5 (144) |
|
| 6 | 3 | 21 | 27.3 ± 0.5 | 16.5 ± 0.1 | 4.0 ± 0.2 | 2.9±0.3 | 6.9 (109) |
| 3а | 23 | 26.5 ± 0.3 | 16.2 ± 0.2 | 3.9 ± 0.4 | 2.7±0.3 | 6.6 (0) |
|
| Fр * | 31.4 | 38.0 | 22.8 | 37.7 | - | ||
| Fт | 38.7 | ||||||
| НСР05 | 0.2 | 0.3 | 0.1 | 0.2 | - | ||
| Scotch pine | |||||||
| 7 | 1 | 73 | 15.5 ± 0,6 | 21.8 ± 0,5 | 2.2 ± 0,2 | 1.7 ± 0,6 | 3.9 |
| 1а | 81 | 15.2 ± 0,5 | 21.3 ± 0,4 | 2.1 ± 0,4 | 1.5 ± 0,2 | 3.6 | |
| 8 | 2 | 74 | 18.3 ± 0,5 | 25.1 ± 0,8 | 2.9 ± 0,2 | 2.8 ± 0,2 | 5.7 (146) |
| 2а | 77 | 19.1 ± 0,2 | 26.7 ± 0,4 | 3.3 ± 0,2 | 3.1 ± 0,4 | 6.4 (178) |
|
| 9 | 3 | 78 | 18.7 ± 0,4 | 22.5 ± 0,4 | 3.1 ± 0,2 | 1.9 ± 0,3 | 5.0 (128) |
| 3а | 71 | 18.1 ± 0,5 | 21.9 ± 0.5 | 2.8 ± 0.2 | 1.8 ± 0.2 | 4.6 (127) |
|
| Fр * | 144.9 | 87.4 | 65.2 | 139.3 | - | ||
| Fт | 155.1 | ||||||
| НСР05 | 0.6 | 0.6 | 0.1 | 0.2 | - | ||
* Experiment option: 1. Control – seedlings, which were once sprayed with water; 1a. Control – seedlings, which were sprayed twice with water; 2. Experimental group 1 – seedlings treated with the Bacterial product LRV at a dose of 10 ml/l once; 2a. Experimental group 2 – seedlings treated with the Bacterial product LRV at a dose of 10 ml/l twice; 3. Experimental group 3 – seedlings treated with the Bacterial product LRV at a dose of 4 ml/l once; 3a. Experimental group 4 – seedlings treated with the Bacterial product LRV at a dose of 4 ml/l twice. Fр * – calculated Fisher coefficient, Fт – tabular Fisher coefficient). **m – mean error; LSD05 – Least significant Difference at 5% significance level.
Table 5.
Characteristics of Scots pine seedlings (sowing in 2020), 2022 *.
| Experiment Option | Seedling height, cm (Xav ± m)* | Seedling height, cm (Xav ± m) | Schutte’s disease, % |
|---|---|---|---|
| Control (no processing) | 22.5 ± 2.3 | 9.8 ± 2.1 | 88.0 |
| Bacterial product LRV 10 ml/l | 23.4 ± 1.4 | 10.2 ± 1.8 | 89.0 |
| Bacterial product LRV 50 ml/l | 25.4 ± 3.3 | 10.4 ± 3.3 | 85.0 |
| Bacterial product LRV 100 ml/l | 21.3 ± 3.5 | 8.5 ± 3.1 | 91.0 |
| Fr * | 7.22 | 2.31 | 5.61 |
| Ft * | 1.44 | ||
* Fr – calculated Fisher criterion, Ft – tabular Fisher criterion.
Table 6.
Characteristics of Pedunculate oak (sowing in 2020) based on research materials in 2022.
* Fr – calculated Fisher criterion, Ft – tabular Fisher criterion.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





