Introduction
Dengue virus (DENV) is a positive stranded RNA virus of the family Flaviviridae and genus Flavivirus that is spread by arthropods. The four different dengue virus serotypes (DENV 1-4) are all spread by Aedes mosquitoes, primarily Aedes aegypti and to a lesser extent Aedes albopictus (1, 2). All serotypes are known to produce the whole spectrum of dengue fever (DF) illnesses and share similar geographic patterns and host/vector interactions (3, 4).
Dengue fever (DF) is a fast growing acute febrile disease with potentially lethal consequences that is a global public health problem, mostly in tropical and subtropical countries (5, 6). This infection can result in a variety of clinical illnesses, from asymptomatic or moderate febrile disease to classic DF to the most serious types of illness, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (7,8). The main clinical signs of each category would be a persistent high fever lasting 2–7 days, bleeding indicated by petechiae, epistaxis, a positive tourniquet test, or thrombocytopenia, and shocks from plasma leakage indicated by hemoconcentration (hematocrit above 20%), pleural effusion, and ascites (9).
The dengue fever is endemic in many countries across the WHO regions of Africa, the Americas, the Eastern Mediterranean, Asia, Australia, and the Western Pacific (4,5), though the Americas, South-East Asia, and the Western Pacific are the most severely affected, Asia accounting for 70% of the global DF disease burden (10). Dengue virus infects 390 million individuals worldwide, with 96 million developing clinical symptoms that result in 500,000 hospitalizations and 25,000 fatalities each year (5, 10).
The epidemiology of DF in Africa is poorly understood, despite the fact that all DENV serotypes circulate in many of the continent's nations, and the vector mosquitoes are abundant in the neighboring Middle East and Sub-Saharan Africa [
9]. With an estimated burden of 25% (21-29%) by IgG, 10% (9-11%) by IgM, and 14% (12-16%) by viral RNA assays, DENV infection appears to be a considerable burden for public health in the region of Sub-Saharan Africa (11). Additionally, numerous countries in the region have suffered DF outbreaks that have been reported to the Africa CDC (12), including Burkina Faso in 2016 and 2017, Côte d'Ivoire in 2017, Cape Verde in 2009, and Egypt in 2015.
Ethiopia has a low level of research on DF despite the fact that the virus is still being transmitted and viral infection rates are rising (13). In the first DF outbreak reported in 2013, which was in the Dire Dawa city administration in the east of the country, 12,000 suspected cases of DF were registered. Of these, 88 cases were confirmed by ELISA and RT-PCR, and 50 of these cases were found to be positive for DENV infection (14). In Dire Dawa city, Godey Town, and the Adar area of the Afar region the next year, numerous further outbreaks were noted year-round fashion (15, 16). In a few serological studies, DENV infections from various regions of the country were reported, including 7.5% current and 13.0% past DENV infections from northwest Ethiopia (7), 22.9% anti-IgG and 7.9% anti-IgM DENV infections from the Borena zone in southern Ethiopia (17), and 25.1% anti-IgG and 8.1% anti-IgM DENV infections from the Arba Minch district (8). Overall the prevalence of DENV infection is vary in Ethiopia (18-23).
Due to its global distribution, DENV infections should be closely monitored and any outbreaks should be quickly identified in order to reduce the resulting mortality and morbidity. However, it is well known that low-income countries like Ethiopia lack the infrastructure and resources required for effective surveillance of this disease. This systematic review and meta-analysis was aimed to estimate the pooled prevalence of DENV infection markers; DENV-specific Immunoglobulin G (IgG), Immunoglobulin M (IgM), and DENV RNA in Ethiopia.
Methods
This systematic review and Meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (24). The review protocol was registered on Prospero (CRD42023445570).
Eligibility Criteria
This review was considered studies that have reported the prevalence of Dengue virus infection in Ethiopia. The study participants were both sex and any age range. We considered observational studies (cross-sectional and Case control) in both outbreak and out of outbreak periods. Studies that had report to a prevalence of at least one of the three biological markers of DENV-RNA, IgM, or IgG were included. We considered all studies written in English language and published before July, 2023. Review studies, studies with only abstracts, studies report infection rates in non-human animals, entomologic and vector related studies were excluded.
Dengue infection was defined as febrile illness presenting with fever and at least two of the following clinical manifestations: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, and leukopenia confirmed by laboratory criteria through the detection of DENV RNA using reverse transcription polymerase chain reaction (RT-PCR) or NS1 antigens using enzyme-linked immunosorbent assay (ELISA) and/or rapid tests (25).
Search Strategy
To find pertinent literature published on DENV infection in Ethiopia, a comprehensive search of PubMed, Hinari, and Google Scholar was carried out. The databases were queried search by using keywords “Dengue”, “Dengue virus”, “Dengue infection”, “Dengue fever” and “DENV” in combination with Ethiopia context. A manual search that consists of scanning reference lists of eligible studies and relevant reviews was performed. The search strategy used in PubMed and Hinari is demonstrated in Supplementary Text S1.
Study Selection
The titles and abstracts of all retrieved citations were imported into EndNote reference manager. Duplicates were removed using this software. Two investigators independently screened titles and abstracts for eligibility based on predetermined selection criteria. The full-texts of articles thought to be potentially eligible were retrieved for further assessment. Further, the investigators independently assessed the full-text of each study for eligibility, and consensually retained studies to be included. Any discrepancies that arise between the reviewers at each level of the selection process were solved through conversation.
Quality Assessment
Two reviewers assessed the quality and risk of bias of all articles included in this review using a modified version of the Critical Appraisal Tool for prevalence studies designed by the Joanna Briggs Institute (JBI) (26). The risk of selection bias, confounding bias, and bias relating to measurement and data analysis were all assessed using JBI tool. Each question was answered either with “yes”, “no”, “unclear” or “not/applicable”. The number of questions for each study that had a "yes" response was used to determine a score. According to this score, studies were categorized into three groups based on their risk of bias; high risk (a score of 0-3), intermediate risk (a score of 4-6), and low risk (a score of 7-9).
Statistical Analysis
To determine the pooled prevalence of DENV markers, the retrieved data were analyzed using Stata software (version 17, StataCorp LLC, Texas, USA). Multiple markers were provided by some researches, and the estimates were input independently in the meta-analysis. In order to quantify the effect, we used the prevalence estimates from each study. The stated prevalence estimates and the sample size of each study were used to compute the standard error (SE). Fixed-effects model (for DENV-IgG) and random-effects model (for DENV-IgM and RNA) were used to generate summary pooled prevalence data with 95% confidence intervals (CI) and the result present in forest plots. Heterogeneity was assessed using the Inconsistency Index (I2). In order to check for potential publication bias, funnel plots were created and visually examined. Egger tests was used to confirm heterogeneity.
Results
Study Selection and Characteristics
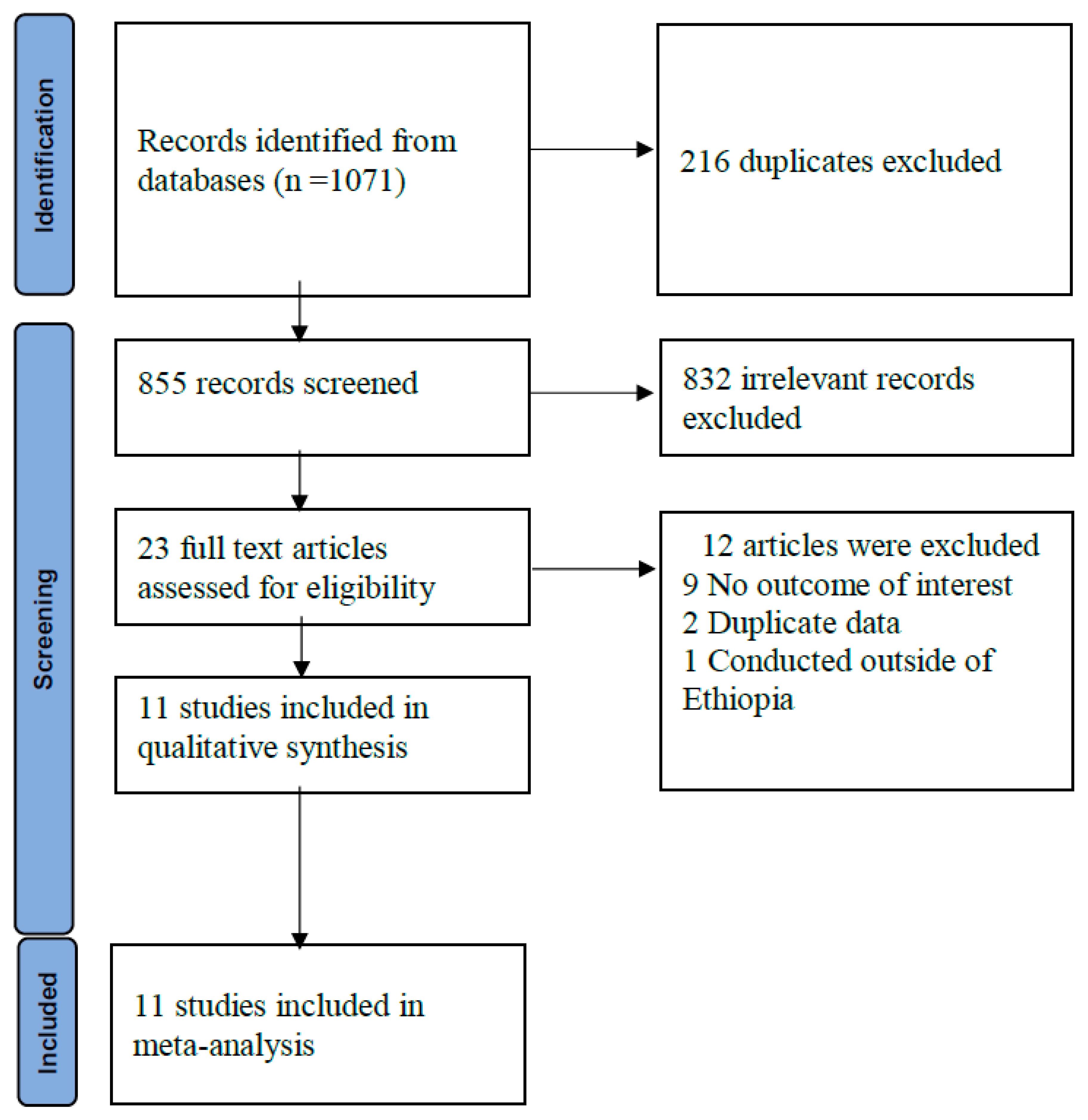
A total of 1,071 records were retrieved from database searches
. After removal of duplicates and screening, 11 studies were finally included in the review (
Figure 1). The methodological quality of studies ranged from intermediate to low risk of bias. Four (36.4%) studies had low, 7(63.6%) had moderate and no study had a high risk of bias. Majority of the included studies were cross-sectional and two were case-control studies. Six studies were conducted in dengue fever suspected participants whereas five were among acute febrile participants (Supplementary Table S1).
Prevalence of Dengue Virus infection
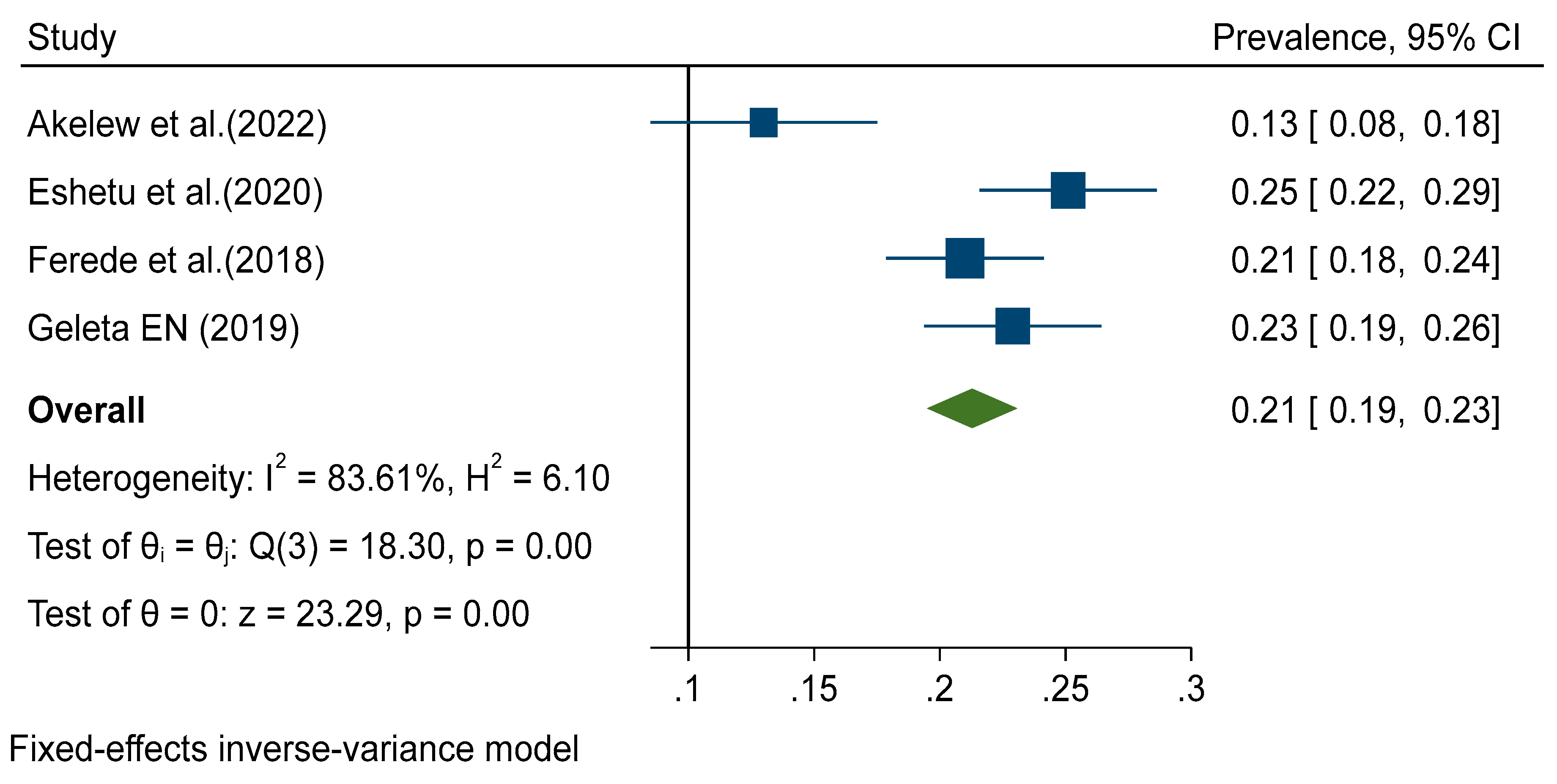
Data from all included studies were analyzed in the quantitative meta-analysis to estimate pooled prevalence. Meta-analysis was performed separately for each of the DENV infection markers (IgG, IgM and RNA). Fixed effects analysis estimated a pooled prevalence of IgG 21% (95% CI: 19-23) (
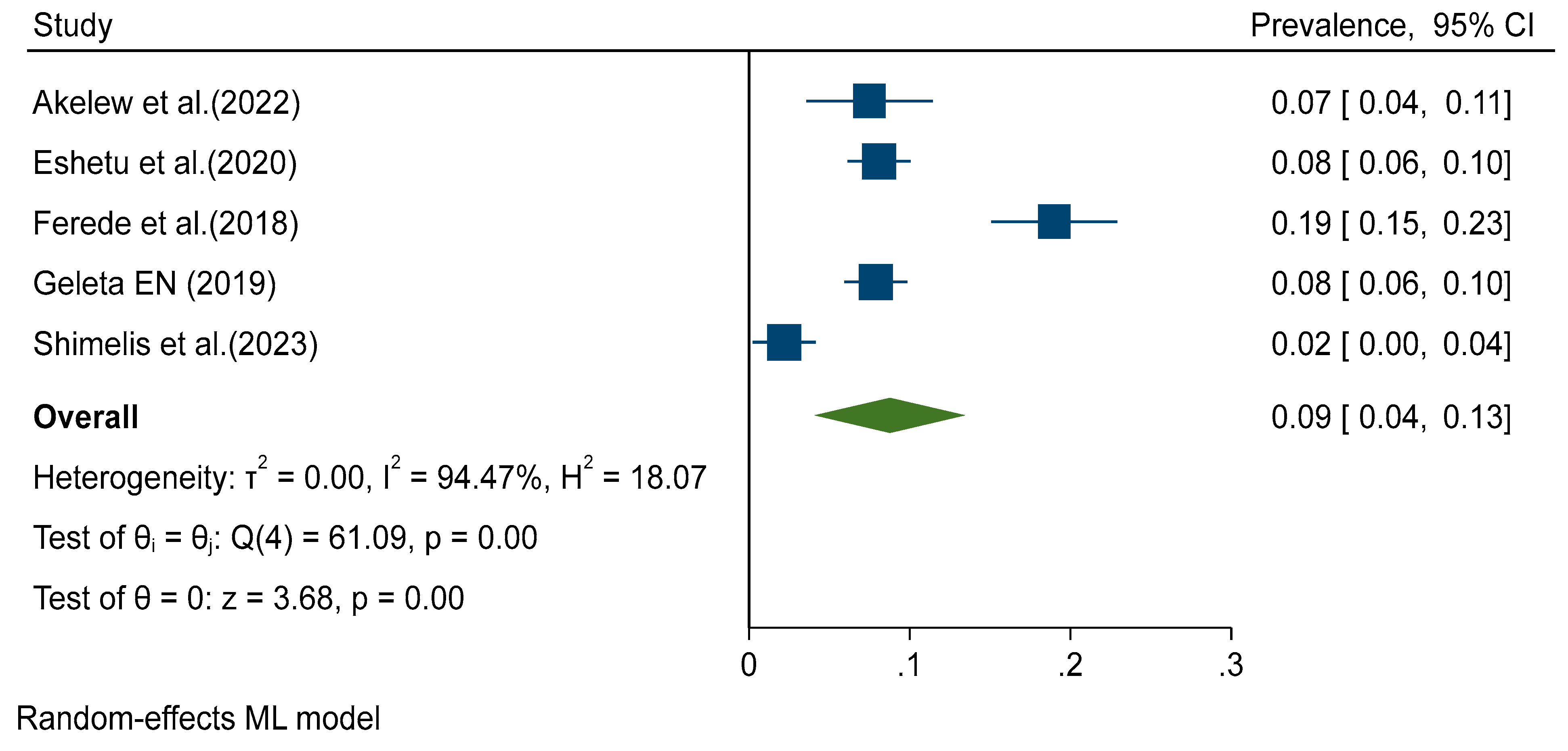
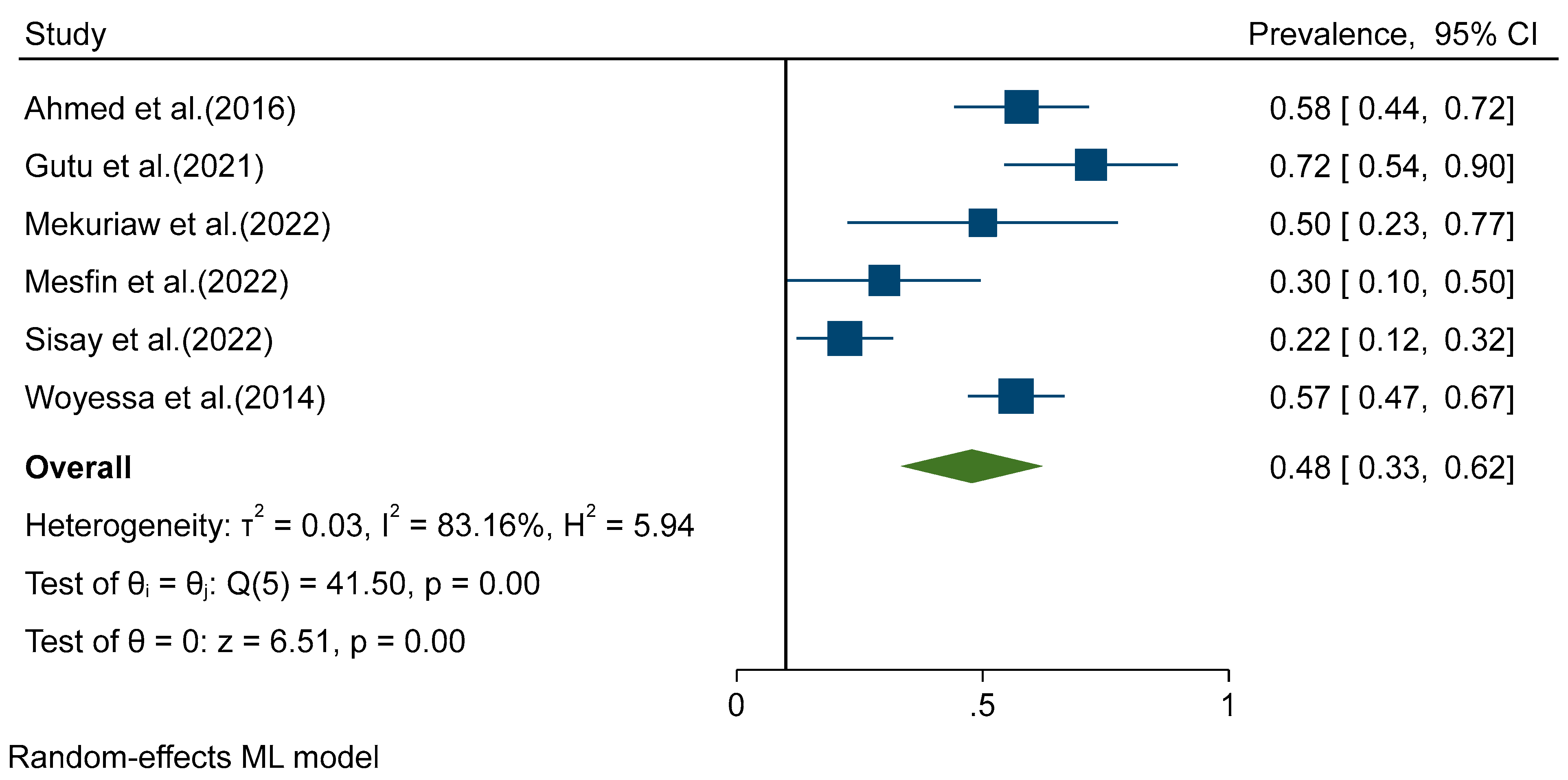
Figure 2). Random effects analysis estimated a pooled prevalence of IgM 9% (95%CI: 4-13) and a pooled DENV-RNA prevalence of 48% (95% CI: 33-62) (
Figure 3 and
Figure 4). Significant levels of heterogeneity between studies were found in all three meta-analysis, 83.61%, 94.74%, and 83.16% for IgG, IgM and RNA respectively (
Figure 2,
Figure 3 and
Figure 4). Visual inspection of the generated funnel plots revealed evidence of potential publication bias in all three meta-analyses (Supplementary Figure S5 and S7). But, egger regression test showed that there is publication bias in DENV IgG studies (
p = 0.002), but no publication bias was detected among DENV-RNA (
p = 0.7) and IgM (
p = 0.052) markers.
Discussions
The epidemiological patterns of dengue virus infections are recognized to generally mimic those of other re-emerging infections, including other arboviral diseases. This trend causes apparent fluctuations in the clinical and serological prevalence estimates of these diseases because of the spikes in prevalence that are frequently observed during outbreaks and the sharp decline that immediately follows. As a result, it is better to explain the epidemiology of emerging and re-emerging infectious diseases over an extended period of time, as a point estimate rarely captures the real burden of these conditions in a given area. This systematic review and meta-analysis of the prevalence of DENV infection in Ethiopia showed high prevalence estimates with a meaningfully high level of heterogeneity that differs according to the infection marker of interest.
Meta-analysis results have showed that the prevalence of dengue virus in Ethiopia is 9%, 21%, and 48% for DENV- IgM, IgG, and DENV-RNA respectively. According to the finding of this review 9% of Ethiopian population has developed acute DENV infection and 21% have had a history of exposure to DENV at some time in their life. The seroprevalence of DENV-RNA was significantly higher than DENV-IgM and IgG, this might be most studies those determine DENV-RNA were at outbreak periods. The estimated IgM prevalence is lower compared to IgG and RNA, RNA is detectable in patients only from infection up to six days after the onset of the fever. IgM are detectable five days after the onset of fever up to three months while IgG appear on the tenth day after the onset of fever and can continue for many years (27).
The prevalence of DENV-IgG was in agreement with results reported from Sub-Saharan Africa (11), and Africa (28). In Contrast, the finding of this review is lower than result reported from Sudan (29). An estimated IgM prevalence is similar with reviews reported from Africa (28) and Sub-Saharan Africa (11) and lower than report from Sudan (29). In addition DENV-RNA finding of this review is higher than reviews reported from elsewhere (11, 28). This discrepancy might be due to the selection and time frame of included studies. For instance, studies conducted during ongoing epidemics or following outbreaks are likely to contribute to a higher number of dengue positive cases (30). Overall the significant difference in prevalence estimate might be due to sample size, geographical variation, time frame of sample collection, type of assay methods, and way of life hood of community (31).
We found that the pooled prevalence estimate of all DENV markers are high in Ethiopia. The DENV-specific RNA can record a four to five fold increase during outbreak. This evidence indicate that there is circulation of the virus in this country, as a result the residents are at high risk of infections such as Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS).
The high prevalence outcome of this review may be an indication of the ineffectiveness of current public health efforts to control DENV vector transmission. Weak health systems in low-income settings can be attributed to the failure to sustain effective and ongoing interventions to manage vector-borne disease (32-34). The growing speed of urbanization with poor infrastructure increase mosquitoes breeding sites. The ecological environment and climatic patterns of tropical and subtropical regions where countries like Ethiopia located favor vector long-life and eggs conservation and development (35-39). Additionally, appearance of insecticides resistance of DENV vectors and human mobility in the country as well as in the region increase challenge of controlling spread of those vectors (38). The majority of studies included in this review were reported from eastern part of the country as well as South and Northwest of Ethiopia. This may suggest that there is dengue virus circulating in Ethiopian neighboring countries including Sudan, Kenya and Djibouti, which is raising possibility of cross-border transmission, particularly with the open borders and free mobility between these countries.
The finding of this review contain important information for researchers, health policy makers as well as clinical practices. Such studies will generate base line information for development of effective public health policies for dengue fever prevention and control. And also, a key information that a surveillance program is required to investigate actual prevalence and burden of DENV, vector distribution, the virus serotypes and genotypes. Currently, there is no effective antiviral and vaccination for dengue fever (39). Proper implementation of routine diagnosis and management of early diagnosed illnesses reduces morbidity and fatality rates. It would be interesting to explore differential diagnosis with other infectious diseases with close clinical presentations, such as malaria. There is also issue of cross-reactivity of serological diagnostic techniques among arboviruses including DENV (40, 41). As a result, confirmatory tests such as real-time (quantitative) polymerase chain reaction (qPCR) and plaque reduction neutralization test (PRNT) are required to validate the findings of these serologic assays. Cost-effective control measures are required. Because treatment and immunization are ineffective, vector management is the primary strategy for reducing the burden of DENV infection. The WHO integrated vector control strategy is useful in combating vector-borne diseases. This mechanism is environmentally safe when using insecticides for the elimination of mosquito’s larva from their artificial and natural habitats (42).
This is the first systematic review and meta-analysis of DENV seroprevalence estimates in Ethiopia. We attempted to summarize the pooled prevalence of studies conducted in Ethiopia those were reported prevalence of DENV. However, this review has certain limitations. There is substantial heterogeneity between studies, it may be due to diagnostic techniques, time frame of sample collection or population. We were not conduct sub-group analysis because the studies were investigated different types of DENV markers of interest using different diagnostic techniques, time frames and populations. This review does not cover all part of the country. This may have an impact on the generalizability of our findings.
Conclusion
The prevalence of DENV infection is high and the virus is spreading in Ethiopia. This suggests that healthcare providers, researchers and policymakers in the country should give more attention to dengue fever. Routine and differential laboratory dengue virus diagnosis is required to detect cases early, to manage cases appropriately and plan for outbreak. Since, there is no effective treatment or vaccine, prevention and vector control should be the primary DENV control mechanism. Mosquito surveillance is essential for identifying mosquito breeding areas, and health education and environmental control can help to limit the spread of dengue virus. To track DENV epidemiology in Ethiopia, a national arbovirus surveillance program should be implemented.
Abbreviations
| DENV |
Dengue virus |
| DF |
Dengue fever |
| DHF |
Dengue Hemorrhagic Fever |
| DSS |
Dengue Shock Syndrome |
| ELISA |
Enzyme-linked immunosorbent assay |
| IFA |
Immunofluorescence assay |
| IgG |
Immunoglobulin G |
| IgM |
Immunoglobulin M |
| RNA |
Ribonucleic acid |
| RT-PCR |
Real-time Polymerase Chain Reaction |
| WHO |
World Health Organization |
References
- WHO. Dengue and severe dengue. World Health Organization. 2014. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nature reviews Disease primers. 2016 Aug 18;2(1):1-25. [CrossRef]
- Mustafa MS, Rasotgi V, Jain S, Gupta VJ. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Medical journal armed forces India. 2015 Jan 1;7 1(1):67-70. [CrossRef]
- Zerfu B, Kassa T, Legesse M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Tropical Medicine and Health. 2023 Feb 24;51(1):11. [CrossRef]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF. The global distribution and burden of dengue. Nature. 2013 Apr 25;496(7446):504-7. [CrossRef]
- Harapan H, Michie A, Sasmono RT, Imrie A. Dengue: A Minireview. Viruses. 2020: 12 (8), 829. [CrossRef]
- Akelew Y, Pareyn M, Lemma M, Negash M, Bewket G, Derbew A, et al. Aetiologies of acute undifferentiated febrile illness at the emergency ward of the University of Gondar Hospital, Ethiopia. Tropical Medicine & International Health. 2022 Mar;27(3):271-9. [CrossRef]
- Eshetu D, Shimelis T, Nigussie E, Shumie G, Chali W, Yeshitela B, et al. Seropositivity to dengue and associated risk factors among non-malarias acute febrile patients in Arba Minch districts, southern Ethiopia. BMC Infectious Diseases. 2020 Dec;20(1):1-6. [CrossRef]
- Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136(3):373–90.
- WHO. Dengue and severe dengue. Fact Sheet [Internet]. World Health Organization. 2022. Available from: https://www.who. int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Eltom K, Enan K, El Hussein ARM, Elkhidir IM. Dengue virus infection in Sub-Saharan Africa between 2010 and 2020: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2021;11: 678945. [CrossRef]
- CDC Africa. Dengue Fever [Internet]. Africa Centres for Disease Control and Prevention. 2022. Available from: https://afric acdc.org/disease/dengue-fever/.
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specifc mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–88. [CrossRef]
- Woyessa AB, Mengesha M, Kassa W, Kife E, Wondabeku M, Girmay A, et al. The frst acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: a descriptive analysis. Ethiop J Health Dev. 2014;28(3).
- Degife LH, Worku Y, Belay D, Bekele A, Hailemariam Z. Factors associated with dengue fever outbreak in Dire Dawa administration city, October 2015, Ethiopia—case control study. BMC Public Health [Internet]. 2019;19(1):650. [CrossRef]
- Gutu MA, Bekele A, Seid Y, Mohammed Y, Gemechu F, Woyessa AB, et al. Another dengue fever outbreak in Eastern Ethiopia-An emerging public health threat. PLoS Negl Trop Dis. 2021;15(1): e0008992. [CrossRef]
- Geleta EN. Serological evidence of dengue fever and its associated factors in health facilities in the Borena Zone, South Ethiopia. Res Rep Trop Med. 2019;10:129–36. [CrossRef]
- Mesfin Z, Ali A, Abagero A, Asefa Z. Dengue Fever Outbreak Investigation in Werder Town, Dollo Zone, Somali Region, Ethiopia. Infection and Drug Resistance. 2022 Jan 1:7207-17. [CrossRef]
- Shimelis T, Mulu A, Mengesha M, Alemu A, Mihret A, Tadesse BT, et al. Detection of dengue virus infection in children presenting with fever in Hawassa, southern Ethiopia. Scientific Reports. 2023 May 17;13(1):7997. [CrossRef]
- Sisay C, Waldetensai A, Seyoum M, Tayachew A, Wossen M, Keneni D, et al. Detection of serotype 1-Dengue fever outbreak in Dire Dawa city, Eastern Ethiopia. Ethiopian Journal of public health and nutrition. 2022 Jan 27;5(1):49-54.
- Mekuriaw W, Kinde S, Kindu B, Mulualem Y, Hailu G, Gebresilassie A, et al. Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia. Insects. 2022 Nov 18;13(11):1066. [CrossRef]
- Ahmed YM, Salah AA. Epidemiology of dengue fever in Ethiopian Somali region: retrospective health facility based study. Cent Afr J Public Health. 2016;2(2):51-6. [CrossRef]
- Ferede G, Tiruneh M, Abate E, Wondimeneh Y, Damtie D, Gadisa E, et al. A serologic study of dengue in northwest Ethiopia: Suggesting preventive and control measures. PLoS neglected tropical diseases. 2018 May 31;12(5):e0006430. [CrossRef]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009 Aug 18;151(4):W-65.
- WHO-TDR. Dengue: guidelines for diagnosis, treatment, prevention, and control- New Edition. Geneva, Switzerland. World Health Organisation. 2009.
- Munn Z, Tufanaru C, Aromataris E. JBI's systematic reviews: data extraction and synthesis. AJN The American Journal of Nursing. 2014 Jul 1;114(7):49-54. [CrossRef]
- Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. New England Journal of Medicine. 2012 Apr 12;366(15):1423-32.
- Simo FB, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Scientific reports. 2019 Sep 20;9(1):13626. [CrossRef]
- Elduma AH, LaBeaud AD, A. Plante J, Plante KS, Ahmed A. High seroprevalence of dengue virus infection in Sudan: Systematic review and meta-analysis. Tropical medicine and infectious disease. 2020 Jul 18;5(3):120. [CrossRef]
- Hunsperger EA, Sharp TM, Lalita P, Tikomaidraubuta K, Cardoso YR, Naivalu T, et al. Use of a rapid test for diagnosis of dengue during suspected dengue outbreaks in resource-limited regions. Journal of clinical microbiology. 2016 Aug;54(8):2090-5. [CrossRef]
- Mwanyika GO, Mboera LE, Rugarabamu S, Ngingo B, Sindato C, Lutwama JJ, Paweska JT, Misinzo G. Dengue virus infection and associated risk factors in Africa: a systematic review and meta-analysis. Viruses. 2021 Mar 24;13(4):536. [CrossRef]
- Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Frontiers in cellular and infection microbiology. 2017 Jul 12;7:317. [CrossRef]
- Villar, L. A., Rojas, D. P., Besada-Lombana, S. & Sarti, E. Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS Negl Trop Dis 9, e0003499. [CrossRef]
- Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa: a systematic review. PLoS Neglected Tropical Diseases. 2016 Dec 7;10(12):e0005194.
- Hanson SM, Craig Jr GB. Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. Journal of Medical Entomology. 1995 Sep 1;32(5):599-604. [CrossRef]
- Hawley WA, Pumpuni CB, Brady RH, Craig Jr GB. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. Journal of Medical Entomology. 1989 Mar 1;26(2):122-9. [CrossRef]
- Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS neglected tropical diseases. 2019 Mar 28;13(3):e0007213. [CrossRef]
- Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annual review of entomology. 2015 Jan 7;60:537-59. [CrossRef]
- WHO. Dengue and severe dengue, 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Papa A, Karabaxoglou D, Kansouzidou A. Acute West Nile virus neuroinvasive infections: Cross-reactivity with dengue virus and tick-borne encephalitis virus. Journal of medical Virology. 2011 Oct; 83(10):1861-5. [CrossRef]
- Zaidi MB, Cedillo-Barron L, Almeida ME, Garcia-Cordero J, Campos FD, Namorado-Tonix K, et al. Serological tests reveal significant cross-reactive human antibody responses to Zika and Dengue viruses in the Mexican population. Acta tropica. 2020 Jan 1;201:105201. [CrossRef]
- WHO. Dengue control: Control strategies. 2019.
- Available at: https://www.who.int/denguecontrol/control_strategies/en/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).