Introduction
Endodontically managed teeth presenting a considerable coronal structure loss after endodontic treatment imposes the necessity of fiberglass posts (FP) associated with resin cements to restore the biomechanical form and function [
1]. The recommendation of these materials is based on the characteristics regarding durability, aesthetics, and operational cost, which makes them a very favorable option for the restoration of the teeth in question [
2]. However, it must be considered that during the preparation for the intraradical retainer, the formation smear residue resulting from the infected dentin debris, remains of gutta-percha and obturator cement can negatively influence the polymerization processes of resin cement and consequently the final cementation quality of the FP which is essential for successful adhesion [
3].
The union of demineralized intrarradicular dentin with the cement and the FP is based on micromechanical retention and therefore, dentine cleaning walls of the root canal is crucial for ideal retention of the post [
4]. In this sense, the efficiency of endodontic irrigation in the sanitization and cleaning stages of the root canal becomes fundamental in reaching areas with difficult access that were untouched during the instrumentation stage, such as isthmuses and lateral canals [
5]. Technological advances have allowed irrigating solutions to be agitated within the root canal for more effective smear layer clearance through mechanical, sonic, or ultrasonic agitation methods [
6]. Among the solutions tested to remove this smear layer, EDTA and its combinations (mainly sodium hypochlorite), are the most used due to their chelating properties [
7]. However, the prolonged use of EDTA can cause erosion in the dentin matrix, thus compromising the bond strength between post and dentin [
8,
9]. Thus, there is a search for alternative solutions that are
more biocompatible than EDTA, in an effort to reduce these possible damages [
7,
10]. As an alternative, chitosan has been increasingly studied because it is a natural solution, biocompatible with tissues, and has adequate properties of biodegradability, bioadhesion, and low cytotoxicity [
11,
12].
There is still a notorious absence of consensus about the real influence of post preparation cleaning procedures on the adhesion of FP to radicular dentin [
4,
13]. So far, there have been a lack of studies evaluating the influence of chitosan in association with various agitation methods, on the adhesion process and biomechanical bond strength of FP. Therefore, it seems opportune to investigate the influence of the final cleaning method on the adhesive force between the post and luting agent and intrarradicular dentin. The null hypotheses assessed were that there would no difference in the level of bond strength depending on (i) the chelating solution, (ii) the chelator activation method, and (iii) the third of the radicular canal.
2. Materials and Methods
The research protocol was approved by the Animal Use Ethics Committee (CEUA) of the Universidade Evangélica de Goiás, Anápolis, Brazil (#001/2021). Three hundred extracted bovine lower incisors with fully developed roots, morphologically similar in size and shape [
14,
15,
16] were obtained and stored in 0.2% thymol solution (Fitofarma, Brazil). Periapical radiographs were obtained to verify the samples normality, and only teeth with a unique root canal without obliterations were included in the study. In total, 90 teeth were used.
2.1. Root canal instrumentation and Obturation
The crowns of the teeth were sectioned using a dual-sided diamond disc (KG Sorensen, Brazil), perpendicular to its long axis getting standardized roots 18 mm in length from the apical end. A #15 K-file (Dentsply Maillefer, Switzerland) was used to verify the patency of all root canals.
The working length (WL) was established using a #15 K file (Dentsply Maillefer), which was introduced into the root canal until it was visible in the apical foramen. The WL was set 1 mm short of this measurement. To simulate clinical conditions, the root apexes were sealed with flow composite (Top Dam, FGM, Dental Products, Brazil).
ProTaper® Gold instruments (Dentsply Maillefer) were used for root canal preparation. The channels were instrumented until the instrument F5 (50/.05). Each instrument was used in the instrumentation of just five root canals through X-Smart Plus endodontic motor (Dentsply Maillefer), with speed and torque standards established by the manufacturer. During instrumentation, the canals were irrigated with 4 mL of 2.5% sodium hypochlorite (Fitofarma, Brazil). The root canals were irrigated with 17% EDTA (Biodinamica, Brazil) for 3 min to remove the smear layer.
The roots were subsequently dried using absorbent paper points (Dentsply Maillefer) and then filled with gutta-percha cones and epoxy resin-based cement (AH Plus; Dentsply Maillefer), mixed according to the instructions of the manufacturer, using Tagger's hybrid technique. The canal access was sealed with micro-hybrid composite resin (TPH Spectrum, Dentsply Brazil). All roots were stored at 37⁰C and 100% humidity for 7 days to allow the cement to light cure.
2.2. Post-space preparation
After the root end-filling, heated condensers (Paiva; SS White) were used to remove the initial portion of the root canal filling mass. The conduits were prepared to a depth of 14 mm using Largo drills #3-5 (Dentsply Maillefer), corresponding fiber posts of 1.5 mm in diameter (Reforpost #3; Angelus, Brazil) [
1,
14]. Root canals were irrigated with 4 mL of 2.5% NaOCl after each drill change and dried with absorbent paper cones.
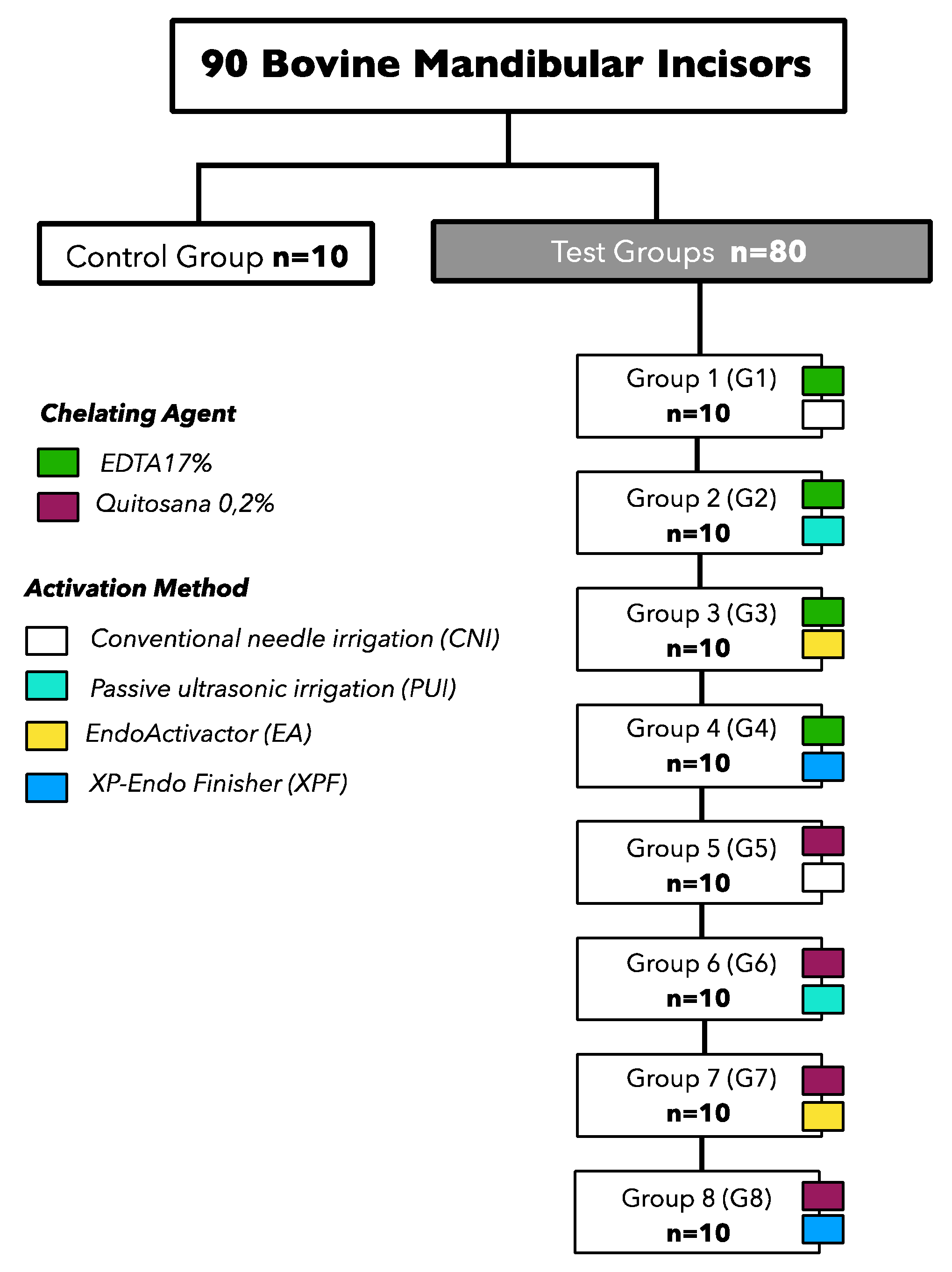
2.3. Experimental groups
The specimens were randomly distributed into eight experimental groups (n=10) and a control group, according to the chelating solution tested and the method of activation of irrigant (
Figure 1).
2.4. Formulation of chelating solutions
The solutions were formulated in a compounding pharmacy (Fitofarma) and were prepared with analytical grade reagents and water purified by a Reverse Osmosis system with Ultraviolet Light (Quimis, Brazil) with electrical conductivity lower than 1μS mm -two. The pH of the solutions was determined with a digital pH meter (Analion, Brazil). The 0.2% chitosan solution was prepared with 0.2 g of chitosan (ACROS Organics Gell, Belgium; degree of deacetylation > 90%) in 100 mL of 1% acetic acid. The mixture was stirred using a magnetic stirrer for 2 h [
7,
11].
A total volume of 4 mL of 2.5% NaOCl, 4 mL of each chelating solution, and another 4 mL of NaOCl was introduced into the root canals using a 5 mL disposable syringe (Ultradent) and a 29-gauge needle (NaviTip; Ultradent). The needle was inserted 1 mm short of the cementoenamel junction (CT) without coming into contact with the canal walls. Each chelating solution was left in the canal for 3 minutes without any activation.
PUI was conducted in 3 cycles of 20 s each with 2 mL of the solution per cycle. The solutions were passively activated using EMS PM 200 ultrasound (EMS, Switzerland) and E1-Irrisonic tip (Helse, Brazil) positioned 1 mm short of the WL, without touching the walls of the root canals, so that it vibrated freely. The ultrasonic unit was set to 10% power.
Three activation cycles were performed as previously described. The solutions were activated with the EndoActivator system (Dentsply Maillefer) and a medium activator tip (25/.04), which was inserted 1 mm from the WL for 20 s (each cycle with 2 mL of the solution) at 10,000 cycles per minute.
Three activation cycles were performed as previously described. The solutions were activated with the XP-Endo Finisher (25/.00) instrument (FKG Dentaire, Switzerland), which was inserted 1 mm short of the WL. The instrument operated at a speed of 800 rpm and torque of 1 Ncm. Slow and smooth movements of penetration and withdrawal were performed for 20 seconds (each cycle with 2 mL of the solution). The cleaning methods were completed, the canals were washed with 4 mL of saline solution and dried with absorbent paper tips.
2.5. Fiber post cementation
After applying a thin layer of utilitarian wax on the external surfaces of the roots to prevent lateral polymerization resulting from the photoactivation of the cement, the post underwent a 15-second cleaning with 70% alcohol. Subsequently, the silane (Silane, Angelus) was applied for 1 minute using a micro brush (KG Sorensen). The self-adhesive resin cement (RelyX U200; 3M-ESPE, USA) was manipulated according to the manufacturer's instructions and inserted into each root canal with the assistance of a lentulo spiral instrument (Dentsply Maillefer) and applied to the surface of the fiber glass post. The post was inserted into the canal with appropriate digital pressure, removing excess cement with a clean micro brush (KG Sorensen) after 1 minute.
Three minutes later, the cement was light-cured using a 1200 mW/cm2 intensity source (Radii-Cal; SDI, Australia) for 40 seconds on the cervical region, along the long axis of the root, and obliquely on the buccal and lingual surfaces, totaling 120 seconds per root. The dentin-cement-post interface was sealed with composite resin to ensure a hermetic seal of the root canal, ensuring the integrity and stability of the procedure.
2.6. Root Sectioning Procedure
In the meticulous process of root sectioning, each root underwent careful transverse cutting using a double-sided diamond disc (4" diameter × 0.012" thickness × 1/2"; Arbor, Extec, Enfield, CT, USA) mounted on a specialized hard tissue microtome (Isomet 1000, Buehler, Lake Bluff, IL, USA) set at a low speed, ensuring precision and accuracy. Throughout the procedure, a continuous flow of water provided effective cooling. From the cervical, middle, and apical regions of the root, two 1 mm thick discs were expertly extracted, resulting in a total of 6 discs per root. These dentin discs were precisely cut at distinct measurements: 11 and 12 mm from the root apex for the cervical region, 8 and 9 mm for the middle region, and 5 and 6 mm for the apical region, ensuring comprehensive representation of each third of the root. This standardized method of sectioning, utilizing advanced equipment and precise measurements, guaranteed the consistency and reliability of the obtained samples, forming the foundation for subsequent analyses and evaluations.
2.7. Micro push-out mechanical test
To perform the micro push-out test, a device developed specifically for this test was used, consisting of stainless-steel metal bases 3 cm in diameter, containing a central hole of 2, 3.5, and 4.5 mm (
Figure 2A) and load applicator tips 1, 1.75 and 2 mm in diameter (
Figure 2B). After positioning the set on the base of the mechanical testing machine (Microtensile OM150, Odeme Dental Research, Brazil) (
Figure 2C) containing a 10 Kgf load cell, the discs were positioned in the hole of the metal base and the set was aligned to the tip load applicator (
Figure 2D). They were then subjected to compression loading in the apex/crown direction at a speed of 0.5 mm/minute until failure occurred. The displacement force values were obtained in KgF which was transformed into Newton. The bond strength, in MPa, was calculated by dividing the force (N) by the area of the adhesive interface. The area of the adhesive interface was calculated by multiplying the height of the disc by the perimeter of the channel lumen, which was considered an ellipse:
where
h is the height of the disk,
a is the largest radius, and
b is the smallest radius.
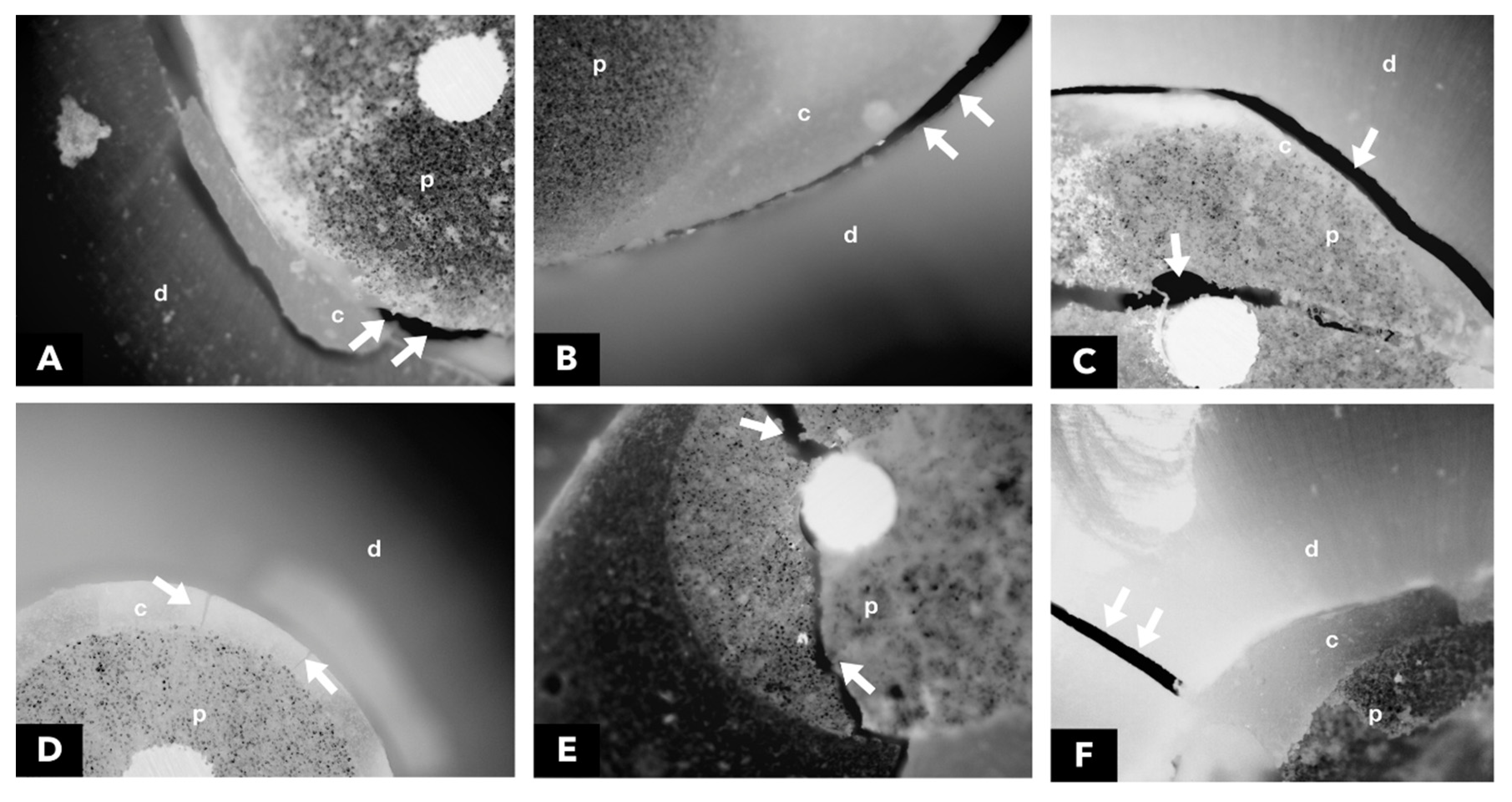
2.8. Analysis of the failure mode by optical microscopy
After the mechanical test, each specimen was stored individually in Eppendorf-type microtubes with distilled water, for later analysis of the fracture pattern by using 40x optical microscopy, without any type of treatment or previous preparation. All samples were analyzed with the aid of an optical microscope (Carl Zeiss Laser Scanning Systems - LSM510, META, Germany. The images were processed with the help of the Zeiss LSM Image Browser software, (META, Germany). Photomicrographs were always obtained with the same increase for all specimens. Failure modes were classified into six categories: (1) adhesive between the post and resin cement, (2) adhesive between resin cement and intrarradicular dentine, (3) mixed, between post, resin cement, and intrarradicular dentine, (4) cohesive in cement, (5) cohesive in the post and (6) cohesive in dentine (
Figure 3).
2.9. Statistical Analysis
The statistical analysis was conducted using Jamovi 1.1.9 software (The Jamovi Project, 2019). Various cleaning methods were compared by examining the frequency of failure modes expressed as a percentage within each tested group. The bond strength values (in MPa) obtained from the micro push-out mechanical test underwent rigorous assessment through the Kruskal-Wallis test, followed by the Dwass-Steel-Critchlow-Fligner test for detailed multiple comparisons. A significance level of α = 5%. Intra-examiner agreement was meticulously evaluated using the kappa coefficient, applied to 10% of the sample, ensuring the reliability of the results.
4. Discussion
In the current investigation, exploring the impact of 0.2% chitosan in various final cleaning methods on the adhesion of glass fiber post (FP) to intrarradicular dentin produced convincing results. The study revealed significant disparities in bond strength between groups G8 and G1, as well as G4 and G6, highlighting the substantial influence of the cleaning protocol on adhesion. These findings led to the partial rejection of previously raised null hypotheses, shedding new light on the intricate dynamics of the FP dentin-root bond.
The micro push-out mechanical test, a well-established and widely recognized method for assessing the bond strength between dentin and FP. This methodology has been extensively highlighted in prior research [
8,
13,
17]. Its widespread use is attributed to its ability to ensure a more uniform distribution of stresses, minimizing data distortion, and reducing the likelihood of premature failures [
18,
19]. One of its significant advantages lies in enabling the assessment of multiple specimens from the same root, a factor crucial for robust comparative analyses. Moreover, this technique facilitates the exploration of regional variations within the root thirds, providing results that closely mirror real-world clinical scenarios [
1,
20,
21].
The choice of using these teeth was justified by easier acquisition when compared to human teeth, and also because they allow a better standardization of the age of the teeth and the space of the root canal [
14,
15,
22]. The very similar characteristics between bovine and human teeth, especially in mechanical tests that evaluate the bond strength to dentin and enamel, provide a solid scientific basis, affirming the relevance and suitability of the chosen experimental model.[
22,
23,
24].
A wide range of materials has been made available on the market for cementing FP [
8]. The resin cement used in the study have the capacity to adhere to the tooth structure, by two mechanisms, the acidic monomers hybridize the dentin, and the resin chemically interacts with the hydroxyapatite [
3,
17,
20,
22]. Prior research has demonstrated that the presence of the smear layer, which forms during the intrarradicular preparation, can prevent the demineralization process promoted by the cement. This interference adversely affects the adhesive capacity of the cement, compromising its ability to form a strong and enduring bond [
25].
EDTA 17% is an important chelator in removing smear layer [
7,
10].However, prolonged use can result in erosive effects on the dentin, leading to a reduction in its microhardness. This can potentially harm the periapical tissues surrounding the tooth [
26]. Studies have focused on more biocompatible alternatives to minimize their interference with adhesive and restorative procedures [
13,
17,
20].
Chitosan, derived from the deacetylation process of chitin found in crab and shrimp shells, is a chemical substance of significant interest in dental research. Its cost-effectiveness, biocompatibility, and minimal cytotoxicity have made it a focal point in the exploration of natural polysaccharides for dental applications [
7,
12,
27]. In comparisons between cleaning methods, differences in bond strength values were found only in the cervical third, as reported in previous investigations [
1,
14,
15].
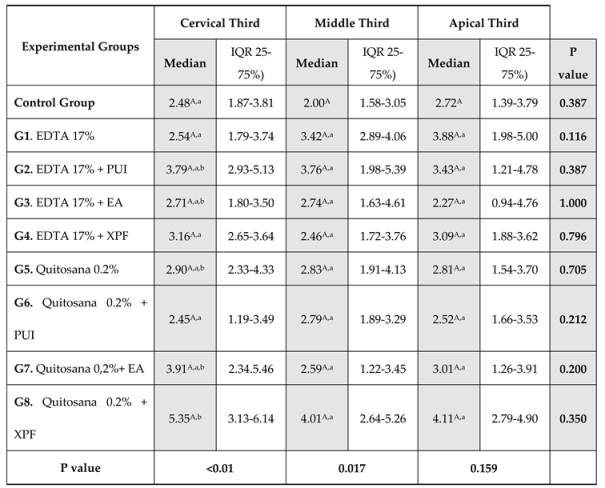
The use of 0.2% chitosan + XPF as a final cleaning protocol positively influenced the the bond strength of fiber post to intrarradicular dentin, with the highest bond strength values in the cervical third, but with no differences for the other thirds (
Table 1). These findings align with a previous study [
17] that compared the impact of 0.2% chitosan and 17% EDTA utilizing various techniques, including conventional and passive ultrasonic irrigation (PUI). The study revealed higher values for 0.2% chitosan + PUI in the cervical third of the root canal.
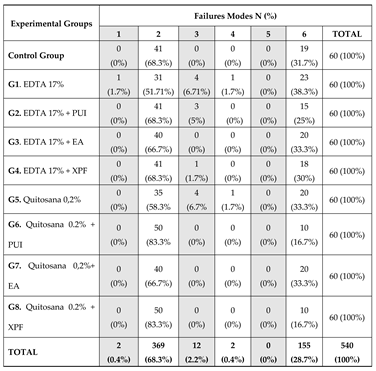
Failure modes were analyzed using the serial root sectioning method, from which two slices were obtained per root third, allowing direct inspection of the cement-dentin interface. As for the mode of adhesive failure, it was found that this most frequently occurred, followed by cohesive failure in the dentin (
Table 2). This corroborates previous studies, which demonstrated greater fragility at the cement-dentin interface [
8,
14,
15,
17,
20] which can be justified by the presence of remaining obturators adhered to the walls of the root canal and within the dentinal tubules [
4].
Certain limitations of the present study deserve attention in future research efforts. Notably, the samples in this study were not exposed to thermal and mechanical factors, which could better reproduce oral conditions, offering more authentic results [
28]. It is imperative to carry out clinical studies to validate the results of this research and evaluate the effectiveness of new substances and technologies in cleaning the root substrate in post-canal preparation, especially with regard to restorations involving fiberglass posts.