1. Introduction
Hypoxia is a medical condition characterized by an inadequate/insufficient oxygen supply to tissues and organs, leading to potential harm or dysfunction. It results from various pathological phenomena including diminished cardiac output, reduced hemoglobin concentration, and atypical vasculature network. Currently, there is a widely acknowledged consensus that restricted oxygen availability jeopardizes not only cell viability but also cellular phenotype and functionality [
1]. Furthermore, inadequate oxygen supply (
hypoxia: different levels of oxygen deprivation, from mild to severe;
hypoxic stress: in cellular or molecular biology research, it specifically refers to a situation where cells or tissues are exposed to lower-than-optimal oxygen levels, causing them to adapt or respond to this oxygen deficiency – where they may activate specific molecular pathways, like the hypoxia-inducible factor or HiF pathway to adapt to the low oxygen environment) may disturb numerous biological processes, particularly those requiring high energy demands, such as tissue repair, restoration, and regeneration. Upon establishing hypoxia, it could lead to a grave metabolic crisis, especially in individuals with metabolic syndromes, cardiovascular diseases, acute traumas, chronic diseases, and cancer, to list a few [
2,
3,
4,
5].
Today, it is widely recognized that exposing human blood to free oxygen gas bubbles causes hemolysis [
6]. Nonetheless, various approaches have been employed to increase oxygen levels and overcome hypoxia; including the utilization of oxygen microparticles, hydrogen peroxide solutions, hyperbaric oxygenation, and inhalation of gas mixtures such as carbogen (composed of 5% CO
2 and 95% O
2) [
7]. However, are considered ineffective, mainly due to clinical and logistical limitations. These include limited gas solubility, cytotoxicity, instability, low gas pay-load, ill-defined gas-release profiles, and low cellular uptake, thereby, rendering them unsuitable for gas delivery purposes. Consequently, arises an imminent need and necessity for novel strategies that can offer and/or provide oxygen to tissues and organs in a manner that is both, safe/biocompatible as well as cost-effective, whilst being
highly biodegradable and easily deployable or user-friendly too [
7].
Herein,
nanobubbles, also known as ultra-fine bubbles or sub-micron bubbles, are extremely small gas-filled spherical bodies enclosed by an interface (gas–liquid or gas–solid) with a diameter of less than 1000 nm (
i.e. ranging in size from tens to hundreds of nanometers in diameter) [
8,
9]. In other words, nanobubbles are composed of gas, such as oxygen or nitrogen, encapsulated within a liquid, typically water, and stabilized by a thin layer of molecules at the gas-liquid interface. Nanobubbles have gained significant attention in various fields, including medicine, materials science, and environmental science, due to their unique properties and potential applications. Indeed, several remarkable properties of these nanobubbles have been widely studied such as their long-term stability and longevity [
10,
11,
12], enhanced dissolution of gases and increased gas interfacial diffusion [
12,
13]. In addition, nanobubbles can be biocompatible and have demonstrated interesting potential as suitable candidates for drug delivery purposes (drugs, genes, or other therapeutic agents, with improved release and delivery to specific tissues or cells.) [
7,
15]. Given that an optimal oxygen-release material must demonstrate compatibility with biological systems, strategies for oxygen delivery, such as nanobubbles, have garnered substantial attention as robust mechanisms to alleviate the hypoxic stress response(s) [
16,
17]; exciting area of research and innovation in various scientific, medical and industrial fields.
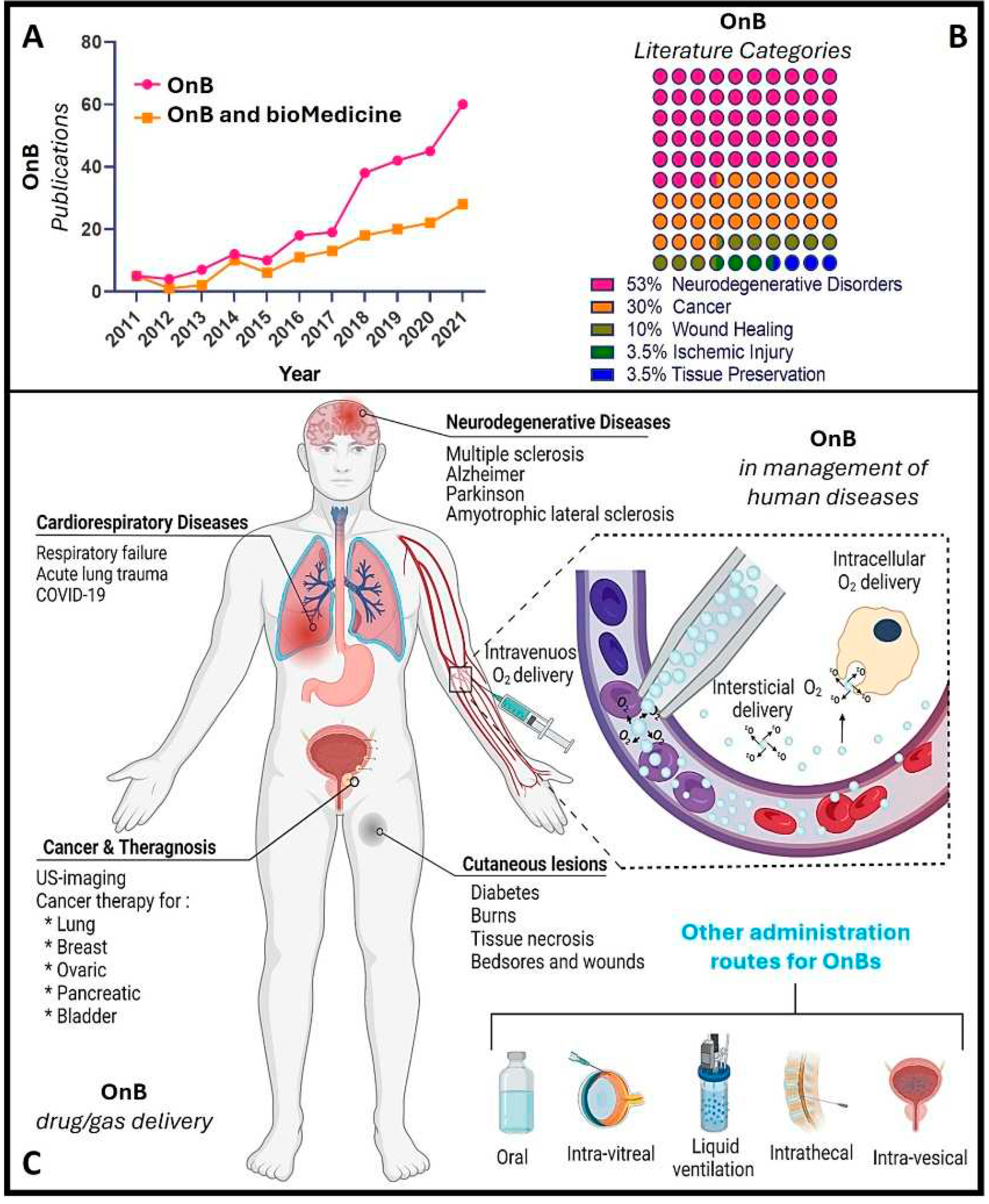
Notably, during the past decade, there has been a remarkable increase in scientific (and technological) interest and a surge in publications in this emerging field (
Figure 1), particularly concerning
oxygen nanobubbles (OnB) and
OnB-mediated oxygenation. OnB are being considered as a supplementary or adjuvant approach for drug (and gene) delivery in various conditions marked or induced by hypoxia and mitochondrial dysfunction. However, the existing literature, to the best of our knowledge, seems to lack a fair presentation and utter/comprehensive understanding of the underlying governing mechanisms.
Consequently, this review article opted to explore, in-depth, the therapeutic potential of oxygen itself, oxygenation and the myriad of diverse possibilities offered by oxygen-infused nanobubbles; deemed critical for designing, developing, characterizing, testing, fine-tuning/optimizing and eventually translating comprehensive, safe, and effective OnB-mediated oxygenation strategies, from bench to clinic. Herein, we emphasize that oxygen can serve a dual role: firstly, in its gaseous form, it can act as a therapeutic agent, promoting the restoration of oxygen dynamics, metabolism, and inflammatory balance; and secondly, an excess of oxygen availability, locally, can disrupt the redox balance; an intriguing aspect harnessed by OnB to counter the hypoxic microenvironment within carcinogenic tumors (i.e. tumor microenvironment or TME). Indeed, this intervention reduces HIF-1 alpha expression, thereby enhancing treatment effectiveness, and micro-/nano-sized bubbles have been employed as supplementary treatments to mitigate tumor cell resistance and metastasis. Henceforth, we have conducted a thorough examination of the impact of OnB systems and and OnB-mediated oxygenation strategies on the pathogenesis and prognosis of various conditions, with a particular focus on cancer and neuro-degenerative disorders, among other relevant ailments, diseases, conditions, and pathologies [5, 7,18–20]. We critically examine the potential applications of OnB in the field of biomedicine via analyzing pivotal research articles. Essentially, our analytical literature study aims to provide an interdisciplinary overview of the fundamental principles of nanobubbles. We then explore their utilization for oxygen delivery as well as highlight broader applications in the biomedical context. Additionally, we delve into investigating the cellular and molecular impact of OnB on mitochondrial functionality and the signaling pathways associated with hypoxic cells; possibly contributing to the comprehension of the underlying biological effects of OnB systems across various eukaryotic cell types, including neurons, glial cells, tumorigenic, as well as cardio-respiratory cells, to list a few. Finally, a special focus is dedicated to elucidating the cross-talk between the mitochondrial metabolism and hypoxic cell response(s), which serve, thus far, as the principal oxygen sensing mechanism(s) in eukaryotic (possessing a clearly defined nucleus) cells; a type of highly diverse, specialized and multi-functional cells in multi-cellular organisms.
2. Hypoxia and Mitochondrial Dysfunction: Interplay and Implications in Pathologies.
The interplay between hypoxia (oxygen deficiency) and mitochondrial dysfunction (perturbing the cellular energy-producing organelles) is a pivotal factor in the development and progression of various human diseases. These intertwined processes, once considered distinct, have emerged as key players in conditions spanning cancer, cardiovascular diseases, neuro-degenerative disorders, and more. Hypoxia disrupts the balance of oxygen supply to tissues, triggering a cascade of cellular responses. Simultaneously, mitochondrial dysfunction, characterized by impaired energy production and oxidative stress, contributes to cellular damage and dysfunction. The dynamic interaction between these two phenomena amplifies disease mechanisms and influences treatment outcomes. Understanding the molecular intricacies of hypoxia-mitochondrial dysfunction crosstalk offers insights into disease pathogenesis and potential therapeutic targets. Exploring this complex interplay offers a promising avenue for advancing our understanding of disease mechanisms and developing novel interventions. Indeed, researchers are designing, developing, and evaluating innovative diagnostic and treatment strategies that target this nexus, providing hope for improved outcomes in a broad spectrum of human pathologies.
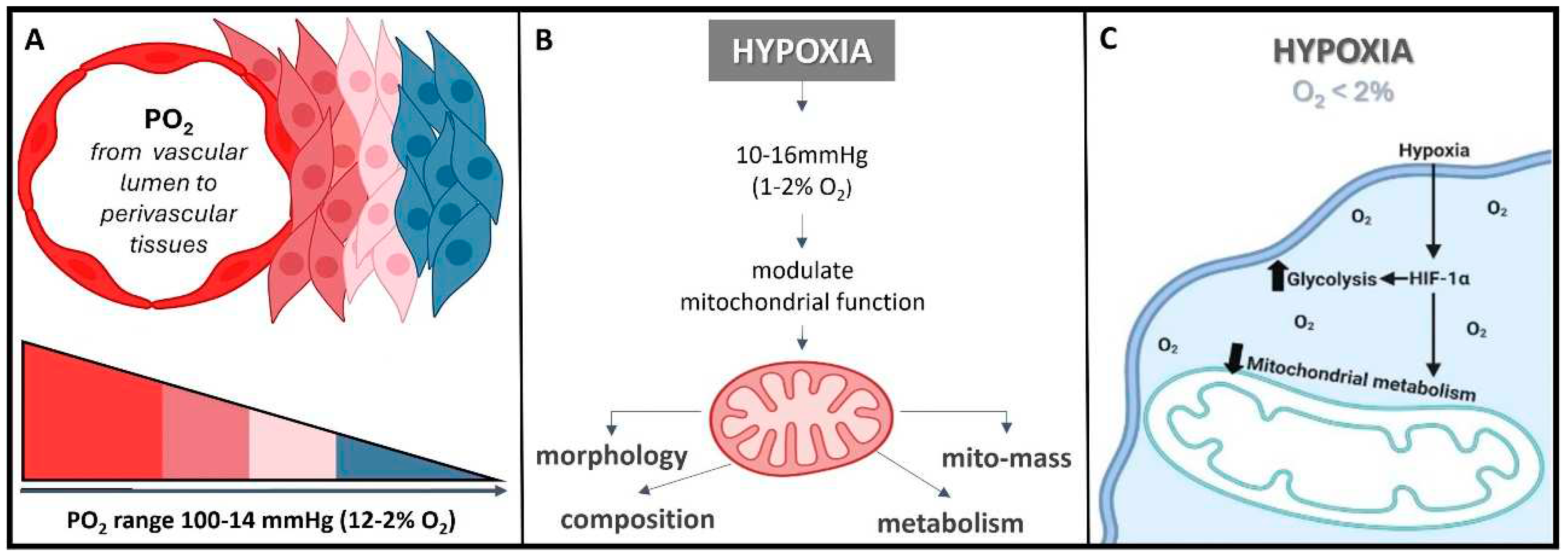
The normal partial pressure of oxygen (PaO
2) in arterial blood typically falls within the range of 75 to 100 millimeters of mercury (mmHg) when measured at sea level and at standard atmospheric pressure; exhibiting variability across various tissues. Normal
tissue oxygen levels can range from 0 to around 60 mmHg, but these values can fluctuate significantly depending on the specific tissue type and its physiological conditions. Measuring PaO
2 directly within cells can be challenging and typically requires specialized techniques, such as microelectrode oxygen sensors or imaging methods. These measurements are valuable for understanding tissue oxygenation in various physiological and pathological conditions and can guide treatment strategies in clinical settings. In most cases, the physiological range spans from 14 to 65 mmHg, corresponding to atmospheric oxygen levels of 2-9% [
21]. Distinct PaO
2 gradients are also evident across both superficial and deep cellular layers, displaying diverse characteristics across from vascular lumen to perivascular tissues (
Figure 2A) [
21,
22] . On the other hand, cellular hypoxia occurs when the oxygen level is between 10-16mmHg (1-2%), and it depends on the type of tissue [
23]. Once oxygen demand exceeds its supply, oxygen level decreases (known as hypoxia) in local tissues or peripherical tissues, leading to a metabolic crisis [
24,
25] (
Figure 2B and C).
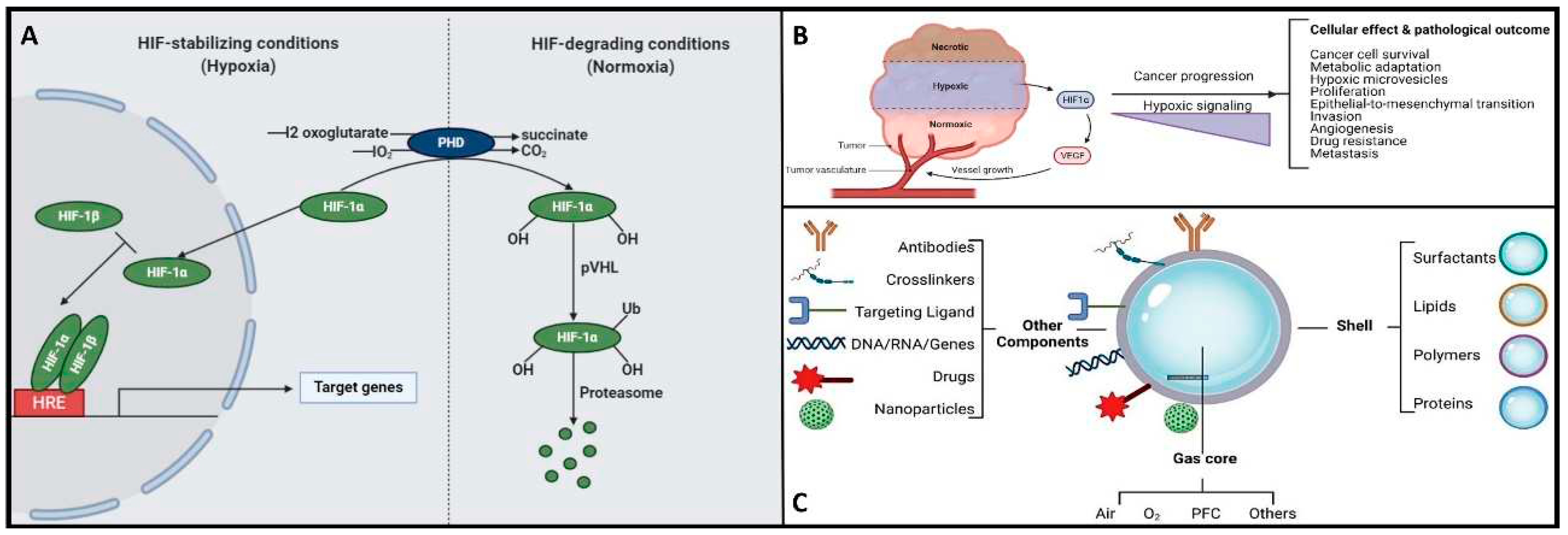
Herein, the hypoxia-inducible factor or HiF, is a heterodimeric transcription factor that plays a central role in cellular adaptation to low oxygen levels. It is considered a master regulator of the cellular response to hypoxia and plays a crucial role in maintaining oxygen homeostasis in various tissues and cells [
26]. Briefly, the HiF is composed of two sub-units: HiF-α (alpha) and HiF-β (beta). The alpha subunit is oxygen-sensitive and exists in different isoforms, with HiF-1α and HiF-2α being the most well-known. In normoxic (oxygen-rich) conditions, prolyl hydroxylase enzymes modify HiF-α, marking it for degradation. However, in hypoxic conditions, this degradation is inhibited, allowing HiF-α to accumulate and form a heterodimer with HiF-β. This active HiF complex then translocates to the cell nucleus, where it binds to specific DNA sequences known as hypoxia-response elements or HREs and initiates the transcription of genes involved in various adaptive responses to low oxygen, including angiogenesis, erythropoiesis, glycolysis, and more. It is noteworthy to mention that HiF's ability to regulate the expression of genes involved in oxygen homeostasis and adaptation to hypoxia makes it a critical factor in various physiological and pathological processes, including embryogenesis, ischemic diseases, cancer, and metabolic disorders. Understanding the role of HiF and its downstream targets is essential for gaining insights into how cells and organisms respond to changing oxygen levels and for developing therapeutic strategies to address related diseases and conditions [
26]. Remember that, once the oxygen decreases below physiological levels, cells trigger a hypoxic cell response, characterized by the HiF-1α stabilization and its nuclear transcription (
Figure 3A). Consequently, the translocation of HiF-1α leads to the activation of approximately 400 target genes. This activation influences metabolic processes by upregulating proteins associated with glycolysis while concurrently suppressing oxygen-dependent pathways, particularly those related to mitochondrial metabolism [
27,
28] These findings establish a direct connection between mitochondria and the HiF pathway, highlighting their interdependence in response to changes in oxygen supply and demand.
Hypoxia exerts diverse effects on mitochondria, but mitochondria also play a role in modulating the cellular response to hypoxia [
24,
29]. Mitochondria serve as the primary oxygen sensors within cells, and their function is profoundly impacted by decreased oxygen levels. Concurrently, hypoxia influences various mitochondrial processes, including fusion, fission, mitophagy, and oxidative phosphorylation (OXPHOS) [
24]. Intriguingly, the inhibition of mitochondrial function can reverse the activation of the HiF pathway induced by hypoxia [
29]. In essence, mitochondria and the cellular response to hypoxia are two inter-connected systems sensitive to oxygen levels. When HIF-1α is degraded, it promotes mitochondrial metabolism, typically observed in normoxic conditions [
29]. Conversely, when HiF-1α is stabilized, it triggers the expression of proteins that, in turn, suppress the mitochondrial metabolism, a characteristic response in a hypoxic condition.
On the other hand, hypoxia is also implicated in the development and severity of numerous age-related and chronic diseases, including cancer and neurodegenerative conditions [
5,
7,
18,
19,
20]. In the context of cancer, cellular responses to hypoxia play a pivotal role in various aspects of tumor progression, encompassing cancer cell survival, proliferation, epithelial-to-mesenchymal transition (EMT), invasion, angiogenesis, drug resistance, and metastasis [
30,
31]. Additionally, hypoxic tumors display greater resistance to chemotherapy, radiotherapy, and photodynamic therapy, ultimately resulting in a poorer prognosis and increased patient mortality [
30,
31]. Please refer to
Figure 3B for further insights. Conversely, the central nervous system (CNS) is particularly susceptible to acute hypoxia and can become compromised rapidly, triggering an ischemic cascade. The brain is highly vulnerable to oxidative stress and mitochondrial impairment in hypoxic conditions. Consequently, it experiences neurodegeneration due to energy deficits. Recent research has shed light on significant alterations in mitochondrial balance within the CNS of individuals suffering from chronic neurodegenerative disorders [
20,
32,
33,
34,
35,
36]. As a result, neurodegeneration is characterized by mitochondrial dysfunction, including reduced activity of complex I, cytochrome oxidase, the electron transport chain (ETC), and the occurrence of mitochondrial DNA mutations. This neuroinflammatory degeneration has been extensively studied in the context of conditions such as Parkinson's disease (PD), Alzheimer's disease (AD), Huntington's disease, and hereditary neuropathy [
20,
32,
33,
34,
35,
36]. Herein, as various neurodegenerative diseases share a common association with mitochondrial dysfunction, there is a pressing need to address, repair, and enhance mitochondrial biogenesis. Within this context, strategies centered on oxygen delivery, such as nanobubbles, have attracted significant attention due to their potential to mitigate hypoxic stress responses and rejuvenate mitochondrial function. To gain a good understanding of the mechanisms and potential applications of nanobubbles in fields like biomedicine, neuroscience, and oncology, it is essential to embark on the fundamental principles governing the hypoxia-inducible factor (HiF) pathway and mitochondrial metabolism. Likewise, nanobubbles hold promise in various applications, primarily due to their stability in oxygenation processes, enabling efficient gas exchange within a defined volume, as aforementioned. This capacity has the potential to enhance mitochondrial metabolism, alleviate hypoxia, and augment oxygen supply, particularly valuable in chronic conditions like cancer, neurodegenerative diseases, and chronic inflammation, amongst others. To unlock the full potential and grasp the mechanism underlying the behavior of OnB in biomedicine, the next section presents foundational principles that govern their bio-performance.
3. NanoBubbles in bioMedicine: Bridging Basic Fundamentals to Practical Applications.
Nanobubbles are minuscule gas-filled spherical entities enclosed by an interface (gas–liquid or gas–solid) with diameters typically in the nanometer range, spanning from tens to hundreds of nanometers [
8,
9]. They represent a distinct class of bubbles, significantly smaller than conventional microbubbles, and are characterized by their unique properties at the nanoscale. In the realm of nanotechnology, nanobubbles have recently garnered considerable attention, mainly attributed to their
exceptional stability and longevity. This remarkable stability arises from the presence of a thin molecular layer at the gas-liquid interface, which effectively confines the gas within the liquid medium. As a result, nanobubbles exhibit prolonged lifetimes, making them pertinent for biomedicine in various scientific and technological applications, including drug delivery [
37,
38], gene delivery [
39], cancer immunotherapy and chemotherapy [
30,
31], wound healing and tissue regeneration, through the intravenous delivery of oxygen carriers such as OnB [
40]. Furthermore, nanobubbles have displayed proficiency in surface modification and cleaning at the nanoscale. Their presence can facilitate the detachment of nanosized contaminants or particles from solid surfaces, offering potential applications in surface engineering, nanofabrication, and precision cleaning processes. In the field of medical imaging, nanobubbles serve as contrast agents for advanced ultrasound imaging techniques. Their nanoscale dimensions allow for improved resolution and specificity in visualizing biological tissues, vasculature, and cellular structures. Beyond the biomedical realm, it is perhaps worth mentioning to the interested reader that nanobubbles have promising environmental applications, including groundwater remediation and wastewater treatment, where their ability to facilitate gas transfer and enhance chemical reactions is leveraged to address various environmental challenges. Henceforth, the multifaceted properties and applications of nanobubbles will do continue to be explored, fueling ongoing research endeavors and innovation across diverse scientific and technological disciplines [
37,
38,
39,
40].
Various techniques enable the synthesis of nanobubbles, including hydrodynamic cavitation, acoustic cavitation, microfluidics, intense mechanical agitation, and electrolysis, among others. What sets nanobubbles apart from other bubbles are several defining parameters, with bubble diameter being the most critical classification criterion, as outlined by ISO/TC 281 and ISO 20480-1:2017 standards. The growing interest in nanobubbles across numerous fields stems not only from their expansive surface area and reactivity when compared to macrobubbles and microbubbles but also from their remarkable attributes, including a heightened gas diffusion rate, enhanced cellular uptake, and robust stability against coalescence, collapse, or rupture. These qualities enable nanobubbles to persist in liquid environments for extended periods, spanning several weeks [
12,
41]. In the ensuing discussion, we delve into the role of physico-chemical features, particularly bubble size, and their interplay with stability. Additionally, we briefly touch upon the significance of factors such as the gas-liquid interface and gas core. Our analysis extends to the implications these factors hold for engineering nanobubbles in diverse biomedical areas.
3.1. Bubble size and physico-chemico-mechanical properties
As mentioned previously, the size of nanobubbles, typically falling within the range of 100 to 250 nanometers, serves as a fundamental parameter that imparts them with inherent and advantageous physico-chemical characteristics [
10,
41]. This size distribution proves highly advantageous in nanobubble preparations, as it aids in overcoming infiltration limitations and facilitates the disruption of the blood-brain barrier [
42]. A noteworthy feature of nanobubbles is their ability to effectively target sites of inflammation, such as those observed in cancer. This efficacy is attributed to the pore cutoff size of the fenestrated endothelium, which ranges from 380 to 780 nanometers. This mechanism, known as the enhanced permeability and retention effect (EPR - describes the abnormal characteristics of the blood vessels associated with pathological conditions, particularly in cancer and inflammation), represents a passive targeting strategy that capitalizes on the efficient accumulation of nanobubbles within tissues featuring elevated vascular permeability (7,15). To simplify, in the context of EPR (leaky blood vessels and the unique properties of the TME to allow therapeutic agents to accumulate preferentially in cancerous tissue while sparing healthy tissue) and nanobubbles, passive targeting means that nanobubbles, due to their size and other properties, can effectively accumulate in areas of disease, such as tumors or inflamed tissues, without the need for active targeting mechanisms. This passive accumulation can enhance the delivery of therapeutic agents or imaging agents to the specific site of interest, contributing to the effectiveness of various medical interventions.
Furthermore, their mechanical properties also contribute significantly to their unique characteristics and potential applications. One notable mechanical property is the elevated internal pressure within nanobubbles. Due to their extremely small size, nanobubbles can contain gas at significantly higher pressures compared to larger bubbles. This heightened internal pressure is a result of the Laplace overpressure, which is inversely proportional to the radius of the bubble. Despite this theoretical thermos-dynamic instability, nanobubbles often exhibit impressive stability, remaining intact for extended periods. The mechanical robustness of nanobubbles, particularly their resistance to coalescence, collapse, or rupture, is another noteworthy trait. This resilience against external forces makes them suitable for various applications where durability is crucial. Indeed, understanding the stability of nanosized bubbles can be partially elucidated through their underlying physical behavior, as elaborated below. When any bubble forms within a solution, it creates an interface, defined by the surface tension (γ) [
43]. The existence of nanobubbles has generated extensive discussion, partly due to the predicted Laplace overpressure associated with them, which can reach several atmospheres, theoretically rendering them thermos-dynamically unstable [
43]. However, in contrast to macro- or microbubbles, nanobubbles often exhibit robust persistence characterized by reduced buoyancy, enabling them to remain intact for days, weeks, or even months [
12,
44]. Additionally, nanobubbles exhibit unique acoustic properties, which have implications in diagnostic imaging and therapeutic ultrasound. Their response to acoustic waves differs from that of microbubbles, opening new opportunities for innovative ultrasound-based techniques. The remarkable attributes defining nanobubbles, including their diminutive size, high surface-to-volume ratio, elevated internal pressure, prolonged stability, electrostatic charge properties, acoustic characteristics, and biocompatibility, have spurred intensive research into their potential across various biomedical applications [
45]. In the realm of biomedicine, the combination of nanobubbles' mechanical attributes, such as their size, stability, and response to external forces, along with their biocompatibility, has led to intensive research into their potential across diverse applications. These mechanical properties, along with their other aforementioned physico-chemical characteristics, position nanobubbles as a promising platform for advancing various fields, from drug delivery to medical imaging.
3.2. Structural composition and electrostatic charge of nanobubbles affects gas core and diffusion
The gas core of a nanobubble is the central part filled with a gas, typically oxygen or nitrogen [
6]. This gas core is encapsulated by a thin layer of liquid, often water, which forms the bubble's shell. The gas core plays a crucial role in the unique properties and applications of nanobubbles. Indeed, (
1)
High Internal Pressure: Nanobubbles exhibit significantly higher internal pressure compared to their larger counterparts, such as microbubbles. This increased pressure is a consequence of the Laplace overpressure, which is inversely proportional to the radius of the bubble. The small size of nanobubbles results in elevated internal pressures, which can have implications for their stability and behavior. (
2)
Gas Diffusion: The gas core of nanobubbles allows for enhanced gas diffusion in liquids. This property is particularly relevant in applications where gas delivery, such as oxygen, to specific biological targets is essential. Nanobubbles can serve as carriers for gases, facilitating their transport to desired locations. The composition of the gas core is another critical factor influencing the stability of nanobubbles [
6]. The introduction of gases like perfluorocarbons (PFCs), especially when combined with oxygen, leads to a reduced efflux from the nanobubble, resulting in a prolonged residence time [
46]. In parallel research efforts, studies have explored combinations of PFCs, sulfur hexafluoride (SF6), and nitric oxide (NO) with oxygen to formulate micro-nanobubble systems [
47,
48,
49]. On a different note, within the realm of nanodroplets (
i.e. an extremely tiny liquid droplet significantly smaller than conventional microdroplets and hence, due to their very small size, can offer more advantages such as increased surface area-to-volume ratios and the ability to penetrate biological barriers or tissues more effectively; growingly being considered as valuable tools in challenging applications including drug delivery, medical imaging, and nanomedicine), liquid PFCs found application as oxygen carriers, capitalizing on their higher oxygen release capacity [
44,
50].
Nanobubbles have a unique structural composition that sets them apart from larger bubbles. While their precise composition can vary depending on the preparation method and the surrounding medium, here is a general overview: (1) Gas Core: At the center of a nanobubble is the gas core, typically composed of gases like oxygen, nitrogen, or a mixture of gases. The choice of gas can influence the bubble's behavior and applications. (2) Liquid Shell: Surrounding the gas core is a thin shell of liquid, which acts as a stabilizing layer. The liquid is often water but can include other liquids or solutions depending on the specific application. (3) Surface Molecules: The interface between the gas core and the liquid shell is defined by surface molecules. These molecules play a critical role in stabilizing the nanobubble and preventing its premature collapse or coalescence. Surface molecules can be surfactants or other stabilizing agents that reduce surface tension. (4) Electrostatic Charge: Nanobubbles may carry an electrostatic charge on their surface due to interactions with the surrounding medium or the presence of charged molecules. This surface charge can influence their behavior, stability and interactions with other particles and/or surfaces.
Henceforth, understanding the structural composition of nanobubbles is crucial for customizing their properties to suit specific applications. Researchers and engineers can manipulate these structural elements to craft nanobubbles with tailored characteristics, catering to a wide array of fields like medicine, materials science, and environmental science. For instance, uncoated nanobubbles, lacking a shell structure, have primarily found utility in applications like water treatment and select human applications such as oral drinks and liquid ventilation (
Table 1,
Table 2,
Table 3 and
Table 4). These uncoated nanobubbles maintain stability through charge stabilization, interacting with electrolytes in water, ultrapure water, distilled water, or saline solutions. Conversely, most engineered nanobubbles adopt a core-shell structure, where the core predominantly contains the chosen gas content, strategically selected for its intended purpose. The shell component exhibits varying compositions and structures, which may include lipids, proteins, polymers, or surfactants. This core-shell configuration has traditionally been employed in the realm of biomedical applications, serving as a contrast agent for ultrasound or as platforms for oxygenation [
51]. The outer shell of nanobubbles functions as a protective layer surrounding the gas core, exerting control over crucial characteristics such as stiffness, elasticity, half-life, and the rate of gas exchange. By encapsulating the gas core, the shell effectively prevents direct contact between the gas and the surrounding solvent, minimizing premature interfacial gas diffusion [
44,
52]. In earlier studies, it was argued that a shell structure was not necessarily a fundamental requirement for bubble formation [
11,
53]. Consequently, both uncoated and coated nanobubbles, or unshelled and shelled nanobubbles, have been utilized in the development of therapeutic strategies for various medical applications. However, increased attention has been directed toward coated nanobubbles, primarily because of their ability to incorporate a versatile array of core and shell materials, manipulation options, and bio-conjugation. This versatility extends beyond gases and drugs allowing for the inclusion of a diverse range of molecules such as proteins, DNA, and ligands [
54], enabling precise
in vivo targeted delivery (
Figure 3C). It is perhaps worth mentioning for the reader herein that shelled micro/nanobubbles (tiny gas-filled bubbles with a protective thin outer shell or coating that is typically composed of various materials including lipids, proteins, polymers, surfactants, or other biocompatible substances) exhibit a more gradual external diffusion of internal gases [
7,
52,
55,
56,
57], contributing their unique characteristics.
4. NanoBubbles as a Platform for O2 Delivery: Innovative OnB-mediated Oxygenation.
In the ever-evolving landscape of medical science and biotechnology, the emergence of nanobubbles as a platform for oxygen delivery has sparked innovation and excitement. These minuscule nano-scaled bubbles have paved the way for groundbreaking approaches to oxygenation, offering a promising solution to address critical challenges in various fields, from medicine to environmental science. Indeed, the rising incorporation of nanobubbles into biomedicine is underpinned by a range of highly beneficial characteristics, as were detailed above. To re-emphasize, these
versatile physico-chemico-mechanical properties encompass stability, the ability to provide a larger surface area for interaction, the availability of uniform or varied size distributions, increased payload capacity, efficient interfacial gas diffusion for precise gas delivery, biodegradability, biocompatibility, accelerated cellular uptake, and the strategic utilization of the EPR effect. The potential of harnessing these nanobubble platforms within clinical settings, to encapsulate and deliver oxygen with exceptional precision (targeted oxygen delivery), is increasingly feasible. Indeed, their diminutive size allows them to navigate intricate biological pathways and reach specific cellular or tissue targets with unparalleled efficiency. These advancements are driven by the development of enhanced formulations of stable and versatile OnB, that can be tailored for a myriad of applications, ranging from enhancing oxygen levels in biological systems to serving as contrast agents in medical imaging. As research, development and innovation efforts continue to advance, the practical integration of nanobubbles into the clinic is becoming increasingly plausible with the translation of novel formulations, including coated and core-shell nanobubbles, have been and are being developed, including our labs, to enhance gas solubility and extend oxygen retention durations, all while avoiding the formation of problematic macro-bubbles [
12], [
41]. In this era of innovation, the exploration of OnB as a platform for oxygen delivery represents a promising frontier. Their ability to navigate the complexities of biological systems and precisely deliver oxygen opens new avenues for addressing hypoxia-related conditions, improving medical diagnostics, and revolutionizing drug delivery strategies. As we delve deeper into the realm of OnB, their potential to reshape the landscape of oxygenation in various fields is an exciting prospect with immense promise for the future.
O2 release strategies involving OnB technologies: The efficient release of oxygen from nanobubbles into a liquid is a spontaneous process driven by the pre-existing concentration gradient between the gas core and the surrounding liquid phase. Moreover, OnB serves a dual purpose as contrast-enhancement agents for ultrasound (US) imaging, offering the distinct advantage of enabling guided and real-time monitoring of oxygen delivery [
58]. In scenarios involving a UltraSound(US)-triggered release mechanism, the emission of high-intensity US waves gives rise to distinct high- and low-pressure resonance zones within the propagating wave. These zones result from resonant phenomena, ultimately leading to the disruption of bubbles and the subsequent liberation of the encapsulated gas core. We present a current summary of studies and potential applications of both uncoated and coated OnB in the field of biomedicine (
Table 1,
Table 2,
Table 3 and
Table 4). The intravascular (IV) delivery of oxygen using OnB has emerged as a prominent administration route in cancer therapies and for conditions characterized by microcirculation dysfunction [
40]. Additionally, OnB show promise as a secure platform for blood oxygenation, achieving oxygen saturation levels of up to 95% without activating the blood complement system or causing hemolysis. Moreover, their minimal IV fluid injection volume requirement underscores their practicality and feasibility [
6]. Research indicates that the cellular uptake of OnB involves complex endocytosis processes, thus preserving cellular functionality and supporting the achievement of desired therapeutic outcomes [
59]. In essence, oxygen release from OnB can occur through gentle methods such as US cavitation or diffusion mechanisms. While alternative oxygen release mechanisms have been explored, such as those involving OnB assisted by photodynamic processes [
60] or pH variations [
61], a significant gap persists in our molecular understanding of how OnB behave within cells; extending to the intricate processes by which mitochondria and HiF- α sense OnB presence.
5. Molecular Insights into the Mechanism of OnB in Chronic Diseases and Disorders
Chronic diseases, ranging from neurodegenerative disorders to cancer, pose significant challenges to both patients and healthcare providers. Hypoxia, a condition characterized by reduced oxygen levels in tissues, plays a pivotal role in the progression and severity of these diseases. Understanding the molecular mechanisms involved in hypoxia-related conditions and the emerging role of OnB is shedding light on innovative therapeutic strategies. Indeed, the exploration and study of the mechanisms underlying the impact of OnB in biomedical contexts has spurred numerous investigations at the cellular and molecular levels. Presently, our understanding of the biological consequences of OnB is most comprehensive in studies related to neurodegenerative disorders and cancer. OnB have been linked to the enhancement of mitochondrial activity, the activation of phosphatidylinositol-3-kinase (PI3K), and the reversal of hypoxic cell responses [
7,
15]. Here are some notes: (
1)
Oxygen Delivery: OnB function as carriers of oxygen, releasing it through various mechanisms, including diffusion, ultrasound-induced cavitation, photodynamic processes, or pH-responsive triggers. This controlled oxygen release is critical for replenishing oxygen-deprived tissues. (
2)
Mitochondrial Regulation: Mitochondria, the cellular powerhouses, are profoundly affected by oxygen levels. Reduced oxygen availability triggers a metabolic crisis marked by mitochondrial dysregulation. OnB can provide oxygen directly to mitochondria, supporting their function and cellular metabolism. (3)
Hypoxia-Inducible Factor (HiF) Pathway: HiF is a key transcription factor that orchestrates cellular responses to low oxygen levels. OnB can modulate the HiF pathway, affecting the expression of genes involved in metabolism, glycolysis, and mitochondrial function. This interplay between OnB and HiF offers insights into how oxygen nanobubbles impact cellular responses in hypoxic conditions. (
4)
Targeted Oxygenation: OnB can navigate complex biological environments to reach specific cells or tissues, providing localized oxygenation. This targeted delivery approach minimizes potential side effects and enhances therapeutic O
2 precision. To summarize, the molecular mechanisms of OnB in chronic diseases involve precise oxygen delivery, regulation of mitochondrial function, and modulation of the HiF pathway. These mechanisms offer promising avenues for therapeutic interventions in a wide range of chronic diseases, with the potential to improve patient outcomes and quality of life. Ongoing research, development and innovation aims to further elucidate these underlying molecular mechanisms and to optimize OnB-based therapies for clinical applications.
5.1. OnB and neurodegenerative disorders/diseases
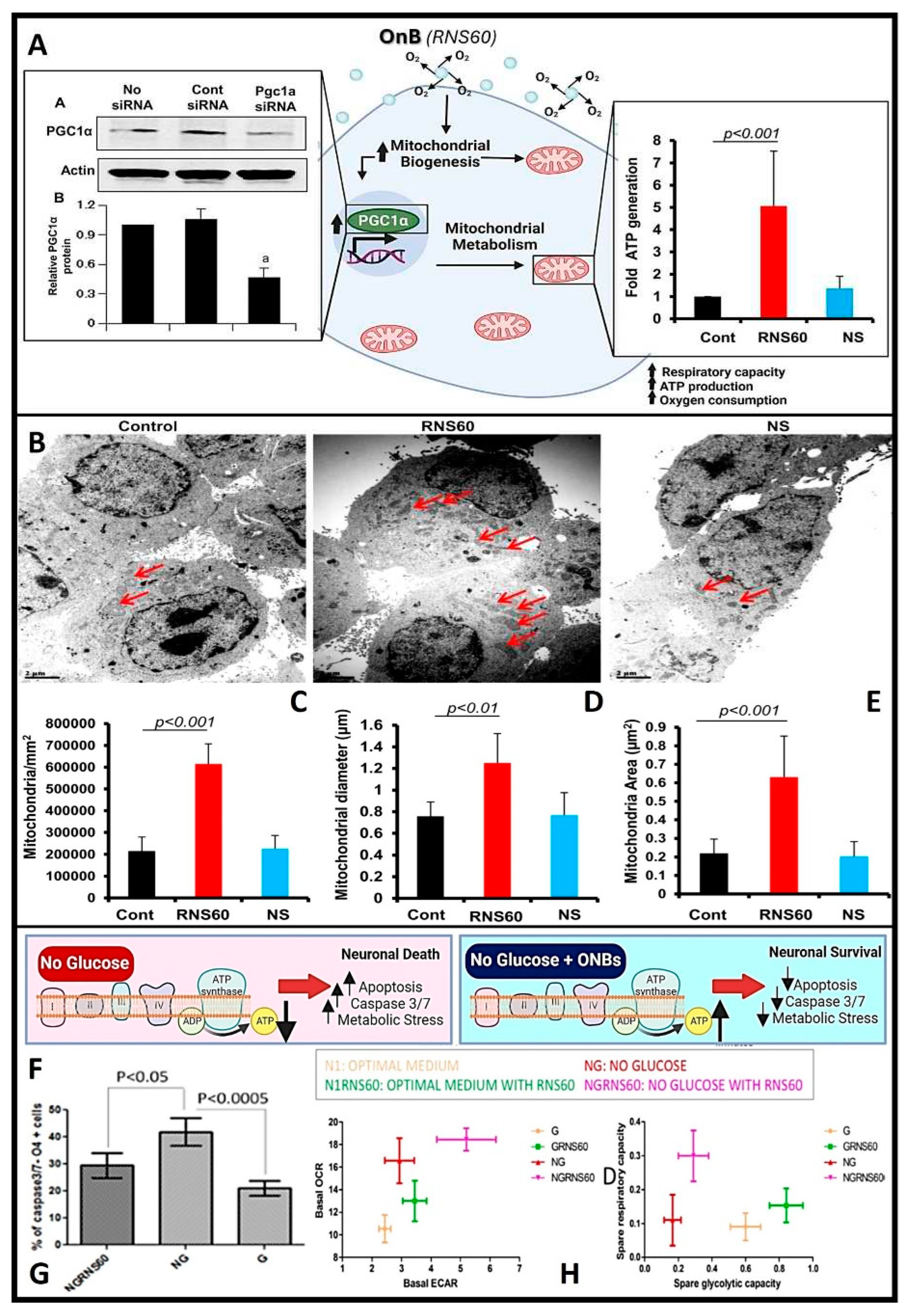
In conditions like Alzheimer's and Parkinson's disease, where mitochondrial dysfunction and hypoxia play significant roles, OnB present promising therapeutic interventions by bolstering neuronal health and function. Mitochondria, pivotal organelles in cellular function, play a central role in energy metabolism through the generation of ATP via oxidative phosphorylation (OXPHOS). Maintaining tissue homeostasis demands substantial energy across various cell types, exemplified by the crucial role of oligodendrocytes (OL) in upholding myelin integrity, which entails significant metabolic activity and ATP consumption. Exposure to OnB results in heightened mitochondrial activity in both neurons and glial cells, marked by increased levels of PGC1α. This enhancement in mitochondrial dynamics translates to amplified respiratory capacity, ATP production, and augmented mitochondrial content in terms of cell size and area (as illustrated in
Figure 4A-E). Similar outcomes have been observed in various other cell types, indicating an improved mitochondrial bioenergetic state [
33,
62]. Neurons treated with OnB exhibit a notable increase in mitochondrial ATP production compared to untreated cells. Significantly, blocking ATP synthesis nullifies the positive effects of OnB. Notably, even the mere exposure to OnBs has shown the remarkable ability to enhance the survival rates of neurons and oligodendrocytes by actively suppressing apoptosis through the downregulation of caspase 3/7 activity, even under conditions of metabolic stress such as glucose deprivation (
Figure 4F-G). This phenomenon is characterized by a higher oxygen consumption rate (OCR), confirming that OnB primarily enhances mitochondrial bio-energetic state under both basal and stress-induced conditions (
Figure 4H). For instance, the measured basal OCR in neurons was higher in the presence of OnB-RNS60 compared to an optimal glucose-containing medium. The correlation of OCR with mitochondrial respiration and extracellular acidification rate (ECAR) with glycolysis reveals a pronounced upward and rightward shift in the presence of RNS60 under glucose-deprived medium conditions (
Figure 4H). This observed shift signifies simultaneous enhancements in both oxidative phosphorylation and glycolysis. This alignment with the shift in oxidative phosphorylation corroborates the modulatory influence exerted by this type of uncoated OnB, which has been shown to modify the electro-physiological properties of the
Xenopus laevis oocyte membrane (the cellular/plasma membrane of oocytes or immature ovarian cells from the African clawed frog,
Xenopus laevis) thru increasing mitochondrial-based ATP synthesis [
84].
These findings highlight the potential of OnB to enhance neuronal survival and function, particularly in the face of metabolic stress conditions, potentially serving as a source of fuel for mitochondrial-related ATP processes [
62]. In addition to the outlined benefits and mechanisms, it's worth mentioning some additional important points related to the use of OnB in addressing mitochondrial dysfunction and hypoxia-related conditions: (
1)
Reduced Oxidative Stress: OnB-mediated oxygenation can help mitigate oxidative stress, a common feature in neurodegenerative diseases. By enhancing mitochondrial function and ATP production, OnB may indirectly reduce the production of harmful reactive oxygen species (ROS), thus contributing to neuroprotection. (
2)
Neuro-inflammation Modulation: OnB treatment has been associated with the suppression of neuroinflammatory responses. Mitochondrial dysfunction and oxidative stress often trigger inflammation in neurodegenerative conditions. The OnB ability to improve mitochondrial health can help dampen neuroinflammation, potentially slowing down disease progression. (
3)
Enhanced Drug Delivery: OnB can serve as carriers for various therapeutic agents, including drugs and gene therapies. This capability allows for targeted drug delivery to specific cell types or tissues affected by neurodegenerative diseases. It can improve the efficacy of treatments while minimizing off-target effects. (
4)
Potential for Combination Therapies: OnB-based interventions can be combined with other treatment modalities, such as photodynamic therapy or immunotherapy, to create synergistic effects in combating neurodegenerative diseases. These multimodal approaches hold promise for more comprehensive disease management. (
5)
Non-Invasive Delivery: OnB can be administered via non-invasive routes like intravenous injection, minimizing the need for invasive procedures. This characteristic is advantageous, especially for elderly patients or those with advanced stages of neurodegenerative diseases. (
6)
Clinical Translation: While much research remains to be done, the potential clinical translation of OnB-based therapies offers hope for improving the quality of life for individuals affected by neurodegenerative diseases. Further preclinical and clinical studies are needed to validate the bio-safety and efficacy of OnB interventions.
5.1.1. Role of OnB in increasing/enhancing mitochondrial bio-genesis and cellular energy
Mitochondrial bio-genesis refers to the process by which new mitochondria are formed within cells, and it plays a crucial role in maintaining cellular energy production and overall cellular health. Cellular metabolism is a controlled biological process that involves numerous signaling pathways and transcriptional networks. As elucidated earlier, PGC1α is a pivotal and orchestrator governing metabolism and mitochondrial bio-genesis [
18]. Functioning as a transcriptional factor, PGC1α serves as transcriptional factor that links external stimuli with mitochondrial metabolism [
63]. However, the mechanism underpinning PGC1α activation, and the subsequent upregulation of mitochondrial bio-genesis remains unclear. Nanobubble-based oxygen delivery systems act as an alternative strategy to not only amplify ATP production, yet also to augment mitochondrial size and mitogenesis through a series of mechanisms, encompassing the following two main actions [
18,
63]:
OnB stimulate mitochondrial bio-genesis by inducing the upregulation of PGC1α (
Figure 5).
OnB upregulate the expression of nuclear respiratory factor 1 (NRF1), mitochondrial transcription factor A (TFAM), and genes closely associated with mitochondrial biogenesis (
Figure 5).
To recap, OnB may contribute to increasing mitochondrial bio-genesis via: (1) Enhanced Oxygen Supply: OnB serves as a source of supplemental oxygen to cells, especially in oxygen-deprived or hypoxic environments. Mitochondria are the primary sites for cellular respiration, where oxygen is used to generate ATP (adenosine triphosphate), the cell's energy currency. By providing additional oxygen, OnB can help ensure that mitochondria have an adequate oxygen supply for efficient ATP production. (2) Activation of Cellular Signaling Pathways: OnB treatment has been associated with the activation of cellular signaling pathways that regulate mitochondrial bio-genesis. One key player in this process is peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), a master regulator of mitochondrial bio-genesis. OnB treatment may increase the expression and activity of PGC1α, leading to the synthesis of new mitochondria. (3) Improved Respiratory Capacity: OnB-induced oxygenation can enhance the respiratory capacity of mitochondria. Mitochondrial bio-genesis involves not only the formation of new mitochondria but also the optimization of the function of the existing mitochondria. OnB may promote the efficient use of oxygen in the electron transport chain, further boosting ATP production. (4) Neuroprotection: In conditions like neurodegenerative diseases, where mitochondrial dysfunction is a hallmark, the ability of OnB to increase mitochondrial bio-genesis is of particular importance. Mitochondrial health is crucial for neuronal survival and function. OnB-induced mitochondrial bio-genesis may help protect neurons from degeneration and maintain their energy demands. (5) Potential Therapeutic Applications: Harnessing OnB to promote mitochondrial biogenesis holds promise for the development of novel therapeutic interventions. Henceforth, by supporting the mitochondrial health and function, OnB-based treatments may benefit individuals diagnosed with and suffering from conditions ranging from neurodegenerative to cardiovascular disorders as well as cancer.
5.1.2. Mitochondrial biogenesis via the phosphatidylinositol 3-kinase (PI3K) enzyme
Beyond the promotion of mitochondrial growth by OnB via the increased expression of PGC1α [
18], emerging scientific evidence underscores the pivotal role of a key signaling molecule, namely PI3K, in mediating numerous beneficial effects attributed to these nanobubbles (32, 86). PI3K is a vital signaling enzyme that governs a broad spectrum of biological responses, encompassing cell proliferation, cell viability, and programmed cell death, known as apoptosis [
64]. Class IA PI3K consists of a regulatory 85-kDa subunit and a catalytic 110-kDa subunit (p85:p110α/β), regulated by multiple receptor tyrosine kinases (RTK) and G protein-coupled receptors (GPCR). Conversely, class IB PI3K comprises a dimer consisting of a 101-kDa regulatory subunit and a p110γ catalytic subunit (p101/p110γ). Recent investigations have corroborated the hypothesis that OnB might as well induce a heightened mitochondrial bio-genesis in neurons by activating PI3K and engaging transcriptional co-activators (
Figure 5) such as CREB (
knowingly to be induced by a variety of growth factors and inflammatory signals prior to mediating the transcription of genes).
This activation of the PI3K has been closely linked to the suppression of pro-inflammatory responses in microglia, achieved through the downregulation of nuclear factor-Κb (86). Furthermore, it has been implicated in enhancing T regulatory cell activity, resulting in reduced nitric oxide production, and subsequently restraining the differentiation of Th17 and Th1 cells [
32]. Consequently, the effects of OnB observed in T cells, glial cells, or neurons can be inhibited by blocking the PI3K pathway [
18,
32,
65]. Nevertheless, several significant questions remain actively under investigation, including the precise mechanism through which charge-stabilized nanobubbles activate PI3K and the identification of other downstream pathways contributing to this activation.PGC1α exerts regulatory control over various mitochondrial functions and essential mitochondrial transcription factors like TFAM. PGC1α's activity is subject to direct modulation through various post-translational modifications, including acetylation, methylation, ubiquitination, and phosphorylation [
66]. However, the underlying mechanisms governing the expression and functional regulation of PGC1α, particularly its intricate interactions with proteins such as SIRT, AMPK, MAPK, and Akt, remain incompletely understood [
66]. In parallel, the multifaceted role of TFAM in terms of its impact on mtDNA, the mitochondrial network (including mitophagy, mito-fission, and mito-fusion), and functional assays for mitochondria remains relatively unexplored territory. Conversely, the upregulation of PGC1α is indirectly controlled by a mechanism involving the expression of NRF1 and CREB [
66,
67]. Recent studies conducted on dopaminergic neuronal cells and glial cells have shed light on the role of Akt, a downstream effector of PI3K, in orchestrating the activation of CREB in the context of O
nB treatment [
18,
33]. These findings suggest a model in which O
nB increase the expression of PGC1α through class IA PI3K-mediated activation of CREB, subsequently leading to enhanced mitochondrial bio-genesis. Notably, experiments utilizing siRNA knockdown targeting both PGC1α and CREB or the inhibition of PI3K have demonstrated the blockade of O
nB-mediated expression of both, TFAM and NRF1 [
18,
67].
To recap, the critical importance of regulating mitochondrial bio-genesis for maintaining cellular energy homeostasis and responding to metabolic demands, the following summarizes how PI3K is involved in the process: (1) Activation of PI3K: Phosphatidylinositol 3-kinase (PI3K) is an enzyme that phosphorylates the lipid molecule phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). This lipid modification triggers a cascade of downstream signaling events. (2) Activation of Akt (Protein Kinase B): PIP3 generated by PI3K activates a protein kinase called Akt or Protein Kinase B (PKB). Akt is a central player in cell signaling pathways that regulate cell growth, survival, and metabolism. (3) Akt-Mediated Signaling: Activated Akt can phosphorylate and regulate various downstream targets, including transcription factors and other proteins involved in mitochondrial biogenesis. One of the key targets is the transcriptional coactivator PGC-1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha). (4) Role of PGC-1α: PGC-1α is a master regulator of mitochondrial biogenesis. When activated by Akt, PGC-1α promotes the expression of genes responsible for mitochondrial DNA replication, transcription, and protein synthesis. It also enhances the formation of new mitochondria by coordinating the synthesis of mitochondrial components. (5) Increased Mitochondrial Mass: The activation of PI3K-Akt signaling and subsequent phosphorylation of PGC-1α result in the increased expression of mitochondrial genes and the growth of existing mitochondria. This leads to an overall increase in mitochondrial mass and function. (6) Enhanced Cellular Energy Production: As mitochondrial biogenesis progresses, the cell becomes more equipped to produce ATP through oxidative phosphorylation, a process that occurs within the mitochondria. This enhanced ATP production supports the cell's energy needs and metabolic processes. (7) Cellular Adaptation: The activation of PI3K-Akt signaling and mitochondrial bio-genesis can be a response to various cellular stimuli, including changes in energy demands, exercise, and environmental factors. Cells adapt to distinct metabolic needs thru modulating their mitochondrial content.
Henceforth, the PI3K-Akt pathway and its involvement in mitochondrial bio-genesis have significant implications in various physiological and pathological conditions. Dysregulation of this pathway can contribute to metabolic disorders, neurodegenerative diseases, cancer, and other health-related issues. Understanding the molecular mechanisms underlying mitochondrial biogenesis via PI3K signaling provides valuable insights into cellular physiology and the development of potential therapeutic interventions for diseases characterized by mitochondrial dysfunction. It is crucial to acknowledge, herein, the complexity of transcriptional regulation and its multi-faceted role. Henceforth, further discovery and experimental research studies are truly desirable to comprehensively understand the intricacies of this transcriptional regulation and its broader effects on the various mitochondrial processes, including the electron transport chain, oxidative stress response, ROS detoxification, tricarboxylic acid cycle or TCA, and mitochondrial bio-genesis regulation.
5.2. OnB and overcoming hypoxia and hypoxic conditions
In a normoxic environment (conditions characterized by normal or standard or adequate levels of oxygen and oxygen concentration), human cells predominantly allocate more than 90% of the available oxygen to mitochondrial respiration [
29]. This leaves approximately 10% of the oxygen in the cytosol, which is adequate for triggering the degradation of HiF-1α. However, under sustained or prolonged hypoxic conditions, mitochondria intensify their oxygen consumption, nearly depleting the cytosolic oxygen pool and consequently stabilizing HiF-1α [
29]. Once activated, HiF-1α plays a pivotal role in the transcription of numerous genes, including TGF- α, VEGF and VEGF Receptor FLT1, GLUT1, MMPs, EPO, HK2, CA9 and iNOS [
29]. In recent years, considerable attention has been focused on unraveling the complex role of HiF-1α in the context of cancer. The upregulation and stabilization of HiF-1α within cancer cells have been closely associated with increased tumor and cell survival, heightened angiogenesis, enhanced proliferation of cancer cells, and elevated resistance to both chemotherapy and radiation therapies [
68,
69]. Modern research efforts have shed light on the significant influence of OnB on HiF-1α dynamics. These nanobubbles have exhibited the ability not only to inhibit HiF-1α expression [
70] but also to substantially reduce hypoxia-induced resistance to radiation in various cancer cell lines, such as EBC-1 and MDA-MB-231 cells (human lung and breast cancer cells) [
69]. Significantly, OnB have shown the potential to serve as a sensitizing adjuvant when administered in combination with low-dose radiation therapy, potentially enhancing treatment bio-efficacy in cancer patients without increasing toxicity. This is due to the ability of OnB in helping to increase the O
2 availability within the tumor microenvironment (tumors often have regions of low oxygen or hypoxia, which can make them more resistant to radiation therapy, and radiation therapy relies on the presence of oxygen to generate free radicals and induce DNA damage in cancer cells ; hence, when tumor cells are hypoxic, radiation therapy becomes less effective). Therefore, by delivering oxygen directly to the tumor site, OnB can potentially alleviate tumor hypoxia, making the cancer cells more susceptible to the damaging effects of radiation, thereby enhancing its efficacy).
5.2.1. Molecular bases to downregulate/suppress HiF-1α post-OnB application/treatment
OnB are designed to release oxygen when they reach the hypoxic regions within tissues or tumors. Such a localized oxygen delivery can increase the oxygen tension in these areas, which, in turn, reduces the stabilization of HiF-1α. HiF-1α (sensitive to oxygen levels), and in normoxic conditions, undergoing hydroxylation and proteasomal bio-degradation.
Hence why HiF-1α serves as a crucial physiological indicator of oxygen delivery and is studied as the primary physiological probe for oxygen delivery due to its rapid degradation in the presence of oxygen. Both
in vitro and
in vivo studies have consistently demonstrated a reduction in HiF-1α expression following OnB exposure [
7,
71,
72]. When tumor cells are exposed to OnB
in vitro, the hypoxic conditions are reversed, accompanied by the suppression of HiF-1α levels
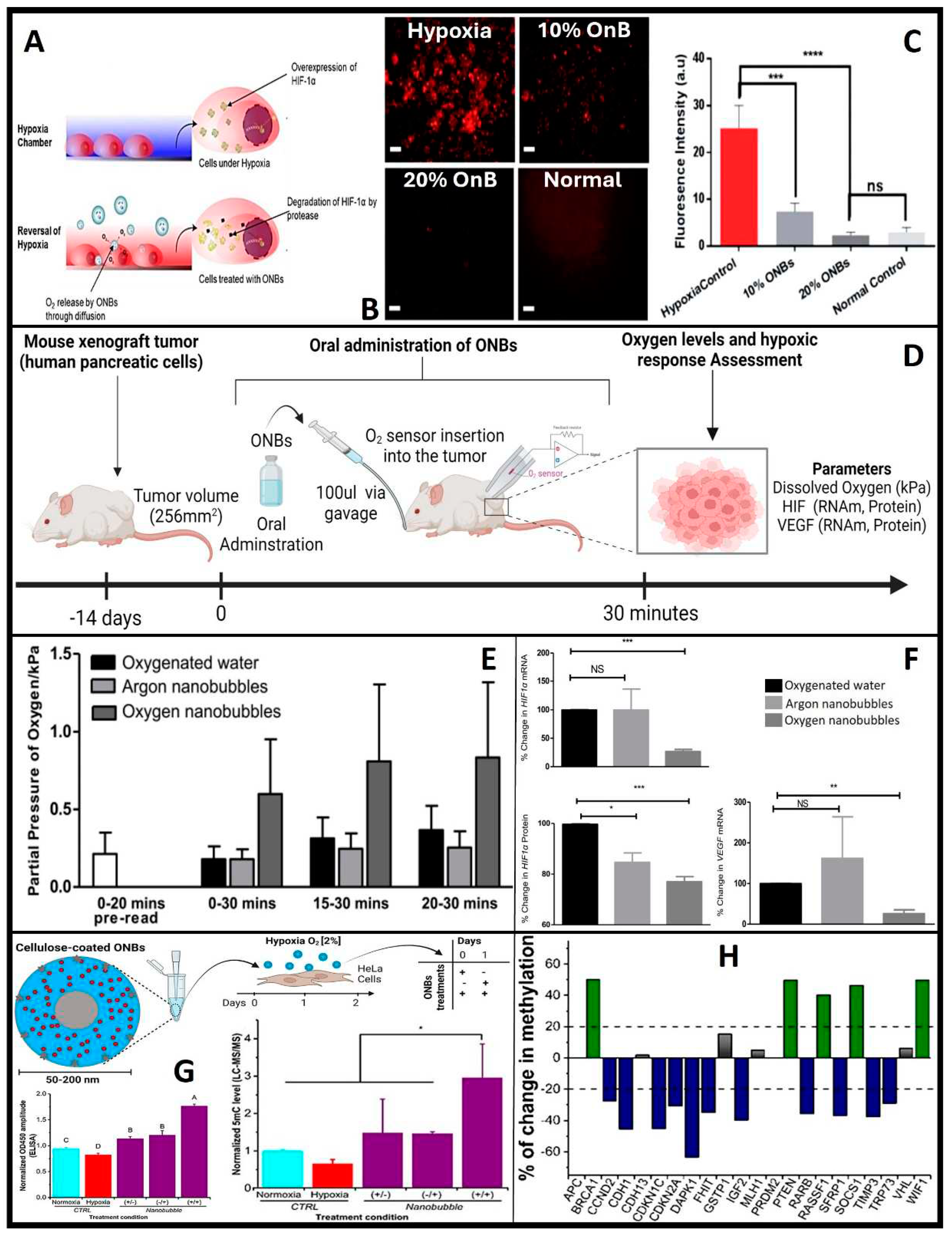
(Figure 6A,B) [
7]. Similarly, in a mouse model, a significant decrease of 75% in mRNA levels and 25% in protein levels of HiF-1α has been observed (
Figure 6C-E) [
70]. These oxygen-induced changes within tumors have also led to a notable reduction in VEGF expression in tumors treated with the oxygen nanobubble suspension. Furthermore, lung cancer and breast cancer cells have exhibited clear suppression of hypoxia-induced HiF-1α expression when cultured in media containing OnB compared to cells cultured in distilled water-based media [
69,
70]. Herein, remember that OnB, while reducing ROS levels within hypoxic regions and interfering with the signaling pathways that activate HiF-1α under hypoxic conditions, they might inhibit the activation of receptor tyrosine kinases (RTKs) or other upstream regulators involved in HiF-1α stabilization. Further, OnB treatment may activate cellular pathways that antagonize HiF-1α expression or stability. These pathways could involve negative feedback loops, post-translational modifications, or competitive binding to HiF-1α. Also, OnB could induce apoptosis (programmed cell death) in hypoxic cells, which may lead to the degradation of HiF-1α. Apoptosis can be triggered in response to prolonged hypoxia and is associated with the degradation of various cellular proteins, including HiF-1α. Finally, to re-emphasize, OnB may indirectly contribute to the downregulation of HIF-1α via reducing the ROS levels locally.
5.2.2. OnB and epigenetic modulation in tumors
Briefly, epigenetic modifications are heritable changes in gene expression that do not involve alterations in the underlying DNA sequence but can influence gene activity. In the context of neoplasia, where tumoral cells exhibit uncontrolled proliferation and insufficient angiogenesis, the tumor's microenvironment often becomes hypoxic. In response to this prevailing hypoxia, various oncogenic mechanisms come into play, with epigenetic modifications taking a prominent role in promoting increased tumor growth and enhancing the survival of cancer cells [
73]. These epigenetic changes encompass a wide spectrum of alterations, including hypomethylation, primarily activating oncogenes [
74], gene-specific hypermethylation of CpG islands in the promoter regions of tumor suppressor genes, rendering them inactive, and contributing to aberrant cell proliferation [
75]. Central to these processes are the Ten-eleven translocation (TET) enzymes, a class of dioxygenases dependent on Fe2+ and α-ketoglutarate. These enzymes facilitate the conversion of 5mC to 5hmC and its downstream derivatives [
76,
77]. The quantification of 5mC levels presents a clear and statistically significant reduction (α=0.05) in DNA methylation within hypoxic cells when exposed to OnB, as compared to control cells (
Figure 6F) [
72]. This observation is further supported and validated through enzyme immunoassay (EIA) and liquid chromatography-mass spectrometry (LC-MS/MS) (
Figure 6G-H) analyses. OnB demonstrate their capacity to effectively reprogram crucial genes associated with hypoxia and tumor suppression, such as MAT2A and PDK-1. Furthermore, a notable negative correlation emerges between the expression levels of PDK-1 and MAT2A and both tumor size and metastasis [
72]. Concurrently, the methylation status of a panel of 22 tumor suppressor genes has been meticulously profiled [
72]. Remarkably, treatment with nanobubbles leads to a significant reduction in promoter methylation for 11 of these genes (
Figure 6G). This intriguing observation suggests that OnB have the potential to reverse hypoxia-induced epigenetic changes, steering the cancerous DNA methylation landscape toward a more normalized state. This process entails both global re-methylation and the selective demethylation of specific tumor suppressor genes. A notable example is the reactivation of cell cycle regulation mediated by genes such as p57 (CDKN1C), p21 (CDKN2A), and p73 (TRP73) upon nanobubble treatment. This observation suggests that the reversal of hypoxia by nanobubble treatment has the potential to reshape the cancerous DNA methylation landscape towards a relatively normal status. It involves global re-methylation and the targeted demethylation of specific tumor suppressor genes. Notably, nanobubble treatment leads to the reactivation of essential cell cycle control mechanisms mediated by p57 (CDKN1C), p21 (CDKN2A), and p73 (TRP73). These genes play crucial roles in suppressing abnormal cell proliferation and inducing apoptosis in cancers [
78,
79]. Importantly, their functions are often silenced through aberrant hypermethylation across a range of various cancer types [
80,
81]. Consequently, the epigenetic modulation of these genes using OnB may contribute to the observed inhibition of tumor progression
in vivo.
To recap, while the specific mechanisms of how OnB may modulate epigenetic changes in tumors are not yet fully understood, some of the potential ways in which OnB could impact epigenetics, include: (1) DNA Methylation: OnB could potentially influence DNA methylation patterns in tumor cells. DNA methylation involves the addition of methyl groups to DNA, often resulting in gene silencing. Changes in oxygen levels can affect the activity of DNA methyltransferases, enzymes responsible for DNA methylation. OnB may influence the activity of these enzymes, thereby altering DNA methylation patterns. (2) Histone Modifications: OnB might affect histone modifications, which are chemical changes to histone proteins around which DNA is wrapped. These modifications can influence chromatin structure and gene expression. Changes in oxygen levels can impact the activity of enzymes that add or remove these chemical groups from histones, and OnB could potentially play a role in this process. (3) Non-Coding RNAs: OnB could influence the expression of non-coding RNAs, such as microRNAs and long non-coding RNAs, which are known to have epigenetic regulatory functions. Changes in oxygen levels within tumor cells might affect the production and function of these non-coding RNAs, leading to epigenetic changes. (4) Cellular Signaling Pathways: OnB have been shown to influence cellular signaling pathways, including those involved in cell survival, proliferation, and apoptosis. These pathways can intersect with epigenetic regulatory mechanisms, and changes in signaling pathways induced by OnB may contribute to epigenetic alterations in tumor cells. Finally, remember that the precise mechanisms through which OnB modulate epigenetic changes in tumors are an active area of research, and more studies are needed to fully understand these processes. Epigenetic modifications are complex and can have profound effects on gene expression and cellular behavior, thus, uncovering the specific epigenetic consequences of OnB treatment in tumors is an intriguing avenue of investigation.
6. Conclusions
The use of ultrafine bubbles as customizable nanocarriers or platforms infused with gas/drug-based nanotechnology represents a highly promising frontier, particularly in the context of conditions related to oxygen deficiency, such as neurodegenerative disorders, cardiovascular diseases, and cancer. Nanobubbles offer a range of advantages, including their stability for oxygenation, efficient gas exchange within confined volumes, and prolonged gas delivery compared to conventional micro/macro bubbles. These attributes encompass negative electric surface charge density, extended lifespan, reduced buoyancy, the ability to carry multiple gases, and strong echogenicity. Both coated and uncoated OnB have shown significant potential in enhancing mitochondrial metabolism, overcoming hypoxia, and increasing oxygen availability across various diseases, including cancer, neurodegenerative disorders, and chronic inflammation. Coated nanobubbles, in particular, have garnered specific attention for their versatility in carrying not only gases but also various molecules, including drugs, proteins, DNA, and ligands for precise in vivo delivery. While existing evidence mainly consists of in vitro studies highlighting the robustness of OnB in cellular oxygenation and their ability to downregulate HiF-1α while enhancing mitochondrial function through PI3K-mediated increases in PGC1α and TFAM transcription, several aspects require further exploration. These include the measurement of oxygen dynamics within cells, investigations into antioxidant cell signaling (such as SOD2), assessments of mitochondrial health and fitness, and comprehensive evaluations of biosafety and targeting precision in preclinical and clinical trials, especially for intravenous OnB applications. Additionally, uncharted territories such as muscular recovery, cutaneous lesion oxygenation, and tissue regeneration offer exciting prospects for future research. Looking ahead, nanobubbles present a range of exogenously responsive properties sensitive to environmental or external stimuli like ultrasonic waves, light, temperature changes, electric and magnetic fields, as well as endogenous triggers like pH and redox states. These features align well with the versatile array of biomaterials (lipids, surfactants, polymers, proteins), opening doors to diverse applications in theragnosis, drug design, and delivery. The amphiphilic nature of nanobubbles, featuring a hydrophobic core and a hydrophilic shell, facilitates their dual functionality in both gas and drug delivery. Consequently, the scope of OnB applications continues to expand, spanning critical domains of biomedicine such as tissue engineering, material science, bio-fabrication and -printing, pharmaceutics and drug delivery, molecular imaging, and theragnosis; paving the way for potential significant advancements and interdisciplinary breakthroughs in the future.
Table 1.
OnB Application in Cancer Studies.
Table 1.
OnB Application in Cancer Studies.
Clinical
Indication |
Study
Type |
Oxygen Release Strategy |
Delivery |
Main Results and Conclusions |
| Lung cancer |
in vitro |
Diffusion |
Cell Culture Media |
Un-coated OnB reduce the hypoxia-induced resistance in cancer cells [69]. |
Pancreatic
cancer |
in vitro/in vivo |
UltraSound
mediated |
Oral |
Surfactant-stabilized OnB reduce the transcriptional and protein levels of HIF1α [70]. Lipid- stabilized oxygen micro-bubbles and US reveal a 45% reduction in tumor volume five days after treatment [82]. |
Ovarian
cancer |
in vivo |
UltraSound
mediated |
IntraPeritoneal |
Oxygen- and Paclitaxel (PTX)-loaded lipid micro-bubbles down-regulate HiF-1α, and increase PTX effectivity [83]. |
| Breast cancer |
in vitro |
Diffusion |
Cell Culture Media |
OnB revert hypoxia, downregulate HiF-1a and improve cellular conditions, leading to further medical applications [7]. |
| in vivo |
UltraSound
mediated |
IntraPeritoneal |
Oxygen micro-bubbles strongly enhance echo intensity in tumor and significantly enhance PO2, after US irradiation [47]. |
Chorio-
carcinoma |
in vitro |
Diffusion |
Cell Culture Media |
Human JEG-3 cells showed a reduction (2-fold decrease—50%) in HiF-1α transcript levels at 3% O2 incubation [84]. |
| NasoPharyngeal carcinoma |
in vitro/in vivo |
pH responsive |
IntraVenous |
pH-responsive OnB increase the intra-tumoral oxygen concentration six-fold, suggesting great potential for overcoming the hypoxia-induced resistance [61]. |
Bladder/Colon
Tumor Cell lines |
in vitro/in vivo |
UltraSound
mediated |
IntraVesical
IntraVascular |
OnB is a promising multi-modal and multifunctional strategy for imaging and targeting hypoxic tumoral micro-environment [85,86]. The bladder tumoral PO2 increased by around 140% after the injection of OnB [72]. Biogenic nanobubbles (gas vesicles) enhance US contrast signal when compared to/with synthetic nanobubbles, enhancing tumor penetration with a range size of 2-200 nm [87]. |
| Glioma |
in vitro/in vivo |
Photodynamic (PDT) |
IntraVenous |
OnB stimulated by function as an oxygen self-supplement agent, enhances the survival rate on glioma-bearing mice model [60]. |
Table 2.
OnB Application in Neuro-Science Studies.
Table 2.
OnB Application in Neuro-Science Studies.
Clinical
Indication |
Study
Type |
Oxygen
Release Strategy |
Delivery |
Main Results and Conclusions |
Amyotrophic Lateral
Sclerosis (ALS) |
in vitro/in vivo |
Diffusion |
IntraPeritoneal |
RNS60 protects neurons, decreasing ALS progression [33,88] and demonstrating the feasibility, safety, and tolerability of long-term administration of RNS60 in patients with ALS [89]. |
| Clinical Trial |
Diffusion |
IntraVenous |
The effect of RNS60 treatment on selected pharmacodynamic biomarkers in ALS patients was concurrently treated with riluzole (NCT03456882). |
| Multiple Sclerosis (MS) |
in vitro/in vivo |
Diffusion |
IntraPeritoneal and
Nebulization |
RNS60 induce the activation of PI3K, promoting myelin genes transcription in oligodendrocytes (OL) and glial cells [90]. RNS60 enhanced OL spare respiratory capacity (SRC) in response to metabolic stress (glucose-nutrient deprivation) [5]. RNS60 led to the enrichment of anti-autoimmune regulatory T cells (Tregs) suppression of autoimmune Th17 cells [91]. |
| Alzheimer’s disease (AD) |
in vivo |
Diffusion |
IntraPeritoneal |
RNS60 suppressed the hippocampus neuronal apoptosis, attenuated Tau phosphorylation, and the burden of Ab [20]. RNS60 upregulated the plasticity-related proteins (PSD95 and NR2A) and NMDA-dependent hippocampal calcium influx [18]. |
| Parkinson’s disease (PD) |
in vitro/in vivo |
Diffusion |
IntraPeritoneal |
RNS60 enhance mitochondrial bio-genesis via PI3K/CREB and PGC1alpha in PD model [65]. Moreover, RNS60 inhibited the activation of NF-κB in the SNpc of MPTP-intoxicated mice [92]. |
| Spinal Cord Diseases, Injuries, and Compression |
Clinical Trial |
US
Contrast |
Inthathecal |
Use of OnB and US improve the identification of discrete areas of perfusion changes in the spinal cord in subjects undergoing spinal cord decompression (NCT05530798). |
Table 3.
OnB Application in Cardio-Respiratory Studies.
Table 3.
OnB Application in Cardio-Respiratory Studies.
Clinical
Indication |
Study
Type |
Oxygen Release Strategy |
Delivery |
Main Results and Conclusions |
| Respiratory Failure and Acute Lung Trauma/Injury |
in vitro/in vivo |
Diffusion |
IntraPeritoneal
IntraVenous |
Peritoneal microbubble oxygenation (PMO) provides extrapulmonary ventilation after a complete tracheal occlusion [93]. PMO is also a promising strategy for other pulmonary diseases [6] and oxygenate blood within 4 sec and do not cause hemolysis or complement activation in hypoxic rabbits [94]. |
Blood
Oxygenation |
in vitro/in vivo |
Diffusion |
Cell Culture
Media |
Oxygen microbubble-containing dextran solutions were effective for improving blood oxygenation [95]. |
| in vivo |
Diffusion |
IntraVenous |
Oxygen microbubbles are safe and effective to deliver more oxygen than human red blood cells (per gram) after being injected in vivo [96]. |
ExtraCorporeal
Membrane Oxygenation (ECMO) |
in vitro |
Diffusion |
De-Oxygenated PBS |
Protein-encapsulated oxygen microbubbles rapidly equilibrate hypoxia by releasing their oxygen core into an oxygen-depleted saline solution [97]. |
| Anti-Thrombotic Effect |
in vitro |
USmediated |
IntraVascular |
PLA-combined Fe3O4-GO-ASA nanobubbles improve the antit-hrombin parameters and significantly inhibit thrombosis within the rabbit blood [98]. |
| MicroFluidic Device for Hypoxemia |
in vitro/in vivo |
Diffusion |
IntraVascular |
OnB from the device were infused into the femoral vein, in vivo, wherein ∼20% of baseline VO2 can be delivered intravenously in real time [99]. |
Ischemic Stroke
Re-Perfusion |
in vivo |
US
mediated |
IntraVascular |
OnB with US stimulation to provide sono-perfusion and local oxygen for the reduction of brain infarct size and neuroprotection after stroke re-perfusion [100]. |
Table 4.
OnB Application in Metabolic diseases, Regenerative Medicine & Molecular Imaging Studies.
Table 4.
OnB Application in Metabolic diseases, Regenerative Medicine & Molecular Imaging Studies.
Clinical
Indication |
Study
Type |
Oxygen Release Strategy |
Delivery |
Main Results and Conclusions |
| Diabetes, Burns, Tissue necrosis, Bedsores and Wounds |
in vitro/in vivo |
US
mediated |
Cell Culture Media |
Dextran and Chitosan nanobubbles might be proposed for the delivery of oxygen which is enhanced by US with a frequency of 45 kHz in hypoxic related diseases [2,101]. |
Clinical Trial
(recruiting) |
Diffusion |
Irrigation |
This micro-/nano-bubble solution is suggested as an irrigation solution to improve wound oxygenation in ischemic tissues (NCT05169814). |
Diabetic
Retinopathy |
in vitro/in vivo |
Diffusion |
Cell Culture
IntraVitreal |
Dextran-OnB release 74.06 µg of O2 after 12 hours at 37°C and mitigate hypoxia during ischemic conditions in the eye, upon timely administration [102]. |
Tissue
Cutaneous Lesions |
in vitro/in vivo |
US
mediated |
Cell Culture Media / Gel formulation topically applied |
The Oxygen-loaded nano-Droplets (OLNDs) are more effective than former oxygen-loaded nanobubbles enhancing oxy-hemoglobin levels by photoacoustic [103]. US-activated chitosan-shelled/DFP-cored OLNDs might be a novel, suitable, and cost-effective way to treat several hypoxia-associated pathologies of the cutaneous tissues [3]. |
Transdermal Drug
Release |
in vitro/in vivo |
US
mediated |
Transdermal micro-needle Gel |
The nanobubbles added into a micro-needle patch and used in addition to US cause better penetration and diffusion of drugs [104]. |
| Cytocompatibility of OnB |
in vitro |
US
mediated |
Cell Culture Media |
Lipid-shelled/-coated OnB show US imaging-responsiveness and enhance cell viability in several cell lines [15]. |
| Drug Delivery in Fluids |
in vitro |
US mediated |
Hypoxic
Solution |
Oxygen release from polysaccharides–peptides, Pingxiao, and chitosan is 94.6%, 75.1%, and 40.2% (respectively) higher than water, especially under the US stimulus [71]. |
On-Chip contrast agent for US
imaging |
in vitro |
US
mediated |
Microfluidic |
Micron-sized lipid shell-based perfluorocarbon gas microbubbles enhance the gas composition for US contrast agents with new shell materials [105]. |
Author Contributions
Conceptualization, S.M.V.G. and Z.S.H.; Validation, M.S.K., Z.S.H. and J.P.A.; Investigation, S.M.V.G. and Z.S.H.; Resources, J.P.A.; Writing—Original Draft Preparation, S.M.V.G.; Writing—Review and Editing, M.S.K., J.P.A. and Z.S.H.; Funding Acquisition, J.P.A. All authors have read and approved the final manuscript.
Funding
This research was funded by Agencia Nacional de Investigación y Desarrollo (ANID) and the Center of Interventional Medicine for Precision and Advanced Cellular Therapy (IMPACT), grant number 21200641 and FB210024, respectively.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors, on request.
Acknowledgments
The figures were created with Biorender.com and the licensing right were powered by Biorender. The authors would like to acknowledge L. Niño for her help/support to S.M.V.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- C. Magliaro, G. Mattei, F. Iacoangeli, A. Corti, V. Piemonte, and A. Ahluwalia, “Oxygen Consumption Characteristics in 3D Constructs Depend on Cell Density,” Front Bioeng Biotechnol, vol. 7, 2019. https://doi.org/10.3389/fbioe.2019.00251. [CrossRef]
- R. Cavalli et al., “Preparation and characterization of dextran nanobubbles for oxygen delivery,” Int J Pharm, vol. 381, no. 2, pp. 160–165, Nov. 2009. https://doi.org/10.1016/j.ijpharm.2009.07.010. [CrossRef]
- C. Magnetto et al., “Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues,” RSC Adv, vol. 4, no. 72, pp. 38433–38441, Sep. 2014. https://doi.org/10.1039/C4RA03524K. [CrossRef]
- C. Wigerup, S. Påhlman, and D. Bexell, “Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer,” Pharmacol Ther, vol. 164, pp. 152–169, Sep. 2016. https://doi.org/10.1016/j.pharmthera.2016.04.009. [CrossRef]
- V. T. S. Rao et al., “Potential Benefit of the Charge-Stabilized Nanostructure Saline RNS60 for Myelin Maintenance and Repair,” Sci Rep, vol. 6, p. 30020, Sep. 2016. https://doi.org/10.1038/srep30020. [CrossRef]
- J. N. Kheir et al., “Bulk manufacture of concentrated oxygen gas-filled microparticles for intravenous oxygen delivery,” Adv Healthc Mater, vol. 2, no. 8, pp. 1131–1141, Aug. 2013. https://doi.org/10.1002/adhm.201200350. [CrossRef]
- M. S. Khan et al., “Engineering oxygen nanobubbles for the effective reversal of hypoxia,” Artif Cells Nanomed Biotechnol, vol. 46, no. sup3, pp. S318–S327, 2018.
- T. Uchida et al., “Transmission electron microscopic observations of nanobubbles and their capture of impurities in wastewater,” Nanoscale Res Lett, vol. 6, no. 1, pp. 1–9, Dec. 2011. https://doi.org/10.1186/1556-276X-6-295. [CrossRef]
- W. B. Zimmerman, V. Tesař, and H. C. H. Bandulasena, “Towards energy efficient nanobubble generation with fluidic oscillation,” Curr Opin Colloid Interface Sci, vol. 16, no. 4, pp. 350–356, 2011. https://doi.org/10.1016/j.cocis.2011.01.010. [CrossRef]
- A. Agarwal, W. J. Ng, and Y. Liu, “Principle and applications of microbubble and nanobubble technology for water treatment,” Chemosphere, vol. 84, no. 9, pp. 1175–1180, Aug. 2011. https://doi.org/10.1016/j.chemosphere.2011.05.054. [CrossRef]
- N. Matsuki et al., “Blood oxygenation using microbubble suspensions,” Eur Biophys J, vol. 41, no. 6, pp. 571–578, Jun. 2012. https://doi.org/10.1007/s00249-012-0811-y. [CrossRef]
- M. Takahashi, K. Chiba, and P. Li, “Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus,” J Phys Chem B, vol. 111, no. 6, pp. 1343–1347, Feb. 2007. https://doi.org/10.1021/jp0669254. [CrossRef]
- J. C. Eriksson and S. Ljunggren, “On the mechanically unstable free energy minimum of a gas bubble which is submerged in water and adheres to a hydrophobic wall,” Colloids Surf A Physicochem Eng Asp, vol. 159, no. 1, pp. 159–163, Nov. 1999. https://doi.org/10.1016/S0927-7757(99)00171-5. [CrossRef]
- S. Ljunggren and J. C. Eriksson, “The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction,” Colloids Surf A Physicochem Eng Asp, vol. 129–130, pp. 151–155, Nov. 1997. https://doi.org/10.1016/S0927-7757(97)00033-2. [CrossRef]
- M. S. Khan et al., “Surface Composition and Preparation Method for Oxygen Nanobubbles for Drug Delivery and Ultrasound Imaging Applications,” Nanomaterials (Basel), vol. 9, no. 1, Jan. 2019. https://doi.org/10.3390/nano9010048. [CrossRef]
- H. Bitterman, “Bench-to-bedside review: oxygen as a drug,” Crit Care, vol. 13, no. 1, p. 205, 2009. https://doi.org/10.1186/cc7151. [CrossRef]
- S. Guo and L. A. DiPietro, “Factors Affecting Wound Healing,” Journal of Dental Research, vol. 89, no. 3. pp. 219–229, Mar. 2010. https://doi.org/10.1177/0022034509359125. [CrossRef]
- G. Chandra et al., “Increase in Mitochondrial Biogenesis in Neuronal Cells by RNS60, a Physically-Modified Saline, via Phosphatidylinositol 3-Kinase-Mediated Upregulation of PGC1α,” J Neuroimmune Pharmacol, vol. 13, no. 2, pp. 143–162, Sep. 2018. https://doi.org/10.1007/s11481-017-9771-4. [CrossRef]
- H.-Y. Qin, R. Mukherjee, E. Lee-Chan, C. Ewen, C. Bleackley, and B. Singh, “A novel mechanism of regulatory T cell-mediated down-regulation of autoimmunity”, Accessed: Sep. 18, 2021. [Online]. Available: http://intimm.oxfordjournals.org/.
- K. K. Modi, A. Jana, S. Ghosh, R. Watson, and K. Pahan, “A physically-modified saline suppresses neuronal apoptosis, attenuates tau phosphorylation and protects memory in an animal model of Alzheimer’s disease,” PLoS One, vol. 9, no. 8, p. e103606, 2014. https://doi.org/10.1371/journal.pone.0103606. [CrossRef]
- C. Mas-Bargues et al., “Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine,” Int J Mol Sci, vol. 20, p. 1195, Mar. 2019. https://doi.org/10.3390/ijms20051195. [CrossRef]
- D. Hirai et al., “Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte,” Microcirculation, vol. 26, no. 5, Jul. 2019.
- D. A. Chan and A. J. Giaccia, “Hypoxia, gene expression, and metastasis,” Cancer and Metastasis Reviews, vol. 26, no. 2, pp. 333–339, Jul. 2007. https://doi.org/10.1007/s10555-007-9063-1. [CrossRef]
- D. C. Fuhrmann and B. Brüne, “Mitochondrial composition and function under the control of hypoxia,” Redox Biol, vol. 12, pp. 208–215, 2017. https://doi.org/10.1016/j.redox.2017.02.012. [CrossRef]
- G. Solaini, A. Baracca, G. Lenaz, and G. Sgarbi, “Hypoxia and mitochondrial oxidative metabolism,” Biochim Biophys Acta Bioenerg, vol. 1797, no. 6–7, pp. 1171–1177, 2010. https://doi.org/10.1016/j.bbabio.2010.02.011. [CrossRef]
- J. Schödel, S. Oikonomopoulos, J. Ragoussis, C. W. Pugh, P. J. Ratcliffe, and D. R. Mole, “High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq,” Blood, vol. 117, no. 23, pp. e207–e217, 2011. https://doi.org/10.1182/blood-2010-10-314427. [CrossRef]
- C. J. Schofield and P. J. Ratcliffe, “Oxygen sensing by HIF hydroxylases,” Nat Rev Mol Cell Biol, vol. 5, no. 5, pp. 343–354, 2004. https://doi.org/10.1038/nrm1366. [CrossRef]
- S. Bhattacharya and P. J. Ratcliffe, “ExCITED about HIF,” Nat Struct Mol Biol, vol. 10, no. 7, pp. 501–503, 2003. https://doi.org/10.1038/nsb0703-501. [CrossRef]
- Y. Eui-Ju, “Hypoxia and aging,” Exp Mol Med, vol. 51, pp. 1–15, 2019.
- E. Y. Lukianova-Hleb, X. Ren, J. A. Zasadzinski, X. Wu, and D. O. Lapotko, “Plasmonic nanobubbles enhance efficacy and selectivity of chemotherapy against drug-resistant cancer cells,” Advanced Materials, vol. 24, no. 28, pp. 3831–3837, Jul. 2012. https://doi.org/10.1002/adma.201103550. [CrossRef]
- W. Um et al., “Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy,” Advanced Materials, vol. 32, no. 16, pp. e1907953-n/a, Apr. 2020, [Online]. Available: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201907953.
- S. Mondal, J. A. Martinson, S. Ghosh, R. Watson, and K. Pahan, “Protection of Tregs, Suppression of Th1 and Th17 Cells, and Amelioration of Experimental Allergic Encephalomyelitis by a Physically-Modified Saline,” PLoS One, vol. 7, no. 12, p. e51869, 2012. https://doi.org/10.1371/journal.pone.0051869. [CrossRef]
- S. Khasnavis et al., “Suppression of nuclear factor-κB activation and inflammation in microglia by physically modified saline,” J Biol Chem, vol. 287, no. 35, pp. 29529–29542, Aug. 2012. https://doi.org/10.1074/jbc.M111.338012. [CrossRef]
- M. T. L. G. S. W. A. B. & W. R. L. Choi J. C., “Electrokinetically Altered Normal Saline Modulates Ion Channel Activity.,” Biophys J, vol. 102, no. 3, p. 683a., 2012. https://doi.org/10.1016/j.bpj.2011.11.3712. [CrossRef]
- S. German et al., “Isotonic Saline Subjected to Taylor-Couette-Poiseuille Flow Demonstrates Anti-Inflammatory Activity In Vitro,” J. Allergy Clin. Immunol., vol. 127, no. 2, pp. AB260–AB260, 2011.
- K. A. A, G. Supurna, and W. R. L, “A SALINE-BASED THERAPEUTIC CONTAINING CHARGE-STABILIZED NANOSTRUCTURES PROTECTS AGAINST CARDIAC ISCHEMIA/REPERFUSION INJURY,” J Am Coll Cardiol, vol. 61, no. 10_Supplement, pp. E106–E106, Mar. 2013. https://doi.org/10.1016/S0735-1097(13)60107-2. [CrossRef]
- D. Batchelor, R. Hassan-Abou-Saleh, L. Coletta, J. McLaughlan, S. Peyman, and S. Evans, “Nested-Nanobubbles for Ultrasound Triggered Drug Release,” 2020.
- A. Prabhakar and R. Banerjee, “Nanobubble Liposome Complexes for Diagnostic Imaging and Ultrasound-Triggered Drug Delivery in Cancers: A Theranostic Approach,” ACS Omega, vol. 4, no. 13, pp. 15567–15580, Sep. 2019. https://doi.org/10.1021/acsomega.9b01924. [CrossRef]
- B. Tayier, Z. Deng, Y. Wang, W. Wang, Y. Mu, and F. Yan, “Biosynthetic nanobubbles for targeted gene delivery by focused ultrasound,” Nanoscale, vol. 11, no. 31, pp. 14757–14768, Aug. 2019. https://doi.org/10.1039/c9nr03402a. [CrossRef]
- L. R. Sayadi et al., “Topical oxygen therapy & micro/nanobubbles: a new modality for tissue oxygen delivery,” Int Wound J, vol. 15, no. 3, pp. 363–374, Jun. 2018. https://doi.org/10.1111/iwj.12873. [CrossRef]
- F. Y. Ushikubo et al., “Evidence of the existence and the stability of nano-bubbles in water,” Colloids Surf A Physicochem Eng Asp, vol. 361, no. 1–3, pp. 31–37, 2010. https://doi.org/10.1016/j.colsurfa.2010.03.005. [CrossRef]
- H. Y. Huang et al., “A multitheragnostic nanobubble system to induce blood-brain barrier disruption with magnetically guided focused ultrasound,” Advanced Materials, vol. 27, no. 4, pp. 655–661, Jan. 2015. https://doi.org/10.1002/adma.201403889. [CrossRef]
- C. Hernandez, L. Nieves, A. C. de Leon, R. Advincula, and A. A. Exner, “Role of Surface Tension in Gas Nanobubble Stability under Ultrasound,” ACS Appl Mater Interfaces, vol. 10, no. 12, pp. 9949–9956, Mar. 2018. https://doi.org/10.1021/acsami.7b19755. [CrossRef]
- M. S. Khan et al., “Oxygen-Carrying Micro/Nanobubbles: Composition, Synthesis Techniques and Potential Prospects in Photo-Triggered Theranostics,” Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry, vol. 23, no. 9, Sep. 2018, [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6225314/.
- D. Lohse, “Bubble puzzles: From fundamentals to applications,” Phys Rev Fluids, vol. 3, no. 11, p. 110504, Nov. 2018. https://doi.org/10.1103/PhysRevFluids.3.110504. [CrossRef]
- M. E. Burkard and H. D. Van-Liew, “Oxygen transport to tissue by persistent bubbles: theory and simulations,” 1994. [Online]. Available: www.physiology.org/journal/jappl.
- C. Yang et al., “Lipid Microbubbles as Ultrasound-Stimulated Oxygen Carriers for Controllable Oxygen Release for Tumor Reoxygenation,” Ultrasound Med Biol, vol. 44, no. 2, pp. 416–425, Sep. 2018. https://doi.org/10.1016/j.ultrasmedbio.2017.08.1883. [CrossRef]
- F. Cavalieri et al., “Polymer Microbubbles As Diagnostic and Therapeutic Gas Delivery Device,” Chemistry of materials, vol. 20, no. 10, pp. 3254–3258, May 2008. https://doi.org/10.1021/cm703702d. [CrossRef]
- J. J. Kwan, M. Kaya, M. A. Borden, and P. A. Dayton, “Theranostic oxygen delivery using ultrasound and microbubbles,” Theranostics, vol. 2, no. 12, pp. 1174–1184, 2012. https://doi.org/10.7150/thno.4410. [CrossRef]
- J. G. Riess, “Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery,” Artif Cells Blood Substit Immobil Biotechnol, vol. 33, no. 1, pp. 47–63, 2005. https://doi.org/10.1081/bio-200046659. [CrossRef]
- E. Stride and M. Edirisinghe, “Novel microbubble preparation technologies,” Soft Matter, vol. 4, no. 12, pp. 235–2359, Nov. 2008. https://doi.org/10.1039/b809517p. [CrossRef]
- B. Park, S. Yoon, Y. Choi, J. Jang, S. Park, and J. Choi, “Stability of Engineered Micro or Nanobubbles for Biomedical Applications,” Pharmaceutics, vol. 12, no. 11, Sep. 2020. https://doi.org/10.3390/pharmaceutics12111089. [CrossRef]
- K. Ebina et al., “Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice,” PLoS One, vol. 8, no. 6, p. e65339, 2013. https://doi.org/10.1371/journal.pone.0065339. [CrossRef]
- E. C. Unger, E. Hersh, M. Vannan, T. O. Matsunaga, and T. McCreery, “Local drug and gene delivery through microbubbles,” Prog Cardiovasc Dis, vol. 44, no. 1, pp. 45–54, Jul. 2001. https://doi.org/10.1053/pcad.2001.26443. [CrossRef]
- F. Kiessling, J. Huppert, and M. Palmowski, “Functional and molecular ultrasound imaging: concepts and contrast agents,” Curr Med Chem, vol. 16, no. 5, pp. 627–642, 2009. https://doi.org/10.2174/092986709787458470. [CrossRef]
- K. Bjerknes, P. C. Sontum, G. Smistad, and I. Agerkvist, “Preparation of polymeric microbubbles: formulation studies and product characterisation,” Int J Pharm, vol. 158, no. 2, pp. 129–136, 1997. https://doi.org/10.1016/S0378-5173(97)00228-7. [CrossRef]
- E. C. Unger, T. Porter, W. Culp, R. Labell, T. Matsunaga, and R. Zutshi, “Therapeutic applications of lipid-coated microbubbles,” Adv Drug Deliv Rev, vol. 56, no. 9, pp. 1291–1314, May 2004. https://doi.org/10.1016/j.addr.2003.12.006. [CrossRef]
- C. Yang et al., “Lipid Microbubbles as Ultrasound-Stimulated Oxygen Carriers for Controllable Oxygen Release for Tumor Reoxygenation,” Ultrasound Med Biol, vol. 44, no. 2, pp. 416–425, Feb. 2018. https://doi.org/10.1016/j.ultrasmedbio.2017.08.1883. [CrossRef]
- M. S. Khan et al., “Anti-tumor drug-loaded oxygen nanobubbles for the degradation of HIF-1α and the upregulation of reactive oxygen species in tumor cells,” Cancers (Basel), vol. 11, no. 10, Oct. 2019. https://doi.org/10.3390/cancers11101464. [CrossRef]
- R. Song, D. Hu, H. Y. Chung, Z. Sheng, and S. Yao, “Lipid-Polymer Bilaminar Oxygen Nanobubbles for Enhanced Photodynamic Therapy of Cancer,” ACS Appl Mater Interfaces, vol. 10, no. 43, pp. 36805–36813, Sep. 2018. https://doi.org/10.1021/acsami.8b15293. [CrossRef]
- R. Song et al., “pH-Responsive Oxygen Nanobubbles for Spontaneous Oxygen Delivery in Hypoxic Tumors,” Langmuir, vol. 35, no. 31, pp. 10166–10172, Aug. 2019. https://doi.org/10.1021/acs.langmuir.8b03650. [CrossRef]
- S. Choi et al., “Enhanced synaptic transmission at the squid giant synapse by artificial seawater based on physically modified saline,” Front Synaptic Neurosci, vol. 6, p. 2, 2014. https://doi.org/10.3389/fnsyn.2014.00002. [CrossRef]
- Q.-L. Cui et al., “Oligodendrocyte Progenitor Cell Susceptibility to Injury in Multiple Sclerosis,” American Journal of Pathology, The, vol. 183, no. 2, pp. 516–525, 2013. https://doi.org/10.1016/j.ajpath.2013.04.016. [CrossRef]
- S. Koyasu, “The role of PI3K in immune cells,” Nat Immunol, vol. 4, no. 4, pp. 313–319, 2003. https://doi.org/10.1038/ni0403-313. [CrossRef]
- S. K. & A. R. & S. G. & R. W. & K. Pahan, “ Protection of dopaminergic neurons in a mouse model of Parkinson’s disease by a physically-modified saline containing charge-stabilized nanobubbles. ,” J Neuroimmune Pharmacol, vol. 9, pp. 218–232, 2014.
- X. Luo et al., “Posttranslational regulation of PGC-1α and its implication in cancer metabolism,” Int J Cancer, vol. 145, no. 6, pp. 1475–1483, Sep. 2019. https://doi.org/10.1002/ijc.32253. [CrossRef]
- H.-C. Lee and Y.-H. Wei, “Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress,” Int J Biochem Cell Biol, vol. 37, no. 4, pp. 822–834, 2005. https://doi.org/10.1016/j.biocel.2004.09.010. [CrossRef]
- A. L. Kung, S. Wang, J. M. Klco, W. G. Kaelin, and D. M. Livingston, “Suppression of tumor growth through disruption of hypoxia-inducible transcription,” Nat Med, vol. 6, no. 12, pp. 1335–1340, 2000. https://doi.org/10.1038/82146. [CrossRef]
- M. Iijima et al., “Development of single nanometer-sized ultrafine oxygen bubbles to overcome the hypoxia-induced resistance to radiation therapy via the suppression of hypoxia-inducible factor-1α,” Int J Oncol, vol. 52, no. 3, pp. 679–686, Sep. 2018. https://doi.org/10.3892/ijo.2018.4248. [CrossRef]
- J. Owen et al., “Reducing Tumour Hypoxia via Oral Administration of Oxygen Nanobubbles,” PLoS One, vol. 11, no. 12, p. e0168088, 2016. https://doi.org/10.1371/journal.pone.0168088. [CrossRef]
- W. Zhao, X. Hu, J. Duan, T. Liu, M. Liu, and Y. Dong, “Oxygen release from nanobubbles adsorbed on hydrophobic particles,” Chem Phys Lett, vol. 608, pp. 224–228, Jul. 2014. https://doi.org/10.1016/j.cplett.2014.05.079. [CrossRef]
- P. N. Bhandari, Y. Cui, B. D. Elzey, C. J. Goergen, C. M. Long, and J. Irudayaraj, “Oxygen nanobubbles revert hypoxia by methylation programming,” Sci Rep, vol. 7, no. 1, pp. 9214–9268, 2017. https://doi.org/10.1038/s41598-017-08988-7. [CrossRef]
- C. Thirlwell, L. Schulz, H. Dibra, and S. Beck, “Suffocating cancer: hypoxia-associated epimutations as targets for cancer therapy,” Clin Epigenetics, vol. 3, no. 1, Dec. 2011. https://doi.org/10.1186/1868-7083-3-9. [CrossRef]
- S. Gonzalo, “HIGHLIGHTED TOPIC Epigenetics in Health and Disease Epigenetic alterations in aging,” J Appl Physiol, vol. 109, pp. 586–597, 2010. https://doi.org/10.1152/japplphysiol.00238.2010.-Aging. [CrossRef]
- K. Zhang et al., “Extravascular gelation shrinkage-derived internal stress enables tumor starvation therapy with suppressed metastasis and recurrence,” Nat Commun, vol. 10, no. 1, Dec. 2019. https://doi.org/10.1038/s41467-019-13115-3. [CrossRef]
- S. Ito et al., “Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine.”.
- B. Thienpont et al., “Tumour hypoxia causes DNA hypermethylation by reducing TET activity,” Nature, vol. 537, no. 7618, pp. 63–68, Aug. 2016. https://doi.org/10.1038/nature19081. [CrossRef]
- H. Cam et al., “p53 family members in myogenic differentiation and rhabdomyosarcoma development,” Cancer Cell, vol. 10, no. 4, pp. 281–293, 2006. https://doi.org/10.1016/j.ccr.2006.08.024. [CrossRef]
- T. Abbas and A. Dutta, “P21 in cancer: Intricate networks and multiple activities,” Nature Reviews Cancer, vol. 9, no. 6. Nature Publishing Group, pp. 400–414, 2009. https://doi.org/10.1038/nrc2657. [CrossRef]
- Y. Zhao et al., “Aberration of p73 promoter methylation in de novo myelodysplastic syndrome,” Hematology, vol. 17, no. 5, pp. 275–282, Sep. 2012. https://doi.org/10.1179/1607845412Y.0000000018. [CrossRef]
- Y. Li et al., “Aberrant DNA methylation of p57KIP2 gene in the promoter region in lymphoid malignancies of B-cell phenotype,” Blood, vol. 100, no. 7, pp. 2572–2577, Oct. 2002. https://doi.org/10.1182/blood-2001-11-0026. [CrossRef]
- C. McEwan et al., “Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours,” J Control Release, vol. 203, pp. 51–56, Sep. 2015. https://doi.org/10.1016/j.jconrel.2015.02.004. [CrossRef]
- T. Luo et al., “Ultrasound-mediated destruction of oxygen and paclitaxel loaded dual-targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts,” Cancer Lett, vol. 391, pp. 1–11, Sep. 2017. https://doi.org/10.1016/j.canlet.2016.12.032. [CrossRef]
- A. Bisazza et al., “Microbubble-mediated oxygen delivery to hypoxic tissues as a new therapeutic device,” Annu Int Conf IEEE Eng Med Biol Soc, vol. 2008, pp. 2067–2070, 2008. https://doi.org/10.1109/IEMBS.2008.4649599. [CrossRef]
- P. Bhandari, G. Novikova, C. J. Goergen, and J. Irudayaraj, “Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy,” Sci Rep, vol. 8, no. 1, pp. 3110–3112, 2018. https://doi.org/10.1038/s41598-018-20363-8. [CrossRef]
- J. Zhang et al., “The optimized fabrication of a novel nanobubble for tumor imaging,” Front Pharmacol, vol. 10, no. MAY, 2019. https://doi.org/10.3389/fphar.2019.00610. [CrossRef]
- Y. Feng et al., “Ultrasound Molecular Imaging of Bladder Cancer via Extradomain B Fibronectin-Targeted Biosynthetic GVs,” Int J Nanomedicine, vol. 18, pp. 4871–4884, 2023. https://doi.org/10.2147/IJN.S412422. [CrossRef]
- A. Vallarola et al., “RNS60 exerts therapeutic effects in the SOD1 ALS mouse model through protective glia and peripheral nerve rescue,” J Neuroinflammation, vol. 15, no. 1, p. 65, Sep. 2018. https://doi.org/10.1186/s12974-018-1101-0. [CrossRef]
- S. Paganoni et al., “A pilot trial of RNS60 in amyotrophic lateral sclerosis,” Muscle Nerve, vol. 59, no. 3, pp. 303–308, Sep. 2019. https://doi.org/10.1002/mus.26385. [CrossRef]
- M. Jana, M. Jana, S. Ghosh, S. Ghosh, K. Pahan, and K. Pahan, “Upregulation of Myelin Gene Expression by a Physically-Modified Saline via Phosphatidylinositol 3-Kinase-Mediated Activation of CREB: Implications for Multiple Sclerosis,” Neurochem Res, vol. 43, no. 2, pp. 407–419, Feb. 2018. https://doi.org/10.1007/s11064-017-2435-1. [CrossRef]
- S. Mondal, S. B. Rangasamy, S. Ghosh, R. L. Watson, and K. Pahan, “Nebulization of RNS60, a Physically-Modified Saline, Attenuates the Adoptive Transfer of Experimental Allergic Encephalomyelitis in Mice: Implications for Multiple Sclerosis Therapy,” Neurochem Res, vol. 42, no. 5, pp. 1555–1570, Sep. 2017. https://doi.org/10.1007/s11064-017-2214-z. [CrossRef]
- N. D. Legband, J. A. Feshitan, M. A. Borden, and B. S. Terry, “Evaluation of peritoneal microbubble oxygenation therapy in a rabbit model of hypoxemia,” IEEE Trans Biomed Eng, vol. 62, no. 5, pp. 1376–1382, May 2015. https://doi.org/10.1109/TBME.2015.2388611. [CrossRef]
- N. D. Legband, J. A. Feshitan, M. A. Borden, and B. S. Terry, “Evaluation of peritoneal microbubble oxygenation therapy in a rabbit model of hypoxemia,” IEEE Trans Biomed Eng, vol. 62, no. 5, pp. 1376–1382, Sep. 2015. https://doi.org/10.1109/TBME.2015.2388611. [CrossRef]
- J. N. Kheir et al., “Oxygen gas-filled microparticles provide intravenous oxygen delivery,” Sci Transl Med, vol. 4, no. 140, p. 140ra88, Sep. 2012. https://doi.org/10.1126/scitranslmed.3003679. [CrossRef]
- N. Matsuki, T. Ishikawa, S. Ichiba, N. Shiba, Y. Ujike, and T. Yamaguchi, “Oxygen supersaturated fluid using fine micro/nanobubbles,” Int J Nanomedicine, vol. 9, pp. 4495–4505, 2014. https://doi.org/10.2147/IJN.S68840. [CrossRef]
- R. P. Seekell et al., “Oxygen delivery using engineered microparticles,” Proc Natl Acad Sci U S A, vol. 113, no. 44, pp. 12380–12385, Sep. 2016. https://doi.org/10.1073/pnas.1608438113. [CrossRef]
- E. J. SWANSON and M. A. BORDEN, “INJECTABLE OXYGEN DELIVERY BASED ON PROTEIN-SHELLED MICROBUBBLES,” Nano Life, vol. 01, no. 03n04, pp. 215–218, Sep. 2010. https://doi.org/10.1142/s1793984410000195. [CrossRef]
- J. Zhang et al., “Ultrasound Imaging and Antithrombotic Effects of PLA-Combined Fe3O4-GO-ASA Multifunctional Nanobubbles,” Front Med (Lausanne), vol. 8, May 2021. https://doi.org/10.3389/fmed.2021.576422. [CrossRef]
- A. Kumar Vutha, R. Patenaude, A. Cole, R. Kumar, J. N. Kheir, and B. D. Polizzotti, “A microfluidic device for real-time on-demand intravenous oxygen delivery,” 2022. https://doi.org/10.1073/pnas. [CrossRef]
- Y.-J. Ho, H.-L. Cheng, L.-D. Liao, Y.-C. Lin, H.-C. Tsai, and C.-K. Yeh, “Oxygen-loaded microbubble-mediated sonoperfusion and oxygenation for neuroprotection after ischemic stroke reperfusion,” Biomater Res, vol. 27, no. 1, p. 65, Jul. 2023. https://doi.org/10.1186/s40824-023-00400-y. [CrossRef]
- R. Cavalli et al., “Ultrasound-mediated oxygen delivery from chitosan nanobubbles,” Int J Pharm, vol. 378, no. 1–2, pp. 215–217, Sep. 2009. https://doi.org/10.1016/j.ijpharm.2009.05.058. [CrossRef]
- V. Messerschmidt, W. Ren, M. Tsipursky, and J. Irudayaraj, “Characterization of Oxygen Nanobubbles and In Vitro Evaluation of Retinal Cells in Hypoxia,” Transl Vis Sci Technol, vol. 12, no. 2, Feb. 2023. https://doi.org/10.1167/tvst.12.2.16. [CrossRef]
- M. Prato et al., “2H,3H-decafluoropentane-based nanodroplets: new perspectives for oxygen delivery to hypoxic cutaneous tissues,” PLoS One, vol. 10, no. 3, p. e0119769, 2015. https://doi.org/10.1371/journal.pone.0119769. [CrossRef]
- S. Shao et al., “Layer-by-Layer Assembly of Lipid Nanobubbles on Microneedles for Ultrasound-Assisted Transdermal Drug Delivery,” ACS Appl Bio Mater, vol. 5, no. 2, pp. 562–569, Feb. 2022. https://doi.org/10.1021/acsabm.1c01049. [CrossRef]
- K. Hettiarachchi, E. Talu, M. L. Longo, P. A. Dayton, and A. P. Lee, “On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging,” Lab Chip, vol. 7, no. 4, pp. 463–468, 2007. https://doi.org/10.1039/b701481n. [CrossRef]
Figure 1.
OnB (oxygen nanobubbles) and biomedicine research. (A) The number of articles published each year retrieved through a scientific search engine (Web of Science) using the terms "oxygen nanobubble" and "biomedical". (B) The percentage of OnB articles published then categorized by indication. (C) Illustrating drug/gas delivery based on OnB and their commonly-investigated administration routes for the management of diseases.
Figure 1.
OnB (oxygen nanobubbles) and biomedicine research. (A) The number of articles published each year retrieved through a scientific search engine (Web of Science) using the terms "oxygen nanobubble" and "biomedical". (B) The percentage of OnB articles published then categorized by indication. (C) Illustrating drug/gas delivery based on OnB and their commonly-investigated administration routes for the management of diseases.
Figure 2.
O2 need depends on cell niche amongst other demands. (A) Sites that are distant from major blood vessels are usually kept at a lower O2 level. However, low PO2 might still be higher than the PO2 of truly hypoxic conditions <16 mmHg (<2% O2). (B) Hypoxia acts on mitochondrial function, affecting the protein composition of the electron transport chain, mitochondrial morphology, and mitochondrial mass. (C) Hypoxia regulates metabolic adaptation, promotes glycolysis, and decreases mitochondrial respiration.
Figure 2.
O2 need depends on cell niche amongst other demands. (A) Sites that are distant from major blood vessels are usually kept at a lower O2 level. However, low PO2 might still be higher than the PO2 of truly hypoxic conditions <16 mmHg (<2% O2). (B) Hypoxia acts on mitochondrial function, affecting the protein composition of the electron transport chain, mitochondrial morphology, and mitochondrial mass. (C) Hypoxia regulates metabolic adaptation, promotes glycolysis, and decreases mitochondrial respiration.
Figure 3.
(A) The functional interplay of the HiF pathway in response to different oxygen concentrations. Herein, under hypoxic conditions, PHD is inhibited and HiF-α is stabilized, which then translocates to the nucleus, dimerizes with its corresponding β-sub-unit, and recognizes the HREs motifs to then induce the transcription of several target genes. On the other hand, when under normoxia, HiF1α-subunit prolyl residues are continuously hydroxylated by PHD. Following hydroxylation, pVHL binds and target HiF-1 α-subunit for ubiquitination and proteasomal degradation. (B) Hypoxic signaling and HiF-1α over-expression: a key hallmark in cancer progression. (C) Schematic representation of the structure and composition of engineering shelled micro-/nano-bubble systems.
Figure 3.
(A) The functional interplay of the HiF pathway in response to different oxygen concentrations. Herein, under hypoxic conditions, PHD is inhibited and HiF-α is stabilized, which then translocates to the nucleus, dimerizes with its corresponding β-sub-unit, and recognizes the HREs motifs to then induce the transcription of several target genes. On the other hand, when under normoxia, HiF1α-subunit prolyl residues are continuously hydroxylated by PHD. Following hydroxylation, pVHL binds and target HiF-1 α-subunit for ubiquitination and proteasomal degradation. (B) Hypoxic signaling and HiF-1α over-expression: a key hallmark in cancer progression. (C) Schematic representation of the structure and composition of engineering shelled micro-/nano-bubble systems.
Figure 4.
O2 nanobubble effect on mitochondrial bio-genesis, apoptosis, and metabolism. (A) Upregulation of PGC1α and ATP production in RNS60-treated MN9D mouse neuronal cells. (B) Cells treated with RNS60 (10% v/v) and NS (10% v/v) under serum-free condition for 24h. (C) RNS60, not NS, upregulated the number of mitochondria and (D) increased the mitochondrial diameter and (E) area in MN9D neuronal cells. (F) Glucose deprivation model in oligodendrocyte progenitor cells (OPCs) to assess OnB effect under metabolic stress. (G) Graph depicts the average % of total cells that were double positive for caspase3/7 and O4, and (H) Graph depicts the influence of RNS60 on OCR vs ECAR; Spare Respiratory Capacity vs. Spare Glycolytic Capacity in OLs cultured under optimal and glucose starvation conditions. NG RNS60: No-glucose with 10% v/v RNS60, NG: No-glucose, G: with glucose. OCR: Oxygen consumption rate, ECAR: Extra-cellular acidification rate.
Figure 4.
O2 nanobubble effect on mitochondrial bio-genesis, apoptosis, and metabolism. (A) Upregulation of PGC1α and ATP production in RNS60-treated MN9D mouse neuronal cells. (B) Cells treated with RNS60 (10% v/v) and NS (10% v/v) under serum-free condition for 24h. (C) RNS60, not NS, upregulated the number of mitochondria and (D) increased the mitochondrial diameter and (E) area in MN9D neuronal cells. (F) Glucose deprivation model in oligodendrocyte progenitor cells (OPCs) to assess OnB effect under metabolic stress. (G) Graph depicts the average % of total cells that were double positive for caspase3/7 and O4, and (H) Graph depicts the influence of RNS60 on OCR vs ECAR; Spare Respiratory Capacity vs. Spare Glycolytic Capacity in OLs cultured under optimal and glucose starvation conditions. NG RNS60: No-glucose with 10% v/v RNS60, NG: No-glucose, G: with glucose. OCR: Oxygen consumption rate, ECAR: Extra-cellular acidification rate.
Figure 5.
Upregulation of PGC1α and mitochondrial bio-genesis via PI3K/CREB in neuronal cells exposed to OnB. Cells were treated with 10% v/v RNS60 or NS for different minute intervals or 2 hrs under serum-free condition followed by monitoring the levels of PI3K (p110), phospho(P)-CREB, PGC1a, NRF1 and TFAM. (A) Illustrating OnB as modulators of transmembrane proteins and cellular signaling pathways. (B) Scheme of PI3K activation and levels of PI3K subunits p110α, p110β and p110γ in cell membranes by Western blot analysis. Pan cadherin was run as a membrane marker. Data values (p110α/pan Cad, p110β/pan Cad and p110γ/pan Cad) are expressed as relative to control: p-value < 0.001 vs control. (C) Pgc1α gene promoter analysis shows the presence of a consensus cAMP response element (CRE) near the transcription start site (TSS). (D) Scheme of the upregulation of PGC1α, its upstream regulation (via PI3K/PDK1/AKT/CREB), their post-translation modifications (phosphorylation, acetylation) and its co-transcription via NRF1/2 to promote mitochondrial bio-genesis following an OnB treatment. (E) The mRNA expression of Pgc1α, NRF1 and TFAM by semi-quantitative RT-PCR. (F) Relative Pgc1a; (G), Relative NRF1; (H) TFAM: limited evidence and further studies are required.
Figure 5.
Upregulation of PGC1α and mitochondrial bio-genesis via PI3K/CREB in neuronal cells exposed to OnB. Cells were treated with 10% v/v RNS60 or NS for different minute intervals or 2 hrs under serum-free condition followed by monitoring the levels of PI3K (p110), phospho(P)-CREB, PGC1a, NRF1 and TFAM. (A) Illustrating OnB as modulators of transmembrane proteins and cellular signaling pathways. (B) Scheme of PI3K activation and levels of PI3K subunits p110α, p110β and p110γ in cell membranes by Western blot analysis. Pan cadherin was run as a membrane marker. Data values (p110α/pan Cad, p110β/pan Cad and p110γ/pan Cad) are expressed as relative to control: p-value < 0.001 vs control. (C) Pgc1α gene promoter analysis shows the presence of a consensus cAMP response element (CRE) near the transcription start site (TSS). (D) Scheme of the upregulation of PGC1α, its upstream regulation (via PI3K/PDK1/AKT/CREB), their post-translation modifications (phosphorylation, acetylation) and its co-transcription via NRF1/2 to promote mitochondrial bio-genesis following an OnB treatment. (E) The mRNA expression of Pgc1α, NRF1 and TFAM by semi-quantitative RT-PCR. (F) Relative Pgc1a; (G), Relative NRF1; (H) TFAM: limited evidence and further studies are required.
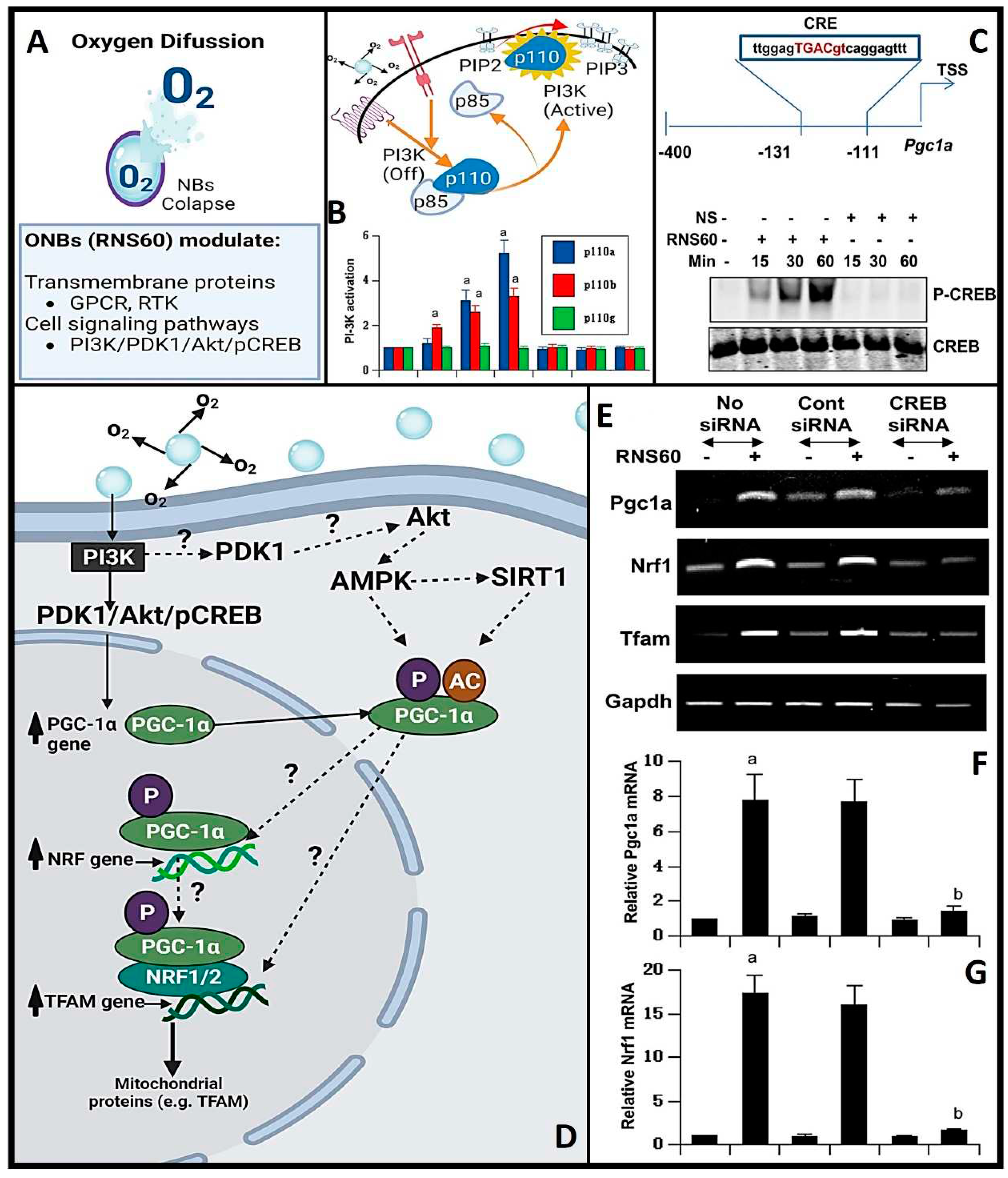
Figure 6.
The in vitro and in vivo effect of OnB on modulating the hypoxic response(s) in/within the tumoral micro-environment (TME). (A) in vitro application of OnB for the reversal of hypoxia and degradation of HiF-1α expression. (B-C) Image-iT fluorescence of MDA-MB-231 cells under hypoxic conditions - scale bar=20 µm. (D-F) Schematic representation of OnB in vivo administration and its effects on Oxygen pressure: HiF-1Alpha and VEGF (mRNA and protein levels). (G) Schematic representation illustrating a lipid-shelled or cellulose-coated OnB and its in vitro application for the reversal of hypoxia and degradation of HiF-1alpha alongside a comparison of HIF-1a expression and Image-iT fluorescence of MDA-MB-231 cells in control and after the hypoxic conditions reversal. (H) OnB therapy triggers a significant reduction in promoter methylation for 11 of these 22 genes.
Figure 6.
The in vitro and in vivo effect of OnB on modulating the hypoxic response(s) in/within the tumoral micro-environment (TME). (A) in vitro application of OnB for the reversal of hypoxia and degradation of HiF-1α expression. (B-C) Image-iT fluorescence of MDA-MB-231 cells under hypoxic conditions - scale bar=20 µm. (D-F) Schematic representation of OnB in vivo administration and its effects on Oxygen pressure: HiF-1Alpha and VEGF (mRNA and protein levels). (G) Schematic representation illustrating a lipid-shelled or cellulose-coated OnB and its in vitro application for the reversal of hypoxia and degradation of HiF-1alpha alongside a comparison of HIF-1a expression and Image-iT fluorescence of MDA-MB-231 cells in control and after the hypoxic conditions reversal. (H) OnB therapy triggers a significant reduction in promoter methylation for 11 of these 22 genes.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).