Submitted:
14 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
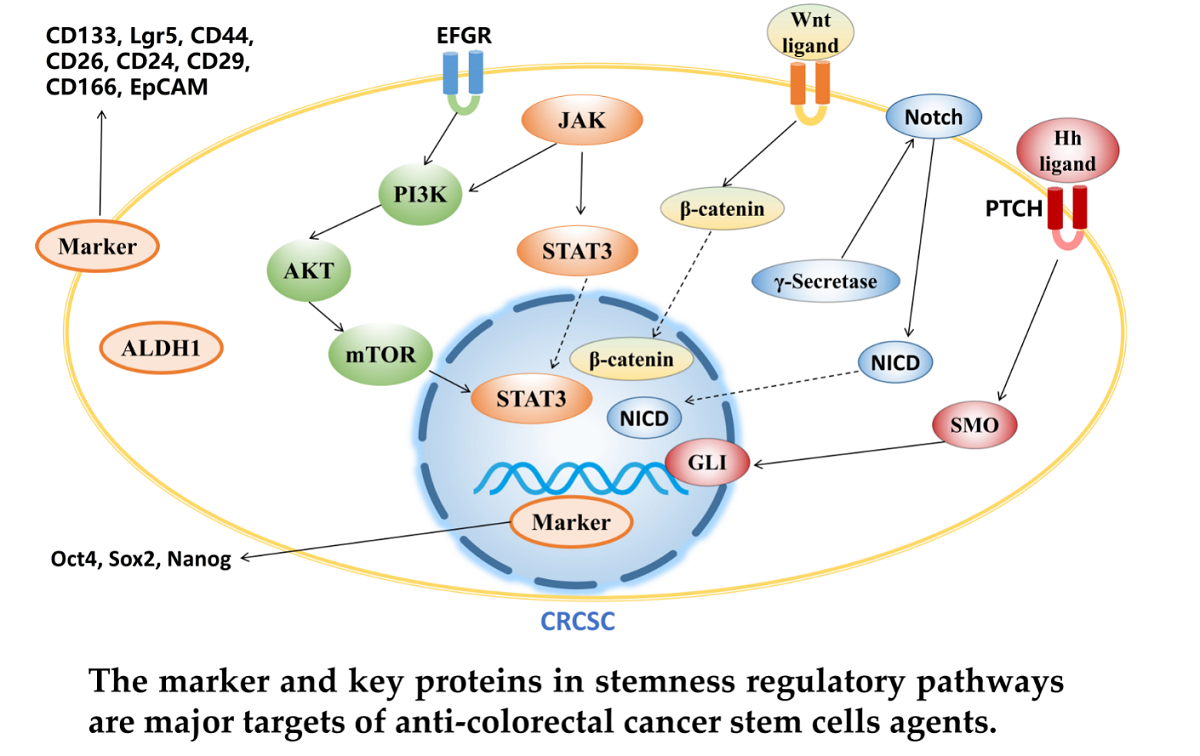
Keywords:
1. Introduction
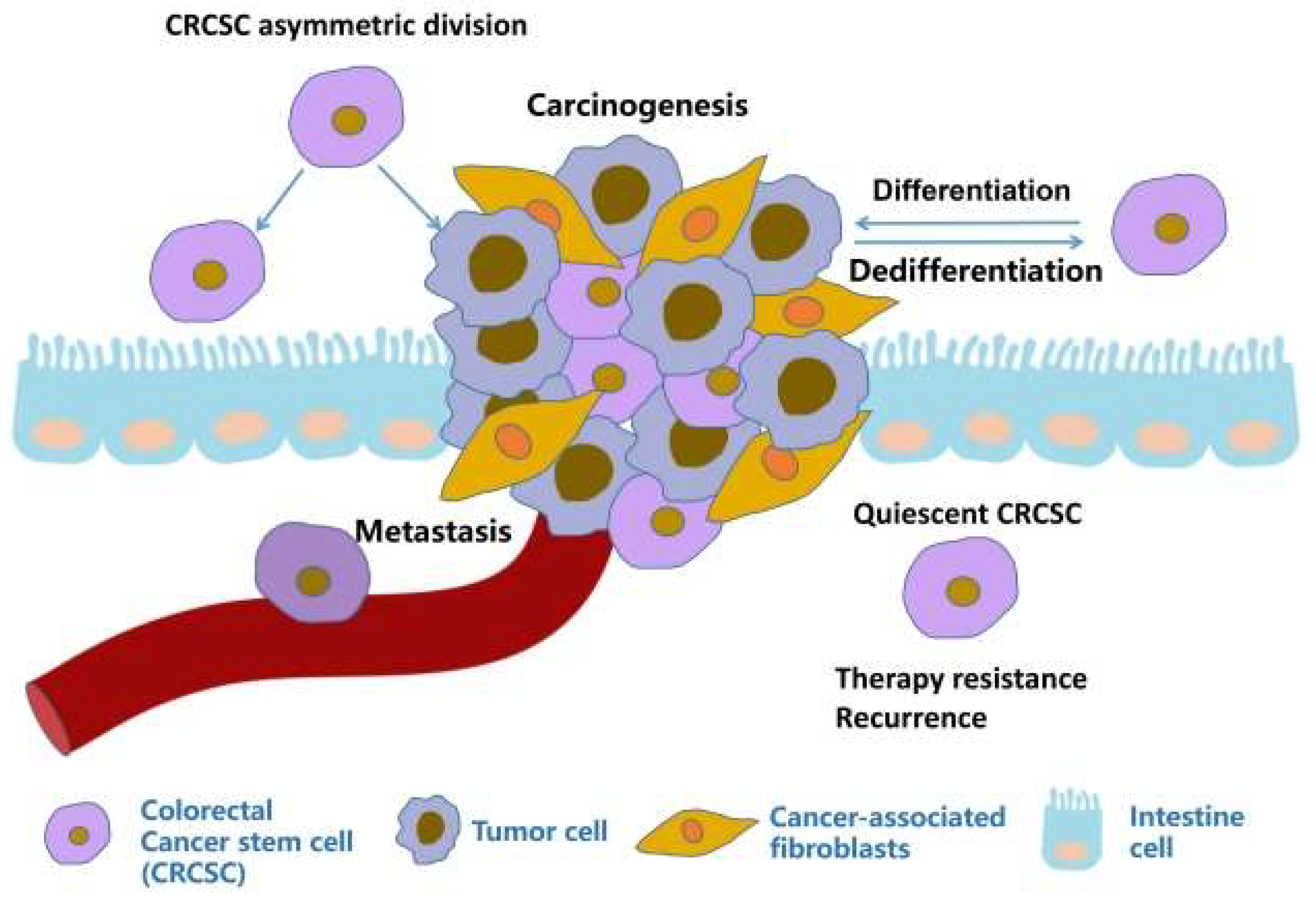
2. Colorectal cancer stem cells
2.1. Origin of CRCSCs
2.2. Identification of CRCSCs
2.3. Cultivation of CRCSCs
3. Agents targeting CRCSCs
3.1. Targeting CRCSC markers
3.2. Targeting signaling pathway
3.2.1. Wnt signaling pathway
3.2.2. Hedgehog signaling pathway
3.2.3. Notch signaling pathway
3.2.4. PI3K/Akt/mTOR signaling pathway
3.2.5. JAK/STAT3 signaling pathway
3.3. Other agents targeting CRCSCs
4. Future prospect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M. A.; Dick, J. E., A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, (6464), 645-8. [CrossRef]
- Dontu, G.; Al-Hajj, M.; Abdallah, W. M.; Clarke, M. F.; Wicha, M. S., Stem cells in normal breast development and breast cancer. Cell proliferation 2003, 36 Suppl 1, (Suppl 1), 59-72.
- Singh, S. K.; Clarke, I. D.; Terasaki, M.; Bonn, V. E.; Hawkins, C.; Squire, J.; Dirks, P. B., Identification of a cancer stem cell in human brain tumors. Cancer research 2003, 63, (18), 5821-8.
- Maitland, N. J.; Collins, A., A tumour stem cell hypothesis for the origins of prostate cancer. BJU international 2005, 96, (9), 1219-23. [CrossRef]
- Lam, J. S.; Yamashiro, J.; Shintaku, I. P.; Vessella, R. L.; Jenkins, R. B.; Horvath, S.; Said, J. W.; Reiter, R. E., Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clinical cancer research : an official journal of the American Association for Cancer Research 2005, 11, (7), 2591-6.
- O'Brien, C. A.; Pollett, A.; Gallinger, S.; Dick, J. E., A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, (7123), 106-10.
- Boman, B. M.; Wicha, M. S., Cancer stem cells: a step toward the cure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008, 26, (17), 2795-9.
- Reya, T.; Morrison, S. J.; Clarke, M. F.; Weissman, I. L., Stem cells, cancer, and cancer stem cells. Nature 2001, 414, (6859), 105-11.
- Chen, K.; Huang, Y. H.; Chen, J. L., Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta pharmacologica Sinica 2013, 34, (6), 732-40. [CrossRef]
- Chaffer, C. L.; Brueckmann, I.; Scheel, C.; Kaestli, A. J.; Wiggins, P. A.; Rodrigues, L. O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; Arendt, L. M.; Kuperwasser, C.; Bierie, B.; Weinberg, R. A., Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, (19), 7950-5.
- Prasetyanti, P. R.; Medema, J. P., Intra-tumor heterogeneity from a cancer stem cell perspective. Molecular cancer 2017, 16, (1), 41. [CrossRef]
- Bütof, R.; Dubrovska, A.; Baumann, M., Clinical perspectives of cancer stem cell research in radiation oncology. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2013, 108, (3), 388-96.
- Rich, J. N., Cancer stem cells in radiation resistance. Cancer research 2007, 67, (19), 8980-4. [CrossRef]
- Hermann, P. C.; Huber, S. L.; Herrler, T.; Aicher, A.; Ellwart, J. W.; Guba, M.; Bruns, C. J.; Heeschen, C., Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell 2007, 1, (3), 313-23.
- Oskarsson, T.; Batlle, E.; Massagué, J., Metastatic stem cells: sources, niches, and vital pathways. Cell stem cell 2014, 14, (3), 306-21.
- Abdullah, L. N.; Chow, E. K., Mechanisms of chemoresistance in cancer stem cells. Clinical and translational medicine 2013, 2, (1), 3. [CrossRef]
- Yao, J.; Liu, Y.; Yang, J.; Li, M.; Li, S.; Zhang, B.; Yang, R.; Zhang, Y.; Cui, X.; Feng, C., Single-Cell Sequencing Reveals that DBI is the Key Gene and Potential Therapeutic Target in Quiescent Bladder Cancer Stem Cells. Frontiers in genetics 2022, 13, 904536. [CrossRef]
- Antonica, F.; Santomaso, L.; Pernici, D.; Petrucci, L.; Aiello, G.; Cutarelli, A.; Conti, L.; Romanel, A.; Miele, E.; Tebaldi, T.; Tiberi, L., A slow-cycling/quiescent cells subpopulation is involved in glioma invasiveness. Nature communications 2022, 13, (1), 4767. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, (3), 209-249.
- Marhaba, R.; Klingbeil, P.; Nuebel, T.; Nazarenko, I.; Buechler, M. W.; Zoeller, M., CD44 and EpCAM: cancer-initiating cell markers. Current molecular medicine 2008, 8, (8), 784-804. [CrossRef]
- Gaiser, M. R.; Lämmermann, T.; Feng, X.; Igyarto, B. Z.; Kaplan, D. H.; Tessarollo, L.; Germain, R. N.; Udey, M. C., Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, (15), E889-97. [CrossRef]
- Cheung, P.; Xiol, J.; Dill, M. T.; Yuan, W. C.; Panero, R.; Roper, J.; Osorio, F. G.; Maglic, D.; Li, Q.; Gurung, B.; Calogero, R. A.; Yilmaz Ö, H.; Mao, J.; Camargo, F. D., Regenerative Reprogramming of the Intestinal Stem Cell State via Hippo Signaling Suppresses Metastatic Colorectal Cancer. Cell stem cell 2020, 27, (4), 590-604.e9.
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T., Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 2017, 545, (7653), 187-192. [CrossRef]
- de Sousa e Melo, F.; Kurtova, A. V.; Harnoss, J. M.; Kljavin, N.; Hoeck, J. D.; Hung, J.; Anderson, J. E.; Storm, E. E.; Modrusan, Z.; Koeppen, H.; Dijkgraaf, G. J.; Piskol, R.; de Sauvage, F. J., A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017, 543, (7647), 676-680. [CrossRef]
- Wang, H.; Gong, P.; Chen, T.; Gao, S.; Wu, Z.; Wang, X.; Li, J.; Marjani, S. L.; Costa, J.; Weissman, S. M.; Qi, F.; Pan, X.; Liu, L., Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2021, 8, (8), 2004320. [CrossRef]
- Ohta, Y.; Fujii, M.; Takahashi, S.; Takano, A.; Nanki, K.; Matano, M.; Hanyu, H.; Saito, M.; Shimokawa, M.; Nishikori, S.; Hatano, Y.; Ishii, R.; Sawada, K.; Machinaga, A.; Ikeda, W.; Imamura, T.; Sato, T., Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature 2022, 608, (7924), 784-794. [CrossRef]
- Perekatt, A. O.; Shah, P. P.; Cheung, S.; Jariwala, N.; Wu, A.; Gandhi, V.; Kumar, N.; Feng, Q.; Patel, N.; Chen, L.; Joshi, S.; Zhou, A.; Taketo, M. M.; Xing, J.; White, E.; Gao, N.; Gatza, M. L.; Verzi, M. P., SMAD4 Suppresses WNT-Driven Dedifferentiation and Oncogenesis in the Differentiated Gut Epithelium. Cancer research 2018, 78, (17), 4878-4890.
- Mani, S. A.; Guo, W.; Liao, M. J.; Eaton, E. N.; Ayyanan, A.; Zhou, A. Y.; Brooks, M.; Reinhard, F.; Zhang, C. C.; Shipitsin, M.; Campbell, L. L.; Polyak, K.; Brisken, C.; Yang, J.; Weinberg, R. A., The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, (4), 704-15.
- Barker, N.; van Es, J. H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P. J.; Clevers, H., Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, (7165), 1003-7.
- Barker, N.; Ridgway, R. A.; van Es, J. H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A. R.; Sansom, O. J.; Clevers, H., Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, (7229), 608-11.
- Llado, V.; Nakanishi, Y.; Duran, A.; Reina-Campos, M.; Shelton, P. M.; Linares, J. F.; Yajima, T.; Campos, A.; Aza-Blanc, P.; Leitges, M.; Diaz-Meco, M. T.; Moscat, J., Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of β-Catenin and Yap by PKCζ. Cell reports 2015, 10, (5), 740-754. [CrossRef]
- Fumagalli, A.; Oost, K. C.; Kester, L.; Morgner, J.; Bornes, L.; Bruens, L.; Spaargaren, L.; Azkanaz, M.; Schelfhorst, T.; Beerling, E.; Heinz, M. C.; Postrach, D.; Seinstra, D.; Sieuwerts, A. M.; Martens, J. W. M.; van der Elst, S.; van Baalen, M.; Bhowmick, D.; Vrisekoop, N.; Ellenbroek, S. I. J.; Suijkerbuijk, S. J. E.; Snippert, H. J.; van Rheenen, J., Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell stem cell 2020, 26, (4), 569-578.e7. [CrossRef]
- Morral, C.; Stanisavljevic, J.; Hernando-Momblona, X.; Mereu, E.; Álvarez-Varela, A.; Cortina, C.; Stork, D.; Slebe, F.; Turon, G.; Whissell, G.; Sevillano, M.; Merlos-Suárez, A.; Casanova-Martí, À.; Moutinho, C.; Lowe, S. W.; Dow, L. E.; Villanueva, A.; Sancho, E.; Heyn, H.; Batlle, E., Zonation of Ribosomal DNA Transcription Defines a Stem Cell Hierarchy in Colorectal Cancer. Cell stem cell 2020, 26, (6), 845-861.e12. [CrossRef]
- Han, T.; Goswami, S.; Hu, Y.; Tang, F.; Zafra, M. P.; Murphy, C.; Cao, Z.; Poirier, J. T.; Khurana, E.; Elemento, O.; Hechtman, J. F.; Ganesh, K.; Yaeger, R.; Dow, L. E., Lineage Reversion Drives WNT Independence in Intestinal Cancer. Cancer discovery 2020, 10, (10), 1590-1609.
- Vasquez, E. G.; Nasreddin, N.; Valbuena, G. N.; Mulholland, E. J.; Belnoue-Davis, H. L.; Eggington, H. R.; Schenck, R. O.; Wouters, V. M.; Wirapati, P.; Gilroy, K.; Lannagan, T. R. M.; Flanagan, D. J.; Najumudeen, A. K.; Omwenga, S.; McCorry, A. M. B.; Easton, A.; Koelzer, V. H.; East, J. E.; Morton, D.; Trusolino, L.; Maughan, T.; Campbell, A. D.; Loughrey, M. B.; Dunne, P. D.; Tsantoulis, P.; Huels, D. J.; Tejpar, S.; Sansom, O. J.; Leedham, S. J., Dynamic and adaptive cancer stem cell population admixture in colorectal neoplasia. Cell stem cell 2022, 29, (8), 1213-1228.e8.
- Makena, M. R.; Ranjan, A.; Thirumala, V.; Reddy, A. P., Cancer stem cells: Road to therapeutic resistance and strategies to overcome resistance. Biochimica et biophysica acta. Molecular basis of disease 2020, 1866, (4), 165339. [CrossRef]
- Sato, T.; Vries, R. G.; Snippert, H. J.; van de Wetering, M.; Barker, N.; Stange, D. E.; van Es, J. H.; Abo, A.; Kujala, P.; Peters, P. J.; Clevers, H., Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, (7244), 262-5.
- Ricci-Vitiani, L.; Lombardi, D. G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R., Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, (7123), 111-5.
- Li, G.; Liu, C.; Yuan, J.; Xiao, X.; Tang, N.; Hao, J.; Wang, H.; Bian, X.; Deng, Y.; Ding, Y., CD133(+) single cell-derived progenies of colorectal cancer cell line SW480 with different invasive and metastatic potential. Clinical & experimental metastasis 2010, 27, (7), 517-27. [CrossRef]
- Greve, B.; Kelsch, R.; Spaniol, K.; Eich, H. T.; Götte, M., Flow cytometry in cancer stem cell analysis and separation. Cytometry. Part A : the journal of the International Society for Analytical Cytology 2012, 81, (4), 284-93.
- Pang, R.; Law, W. L.; Chu, A. C.; Poon, J. T.; Lam, C. S.; Chow, A. K.; Ng, L.; Cheung, L. W.; Lan, X. R.; Lan, H. Y.; Tan, V. P.; Yau, T. C.; Poon, R. T.; Wong, B. C., A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell stem cell 2010, 6, (6), 603-15. [CrossRef]
- Choi, D.; Lee, H. W.; Hur, K. Y.; Kim, J. J.; Park, G. S.; Jang, S. H.; Song, Y. S.; Jang, K. S.; Paik, S. S., Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World journal of gastroenterology 2009, 15, (18), 2258-64. [CrossRef]
- Fujimoto, K.; Beauchamp, R. D.; Whitehead, R. H., Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology 2002, 123, (6), 1941-8. [CrossRef]
- Dalerba, P.; Dylla, S. J.; Park, I. K.; Liu, R.; Wang, X.; Cho, R. W.; Hoey, T.; Gurney, A.; Huang, E. H.; Simeone, D. M.; Shelton, A. A.; Parmiani, G.; Castelli, C.; Clarke, M. F., Phenotypic characterization of human colorectal cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America 2007, 104, (24), 10158-63.
- Munz, M.; Baeuerle, P. A.; Gires, O., The emerging role of EpCAM in cancer and stem cell signaling. Cancer research 2009, 69, (14), 5627-9. [CrossRef]
- Huang, E. H.; Hynes, M. J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J. Z.; Wicha, M. S.; Boman, B. M., Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer research 2009, 69, (8), 3382-9. [CrossRef]
- Chen, Y. C.; Hsu, H. S.; Chen, Y. W.; Tsai, T. H.; How, C. K.; Wang, C. Y.; Hung, S. C.; Chang, Y. L.; Tsai, M. L.; Lee, Y. Y.; Ku, H. H.; Chiou, S. H., Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One 2008, 3, (7), e2637. [CrossRef]
- Tang, Q.; Chen, J.; Di, Z.; Yuan, W.; Zhou, Z.; Liu, Z.; Han, S.; Liu, Y.; Ying, G.; Shu, X.; Di, M., TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. Journal of experimental & clinical cancer research : CR 2020, 39, (1), 232. [CrossRef]
- Yao, C.; Su, L.; Shan, J.; Zhu, C.; Liu, L.; Liu, C.; Xu, Y.; Yang, Z.; Bian, X.; Shao, J.; Li, J.; Lai, M.; Shen, J.; Qian, C., IGF/STAT3/NANOG/Slug Signaling Axis Simultaneously Controls Epithelial-Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer. Stem cells (Dayton, Ohio) 2016, 34, (4), 820-31. [CrossRef]
- Wu, A.; Oh, S.; Wiesner, S. M.; Ericson, K.; Chen, L.; Hall, W. A.; Champoux, P. E.; Low, W. C.; Ohlfest, J. R., Persistence of CD133+ cells in human and mouse glioma cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem cells and development 2008, 17, (1), 173-84. [CrossRef]
- Elkashty, O. A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P. J.; Tran, S. D., Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, (4), 458-466. [CrossRef]
- Liu, L.; Borlak, J., Advances in Liver Cancer Stem Cell Isolation and their Characterization. Stem cell reviews and reports 2021, 17, (4), 1215-1238. [CrossRef]
- Kharchenko, P. V., The triumphs and limitations of computational methods for scRNA-seq. Nature methods 2021, 18, (7), 723-732.
- Frank, M. H.; Wilson, B. J.; Gold, J. S.; Frank, N. Y., Clinical Implications of Colorectal Cancer Stem Cells in the Age of Single-Cell Omics and Targeted Therapies. Gastroenterology 2021, 160, (6), 1947-1960. [CrossRef]
- Zheng, H.; Liu, H.; Li, H.; Dou, W.; Wang, J.; Zhang, J.; Liu, T.; Wu, Y.; Liu, Y.; Wang, X., Characterization of stem cell landscape and identification of stemness-relevant prognostic gene signature to aid immunotherapy in colorectal cancer. Stem cell research & therapy 2022, 13, (1), 244. [CrossRef]
- Chen, S. F.; Chang, Y. C.; Nieh, S.; Liu, C. L.; Yang, C. Y.; Lin, Y. S., Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One 2012, 7, (2), e31864. [CrossRef]
- Zhang, J.; Zhang, Y.; Cheng, L.; Li, C.; Dai, L.; Zhang, H.; Yan, F.; Shi, H.; Dong, G.; Ning, Z.; Xu, W.; Si, C.; Deng, H.; Xiong, H., Enrichment and characterization of cancer stem-like cells in ultra-low concentration of serum and non-adhesive culture system. American journal of translational research 2018, 10, (5), 1552-1561.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P., Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World journal of stem cells 2019, 11, (12), 1065-1083. [CrossRef]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J., Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? International journal of molecular sciences 2018, 19, (1).
- Guo, X.; Chen, Y.; Ji, W.; Chen, X.; Li, C.; Ge, R., Enrichment of cancer stem cells by agarose multi-well dishes and 3D spheroid culture. Cell and tissue research 2019, 375, (2), 397-408. [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M. E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M. P., Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, (7630), 560-564.
- Clevers, H., Modeling Development and Disease with Organoids. Cell 2016, 165, (7), 1586-1597. [CrossRef]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L. B.; Crawford, H. C.; Arrowsmith, C.; Kalloger, S. E.; Renouf, D. J.; Connor, A. A.; Cleary, S.; Schaeffer, D. F.; Roehrl, M.; Tsao, M. S.; Gallinger, S.; Keller, G.; Muthuswamy, S. K., Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature medicine 2015, 21, (11), 1364-71.
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R. T.; Schuh, A. C.; Yeh, S. P.; Daigle, S. R.; Hui, J.; Pandya, S. S.; Gianolio, D. A.; de Botton, S.; Döhner, H., Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. The New England journal of medicine 2022, 386, (16), 1519-1531.
- Herpers, B.; Eppink, B.; James, M. I.; Cortina, C.; Cañellas-Socias, A.; Boj, S. F.; Hernando-Momblona, X.; Glodzik, D.; Roovers, R. C.; van de Wetering, M.; Bartelink-Clements, C.; Zondag-van der Zande, V.; Mateos, J. G.; Yan, K.; Salinaro, L.; Basmeleh, A.; Fatrai, S.; Maussang, D.; Lammerts van Bueren, J. J.; Chicote, I.; Serna, G.; Cabellos, L.; Ramírez, L.; Nuciforo, P.; Salazar, R.; Santos, C.; Villanueva, A.; Stephan-Otto Attolini, C.; Sancho, E.; Palmer, H. G.; Tabernero, J.; Stratton, M. R.; de Kruif, J.; Logtenberg, T.; Clevers, H.; Price, L. S.; Vries, R. G. J.; Batlle, E.; Throsby, M., Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nature cancer 2022, 3, (4), 418-436. [CrossRef]
- Frampton, J. E., Catumaxomab: in malignant ascites. Drugs 2012, 72, (10), 1399-410.
- Bezan, A.; Hohla, F.; Meissnitzer, T.; Greil, R., Systemic effect of catumaxomab in a patient with metastasized colorectal cancer: a case report. BMC cancer 2013, 13, 618. [CrossRef]
- Ströhlein, M. A.; Lordick, F.; Rüttinger, D.; Grützner, K. U.; Schemanski, O. C.; Jäger, M.; Lindhofer, H.; Hennig, M.; Jauch, K. W.; Peschel, C.; Heiss, M. M., Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie 2011, 34, (3), 101-8. [CrossRef]
- Bellone, S.; Black, J.; English, D. P.; Schwab, C. L.; Lopez, S.; Cocco, E.; Bonazzoli, E.; Predolini, F.; Ferrari, F.; Ratner, E.; Silasi, D. A.; Azodi, M.; Schwartz, P. E.; Santin, A. D., Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE), is highly active against primary uterine serous papillary carcinoma cell lines in vitro. American journal of obstetrics and gynecology 2016, 214, (1), 99.e1-8. [CrossRef]
- Herrmann, I.; Baeuerle, P. A.; Friedrich, M.; Murr, A.; Filusch, S.; Rüttinger, D.; Majdoub, M. W.; Sharma, S.; Kufer, P.; Raum, T.; Münz, M., Highly efficient elimination of colorectal tumor-initiating cells by an EpCAM/CD3-bispecific antibody engaging human T cells. PLoS One 2010, 5, (10), e13474. [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; Feng, K. C.; Yang, Q. M.; Li, X. L.; Han, W., CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, (7), e1440169. [CrossRef]
- Silva Galbiatti-Dias, A. L.; Fernandes, G. M. M.; Castanhole-Nunes, M. M. U.; Hidalgo, L. F.; Nascimento Filho, C. H. V.; Kawasaki-Oyama, R. S.; Ferreira, L. A. M.; Biselli-Chicote, P. M.; Pavarino É, C.; Goloni-Bertollo, E. M., Relationship between CD44(high)/CD133(high)/CD117(high) cancer stem cells phenotype and Cetuximab and Paclitaxel treatment response in head and neck cancer cell lines. American journal of cancer research 2018, 8, (8), 1633-1641.
- Sato-Dahlman, M.; Miura, Y.; Huang, J. L.; Hajeri, P.; Jacobsen, K.; Davydova, J.; Yamamoto, M., CD133-targeted oncolytic adenovirus demonstrates anti-tumor effect in colorectal cancer. Oncotarget 2017, 8, (44), 76044-76056. [CrossRef]
- Masuda, M.; Uno, Y.; Ohbayashi, N.; Ohata, H.; Mimata, A.; Kukimoto-Niino, M.; Moriyama, H.; Kashimoto, S.; Inoue, T.; Goto, N.; Okamoto, K.; Shirouzu, M.; Sawa, M.; Yamada, T., TNIK inhibition abrogates colorectal cancer stemness. Nature communications 2016, 7, 12586. [CrossRef]
- Chen, Y.; Wang, X. Q.; Zhang, Q.; Zhu, J. Y.; Li, Y.; Xie, C. F.; Li, X. T.; Wu, J. S.; Geng, S. S.; Zhong, C. Y.; Han, H. Y., (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, (6). [CrossRef]
- Toden, S.; Tran, H. M.; Tovar-Camargo, O. A.; Okugawa, Y.; Goel, A., Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, (13), 16158-71. [CrossRef]
- Wu, X.; Luo, F.; Li, J.; Zhong, X.; Liu, K., Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. International journal of oncology 2016, 48, (4), 1333-40. [CrossRef]
- Chen, Y.; Wang, M. H.; Zhu, J. Y.; Xie, C. F.; Li, X. T.; Wu, J. S.; Geng, S. S.; Han, H. Y.; Zhong, C. Y., TAp63α targeting of Lgr5 mediates colorectal cancer stem cell properties and sulforaphane inhibition. Oncogenesis 2020, 9, (10), 89. [CrossRef]
- Chen, Y.; Li, Y.; Wang, X. Q.; Meng, Y.; Zhang, Q.; Zhu, J. Y.; Chen, J. Q.; Cao, W. S.; Wang, X. Q.; Xie, C. F.; Li, X. T.; Geng, S. S.; Wu, J. S.; Zhong, C. Y.; Han, H. Y., Phenethyl isothiocyanate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Phytotherapy research : PTR 2018, 32, (12), 2447-2455. [CrossRef]
- Wang, Z.; Zhou, L.; Xiong, Y.; Yu, S.; Li, H.; Fan, J.; Li, F.; Su, Z.; Song, J.; Sun, Q.; Liu, S. S.; Xia, Y.; Zhao, L.; Li, S.; Guo, F.; Huang, P.; Carson, D. A.; Lu, D., Salinomycin exerts anti-colorectal cancer activity by targeting the β-catenin/T-cell factor complex. British journal of pharmacology 2019, 176, (17), 3390-3406.
- Wang, L.; Chang, J.; Varghese, D.; Dellinger, M.; Kumar, S.; Best, A. M.; Ruiz, J.; Bruick, R.; Peña-Llopis, S.; Xu, J.; Babinski, D. J.; Frantz, D. E.; Brekken, R. A.; Quinn, A. M.; Simeonov, A.; Easmon, J.; Martinez, E. D., A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nature communications 2013, 4, 2035. [CrossRef]
- Hsu, H. C.; Liu, Y. S.; Tseng, K. C.; Yang, T. S.; Yeh, C. Y.; You, J. F.; Hung, H. Y.; Chen, S. J.; Chen, H. C., CBB1003, a lysine-specific demethylase 1 inhibitor, suppresses colorectal cancer cells growth through down-regulation of leucine-rich repeat-containing G-protein-coupled receptor 5 expression. Journal of cancer research and clinical oncology 2015, 141, (1), 11-21. [CrossRef]
- Yang, W.; Li, Y.; Ai, Y.; Obianom, O. N.; Guo, D.; Yang, H.; Sakamuru, S.; Xia, M.; Shu, Y.; Xue, F., Pyrazole-4-Carboxamide (YW2065): A Therapeutic Candidate for Colorectal Cancer via Dual Activities of Wnt/β-Catenin Signaling Inhibition and AMP-Activated Protein Kinase (AMPK) Activation. Journal of medicinal chemistry 2019, 62, (24), 11151-11164. [CrossRef]
- Fang, L.; Zhu, Q.; Neuenschwander, M.; Specker, E.; Wulf-Goldenberg, A.; Weis, W. I.; von Kries, J. P.; Birchmeier, W., A Small-Molecule Antagonist of the β-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer research 2016, 76, (4), 891-901.
- Shin, J. H.; Jeong, J.; Choi, J.; Lim, J.; Dinesh, R. K.; Braverman, J.; Hong, J. Y.; Maher, S. E.; Amezcua Vesely, M. C.; Kim, W.; Koo, J. H.; Tang, W.; Wu, D.; Blackburn, H. N.; Xicola, R. M.; Llor, X.; Yilmaz, O.; Choi, J. M.; Bothwell, A. L. M., Dickkopf-2 regulates the stem cell marker LGR5 in colorectal cancer via HNF4α1. iScience 2021, 24, (5), 102411. [CrossRef]
- Roy, S.; Zhao, Y.; Yuan, Y. C.; Goel, A., Metformin and ICG-001 Act Synergistically to Abrogate Cancer Stem Cells-Mediated Chemoresistance in Colorectal Cancer by Promoting Apoptosis and Autophagy. Cancers 2022, 14, (5). [CrossRef]
- Bamodu, O. A.; Yang, C. K.; Cheng, W. H.; Tzeng, D. T. W.; Kuo, K. T.; Huang, C. C.; Deng, L.; Hsiao, M.; Lee, W. H.; Yeh, C. T., 4-Acetyl-Antroquinonol B Suppresses SOD2-Enhanced Cancer Stem Cell-Like Phenotypes and Chemoresistance of Colorectal Cancer Cells by Inducing hsa-miR-324 re-Expression. Cancers 2018, 10, (8).
- Chang, T. C.; Yeh, C. T.; Adebayo, B. O.; Lin, Y. C.; Deng, L.; Rao, Y. K.; Huang, C. C.; Lee, W. H.; Wu, A. T.; Hsiao, M.; Wu, C. H.; Wang, L. S.; Tzeng, Y. M., 4-Acetylantroquinonol B inhibits colorectal cancer tumorigenesis and suppresses cancer stem-like phenotype. Toxicology and applied pharmacology 2015, 288, (2), 258-68. [CrossRef]
- Zhang, Q.; Li, X. T.; Chen, Y.; Chen, J. Q.; Zhu, J. Y.; Meng, Y.; Wang, X. Q.; Li, Y.; Geng, S. S.; Xie, C. F.; Wu, J. S.; Zhong, C. Y.; Han, H. Y., Wnt/β-catenin signaling mediates the suppressive effects of diallyl trisulfide on colorectal cancer stem cells. Cancer chemotherapy and pharmacology 2018, 81, (6), 969-977.
- Kumar, B.; Ahmad, R.; Sharma, S.; Gowrikumar, S.; Primeaux, M.; Rana, S.; Natarajan, A.; Oupicky, D.; Hopkins, C. R.; Dhawan, P.; Singh, A. B., PIK3C3 Inhibition Promotes Sensitivity to Colon Cancer Therapy by Inhibiting Cancer Stem Cells. Cancers 2021, 13, (9). [CrossRef]
- Kim, H.; Yu, Y.; Choi, S.; Lee, H.; Yu, J.; Lee, J. H.; Kim, W. Y., Evodiamine Eliminates Colon Cancer Stem Cells via Suppressing Notch and Wnt Signaling. Molecules (Basel, Switzerland) 2019, 24, (24). [CrossRef]
- Husain, K.; Coppola, D.; Yang, C. S.; Malafa, M. P., Farnesyl dimethyl chromanol targets colon cancer stem cells and prevents colorectal cancer metastasis. Scientific reports 2021, 11, (1), 2185. [CrossRef]
- Chen, Y.; Rao, X.; Huang, K.; Jiang, X.; Wang, H.; Teng, L., FH535 Inhibits Proliferation and Motility of Colon Cancer Cells by Targeting Wnt/β-catenin Signaling Pathway. Journal of Cancer 2017, 8, (16), 3142-3153. [CrossRef]
- Lin, D.; Shen, Y.; Liang, T., Oncolytic virotherapy: basic principles, recent advances and future directions. Signal transduction and targeted therapy 2023, 8, (1), 156.
- Zhao, F.; Zhang, R.; Wang, J.; Wu, D.; Pan, M.; Li, M.; Guo, M.; Dou, J., Effective tumor immunity to melanoma mediated by B16F10 cancer stem cell vaccine. International immunopharmacology 2017, 52, 238-244. [CrossRef]
- Hubbard, J. M.; Tőke, E. R.; Moretto, R.; Graham, R. P.; Youssoufian, H.; Lőrincz, O.; Molnár, L.; Csiszovszki, Z.; Mitchell, J. L.; Wessling, J.; Tóth, J.; Cremolini, C., Safety and Activity of PolyPEPI1018 Combined with Maintenance Therapy in Metastatic Colorectal Cancer: an Open-Label, Multicenter, Phase Ib Study. Clinical cancer research : an official journal of the American Association for Cancer Research 2022, 28, (13), 2818-2829.
- Katoh, M., Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). International journal of oncology 2017, 51, (5), 1357-1369. [CrossRef]
- Silva, V. R.; Santos, L. S.; Dias, R. B.; Quadros, C. A.; Bezerra, D. P., Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer communications (London, England) 2021, 41, (12), 1275-1313. [CrossRef]
- Sun, X.; Song, J.; Li, E.; Geng, H.; Li, Y.; Yu, D.; Zhong, C., (-)-Epigallocatechin-3-gallate inhibits bladder cancer stem cells via suppression of sonic hedgehog pathway. Oncology reports 2019, 42, (1), 425-435.
- Jiang, P.; Xu, C.; Zhang, P.; Ren, J.; Mageed, F.; Wu, X.; Chen, L.; Zeb, F.; Feng, Q.; Li, S., Epigallocatechin-3-gallate inhibits self-renewal ability of lung cancer stem-like cells through inhibition of CLOCK. International journal of molecular medicine 2020, 46, (6), 2216-2224.
- Wang, D.; Upadhyaya, B.; Liu, Y.; Knudsen, D.; Dey, M., Phenethyl isothiocyanate upregulates death receptors 4 and 5 and inhibits proliferation in human cancer stem-like cells. BMC cancer 2014, 14, 591. [CrossRef]
- Dong, T. T.; Zhou, H. M.; Wang, L. L.; Feng, B.; Lv, B.; Zheng, M. H., Salinomycin selectively targets 'CD133+' cell subpopulations and decreases malignant traits in colorectal cancer lines. Annals of surgical oncology 2011, 18, (6), 1797-804. [CrossRef]
- Zhang, C.; Tian, Y.; Song, F.; Fu, C.; Han, B.; Wang, Y., Salinomycin inhibits the growth of colorectal carcinoma by targeting tumor stem cells. Oncology reports 2015, 34, (5), 2469-76. [CrossRef]
- Takebe, N.; Miele, L.; Harris, P. J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S. X.; Ivy, S. P., Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews. Clinical oncology 2015, 12, (8), 445-64. [CrossRef]
- Varjosalo, M.; Taipale, J., Hedgehog: functions and mechanisms. Genes & development 2008, 22, (18), 2454-72. [CrossRef]
- Cochrane, C. R.; Szczepny, A.; Watkins, D. N.; Cain, J. E., Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers 2015, 7, (3), 1554-85. [CrossRef]
- Frampton, J. E.; Basset-Séguin, N., Vismodegib: A Review in Advanced Basal Cell Carcinoma. Drugs 2018, 78, (11), 1145-1156. [CrossRef]
- Wu, C.; Hu, S.; Cheng, J.; Wang, G.; Tao, K., Smoothened antagonist GDC-0449 (Vismodegib) inhibits proliferation and triggers apoptosis in colon cancer cell lines. Experimental and therapeutic medicine 2017, 13, (5), 2529-2536. [CrossRef]
- Batsaikhan, B. E.; Yoshikawa, K.; Kurita, N.; Iwata, T.; Takasu, C.; Kashihara, H.; Shimada, M., Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer research 2014, 34, (11), 6339-44.
- Strosberg, J. R.; Yeatman, T.; Weber, J.; Coppola, D.; Schell, M. J.; Han, G.; Almhanna, K.; Kim, R.; Valone, T.; Jump, H.; Sullivan, D., A phase II study of RO4929097 in metastatic colorectal cancer. European journal of cancer (Oxford, England : 1990) 2012, 48, (7), 997-1003. [CrossRef]
- Fischer, M.; Yen, W. C.; Kapoun, A. M.; Wang, M.; O'Young, G.; Lewicki, J.; Gurney, A.; Hoey, T., Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer research 2011, 71, (5), 1520-5.
- Ponnurangam, S.; Mammen, J. M.; Ramalingam, S.; He, Z.; Zhang, Y.; Umar, S.; Subramaniam, D.; Anant, S., Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Molecular cancer therapeutics 2012, 11, (4), 963-72.
- Li, Y.; Wang, Z.; Jin, J.; Zhu, S. X.; He, G. Q.; Li, S. H.; Wang, J.; Cai, Y., Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochemical and biophysical research communications 2020, 523, (4), 947-953. [CrossRef]
- Chandra Boinpelly, V.; Verma, R. K.; Srivastav, S.; Srivastava, R. K.; Shankar, S., α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. Journal of cellular and molecular medicine 2020, 24, (19), 11343-11354.
- Chen, J.; Shao, R.; Li, F.; Monteiro, M.; Liu, J. P.; Xu, Z. P.; Gu, W., PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clinical and experimental pharmacology & physiology 2015, 42, (12), 1317-26. [CrossRef]
- Peng, Y. C.; Lu, S. D.; Zhong, J. H.; Xie, Z. B.; You, X. M.; Peng, N. F.; Li, L. Q., Combination of 5-fluorouracil and 2-morphilino-8-phenyl-4H-chromen-4-one may inhibit liver cancer stem cell activity. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2016, 37, (8), 10943-58. [CrossRef]
- Tanaka, G.; Inoue, K.; Shimizu, T.; Akimoto, K.; Kubota, K., Dual pharmacological inhibition of glutathione and thioredoxin systems synergizes to kill colorectal carcinoma stem cells. Cancer medicine 2016, 5, (9), 2544-57. [CrossRef]
- Cai, Z.; Ke, J.; He, X.; Yuan, R.; Chen, Y.; Wu, X.; Wang, L.; Wang, J.; Lan, P.; Wu, X., Significance of mTOR signaling and its inhibitor against cancer stem-like cells in colorectal cancer. Annals of surgical oncology 2014, 21, (1), 179-88. [CrossRef]
- Seo, Y.; Kim, J.; Park, S. J.; Park, J. J.; Cheon, J. H.; Kim, W. H.; Kim, T. I., Metformin Suppresses Cancer Stem Cells through AMPK Activation and Inhibition of Protein Prenylation of the Mevalonate Pathway in Colorectal Cancer. Cancers 2020, 12, (9). [CrossRef]
- Wang, K.; Huang, W.; Sang, X.; Wu, X.; Shan, Q.; Tang, D.; Xu, X.; Cao, G., Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine : international journal of phytotherapy and phytopharmacology 2020, 68, 153191. [CrossRef]
- Francipane, M. G.; Lagasse, E., Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget 2013, 4, (11), 1948-62. [CrossRef]
- Mangiapane, L. R.; Nicotra, A.; Turdo, A.; Gaggianesi, M.; Bianca, P.; Di Franco, S.; Sardina, D. S.; Veschi, V.; Signore, M.; Beyes, S.; Fagnocchi, L.; Fiori, M. E.; Bongiorno, M. R.; Lo Iacono, M.; Pillitteri, I.; Ganduscio, G.; Gulotta, G.; Medema, J. P.; Zippo, A.; Todaro, M.; De Maria, R.; Stassi, G., PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut 2022, 71, (1), 119-128. [CrossRef]
- Malkomes, P.; Lunger, I.; Luetticke, A.; Oppermann, E.; Haetscher, N.; Serve, H.; Holzer, K.; Bechstein, W. O.; Rieger, M. A., Selective AKT Inhibition by MK-2206 Represses Colorectal Cancer-Initiating Stem Cells. Annals of surgical oncology 2016, 23, (9), 2849-57. [CrossRef]
- Lin, L.; Liu, Y.; Li, H.; Li, P. K.; Fuchs, J.; Shibata, H.; Iwabuchi, Y.; Lin, J., Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. British journal of cancer 2011, 105, (2), 212-20. [CrossRef]
- Jonker, D. J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M. M.; Wei, A. C.; Gao, Y.; Tebbutt, N. C.; Markman, B.; Price, T.; Esaki, T.; Koski, S.; Hitron, M.; Li, W.; Li, Y.; Magoski, N. M.; Li, C. J.; Simes, J.; Tu, D.; O'Callaghan, C. J., Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. The lancet. Gastroenterology & hepatology 2018, 3, (4), 263-270. [CrossRef]
- Chung, S. Y.; Chen, Y. H.; Lin, P. R.; Chao, T. C.; Su, J. C.; Shiau, C. W.; Su, Y., Two novel SHP-1 agonists, SC-43 and SC-78, are more potent than regorafenib in suppressing the in vitro stemness of human colorectal cancer cells. Cell death discovery 2018, 4, 25. [CrossRef]
- Kuramoto, K.; Yamamoto, M.; Suzuki, S.; Togashi, K.; Sanomachi, T.; Kitanaka, C.; Okada, M., Inhibition of the Lipid Droplet-Peroxisome Proliferator-Activated Receptor α Axis Suppresses Cancer Stem Cell Properties. Genes 2021, 12, (1). [CrossRef]
- Yang, B.; Bai, H.; Sa, Y.; Zhu, P.; Liu, P., Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. Journal of Cancer 2020, 11, (8), 2303-2317. [CrossRef]
- Toden, S.; Ravindranathan, P.; Gu, J.; Cardenas, J.; Yuchang, M.; Goel, A., Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Scientific reports 2018, 8, (1), 3335. [CrossRef]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G. S.; Wu, K., Notch signaling: an emerging therapeutic target for cancer treatment. Cancer letters 2015, 369, (1), 20-7. [CrossRef]
- Bray, S. J., Notch signalling in context. Nature reviews. Molecular cell biology 2016, 17, (11), 722-735. [CrossRef]
- BeLow, M.; Osipo, C., Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells 2020, 9, (10). [CrossRef]
- Hoey, T.; Yen, W. C.; Axelrod, F.; Basi, J.; Donigian, L.; Dylla, S.; Fitch-Bruhns, M.; Lazetic, S.; Park, I. K.; Sato, A.; Satyal, S.; Wang, X.; Clarke, M. F.; Lewicki, J.; Gurney, A., DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell stem cell 2009, 5, (2), 168-77. [CrossRef]
- Polivka, J., Jr.; Janku, F., Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacology & therapeutics 2014, 142, (2), 164-75. [CrossRef]
- Chen, S.; Fisher, R. C.; Signs, S.; Molina, L. A.; Shenoy, A. K.; Lopez, M. C.; Baker, H. V.; Koomen, J. M.; Chen, Y.; Gittleman, H.; Barnholtz-Sloan, J.; Berg, A.; Appelman, H. D.; Huang, E. H., Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells reduces tumor growth due to apoptosis. Oncotarget 2017, 8, (31), 50476-50488. [CrossRef]
- Pei, S.; Minhajuddin, M.; Callahan, K. P.; Balys, M.; Ashton, J. M.; Neering, S. J.; Lagadinou, E. D.; Corbett, C.; Ye, H.; Liesveld, J. L.; O'Dwyer, K. M.; Li, Z.; Shi, L.; Greninger, P.; Settleman, J.; Benes, C.; Hagen, F. K.; Munger, J.; Crooks, P. A.; Becker, M. W.; Jordan, C. T., Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. The Journal of biological chemistry 2013, 288, (47), 33542-33558. [CrossRef]
- Song, C. W.; Lee, H.; Dings, R. P.; Williams, B.; Powers, J.; Santos, T. D.; Choi, B. H.; Park, H. J., Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Scientific reports 2012, 2, 362. [CrossRef]
- Saif, M. W.; Rajagopal, S.; Caplain, J.; Goodman, M. D.; Popowich, D.; Orkin, B. A.; Tsichlis, P. N.; Martell, R., The First Study Evaluating the Safety of Pre-Surgery Administration of Metformin in Patients with Colorectal and other Gastrointestinal Cancers and Effect on Cancer Stem Cells. Cancer medicine journal 2021, 4, (Suppl 4), 1-10.
- Brooks, A. J.; Putoczki, T., JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, (7). [CrossRef]
- Quintás-Cardama, A.; Verstovsek, S., Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clinical cancer research : an official journal of the American Association for Cancer Research 2013, 19, (8), 1933-40.
- Park, S. Y.; Lee, C. J.; Choi, J. H.; Kim, J. H.; Kim, J. W.; Kim, J. Y.; Nam, J. S., The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. Journal of experimental & clinical cancer research : CR 2019, 38, (1), 399.
- Li, Y.; Rogoff, H. A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A. B.; Li, C. J., Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, (6), 1839-44.
- Li, Y.; Han, Q.; Zhao, H.; Guo, Q.; Zhang, J., Napabucasin Reduces Cancer Stem Cell Characteristics in Hepatocellular Carcinoma. Frontiers in pharmacology 2020, 11, 597520. [CrossRef]
- Song, L.; Li, Z. Y.; Liu, W. P.; Zhao, M. R., Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer biology & therapy 2015, 16, (1), 1-7. [CrossRef]
- van den Brink, G. R.; Hardwick, J. C., Hedgehog Wnteraction in colorectal cancer. Gut 2006, 55, (7), 912-4.
- Ahmed, M.; Jinks, N.; Babaei-Jadidi, R.; Kashfi, H.; Castellanos-Uribe, M.; May, S. T.; Mukherjee, A.; Nateri, A. S., Repurposing Antibacterial AM404 as a Potential Anticancer Drug for Targeting Colorectal Cancer Stem-Like Cells. Cancers 2019, 12, (1). [CrossRef]
- Narayan, S.; Ramisetti, S.; Jaiswal, A. S.; Law, B. K.; Singh-Pillay, A.; Singh, P.; Amin, S.; Sharma, A. K., ASR352, A potent anticancer agent: Synthesis, preliminary SAR, and biological activities against colorectal cancer bulk, 5-fluorouracil/oxaliplatin resistant and stem cells. European journal of medicinal chemistry 2019, 161, 456-467. [CrossRef]
- Narayan, S.; Jaiswal, A. S.; Sharma, R.; Nawab, A.; Duckworth, L. V.; Law, B. K.; Zajac-Kaye, M.; George, T. J.; Sharma, J.; Sharma, A. K.; Hromas, R. A., NSC30049 inhibits Chk1 pathway in 5-FU-resistant CRC bulk and stem cell populations. Oncotarget 2017, 8, (34), 57246-57264. [CrossRef]
- Takeda, M.; Koseki, J.; Takahashi, H.; Miyoshi, N.; Nishida, N.; Nishimura, J.; Hata, T.; Matsuda, C.; Mizushima, T.; Yamamoto, H.; Ishii, H.; Doki, Y.; Mori, M.; Haraguchi, N., Disruption of Endolysosomal RAB5/7 Efficiently Eliminates Colorectal Cancer Stem Cells. Cancer research 2019, 79, (7), 1426-1437.
- Zhang, Z. Y.; Zheng, S. H.; Yang, W. G.; Yang, C.; Yuan, W. T., Targeting colon cancer stem cells with novel blood cholesterol drug pitavastatin. European review for medical and pharmacological sciences 2017, 21, (6), 1226-1233.
- Huang, T. H.; Wu, S. Y.; Huang, Y. J.; Wei, P. L.; Wu, A. T.; Chao, T. Y., The identification and validation of Trichosstatin A as a potential inhibitor of colon tumorigenesis and colon cancer stem-like cells. American journal of cancer research 2017, 7, (5), 1227-1237.
- Bogachek, M. V.; Park, J. M.; De Andrade, J. P.; Lorenzen, A. W.; Kulak, M. V.; White, J. R.; Gu, V. W.; Wu, V. T.; Weigel, R. J., Inhibiting the SUMO Pathway Represses the Cancer Stem Cell Population in Breast and Colorectal Carcinomas. Stem cell reports 2016, 7, (6), 1140-1151. https://doi.org/10.1016/j.stemcr.2016.11.001. [CrossRef]
- Manic, G.; Signore, M.; Sistigu, A.; Russo, G.; Corradi, F.; Siteni, S.; Musella, M.; Vitale, S.; De Angelis, M. L.; Pallocca, M.; Amoreo, C. A.; Sperati, F.; Di Franco, S.; Barresi, S.; Policicchio, E.; De Luca, G.; De Nicola, F.; Mottolese, M.; Zeuner, A.; Fanciulli, M.; Stassi, G.; Maugeri-Saccà, M.; Baiocchi, M.; Tartaglia, M.; Vitale, I.; De Maria, R., CHK1-targeted therapy to deplete DNA replication-stressed, p53-deficient, hyperdiploid colorectal cancer stem cells. Gut 2018, 67, (5), 903-917. [CrossRef]
- Wu, Z.; Huang, M.; Gong, Y.; Lin, C.; Guo, W., BRAF and EGFR inhibitors synergize to increase cytotoxic effects and decrease stem cell capacities in BRAF(V600E)-mutant colorectal cancer cells. Acta biochimica et biophysica Sinica 2018, 50, (4), 355-361. [CrossRef]
- Quarni, W.; Dutta, R.; Green, R.; Katiri, S.; Patel, B.; Mohapatra, S. S.; Mohapatra, S., Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Scientific reports 2019, 9, (1), 15202. [CrossRef]
- Zhang, S.; Ju, X.; Yang, Q.; Zhu, Y.; Fan, D.; Su, G.; Kong, L.; Li, Y., USP47 maintains the stemness of colorectal cancer cells and is inhibited by parthenolide. Biochemical and biophysical research communications 2021, 562, 21-28. [CrossRef]
- Boothello, R. S.; Patel, N. J.; Sharon, C.; Abdelfadiel, E. I.; Morla, S.; Brophy, D. F.; Lippman, H. R.; Desai, U. R.; Patel, B. B., A Unique Nonsaccharide Mimetic of Heparin Hexasaccharide Inhibits Colon Cancer Stem Cells via p38 MAP Kinase Activation. Molecular cancer therapeutics 2019, 18, (1), 51-61.
- Ferguson, L. P.; Diaz, E.; Reya, T., The Role of the Microenvironment and Immune System in Regulating Stem Cell Fate in Cancer. Trends in cancer 2021, 7, (7), 624-634. [CrossRef]
- Antonio, N.; Bønnelykke-Behrndtz, M. L.; Ward, L. C.; Collin, J.; Christensen, I. J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P., The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. The EMBO journal 2015, 34, (17), 2219-36. [CrossRef]
- Meacham, C. E.; Morrison, S. J., Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, (7467), 328-37. [CrossRef]
- Kobayashi, H.; Gieniec, K. A.; Lannagan, T. R. M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E. M.; Sakai, A.; Suzuki, N.; Ichinose, M.; Wright, J. A.; Vrbanac, L.; Ng, J. Q.; Goyne, J.; Radford, G.; Lawrence, M. J.; Sammour, T.; Hayakawa, Y.; Klebe, S.; Shin, A. E.; Asfaha, S.; Bettington, M. L.; Rieder, F.; Arpaia, N.; Danino, T.; Butler, L. M.; Burt, A. D.; Leedham, S. J.; Rustgi, A. K.; Mukherjee, S.; Takahashi, M.; Wang, T. C.; Enomoto, A.; Woods, S. L.; Worthley, D. L., The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, (3), 890-906. [CrossRef]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L., Colon cancer: cancer stem cells markers, drug resistance and treatment. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2014, 68, (8), 911-6.
- Li, M.; Younis, M. H.; Zhang, Y.; Cai, W.; Lan, X., Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: cancer and beyond. European journal of nuclear medicine and molecular imaging 2022, 49, (8), 2844-2868.
- Drost, J.; van Jaarsveld, R. H.; Ponsioen, B.; Zimberlin, C.; van Boxtel, R.; Buijs, A.; Sachs, N.; Overmeer, R. M.; Offerhaus, G. J.; Begthel, H.; Korving, J.; van de Wetering, M.; Schwank, G.; Logtenberg, M.; Cuppen, E.; Snippert, H. J.; Medema, J. P.; Kops, G. J.; Clevers, H., Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521, (7550), 43-7.
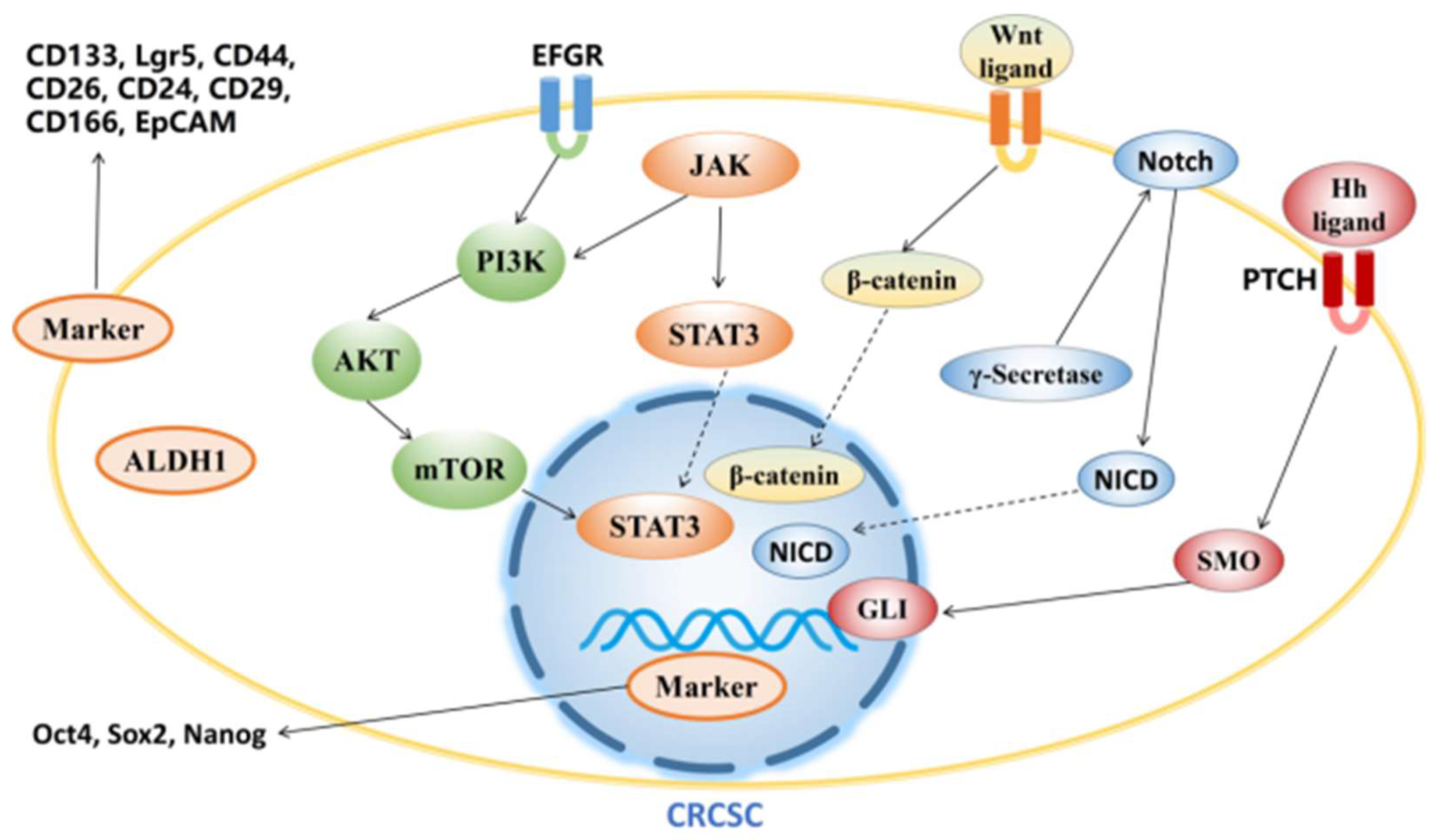
| Agents | Targets of CRCSCs | Reference |
| MCLA-158 | EFGR and LGR5 | [65] |
| Catumaxomab | EpCAM | [67, 68] |
| Solidomab | EpCAM | [69, 70] |
| CD133-directed CAR T cells | CD133 | [71] |
| Cetuximab | EFGR | [72] |
| CD133-targeted oncolytic virus |
CD133 | [73] |
| NCB0846 | Wnt pathway | [74] |
| Epigallocatechin gallate | Wnt pathway | [75, 76] |
| XAV939 | Wnt pathway | [77] |
| Phenethyl isothiocyanate and sulforaphane | Wnt pathway | [78, 79] |
| Salinomycin | Wnt pathway | [80] |
| JIB04 | Wnt pathway | [81] |
| CBB1003 | Wnt pathway | [82] |
| YW2065 | Wnt pathway | [83] |
| LF3 | Wnt pathway | [84] |
| Dickkopf-2 | Wnt pathway | [85] |
| ICG-001 | Wnt pathway | [86] |
| 4-Acetyl-antroquinonol B | Wnt pathway and JAK-STAT pathway | [87, 88] |
| Diallyl trisulfide | Wnt pathway | [89] |
| 36-077 | Wnt pathway | [90] |
| Evodiamine | Wnt and Notch pathway | [91] |
| Farnesyl dimethyl chromanol | Wnt pathway | [92] |
| FH535 | Wnt pathway | [93] |
| Agents | Targets of CRCSCs | Reference |
| Vismodegib | SMO of Hedgehog pathway | [108] |
| Cyclopamine | SMO of Hedgehog pathway | [109] |
| RO4929097 | γ-secretase of Notch pathway | [110] |
| Anti-DLL4 | DLL4 of Notch pathway | [111] |
| Honokiol | γ-secretase of Notch pathway | [112] |
| Quercetin | γ-secretase of Notch pathway | [113] |
| α-Mangostine | Notch pathway | [114] |
| BEZ235 | PI3K/Akt/mTOR pathway | [115] |
| LY294002 | PI3K/Akt/mTOR pathway | [116] |
| Piplartine | PI3K/Akt/mTOR pathway | [117] |
| Rapamycin | mTOR of PI3K/Akt/mTOR pathway | [118] |
| Metformin | mTOR of PI3K/Akt/mTOR pathway | [119] |
| Atractylenolide I | PI3K/Akt/mTOR pathway | [120] |
| Taselisib | PI3K/Akt/mTOR pathway | [121] |
| Buparlisib | Akt of PI3K/Akt/mTOR pathway | [122] |
| Miransertib | Akt of PI3K/Akt/mTOR pathway | [122] |
| MK-2206 | Akt of PI3K/Akt/mTOR pathway | [123] |
| Torkinib | mTOR of PI3K/Akt/mTOR pathway | [118] |
| Curcumin and GO-Y030 | STAT3 of JAK/STAT3 signaling pathway | [124] |
| Napabucasin | STAT3 of JAK/STAT3 signaling pathway | [125] |
| Agents | Targets of CRCSCs | Reference |
| AM404 | FBXL5 | [146] |
| LY2606368 | Checkpoint kinase 1 | [153] |
| ASR352 | Checkpoint kinase 1 | [147] |
| NCS30049 | Checkpoint kinase 1 | [148] |
| Mefloquine | RAB5/7 | [149] |
| Pitavastatin | - | [150] |
| Trichostatin A | histone deacetylase | [151] |
| Dabrafenib | BRAF | [154] |
| Mithramycin A | SP1 | [155] |
| Parthenolide | USP47 | [156] |
| Gambogic acid | ZFP36 | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




