1. Introduction
Wetlands serve as vital habitats for birds, providing essential resources such as nesting sites, food, and shelter [
1,
2]. Not only wetlands harbour a rich diversity of bird species, but they also contribute to the overall ecological balance of the region [
3]. The activity patterns of birds are influenced by various factors, including interspecies competition and circadian rhythms [
4,
5]. Understanding these factors, particularly the importance of competitive interactions, can provide valuable insights into bird ecology, population dynamics, and potential conservation strategies [
6,
7].
Competitive interactions among bird species play a crucial role in determining the structure and function of avian communities [
8]. These interactions affect resource use, habitat selection, and ultimately the survival and reproduction of individual species [
9]. Interspecies competition can lead to niche partitioning, where species adapt their behaviours and activity times to reduce competition and coexist within the same habitat [
10,
11]. Therefore, examining competitive interactions in the context of circadian rhythms can help us better understand the complex relationships among bird species and their environment [
12].
Camera trapping has long been an effective tool for determining species distribution and estimating population density, as well as facilitating various research dimensions, such as studying animals' circadian rhythms and constructing biodiversity databases [
13]. While camera trapping techniques historically focused on the spatial elements of species ecology and population dispersion, recent shifts in research focus have occurred. This shift is largely due to the capacity of timestamped photographs to reveal temporal changes in species' behavioral patterns [
14]. A key area of research in camera trapping and temporal data is the observation of wildlife behavioral patterns. To ensure the precision of results derived from these methods, it becomes essential to leverage a diverse dataset that encompasses both the visual material captured [
15,
16,
17,
18,
19].
We hypothesized that bird activity patterns would be shaped by interspecies competition. Furthermore, we anticipated that habitat characteristics and environmental factors would also play a role in mediating the relationship between interspecies competition and bird activity patterns.
Our primary objectives were to: (1) Identify the bird species present in the wetland. (2) Determine their activity patterns in relation to their circadian rhythms. (3) Assess the role of interspecies competition in shaping these activity patterns. (4) Evaluate the influence of environmental factors and habitat characteristics on bird distribution and activity.
2. Materials and Methods
2.1. Study site
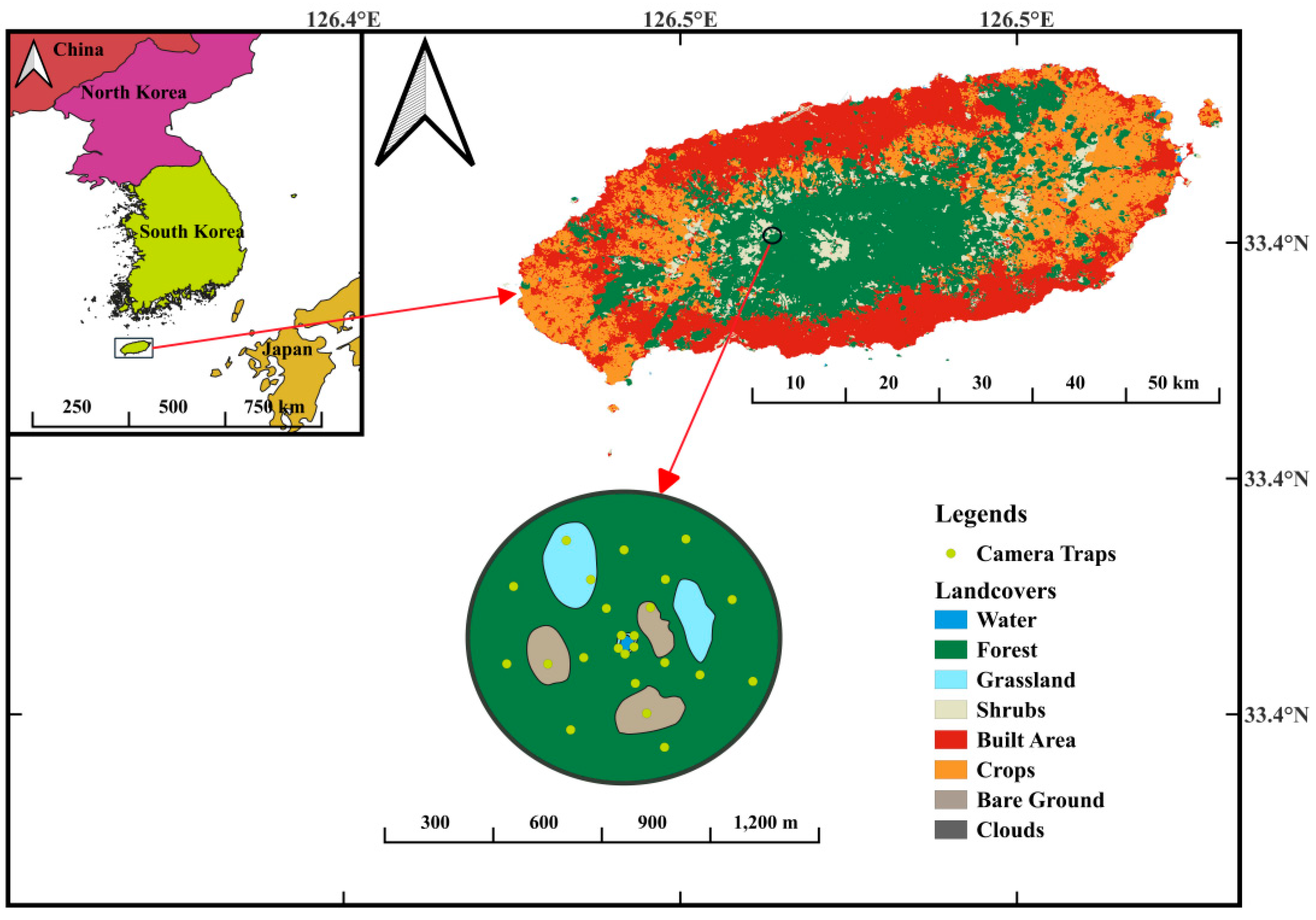
Hallasan National Park, located on Jeju Island, Republic of Korea, is an exceptional and ecologically significant area. It has received several designations, including UNESCO Biosphere Reserve, World Natural Heritage site, and Global Geopark, highlighting its importance for conservation and biodiversity [
20]. The study was carried out in a high-altitude wetland situated at an elevation of 950 meters within Hallasan National Park (33°22'13.97"N, 126°26'54.76"E). This wetland is ephemeral in nature, remaining dry and filling up with water only during rainfall periods. It was selected for investigation due to its underexplored status and potential to offer insights into bird species interactions and their adaptation to dynamic environmental conditions. The study site is home to more than 120 bird species, including the critically endangered Pitta nympha and
Terpsiphome atrocaudata. The vegetation surrounding the wetland is predominantly composed of broad-leaved forests, such as
Carpinus laxiflora,
Styrax japonicus, and
Quercus serrata, with mixed forests that include coniferous species. Throughout the study period, the highest recorded temperature was 28.6°C, the lowest was -20.2°C, and the average precipitation was 563.02mm [
21]. The study site has experienced minimal anthropogenic disturbance, ensuring that bird species interactions can be studied in a relatively pristine environment.
2.2. Filed sampling
A total of 24 camera traps (ROBOT D30, Bushwhacker Shenzhen, China) were deployed throughout the study site from March 2018 to March 2023. The camera traps were strategically placed based on habitat type, ensuring a balanced representation of shrubs, trees, grasslands, and wetland areas. Each camera trap was positioned at least 100 meters apart from each other (
Figure 1). This setup facilitated comprehensive monitoring of bird species interactions and activity patterns across different habitats [
22]. The cameras captured three consecutive photographs and 10-second videos whenever motion was detected.
The Passeriformes were analyzed by categorizing them into nesting guilds and foraging guilds, based on their nesting and feeding locations, respectively. The nesting guilds were divided into groups that breed in the canopy (C), ground-shrub vegetation (GS), and secondary cavity nesters (S) that utilize existing cavities in trees or other structures [
23]. Furthermore, the foraging guilds were classified according to the main foraging locations of forest birds, such as foliage searchers (FS) that explore the canopy for food, ground-shrub foragers (GF) that search for food in the ground and shrub vegetation, and aerial insect pursuers (AI) that catch insects in the air [
24,
25]. It is important to note that the nesting and foraging guilds provided are general information but are applicable only to the habits of the species observed in the study area.
2.3. Activity pattern
The cameras were programmed to follow Korean Standard Time. Consecutive captures of the same species within a one-hour interval were considered redundant and excluded from the analysis. To examine bird activity patterns in relation to circadian rhythms, we categorized observation times into three distinct time zones: morning (6:00-10:00), midday (10:00-14:00), and afternoon (14:00-18:00). Each species was classified into one of these categories if at least 50% of the records corresponded to that time. If the percentage was less than 50%, we considered it as cathemeral (active throughout the day). No separate time zone was designated for nighttime observations, as accurately identifying individual bird species captured during the night was challenging. For the analysis of time zones, species with fewer than 10 samples were not considered in order to reflect time zone variability and accurately identify specific time zone patterns within the data. Regarding the overlap index, analysis was only conducted for species with a sample size of 50 or more to enhance the reliability of overlapping activity patterns between two species. We recorded bird activity patterns every hour, identifying and recording each bird species in the dataset. The number of observations for each Passeriformes species throughout the study period was counted. To calculate the degree of temporal overlap in bird activity among different species, we divided the study period into one-hour intervals. The number of observations for each bird species within each interval was tabulated. The temporal overlap index (TOI) between each pair of bird species was then calculated as the sum of the minimum counts in each one-hour interval divided by the sum of the maximum counts [
26]. This resulted in a TOI score (△) ranging from 0 to 1, with higher scores indicating greater temporal overlap in bird activity [
27]. Statistical analyses were performed using R version 4.0.2 [
28]. The 'table' function was employed to count the number of observations for each bird species, and the 'cut' function was used to divide the study period into one-hour intervals. The temporal overlap index was calculated using the 'pmin' and 'pmax' functions in R.3.
3. Results
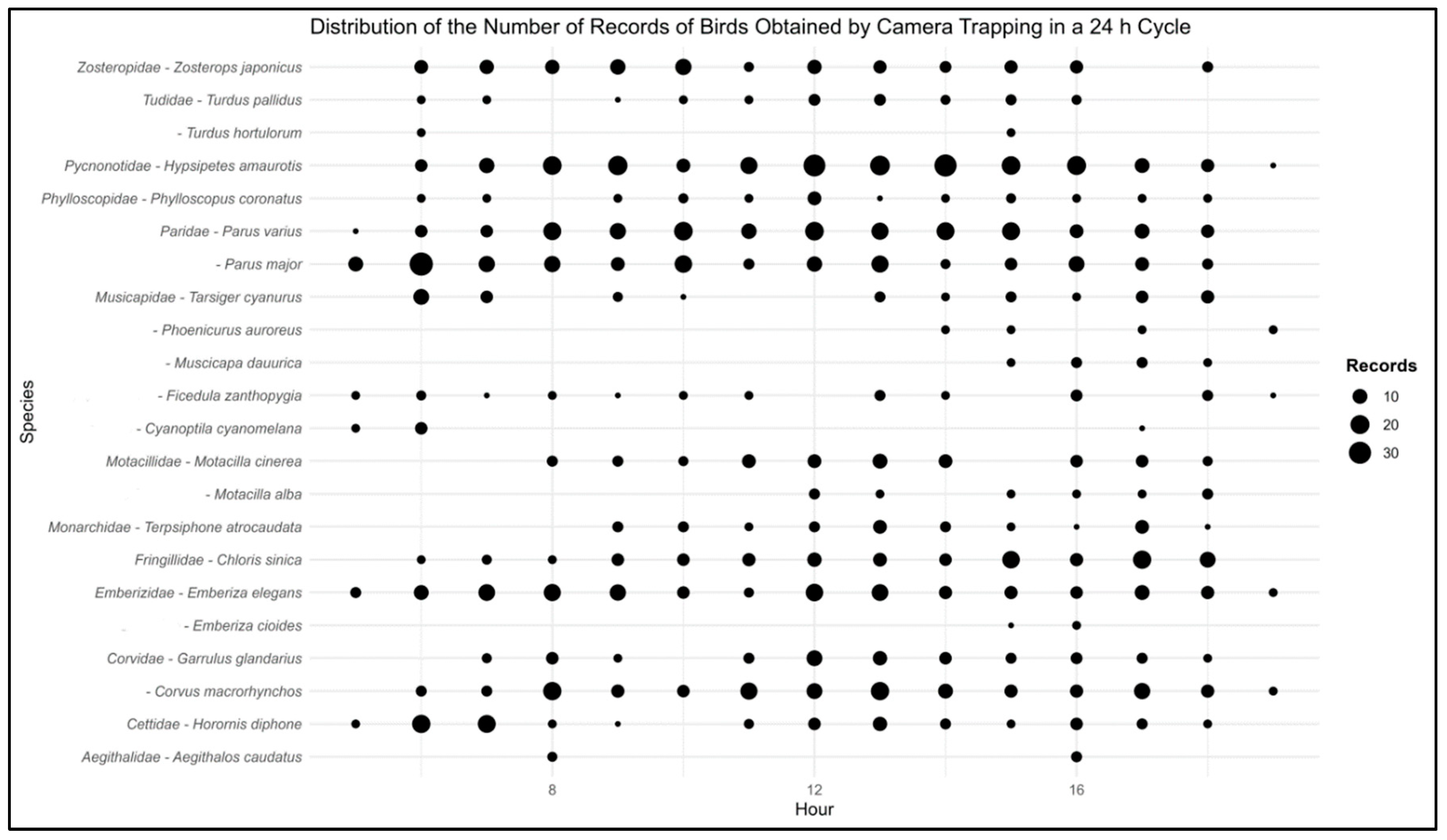
Our ecological investigation, complemented by extensive camera-trap sampling, yielded 5,322 photographs, out of which 1,427 were identified as independent bird sightings from 26 families and 49 species (
Table S1). Among these, a total of 13 families and 22 species of order Passeriformes were included in the analysis of temporal activity (
Figure 2).
Regarding temporal activity patterns, Cyanoptila cyanomelana and Horornis canturians exhibited morning activity, while Muscicapa dauurica, Phoenicurus auroreus, Turdus hortulorum, Aegithalos caudatus, and Emberiza cioides showed heightened activity during the afternoon (
Table 1). Interestingly, although a substantial number of species were observed during midday, they did not constitute more than 50% of the sightings, preventing their classification as midday species. The remaining 15 species were categorized as cathemeral, displaying activity throughout the day.
Among the studied species, twelve were classified as Ground-shrub nesters (GS), building their nests in shrubs or on the ground. Within this group, four species were Foliage searchers (FS), which forage within foliage, five were Ground-shrub foragers (GF), foraging on the ground or in shrubs, and three were Aerial insect pursuers (AI), catching insects in flight. Seven species were categorized as Canopy nesters (C), constructing their nests in the tree canopy. Among these, five species were confirmed as GF, while FS and AI each comprised a single species. Two species were classified as Secondary cavity nesters (S), making their nests in pre-existing cavities or spaces. All of these were FS.
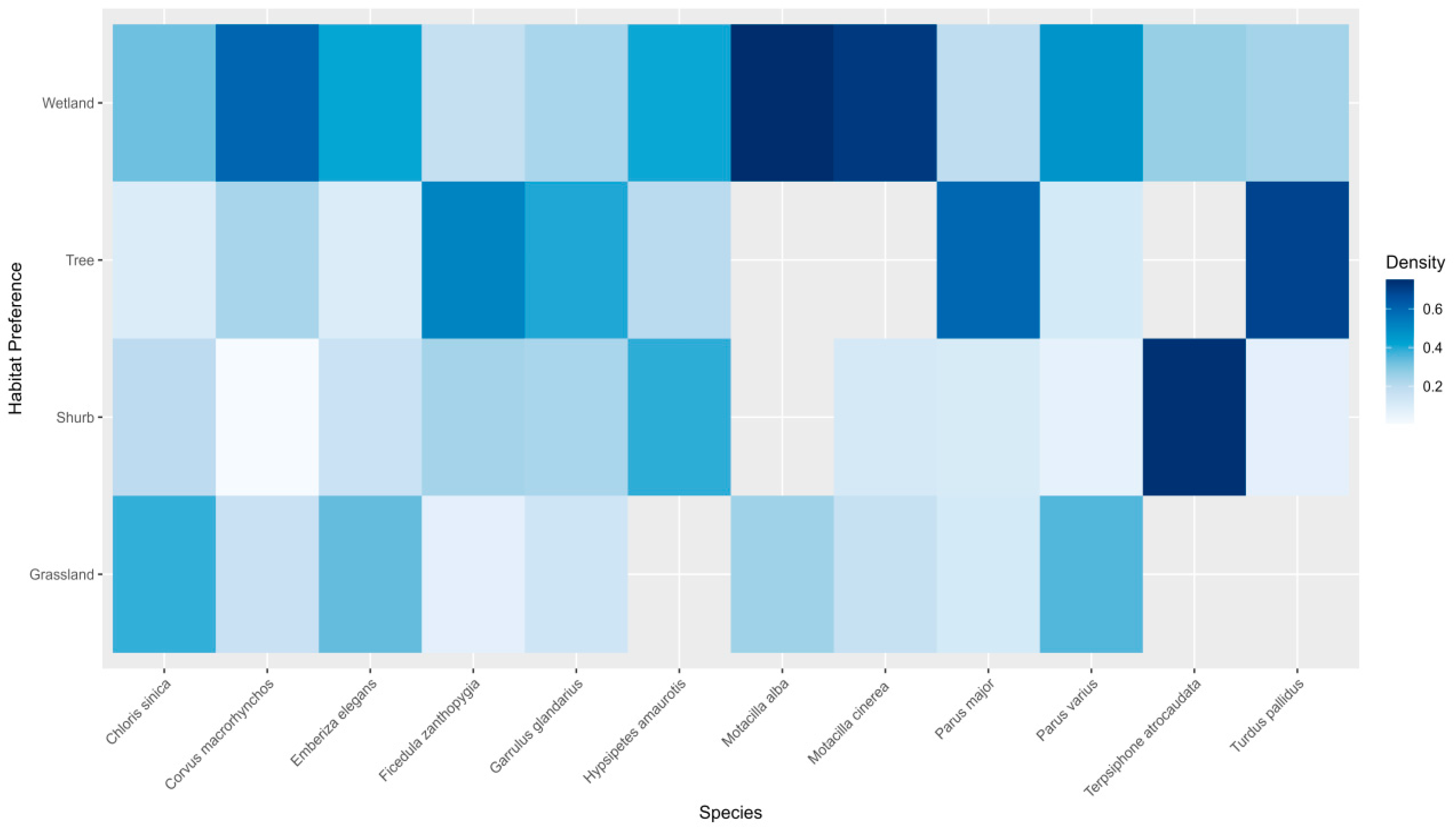
The study also examined habitat preferences across various bird species, revealing distinct predilections for different habitat types (
Figure 3). In grassland habitats, Chloris sinica emerged as a prominent species with a strong affinity for this environment. Other species such as
Parus varius and
Emberiza elegans were also observed to favour grassland habitats.
Corvus macrorhynchos and
Motacilla alba, though less dominant, had a notable presence in these areas. In shrub habitats,
Terpsiphone atrocaudata dominated, being predominantly observed in this type of habitat.
Hypsipetes amaurotis was also commonly found in shrubs, while
Corvus macrorhynchos had a minimal presence and
Motacilla alba had no records in shrub habitats. Tree habitats appealed significantly to species such as
Turdus pallidus,
Parus major, and
Ficedula zanthopygia, which displayed a marked preference for these environments.
Garrulus glandarius demonstrated a modest inclination toward tree habitats, while
Motacilla alba,
Motacilla cinerea, and
Terpsiphone atrocaudata showed no significant presence in these habitats. Regarding wetland habitats, both
Motacilla alba and
Motacilla cinerea exhibited a strong preference. Other species, including
Corvus macrorhynchos,
Emberiza elegans,
Hypsipetes amaurotis, and
Parus varius, also displayed a favourable preference for wetland habitats.
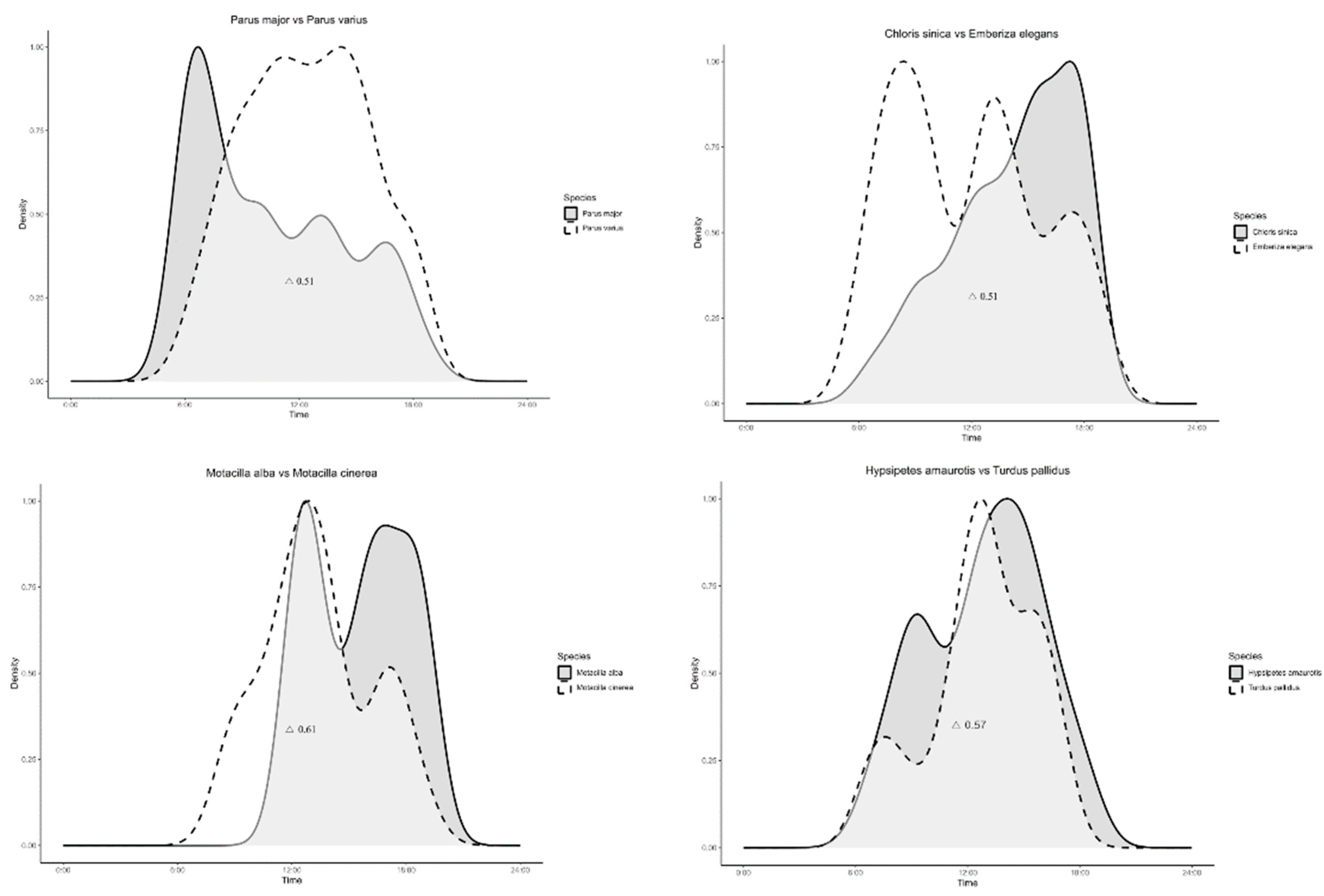
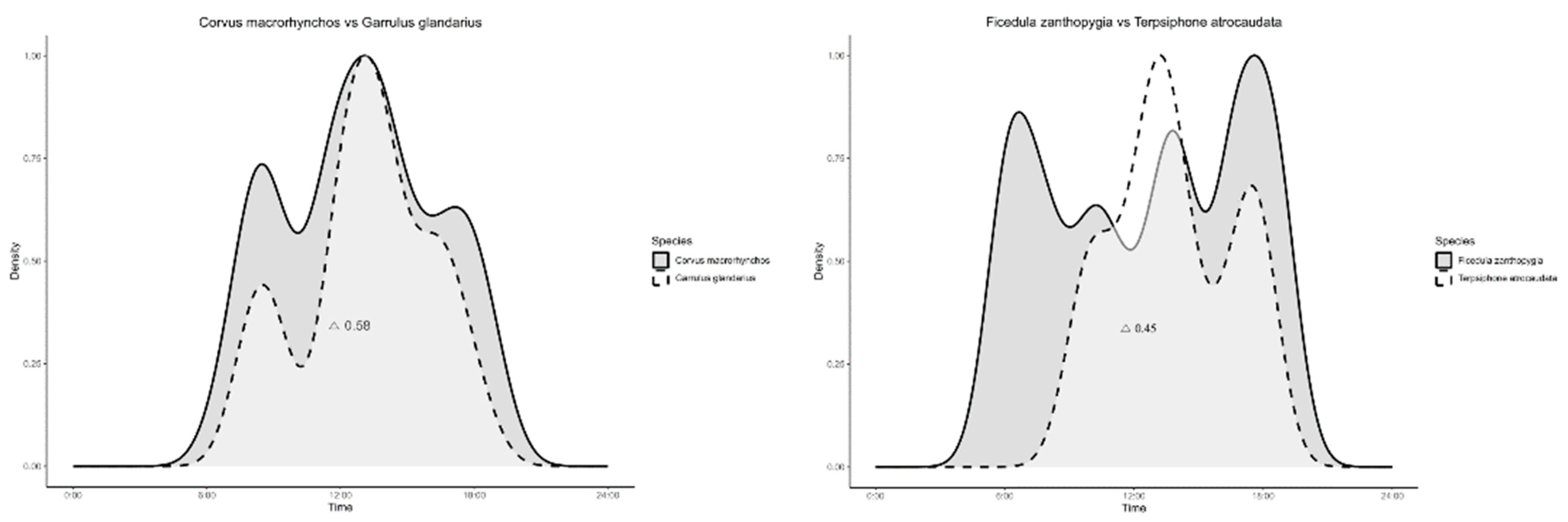
A cross-analysis of 12 species with similar behavioral ecology revealed varying degrees of overlap in their activity patterns.
Terpsiphone atrocaudata and
Ficedula zanthopygia had an overlap coefficient of 0.45,
Parus major and
Parus varius had 0.51,
Chloris sinica and
Emberiza elegans had 0.51,
Hypsipetes amaurotis and
Turdus pallidus had 0.57,
Corvus macrorhynchos and
Garrulus glandarius had 0.58, and
Motacilla cinerea and
Motacilla alba had 0.61 (
Figure 4).
A cross-analysis of 12 species with similar behavioral ecology revealed varying degrees of overlap in their activity patterns.
Terpsiphone atrocaudata and
Ficedula zanthopygia had an overlap coefficient of 0.45,
Parus major and
Parus varius had 0.51,
Chloris sinica and
Emberiza elegans had 0.51,
Hypsipetes amaurotis and
Turdus pallidus had 0.57,
Corvus macrorhynchos and
Garrulus glandarius had 0.58, and
Motacilla cinerea and
Motacilla alba had 0.61 (
Figure 4).
4. Discussion
Passeriformes, commonly known as perching birds, typically exhibit heightened activity during the early morning hours, a behavior referred to as the "dawn chorus" [
29]. This phenomenon is primarily influenced by three key factors: mate attraction and territory defence, light levels, and energy efficiency[
30]. Among the species studied,
Parus major and
Horornis canturians demonstrated pronounced morning activity patterns, aligning with the widely observed avian behavior of the dawn chorus. These species appear to take advantage of the early morning hours for foraging and other activities, likely due to the optimal conditions provided by the quiet morning environment, suitable light levels, and the need to replenish overnight-depleted energy reserves [
31,
32]. Conversely, five species,
Muscicapa dauurica,
Phoenicurus auroreus,
Turdus hortulorum,
Aegithalos caudatus, and
Emberiza cioides, displayed heightened activity levels during the afternoon. This suggests variations in species-specific behavior, possibly driven by factors such as prey availability, predator activity, and temperature fluctuations [
33]. The remaining 15 species, including
Parus major and
Garrulus glandarius, were classified as cathemeral, with their activities spread relatively evenly throughout the day. These species did not exhibit a distinct preference for a particular time zone, suggesting their adaptability to utilize resources and engage in activities regardless of the time [
34,
35].
The overlap coefficients (△) calculated for each pair of species ranged from 0.45 to 0.61. Among the species with similar ecology and behavior, except for
Fiedula zanthophygia and
Terpsiphone atrocaudata, more than 50% overlap in activity was observed. We initially anticipated similar activity patterns among Passeriformes due to their shared behavioral ecology[
36,
37]. However, these results indicate that, despite their similar behavioral ecology, these species exhibit peak activities at different times of the day, resulting in varying degrees of overlap in their activity patterns [
38]. Many passerine species display high behavioral flexibility, adjusting their activity patterns according to environmental conditions [
39]. We propose two possible interpretations for these activity differences:
1. Adaptation to Resource Availability: The disparities in activity periods might be an ecological strategy to optimize resource use [
40]. Closely related species pairs, such as
Chloris sinica and
Emberiza elegans, might exploit the same resources at different times in wetland and grassland habitats to avoid direct competition and promote coexistence [
41]. Similarly,
Hypsipetes amaurotis and
Turdus pallidus, as well as
Corvus macrorhynchos and
Garrulus glandarius, demonstrate a diverse range of habitat preferences. While these species may share certain habitats, the overlap index (0.57, 0.58) suggests a moderate level of overlap, indicating that they are not exclusively bound to the same spaces or resources. Hence, despite overlap in habitat preference, their broad habitat use may reduce direct competition [
42].
2. Thermoregulation: The divergent activity peaks may be attributed to thermoregulatory behavior. Birds modulate their behavior to manage body temperature, as suggested by Ryeland et al. (2021). Species with morning activity peaks, such as
Parus major, may strategize to avoid the heat of the day by seeking shade in trees [
43]. Conversely, species like
Parus varius, which exhibit heightened afternoon activity, may have lower heat tolerance or employ distinct strategies to cope with elevated temperatures [
44].
Furthermore, our results demonstrate similarity between certain species, with
Motacilla alba and
Motacilla cinerea showing relatively high temporal overlap compared to other bird pairs. This similarity can be attributed to ecological adaptations, where these species share behavioral preferences for resting and foraging times [
45]. For instance, both
Motacilla alba and
Motacilla cinerea primarily feed on insects and are often observed being active together in the afternoon at wetlands, coinciding with the peak activity of their insect prey. This ecological similarity suggests a high level of activity overlap among these species, indicating that they might be less sensitive to resource sharing compared to other species [
46,
47].
Overall, this study highlights significant overlaps in activity patterns between certain pairs of species within Passeriformes. Additionally, individual activity trends emphasize the diversity of temporal niches occupied by these birds. However, due to limited sample sizes and the influence of various environmental factors such as temperature, precipitation, wind, humidity, and human presence on birds' circadian rhythms, generalizing these findings can be challenging. Therefore, further data collection on these diverse environmental factors and additional research results are necessary to ensure a comprehensive understanding. This study contributes to the understanding of niche separation within Passeriformes and provides valuable insights into the behavioral ecology of bird species.
5. Conclusions
The study provided valuable insights about the behavioral patterns and habitat preferences of Passeriformes. The data collection of 5,322 camera-trap images enabled us to identify distinctive daily activity pattern, with some Passeriformes being active during mornings and others in afternoons. Notably, many species demonstrated adaptability, being active throughout the day.
Our research unveiled distinct habitat preferences among them, emphasizing the diverse ways in which birds interact with their environment. For example, certain species were predominantly found in grasslands, while others favored shrubs or tree canopies. Additionally, our findings underscored significant overlaps in daily activity patterns among some Passeriformes, indicating potential shared behavioral traits or ecological adaptations.
Two primary interpretations emerge from the data: Passeriformes might adjust their activity patterns to avoid resource competition, and their behaviors might be influenced by thermoregulation needs. A few species displayed high overlap, suggesting ecological similarities and potential adaptability in resource sharing.
Due to varying environmental factors influencing bird behavior, a more exhaustive data collection and analysis is necessary for a holistic understanding. Our results showed a comprehend niche separation among Passeriformes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Y. -H.J. and S. -H.C. conceived the study, field design and methodology. Y. -H.J., S. -H.C., S. -M.P., M.B., S. -D.J. and K.B. carried out the field study, data collection and analysis. Y. -H.J., S. -H.C. (the paper as a co-first author) wrote the original manuscript and was reviewed/edited by H. -S.O.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All used data are included within the manuscript.
Acknowledgments
This work was supported by a grant from the National Institute of Ecology (NIE) of the Republic of Korea (NIE-B-2022-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John wiley & sons: 2015.
- Zedler, J.B.; Kercher, S. Wetland resources: status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39-74. [CrossRef]
- Bateman, P.; Fleming, P.; Wolfe, A. A different kind of ecological modelling: the use of clay model organisms to explore predator–prey interactions in vertebrates. J. Zool. 2017, 301, 251-262. [CrossRef]
- Sellers, L. Rhythms of Life: The Biological Clocks That Control the Daily Lives of Every Living Thing. J. Coll. Sci. Teach. 2006, 35, 57.
- Strauss, S.Y.; Irwin, R.E. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 435-466. [CrossRef]
- Gonzalez, A.; Rayfield, B.; Lindo, Z. The disentangled bank: how loss of habitat fragments and disassembles ecological networks. Am. J. Bot. 2011, 98, 503-516. [CrossRef]
- HilleRisLambers, J.; Harsch, M.A.; Ettinger, A.K.; Ford, K.R.; Theobald, E.J. How will biotic interactions influence climate change–induced range shifts? Ann. Ny. Acad. Sci. 2013, 1297, 112-125.
- Marra, P.P.; Studds, C.E.; Wilson, S.; Sillett, T.S.; Sherry, T.W.; Holmes, R.T. Non-breeding season habitat quality mediates the strength of density-dependence for a migratory bird. Proc. R. Soc. B: Biol. Sci. 2015, 282, 20150624. [CrossRef]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities: positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. Bioscience 2001, 51, 235-246.
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends. in. Ecol. Evol 2003, 18, 119-125. [CrossRef]
- Sillett, T.S.; Holmes, R.T. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 2002, 71, 296-308. [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Ann. Rev. Eco.l S. 2003, 34, 153-181. [CrossRef]
- Ogurtsov, S.S.; Zheltukhin, A.S.; Kotlov, I.P. Daily activity patterns of large and medium-sized mammals based on camera traps data in the Central Forest Nature Reserve, Valdai Upland, Russia. Nature Conservation Research. Запoведная наука 2018, 3, 68-88. [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: an R package for efficient camera trap data management. MEE 2016, 7, 1457-1462. [CrossRef]
- Imbeau, L.; Savard, J.-P.L.; Gagnon, R. Comparing bird assemblages in successional black spruce stands originating from fire and logging. Can. J. Zool. 2000, 77, 1850-1860.
- Martin, T.E. Are microhabitat preferences of coexisting species under selection and adaptive? Ecology 1998, 79, 656-670.
- Klaassen, R.H.; Hake, M.; Strandberg, R.; Alerstam, T. Geographical and temporal flexibility in the response to crosswinds by migrating raptors. Proc. R. Soc. B: Biol. Sci. 2011, 278, 1339-1346. [CrossRef]
- Dokter, A.M.; Shamoun-Baranes, J.; Kemp, M.U.; Tijm, S.; Holleman, I. High altitude bird migration at temperate latitudes: a synoptic perspective on wind assistance. PloS one 2013, 8, e52300. [CrossRef]
- Newton, I. The migration ecology of birds, Vol. 2008.
- Kim, E.-S.; Oh, C.H.; Park, H.C.; Lee, S.-H.; Choi, J.; Lee, S.-H.; Cho, H.-B.; Lim, W.; Kim, H.; Yoon, Y.-K. Disturbed regeneration of saplings of Korean fir (Abies koreana Wilson), an endemic tree species, in Hallasan National Park, a UNESCO Biosphere Reserve, Jeju Island, Korea. J. Mar. Isl. 2016, 5, 68-78. [CrossRef]
- NIFS. National Institute of Forest Science: Hourly Weather Data. Available online: http://mw.nifos.go.kr/SiteData/Site_01Hour.aspx?SearchType=Hour (accessed on 20/03/2023).
- Hutto, R.L.; Young, J.S. Regional landbird monitoring: perspectives from the northern Rocky Mountains. Wildl. Soc. Bull. 2002, 738-750.
- Buckley, L.B.; Urban, M.C.; Angilletta, M.J.; Crozier, L.G.; Rissler, L.J.; Sears, M.W. Can mechanism inform species’ distribution models? Ecol. Lett. 2010, 13, 1041-1054.
- Choi, C.-Y.; Lee, E.-J.; Nam, H.-Y.; Lee, W.-S. Effects of postfire logging on bird populations and communities in burned forests. J. Korean Soc. For. Sci. 2007, 96, 115-123.
- Grimm, V.; Augusiak, J.; Focks, A.; Frank, B.M.; Gabsi, F.; Johnston, A.S.; Liu, C.; Martin, B.T.; Meli, M.; Radchuk, V. Towards better modelling and decision support: Documenting model development, testing, and analysis using TRACE. Ecol. Modell. 2014, 280, 129-139. [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322-337. [CrossRef]
- Efford, M.G. Estimation of population density by spatially explicit capture–recapture analysis of data from area searches. Ecology 2011, 92, 2202-2207. [CrossRef]
- Team, R.D.C. R: A language and environment for statistical computing. (No Title) 2010.
- Catchpole, C.K.; Slater, P.J. Bird song: biological themes and variations; Cambridge university press: 2003.
- Marzluff, J.; Bowman, R.; Donnelly, R. Avian Ecology and Conservation in an Urbanizing World. 2001. [CrossRef]
- Brumm, H.; Zollinger, S.A. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 2011, 148, 1173-1198. [CrossRef]
- Marler, P.R.; Slabbekoorn, H. Nature's music: the science of birdsong; Elsevier: 2004.
- Murakami, M.; Nakano, S. Species-specific foraging behavior of birds in a riparian forest. Eco.l Res. 2001, 16, 913-923. [CrossRef]
- Gill, F.B. Ornithology; W.H. Freeman and Company: 2007.
- Pérez-Irineo, G.; Santos-Moreno, A. Bird activity patterns in the understorey of an evergreen forest in Oaxaca, Mexico. Neotrop. Biol. Conserv. 2021, 16. [CrossRef]
- Lima, S.L. Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009, 84, 485-513. [CrossRef]
- Moller, A. Flight distance and population trends in European breeding birds. Behav. Ecol. 2008, 19, 1095-1102. [CrossRef]
- Smetzer, J.R.; Paxton, K.L.; Hart, P.J.; Paxton, E.H. Activity patterns of Hawaiian forest birds in a fragmented and continuous landscape. J. Avian, Biol. 2022, 2022, e02905.
- Robbins, C.S. Effect of time of day on bird activity. Studies in avian biology 1981, 6, 275-286.
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. PNAS 2009, 106, 19659-19665. [CrossRef]
- Sottas, C.; Reif, J.; Kuczyński, L.; Reifová, R. Interspecific competition promotes habitat and morphological divergence in a secondary contact zone between two hybridizing songbirds. J. Evol. Biol. 2018, 31, 914-923. [CrossRef]
- Ryeland, J.; Weston, M.A.; Symonds, M.R. The importance of wetland margin microhabitat mosaics; the case of shorebirds and thermoregulation. J. Appl. Ecol. 2021, 58, 382-391. [CrossRef]
- Wolf, B.O.; Walsberg, G.E. Thermal effects of radiation and wind on a small bird and implications for microsite selection. Ecology 1996, 77, 2228-2236. [CrossRef]
- Ceia, F.R.; Ramos, J.A. Individual specialization in the foraging and feeding strategies of seabirds: a review. Mar. Biol. 2015, 162, 1923-1938. [CrossRef]
- Seward, A.M.; Beale, C.M.; Gilbert, L.; Jones, T.H.; Thomas, R.J. The impact of increased food availability on reproduction in a long-distance migratory songbird: implications for environmental change? Plos one 2014, 9, e111180.
- Isack, H.A.; Reyer, H.-U. Honeyguides and honey gatherers: interspecific communication in a symbiotic relationship. Science 1989, 243, 1343-1346. [CrossRef]
- Martin, K.; Eadie, J.M. Nest webs: a community-wide approach to the management and conservation of cavity-nesting forest birds. For. Ecol. Manage. 1999, 115, 243-257. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).