1. Introduction
Amyloidosis encompasses a heterogeneous group of diseases, acquired, or inherited, which may occur in a systemic or in a localized form and that share a pathogenetic mechanism of amyloid fibril deposition in the extracellular space of various organs[
1]. Heart involvement, namely cardiac amyloidosis (CA), is the main determinant of long-term outcome [
2].
Although there are more than 30 different precursor proteins, the most frequent forms of CA are caused by transthyretin (ATTR) and immunoglobulin light chain (AL) precursor proteins [
3]. ATTR amyloidosis includes a non-hereditary form due to the accumulation in various organs of wild-type ATTR fibrils (ATTRwt) and a hereditary form due to mutations in the transthyretin gene, variant amyloidosis (ATTRv) [
2]. The disease occurs when the structural integrity and consequently the function of the tissues affected by the deposition of amyloid fibrils is lost. ATTRwt amyloidosis has a greater involvement of the heart while ATTRv amyloidosis leads to both cardiomyopathy and polyneuropathy [
4].
The epidemiology of amyloidosis, once considered a rare and incurable disease, has been rewritten in recent years thanks to important innovations in the diagnostic and therapeutic fields [
5]. The diagnosis of amyloidosis was once reached only by biopsy and usually at an advanced level of pathology[
6]. Currently, due to advances in cardiac magnetic resonance imaging (MRI) and cardiac scintigraphy with bone tracers, cardiac transthyretin amyloidosis (ATTR-CA) can be diagnosed non-invasively and usually at an earlier stage of disease. Cardiac scintigraphy with bone tracers has brought a revolution in the non-invasive diagnosis of ATTR-CA mainly due to the contribution of the algorithm proposed by Gillmore et al.[
7], where scintigraphy achieved excellent sensitivity and specificity values (99% and 86%, respectively) and a positive predictive value of 100% when combined with serum and urinary immunofixation and immunoglobulin light chain assay negative for monoclonal component.
However, despite the enormous progress in the non-invasive diagnosis of ATTR-CA in recent years, cardiac scintigraphy with bone tracers still presents several mysteries: the mechanism by which bone tracers are captured by ATTR-CA patients is still unknown and what further applications of cardiac scintigraphy beyond the diagnosis of amyloidosis might be.
This review addresses this by presenting what we know to date and provides insights into future perspectives in the field of cardiac scintigraphy with bone tracers.
2. Bone Tracers and Cardiac Amyloidosis
2.1. Pathophysiological Mechanisms of Amyloidogenic Cascade
Initially, several molecules, which may be unfolded, partially folded or completely folded, aggregate to generate totally or only partially disordered or structured oligomers. As the aggregation process proceeds, these oligomers may acquire a beta-sheet structure and then continue to grow by aggregating with each other or with other monomers. Amyloid fibrils are insoluble polymers consisting of various protein subunits that are in turn formed from soluble precursors that, through conformational changes, obtain a beta-sheet configuration. The process of fibrillogenesis is facilitated by partial folding or unfolding of the precursors, which is accelerated by acidification, proteolysis and nucleation, primary or secondary. At this point, the toxicity of amyloid fibrils is due to their deposition in the extracellular space of organs and tissues, undermining their structural integrity [
8].
2.2. Cardiac Scintigraphy
The diagnosis of CA was previously established by endomyocardial biopsy (EMB), which was associated with procedural risks and the need to be performed in capable hands. Bone tracer scintigraphy is a nuclear medicine diagnostic method that uses radiation emitted by a radiopharmaceutical labelled with radioactive isotopes previously injected intravenously and detected by a suitable instrument, the gamma-camera, allowing areas of tracer accumulation to be identified. Irradiation is minimal and no side effects are described, so it can be performed in anyone except pregnant or breastfeeding women. Thanks to the recent protocol proposed by Gillmore et al., myocardial scintigraphy with bone tracers allow the diagnosis of ATTR-CA without the need for EMB[
7].
2.3. Bone Tracers
Tracers used for bone scintigraphy are diphosphonates or pyrophosphates bound to metastable technetium-99 (99mTc) and have been used to bind amyloid since the 1980s [
9].
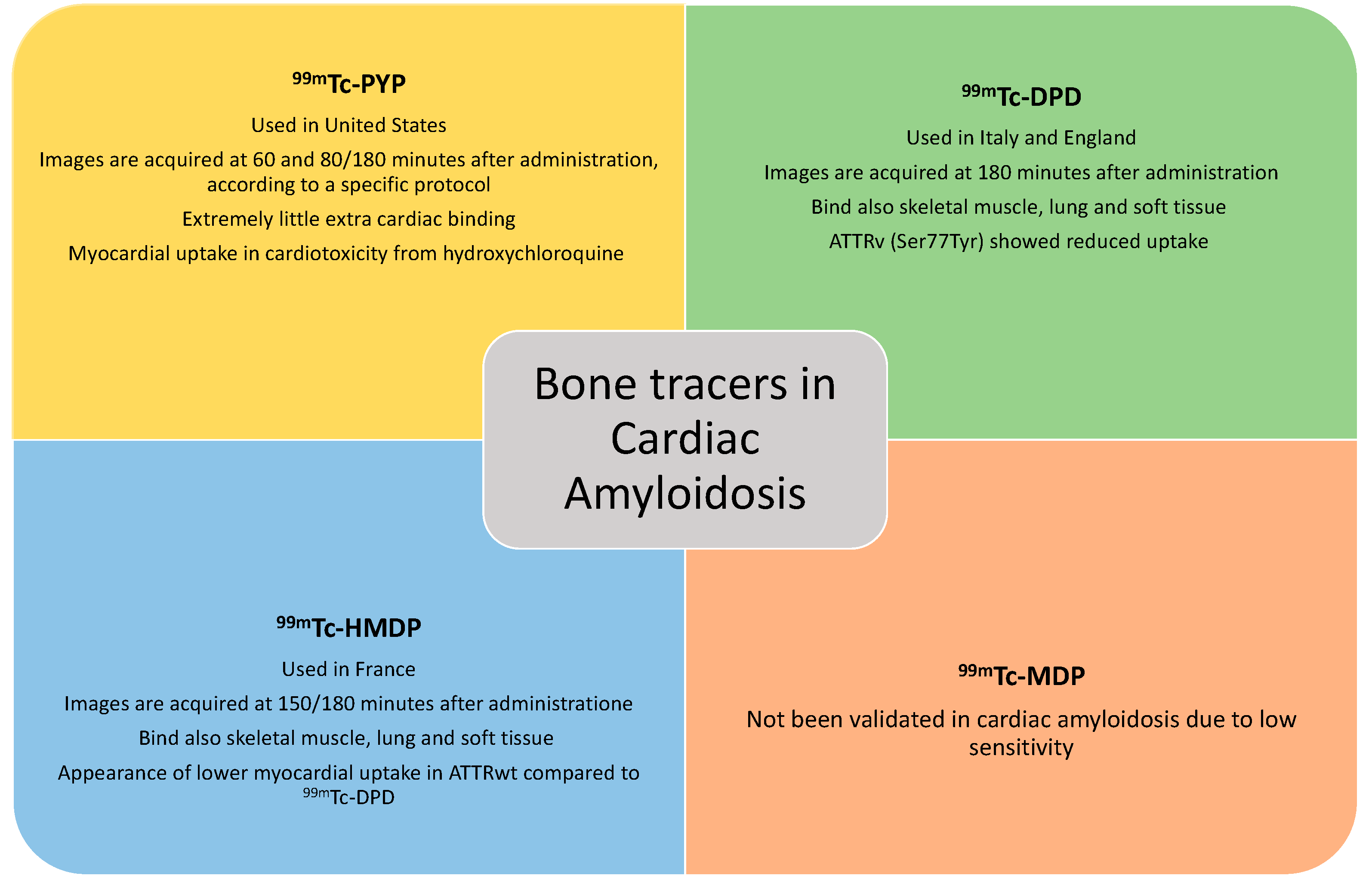
Bone tracers currently used to identify cardiac amyloid deposits are:
99mTc-pyrophosphate (
99mTc-PYP) [
10], the only one employed in America,
99mTc-3,3-diphosphone-1,2-propan-dicarboxylic acid (
99mTc- DPD) [
11], employed in England and Italy, and
99mTc-hydroxymethylene-diphosphonate (
99mTc-HMDP)[
12], being used in France, all essentially analogues from a diagnostic point of view [
13]. Although other bone tracers exist, they are not validated for non-invasive confirmation of ATTR-CA. For example,
99mTc methylene diphosphonate (
99mTc-MDP) has been associated with low sensitivity towards detection of cardiac amyloid infiltration and false negative results (
Figure 1). post-injection,
99mTc-PYP image is acquired at 60 minutes and 80 minutes or 3 hours (based on local protocol), and
99mTc-HMDP is acquired at 2.5 or 3 hours.
However, it is still unknown the mechanism by which these tracers bind amyloid and why uptake is greater in ATTR amyloidosis than in AL. It has been suggested that this difference may be due to a greater presence of microcalcifications in ATTR amyloidosis than in AL [
14][
15] or to the different type of fibrils associated with the C-terminal fragments of the protein (type A or type B) [
16]. Interestingly, in this recent study, the authors demonstrated that these microcalcifications are present in a dust-like form but, more importantly, their presence is not necessarily associated with amyloid deposits at the cardiac level [
14].
Compared to other bone tracers 99mTC-PYP accumulates substantially in myocardial TTR deposits while it has extremely little extracardiac binding [
17]. In contrast, with 99mTc-DPD/HMDP, amyloid deposition was also revealed in skeletal muscle, lung and soft tissue [
18].
There is only a single study designed as a head-to-head comparison of clinical accuracy of different bone tracers in patients with suspected CA and has been conducted at the National Amyloidosis Centre (NAC) in a cohort of patients being scanned with 99mTc-HMDP (locally) and, later, with 99mTc-DPD (at the NAC)[
19]. The median time interval between the first and second radionuclide scan was less than 5 months. Perugini grade differed between 99mTc-HMDP and 99mTc-DPD in 33% patients, all of whom had wild-type ATTR-CA, with lower myocardial uptake on 99mTc-HMDP (grade 1) in all such cases compared with 99mTc-DPD (grade 2). Based on these findings, prospective head-to-head comparison of the three approved radiotracers is required in the near future.
2.4. Cardiac Evaluation Methods
There are several quantitative and qualitative methods utilized to assess the presence and degree of bone tracer accumulation in the heart (
Table 1). Acquisition of both antero-posterior and latero-lateral projection is crucial to discriminate the site of accumulation on planar imaging. The most commonly used is the Perugini score, which qualitatively assesses on planar images the degree of cardiac uptake compared to bone uptake. Tracer uptake is then classified into 4 categories: grade 0: no cardiac uptake; grade 1: mild cardiac uptake, less than bone uptake; grade 2: moderate cardiac uptake accompanied by attenuated bone uptake; grade 3: strong cardiac uptake with mild/absent bone uptake [
20] [
21]. Recently, a new modified Perugini score has been proposed by Dorbala et al. in which radiotracer uptake is compared with bone uptake and in particular with rib uptake; however, clinical application of this modified score in the real world has not been validated yet[
22].
Semi-quantitative methods to assess the degree of myocardial uptake of bone tracers are the heart to contralateral (H/CL) ratio, heart to whole body ratio (H/WB), heart to pelvis ratio (H/P) and heart to skull ratio (H/S).
In particular, the H/CL method, validated only in scintigraphy with
99mTc-PYP, consists in comparing, on planar antero-posterior images, two regions of interest (ROI): the first drawn around the heart avoiding including sternum and lung and the other contralateral, of the same dimensions, not including the right ventricle (heart ROI/ CL ROI) [
20]. An H/CL ratio ≥1.5 at 1h after administration of the bone tracer, in association with a Perugini grade ≥2, has a sensitivity of 97% and a specificity of 100% in distinguishing ATTR amyloidosis from the AL form [
22]. An H/CL index ≥1.6 has been associated with poor outcome[
23].
3. Clinical Application of Cardiac Scintigraphy with Bone Tracers
Cardiac scintigraphy with bone tracers has proven to be a crucial technique to allow non-invasive diagnosis of ATTR CA in about 70% of cases [
24] [
25] as recommended by the position statement of the European Society of Cardiology
[26], and a useful tool in differentiating CA from other disease that cause of increased LV thickness [
7].
In the presence of a strong clinical, echocardiographic or cardiac MRI suspicion of CA, after ruling out the possible presence of a monoclonal component by serum and urinary immunofixation and serum light chain assay, a cardiac scintigraphy with bone tracers demonstrating a Perugini grade 2 or 3 myocardial uptake confirmes the diagnosis of ATTR-CA with an accuracy and specificity of 99% and 100% [
7]. Cardiac or extracardiac histological diagnosis is mandatory in all cases with evidence of monoclonal proteins in serum and/or urine.
Single-photon emission computed tomography (SPECT) should always be performed following acquisition of planar imaging, ideally with a hybrid SPECT/CT technique to increase specificity. SPECT allows three-dimensional images to be obtained to better understand the location of the bone tracer accumulation; planar images is no more recommended in isolation for the work up of patients with suspected CA. For example, SPECT is useful to differentiate myocardial uptake from the persistence of the bone tracer in the ventricular cavity (“blood pool”) or from uptake of thoracic bone structures overlapping the heart (i.e. rib fracture, metastatic bone lesion). [
20] [
27] [
28] In addition, SPECT allows focal myocardial uptake to be identified and helps in cases of falsely negative H/CL index due to patients with previous infarction and thus reduced vital myocardium.
However, there are further scenarios that must be considered to avoid inappropriate applications of scintigraphy or incorrect interpretations with the risk of important clinical implications, such as fatal misdiagnosis or inappropriate utilization of financial and biological resources. [
29] [
30]
The mere presence of uptake on bone tracer scintigraphy, which should always be considered an abnormal finding, is not sufficient in isolation for establishing the diagnosis of ATTR-CA as patients with AL CA and apolipoprotein A-I CA may also present with myocardial uptake on cardiac scintigraphy. Although anecdotical, cardiotoxicity from hydroxychloroquine has been associated to myocardial uptake of tracer on scintigraphy with 99mTc-PYP [
31].
The absence of myocardial uptake (i.e., Perugini grade 0), does not always exclude the diagnosis. In patients with suspected CA based on characteristic non-invasive cardiac imaging, absence of myocardial uptake is still consistent with low sensitivity of cardiac scintigraphy in presence of rare TTR mutations (i.e. S77Y variant), [
32] non-ATTR forms of amyloidosis [
33] [
34] or AL amyloidosis, and further tests should be considered to assess for CA.
4. Future Directions
4.1. Why Do Bone Tracers Work in Cardiac Amyloidosis?
The precise mechanism by which bone tracers function in amyloidosis is still unknown, although various hypotheses have been generated over the years. For example, as previously stated, certain studies support the possibility that bone tracers may bind microcalcifications that are more represented in ATTR CA than in AL, although histologically only a percentage of patients show significant calcifications, in each case more represented at bone level than at cardiac level; and yet the uptake of bone tracers remains greater at the cardiac level. Calcifications are probably not the only mechanism at play, not least because we are not sure of the ultimate ligand of the bone tracer, which could therefore be something else (amyloid? extracellular matrix? other?).
In one study it was shown that cardiac scintigraphy with bone tracers was very sensitive in diagnosing the disease in patients with a greater presence of type A amyloid fibrils, while in patients with only type B fibrils the scintigraphy did not detect them [
16].
4.2. Initial Cardiac Involvement
Through Gillmore's algorithm it is possible to diagnose phenotypically expressed disease, meaning that there must be clinical-instrumental suspicion to initiate the non-invasive diagnostic work-up; however, subjects with cardiac uptake on scintigraphy but asymptomatic and without a clinical context suggestive of amyloidosis are excluded from Gillmore's algorithm. This suggests that there is a difference between simple cardiac amyloid deposition and amyloid deposition in the context of a pathology, that is, amyloidotic cardiomyopathy (AC).
4.3. Negative Scintigraphy but Suspicious Phenotype, When to Perform Further Examinations?
Similarly, there are subjects with echocardiographic and cardiac MRI criteria suggestive of cardiac amyloidosis with, however, reduced or no uptake on cardiac scintigraphy (e.g. Perugini grade 0 or 1). In this case, the instrumental/scintigraphic discrepancy raises the suspicion of rare amyloidosis subtypes such as AL amyloidosis, ApoAI and ApoAIV amyloidosis and certain ATTRv (Val30Met, Phe64Leu, Ser77Tyr).
In a study by Musumeci et al. [
35] it was observed that cardiac scintigraphy with bone tracers was negative in 89% of patients with Phe64Leu ATTRv and had a reduced uptake in Val30Met ATTRv. Furthermore, Ser77Tyr also showed reduced uptake on scintigraphy with 99mTc-DPD despite typical echocardiographic and cardiac MRI features [
9], [
36]. Clearly these findings suggest that in the various ATTR subtypes there may be different pathogenesis, linked to different biological mechanisms [
37].
In these cases, SPECT-TC is very useful because it allows focal/regional radiotracer uptake to be recognized, as cardiac uptake often begins in the basal areas of the inferior interventricular septum and then extends in a baso-apical and septal-lateral direction.
4.4. Is Scintigraphy Useful in Asymptomatic TTR Gene Variant Carriers?
In cases of ATTRv in which an asymptomatic subject without echocardiographic or cardiac MRI criteria suggestive of amyloidosis is a carrier of a known mutation, scintigraphy could be used as a screening method since it detects cardiac involvement early even when the MRI is normal. Even a low-grade cardiac uptake (e.g. Perugini grade 1) configures cardiac involvement at an early stage [
38].
4.5. Is Cardiac Scintigraphy with Bone Tracers only Useful for Diagnosis? And for Tracking Progression of Heart Disease and Assessing Prognosis?
Cardiac scintigraphy with bone tracers also seems to provide rough information for prognosis: patients with Perugini grade 2 or 3 myocardial uptake in ATTR amyloidosis have a poorer outcome compared to those presenting with Perugini grade 1 myocardial uptake [
9] [
39] [
40]. However, the use of different bone tracers in different countries limits the possibility of comparison. Besides scintigraphy cannot be the method of choice in assessing disease progression nor in evaluating the response to specific therapies since it still creates biological damage and especially due to ignorance of the principles guiding cardiac uptake [
41].
Rather the use of SPECT improves diagnostic accuracy and can help in monitoring disease progression and response to specific therapy. In a recent work by Genovesi et al. it was shown that late cardiac uptake of [18F]-florbetaben by PET/CT may be able to discriminate ATTR amyloidosis from AL, possibly paving the way for non-invasive diagnosis even in AL amyloidosis. [
42] However, further studies are needed, also because of the significant variability of findings in individual patients. [
43] [
44]
Recently, Porcari et al proposed that right ventricular uptake on cardiac scintigraphy may be associated with an increased risk of all-cause mortality among patients with ATTR-CA [
45]. Although the reason is unclear, the authors speculate that biventricular uptake (left and right ventricle) may reflect a more advanced disease and a more advanced cardiac amyloid infiltration compared to isolated LV uptake. If confirmed in further dedicated research, characterization of presence and extent of RV uptake might become the first solid nuclear imaging parameter associated with overall survival among patients with either wild-type or variant ATTR-CA.
5. Conclusions
The development of a nonbiopsy algorithm for confirmation of ATTR-CA has deeply transformed the diagnosis approach to patient with suspected disease. Cardiac scintigraphy with bone tracer has entered the clinical arena and is now widely used worldwide as diagnostic tool coupled with exclusion of monoclonal protein in serum and urine. However, the mechanisms of action and the binding site of bone tracers in the amyloid heart still represents a great mystery which is crucial to understand to advancing clinical application of this technique for prognostication and monitoring treatment response.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. , “Systemic amyloidosis,” The Lancet, 2016, vol. 387, no. 10038, pp. 2641–2654. [CrossRef]
- Porcari, A.; Merlo, M.; Rapezzi, C.; Sinagra, G. , “Transthyretin amyloid cardiomyopathy: An uncharted territory awaiting discovery,” Eur. Journal of Int. Medicine, 2020, vol. 82, pp. 7–15. [CrossRef]
- Benson MD; Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee, Amyloid, vol. 25, no. 4, pp. 215–219, Oct. 2018. [CrossRef]
- Porcari, A.; Fontana, M.; Gillmore, J.D. Transthyretin cardiac amyloidosis, Cardiovasc Res, Aug. 2022. [CrossRef]
- Rossi M; Varrà GG, Porcari A, Saro R, Pagura L, Lalario A, Dore F, Bussani R, Sinagra G, Merlo M. Re-Definition of the Epidemiology of Cardiac Amyloidosis, Biomedicines, vol. 10, no. 7. MDPI, Jul. 01, 2022. [CrossRef]
- Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis, Circulation, vol. 140, no. 1, pp. 16–26, Jul. 2019. [CrossRef]
- Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis, Circulation, vol. 133, no. 24, pp. 2404–2412, Jun. 2016. [CrossRef]
- Chiti, F.; Dobson, C.M. , Protein Misfolding, AmyloidFormation, and HumanDisease: A Summary of Progress Over the Last Decade, Annu Rev Biochem, 2017.
- Martinez-Naharro, A.; Baksi, A.J.; Hawkins, P.N.; Fontana, M. , Diagnostic imaging of cardiac amyloidosis, Nature Reviews Cardiology, vol. 17, no. 7. Nature Research, pp. 413–426, Jul. 01, 2020. [CrossRef]
- Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis, JAMA Cardiol, vol. 1, no. 8, pp. 880–889, Nov. 2016. [CrossRef]
- Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, Lane T, Whelan CJ, Lachmann HJ, Gillmore JD, et al. Utility and limitations of 3,3-diphosphono-1, 2-propanodicarboxylic acid scintigraphy in systemic amyloidosis, Eur Heart J Cardiovasc Imaging, vol. 15, no. 11, pp. 1289–1298, Nov. 2014. [CrossRef]
- Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, Dierckx RA, Slart RH, Hazenberg BP. Bone scintigraphy with 99m technetiumhydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis, Amyloid, pp. 21:35-44, 2014. [CrossRef]
- Rapezzi, C.; Gagliardi, C.; Milandri, A. , Analogies and disparities among scintigraphic bone tracers in the diagnosis of cardiac and non-cardiac ATTR amyloidosis, Journal of Nuclear Cardiology, vol. 26, no. 5. Springer New York LLC, pp. 1638–1641, Oct. 01, 2019. [CrossRef]
- Thelander U, Westermark GT, Antoni G, Estrada S, Zancanaro A, Ihse E, Westermark P, Cardiac microcalcifications in transthyretin (ATTR) amyloidosis, Int J Cardiol, vol. 352, pp. 84–91, Apr. 2022. [CrossRef]
- Stats, M.A.; Stone, J.R. , Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis, Cardiovascular Pathology, vol. 25, no. 5, pp. 413–417, Sep. 2016. [CrossRef]
- Pilebro, B.; Suhr, O.B.; Näslund, U.; Westermark, P.; Lindqvist, P.; Sundström, T. , 99mTc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis, Ups J Med Sci, vol. 121, no. 1, pp. 17–24, Jan. 2016. [CrossRef]
- Sperry, B.W.; Gonzalez, M.H.; Brunken, R.; Cerqueira, M.D.; Hanna, M.; Jaber, W.A. , Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis, Journal of Nuclear Cardiology, vol. 26, no. 5, pp. 1630–1637, Oct. 2019. [CrossRef]
- Hutt DF, Gilbertson J, Quigley AM, Wechalekar AD, 99m Tc-DPD scintigraphy as a novel imaging modality for identification of skeletal muscle amyloid deposition in light-chain amyloidosis., Amyloid, pp. 23:134–5., 2016.
- Porcari A, Hutt DF, Grigore SF, Quigley AM, Rowczenio D, Gilbertson J, Patel R, Razvi Y, Ioannou A, Rauf MU, Comparison of different technetium-99mlabelled bone tracers for imaging cardiac amyloidosis, Eur J Prev Cardiol, vol. 30, no. 3, pp. E4–E6, Feb. 2023. [CrossRef]
- Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans AWJM et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2—Diagnostic criteria and appropriate utilization, Journal of Nuclear Cardiology, 2019. [CrossRef]
- Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi-Reggiani L et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy, J Am Coll Cardiol, vol. 46, no. 6, pp. 1076–1084, Sep. 2005. [CrossRef]
- Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans AWJM et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—evidence base and standardized methods of imaging, Journal of Nuclear Cardiology, vol. 26, no. 6, pp. 2065–2123, Dec. 2019. [CrossRef]
- Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis, JAMA Cardiol, vol. 1, no. 8, pp. 880–889, Nov. 2016. [CrossRef]
- Porcari A, Baggio C, Fabris E, Merlo M, Bussani R, Perkan A, Sinagra G. Endomyocardial biopsy in the clinical context: current indications and challenging scenarios, Heart Failure Reviews, vol. 28, no. 1. Springer, pp. 123–135, Jan. 01, 2023. [CrossRef]
- Sinagra, G.; Porcari, A.; Fabris, E.; Merlo, M. , Standardizing the role of endomyocardial biopsy in current clinical practice worldwide, European Journal of Heart Failure, vol. 23, no. 12. John Wiley and Sons Ltd, pp. 1995–1998, Dec. 01, 2021. [CrossRef]
- Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases, Eur Heart J, vol. 42, no. 16, pp. 1554–1568, Apr. 2021. [CrossRef]
- Mattana F, Muraglia L, Girardi F, Cerio I, Porcari A, Dore F, Bonfiglioli R, Fanti S, Clinical application of cardiac scintigraphy with bone tracers: controversies and pitfalls in cardiac amyloidosis, Vessel Plus, 2022. [CrossRef]
- Porcari A, Rossi M, Dore F, Imazio M, Fontana M, Merlo M, Sinagra G, Ten questions for the cardiologist about cardiac scintigraphy with bone tracers, amyloidosis and the heart, G Ital Cardiol (Rome), pp. 23(6):424-432, 2022.
- Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, Hoffman J, Jaber W, Soman P, Witteles RM, Grogan M. Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis: JACC Review Topic of the Week, Journal of the American College of Cardiology, vol. 75, no. 22. Elsevier USA, pp. 2851–2862, Jun. 09, 2020. [CrossRef]
- Porcari, A.; Fontana, M.; Gillmore, J.D. , Letter by Porcari et al Regarding Article, Association Between Atrial Uptake on Cardiac Scintigraphy With Technetium-99m-Pyrophosphate Labeled Bone-Seeking Tracers and Atrial Fibrillation, Circulation: Cardiovascular Imaging, vol. 15, no. 9. Lippincott Williams and Wilkins, p. E014692, Sep. 01, 2022. [CrossRef]
- Ian, C.Y. Chang, John P. Bois, Melanie C. Bois, Joseph J. Maleszewski, Geoffrey B. Johnson, and Martha Grogan, Hydroxychloroquine-Mediated Cardiotoxicity With a False-Positive 99mTechnetium–Labeled Pyrophosphate Scan for Transthyretin-Related Cardiac Amyloidosis, Circ Cardiovasc Imaging. Elsevier USA, p. 2466, 2018. [CrossRef]
- Porcari A, Razvi Y, Masi A, Patel R, Ioannou A, Rauf MU, Hutt DF, Rowczenio D, Gilbertson J, Martinez-Naharro A et al. Prevalence, characteristics and outcomes of older patients with hereditary versus wild-type transthyretin amyloid cardiomyopathy, Eur J Heart Fail, vol. 25, no. 4, pp. 515–524, Apr. 2023. [CrossRef]
- Lucchini E, Merlo M, Ballerini M, Porcari A, Sinagra G, Pagnan L, Rensi M, Romano A, Bussani R, Ballotta L, Zaja F, Case Report: Cardiac Involvement by Lymphoma: Rare but Heterogeneous Condition With Challenging Behaviors, Front Oncol, vol. 11, Apr. 2021. [CrossRef]
- Ioannou A, Porcari A, Patel RK, Razvi Y, Sinigiani G, Martinez-Naharro A, Venneri L, Moon J, Rauf MU, Lachmann H et al. Rare Forms of Cardiac Amyloidosis: Diagnostic Clues and Phenotype in Apo AI and AIV Amyloidosis, Circ Cardiovasc Imaging, vol. 16, no. 7, pp. 523–535, Jul. 2023. [CrossRef]
- Musumeci MB, Cappelli F, Russo D, Tini G, Canepa M, Milandri A, Bonfiglioli R, Di Bella G, My F, Luigetti M, Grandis M et al. Low Sensitivity of Bone Scintigraphy in Detecting Phe64Leu Mutation-Related Transthyretin Cardiac Amyloidosis, JACC Cardiovasc Imaging, vol. 13, no. 6, pp. 1314–1321, Jun. 2020. [CrossRef]
- Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, Kotecha T, Francis R, Hutt DF, Rezk T et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis, J Am Coll Cardiol. 2017 Jul 25;70(4):466-477. [CrossRef] [PubMed]
- Alexander, K.M.; Witteles, R.M. , Bone Scintigraphy Imaging for Transthyretin Cardiac Amyloidosis: Still Much to Learn, JACC: Cardiovascular Imaging, vol. 13, no. 6. Elsevier Inc., pp. 1322–1324, Jun. 01, 2020. [CrossRef]
- Ioannou A, Patel RK, Razvi Y, Porcari A, Knight D, Martinez-Naharro A, Kotecha T, Venneri L, Chacko L, Brown J et al. Multi-Imaging Characterization of Cardiac Phenotype in Different Types of Amyloidosis, JACC Cardiovasc Imaging, Sep. 2022. [CrossRef]
- Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, Ferlini A, Longhi S, Lorenzini M, Reggiani LB et al. Role of 99mTc-DPD Scintigraphy in Diagnosis and Prognosis of Hereditary, JACC Cardiovasc Imaging, p. VOL. 4, NO. 6, 2011.
- Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, Guendouz S, Tissot CM, Hittinger L, Dubois-Randé JL et al. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis., Amyloid, 2015;22(4):210-20. [CrossRef]
- Scully PR, Morris E, Patel KP, Treibel TA, Burniston M, Klotz E, Newton JD, Sabharwal N, Kelion A, Manisty C et al. DPD Quantification in Cardiac Amyloidosis: A Novel Imaging Biomarker, JACC Cardiovasc Imaging, vol. 13, no. 6, pp. 1353–1363, Jun. 2020. [CrossRef]
- Genovesi D, Vergaro G, Giorgetti A, Marzullo P, Scipioni M, Santarelli MF, Pucci A, Buda G, Volpi E, Emdin M. [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. JACC Cardiovasc Imaging. 2021 Jan;14(1):246-255. [CrossRef]
- Ross JC, Hutt DF, Burniston M, Grigore SF, Fontana M, Page J, Hawkins PN, Gilbertson JA, Rowczenio D, Gillmore JD, The role of serial 99mTc-DPD scintigraphy in monitoring cardiac transthyretin amyloidsis, Amyloid. 2022 pp. 29:38-49., 2022. [CrossRef]
- Aldostefano Porcari, Alberto Aimo, Giuseppe Vergaro, Marco Merlo, Michele Emdin, and Gianfranco Sinagra, “Ten perspectives for research and innovation in cardiac amyloidosis,” 2023.
- Porcari A, Pagura L, Canepa M, Biagini E, Cappelli F, Gagliardi C, Longhi S, Tini G, Dore F, Bonfiglioli R et al. Prevalence and prognostic significance of RVuptake (biventricular uptake)at planar scintigraphy in patients with ATTR cardiac amyloidosis, Eur Heart J Suppl, 2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).