1. Introduction
The origin of the Arazá is attributed to the Peruvian Amazon region, more precisely in Loreto (Peru) near the source of the Amazon River where the greatest genetic diversity is reported for its two subspecies (sororia and stipitata). The stipitata variety spread between countries that share Amazonia such as Peru and Brazil [
1].
In the Colombian region its origin comes from the sexual seeds brought from Manaus-Brazil and Iquitos-Peru. This plant is characterized by having a globose and somewhat depressed berry fruit. Climatic factors affect its production and cultivation, such that it requires large amounts of rainwater (annual rainfall of 2900 mm), little sunlight (sunshine of 1650 hours per year), an average temperature of 26 °C and a relative humidity of 84%. Since 1989, the domestication of this fruit began in Colombia, starting in the departments of Amazonas and its proximity to Peru and Brazil. It later expanded to the departments of Caquetá, Guaviare, Meta, Cundinamarca, Caldas, Antioquia, and Putumayo [
2].
The compounds that abound in the fruit and that have been registered through research include flavonoids, carotenoids, and phenolic acids. These are bioactive compounds with great health benefits for people, who can obtain from them antioxidant, anticarcinogenic, antimicrobial, anti-inflammatory, antispasmodic, anti-ulcer, and cardiovascular disease prevention properties [
3].
Given the excellent and diverse climatic characteristics, Colombia has a great potential in a variety of promising native fruits for their exploitation and transformation into different types of products. The consumption of this type of fruit is not exclusively for taste. Currently there is a growing concern for improving health conditions, and there is an interest not only in the nutritional value but also in its ability to prevent or assist in the treatment of various diseases. This is the case of the Eugenia Stipitata fruit, which has been found to have anti-inflammatory and antipyretic properties without showing toxicity in mice [
4,
5].
The objective of this review is to make a compilation of the compounds of the Amazonian fruit that have already been fully identified and to obtain information on the secondary metabolites found to establish their possible preventive and therapeutic effects on human health.
2. Materials and Methods
2.1. Literature search
For this study a search was carried out in repositories of Colombian universities, in the Network of Latin American Repositories, LA REFERENCIA (network of open access repositories), SINCHI (Amazonian Institute of Scientific Research), Alexander von Humboldt (Institutional Repository of Scientific Documentation), in databases to which the University of Caldas has access: Science Direct, Scopus, PubMed (MedLine), Web of Science, Agriculture Collection Gale, SAGE, OXFORD University, DOAJ, as well as open access resources (Scielo and Redalyc).
In addition, ResearchGate and Google Scholar databases were examined. These searches were conducted between May 17 and September 30, 2021. The keywords used were, Eugenia stipitata, bioactive compounds, polyphenols, flavonoids. The search strategies were “Eugenia stipitata, AND compounds bioactive, OR polyphenols, OR flavonoids”. “Repository:”, symbol “+”, “Eugenia Stipitata”, “Arazá”, “Eugenia Stipitata AND Bioactive”, “Flavonoids AND Eugenia Stipitata”, “Eugenia Stipitata AND polyphenols”.
Google Scholar included words such as: “Eugenia stipitata “+”bioactive compounds “+”polyphenols”; repository: “eugenia stipitata “+”bioactive “+”polyphenols”; repository: “eugenia stipitata”. Studies in Portuguese, English and Spanish were included.
2.2. Inclusion and Exclusion Criteria
Articles identifying and quantifying bioactive compounds present in Eugenia Stipitata Mc Vaugh were included. We did not include articles that presented logical inconsistencies related to the instruments and analytical methods for obtaining bioactive compounds, nor those that studied species other than Eugenia Stipitata Mc Vaugh.
2.3. Inclusion and Exclusion Criteria
The articles that met the inclusion criteria were recorded in an Excel sheet along with the identification and quantification data of the compounds according to the different parts of the fruit (pulp, peel, seed). In addition, moisture, number of samples treated, type of compound, chemical formula and a confidence code of the references were included. The data were validated by two of the study investigators.
3. Results
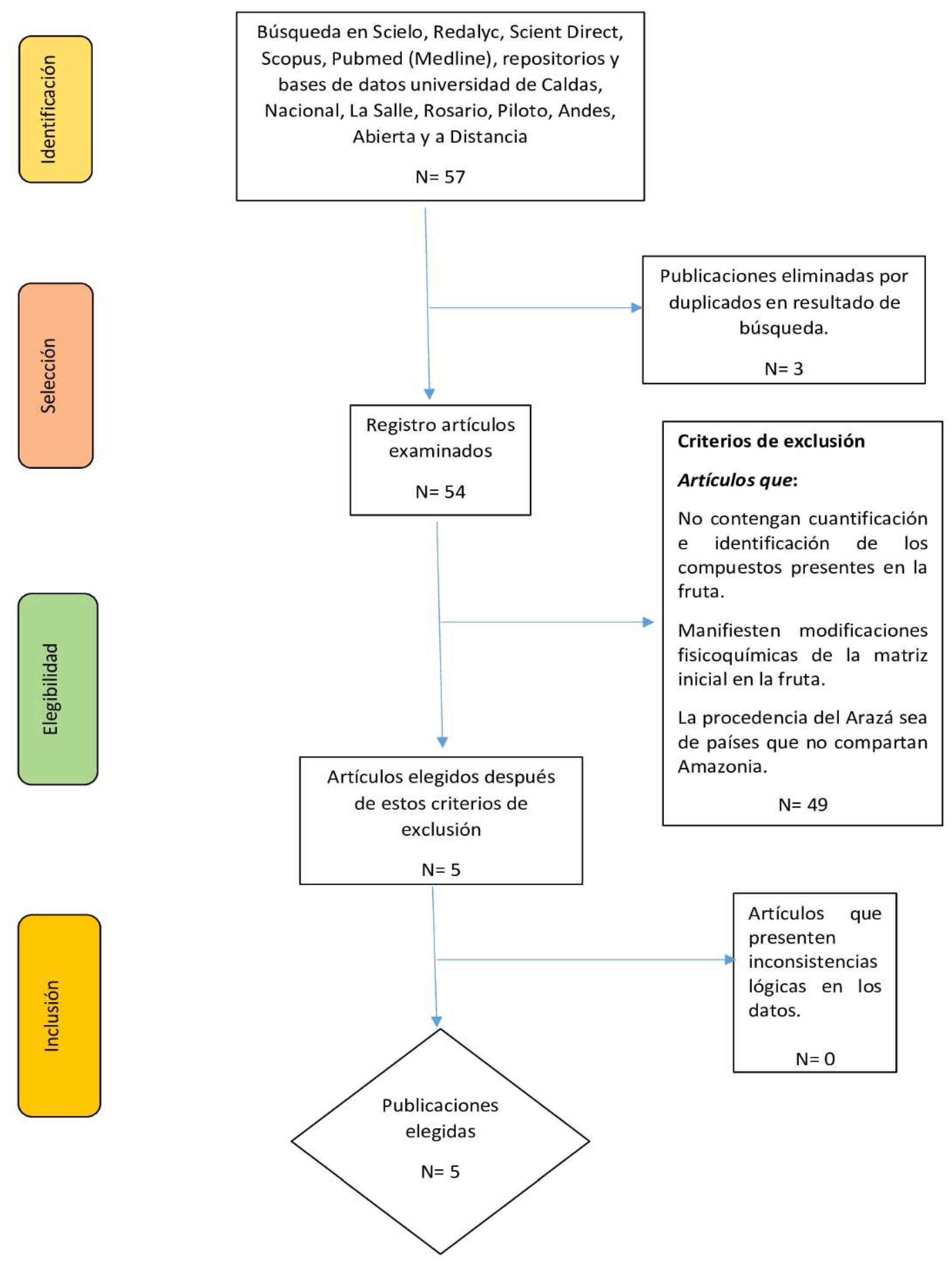
Figure 1 shows the PRISMA diagram with the different phases of the literature review. Following the identification, screening, and eligibility phases, 5 studies that met the inclusion criteria were included. In this study, 82 compounds were found, of which 24 were identified and quantified and 58 were only identified (
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5). Based on these results, it was possible to obtain an important compendium of bioactive compounds whose totality had not been reported in previous studies [
6].
The samples treated for fruit moisture calculation by [
7] for both pulp and peel are 6. The compounds reported by [
8] are part of the combination of pulp and peel. The quercetin and kaempferol values contributed by [
9,
10] were reported as the average of both authors. The values selected by [
11] were taken from the ripe fruit. The compounds identified by [
12] are part of the pulp and peel mixture.
3.1. Carotenoids
3.1.1. Identified and quantified
This group has the highest number of compounds reported with identification and quantification. [
7] found 14 compounds in both pulp and peel. In relation to the total concentration, it was possible to identify that the carotenoids in peel are approximately 3 times more than the content found in the pulp. Due to the high concentration of carotenoids in the peel, a higher antioxidant power is attributed to this part of the fruit.
Another study [
8] found 4 new compounds (violaxanthin, 15-cis-β-carotene, 13-cis-β-carotene, 9-cis-β-carotene) from the homogenization of pulp and peel. High percentages (45%) of bioaccessibility were also recorded for zeaxanthin, 15-cis-β-carotene, and α-carotene compounds, i.e., those components that are released from the food matrix in greater proportion during digestion and become available for absorption.
The most abundant carotenoids are lutein (18.1%–18.2%), β-cryptoxanthin (5.56%-27.6%), β-carotene (5.21%–23.8%) and violaxanthin (11.8%).
3.1.2. Identified and unquantified
According to [
7] the study reported only one unquantified compound called anhydro zeaxanthin found in pulp and peel. In turn, the study describes the presence of an unknown compound possibly rubixanthin-palmitate. This would indicate the possible existence of other carotenoids that have not been reported.
Table 1.
Compounds Identified and Quantified (carotenoids pulp).
Table 1.
Compounds Identified and Quantified (carotenoids pulp).
| N° |
Average humidity |
Class |
Type |
Chemical Formula |
Result |
SD |
Quantity |
† |
| Min |
Max |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Lutein |
C40H56O2 |
0,15 |
0,11 |
0,05 |
0,26 |
[7] |
| 3 |
93,4 |
Xanthophyll |
Lutein |
C40H56O2 |
0,16 |
NA |
- |
- |
[8] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Lutein myristate palmitate |
C70H112O4 |
0,10 |
0,017 |
0,082 |
0,12 |
[7] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
β-Cryptoxanthin palmitate |
C56H86O2 |
0,092 |
0,038 |
0,054 |
0,13 |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Lutein dipalmitate |
C72H116O4 |
0,091 |
0,018 |
0,073 |
0,11 |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Anhydroluteina |
C40H54O |
0,063 |
0,024 |
0,039 |
0,087 |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Zeinoxanthin Palmitate |
C56H86O3 |
0,054 |
0,022 |
0,032 |
0,076 |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
β-Cryptoxanthin |
C40H56O |
0,047 |
0,027 |
0,020 |
0,074 |
| 3 |
93,4 |
Xanthophyll |
β-Cryptoxanthin |
C40H56O |
0,24 |
NA |
- |
- |
[8] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Lutein Dimyristate |
C68H108O4 |
0,047 |
0,0090 |
0,038 |
0,056 |
[7] |
| 3 |
93,6 ± 1,0 |
Carotene |
β-Carotene |
C40H56 |
0,044 |
0,016 |
0,028 |
0,060 |
| 3 |
93,4 |
Carotene |
Todo-trans-β-Caroteno |
C40H56 |
0,21 |
NA |
- |
- |
[8] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
β-Cryptoxanthin myristate |
C54H82O2 |
0,043 |
0,022 |
0,021 |
0,065 |
[7] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Zeinoxanthin myristate |
C54H82O2 |
0,038 |
0,016 |
0,022 |
0,054 |
| 3 |
93,6 ± 1,0 |
Carotene |
α-Carotene |
C40H56 |
0,031 |
0,014 |
0,017 |
0,045 |
| 3 |
93,4 |
Carotene |
Todo-trans-α-Carotene |
C40H56 |
0,065 |
NA |
- |
- |
[8] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Zeaxanthin |
C40H56O |
0,025 |
0,020 |
0,0050 |
0,045 |
[7] |
| 3 |
93,6 ± 1,0 |
Xanthophyll |
Zeaxanthin |
C40H56O2 |
0,017 |
0,0090 |
0,0080 |
0,026 |
| 3 |
93,4 |
Xanthophyll |
Zeaxanthin |
C40H56O2 |
0,055 |
NA |
- |
- |
[8] |
| 3 |
93,4 |
Xanthophyll |
Violaxanthin |
C40H56O4 |
0,10 |
NA |
- |
- |
| 3 |
93,4 |
Carotene |
15-cis-β Carotene |
C40H56 |
0,011 |
NA |
- |
- |
| 3 |
93,4 |
Carotene |
13-cis-β Carotene |
C40H56 |
0,016 |
NA |
- |
- |
| 3 |
93,4 |
Carotene |
9-cis-β Carotene |
C40H56 |
0,018 |
NA |
- |
- |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Anhydroluteina |
C40H54O |
0,14 |
0,025 |
0,11 |
0,16 |
Table 2.
Compounds Identified and Quantified (carotenoids peel).
Table 2.
Compounds Identified and Quantified (carotenoids peel).
| N° |
Average humidity |
Class |
Type |
Chemical Formula |
Result |
SD |
Quantity |
† |
| Min |
Max |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Lutein |
C40H56O2 |
0,76 |
0,12 |
0,64 |
0,87 |
[7] |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Lutein dipalmitate |
C72H116O4 |
0,26 |
0,046 |
0,21 |
0,30 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Lutein myristate palmitate |
C70H112O4 |
0,24 |
0,032 |
0,20 |
0,27 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Zeinoxanthin palmitate |
C56H86O3 |
0,19 |
0,024 |
0,16 |
0,21 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
β-Criptoxantina palmitato |
C56H86O2 |
0,15 |
0,032 |
0,12 |
0,19 |
| 3 |
89,0 ± 2,4 |
Carotene |
β-Carotene |
C40H56 |
0,14 |
0,025 |
0,12 |
0,17 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
β-Criptoxantina |
C40H56O |
0,14 |
0,045 |
0,10 |
0,19 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Anhydroluteina |
C40H54O |
0,14 |
0,025 |
0,11 |
0,16 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Zeinoxanthin |
C40H56O2 |
0,11 |
0,049 |
0,065 |
0,16 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Zeinoxanthin myristate |
C54H82O2 |
0,10 |
0,006 |
0,10 |
0,11 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Lutein Dimyristate |
C68H108O4 |
0,10 |
0,014 |
0,087 |
0,12 |
| 3 |
89,0 ± 2,4 |
Carotene |
α-Carotene |
C40H56 |
0,096 |
0,020 |
0,076 |
0,12 |
| 3 |
89,0 ± 2,4 |
Xanthophyll |
Zeinoxanthin |
C40H56O |
0,081 |
0,023 |
0,058 |
0,10 |
| 3 |
89,0 ± 2,5 |
Xanthophyll |
β-Cryptoxanthin myristate |
C54H82O2 |
0,077 |
0,010 |
0,067 |
0,09 |
3.2. Phenolic acids
3.2.1. Identified and quantified
[
11] determined that the pulp and peel of ripe Arazá have a high antioxidant power thanks to this group of compounds. These authors identified and quantified chlorogenic acid, gallic acid and caffeic acid as the main phenolic acids. It has also been found that chlorogenic acid in pulp increases its concentration as fruit ripening progresses, its concentration then goes from 4.41 mg/100g (green state) to 7.56 mg/100 g (ripe state). The same happens with gallic acid in the peel, which increases from 2.32 mg/100 g (green state) to 3.91 mg/100 g (ripe state). Chlorogenic acid is found in higher concentration in the ripe state in peel (39.6 mg/100 g) and pulp (7.56 mg/100g) followed by gallic acid respectively (3.91 mg/100g and 2.94 mg/100g). Of these 3 compounds chlorogenic acid, given its high concentration, has the highest antioxidant capacity, followed by gallic acid. The presence of ellagic acid is also relevant since it has a high free radical scavenging capacity [
13,
14].
3.2.2. Identified and unquantified
[
15] described 9 compounds present in the mixture of peel and pulp. They also reported 6 phenolic acids in the seed, describing that the phenolic content in the seed is 15 times higher than that found in the peel and pulp, which would indicate a greater antioxidant potential. This finding was confirmed by [
16] who also reported for the first time the presence of p-coumaric acid and sinapic acid. On the other hand, [
13] provide 4 different compounds in the pulp (Ellagic acid hexoside, Vanillic acid-O-hexoside II, Coumaric acid-O-Hexoxide I, Pinoresinol hexoside).
Table 3.
Compounds Identified and Quantified (Phenolic acids pulp).
Table 3.
Compounds Identified and Quantified (Phenolic acids pulp).
| N° |
Average humidity |
Class |
Type |
Chemical Formula |
Result |
SD |
Quantity |
† |
| Min |
Max |
| 3 |
NA |
Hydroxycinnamic acid |
Chlorogenic acid |
C16H18O9 |
7,6 |
NA |
- |
- |
[11] |
| 3 |
NA |
Hydroxybenzoic acid |
Gallic acid |
C7H6O5 |
2,9 |
NA |
- |
- |
| 3 |
NA |
Hydroxycinnamic acid |
Caffeic acid |
C9H8O4 |
0,40 |
NA |
- |
- |
Table 4.
Compounds Identified and Quantified (Phenolic acids peel).
Table 4.
Compounds Identified and Quantified (Phenolic acids peel).
| N° |
Average humidity |
Class |
Type |
Chemical Formula |
Result |
SD |
Quantity |
† |
| Min |
Max |
| 3 |
NA |
Hydroxycinnamic acids |
Chlorogenic acid |
C16H18O9 |
40 |
NA |
- |
- |
[11] |
| 3 |
NA |
Hydroxycinnamic acids |
Gallic acid |
C7H6O5 |
3,9 |
NA |
- |
- |
| 3 |
NA |
Hydroxycinnamic acids |
Caffeic acid |
C9H8O4 |
1,1 |
NA |
- |
- |
3.3. Flavonoids
3.3.1. Identified and quantified
For this group the pulp was evaluated in which myricetin, quercetin and kaempferol were found. Myricetin being the major compound with (17 mg/100 g) in pulp [
9,
10].
3.3.2. Identified and unquantified
[
13] found 6 compounds in the pulp that had not been previously reported. On the other hand, [
12] recently found 9 positive ion compounds in the pulp and peel that also had not been previously reported. These same authors evaluated the seeds of the fruit and found 11 compounds, 1 of them eugenitin as a negative ion and the remaining as positive ions (
Table 6).
Table 5.
Compounds Identified and Quantified (flavonoids pulp).
Table 5.
Compounds Identified and Quantified (flavonoids pulp).
| N° |
Average humidity |
Class |
Type |
Chemical Formula |
Result |
SD |
Quantity |
† |
| Min |
Max |
| 3 |
87,9 ± 0,1 |
Flavonol |
Myricetin |
C15H10O8 |
17 |
0,50 |
17 |
18 |
[9] |
| 3 |
87,9 ± 0,1 |
Flavonol |
Quercetin |
C15H14O6 |
9,8 |
0,42 |
9,4 |
10 |
[9,10] |
| 3 |
87,9 ± 0,1 |
Flavonol |
Kaempferol |
C15H10O6 |
3,1 |
1,1 |
2,0 |
4,2 |
Table 6.
Compounds Identified and unquantified (pulp, peel, and seed).
Table 6.
Compounds Identified and unquantified (pulp, peel, and seed).
| Carotenoids (pulp) |
| N° |
Class |
Type |
Chemical Formula |
† |
| 1 |
Xanthophyll |
Anhydrozeaxanthin |
C40H56O2 |
[7] |
| Flavonoid (pulp) |
| 1 |
Flavan-3-ol |
Gallocatechin-Gallate |
C22H18O11 |
[13] |
| 2 |
Flavonol |
Dihydroquercetin glycoside |
C21H22O12 |
| 3 |
Flavanone |
Hydrated naringin |
C27H34O15 |
| 4 |
Flavanone |
Eriodictyol-7-O-glucoside VI |
C21H22O11 |
| 5 |
Flavan-3-ol |
Epicatechin-gallate |
C22H18O10 |
| 6 |
Flavan-3-ol |
Epicatechin |
C15H14O6 |
| 7 |
Flavone |
Apigenin hexoside (positive ion) [M + H - H2O] + |
C21H20O10 |
[12] |
| 8 |
Flavone |
Apigenin Hexoside Caffeate (positive Ion) [M + H - H2O] |
C36H36O17 |
| 9 |
Flavone |
Methylapigenin hexoside (positive ion) [M + Na] + |
C22H22O9 |
| 10 |
Flavan-3-ol |
Catechin dihexoside (positive ion) [M + H] + |
C27H34O16 |
| 11 |
Flavan-3-ol |
Gallocatechin (positive ion) [M + H] + |
C15H14O7 |
| 12 |
Flavone |
Luteolin hexoside (positive ion) [M + H] + |
C20H18O10 |
| 13 |
Flavone |
Luteolin Malonyldihexoside (positive Ion)) [M + Na] + |
C30H32O18 |
| 14 |
Flavonol |
Kaempferol hydroxypropionylhexoside (positive ion) [M + K] + |
C30H34O18 |
| 15 |
Flavonol |
Quercetin hexopyranosyl -hexoside (positive ion) [M + Na] + |
C26H28O16 |
| Phenolic Acids (pulp) |
| 1 |
Hydroxybenzoic acid |
Glycogalic acid (negative ion) [M-H]- |
C13H16O10 |
[12] |
| 2 |
Hydroxycinnamic acid |
Caffeoylquinic Acid (positive Ion) [M + K] + |
C16H18O9 |
| 3 |
Hydroxycinnamic acid |
Caffeoyl tartaric acid (positive Ion) [M + H] + |
C13H12O9 |
| 4 |
Hydroxycinnamic acid |
Caffeoyl-hexose (positive Ion) [M + K] + |
C15H18O9 |
| 5 |
Hydroxycinnamic acid |
Caffeoyl methylquinic acid (positive ion) [M + Na] + |
C17H20O9 |
| 6 |
Hydroxycinnamic acid |
Coumaroyl tartaric acid (positive ion) [M + H +H2O] |
C13H12O8 |
| 7 |
Hydroxybenzoic acid |
Fertaric acid (positive ion) [M + K] + |
C14H14O9 |
| 8 |
Hydroxybenzoic acid |
Gallic acid hexoside (positive ion) [M + K] + |
C13H16O10 |
| 9 |
Hydroxybenzoic acid |
Vanillic Acid (positive ion) [M + K] + |
C8H8O4 |
| 10 |
Hydroxybenzoic acid |
Ellagic acid hexoside |
C20H16O13 |
[13] |
| 11 |
Hydroxybenzoic acid |
Vanillic acid-O-hexoside II |
C14H18O9 |
| 12 |
Hydroxycinnamic acid |
Coumaric acid - O - Hexoside I |
C15H18O8 |
| 13 |
Hydroxycinnamic acid |
Hexoside Pinoresinol |
C26H30O11 |
| Hydrolyzable Tannins (pulp) |
| 1 |
Elagitanino |
Telimagrandin II/pterocaryanin C |
C41H30O26 |
[13] |
| 2 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) I |
C41H28O26 |
| 3 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) II |
C41H28O26 |
| 4 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) Derivative I |
C41H28O26 |
| 5 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) Derivative II |
C41H28O26 |
| 6 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) Derivative III |
C41H28O26 |
| 7 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) Derivative IV |
C41H28O26 |
| 8 |
Di-HHDP-galloyl-glucose (casuarictin/potentilin) Derivative V |
C41H28O26 |
| Flavonoid (seed) |
| 1 |
Chromone |
Eugenitine (Negative Ion) [M + Cl]- |
C12H12O4 |
[12] |
| 2 |
Flavona |
Apigenin hexoside (positive Ion) [M + H - H2O] |
C21H20O10 |
| 3 |
Flavona |
Apigenin caffeate hexoside (positive Ion) [M + H - H2O] |
C36H36O17 |
| 4 |
Flavona |
Methylapigenin hexoside (positive ion) [M + Na] + |
C22H22O9 |
| 5 |
Flavan-3-ol |
Catechin dihexoside (positive ion) [M + H] + |
C27H34O16 |
| 6 |
Flavan-3-ol |
Catechin hexoside (positive ion) [M + H] + |
C21H24O10 |
| 7 |
Flavonol |
Kaempferol diacetyl dicoumaroylhexoside (positive ion) [M + K] + |
C43H36O17 |
| 8 |
Flavonol |
Kaempferol dihexoside (positive Ion) [M + H - H2O] |
C39H50O23 |
| 9 |
Flavona |
Luteolin hexoside (positive ion) [M + H] + |
C20H18O10 |
| 10 |
Flavonol |
Myricetin coumaridihexoside hexoside (positive on) [M + K] + |
C42H46O23 |
| 11 |
Flavonol |
Quercetin hexopyranosyl-hexoside (positive ion) [M + Na] + |
C26H28O16 |
| 12 |
Flavonol |
Isoquercitrin |
C21H20O12 |
[16] |
| 13 |
Dihydrochalcone |
Floridzin |
C21H24O10 |
| Phenolic Acids (seed) |
| 1 |
Hydroxybenzoic acid |
Gallic acid (negative ion) [M-H]- |
C7H6O5 |
[12] |
| 2 |
Hydroxycinnamic acid |
Caffeoylquinic Acid (positive Ion) [M + K] + |
C16H18O9 |
| 3 |
Hydroxycinnamic acid |
Caffeoyl methylquinic acid (positive ion) [M + Na] + |
C17H20O9 |
| 4 |
Hydroxycinnamic acid |
Coumaroyl tartaric acid (positive Ion) [M + H - H2O] + |
C13H1208 |
| 5 |
Hydroxybenzoic acid |
Gallic acid hexoside (positive ion) [M + K] + |
C13H16O10 |
| 6 |
Hydroxybenzoic acid |
Vanillic acid (positive ion) [M + K] + |
C8H8O4 |
| 7 |
Hydroxycinnamic acid |
P-coumaric acid |
C9H8O3 |
[16] |
| 8 |
Hydroxycinnamic acid |
Synapic acid |
C11H12O5 |
| Carotenoids (peel) |
| 1 |
Xanthophyll |
Anhydrozeaxanthin |
C40H56O2 |
[7] |
3.4. Tannins
3.4.1. Identified and quantified
[
13] found 8 compounds of the hydrolyzable tannin family named as ellagitannins. This group of compounds is formed by single molecules of phenolic acids within which include gallic acid, chlorogenic acid, caffeic acid and other basic structures of phenolic acids mentioned above [
17].
Thus, [
9], classified Eugenia stipitata Mc Vaugh as a medium category fruit in terms of phenolic content. According to
Table 3 and
Table 4 the fruit would be in the low-medium range of total phenolic content. The diversity of results has to do with environmental conditions, geographical area, and management during harvest and postharvest.
Table 7 shows the comparison of the total phenolic content reported in the literature and
Table 8 describes the evaluation range of total phenolic compounds depending on the concentration by means of the analysis proposed by [
18].
Table 7.
Classification of Arazá according to total phenol content.
Table 7.
Classification of Arazá according to total phenol content.
| Total phenolics (mg gallic acid (GAE)/100 g wet weight in pulp) |
Source |
| 184,08 ± 8,25 |
[9] |
| 144,0 ± 0,06 |
[19] |
| 122,78 ± 2,52 |
[20] |
| 111 ± 3,64 |
[21] |
| 87 ± 2 |
[22] |
| 19,3 ± 5,1 |
[7] |
Table 8.
Evaluation range of total phenolic compounds.
Table 8.
Evaluation range of total phenolic compounds.
| Total phenolics level |
Amount in (mg gallic acid (GAE)/100 g wet weight in pulp) |
| Alto |
> 500 |
| Medio |
100–500 |
| Bajo |
< 100 |
4. Discussion
The present study aimed to characterize the phenolic compounds present in the fruit of Eugenia Stipitata Mac Vaugh (Arazá). After conducting a literature review, a total of 82 compounds were identified and quantified, of which 24 were successfully identified and quantified, while 58 were identified without quantification.
The review of carotenoids provided significant insights into the antioxidant potential of the Arazá fruit. The peel exhibited notably higher concentrations of carotenoids compared to the pulp, indicating enhanced antioxidant capacity. These findings align with previous studies such as [
23], who reported higher antioxidant capacity in the mature fruit peel attributed to the presence of β-carotene.
Furthermore, the identification of new compounds such as violaxanthin, 15-cis-β-carotene, 13-cis-β-carotene, and 9-cis-β-carotene in both the pulp and peel further contributes to expanding the catalog of known bioactive compounds in Arazá. Importantly, the notable bioaccessibility of certain carotenoids emphasizes their potential health benefits upon consumption [
24]. This assertion is shared by various authors, who have concluded in their studies that carotenoids like lutein, cryptoxanthin, and zeaxanthin, which were found to be the most representative in the fruit, provide ocular health benefits with sun-protective properties, in addition to scavenging free radicals and preventing cellular aging of the heart and liver [
25,
26].
Compounds belonging to the phenolic acid family are attributed with biological properties such as antimicrobial and anticancer activity. Therefore, food industries have utilized these compounds as additives for their antibacterial activity. These benefits are linked to their antioxidant capacity, which, as explained by [
25], could be attributed to the high phenolic content of the seed, which they reported to be 15 times higher than that of the peel or fruit. Additionally, they identified six phenolic acids in the seed, a finding that aligns with [
16], who also noted the presence of p-coumaric acid and sinapic acid for the first time.
Similarly, flavonoid compounds such as myricetin, quercetin, and kaempferol hold nutraceutical value, aiding in glycemic control, reducing inflammation, lowering the risk of cardiovascular diseases, and exerting anticancer effects for consumers. The concentration of myricetin as a key compound, with notable levels reported by [
10] contributes to the nutritional value of Arazá. Recent studies by [
13] and [
15] expanded the list of flavonoids found in both the pulp and peel, highlighting the fruit’s complexity and the potential for additional health benefits.
The phenolic content of the fruit falls within a moderate range, with variability attributed to factors such as environmental conditions, geographical location, and post-harvest practices. These results underscore the influence of multiple factors on phenolic content and provide valuable insights for future studies and industrial applications [
9].
In terms of strengths, it’s important to mention that this article provides information about identified and quantified compounds related to the Eugenia stipitata Mac Vaughn fruit. Compounds were identified not only in the edible part of the fruit but also in the peel and seed. The literature review revealed that some studies do not explore the entire fruit (pulp, peel, and seed), focusing on one or two parts. Since this review aimed to collect data from different parts of the fruit, the compiled information surpasses what is reported in other literature articles. This is the case with [
27], who reported 11 carotenoids and 14 bioactive phenolic compounds from Eugenia stipitata Mac Vaughn, highlighting only the fruit’s pulp. However, the results described in this study cover the peel, pulp, and seed, providing a complete profile and expanding the number of identified compounds to 82.
Another noteworthy aspect is the investigation of a fruit variety of international interest for Amazonian countries such as Brazil, Peru, Colombia, Venezuela, Ecuador, Bolivia, Suriname, and Guyana.
Regarding limitations, it’s worth mentioning that some standard deviation data are not reported throughout this review, as they were not provided in the evaluated studies. Despite attempts to contact the authors, satisfactory responses for complete records were not obtained.
It’s important to highlight that a strength of this work was the inclusion of polyphenol analysis of the seeds, an area that had received limited attention in previous research. This innovative approach opens avenues to explore potential industrial applications of these compounds.
5. Conclusion
This review was able to identify and report the concentration, and in some cases the amount, of phenolic compounds with antioxidant and anti-inflammatory capacity of Eugenia stipitata Mac Vaugh. With this scope, it was possible to have a more complete picture of the potential of the fruit to possibly improve health aspects, and to give a greater enhancement and added value to this product so that it can be integrated more frequently in the menu of the people who access it and even to be exported.
We call on researchers to try to quantify the polyphenols in future studies, for which no information was found in the literature reviewed, to give this fruit all its nutritional and therapeutic value that it presents in its compounds.
Author Contributions
Conceptualization, A.A.L., D.F.M.R. and C.H.G.C.; methodology, A.A.L., D.F.M.R. and C.H.G.C.; software, A.A.L. and D.F.M.R.; validation, A.A.L., D.F.M.R. and C.H.G.C.; formal analysis, A.A.L. and D.F.M.R.; investigation, A.A.L., D.F.M.R. and C.H.G.C.; data curation, C.H.G.C.; writing—original draft preparation, A.A.L., D.F.M.R. and C.H.G.C.; writing—review and editing, C.H.G.C.; visualization, A.A.L., D.F.M.R. and C.H.G.C.; supervision, C.H.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Escobar Acevedo CJ, Zuluaga Pelaez JJ, Cardenas Guzman CA, Rivas Cenon EH. El Cultivo de Araza (Eugenia stipitata McVaugh). 1999.
- Hernández MS, Barrera JA, Carrillo M. Arazá [Internet]. 2006. 1–146 p. Available from: www.sinchi.org.co.
- Martín Gordo, DA. Los Compuestos Fenólicos, Un Acercamiento A Su Biosíntesis, Síntesis Y Actividad Biológica. Revista de Investigación Agrarian y Ambiental. 2018, 9, 81–104. [Google Scholar] [CrossRef]
- Costa, W.K.; de Oliveira JR, S.; de Oliveira, A.M.; da Silva Santos, I.B.; da Cunha, R.X.; de Freitas, A.F.S.; et al. Essential oil from Eugenia stipitata McVaugh leaves has antinociceptive, anti-inflammatory and antipyretic activities without showing toxicity in mice. Ind Crops Prod. 2020, 144, 112059. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant Properties of Amazonian Fruits: A Mini Review of In Vivo and In Vitro Studies. Oxidative Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Farias, D.d.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; Sacramento, C.K.D.; Pastore, G.M. Chemical characterization of Eugenia stipitata: A native fruit from the Amazon rich in nutrients and source of bioactive compounds. Food Res. Int. 2020, 139, 109904. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Kopec, R.E.; Barry, A.M.; Riedl, K.M.; Schwartz, S.J. Determination of carotenoids, total phenolic content, and antioxidant activity of Arazá (Eugenia stipitata McVaugh), an amazonian fruit. Journal of Agricultural and Food Chemistry. 2012, 60, 4709–4717. [Google Scholar] [CrossRef]
- Berni, P.; Campoli, S.S.; Negri, T.C.; de Toledo, N.M.V.; Canniatti-Brazaca, S.G. Non-conventional Tropical Fruits: Characterization, Antioxidant Potential and Carotenoid Bioaccessibility. Plant Foods Hum. Nutr. 2019, 74, 141–148. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; et al. Evaluation of the antioxidant, antiproliferative and antimutagenic potential of araçá-boi fruit (Eugenia stipitata Mc Vaugh - Myrtaceae) of the Brazilian Amazon Forest. Food Research International. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Gonçalves, A.E.D.S.S.; Lajolo, F.M.; Genovese, M.I. Chemical Composition and Antioxidant/Antidiabetic Potential of Brazilian Native Fruits and Commercial Frozen Pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. [Google Scholar] [CrossRef]
- Fabio A. Cuellar, Edna Ariza, Cecilia Anzola, Patricia Restrepo. Capacidad antioxidante del arazá (Eugenia stipitata Mc Vaugh) durante la maduración. Revista Colombiana de Química. 2013, 42, 1–8.
- de Araújo, F.F.; Farias, D.d.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; Sacramento, C.K.D.; Pastore, G.M. Chemical characterization of Eugenia stipitata: A native fruit from the Amazon rich in nutrients and source of bioactive compounds. Food Res. Int. 2020, 139, 109904. [Google Scholar] [CrossRef]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive characterization of bioactive phenols from new Brazilian superfruits by LC-ESI-QTOF-MS, and their ROS and RNS scavenging effects and anti-inflammatory activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; et al. Influence of high-intensity ultrasound on color, chemical composition and antioxidant properties of araçá-boi pulp. Food Chem. 2021, 338, 1–8. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Farias, D.d.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; Sacramento, C.K.D.; Pastore, G.M. Gastrointestinal bioaccessibility and bioactivity of phenolic compounds from araçá-boi fruit. LWT-Food Science and Technology 2020, 135, 110230. [Google Scholar] [CrossRef]
- Álvarez, A.; Jiménez, Á.; Méndez, J.; Murillo, E. Chemical and biological study of Eugenia Stipitata mc vaugh collected in the Colombian Andean region. Asian J. Pharm. Clin. Res. 2018, 11, 362–369. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Wall-Medrano, A.; González-Aguilar, G.A.; López-Díaz, J.A.; Álvarez-Parrilla, E.; De La Rosa, L.A.; et al. Taninos hidrolizables; bioquímica, aspectos nutricionales y analíticos y efectos en la salud. Nutricion Hospitalaria. Grupo Aula Medica S.A. 2015, 31, 55–66. [Google Scholar]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Rosan Fortunato Seixas F, Kempfer Bassoli B, Borghi Virgolin L, Chancare Garcia L, Soares Janzantti N. Physicochemical properties and effects of fruit pulps from the amazon biome on physiological parameters in rats. Nutrients. 2021, 13, 1–11.
- Virgolin, L.B.; Seixas, F.R.F.; Janzantti, N.S. Composition, content of bioactive compounds, and antioxidant activity of fruit pulps from the Brazilian Amazon biome. Pesqui Agropecu Bras. 2017, 52, 933–941. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Genovese, M.; Pinto, M.D.S.; Gonçalves, A.D.S.S.; Lajolo, F. Bioactive Compounds and Antioxidant Capacity of Exotic Fruits and Commercial Frozen Pulps from Brazil. Food Sci. Technol. Int. 2008, 14, 207–214. [Google Scholar] [CrossRef]
- Cuellar, F.A.; Ariza, E.; Anzola, C.; Restrepo, P. Capacidad antioxidante del arazá (Eugenia stipitata Mc Vaugh) durante la maduración. Revista Colombiana de Química. 2013, 42, 1–8. [Google Scholar]
- Leite-Legatti, A.V.; Batista, G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; de Carvalho-Silva, L.B.; Ruiz, A.L.T.G.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Neri-Numa, I.A.; Farias, D.d.P.; da Cunha, G.R.M.C.; Pastore, G.M. Wild Brazilian species of Eugenia genera (Myrtaceae) as an innovation hotspot for food and pharmacological purposes. Food Res. Int. 2019, 121, 57–72. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef]
- Sousa, H.; Leal, G.; Damiani, C.; Borges, S.; Freitas, B.; Martins, G. Some wild fruits from amazon biodiversity: Composition, bioactive compounds, and characteristics. Food Res. 2021, 5, 17–32. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).