Submitted:
16 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
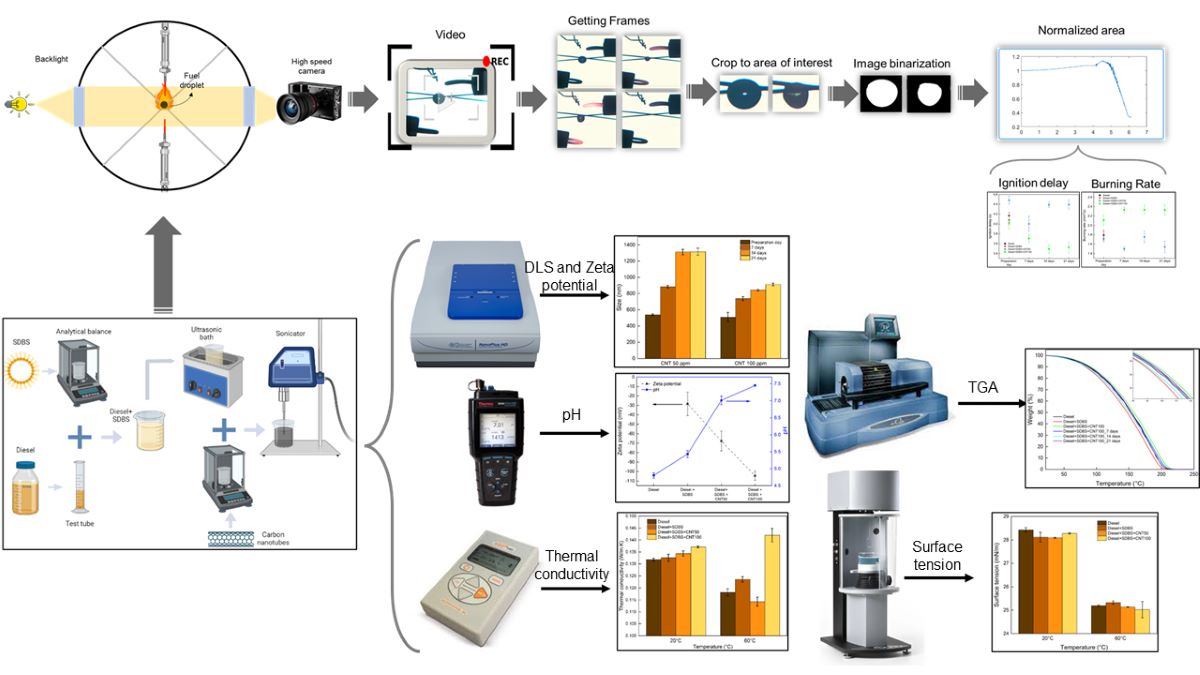
2. Methodology
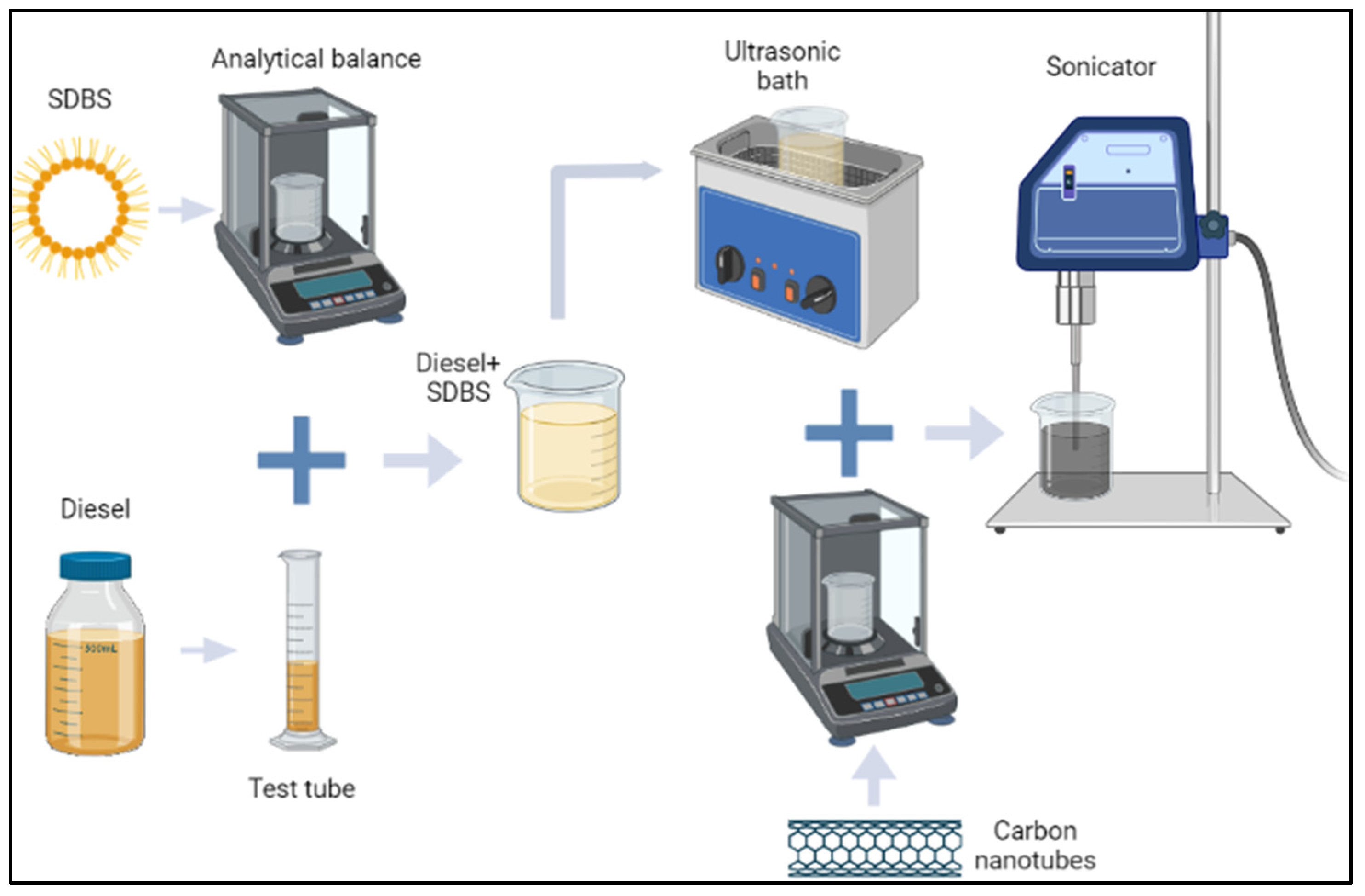
2.1. Nanofuels preparation and characterization
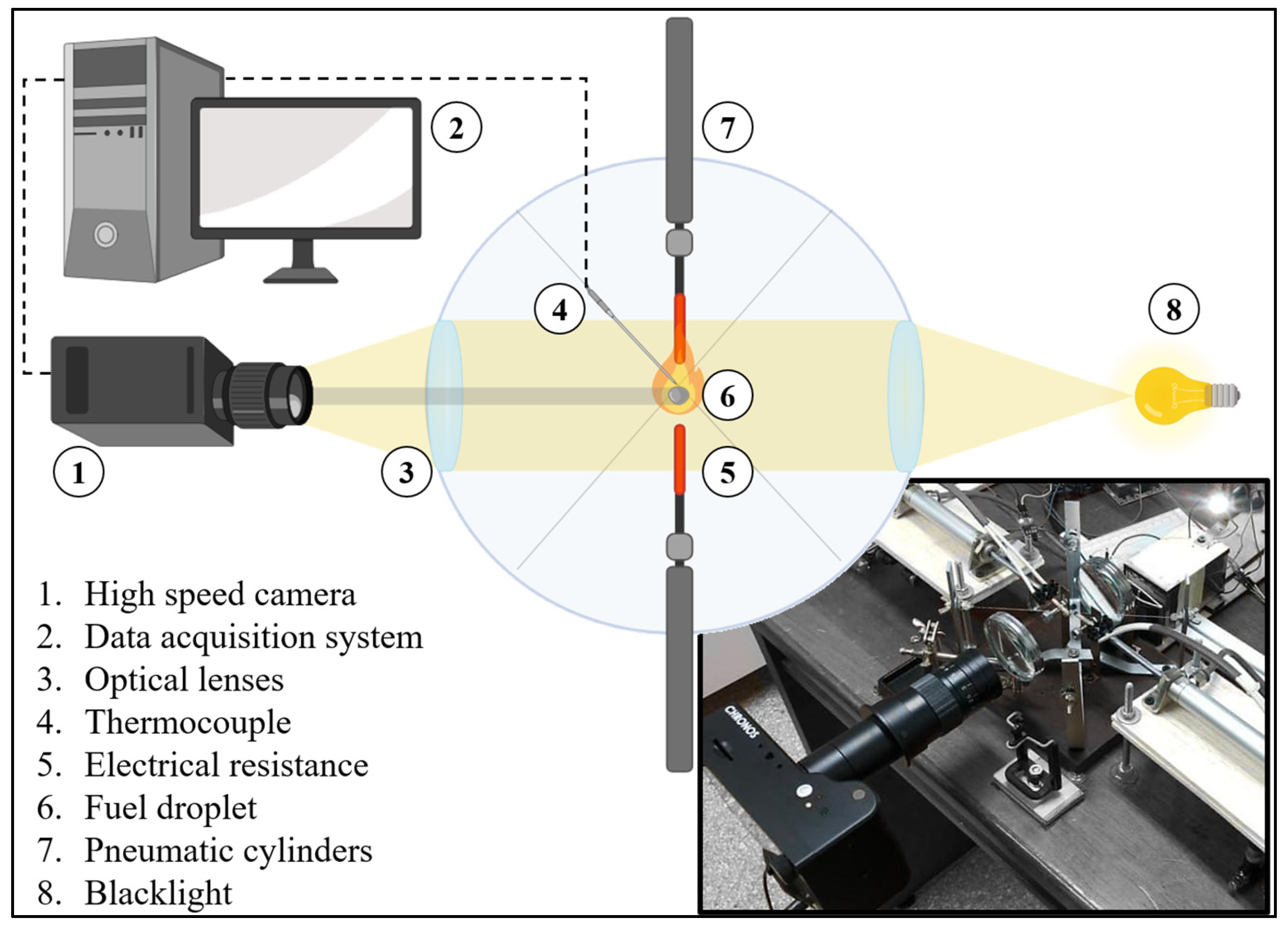
2.2. Experimental procedure
2.3. Data reduction
3. Results and discussion
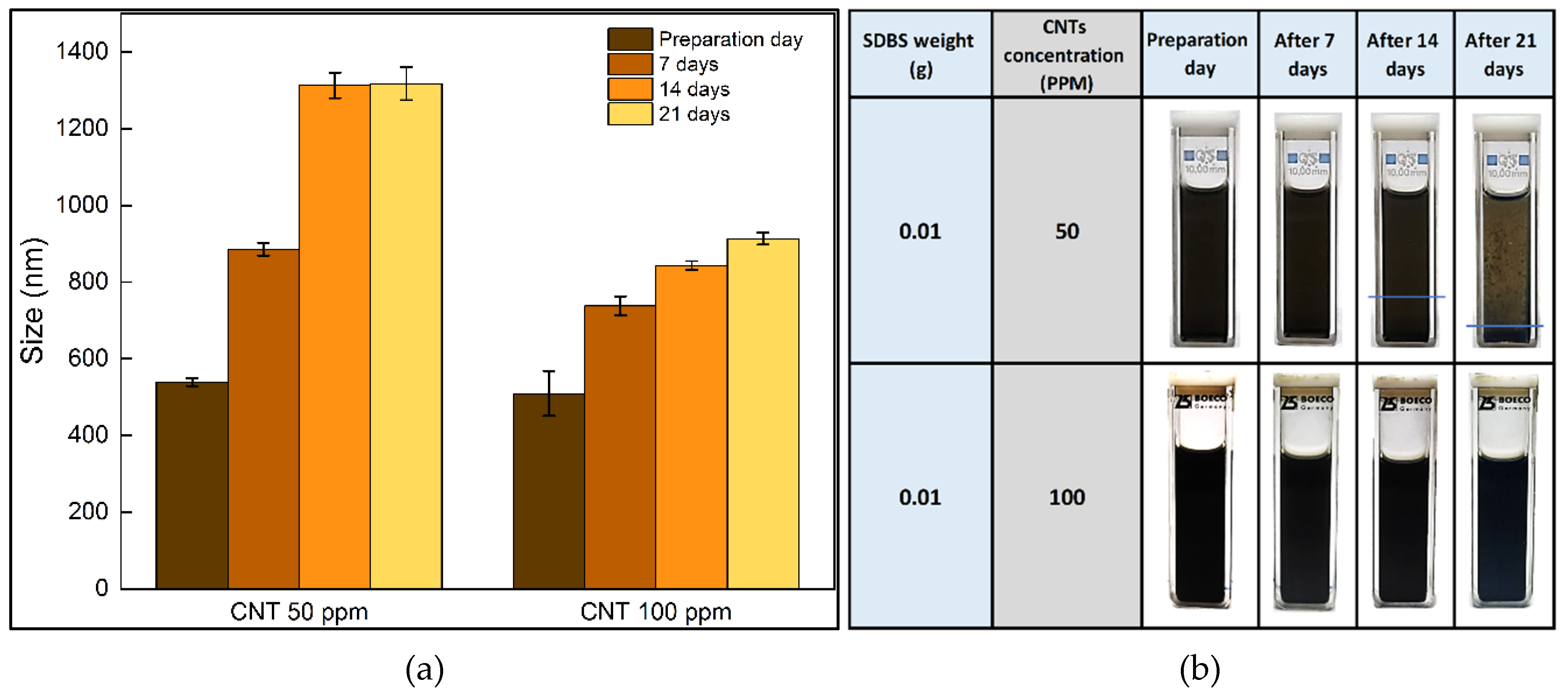
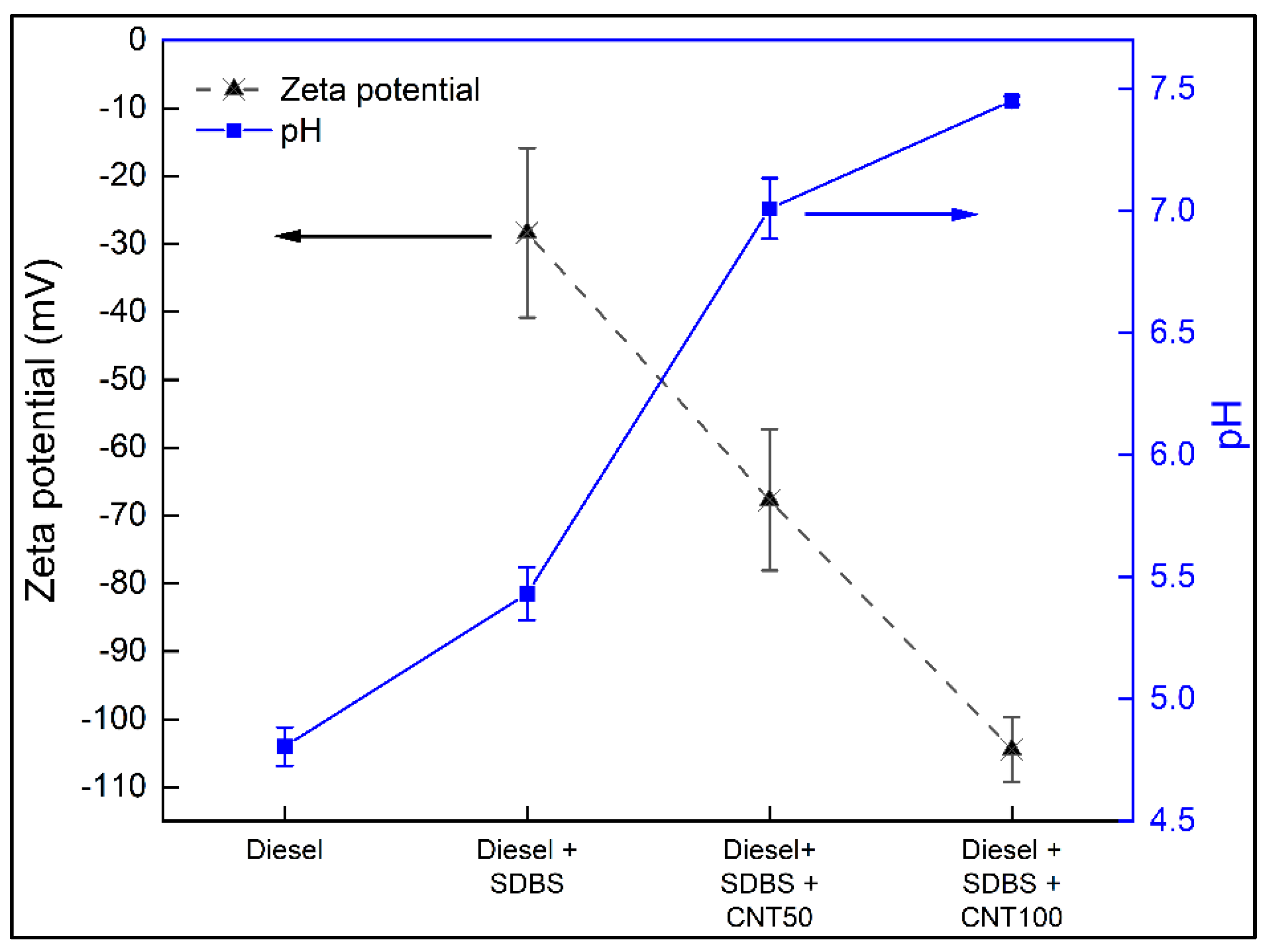
3.1. Stability analysis
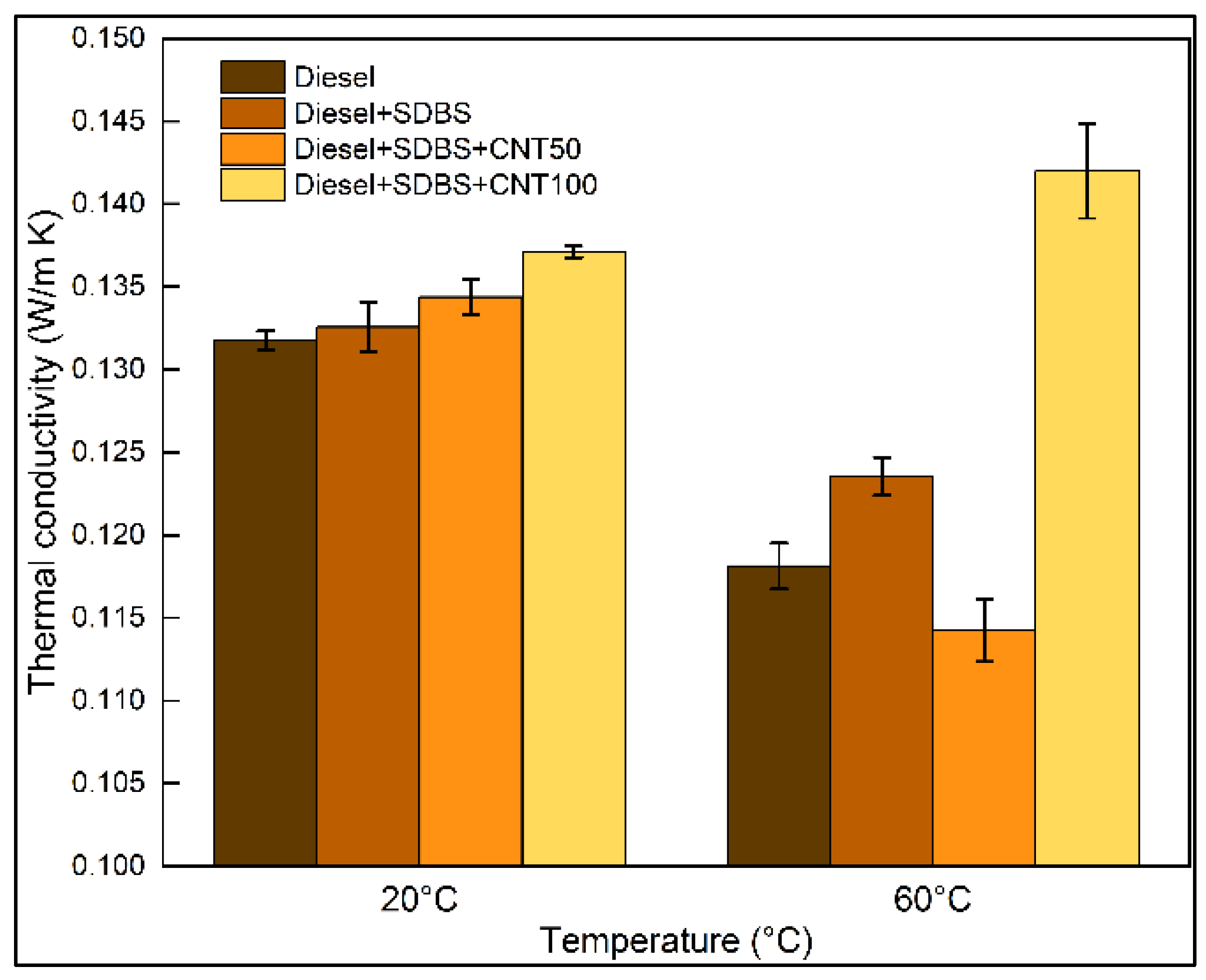
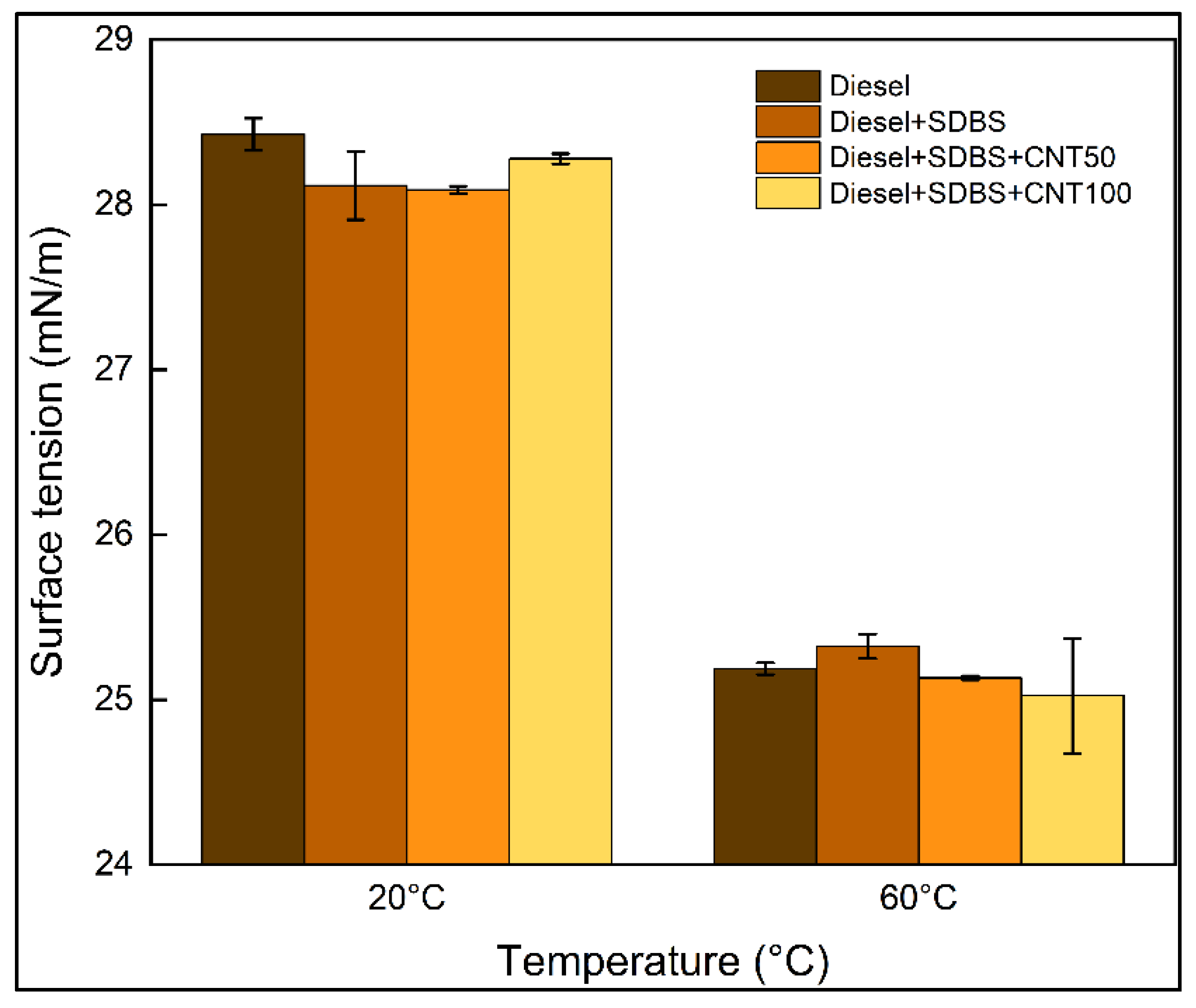
3.2. Thermal conductivity and surface tension of nanofuels
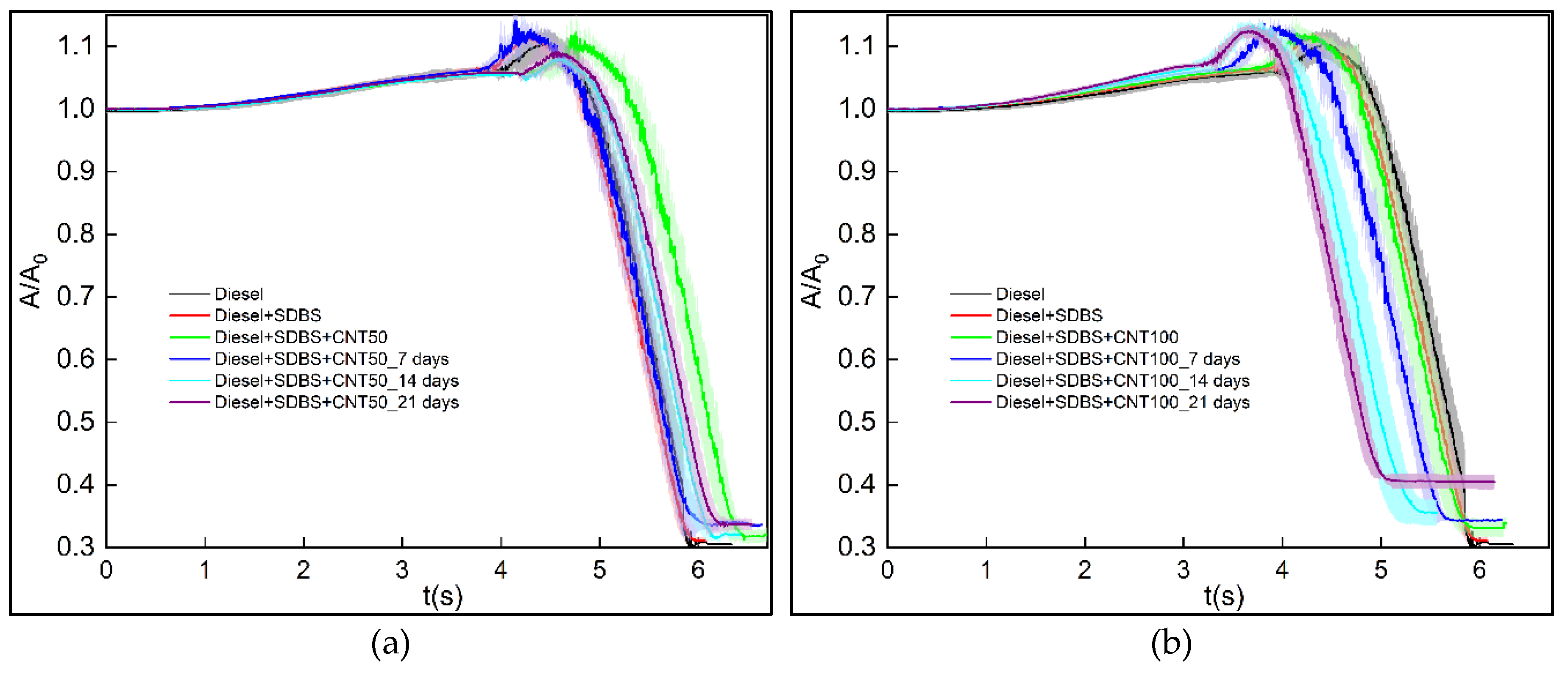
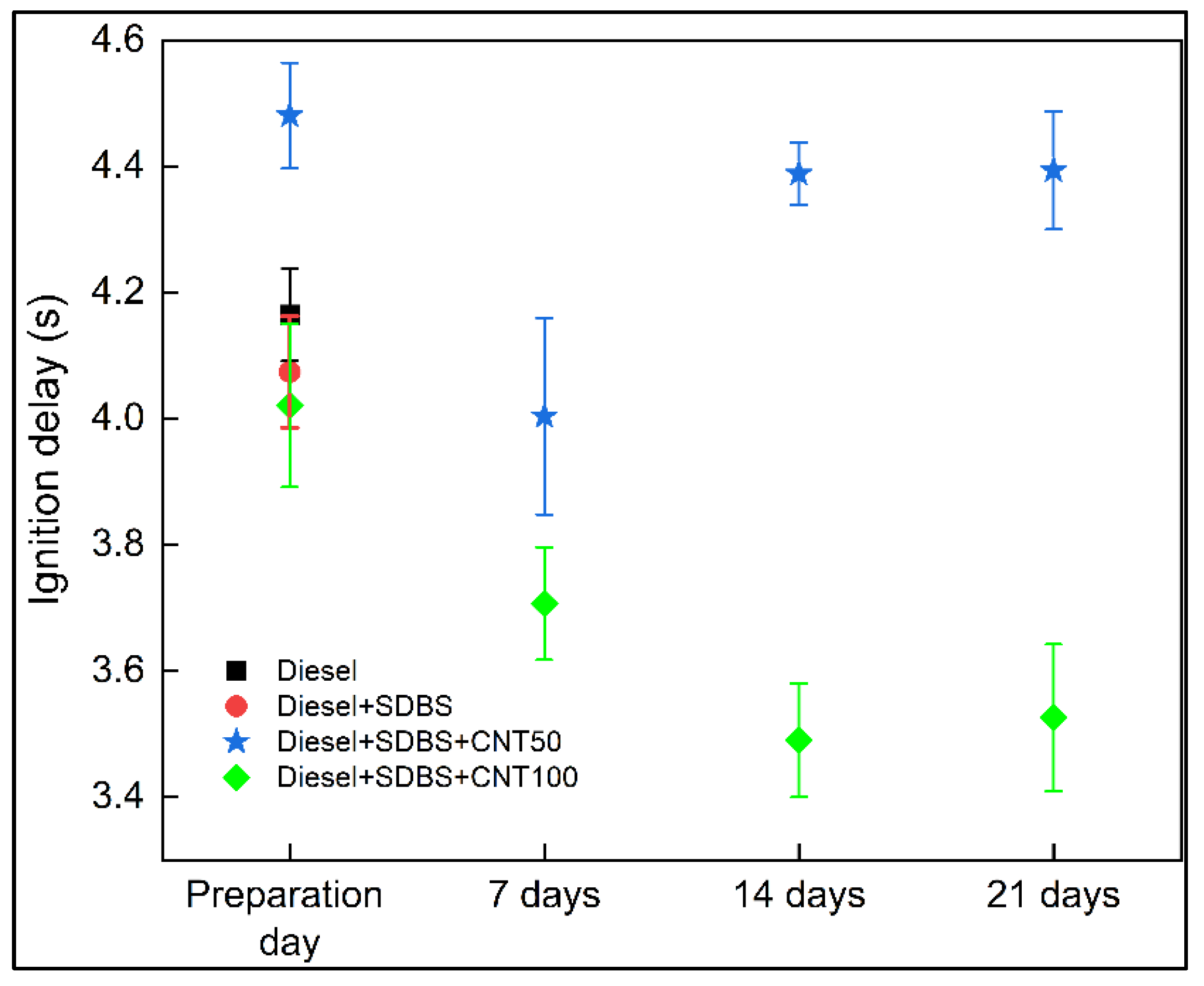
3.3. Evolution of normalized area and ignition delay
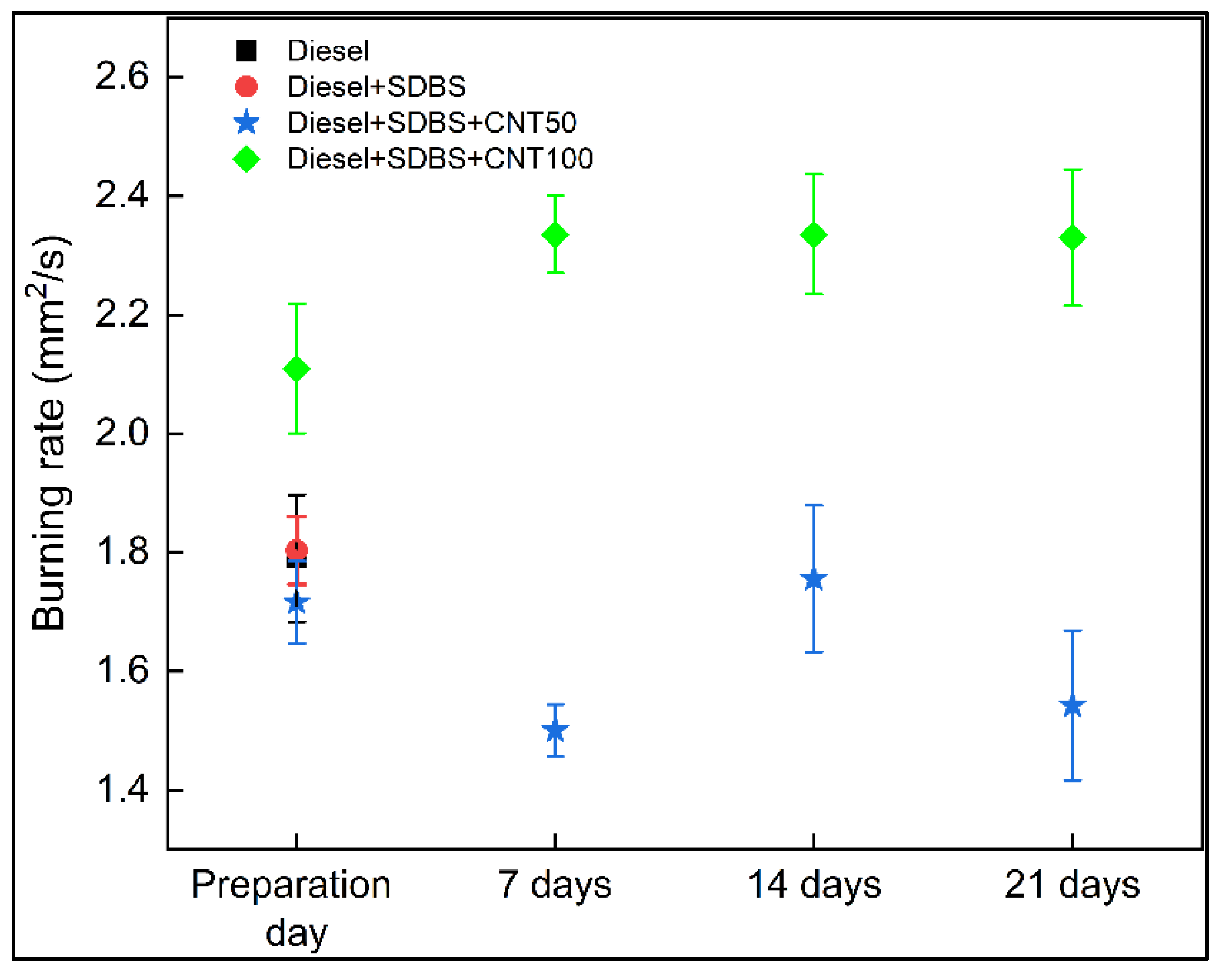
3.4. Burning rate analysis
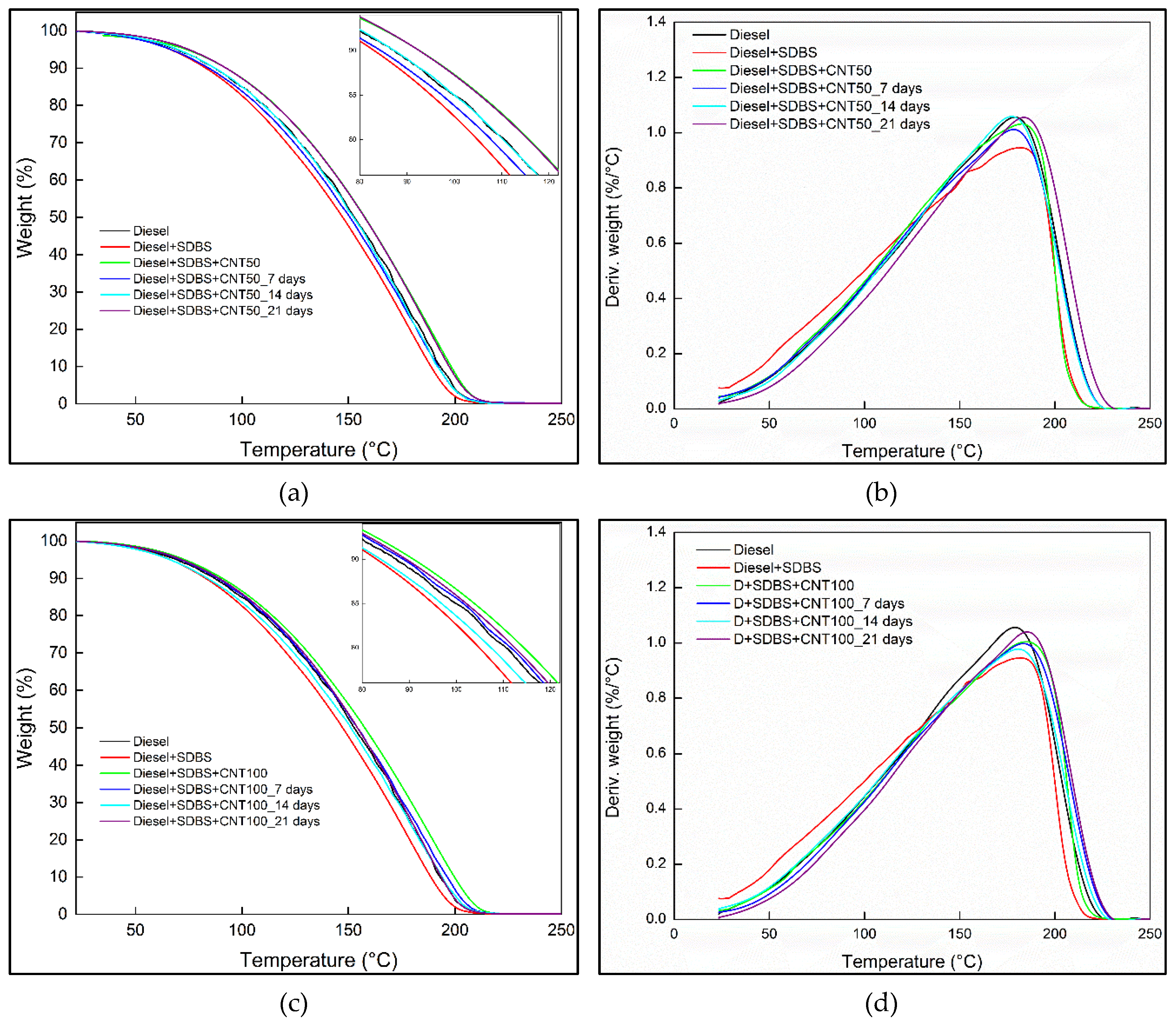
3.5. Thermogravimetric analysis
4. Conclusions
- Through the use of DLS, visual inspection, pH, and Zeta potential measurement, the nanofuels at a concentration of CNTs at 100 ppm were demonstrated to be more stable than those at 50 ppm. These findings might be explained by the presence of SDBS, which at a CNTs concentration of 50 ppm is probable to create macromolecules which promoted a decrease in stability. On the other hand, it is possible the existence of interactions between CNTs and SDBS at a concentration of 100 ppm because SDBS attaches to the surface of CNTs and forms an absorption layer that surrounds the nanomaterials and inhibits the formation of macromolecules, which significantly enhances temporal stability at this concentration.
- At 20 °C, the SDBS at a concentration of 0.02 w/v% in commercial diesel had a minimal impact on the thermal conductivity, while utilizing CNTs at a concentration of 50 ppm and 100 ppm, thermal conductivity increased 2% and 4%, respectively. However, at 60 °C, a concentration of 50 ppm showed negative effects on thermal conductivity, whereas with a concentration of 100 ppm, the thermal conductivity increased around 20%. On the other hand, the results indicate that when diesel -SDBS-CNT blends are used at temperatures between 20 °C and 60 °C, the surface tension has negligible changes in comparison to raw diesel.
- The duration of the combustion process is influenced by the stability of nanofuels at a concentration of 50 ppm, with greater durations for the first day and minimal changes after seven days for diesel and diesel - SDBS. However, concentration of 100 ppm provided better results, since the combustion of diesel and diesel-SDBS took less time after each interval of seven days. This is because, compared to a concentration of 50 ppm, nanoparticles aggregate more slowly and have higher porosity, giving them more time to improve combustion by acting as thermal bridges and having numerous heterogeneous nucleation sites within the droplet. Therefore, adding 50 ppm of CNTs to diesel causes the ignition delay time to increase by roughly 7.6%, and adding 100 ppm causes a decrease by roughly 16.2%.
- According to the data, the combustion rate is lowered by up to 16% at concentrations of 50 ppm. This is a result of the nanofuels decreased stability at this concentration, their limited increase in thermal conductivity, and their decreased porosity. On the contrary, at a concentration 100 ppm of CNT, the burning rate of diesel and diesel - SDBS was increased between 18% and 30.5%.
Author Contributions
Acknowledgements
Conflicts of Interest
Nomenclature
| A/A0 | Normalized area of the drop |
| CNT | Carbon nanotubes |
| DLS | Dynamic light scattering |
| K | Burning rate constant |
| SDBS | Sodium dodecylbenzene sulfonate |
| TGA | Thermogravimetric analysis |
References
- M. Shekofteh, T. M. Gundoshmian, A. Jahanbakhshi, and A. Heidari-Maleni, “Performance and emission characteristics of a diesel engine fueled with functionalized multi-wall carbon nanotubes (MWCNTs-OH) and diesel–biodiesel–bioethanol blends,” Energy Reports, vol. 6, pp. 1438–1447, 2020. [CrossRef]
- J. Sadhik Basha, “Impact of Carbon Nanotubes and Di-Ethyl Ether as additives with biodiesel emulsion fuels in a diesel engine – An experimental investigation,” Journal of the Energy Institute, vol. 91, no. 2, pp. 289–303, 2018. [CrossRef]
- H. Griffiths, “Euro 7 standards: EU considers lifetime surveillance of every new car,” Londres, Mar. 05, 2021. [Online]. Available: https://www.autoexpress.co.uk/news/354437/euro-7-standards-eu-considers-lifetime-surveillance-every-new-car.
- X. Guo, C. Liang, M. Umar, and N. Mirza, “The impact of fossil fuel divestments and energy transitions on mutual funds performance,” Technol Forecast Soc Change, vol. 176, p. 121429, Mar. 2022. [CrossRef]
- S. P. Jena, S. Mahapatra, and S. K. Acharya, “Optimization of performance and emission characteristics of a diesel engine fueled with Karanja biodiesel using Grey-Taguchi method,” Mater Today Proc, vol. 41, pp. 180–185, 2021. [CrossRef]
- V. Saxena, N. Kumar, and V. K. Saxena, “Multi-objective optimization of modified nanofluid fuel blends at different TiO2 nanoparticle concentration in diesel engine: Experimental assessment and modeling,” Appl Energy, vol. 248, no. December 2018, pp. 330–353, 2019. [CrossRef]
- A. Yousefi, H. Guo, and M. Birouk, “Effect of diesel injection timing on the combustion of natural gas/diesel dual-fuel engine at low-high load and low-high speed conditions,” Fuel, vol. 235, no. August 2018, pp. 838–846, 2019. [CrossRef]
- Q. Wu, X. Xie, Y. Wang, and T. Roskilly, “Effect of carbon coated aluminum nanoparticles as additive to biodiesel-diesel blends on performance and emission characteristics of diesel engine,” Appl Energy, vol. 221, pp. 597–604, Jul. 2018. [CrossRef]
- A. I. El-Seesy, A. M. A. Attia, and H. M. El-Batsh, “The effect of Aluminum oxide nanoparticles addition with Jojoba methyl ester-diesel fuel blend on a diesel engine performance, combustion and emission characteristics,” Fuel, vol. 224, pp. 147–166, Jul. 2018. [CrossRef]
- H. Hosseinzadeh-Bandbafha et al., “Effects of aqueous carbon nanoparticles as a novel nanoadditive in water-emulsified diesel/biodiesel blends on performance and emissions parameters of a diesel engine,” Energy Convers Manag, vol. 196, pp. 1153–1166, Sep. 2019. [CrossRef]
- C. S. Aalam, C. G. Saravanan, and M. Kannan, “Experimental investigations on a CRDI system assisted diesel engine fuelled with aluminium oxide nanoparticles blended biodiesel,” Alexandria Engineering Journal, vol. 54, no. 3, pp. 351–358, 2015. [CrossRef]
- J. B. Ooi, H. M. Ismail, V. Swamy, X. Wang, A. K. Swain, and J. R. Rajanren, “Graphite Oxide Nanoparticle as a Diesel Fuel Additive for Cleaner Emissions and Lower Fuel Consumption,” Energy and Fuels, vol. 30, no. 2, pp. 1341–1353, 2016. [CrossRef]
- S. Kumar, P. Dinesha, and M. A. Rosen, “Effect of injection pressure on the combustion, performance and emission characteristics of a biodiesel engine with cerium oxide nanoparticle additive,” Energy, vol. 185, pp. 1163–1173, 2019. [CrossRef]
- S. Manigandan et al., “Comparative study of nanoadditives TiO2, CNT, Al2O3, CuO and CeO2 on reduction of diesel engine emission operating on hydrogen fuel blends,” Fuel, vol. 262, no. November 2019, 2020. [CrossRef]
- T. Kegl, A. Kovač Kralj, M. Kegl, and B. Kegl, Nanomaterials for Environmental Application, vol. 10, no. July. in Green Energy and Technology, vol. 10. Cham: Springer International Publishing, 2020. [CrossRef]
- R. A. Alenezi, A. M. Norkhizan, R. Mamat, Erdiwansyah, G. Najafi, and M. Mazlan, “Investigating the contribution of carbon nanotubes and diesel-biodiesel blends to emission and combustion characteristics of diesel engine,” Fuel, vol. 285, no. July 2020, p. 119046, 2021. [CrossRef]
- Y. H. Bello et al., “Investigating the engine performance, emissions and soot characteristics of CI engine fueled with diesel fuel loaded with graphene oxide-titanium dioxide nanocomposites,” Fuel, vol. 269, p. 117436, Jun. 2020. [CrossRef]
- S. Tanvir and L. Qiao, “Droplet burning rate enhancement of ethanol with the addition of graphite nanoparticles: Influence of radiation absorption,” Combust Flame, vol. 166, pp. 34–44, Apr. 2016. [CrossRef]
- S. Mosadegh, A. Ghaffarkhah, C. van der Kuur, M. Arjmand, and S. Kheirkhah, “Graphene oxide doped ethanol droplet combustion: Ignition delay and contribution of atomization to burning rate,” pp. 1–45, 2021.
- J. B. Ooi, H. M. Ismail, V. Swamy, X. Wang, A. K. Swain, and J. R. Rajanren, “Graphite Oxide Nanoparticle as a Diesel Fuel Additive for Cleaner Emissions and Lower Fuel Consumption,” Energy and Fuels, vol. 30, no. 2, pp. 1341–1353, 2016. [CrossRef]
- J. B. Ooi, J. R. Rajanren, H. M. Ismail, V. Swamy, and X. Wang, “Improving combustion characteristics of diesel and biodiesel droplets by graphite oxide addition for diesel engine applications,” Int J Energy Res, vol. 41, no. 14, pp. 2258–2267, Nov. 2017. [CrossRef]
- A. F. Abdul Rasid and Y. Zhang, “Comparison of the burning of a single diesel droplet with volume and surface contamination of soot particles,” Proceedings of the Combustion Institute, vol. 38, no. 2, pp. 3159–3166, Jan. 2021. [CrossRef]
- G. Singh, M. Esmaeilpour, and A. Ratner, “Effect of carbon-based nanoparticles on the ignition, combustion and flame characteristics of crude oil droplets,” Energy, vol. 197, Apr. 2020. [CrossRef]
- X. Wang et al., “A comprehensive review on the properties of nanofluid fuel and its additive effects to compression ignition engines,” Appl Surf Sci, vol. 504, no. January 2019, 2020. [CrossRef]
- T. Mesri Gundoshmian, A. Heidari-Maleni, and A. Jahanbakhshi, “Evaluation of performance and emission characteristics of a CI engine using functional multi-walled carbon nanotubes (MWCNTs-COOH) additives in biodiesel-diesel blends,” Fuel, vol. 287, Mar. 2021. [CrossRef]
- M. Dai, J. Wang, N. Wei, X. Wang, and C. Xu, “Experimental study on evaporation characteristics of diesel/cerium oxide nanofluid fuel droplets,” Fuel, vol. 254, no. February, p. 115633, 2019. [CrossRef]
- R. N. Mehta, U. More, N. Malek, M. Chakraborty, and P. A. Parikh, “Study of stability and thermodynamic properties of water-in-diesel nanoemulsion fuels with nano-Al additive,” Appl Nanosci, vol. 5, no. 8, pp. 891–900, Nov. 2015. [CrossRef]
- X. Wang, N. Wei, J. Gao, J. Yan, and G. Jiang, “Evaporation Characteristics of Ethanol Diesel Droplets Containing Nanoparticles,” J Shanghai Jiaotong Univ Sci, vol. 26, no. 2, pp. 201–209, Apr. 2021. [CrossRef]
- Muthukumar M, Senthil Kumar A P, Sasikumar C, Yuvaraj S, and T. S. Singh, “Effect of nanoparticles on the droplet combustion of rice bran oil biodiesel,” Biomass Convers Biorefin, vol. 11, no. 4, pp. 1375–1393, Aug. 2021. [CrossRef]
- X. Wang, M. Dai, Y. Xie, J. Han, Y. Ma, and C. Chen, “Experimental investigation of evaporation characteristics of biodiesel-diesel blend droplets with carbon nanotubes and nanoceria as nanoadditives,” Appl Surf Sci, vol. 505, no. January 2019, p. 144186, 2020. [CrossRef]
- K. Kannaiyan and R. Sadr, “The effects of alumina nanoparticles as fuel additives on the spray characteristics of gas-to-liquid jet fuels,” Exp Therm Fluid Sci, vol. 87, pp. 93–103, Oct. 2017. [CrossRef]
- R. Küçükosman, A. A. Yontar, and K. Ocakoglu, “Nanoparticle additive fuels: Atomization, combustion and fuel characteristics,” J Anal Appl Pyrolysis, vol. 165, p. 105575, Aug. 2022. [CrossRef]
- M. S. Gad and S. Jayaraj, “A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel,” Fuel, vol. 267, p. 117168, May 2020. [CrossRef]
- S. N. A. Yusof, N. A. C. Sidik, Y. Asako, W. Mohd. A. A. Japar, S. B. Mohamed, and N. M. Muhammad, “A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE),” Nanotechnol Rev, vol. 9, no. 1, pp. 1326–1349, Dec. 2020. [CrossRef]
- D. Liang et al., “Effects of solids’ concentration and oleic acid dispersant on the stability and combustion characteristics of aluminum/bioethanol nanofluid fuel,” Powder Technol, vol. 398, p. 117108, Jan. 2022. [CrossRef]
- K. Pandey, K. Chattopadhyay, and S. Basu, “Combustion dynamics of low vapour pressure nanofuel droplets,” Physics of Fluids, vol. 29, no. 7, p. 074102, Jul. 2017. [CrossRef]
- F. Dong, R. T. Koodali, H. Wang, and W. Ho, “Nanomaterials for Environmental Applications,” J Nanomater, vol. 2014, pp. 1–4, 2014. [CrossRef]
- V. Saxena, N. Kumar, and V. K. Saxena, “Multi-objective optimization of modified nanofluid fuel blends at different TiO2 nanoparticle concentration in diesel engine: Experimental assessment and modeling,” Appl Energy, vol. 248, pp. 330–353, Aug. 2019. [CrossRef]
- S. Sayyed, R. K. Das, and K. Kulkarni, “Experimental investigation for evaluating the performance and emission characteristics of DICI engine fueled with dual biodiesel-diesel blends of Jatropha, Karanja, Mahua, and Neem,” Energy, vol. 238, p. 121787, Jan. 2022. [CrossRef]
- A. I. EL-Seesy, M. S. Waly, H. M. El-Batsh, and R. M. El-Zoheiry, “Enhancement of the waste cooking oil biodiesel usability in the diesel engine by using n-decanol, nitrogen-doped, and amino-functionalized multi-walled carbon nanotube,” Energy Convers Manag, vol. 277, p. 116646, Feb. 2023. [CrossRef]
- S. Mosadegh, A. Ghaffarkhah, C. van der Kuur, M. Arjmand, and S. Kheirkhah, “Graphene oxide doped ethanol droplet combustion: Ignition delay and contribution of atomization to burning rate,” pp. 1–45, 2021, [Online]. Available: http://arxiv.org/abs/2104.13544. [CrossRef]
- J. Wang et al., “Flame spread and combustion characteristics of two adjacent jatropha oil droplets,” Fuel, vol. 285, no. August 2020, p. 119077, 2021. [CrossRef]
- J. Rentería, A. Gallego, D. Gamboa, K. Cacua, and B. Herrera, “Effect of amide-functionalized carbon nanotubes as commercial diesel and palm-oil biodiesel additives on the ignition delay: A study on droplet scale,” Fuel, vol. 338, Apr. 2023. [CrossRef]
- A. Braeuer, In situ Spectroscopic Techniques at High Pressure. in ISSN. Elsevier Science, 2015.
- C. K. Law, “Recent advances in droplet vaporization and combustion,” Prog Energy Combust Sci, vol. 8, no. 3, pp. 171–201, 1982. [CrossRef]
- S. Balamurugan and V. Sajith, “Experimental investigation on the stability and abrasive action of cerium oxide nanoparticles dispersed diesel,” Energy, vol. 131, pp. 113–124, 2017. [CrossRef]
- H. Dautzenberg, “Polymeric stabilization of colloidal dispersions.,” Acta Polymerica, vol. 36, no. 8, pp. 457–457, Aug. 1985. [CrossRef]
- R. Küçükosman, A. A. Yontar, and K. Ocakoglu, “Nanoparticle additive fuels: Atomization, combustion and fuel characteristics,” J Anal Appl Pyrolysis, vol. 165, p. 105575, Aug. 2022. [CrossRef]
- J. Liu et al., “Applications of functional nanoparticle–stabilized surfactant foam in petroleum-contaminated soil remediation,” J Hazard Mater, vol. 443, p. 130267, Feb. 2023. [CrossRef]
- N. Ali, J. A. Teixeira, and A. Addali, “A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties,” Journal of Nanomaterials, vol. 2018. Hindawi Limited, 2018. [CrossRef]
- G. Hwang et al., “Analysis of stability behavior of carbon black nanoparticles in ecotoxicological media: Hydrophobic and steric effects,” Colloids Surf A Physicochem Eng Asp, vol. 554, no. June, pp. 306–316, 2018. [CrossRef]
- Q. Lin, M. Xu, Z. Cui, X. Pei, J. Jiang, and B. Song, “Structure and stabilization mechanism of diesel oil-in-water emulsions stabilized solely by either positively or negatively charged nanoparticles,” Colloids Surf A Physicochem Eng Asp, vol. 573, pp. 30–39, Jul. 2019. [CrossRef]
- M. L. Huber, A. Laesecke, and R. Perkins, “Transport Properties of n-Dodecane,” Energy & Fuels, vol. 18, no. 4, pp. 968–975, Jul. 2004. [CrossRef]
- A. Gallego, K. Cacua, B. Herrera, D. Cabaleiro, M. M. Piñeiro, and L. Lugo, “Experimental evaluation of the effect in the stability and thermophysical properties of water-Al2O3 based nanofluids using SDBS as dispersant agent,” Advanced Powder Technology, vol. 31, no. 2, pp. 560–570, Feb. 2020. [CrossRef]
- L. Liu, X. Zhang, and X. Lin, “Experimental investigations on the thermal performance and phase change hysteresis of low-temperature paraffin/MWCNTs/SDBS nanocomposite via dynamic DSC method,” Renew Energy, vol. 187, pp. 572–585, Mar. 2022. [CrossRef]
- M. E. M. Soudagar, N. N. Nik-Ghazali, M. Abul Kalam, I. A. Badruddin, N. R. Banapurmath, and N. Akram, “The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics,” Energy Convers Manag, vol. 178, no. September, pp. 146–177, 2018. [CrossRef]
- E. v. Timofeeva et al., “Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory,” Phys Rev E, vol. 76, no. 6, p. 061203, Dec. 2007. [CrossRef]
- B. Esteban, J. R. Riba, G. Baquero, R. Puig, and A. Rius, “Characterization of the surface tension of vegetable oils to be used as fuel in diesel engines,” Fuel, vol. 102, pp. 231–238, Dec. 2012. [CrossRef]
- X. Wang, M. Dai, J. Wang, Y. Xie, G. Ren, and G. Jiang, “Effect of ceria concentration on the evaporation characteristics of diesel fuel droplets,” Fuel, vol. 236, no. September 2018, pp. 1577–1585, 2019. [CrossRef]
- M. H. U. Bhuiyan, R. Saidur, R. M. Mostafizur, I. M. Mahbubul, and M. A. Amalina, “Experimental investigation on surface tension of metal oxide-water nanofluids,” International Communications in Heat and Mass Transfer, vol. 65, pp. 82–88, Jul. 2015. [CrossRef]
- D. Mei, Y. Fang, Z. Zhang, D. Guo, Z. Chen, and C. Sun, “Analysis of surface tension for nano-fuels containing disparate types of suspended nanoparticles,” Powder Technol, vol. 388, pp. 526–536, Aug. 2021. [CrossRef]
- M. Kubo, Y. Ishihara, Y. Mantani, and M. Shimada, “Evaluation of the factors that influence the fabrication of porous thin films by deposition of aerosol nanoparticles,” Chemical Engineering Journal, vol. 232, pp. 221–227, 2013. [CrossRef]
- J. Liu, J. J. Swanson, D. B. Kittelson, D. Y. H. Pui, and J. Wang, “Microstructural and loading characteristics of diesel aggregate cakes,” Powder Technol, vol. 241, pp. 244–251, 2013. [CrossRef]
- L. Wang, K. J. Dong, C. C. Wang, R. P. Zou, Z. Y. Zhou, and A. B. Yu, “Computer simulation of the packing of nanoparticles,” Powder Technol, vol. 401, pp. 1–10, 2022. [CrossRef]
- M. R. Chow et al., “Effects of ethanol on the evaporation and burning characteristics of palm-oil based biodiesel droplet,” Journal of the Energy Institute, vol. 98, no. May, pp. 35–43, 2021. [CrossRef]
- G. Jiang, J. Yan, G. Wang, M. Dai, C. Xu, and J. Wang, “Effect of nanoparticles concentration on the evaporation characteristics of biodiesel,” Appl Surf Sci, vol. 492, no. June, pp. 150–156, 2019. [CrossRef]
- S. Gumus, H. Ozcan, M. Ozbey, and B. Topaloglu, “Aluminum oxide and copper oxide nanodiesel fuel properties and usage in a compression ignition engine,” Fuel, vol. 163, pp. 80–87, 2016. [CrossRef]
- A. K. Agarwal, J. G. Gupta, and A. Dhar, “Potential and challenges for large-scale application of biodiesel in automotive sector,” Prog Energy Combust Sci, vol. 61, pp. 113–149, 2017. [CrossRef]
- R. Dubey, D. Dutta, A. Sarkar, and P. Chattopadhyay, “Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences,” Nanoscale Adv, vol. 3, no. 20, pp. 5722–5744, 2021. [CrossRef]
- J. Sadhik Basha and R. B. Anand, “The influence of nano additive blended biodiesel fuels on the working characteristics of a diesel engine,” Journal of the Brazilian Society of Mechanical Sciences and Engineering, vol. 35, no. 3, pp. 257–264, Oct. 2013. [CrossRef]
- D. Mei, X. Li, Q. Wu, and P. Sun, “Role of Cerium Oxide Nanoparticles as Diesel Additives in Combustion Efficiency Improvements and Emission Reduction,” Journal of Energy Engineering, vol. 142, no. 4, Dec. 2016. [CrossRef]
- S. Tanvir and L. Qiao, “Effect of addition of energetic nanoparticles on droplet-burning rate of liquid fuels,” in Journal of Propulsion and Power, American Institute of Aeronautics and Astronautics Inc., Jan. 2015, pp. 408–415. [CrossRef]
- Y. Gan, Y. S. Lim, and L. Qiao, “Combustion of nanofluid fuels with the addition of boron and iron particles at dilute and dense concentrations,” Combust Flame, vol. 159, no. 4, pp. 1732–1740, Apr. 2012. [CrossRef]
- Y. Gan and L. Qiao, “Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles,” Combust Flame, vol. 158, no. 2, pp. 354–368, Feb. 2011. [CrossRef]
- M. Nour, A. M. A. Attia, and S. A. Nada, “Improvement of CI engine combustion and performance running on ternary blends of higher alcohol (Pentanol and Octanol)/hydrous ethanol/diesel,” Fuel, vol. 251, no. February, pp. 10–22, 2019. [CrossRef]
- H. Li, S. Pokhrel, M. Schowalter, A. Rosenauer, J. Kiefer, and L. Mädler, “The gas-phase formation of tin dioxide nanoparticles in single droplet combustion and flame spray pyrolysis,” Combust Flame, vol. 215, pp. 389–400, 2020. [CrossRef]

| Type | Specification |
|---|---|
| Purity | >95 % |
| External diameter | 20-30 nm |
| Internal diameter | 5-10 nm |
| Length | 10-30 µm |
| Surface area | >110 m2/g |
| Density | 2.1 g/cm3 |
| Property | Unit | Diesel | Diesel + SDBS | Diesel + SDBS + CNT 100 ppm | Standard |
|---|---|---|---|---|---|
| Kinematic viscosity at 40 ◦C | mm²/s | 3.771 | 4.374 | 4.469 | ASTM D445 |
| Cetane index | - | 48.68 | 48.90 | 48.80 | ASTM D976 |
| Heating value | MJ/kg | 45.14 | 44.94 | 44.92 | ASTM D240 |
| API gravity of petroleum products at 15.6 °C | °API | 33.2 | 31.8 | 31.8 | ASTM D287 |
| Gum content in fuel by evaporation jet | mg/100 ml | 49.5 | 35.5 | 15.5 | ASTM D381 |
| Pour point | °C | -15 | -12 | -12 | ASTM D97 |
| Flashpoint | °C | 71 | 72 | 73 | ASTM D93 |
| Cloud point | °C | 2 | -1 | -6 | ASTM D2500 |
| Parameter | Instrument | Uncertainty |
|---|---|---|
| Thermogravimetric analysis | TA Instrument SDTQ 600 | ± 1 x 10-7 g and ± 1 °C |
| Scanning Electron Microscopy | JEOL JSM-7100F | ± 1.2 nm |
| Dynamic Light Scattering (DLS) | Micromeritics Nanoplus HD | ± 0.1 nm |
| Carbon nanotubes weight | Precisa EP225-DR | ± 1 x 10-6 g |
| Fuel/nanofuel | Day of the tests | Coefficient of variation of A/A0 | ||
|---|---|---|---|---|
| Maximum | Minimum | Mean | ||
| Diesel | - | 19.63% | 0.04% | 2.11% |
| Diesel + SDBS | - | 12.70% | 0.01% | 1.56% |
| Diesel + SDBS + CNT 50 ppm | Preparation day | 20.40% | 0.02% | 2.49% |
| 7 days | 18.64% | 0.01% | 2.21% | |
| 14 days | 18.76% | 0.01% | 1.93% | |
| 21 days | 15.46% | 0.01% | 1.86% | |
| Diesel + SDBS + CNT 100 ppm | Preparation day | 19.52% | 0.02% | 2.86% |
| 7 days | 14.86% | 0.02% | 1.89% | |
| 14 days | 23.24% | 0.02% | 3.50% | |
| 21 days | 12.92% | 0.02% | 1.96% | |
| Fuel/nanofuel | Day of the tests | Coefficient of variation of the ignition delay | Coefficient of variation of the Burning rate |
|---|---|---|---|
| Diesel | - | 1.76% | 5.98% |
| Diesel + SDBS | - | 2.17% | 3.16% |
| Diesel + SDBS + CNT 50 ppm | Preparation day | 1.87% | 4.03% |
| 7 days | 3.89% | 2.92% | |
| 14 days | 1.12% | 7.00% | |
| 21 days | 2.13% | 8.18% | |
| Diesel + SDBS + CNT 100 ppm | Preparation day | 3.22% | 5.16% |
| 7 days | 2.41% | 2.80% | |
| 14 days | 2.59% | 4.33% | |
| 21 days | 3.30% | 4.92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





