Submitted:
16 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
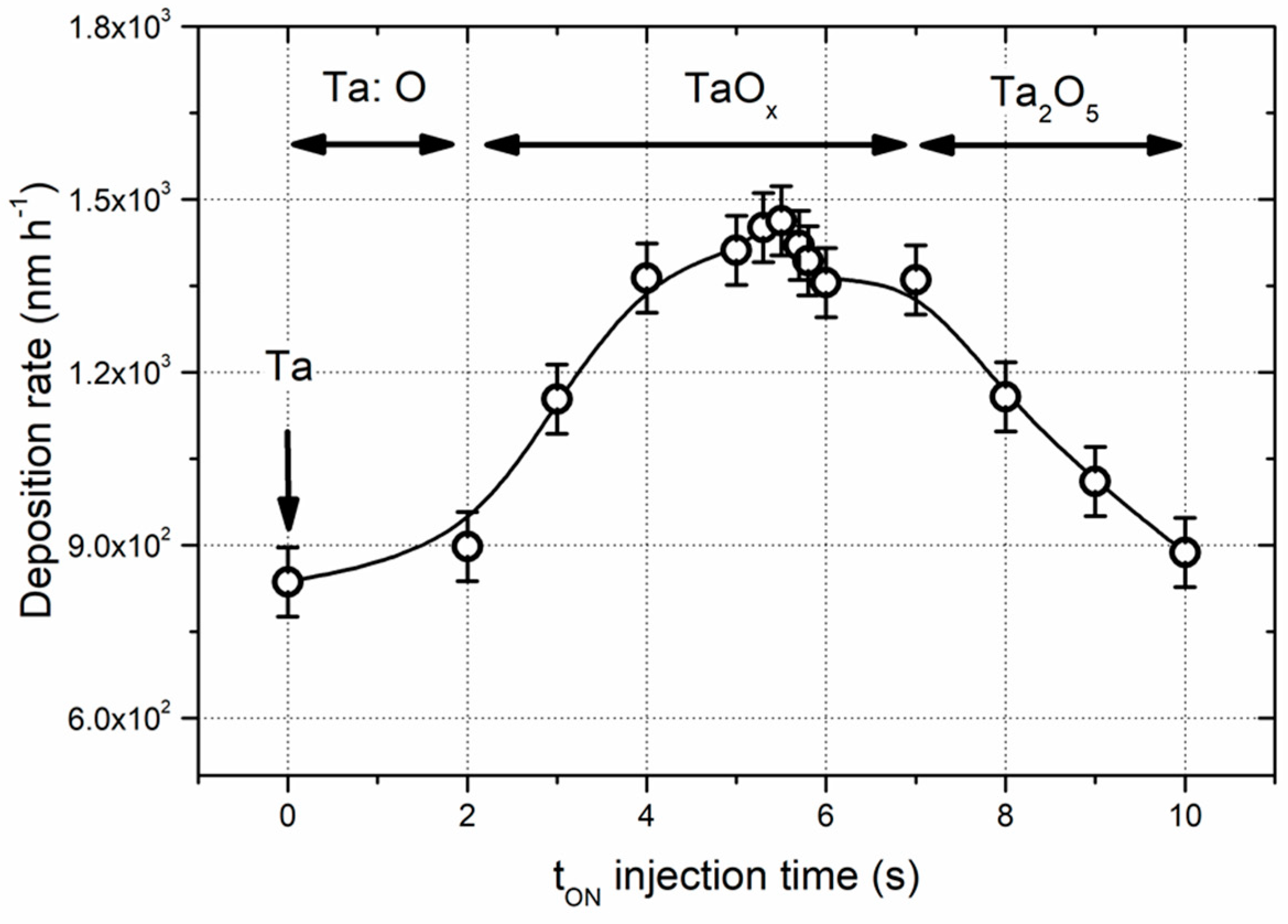
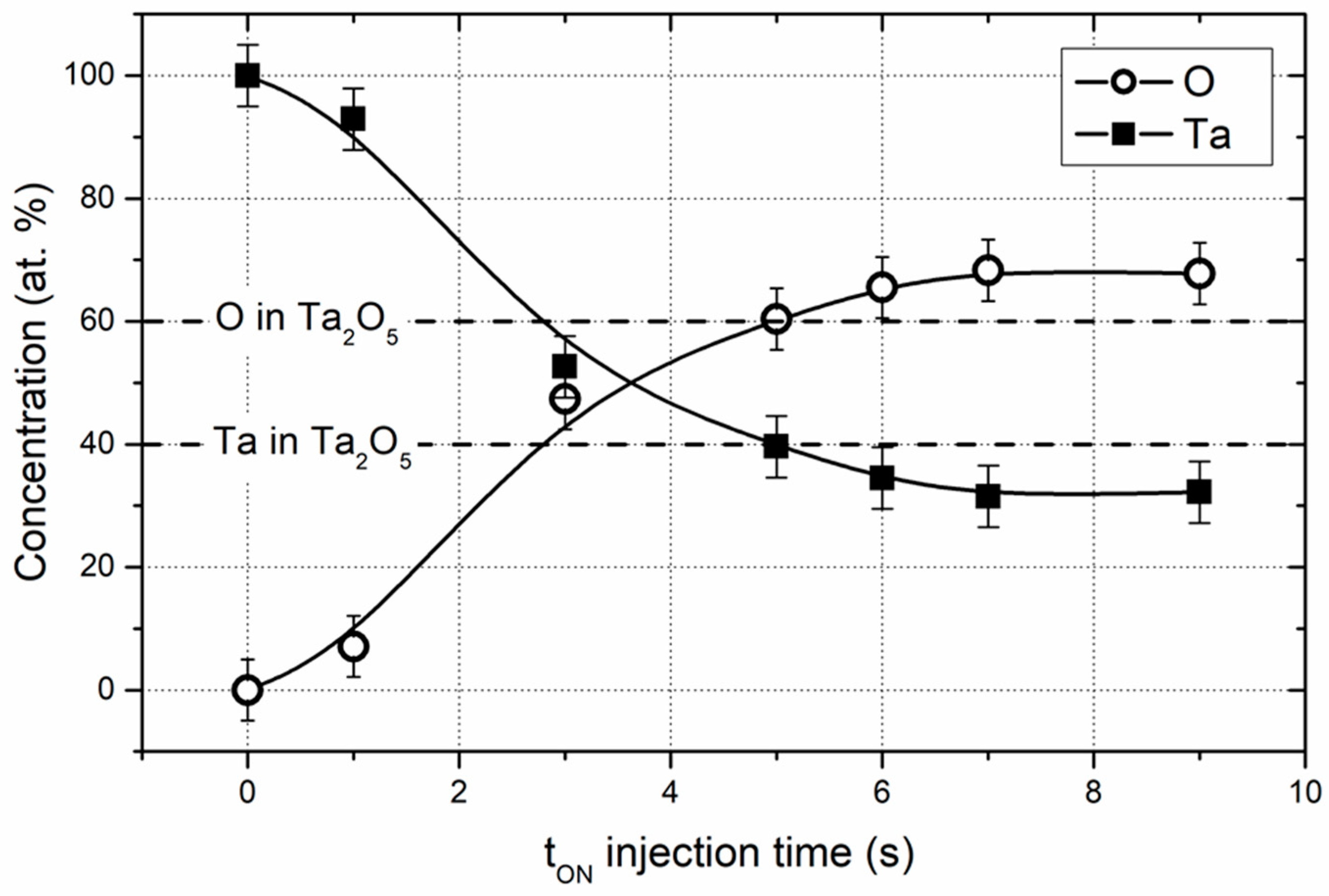
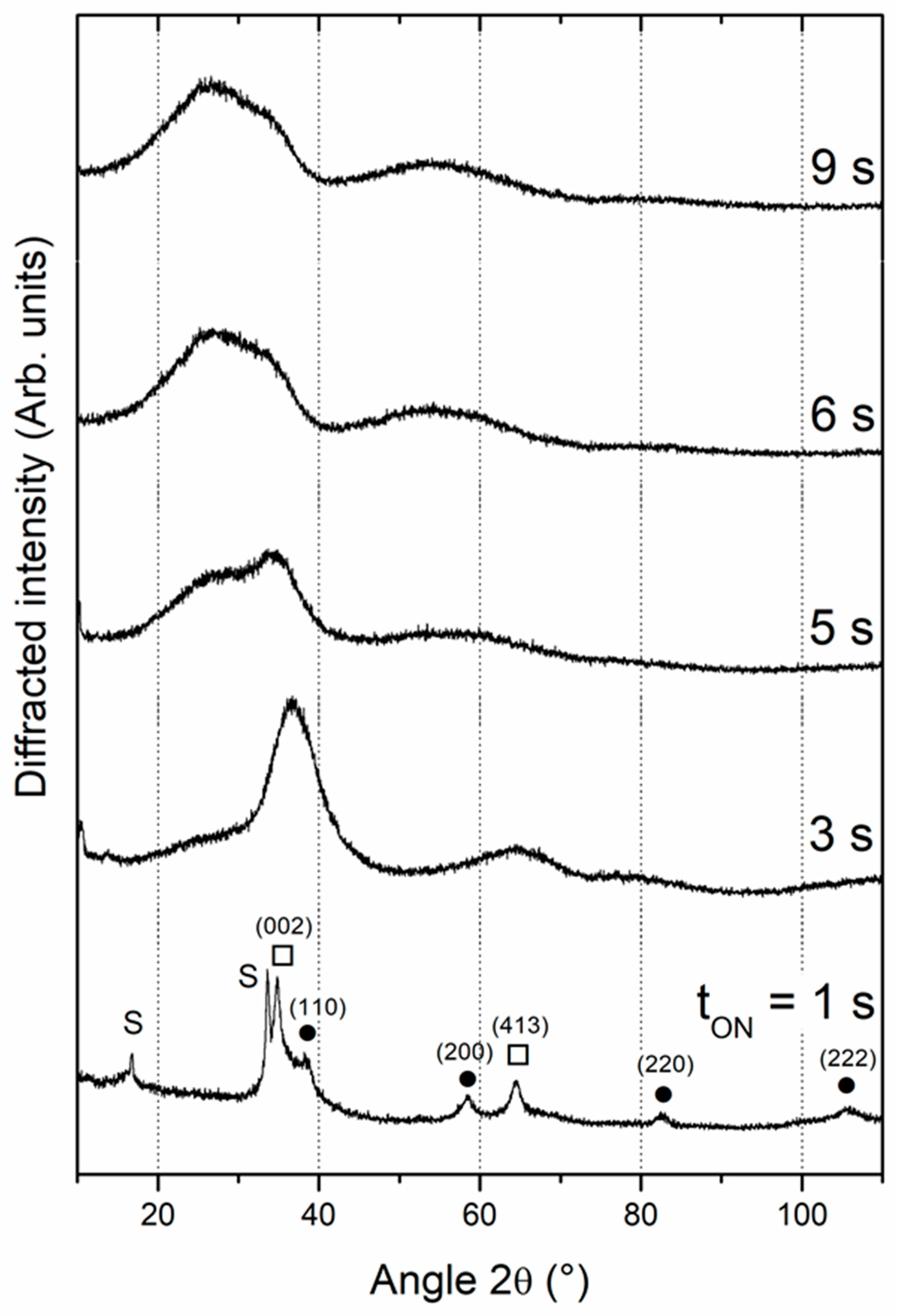
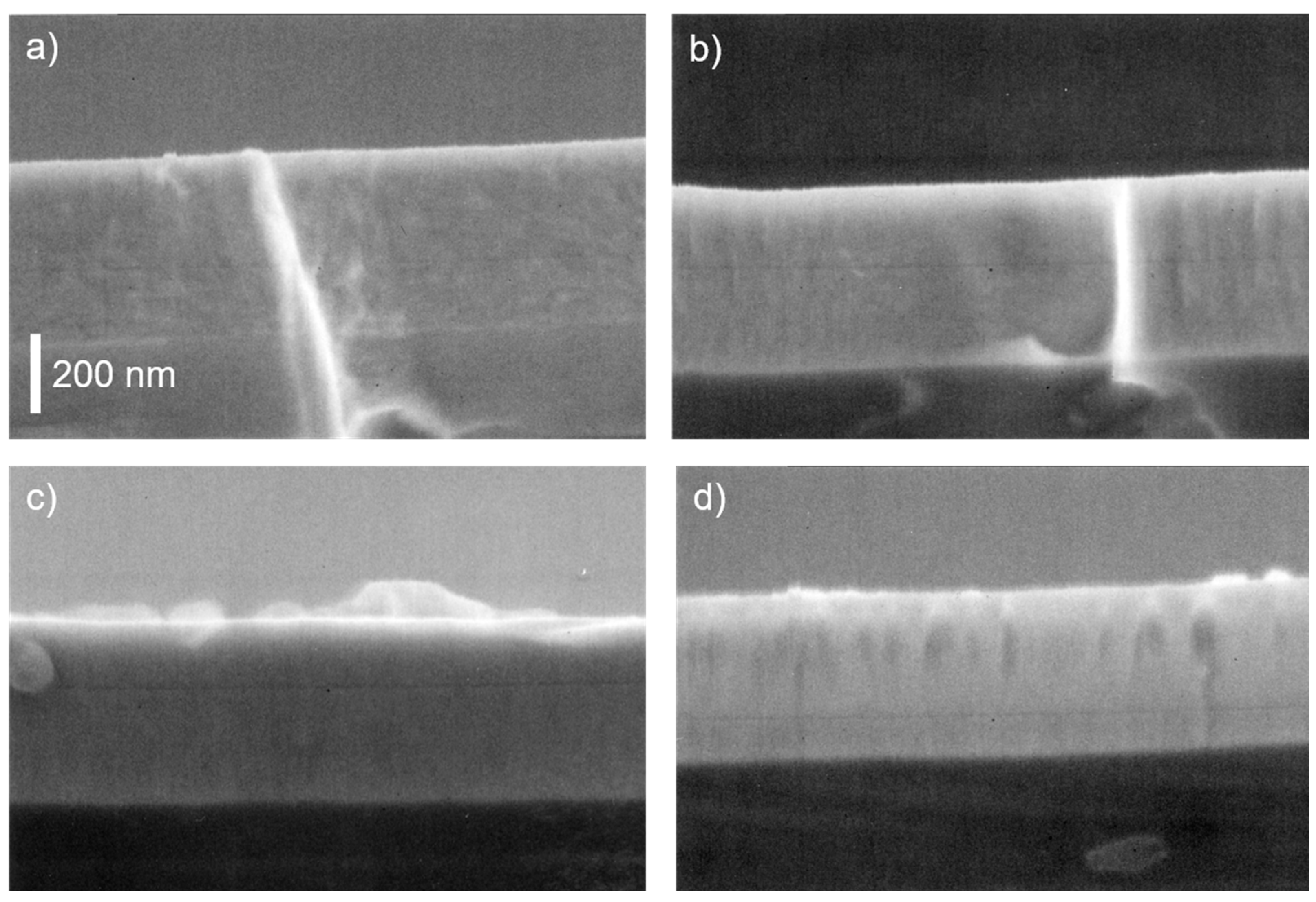
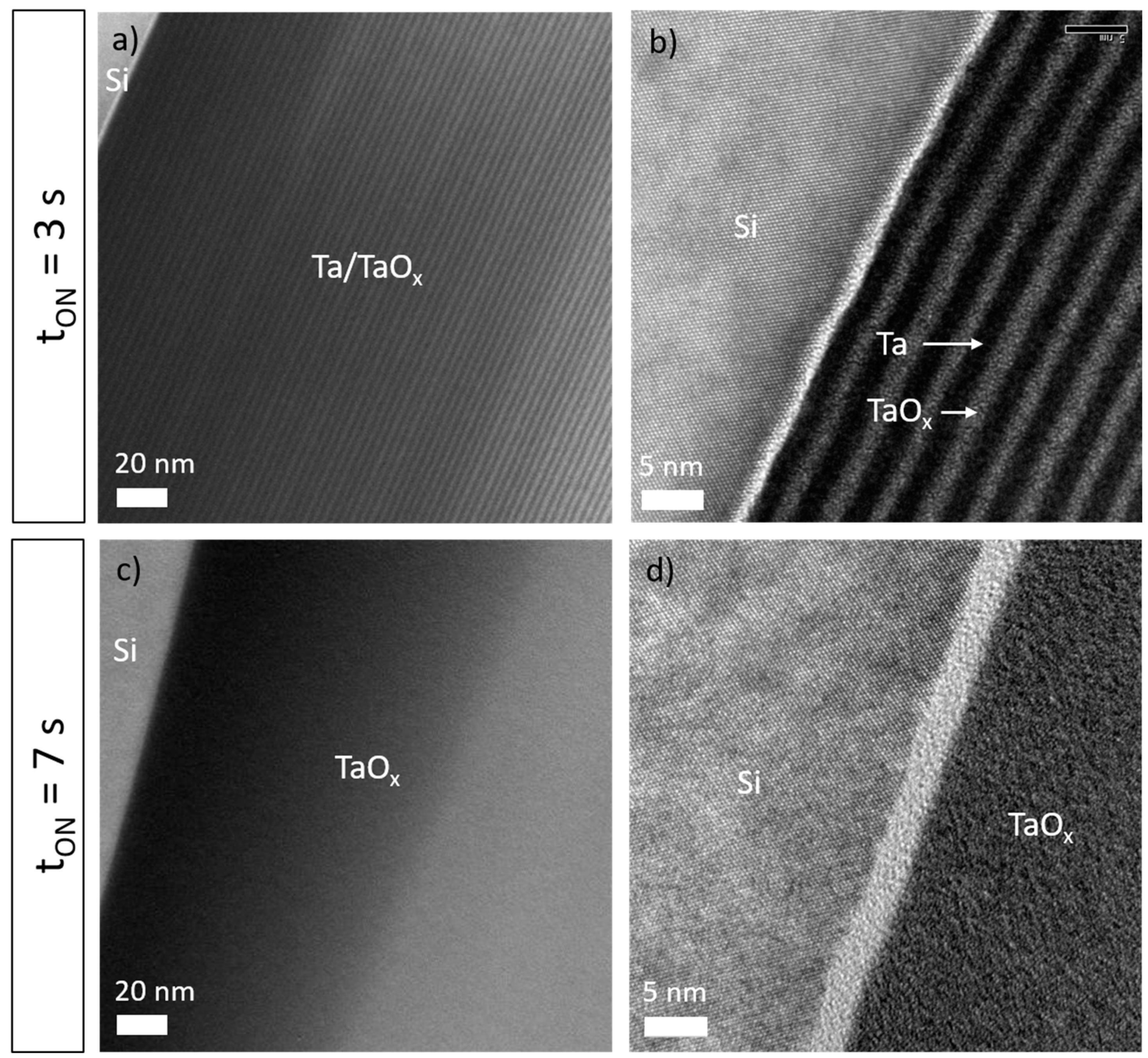
3.1. Composition and structure
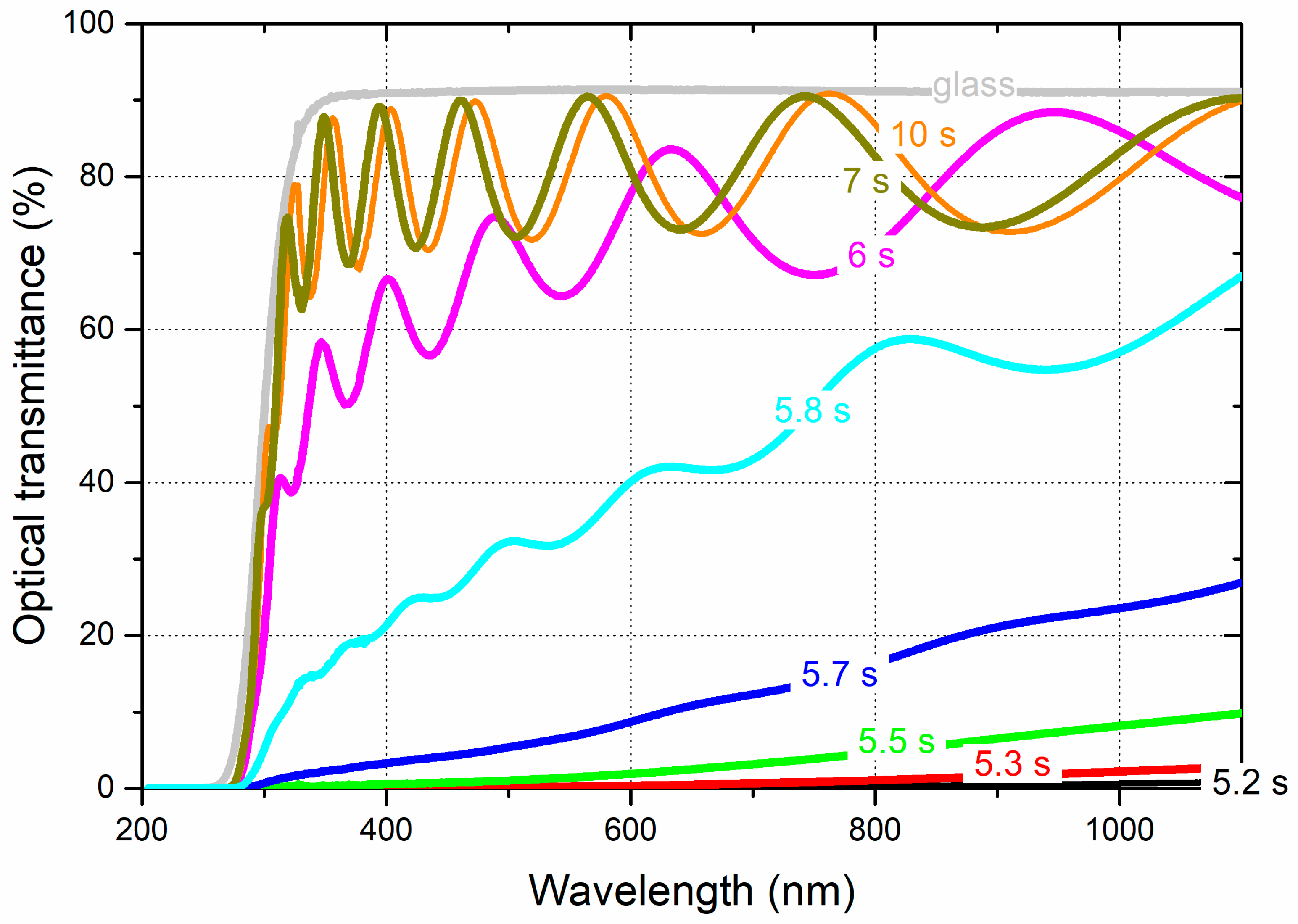
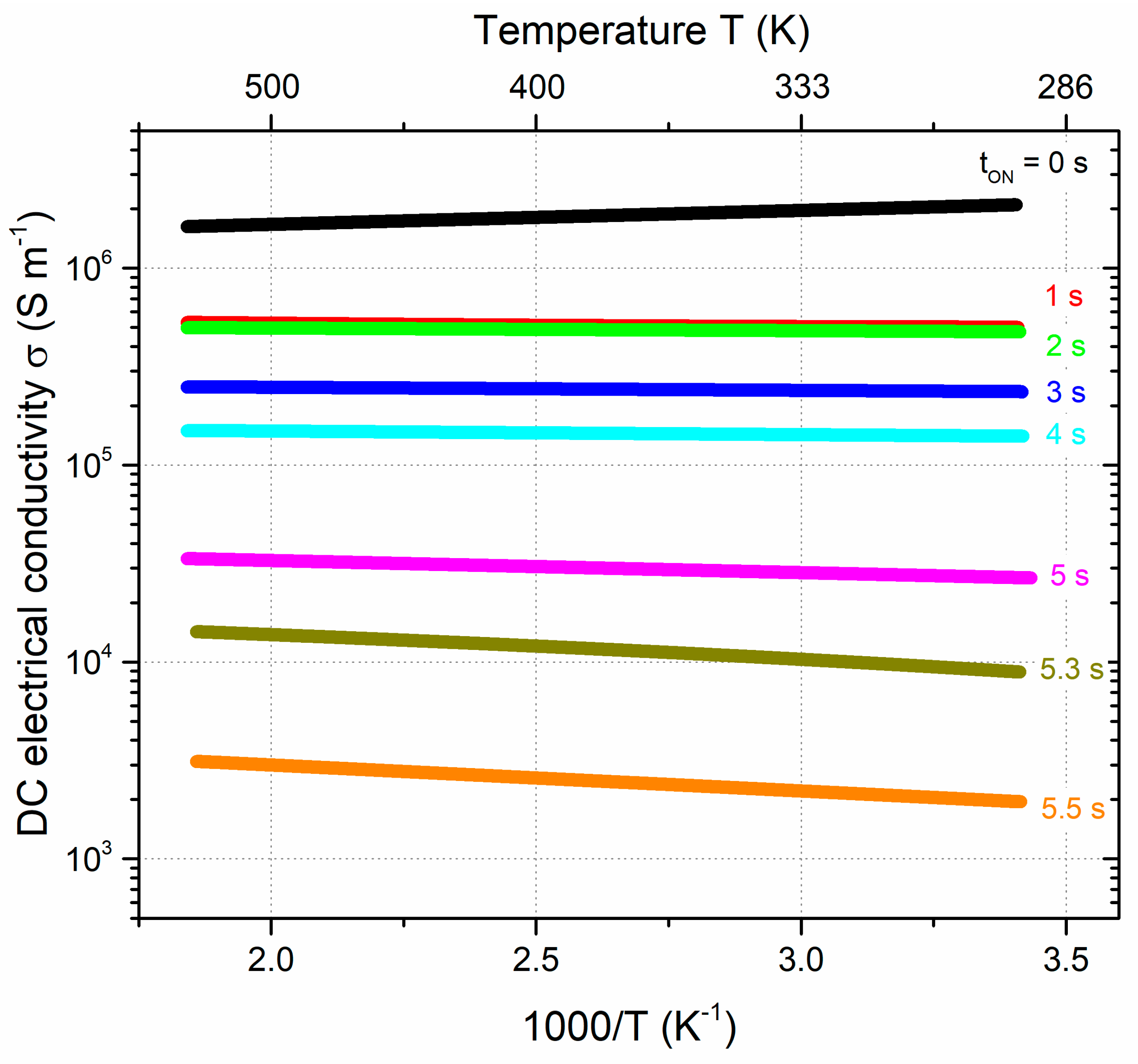
3.2. Optical and electrical properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Park, K.; Kim, H.J. High-performance vacuum-processes metal oxide thin-film transistors: A review of recent developments. J. Soc. Inf. Display 2020, 28, 591–622. [Google Scholar] [CrossRef]
- Chen, R.; Lan, L. Solution-processed metal-oxide thin-film transistors: A review of recent developments. Nanotechnology 2019, 30, 312001–35. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuator. B-Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductive electrodes for electrochromic devices: A review. Appl. Phys. A 1993, 57, 19–24. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1702774–26. [Google Scholar] [CrossRef]
- Losego, M.D.; Efremenko, A.Y.; Rhodes, C.L.; Cerruti, M.G.; Franzen, S.; Maria, J.P. Conductive oxide thin films: Model systems for understanding and controlling surface plasmon resonance. J. Appl. Phys. 2009, 106, 024903–024908. [Google Scholar] [CrossRef]
- Chittinan, D.; Buranasiri, P.; Lertvanithphol, T.; Eiamchai, P.; Tantiwanichapan, K.; Sathukarn, A.; Limwichean, S.; Klamchuen, A.; Wutikhun, T.; Limsuwan, P. , Nakajima, H.; Phae-ngam, W.; Triamnak, N.; Horprathum, M. Tailoring the structural and optical properties of fabricated TiO2 thin films by O2 duty cycle in reactive gas-timing magnetron sputtering. Vacuum 2023, 214, 112205–112211. [Google Scholar] [CrossRef]
- Medvedeva, J.E.; Buchholz, D.B.; Chang, R.P.H. Recent advances in understanding the structure and properties of amorphous oxide semiconductors. Adv. Electron. Mater. 2017, 3, 1700082. [Google Scholar] [CrossRef]
- Moreira, H.; Costa-Barbosa, A.; Mariana Marques, S.; Sampaio, P.; Carvalho, S. Evaluation of cell activation promoted by tantalum and tantalum oxide coatings deposited by reactive DC magnetron sputtering. Surf. Coat. Technol. 2017, 330, 260–269. [Google Scholar] [CrossRef]
- Sharath, S.U.; Joseph, M.J.; Vogel, S.; Hildebrandt, E.; Komissinskiy, P.; Kurian, J.; Schroeder, T.; Alff, L. Impact of oxygen stoichiometry on electroforming and multiple switching modes in TiN/TaOx/Pt based ReRAM. Appl. Phys. Lett. 2016, 109, 173503–173504. [Google Scholar] [CrossRef]
- Franke, E.; Schubert, M.; Trimble, C.L.; Devries, M.J.; Woollam, J.A. Optical properties of amorphous and polycrystalline tantalum oxide measured by spectroscopic ellipsometry. Thin Solid Films 2001, 388, 283–289. [Google Scholar] [CrossRef]
- Kazuki, T.; Yasusei, Y.; Shanhu, B.; Masahisa, O.; Kazuki, Y. Solid electrolyte of tantalum oxide thin film deposited by reactive DC and RF magnetron sputtering for all-solid-state switchable mirror glass. Sol. Energy Mater. Sol. Cells 2008, 92, 120–125. [Google Scholar] [CrossRef]
- Borisenko, V.E.; Parkhutik, V.P. RBS study of transient thermal anodic oxidation of tantalum films. Phys. Status Solidi A-Appl. Mat. 1986, 93, 123–130. [Google Scholar] [CrossRef]
- Gangloff, D.; Shi, M.; Wu, T.; Bylinskii, A.; Braverman, B.; Gutierrez, M.; Nichols, R.; Li, J.; Aichholz, K.; Cetina, M. Karpa, L.; Jelenkovic, B.; Chuang, I.; Vuletic, V. Preventing and reversing vacuum-induced optical losses in high-finesse tantalum (V) oxide mirror coatings. Opt. Express 2015, 23, 18014–18028. [Google Scholar] [CrossRef]
- Yun, S.N.; Wang, L.; Guo, W.; Ma, T.L. Non-Pt counter electrode catalysts using tantalum oxide for low-cost dye-sensitized solar cells. Electrochem. Commun. 2012, 24, 69–73. [Google Scholar] [CrossRef]
- Schmitt, K.; Oehse, K.; Sulz, G.; Hoffmann, C. Evanescent field sensors based on tantalum pentoxide waveguides – A review. Sensors, 2007, 8, 711–738. [Google Scholar] [CrossRef]
- Rouahi, A.; Challali, F.; Dakhlaoui, I.; Vallee, C.; Salimy, S.; Jomni, F.; Yangui, B.; Besland, M.P.; Goullet, A.; Sylvestre, A. Structural and dielectric characterization of sputtered tantalum titanium oxide thin films for high temperature capacitor applications. Thin Solid Films, 2016, 606, 127–132. [Google Scholar] [CrossRef]
- Kimura, H.; Mizuki, K.; Kamiyama, S.; Suzuki, H. Extended X-ray absorption fine-structure analysis of the difference in local-structure of tantalum oxide capacitor films produced by various annealing methods. Appl. Phys. Lett. 1995, 66, 2209–2221. [Google Scholar] [CrossRef]
- Lau, W.S.; Tan, T.S.; Babu, P.; Sandler, N.P. Mechanism of leakage current reduction of tantalum oxide capacitors by titanium doping. Appl. Phys. Lett. 2007, 90, 112903. [Google Scholar] [CrossRef]
- Yu, H.B.; Zhu, S.Y.; Yang, X.; Wang, X.H.; Sun, H.W.; Huo, M.X. Synthesis of coral-like tantalum oxide films via anodization in mixed organic-inorganic electrolytes. PLoS ONE 2013, 8, e66447. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Samukawa, S. Resistive switching in a few nanometers thick tantalum oxide thin film formed by a metal oxidation. Appl. Phys. Lett. 2015, 106, 173110–173114. [Google Scholar] [CrossRef]
- He, X.M.; Wu, J.H.; Zhao, L.L.; Mang, J.; Gao, X.D.; Li, X.M. Synthesis and optical properties of tantalum oxide films prepared by ionized plasma-assisted pulsed laser deposition. Solid State. Commun. 2008, 147, 90–93. [Google Scholar] [CrossRef]
- Kim, I.; Ahn, S.D.; Cho, B.W.; Ahn, S.T.; Lee, J.Y.; Chun, J.S.; Lee, W.J. Microstructure and electrical properties of tantalum oxide thin film prepared by electron cyclotron resonance plasma enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 1994, 33, 6691–6698. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W.A.B.W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Wang, S.C.; Liu, K.Y. , Huang, J.L. Tantalum oxide film prepared by reactive magnetron sputtering deposition for all-solid-state electrochromic device. Thin Solid Films 2011, 520, 1454–1459. [Google Scholar] [CrossRef]
- Ngaruiya, J.M. , Venkataraj, S.; Drese, R.; Kappertz, O.; Leervad Pedersen, T.P.; Wuttig, M. Preparation and characterization of tantalum oxide films produced by reactive DC magnetron sputtering. Phys. Stat. Sol. 2003, 198, 99–110. [Google Scholar] [CrossRef]
- Depla, D. De Gryse, R. Target poisoning during reactive magnetron sputtering: Part I: The influence of ion implantation. Surf. Coat. Technol. 2004, 183, 184–189. [Google Scholar] [CrossRef]
- Berg, S.; Särhammar, E.; Nyberg, T. Upgrading the “Berg-model” for reactive sputtering processes. Thin Solid Films 2014, 565, 186–192. [Google Scholar] [CrossRef]
- Billard, A.; Frantz, C. Attempted modelling of thickness and chemical heterogeneity in coatings prepared by dc reactive magnetron sputtering. Surf. Coat. Technol. 1993, 59, 41–47. [Google Scholar] [CrossRef]
- Martin, N.; J. Lintymer, J.; Gavoille, J.; Chappé, J.M.; Sthal, F.; Takadoum, J.; Vaz, F.; Rebouta, L. Reactive sputtering of TiOxNy coatings by the reactive gas pulsing process – Part II : The role of the duty cycle. Surf. Coat. Technol. 2007, 201, 7727–7732. [Google Scholar] [CrossRef]
- Martin, N.; Sanjinès, R.; Takadoum, J.; Lévy, F. Enhanced sputtering of titanium oxide, nitride and oxynitride thin films by the reactive gas pulsing technique. Surf. Coat. Technol. 2007, 142–144, 615–620. [Google Scholar] [CrossRef]
- Petitjean, C.; Rousselot, C.; Pierson, J.F.; Billard, A. Reactive sputtering of iron in Ar-N2 and Ar-O2 mixtures. Surf. Coat. Technol. 2005, 200, 431–434. [Google Scholar] [CrossRef]
- Martin, N.; Bally, A.R.; Hones, P.; Sanjinès, R.; Lévy, F. High rate and process control of reactive sputtering by reactive gas pulsing : The Ti—O system. Thin Solid Films 2000, 377–378, 550–556. [Google Scholar] [CrossRef]
- Khemasiri, N.;Chananonnawathorn, C.; Klamchuen, A.; Jessadaluk, S.; Pankiew, A.; Vuttivong, S.; Eiamchai, P.; Horprathum, M.; Pornthreeraphat, S.; Kasamechonchung, P.; Tantisantisom, K.; Boonkoom, T.; Songsiririthigul, P.; Nakajima, H.; Nukeaw, J. Crucial role of reactive pulse-gas on a sputtered Zn3N2 thin film formation. RSC Adv. 2016, 6, 94905–94910. [Google Scholar] [CrossRef]
- Xu, X.; Arab Pour Yazdi, M.; Salut, R.; Cote, J.M.; Billard, A.; Martin, N. Structure, composition and electronic transport properties of tungsten oxide thin films sputter-deposited by the reactive gas pulsing process. Mater. Chem. Phys. 2018, 205, 391–400. [Google Scholar] [CrossRef]
- Oechsner, H.; Schoof, H.; Stumpe, E. Sputtering of Ta2O5 by Ar+ ions at energies below 1 keV. Surf. Sci. 1978, 76, 343–354. [Google Scholar] [CrossRef]
- Rousselot, C.; Martin, N. Hysteresis effect in DC and RF reactive magnetron sputtering. Vide-Sci. Technol. Appl. 1999, 54, 481–490. [Google Scholar]
- Djeffal, F.; Martin, N. , Ferhati, H.; Benhaya, A. Tunable band-selective photodetector based on sputter-deposited SnOx thin-films: Effect of reactive gas pulsing process. J. Alloy. Compd. 2023, 968, 171851–171858. [Google Scholar] [CrossRef]
- Xu, X.; Arab Pour Yazdi, M.; Salut, R.; Cote, J.M.; Billard, A.; Martin, N. Structure, composition and electronic transport properties of tungsten oxide thin film sputter-deposited by the reactive gas pulsing process. Mater. Chem. Phys. 2018, 205, 391–400. [Google Scholar] [CrossRef]
- Sproul, W.D. High-rate reactive sputtering process-control. Surf. Coat. Technol. 1987, 33, 73–81. [Google Scholar] [CrossRef]
- Hmiel, A.F. Partial-pressure control of reactively sputtered titanium nitride. J. Vac. Sci. Technol. 1985, A3, 592–595. [Google Scholar] [CrossRef]
- Larsson, T.; Blom, H.O.; Nender, C.; Berg, S. A physical model for eliminating instabilities in reactive sputtering. J. Vac. Sci. Technol. 1988, A6, 1832–1836. [Google Scholar] [CrossRef]
- Baker, P.N. Preparation and properties of tantalum thin films. Thin Solid Films 1972, 14, 3–25. [Google Scholar] [CrossRef]
- Knepper, R. , Stevens, B., Baker, S.P. Effect of oxygen on the thermomechanical behavior of tantalum thin films during the β-α phase formation. J. App. Phys. 2006, 100, 123508. [Google Scholar] [CrossRef]
- Feinstein, L.G. , Huttemann, R.D. Factors controlling the structure of sputtered Ta films. Thin Solid Films 1973, 16, 129–145. [Google Scholar] [CrossRef]
- Garg, S.P.; Krishnamurty, N.; Awasthi, A.; Venkatraman, M. The Ta-O (Oxygen-Tantalum) system. J. Phase Equilib. 1996, 17, 63–77. [Google Scholar] [CrossRef]
- Colin, J.J.; Abadias, G.; Michel, A.; Jaouen, C. On the origin of the metastable β-Ta phase in tantalum sputtered thin films. Acta Mater. 2017, 126, 481–493. [Google Scholar] [CrossRef]
- Cacucci, A.; Loffredo, S.; Potin, V.; Imhoff, L.; Martin, N. Interdependence of structural and electrical properties in tantalum/tantalum oxide multilayers. Surf. Coat. Technol. 2013, 227, 38–41. [Google Scholar] [CrossRef]
- Zhou, J.C.; Luo, D.T.; Li, Y.Z.; Liu, Z. Effect of sputtering pressure and rapid thermal annealing on optical properties of Ta2O5 thin films. Trans. Nonferrous Met. Soc. China 2009, 19, 359–363. [Google Scholar] [CrossRef]
- Vlcek, J.; Rezek, J.; Houska, J.; Cerstvy, R.; Bugyi, R. Process stabilization and a significant enhancement of the deposition rate in reactive high-power impulse magnetron sputtering of ZrO2 and Ta2O5 films. Surf. Coat. Technol. 2013, 236, 550–556. [Google Scholar] [CrossRef]
- Ngaruiya, J.; Venkataraj, S.; Drese, R.; Kappertz, O.; Leervad Perdersen, T.P.; Wuttig, M. Preparation and characterization of tantalum oxide thin films produced by reactive DC magnetron sputtering. Phys. Stat. Sol. 2003, 198, 99–110. [Google Scholar] [CrossRef]
- Chittinan, D.; Buranasiri, P.; Lertvanithphol, T.; Eiamchai, P.; Patthanasettakul, V.; Chananonnawathorn, C.; Limwichean, S.; Nuntawong, N.; Klamchuen, A.; Muthitamongkol, P.; Limsuwan, P.; Chindaudom, P.; Nukeaw, J.; Nakajima, H.; Horprathum, M. Observations of the initial stage on reactive gas-timing sputtered TaO thin films by dynamic in situ spectroscopic ellipsometry. Optic. Mater. 2019, 92, 223–232. [Google Scholar] [CrossRef]
- Ito, Y.; Abe, Y.; Kawamura, M.; Kim, K.H.; Kiba, T. Influence of substrate cooling on ion conductivity of tantalum oxide thin films prepared by reactive sputtering using water vapor injection. Thin Solid Films 2020, 710, 138276. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Parreira, N.; Polcar, T. Cavaleiro, A. Characterization of W-O coatings deposited by magnetron sputtering with reactive gas pulsing. Surf. Coat. Technol. 2007, 201, 5481–5486. [Google Scholar] [CrossRef]
- Potin, V.; Cacucci, A.; Martin, N. Correlations between structure, composition and electrical properties of tungsten/tungsten oxide periodic multilayers sputter deposited by gas pulsing. Superlattices Microstruct. 2017, 101, 127–137. [Google Scholar] [CrossRef]
- El Mouatassim, A.; Pac, M.J.; Pailloux, F.; Amiard, G.; Henry, P.; Rousselot, C.; Eyidi, D.; Tuilier, M.H.; Cabioc’h, T. On the possibility of synthesizing multilayered coatings in the (Ti, Al)N system by RGPP: A microstructural study. Surf. Coat. Technol. 2019, 374, 845–851. [Google Scholar] [CrossRef]
- Fenker, M.; Kappl, H.; Sandu, C.S. Precise control of multilayered structures of Nb-O-N thin films by the use of reactive gas pulsing process in DC magnetron sputtering. Surf. Coat. Technol. 2008, 202, 2358–2362. [Google Scholar] [CrossRef]
- Springer, S.G.; Schmid, P.E.; Sanjinès, R.; Lévy, F. Morphology and electrical properties of titanium oxide nanometric multilayers deposited by DC reactive sputtering. Surf. Coat. Technol. 2002, 151–152, 51–54. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Wei, Y.; Yan, Y. Effect of oxygen content on the properties of sputtered TaOx electrolyte film in all-solid-state electrochromic devices. Coatings 2022, 12, 183–15. [Google Scholar] [CrossRef]
- Martin, N.; Besnard, A.; Sthal, F.; Takadoum, J. The reactive gas pulsing process for tuneable properties of sputter deposited titanium oxide, nitride and oxynitride coatings. Inter. J. Mater. Prod. Technol. 2010, 39, 159–177. [Google Scholar] [CrossRef]
- Cacucci, A.; Tsiouassis, I.; Potin, V.; Imhoff, L.; Martin, N.; Nyberg, T. The interdependence of structural and electrical properties in TiO2/TiO/Ti periodic multilayers. Acta Mater. 2013, 61, 4215–4225. [Google Scholar] [CrossRef]
- Desal, P.D.; Chu, T.K.; James, H.M.; Ho, C.Y. Electrical resistivity of selected elements. J. Phys. Chem. Ref. Data 1984, 13, 1069–1096. [Google Scholar] [CrossRef]
- Reiss, G.; Vancea, J.; Hoffman, H. Grain-boundary resistance in polycrystalline metals. Phys. Rev. Lett. 1986, 56, 2100–2103. [Google Scholar] [CrossRef]
- Schwartz, N.; Reed, W.A.; Polash, P.; Read, M.H. Temperature coefficient of resistance of beta-tantalum films and mixtures with b.c.c.-tantalum. Thin Solid Films 1972, 14, 333–347. [Google Scholar] [CrossRef]
- Baker, A.; Engwall, A.M.; Bayu-Aji, L.B.; Bae, J.H.; Shin, S.J.; Moody, J.D.; Kucheyev, S.O. Tantalum suboxide films with tunable composition and electrical resistivity deposited by reactive magnetron sputtering. Coatings 2022, 12, 917–910. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




