Submitted:
16 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
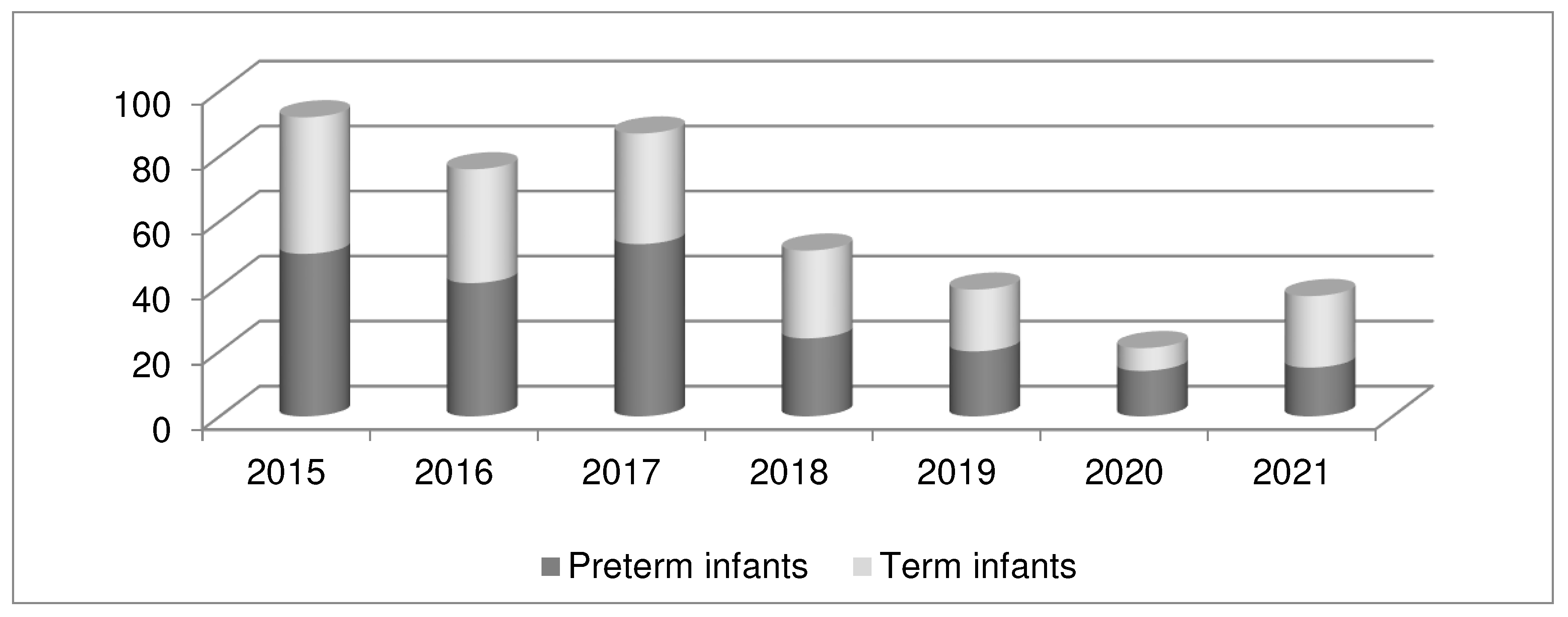
3.1. Group and subgroup analysis
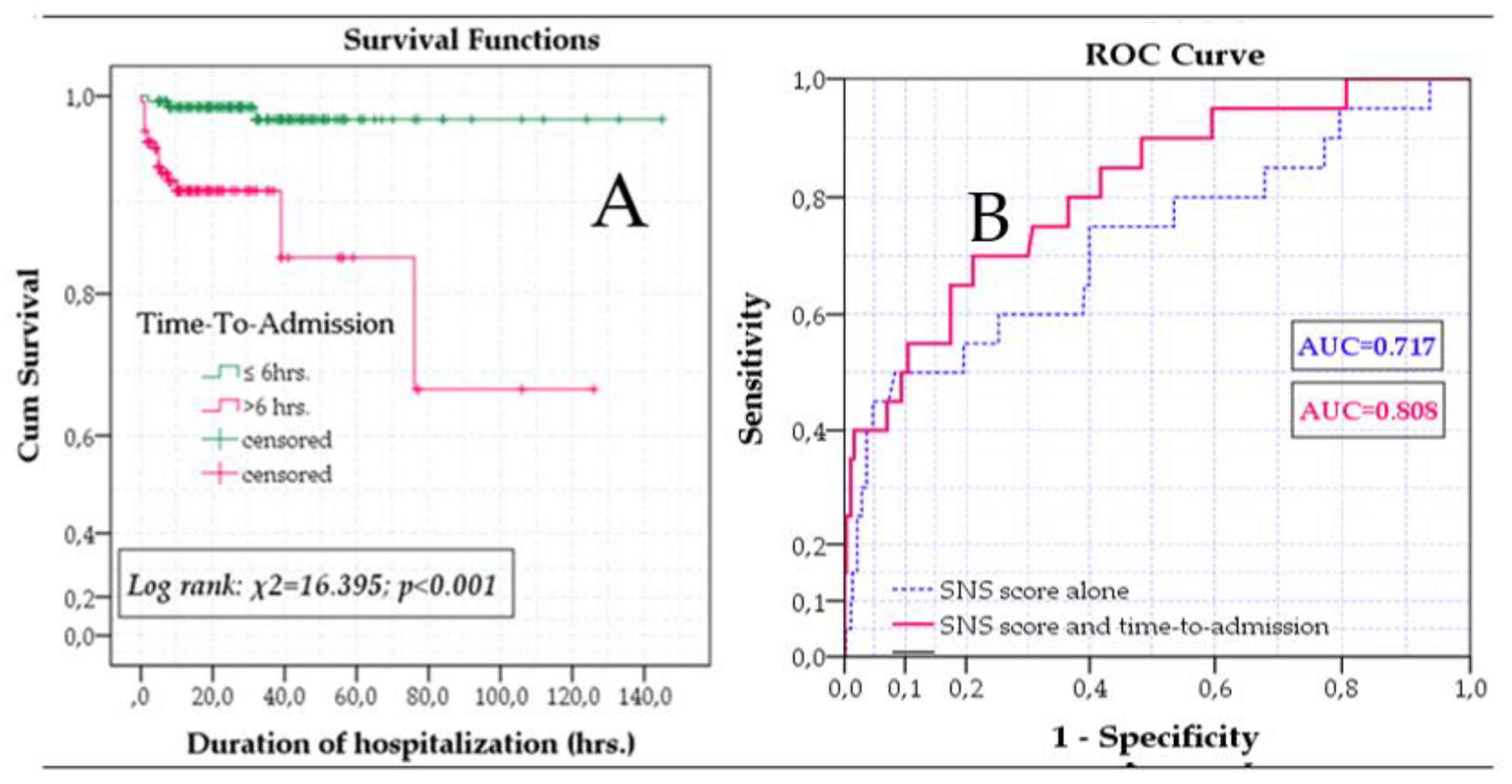
3.2. SNS score to predict mortality
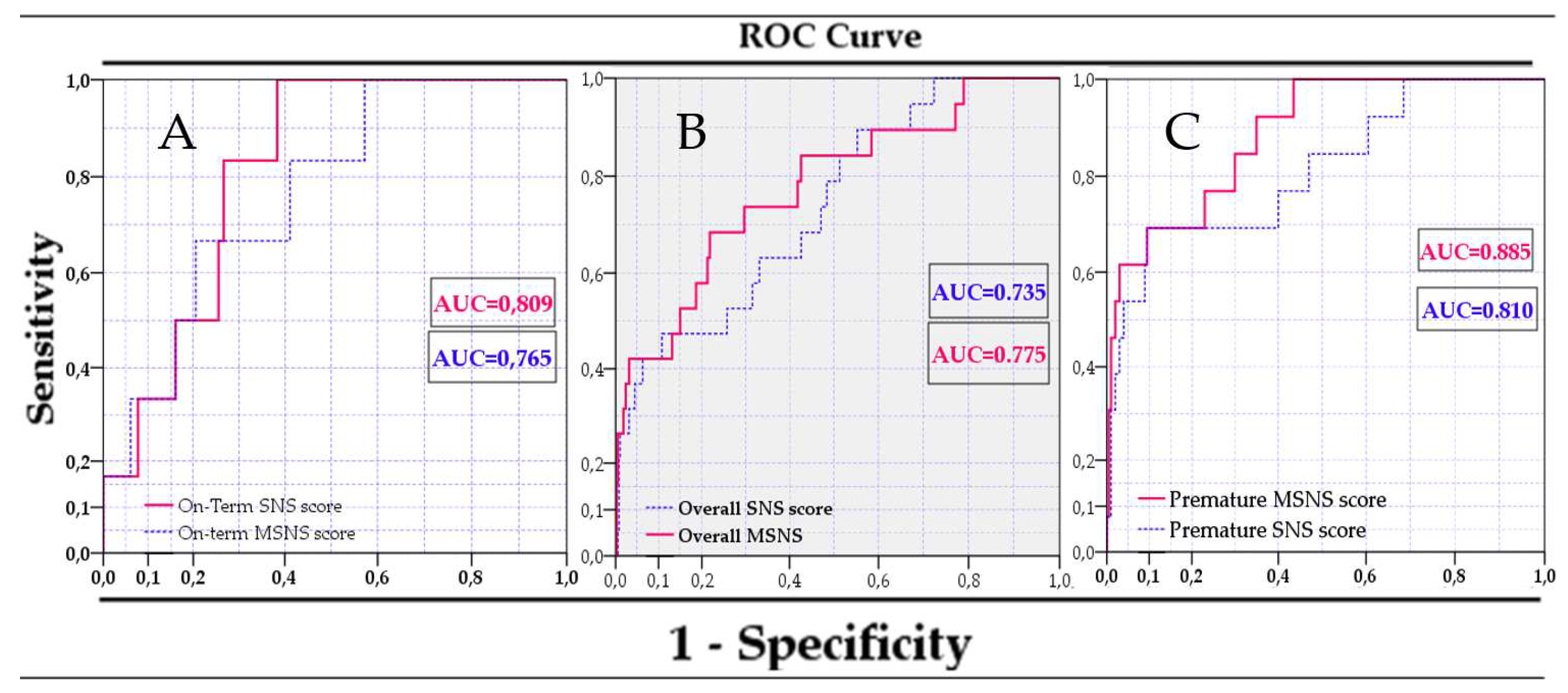
3.3. Use of an improved SNS score for predicting mortality
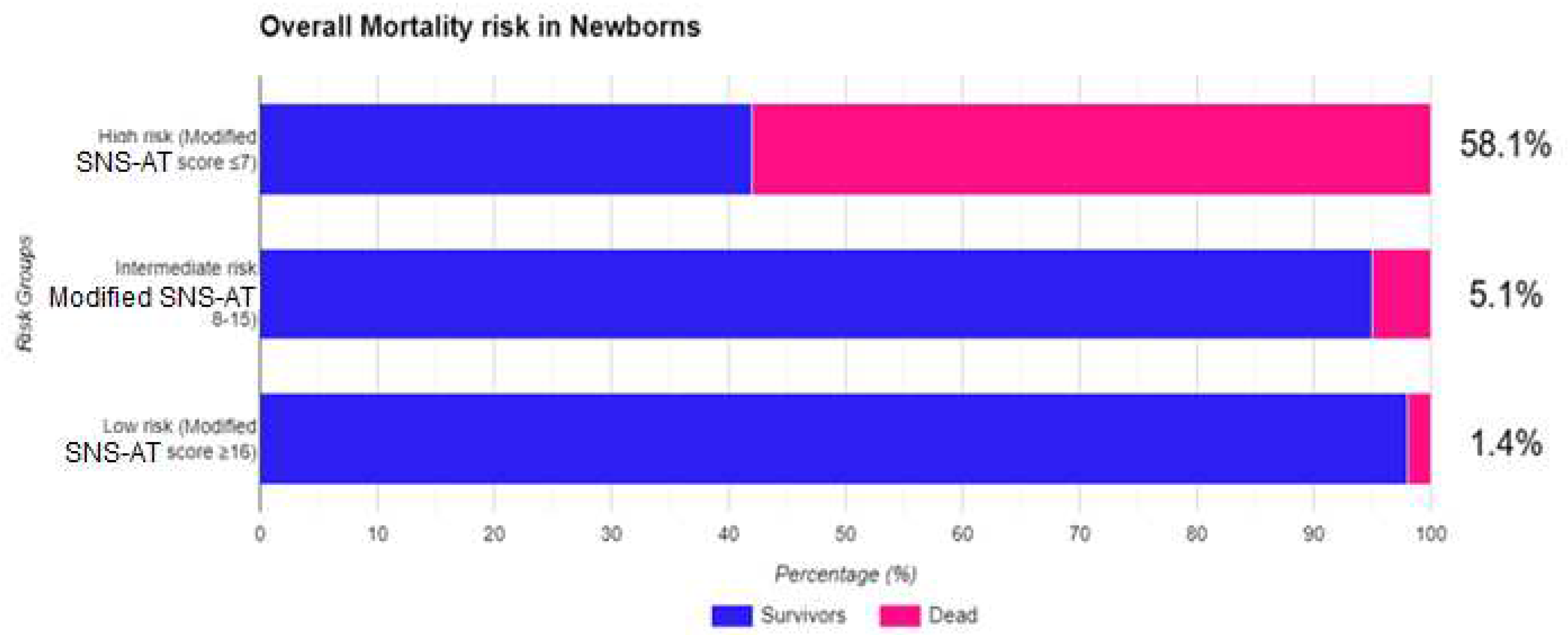
3.4. Evaluation of mortality risk with MSNS-AT score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Standards For Improving Quality Of Maternal and Newborn Care in Health Facilities. World Health Organisation. 2016. Available online: http://www.who.int (accessed on 3 September 2023).
- GBD 2019 Under-5 Mortality Collaborators. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet. 2021, 398, 870–905. [Google Scholar] [CrossRef]
- Qu, W., Shen, Y., Qi, Y., Jiang, M., Zheng, X., Zhang, J., Wu, D., He, W., Geng, W., Hei., M. Comparison of four neonatal transport scoring methods in the prediction of mortality risk in full-term, out-born infants: a single-center retrospective cohort study. Eur J Pediatr. 2022, 181, 3005–3011.
- Neonatal Mortality-UNICEF Data. Available online: https://data. unicef.org/topic/child-survival/neonatal-mortality/ (accessed on 30 August 2023).
- Meshram, R.M., Nimsarkar, R.A., Nautiyal, A.P. Role of modified sick neonatal score in predicting the neonatal mortality at limited-resource setting of central India. J Clin Neonatol 2023, 12, 1–6. [CrossRef]
- Cavallin, F., Contin, A., Alfeu, N., Macmillian, B., Seni, A.H.A., Cebola, B.R., Calgaro, S., Putoto, G., Trevisanuto, D. Prognostic role of TOPS in ambulance-transferred neonates in a low-resource setting: a retrospective observational study. BMC Pregnancy Childbirth. 2022, 22, 726.
- Guidelines for Perinatal Care. American Academy of Pediatrics. Committee on Fetus and Newborn. Edited by American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. 2012.
- Chang, A.S., Berry, A., Jones, L.J., Sivasangari, S. Specialist teams for neonatal transport to neonatal intensive care units for prevention of morbidity and mortality. Cochrane Database Syst Rev. 2015, 2015, CD007485.
- Chheda, A., Khadse, S., Valvi, C., Kulkarni, R., Hiremath, A. Importance of Temperature, Oxygen Saturation, Perfusion, Sugar (TOPS) Parameters and the Concept of TOPS Score for Neonatal Transport in India – A Pilot Project. Pediatric Oncall Journal. 2018, 15, 69–72.
- Amer, R., Moddemann, D., Seshia, M., Alvaro, R., Synnes, A., Lee, K.S., Lee, S.K., Shah, P.S.; Canadian Neonatal Network and Canadian Neonatal Follow-up Network Investigators. Neurodevelopmental Outcomes of Infants Born at <29 Weeks of Gestation Admitted to Canadian Neonatal Intensive Care Units Based on Location of Birth. J Pediatr. 2018, 196, 31–37.
- Whyte, H.E., Jefferies, A.L.; Canadian Paediatric Society, Fetus and Newborn Committee. The interfacility transport of critically ill newborns. Paediatr Child Health. 2015, 20, 265–275.
- Chen, W.H., Su, C.H., Lin, L.C., Lin, H.C., Lin, Y.J., Hsieh, H.Y., Sheen, J.M., Lee, C.T. Neonatal mortality among outborn versus inborn babies. Pediatr Neonatol. 2021, 62, 412–418.
- Mohan, K.R., Kumar, R. Study of indications, complications and outcomes of neonatal transport by a skilled team. Int J Contemp Pediatr. 2019, 6, 2402–2405. [CrossRef]
- Rathod, D., Adhisivam, B., Bhat, B.V. Transport of sick neonates to a tertiary care hospital, South India: condition at arrival and outcome. Trop Doct. 2015, 45, 96–99. [CrossRef]
- O'Brien, E.A., Colaizy, T.T., Brumbaugh, J.E., Cress, G.A., Johnson, K.J., Klein, J.M., Bell, E.F. Body temperatures of very low birth weight infants on admission to a neonatal intensive care unit. J Matern Fetal Neonatal Med. 2019, 32, 2763–2766. [CrossRef]
- Tay, V.Y., Bolisetty, S., Bajuk, B., Lui, K., Smyth, J.; the New South Wales and the Australian Capital Territory Neonatal Intensive Care Units' Data Collection. Admission temperature and hospital outcomes in extremely preterm infants. J Paediatr Child Health. 2019, 55, 216–223.
- Standards for improving quality of care for small and sick newborns in health facilities. Geneva: World Health Organization; 2020. Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo (accessed on 3 September 2023).
- Stroud, M.H., Trautman, M.S., Meyer, K., Moss, M.M., Schwartz, H.P., Bigham, M.T., Tsarouhas, N., Douglas, W.P., Romito, J., Hauft, S., Meyer, M.T., Insoft, R. Pediatric and neonatal interfacility transport: results from a national consensus conference. Pediatrics 2013, 132, 359–366.
- Das, R.R., Sankar, J., Sankar, M.J. Sick Neonate Score: Better than Others in Resource Restricted Settings? Indian J Pediatr. 2016, 83, 97–98. [CrossRef]
- Ravikumar, S.P., Kaliyan, A., Jeganathan, S., Manjunathan, R. Post-transport TOPS score as a predictive marker of mortality among transported neonates and its comparative analysis with SNAP-II PE. Heliyon. 2022, 8, e10165. [CrossRef]
- Chellani, H., Arya, S. Scoring Tools to Predict Neonatal Mortality: Where Do We Stand Today? Indian J Pediatr. 2023, 90, 323. [CrossRef]
- Garg, B., Sharma, D., Farahbakhsh, N. Assessment of sickness severity of illness in neonates: review of various neonatal illness scoring systems. J Matern Fetal Neonatal Med. 2018, 31, 1373–1380. [CrossRef] [PubMed]
- Lee, K.S. Neonatal transport metrics and quality improvement in a regional transport service. Transl Pediatr. 2019, 8, 233–245. [Google Scholar] [CrossRef]
- Adhisivam, B. Clinical Scores for Sick Neonates. Indian J Pediatr. 2023, 90, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.S., Field, D.J., Manktelow, B. Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed. 2005, 90, F11–F16. [CrossRef] [PubMed]
- Padar, C., Rajan, A., Shriyan, A., Oommen, R.A. Modified Sick Neonatal Score and Delta: Modified Sick Neonatal Scores As Prognostic Indicators in Neonatal Intensive Care Units. Cureus. 2022, 14, e28414.
- Reddy, P., Gowda, B.R. A. A Study of the Prediction of Mortality in a Tertiary Care Hospital Using the Modified Sick Neonatal Score (MSNS): An Observational Cross-Sectional Study. Cureus. 2023, 15, e38484.
- Shivaramakrishnababji, N., Rajesh, C., Mekala, A., Siddani, B.R. Validation of modified sick neonatal score, a simple clinical score for assessment of severity of illness and outcome in new-borns for resource poor settings. Int J Contemp Pediatr 2022, 9, 53–57.
- Agrawal, J. Sick Neonate Score: Role in Predicting Neonatal Mortality. Clinics Mother Child Health. 2020, 17, 355. [Google Scholar]
- Rathod, D., Adhisivam, B., Bhat, B.V. Sick Neonate Score--A Simple Clinical Score for Predicting Mortality of Sick Neonates in Resource Restricted Settings. Indian J Pediatr. 2016, 83, 103–106. [CrossRef] [PubMed]
- WHO. Newborn mortality. 2021. Available online: https://www.who.int/news-room/factsheets/detail/levels-and-trends-in-child-mortality-report-2021 (accessed on 12 August 2023).
- Hirata, K., Ueda, K., Wada, K., Ikehara, S., Tanigawa, K., Kimura, T., Ozono, K., Iso, H; Japan Environment and Children’s Study Group. Long-term outcomes of children with neonatal transfer: the Japan Environment and Children's Study. Eur J Pediatr. 2022, 181, 2501–2511. [CrossRef]
- Ashokcoomar, P., Bhagwan, R. Towards a safer and more efficient neonatal transfer system in South Africa: A qualitative inquiry with ALS paramedics. Australasian Journal of Paramedicine 2021, 18, 1–9.
- Dempsey, E.M., Barrington, K.J. Evaluation and treatment of hypotension in the preterm infant. Clin Perinatol. 2009, 36, 75–85. [CrossRef]
- Ordinul Ministrului Sănătăţii şi Familiei nr. 910 privind criteriile de ierarhizare a secţiilor de spital de specialitate obstetrică, ginecologie şi neonatologie, publicat în Monitorul Oficial al României, 18.11.2002.
- Bivoleanu, A., Avasiloaiei, A., Voicilă, C., Stamatin, M., Stoicescu, S.M. Echilibrarea nou-născutului pentru transport și transportul neonatal, aprobat prin Ordinul Ministerului Sănătății 1232/2.08.2011, pulicat în Monitorul Oficial al României, Partea I, nr. 586/18.08.2011, Editura Alma Mater, Sibiu, 2011.
- Ordinul Ministrului Sănătăţii nr. 417 privind Înfiinţarea Unităţii de transport neonatal specializat, publicat în Monitorul Oficial al României, Partea I, nr. 349; 21.04.2004.
- Odinul Ministrului Sănătății privind aprobarea metodologiei şi criteriilor minime obligatorii de ierarhizare a structurilor de obstetrică-ginecologie, neonatologie şi pediatrie care asigură servicii de terapie intensivă pentru nou-născuți și pentru modificarea și completarea Ordinului ministrului sănătăţii nr. 323/2011 privind aprobarea metodologiei şi a criteriilor minime obligatorii pentru clasificarea spitalelor în funcţie de competenţă. Available online: https://www.ms.ro/ro/transparenta-decizionala/acte-normative-in-transparenta/ordin-privind-aprobarea-metodologiei-%C5%9Fi-criteriilor-minime-obligatorii-de-ierarhizare-a-structurilor-de-obstetric%C4%83-ginecologie-neonatologie-%C5%9Fi-pediatrie-care-asigur%C4%83-servicii-de-terapie-intensiv%C4%83-pentru-nou-n%C4%83scu%C8%9Bi/ (accessed on 25 September 2023).
- Ordinului Ministrului Sănătăţii nr. 323/18.04.2011 privind aprobarea metodologiei şi a criteriilor minime obligatorii pentru clasificarea spitalelor în funcţie de competenţă, publicat în Monitorul Oficial al României nr. 274/19.04.2011.
- Broughton, S.J., Berry, A., Jacobe, S., Cheeseman, P., Tarnow-Mordi, W.O., Greenough, A; Neonatal Intensive Care Unit Study Group. The mortality index for neonatal transportation score: a new mortality prediction model for retrieved neonates. Pediatrics. 2004, 114, e424–e428. [CrossRef]
- Mathur, N.B., Arora, D. Role of TOPS (a simplified assessment of neonatal acute physiology) in predicting mortality in transported neonates. Acta Paediatr. 2007, 96, 172–175. [CrossRef]
- Hermansen, M.C., Hasan, S., Hoppin, J., Cunningham, M.D. A validation of a scoring system to evaluate the condition of transported very-low-birthweight neonates. Am J Perinatol. 1988, 5, 74–78. [CrossRef] [PubMed]
- Dorling, J.S., Field, D.J. Value and validity of neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed. 2008, 93, F80–F82.
- Ray, S., Mondal, R., Chatterjee, K., Samanta, M., Hazra, A., Sabui, T.K. Extended Sick Neonate Score (ESNS) for Clinical Assessment and Mortality Prediction in Sick Newborns referred to Tertiary Care. Indian Pediatr. 2019, 56, 130–133. [CrossRef]
- Dammann, O., Shah, B., Naples, M., Bednarek, F., Zupancic, J., Allred, E.N., Leviton, A; ELGAN Study Investigators. Interinstitutional variation in prediction of death by SNAP-II and SNAPPE-II among extremely preterm infants. Pediatrics. 2009, 124, e1001–e1006. [CrossRef]
- Cole, T.J., Hey, E., Richmond, S. The PREM score: a graphical tool for predicting survival in very preterm births. Arch Dis Child Fetal Neonatal Ed. 2010, 95, F14–F19. [CrossRef]
- Helenius, K., Longford, N., Lehtonen, L., Modi, N., Gale, C; Neonatal Data Analysis Unit and the United Kingdom Neonatal Collaborative. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019, 367, l5678.
- Fang, J.L., Mara, K.C., Weaver, A.L., Clark, R.H., Carey, W.A. Outcomes of outborn extremely preterm neonates admitted to a NICU with respiratory distress. Arch Dis Child Fetal Neonatal Ed. 2020, 105, 33–40. [CrossRef] [PubMed]
- Shipley, L., Gyorkos, T., Dorling, J., Tata, L.J., Szatkowski, L., Sharkey, D. Risk of Severe Intraventricular Hemorrhage in the First Week of Life in Preterm Infants Transported Before 72 Hours of Age. Pediatr Crit Care Med. 2019, 20, 638–644. [CrossRef] [PubMed]
- Jensen, E.A., Lorch, S.A. Effects of a Birth Hospital's Neonatal Intensive Care Unit Level and Annual Volume of Very Low-Birth-Weight Infant Deliveries on Morbidity and Mortality. JAMA Pediatr. 2015, 169, e151906. [CrossRef] [PubMed]
- Gupta, N., Shipley, L., Goel, N., Browning Carmo, K., Leslie, A., Sharkey, D. Neurocritical care of high-risk infants during inter-hospital transport. Acta Paediatr. 2019, 108, 1965–1971. [CrossRef]
- Redpath, S., Shah, P.S., Moore, G.P., Yang, J., Toye, J., Perreault, T., Lee. K.S; Canadian Neonatal Transport Network and Canadian Neonatal Network Investigators. Do transport factors increase the risk of severe brain injury in outborn infants <33 weeks gestational age? J Perinatol. 2020, 40, 385–393.
- Behera, B., Lal Meena, B. Outcomes of Sick Neonates Transported to a Tertiary Care Hospital by a Trained Team, in Northern India. Indian Journal of Neonatal Medicine and Research. 2021, 9, PO10–PO15.
- Behera, B., Archana, B.R. SNAPPE-II (Score for Neonatal Acute Physiology with Perinatal Extension-II) in Predicting Mortality and Morbidity in NICU. J Clin Diagn Res. 2015, 9, SC10–SC12.
- Morse, S., Groer, M., Shelton, M.M., Maguire, D., Ashmeade, T. A Systematic Review: The Utility of the Revised Version of the Score for Neonatal Acute Physiology Among Critically Ill Neonates. J Perinat Neonatal Nurs. 2015, 29, 315–344. [CrossRef] [PubMed]
- Fleisher, B.E., Murthy, L., Lee, S., Constantinou, J.C., Benitz, W.E., Stevenson, D.K. Neonatal severity of illness scoring systems: a comparison. Clin Pediatr (Phila). 1997, 36, 223–227. [CrossRef] [PubMed]
- Mansoor, K.P., Ravikiran, S.R., Kulkarni, V., Baliga, K., Rao, S., Bhat, K.G., Baliga, B.S., Kamath, N. Modified Sick Neonatal Score (MSNS): A Novel Neonatal Disease Severity Scoring System for Resource-Limited Settings. Crit Care Res Pract 2019, 2019, 9059073.
- Mori, R., Fujimura, M., Shiraishi, J., Evans, B., Corkett, M., Negishi, H., Doyle, P. Duration of inter-facility neonatal transport and neonatal mortality: systematic review and cohort study. Pediatr Int. 2007, 49, 452–458. [CrossRef]
| Variables | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Respiratory effort | Apnea or grunting | Tachypnea (>60/min) +/- retractions | Normal (40-60/min) |
| Heart rate | Bradycardia/ Asystole |
Tachycardia (>160/min) | Normal (100-160/min) |
| Mean blood pressure (mmHg) | <30 | 30-39 | >39 |
| Axillary temperature (0C) | <36 | 36-36.5 | 36.5-37.5 |
| Capillary filling time (s) | >5 | 3-5 | <3 |
| Random blood sugar (mg/dL) | <40 | 40-60 | >60 |
| SpO2 in room air (%) | <85 | 85-92 | >92 |
| Preterm Infants (N 217) | Term Infants (N 186) |
All Infants (N 403) |
|
|---|---|---|---|
| Gestational age (weeks)(mean SD) | 35.56±2.87 | 38.73±1.31 | 35.40±3.83 |
| Birth weight (grams) (mean SD) | 1788.55±561.90 | 3119.30±631.25 | 2402.74±891.80 |
| Male gender (N/%) | 113/52.1 | 111/59.7 | 224/55.6 |
| Time to admission (hours) (mean SD) | 17.33±65.07 | 27.06±32.44 | 21,82±52.75 |
| Apgar score/1 min. (mean SD) | 6.60±2.12 | 7.30±2.32 | 6.92±2.24 |
| Apgar score< 3/1 min. (N/%) | 22/10.1 | 19/10.2 | 41/10.2 |
| Early onset sepsis (N/%) | 37/17.1 | 75/40.3 | 112/27.8 |
| Place of birth | |||
| Home (N/%) | 8/3.7 | 5/2.7 | 13/3.2 |
| Level I (N/%) | 125/57.6 | 126/67.7 | 251/62.3 |
| Level II (N/%) | 82/38.2 | 55/29.6 | 138/34.2 |
| Level III (N/%) | 1/0.5 | - | 1/0.2 |
| Death (N/%) | 14/6.5 | 6/3.2 | 20/5.0 |
| SNS score (mean SD) | 10.04±2.67 | 11.85±2.20 | 10.87±2.63 |
| Preterm Infants | Term Infants | ALL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors (mean/SD) |
Non-survivors (mean/SD) |
p* | Survivors (mean/SD) |
Non-survivors (mean/SD) |
p* | Survivors (mean/SD) |
Non-survivors (mean/SD) |
p* | |
| Gestational Age (weeks) | 32.7/2.7 | 30.7/4.4 | 0.007 | 38.7/1.3 | 38.3/1.2 | 0.457 | 35.5/3.7 | 32.9/5.1 | 0.003 |
| Birth weight (grams) | 1804/543 | 1557/767 | 0.112 | 3128/638 | 2853/261 | 0.295 | 2426/885 | 1946/889 | 0.010 |
| Sns score | 10.4/2.4 | 5.3/2.1 | <0.001 | 11.9/2.2 | 10.2/2.2 | 0.057 | 11.1/2.4 | 6.8/3.0 | <0.001 |
| Preterm Infants (N 217) |
Term Infants (N 186) |
All Infants (N 403) |
||||
|---|---|---|---|---|---|---|
| Median (IQR) | p* | Median (IQR) | p* | Median (IQR) | p* | |
| Gestational age (weeks) | 36 (31-35) | 0.077 | 39 (38-39) | 0.525 | 36(33-39) | 0.026 |
| Birth weight (grams) | 1750 (1400-2162.5) |
0.164 | 3100 (2800-3422.5) |
0.135 | 2330 (1700-3100) |
0.032 |
| Time to admission (hours) | 4 (3-7) | <0.001 | 12.5 (5-36) | 0.343 | 6.0 (3-21) | 0.013 |
| Apgar score/1 min. | 7 (6-8) | <0.001 | 8 (6.75-9) | 0.038 | 7 (6-8.75) | <0.001 |
| Sns score | 11.0 (8-12) | <0.001 | 12.5 (10-14) | 0.038 | 11.0 (11-13) | <0.001 |
| Preterm Infants | Term Infants | ALL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (N/%) |
Non-survivors (N/%) |
p-value |
OR (95%CI) |
Survivors (N/%) |
Non-survivors (N/%) |
p-value | OR (95%CI) |
Survivors (N/%) |
Non-survivors (N/%) |
p-value | OR (95%CI) |
|
| Male gender | 105/51.7 | 8/57.1 | 0.696 |
1.23(0.44-3.42) | 106/58.9 | 5/83.3 | 0.232 | 3.38(0.46-28.34) | 211/55.1 | 13/65.0 | 0.386 | 1.48(0.60-3.64) |
| Apgar score <3 | 20/9.9 | 2/15.4 | 0.529 | 1.65(0.34-7.99) | 19/10.6 | 0/0 | 0.404 | - | 39/10.2 | 2/10.5 | 0.995 | 1.03(0.23-4.65) |
| Early sepsis | 34/16.7 | 3/21.4 | 0.654 | 1.36(0.36-5.12) | 72/40.0 | 3/50.0 | 0.626 | 1.50(0.29-7.64) | 106/27.7 | 6/30.0 | 0.822 | 1.12(0.42-2.99) |
| Sns score≤8 | 48/23.6 | 14/100 | <0.001 | 1.29(1.13-1.48) | 18/10.0 | 1/16.7 | 0.598 | 1.80(0.20-16.27) | 66/17.2 | 15/75.0 | <0.001 | 14.41(5.06-41.02) |
| Place of birth home | 7/3.4 | 1/7.1 | 0.013 | - | 5/2.8 | 0/0 | 0.750 | 12/3.1 | 1/5.0 | 0.014 | - | |
| Level i | 123/60.6 | 2/14.3 | 122/67.8 | 4/66.7 | 245/64.0 | 6/30.0 | ||||||
| Level ii | 72/35.5 | 11/78.6 | 53/29.4 | 2/33.3 | 125/32.6 | 13/65.0 | ||||||
| Level iii | 1/0.5 | 0/0 | 0/0 | 0/0 | 1/0.3 | 0/0 | ||||||
| 0 points | 1 points | 2 points | 3 points | |
| Gestational age (weeks) | <32 | 32-36 | ≥37 | - |
| Birth weight (g) | < 1500 | 1500-2499 | ≥2500 | - |
| Time from birth to admission (h) | ≥12 | 6-12 | - | <6 |
| Final MSNS-AT Score | Points granted on the above variables are added to the SNS score | |||
| Model | AUC | p-Value | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sns score in term infants | 0.809 | 0.010 | 0.698 | 0.920 |
| Msns-at in term infants | 0.765 | 0.027 | 0.601 | 0.929 |
| Sns score in preterm infants | 0.810 | 0.000 | 0.676 | 0.945 |
| Msns-at in preterm infants | 0.885 | 0.000 | 0.800 | 0.970 |
| Sns score in all infants | 0.735 | 0.001 | 0.622 | 0.848 |
| Msns-at in all infants | 0.775 | 0.000 | 0.659 | 0.890 |
| Multivariable Cox Regression | p-Value | HR | 95.0% CI for HR | |
|---|---|---|---|---|
| Variables | Lower | Upper | ||
| Gestational age | 0.920 | 1.012 | 0.800 | 1.280 |
| Birthweight | 0.904 | 1.000 | 0.999 | 1.001 |
| Apgar 1 min. | 0.563 | 1.113 | 0.774 | 1.600 |
| Apgar <3 | 0.966 | 1.054 | 0.093 | 11.918 |
| Early sepsis | 0.871 | 0.917 | 0.322 | 2.610 |
| Time-to-admission | 0.575 | 1.001 | 0.997 | 1.006 |
| Msns-at ≥16 | <0.001 | |||
| Msns-at 8-15 | 0.048 | 3.607 | 1.023 | 14.653 |
| Msns-at ≤7 | <0.001 | 47.120 | 9.593 | 231.459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





