Submitted:
17 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
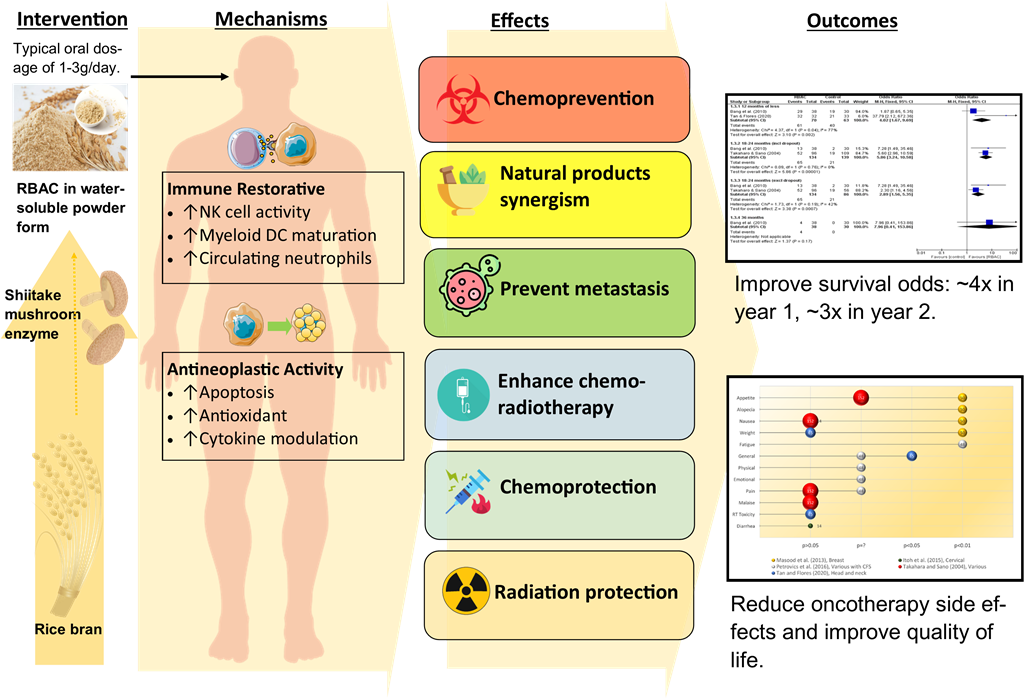
Introduction
Methods
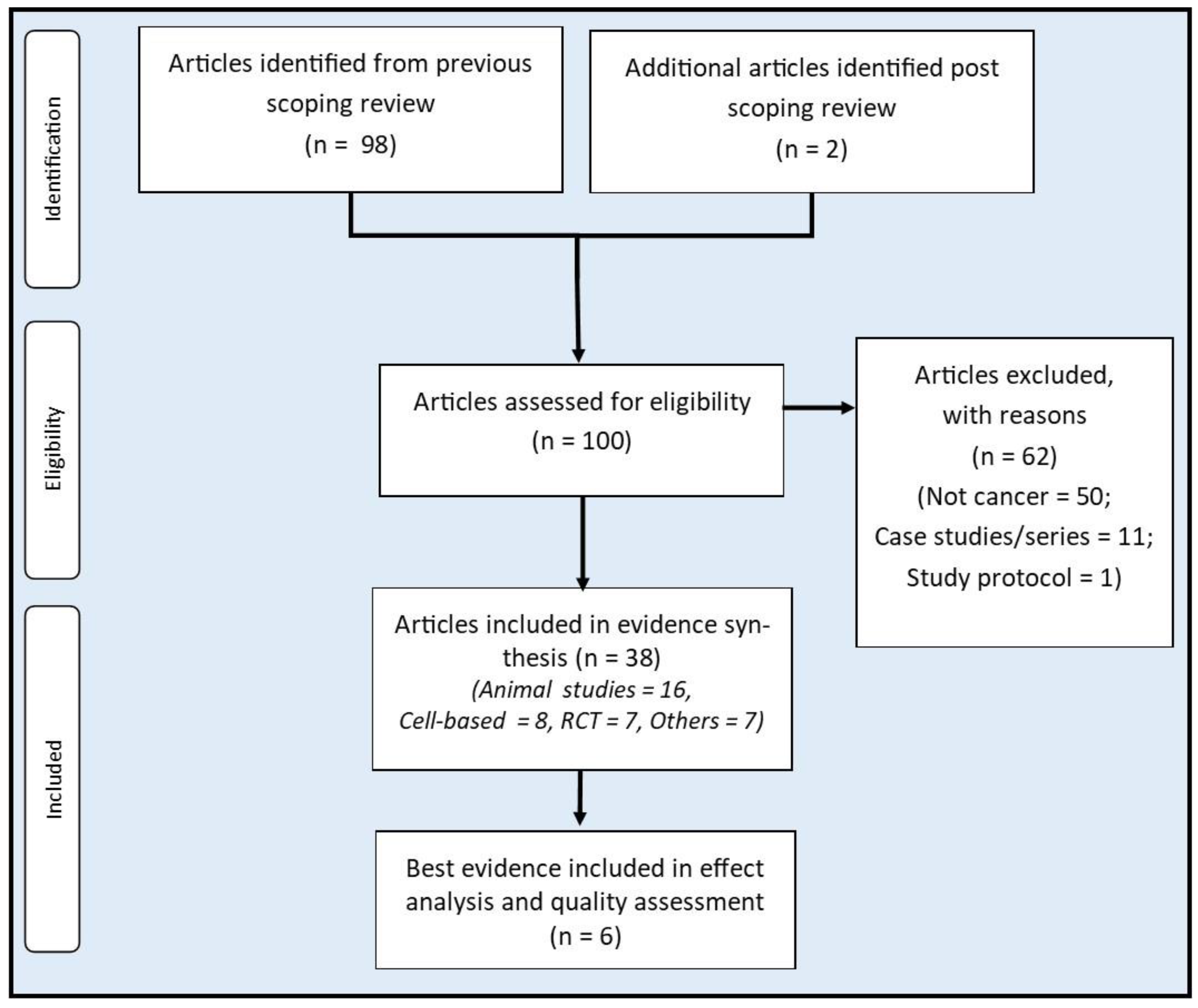
Sources of evidence
Selection criteria
Evidence synthesis, analysis, and presentation
Quality assessment
Results
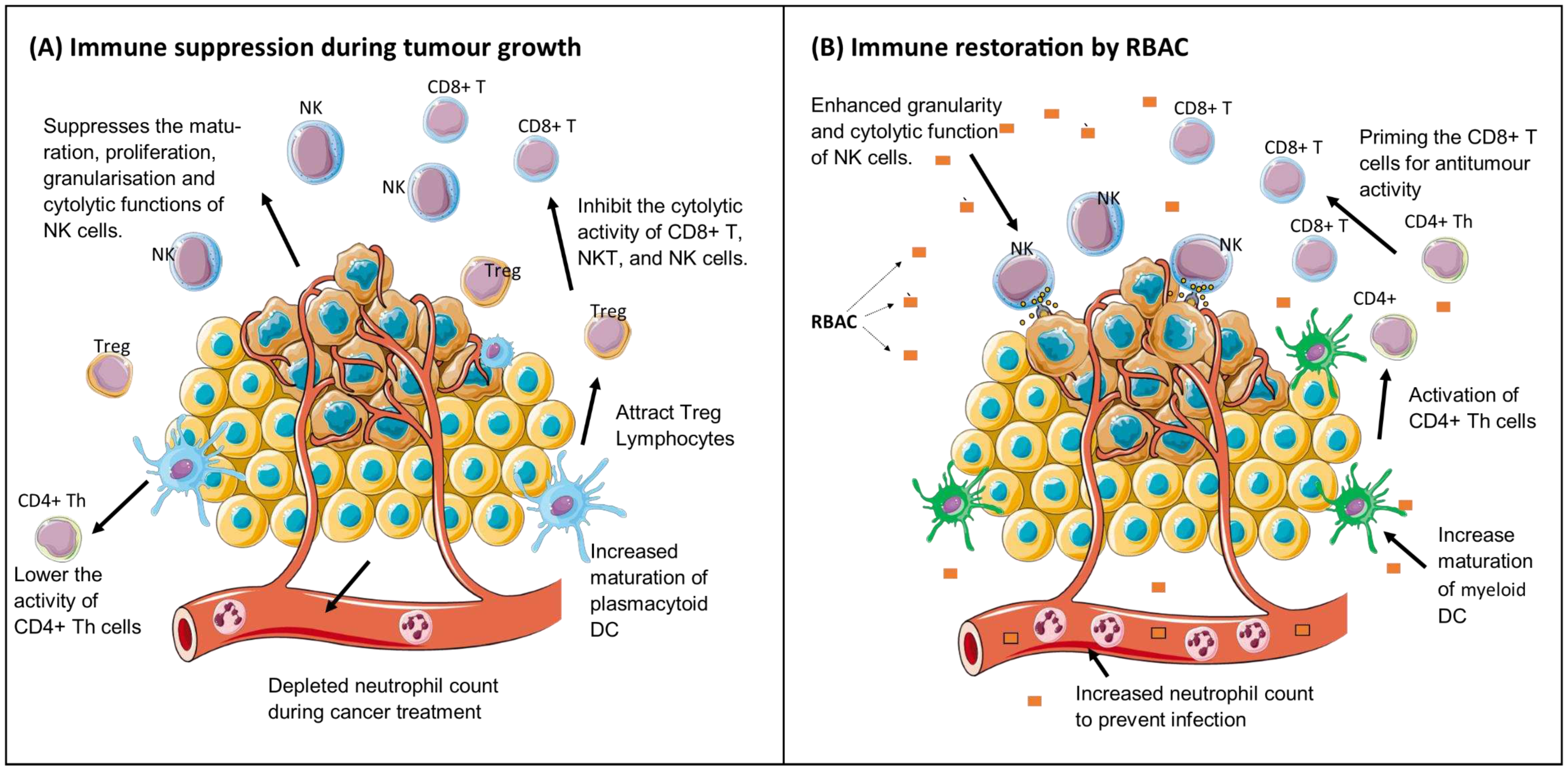
Immune restorative effects
Anticancer effects and pathways
Anticancer effects in vivo
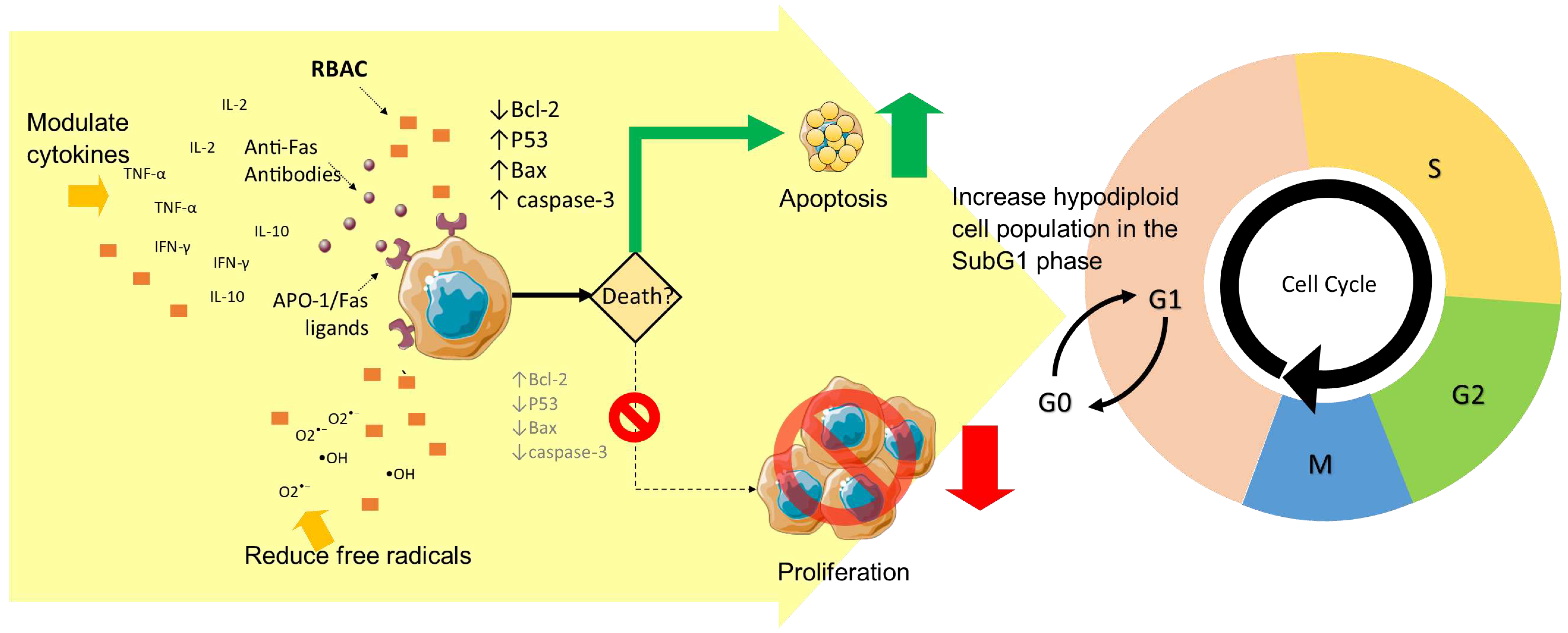
Promote cancer cell apoptosis
Prevent oxidative stress
Modulate cytokine production
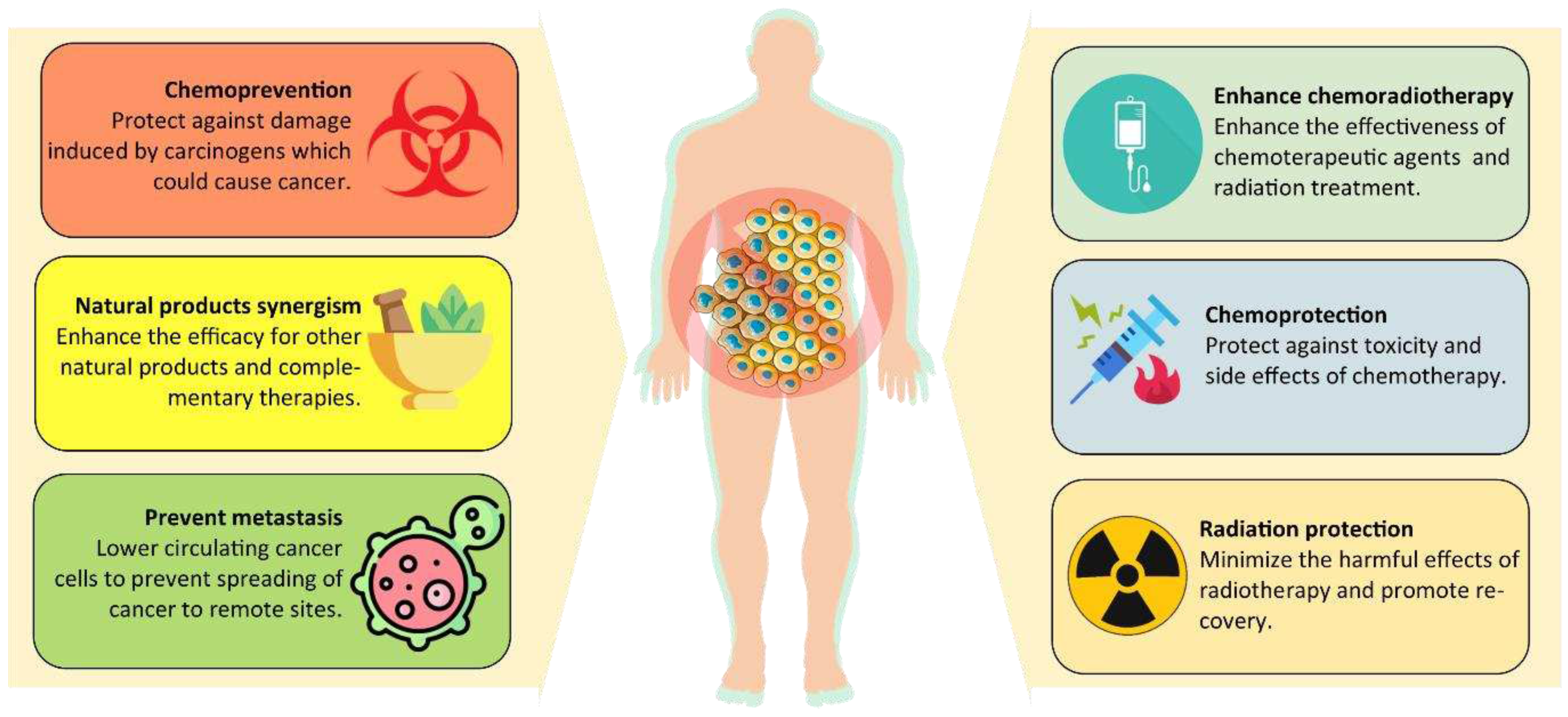
Chemoprevention
Enhance chemotherapy
Chemoprotection
Radioprotection and radiotherapy enhancement
Synergistic effects with other natural products and complementary therapies
Metastasis prevention
Best available evidence of RBAC treatment in cancer patients from clinical trials
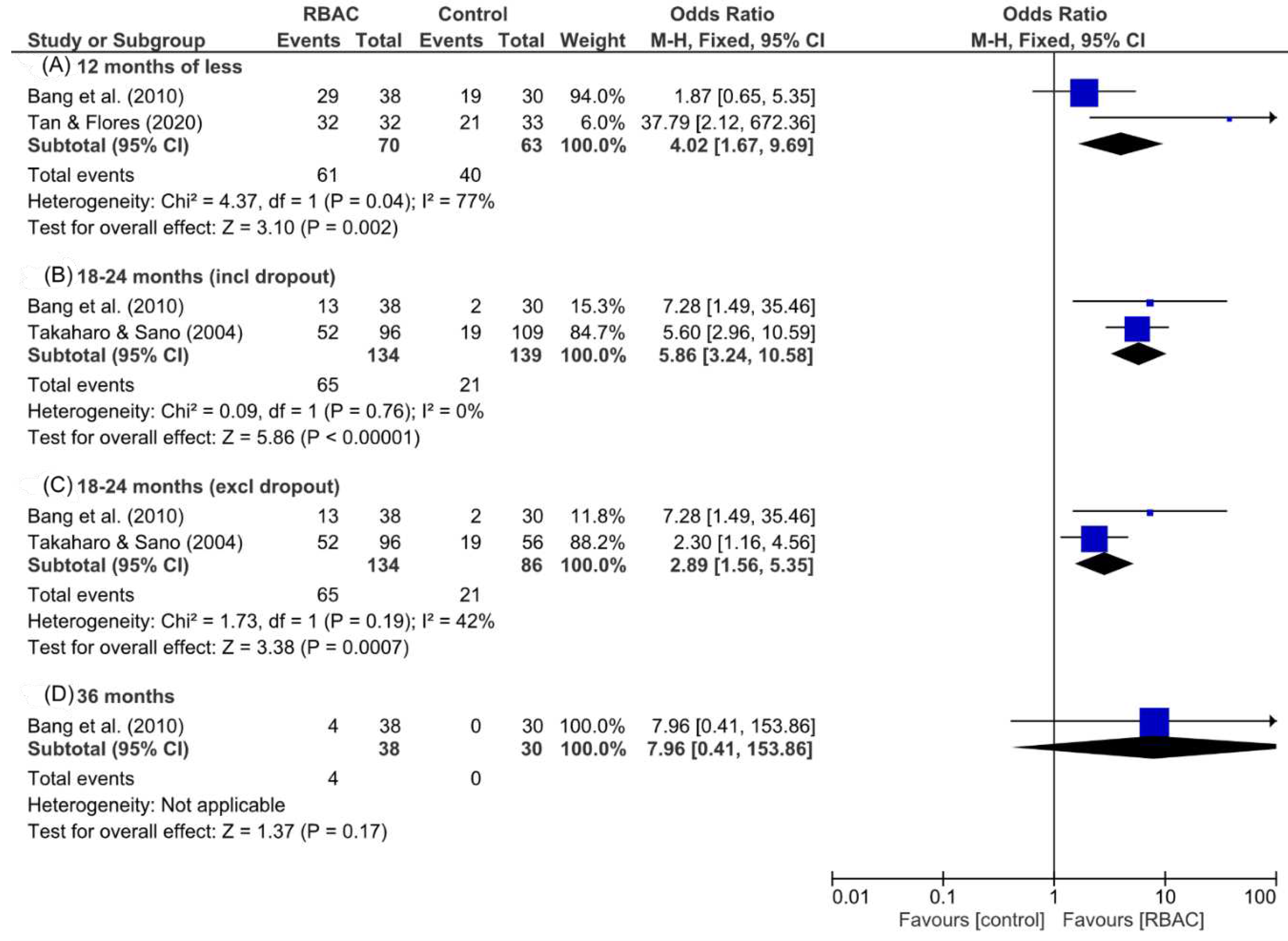
Survival rate analysis
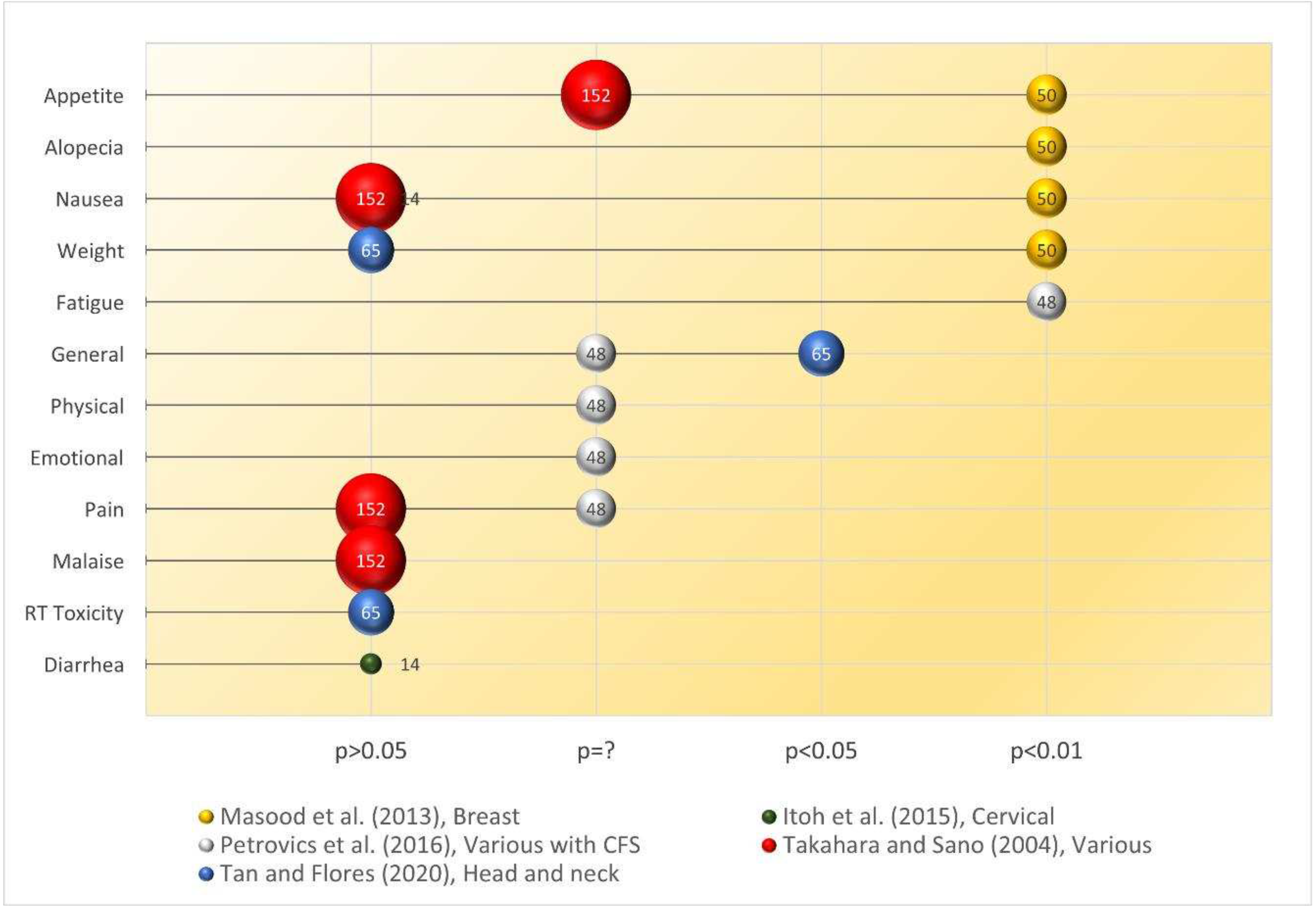
Quality of life assessment
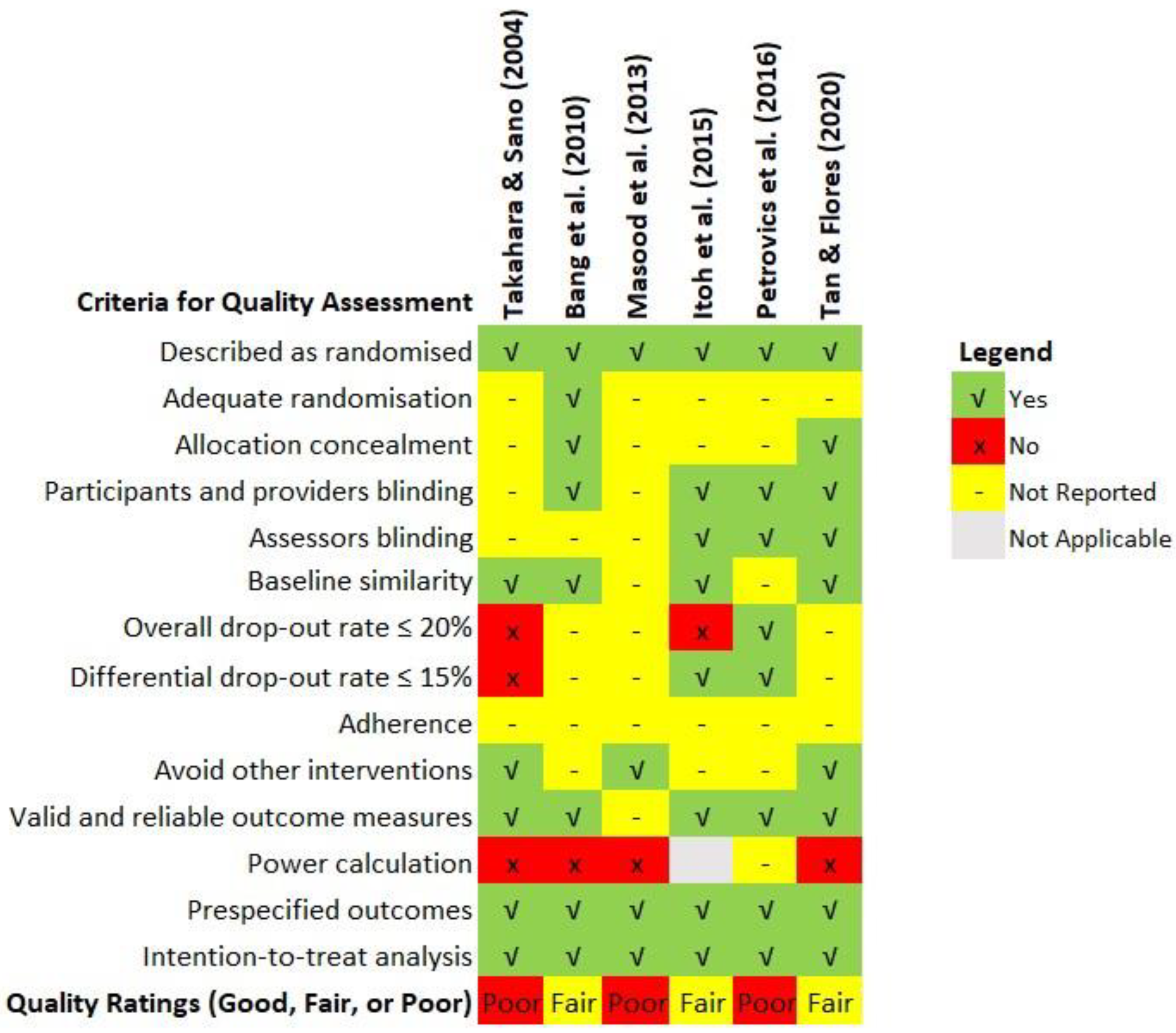
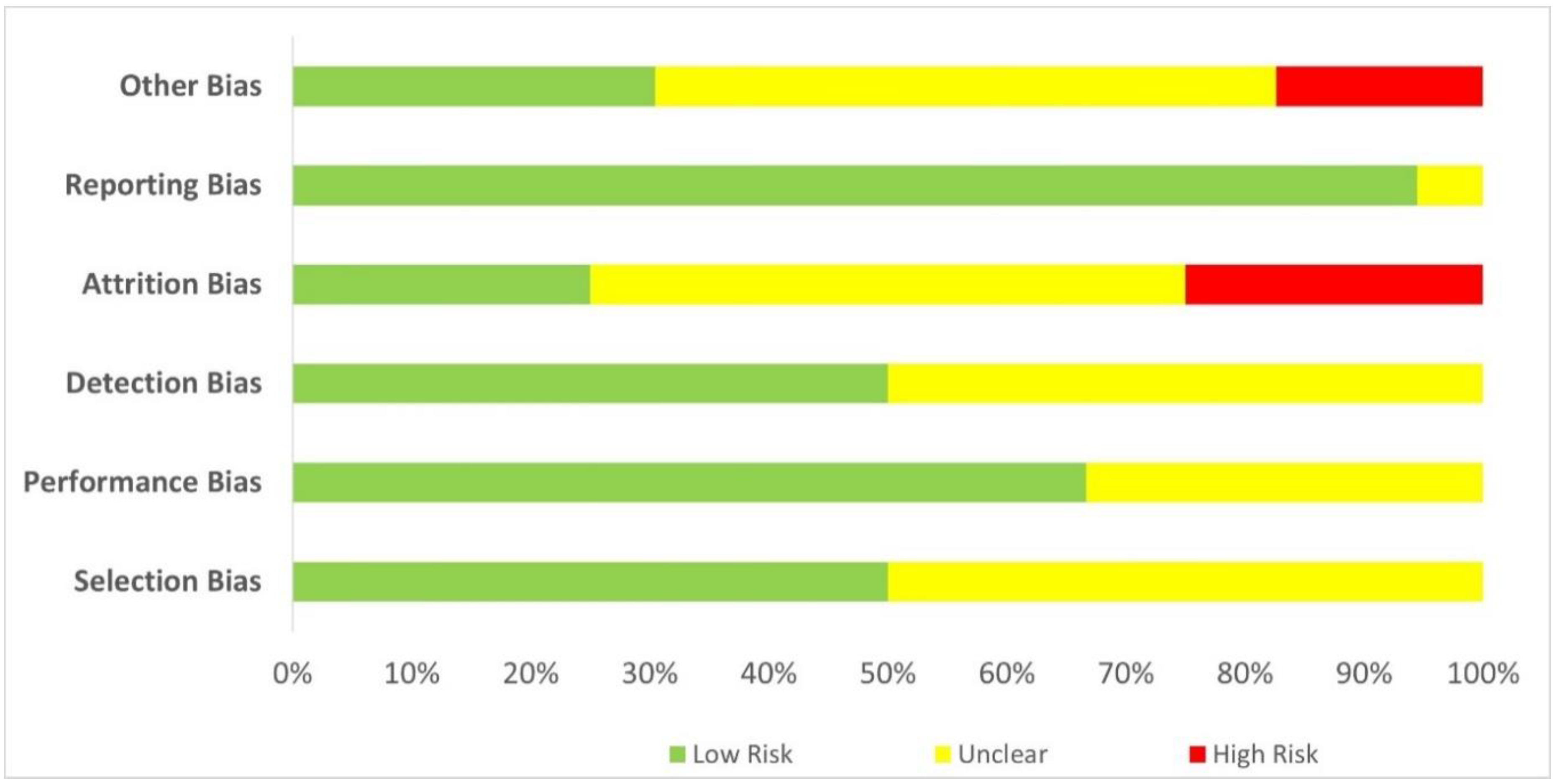
Quality assessment of the included studies
Discussion
Conclusion
Supplementary Materials
Author Contributions
Funding
Declarations
Conflicts of Interest
References
- Abdalla, Y.O.A.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Alshaibi, H.F.; Al-Shehri, B.; Hassan, B.; Al-Zahrani, R.; Assiss, T. Modulated Electrohyperthermia: A New Hope for Cancer Patients. BioMed Res. Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Altun, I.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. 2018, 47, 1218–1219.
- Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J. Clin. Oncol. 1996, 14, 671–679. [Google Scholar] [CrossRef]
- Amjad, M. T. , Chidharla, A., & Kasi, A. (2023, 27 Feb, 2023). Cancer chemotherapy. StatPearls Publishing. https://www.ncbi.nlm.nih. 5643. [Google Scholar]
- An, S. Y. (2011). Immune-enhance and anti-tumor effect of exo-biopolymer extract from submerged culture of Lentinus edodes with rice bran [Doctoral Thesis, Korea University]. Seoul, Republic of Korea.
- Andocs, G.; Szasz, O.; Szasz, A. Oncothermia Treatment of Cancer: From the Laboratory to Clinic. Electromagn. Biol. Med. 2009, 28, 148–165. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Fattah, S.M.A.; Pan, D.; Tolentino, L.; Ghoneum, M. Chemopreventive Activity of MGN-3/Biobran Against Chemical Induction of Glandular Stomach Carcinogenesis in Rats and Its Apoptotic Effect in Gastric Cancer Cells. Integr. Cancer Ther. 2016, 15, NP26–NP34. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Ali, D.A.; El-Dein, M.A.; Ghoneum, M. Enhancing the Apoptotic Effect of a Low Dose of Paclitaxel on Tumor Cells in Mice by Arabinoxylan Rice Bran (MGN-3/Biobran)*. Nutr. Cancer 2016, 68, 1010–1020. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Ali, D.A.; Othman, R.M. INHIBITION OF EXPERIMENTAL CARCINOGENESIS BY THE BIOACTIVE NATURAL PRODUCT BIOBRAN. J. Plant Prot. Pathol. 2016, 7, 85–91. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Ali, D.A.; Othman, R.; French, S.W.; Ghoneum, M. Chemopreventive role of arabinoxylan rice bran, MGN-3/Biobran, on liver carcinogenesis in rats. Biomed. Pharmacother. 2020, 126, 110064. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Areida, S.K.; O Ahmed, K.; Ghoneum, M. Arabinoxylan rice bran (MGN-3/Biobran) enhances radiotherapy in animals bearing Ehrlich ascites carcinoma†. J. Radiat. Res. 2019, 60, 747–758. [Google Scholar] [CrossRef] [PubMed]
- El-Din, N.K.B.; Mahmoud, A.Z.; Hassan, T.A.; Ghoneum, M. Baker’s Yeast Sensitizes Metastatic Breast Cancer Cells to Paclitaxel In Vitro. Integr. Cancer Ther. 2017, 17, 542–550. [Google Scholar] [CrossRef] [PubMed]
- El-Din, N.K.B.; Noaman, E.; Ghoneum, M. In Vivo Tumor Inhibitory Effects of Nutritional Rice Bran Supplement MGN-3/Biobran on Ehrlich Carcinoma-Bearing Mice. Nutr. Cancer 2008, 60, 235–244. [Google Scholar] [CrossRef]
- The Effects of the Arabinoxylane and the Polysaccharide Peptide (PSP) on the Antiallergy, Anticancer. J. Korean Soc. Food Sci. Nutr. 2004, 33, 469–474. [CrossRef]
- Bang, M.H.; Van Riep, T.; Thinh, N.T.; Song, L.H.; Dung, T.T.; Van Truong, L.; Van Don, L.; Ky, T.D.; Pan, D.; Shaheen, M.; et al. Arabinoxylan rice bran (MGN-3) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: a three-year randomized clinical trial. . 2010, 30, 5145–51. [Google Scholar]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Brush, T.P.; Trinh, S.; Brawner, C.M.; Ali, K.H.; Hauke, R.J.; Elkahwaji, J.E. Abstract 5662: RBAC, a modified form of Arabinoxylan from rice bran, impairs prostate cancer cell line proliferation, adhesion, and invasion in vitro. Cancer Res 2010, 70, 5662–5662. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Lagana, D.; Catford, J.; Shaw, D.; Bak, N. Bloodstream infections in neutropenic patients with haematological malignancies. Infect. Dis. Heal. 2019, 25, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Myeloid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Immunobiology 2015, 220, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Cholujova, D.; Jakubikova, J.; Czako, B.; Martisova, M.; Hunakova, L.; Duraj, J.; Mistrik, M.; Sedlak, J. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol. Immunother. 2012, 62, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cholujova, D.; Jakubikova, J.; Sedlak, J. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma 2009, 56, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Clark, H. R. (1999, May-1999). The natural pharmacy. Winning the war against cancer: MGN-3: mushroom ammunition. Alternative Medicine Magazine, (29), 26-28.
- Cooper, G. M. (2000). The development and causes of cancer. In The Cell: A Molacular Approach. (2nd ed.). Sinauer Associates.
- Dare, A. J. , Anderson, B. O., Sullivan, R., Pramesh, C. S., Andre, I., Adewole, I. F., Badwe, R. A., & Gauvreau, C. L. (2015). Surgical services for cancer care. In H. Gelband, P. Jha, R. Sankaranarayanan, & S. Horton (Eds.), Cancer: Disease Control Priorities (3rd ed., Vol. 3). The International Bank for Reconstruction and Development / The World Bank. [CrossRef]
- Deeks, J. J. , Higgins, J. P., & Altman, D. G. (2021). Chapter 10: Analysing data and undertaking meta-analyses. In J. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. Page, & V. Welch (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated 21). Cochrane. 20 February.
- Delaney, G.; Barton, M. Evidence-based Estimates of the Demand for Radiotherapy. Clin. Oncol. 2014, 27, 70–76. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Endo, Y.; Kanbayashi, H. Modified Rice Bran Beneficial for Weight Loss of Mice as a Major and Acute Adverse Effect of Cisplatin. Basic Clin. Pharmacol. Toxicol. 2003, 92, 300–303. [Google Scholar] [CrossRef]
- Fan, H.; Walters, C.S.; Dunston, G.M.; Tackey, R. IL-12 PLAYS A SIGNIFICANT ROLE IN THE APOPTOSIS OF HUMAN T CELLS IN THE ABSENCE OF ANTIGENIC STIMULATION. Cytokine 2002, 19, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Sakamuru, S.; Huang, R.; Teneva, N.; Simmons, S.O.; Xia, M.; Tice, R.R.; Austin, C.P.; Myung, K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. 2012, 109, 5423–5428. [Google Scholar] [CrossRef]
- Fridrichova, I.; Kalinkova, L.; Ciernikova, S. Clinical Relevancy of Circulating Tumor Cells in Breast Cancer: Epithelial or Mesenchymal Characteristics, Single Cells or Clusters? Int. J. Mol. Sci. 2022, 23, 12141. [Google Scholar] [CrossRef]
- G. , M.S.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2022, 12, 809308. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, G.; Szasz, O.; Szasz, A. A New Paradigm and Promising Method in Cancer Therapies Oncothermia: a New Paradigm and Promising Method in Cancer Therapies. Acupunct. Electro-Therapeutics Res. 2013, 38, 161–197. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M. (1998). Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). International Journal of Immunotherapy, 14(2), 89-99.
- Ghoneum, M. (1999, Dec 11-13). Immunostimulation and cancer prevention [Abstract]. 7th International Congress on Anti-Aging & Biomedical Technologies, Las Vegas, NV, USA.
- Ghoneum, M. (2000, Jan). One sizeable step for immunology, one giant leap for cancer patients. Townsend Letter for Doctors and Patients, (198), 58-62.
- Ghoneum, M. (2016). From bench to bedside : The growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunology, 1(2), 1006.
- Ghoneum, M.; Abdulmalek, S.; Fadel, H.H. Biobran/MGN-3, an Arabinoxylan Rice Bran, Protects against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An In Vitro and In Silico Study. Nutrients 2023, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Abedi, S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J. Pharm. Pharmacol. 2004, 56, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Agrawal, S. Activation of Human Monocyte-Derived Dendritic Cells in Vitro by the Biological Response Modifier Arabinoxylan Rice Bran (MGN-3/BIOBRAN). Int. J. Immunopathol. Pharmacol. 2011, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Agrawal, S. MGN-3/Biobran Enhances Generation of Cytotoxic CD8+ T Cells VIA Upregulation of DEC-205 Expression on Dendritic Cells. Int. J. Immunopathol. Pharmacol. 2014, 27, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; El-Din, N.K.B.; Fattah, S.M.A.; Tolentino, L. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body -irradiation in mice via restoration of hematopoietic tissues. J. Radiat. Res. 2013, 54, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; El-Din, N.K.B.; Ali, D.A.; El-Dein, M.A. Modified arabinoxylan from rice bran, MGN-3/biobran, sensitizes metastatic breast cancer cells to paclitaxel in vitro. . 2014, 34, 81–7. [Google Scholar]
- Ghoneum, M. , & Brown, J. (1999). NK Immunorestoration and cancer patients by MGN-3, a modified arabinoxylan rice bran (Study of 32 patients followed for up to 4 years). In R. M. Klatz & R. Goldman (Eds.), Anti-aging Medical Therapeutics (Vol. III, pp. 217-226). Health Quest Publications.
- Ghoneum, M.H.; El Sayed, N.S. Protective Effect of Biobran/MGN-3 against Sporadic Alzheimer’s Disease Mouse Model: Possible Role of Oxidative Stress and Apoptotic Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Ghoneum, M.; Gollapudi, S. Modified arabinoxylan rice bran (MGN-3/Biobran) sensitizes human T cell leukemia cells to death receptor (CD95)-induced apoptosis. Cancer Lett. 2003, 201, 41–49. [Google Scholar] [CrossRef]
- Ghoneum, M. , & Gollapudi, S. (2004). Induction of apoptosis in breast cancer cells by saccharomyces cerevisiae the baker’s yeast, in vitro. Anticancer Research, 24(3A), 1455.
- Ghoneum, M. , & Gollapudi, S. (2005a). Modified arabinoxylan rice bran (MGN-3/Biobran) enhances yeast-induced apoptosis in human breast cancer cells in vitro. Anticancer Research, 25(2a), 859-870.
- Ghoneum, M.; Gollapudi, S. Synergistic role of arabinoxylan rice bran (MGN-3/Biobran) in S. cerevisiae-induced apoptosis of monolayer breast cancer MCF-7 cells.. 2005, 25, 4187–96. [Google Scholar]
- Ghoneum, M.; Gollapudi, S. Synergistic apoptotic effect of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (turmeric) on human multiple myeloma cell line U266 in vitro. Neoplasma 2011, 58, 118–123. [Google Scholar] [CrossRef]
- Ghoneum, M.; Jewett, A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. . 2000, 24, 314–24. [Google Scholar]
- Ghoneum, M.; Matsuura, M.; Gollapudp, S. Modified Arabinoxylan Rice Bran (Mgn-3/Biobran) Enhances Intracellular Killing of Microbes by Human Phagocytic Cellsin Vitro. Int. J. Immunopathol. Pharmacol. 2008, 21, 87–95. [Google Scholar] [CrossRef]
- Ghoneum, M. , Tachiki, K. H., Ueyama, K., Makinodan, T., Makhijani, N., & Yamaguchi, D. (2000, Dec 14-17). Natural biological response modifier (MGN-3) shown to be effective against tumor cell growth [Abstract]. 8th International Congress on Anti-Aging & Biomedical Technologies, Las Vegas, NV, USA.
- Ghoneum, M. H. (2023). Enhancing natural killer cell activity. In S. C. Pak, S. L. Ooi, P. S. Micalos, & M. H. Ghoneum (Eds.), Modified Rice Bran Arabinoxylan : Therapeutic Applications in Cancer and Other Diseases (pp. 15-25). Springer Nature. [CrossRef]
- Giese, S.; Sabell, G.R.; Coussons-Read, M. Impact of Ingestion of Rice Bran and Shitake Mushroom Extract on Lymphocyte Function and Cytokine Production in Healthy Rats. J. Diet. Suppl. 2008, 5, 47–61. [Google Scholar] [CrossRef]
- Gollapudi, S.; Ghoneum, M. MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin. Cancer Detect. Prev. 2008, 32, 1–6. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Addition of Rice Bran Arabinoxylan to Curcumin Therapy May Be of Benefit to Patients With Early-Stage B-Cell Lymphoid Malignancies (Monoclonal Gammopathy of Undetermined Significance, Smoldering Multiple Myeloma, or Stage 0/1 Chronic Lymphocytic Leukemia). Integr. Cancer Ther. 2016, 15, 183–189. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support. Care Cancer 2019, 28, 211–220. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: a state of the art review. Anal. 2017, 142, 2273–2321. [Google Scholar] [CrossRef]
- Hajto, T. Can a standardized plant immunomodulator (rice bran arabinoxylan concentrate/MGN-3) increase the effects of MEK and BRAF inhibitors with clinical benefit? Case report of a patient with carcinoma in biliary duct. Res. Rev. Insights 2017, 1. [Google Scholar] [CrossRef]
- Hajtó, T. (2018). New perspectives to improve the MHC-I unrestricted immune mechanisms against malignant tumors. Advances in Clinical and Translational Research, 2(3), 100014.
- Hajtó, T. (2023). The therapeutic application of RBAC in cancer. In S. C. Pak, S. L. Ooi, P. S. Micalos, & M. H. Ghoneum (Eds.), Modified Rice Bran Arabinoxylan : Therapeutic Applications in Cancer and Other Diseases (pp. 67-78). Springer Nature. [CrossRef]
- Hajtó, T. , Adámy, A., Langmár, Z., Kirsch, A., Ábrahám, L., Perjési, P., & Németh, P. (2013). Enhanced effectiveness of conventional oncotherapy with plant immunomodulators: Overview of recent advances. Advancement in Medicinal Plant Research, 1(3), 56-65.
- Hajto, T.; Baranyai, L.; Kirsch, A.; Kuzma, M.; Perjési, P. Can a synergistic activation of pattern recognition receptors by plant immunomodulators enhance the effect of oncologic therapy? Case Report of a patient with uterus and ovary sarcoma. Clin. Case Rep. Rev. 2015, 1. [Google Scholar] [CrossRef]
- Hajto, T.; Horváth, A.; Baranyai, L.; Kuzma, M.; Perjési, P. Can the EGFR inhibitors increase the immunomodulatory effects of standardized plant extracts (mistletoe lectin and arabonoxylan) with clinical benefit? Case report of a patient with lung adenocarcinoma. Clin. Case Rep. Rev. 2016, 2. [Google Scholar] [CrossRef]
- Hajto, T.; Horvath, A.; Papp, S. Improvement of Quality of Life in Tumor Patients after an Immunomodulatory Treatment with Standardized Mistletoe Lectin and Arabinoxylan Plant Extracts. Int. J. Neurorehabilit. 2016, 03. [Google Scholar] [CrossRef]
- Hajto, T.; Kirsch, A. Case Reports of Cancer Patients with Hepatic Metastases Treated by Standardized Plant Immunomodulatory Preparations. J. Cancer Res. Updat. 2013, 2. [Google Scholar] [CrossRef]
- Tibor, H.; László, P.; Monika, K.; Edina, H.; Tamás, O.; Pál, J.; Lilla, B.; Zoltán, K. Enhancing effect of streptozotocin-induced insulin deficit on antitumor innate immune defense in rats. Res. Rev. Insights 2021, 6. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: an update with new promising compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—the gold standard for effectiveness research: Study design: Randomised controlled trials. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J. Hematol. Oncol. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- Igari, N. (2020). Biobran, immuno modulating rice bran arabinoxylan compound: functions and evidence. Food processing and ingredients [食品と開発], 55(10), 24-26.
- Itoh, Y.; Mizuno, M.; Ikeda, M.; Nakahara, R.; Kubota, S.; Ito, J.; Okada, T.; Kawamura, M.; Kikkawa, F.; Naganawa, S. A Randomized, Double-Blind Pilot Trial of Hydrolyzed Rice Bran versus Placebo for Radioprotective Effect on Acute Gastroenteritis Secondary to Chemoradiotherapy in Patients with Cervical Cancer. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Jacoby, H.I.; Wnorowski, G.; Sakata, K.; Maeda, H.; D, P.; Mba, G.W.B.; Mba, B. The Effect of MGN-3 on Cisplatin and Doxorubicin Induced Toxicity in the Rat. J. Nutraceuticals, Funct. Med Foods 2001, 3, 3–11. [Google Scholar] [CrossRef]
- Robb, K.A.; Simon, A.E.; Miles, A.; Wardle, J. Public perceptions of cancer: a qualitative study of the balance of positive and negative beliefs. BMJ Open 2014, 4, e005434–e005434. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-J.; Ryu, S.-N.; Han, S.-J.; Kim, H.-Y.; Kim, J.-H.; Hong, S.-G. In Vivo Immunological Activity in Fermentation with Black Rice Bran. Korean J. Food Nutr. 2011, 24, 273–281. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.-H.; Yang, S.-B.; Hong, S.-G.; Lee, S.-A.; Hwang, S.-J.; Shin, K.-S.; Suh, H.-J.; Park, M.-H. A Polysaccharide Extracted from Rice Bran Fermented withLentinus edodesEnhances Natural Killer Cell Activity and Exhibits Anticancer Effects. J. Med. Food 2007, 10, 25–31. [Google Scholar] [CrossRef]
- Kim, J.-M.; Hong, S.-G.; Song, B.-S.; Sohn, H.-J.; Baik, H.; Sung, M.-K. Efficacy of Cereal-based Oral Nutrition Supplement on Nutritional Status, Inflammatory Cytokine Secretion and Quality of Life in Cancer Patients Under Cancer Therapy. J. Cancer Prev. 2020, 25, 55–63. [Google Scholar] [CrossRef]
- Kim, K.; Khang, D. Past, Present, and Future of Anticancer Nanomedicine. Int. J. Nanomed. 2020, ume 15, 5719–5743. [Google Scholar] [CrossRef]
- Knudson, A.G. Cancer genetics. Am. J. Med Genet. 2002, 111, 96–102. [Google Scholar] [CrossRef]
- Sjøgren, K.; Jacobsen, K.A.; Grønberg, B.H.; Halvorsen, T.O. Timing of Severe Toxicity from Chemotherapy in Patients With Lung Cancer. Anticancer. Res. 2020, 40, 6399–6406. [Google Scholar] [CrossRef]
- Lemoine, M. Anya Plutynski’s Explaining Cancer: Finding Order in Disorder. Philos. Med. 2022, 3. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. , Niu, Z., Zhang, H., Kong, Y., Wang, Z., Yang, X., & Yuan, F. (2019). Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. International Journal of Clinical and Experimental Pathology, 12(9), 3604-3612.
- Lissoni, P. , Messina, G., Brivio, F., Fumagalli, L., Rovelli, F., Maruelli, L., Miceli, M., Marchiori, P., Porro, G., Held, M., Fede, G., & Uchiyamada, T. (2008). Modulation of the anticancer immunity by natural agents: inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors. Cancer Therapy, 6(2), 1011-1016.
- Liu, S.; Edgerton, S.M.; Moore, D.H.; Thor, A.D. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. . 2001, 7, 1716–23. [Google Scholar] [PubMed]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: a systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent Phagocytic Activity Discriminates Metastatic and Primary Human Malignant Melanomas: A Key Role of Ezrin. Mod. Pathol. 2003, 83, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Ai, L. Plant Natural Products: Promising Resources for Cancer Chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Mistletoe lectins: From interconnecting proteins to potential tumour inhibiting agents. Phytomedicine Plus 2021, 1, 100039. [Google Scholar] [CrossRef]
- Martins-Teixeira, M.B.; Carvalho, I. Antitumour Anthracyclines: Progress and Perspectives. ChemMedChem 2020, 15, 933–948. [Google Scholar] [CrossRef]
- Masood, A. I. , Sheikh, R., & Anwer, R. A. (2013). “BIOBRAN MGN-3”; Effect of reducing side effects of chemotherapy in breast cancer patients. The Professional Medical Journal, 20(1), 13-16.
- Mazzotti, E.; Cappellini, G.C.A.; Buconovo, S.; Morese, R.; Scoppola, A.; Sebastiani, C.; Marchetti, P. Treatment-related side effects and quality of life in cancer patients. Support. Care Cancer 2012, 20, 2553–2557. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Miura, T. , Chiba, M., Miyazaki, Y., Kato, Y., & Maeda, H. (2004/2013). Chemical structure of the component involved in immunoregulation. In BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): Basic and clinical application to integrative medicine (2nd ed., pp. 14-22). BioBran Research Foundation. (Reprinted from a report of 2004 Annual Meeting of the Japanese society of Applied Glycoscience.
- Morse, W.; Nawaz, H.; Choudhry, A.A. Combination of chemotherapeutic agents and biological response modifiers (immunotherapy) in triple-negative/Her2( +) breast cancer, multiple myeloma, and non-small-cell lung cancer. J. Egypt. Natl. Cancer Inst. 2022, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The Role of Natural Products and Their Multitargeted Approach to Treat Solid Cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Jain, R.; Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 2021, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. (n.d.). Cancer. In Dictionary of Cancer Terms. https://www.cancer.
- National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 23 February 2022).
- Nesher, L.; Rolston, K.V.I. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2013, 42, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Noaman, E.; El-Din, N.K.B.; Bibars, M.A.; Mossallam, A.A.A.; Ghoneum, M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008, 268, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Nordvig, J.; Aagaard, T.; Daugaard, G.; Brown, P.; Sengeløv, H.; Lundgren, J.; Helleberg, M. Febrile Neutropenia and Long-term Risk of Infection Among Patients Treated With Chemotherapy for Malignant Diseases. Open Forum Infect. Dis. 2018, 5, ofy255. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Ooi, S.L.; McMullen, D.; Golombick, T.; Nut, D.; Pak, S.C. Evidence-Based Review of BioBran/MGN-3 Arabinoxylan Compound as a Complementary Therapy for Conventional Cancer Treatment. Integr. Cancer Ther. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Record #1671 is using a reference type undefined in this output style.
- Ooi, S.L.; Pak, S.C.; Micalos, P.S.; Schupfer, E.; Lockley, C.; Park, M.H.; Hwang, S.-J. The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review. Molecules 2021, 26, 2539. [Google Scholar] [CrossRef]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef]
- Pasiarski, M.; Grywalska, E.; Kosmaczewska, A.; Góźdź, S.; Roliński, J. The frequency of myeloid and lymphoid dendritic cells in multiple myeloma patients is inversely correlated with disease progression. 67. [CrossRef]
- Pérez-Martínez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E.; Zerbes, P.; Schwörer, E.; Nuñéz, F.; Martín, I.G.; Sallis, H.; et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell–mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2014, 17, 601–612. [Google Scholar] [CrossRef]
- Pescatore, F. M. , Přestáno, C. ( 28(1), 8–10. [PubMed]
- Petrovics, G.; Szigeti, G.; Hamvas, S.; Máté. ; Betlehem, J.; Hegyi, G. Controlled pilot study for cancer patients suffering from chronic fatigue syndrome due to chemotherapy treated with BioBran (MGN-3-Arabinoxylane) and targeted radiofrequency heat therapy. Eur. J. Integr. Med. 2016, 8, 29–35. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D. H. , & Arlt, V. M. (2009). Genotoxicity: damage to DNA and its consequences. In A. Luch (Ed.), Molecular, Clinical and Environmental Toxicology: Volume 1: Molecular Toxicology (pp. 87-110). Birkhäuser Basel. [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Riggan, L.; Shah, S.; E O’sullivan, T. Arrested development: suppression of NK cell function in the tumor microenvironment. Clin. Transl. Immunol. 2021, 10. [Google Scholar] [CrossRef]
- Rosenberg, J.; Huang, J. CD8+ T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef]
- Salako, O.; Okunade, K.S.; Adeniji, A.A.; Fagbenro, G.T.; Afolaranmi, O.J. Chemotherapy induced neutropenia and febrile neutropenia among breast cancer patients in a tertiary hospital in Nigeria. ecancermedicalscience 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Schegoleva, A.A.; Khozyainova, A.A.; Gerashchenko, T.S.; Zhuikova, L.D.; Denisov, E.V. Metastasis prevention: targeting causes and roots. Clin. Exp. Metastasis 2022, 39, 505–519. [Google Scholar] [CrossRef]
- Schmidt, M.; Lügering, N.; Pauels, H.-G.; Schulze-Osthoff, K.; Domschke, W.; Kucharzik, T. IL-10 induces apoptosis in human monocytes involving the CD95 receptor/ligand pathway. Eur. J. Immunol. 2000, 30, 1769–1777. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic Effect of Saccharomyces cerevisiae on Human Colon Cancer SW480 Cells by Regulation of Akt/NF-ĸB Signaling Pathway. Probiotics Antimicrob. Proteins 2019, 12, 311–319. [Google Scholar] [CrossRef]
- Koyama, S.; Nishikawa, H. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J. Immunother. Cancer 2021, 9, e002591. [Google Scholar] [CrossRef]
- Sojka, D.K.; Huang, Y.; Fowell, D.J. Mechanisms of regulatory T-cell suppression—a diverse arsenal for a moving target. Immunology 2008, 124, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Takahara, K. , & Sano, K. (2004). The life prolongation and QOL improvement effect of rice bran arabinoxylan derivative (MGN-3, Biobran) for progressive cancer. Clinical Pharmacology and Therapy, 14(3), 267-271.
- Tan, D. F. S. , & Flores, J. A. S. (2020). The immunomodulating effects of arabinoxylan rice bran ( Lentin ) on hematologic profile, nutritional status and quality of life among head and neck carcinoma patients undergoing radiation therapy: A double blind randomized control trial. Radiology Journal, The Official Publication of the Philippine College of Radiology, 12(February), 11-16.
- Tazawa, K. , Namikawa, H., Oida, N., Itoh, K., Yatsuzuka, M., Koike, J., Masada, M., & Maeda, H. (2000). Scavenging activity of MGN-3 (arabinoxylane from rice bran) with natural killer cell activity on free radicals. Biotherapy: Official journal of Japanese Society of Biological Response Modifiers, 14(5), 493-495.
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of Cancer. CA: A Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Wu, C.-F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell. Physiol. Biochem. 2018, 51, 2647–2693. [Google Scholar] [CrossRef]
- Weston, A. , & Harris, C. C. (2003). Multistage carcinogenesis. In D. W. Kufe, R. E. Pollock, R. R. Weichselbaum, R. C. J. Bast, T. S. Gansler, J. F. Holland, & E. Frei (Eds.), Holland-Frei Cancer Medicine (6th ed.). BC Decker.
- E Wilson, B.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Wu, Z.; Li, S.; Zhu, X. The Mechanism of Stimulating and Mobilizing the Immune System Enhancing the Anti-Tumor Immunity. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, F.; Li, H.; Yang, L.; Lv, T.; Gu, W.; Song, Y. Circulating Tumor Cells Were Associated with the Number of T Lymphocyte Subsets and NK Cells in Peripheral Blood in Advanced Non-Small-Cell Lung Cancer. Dis. Markers 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid dendritic cell biology and its role in immune-mediated diseases. Clin. Transl. Immunol. 2020, 9. [Google Scholar] [CrossRef]
- Ba, Y.; Shi, Y.; Jiang, W.; Feng, J.; Cheng, Y.; Xiao, L.; Zhang, Q.; Qiu, W.; Xu, B.; Xu, R.; et al. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges. Cancer Biol. Med. 2020, 17, 896–909. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef]
- Zabor, E.C.; Kaizer, A.M.; Hobbs, B.P. Randomized Controlled Trials. Chest 2020, 158, S79–S87. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Ge, S.; Chen, C.; Li, S.; Wu, X.; Feng, X.; Wang, Y.; Cai, D. Saikosaponin A Inhibits Breast Cancer by Regulating Th1/Th2 Balance. Front. Pharmacol. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cheng, W.; Qu, W.; Wang, K. Arabinoxylan rice bran (MGN-3/Biobran) alleviates radiation-induced intestinal barrier dysfunction of mice in a mitochondrion-dependent manner. Biomed. Pharmacother. 2020, 124, 109855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lawrence, T.; Liang, Y. The Role of Plasmacytoid Dendritic Cells in Cancers. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
| # | RBAC (dose) | Study Design | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (3g/day for 1 to 2 weeks) |
Before & after study. Various malignancies (n=32) | Biobran MGN-3 significantly increased (p<0.001) NKC cytolytic activity up to 10-fold compared to baseline. Increased NKC granularity with enhanced tumour-killing capacity was observed posttreatment. | (Ghoneum & Brown, 1999) |
| 2 | Biobran MGN-3 (3g/day for 1 month) | Before & after study. Various malignancies (n=5) | Statistically significant (p<0.001) increases in responses with T & B cell mitogen tests compared to baseline demonstrated signs of restoration of the adaptive immunity. | (Ghoneum & Brown, 1999) |
| 3 | Biobran MGN-3 (3g/day for 1 to 2 weeks) | Before & after study. Various malignancies (n=90) | 95.5% of patients demonstrated 2 to 10-fold increases in NKC cytolytic activity at 1-2 weeks posttreatment. | (Ghoneum, 1999) |
| 4 | Biobran MGN-3 (3g/day for 18 months) | RCT. Patients with progressive cancer of late stages (n=152, RBAC=96, Control=56). | A significantly higher portion of participants with low or medium NKC activity in the RBAC group survived than the control group (Low: 42.5% vs 12.5%, p<0.01; Medium: 51.4% vs 28.0%, p<0.05). | (Takahara & Sano, 2004) |
| 5 | Biobran MGN-3 (2g/day for 1st month, 1g/day for 2nd month) | Before & after study. Various malignancies (n=22) | A statistically significant change in the ratio of Th/Treg was detected (p=0.025), and the increase in Th/Treg was more pronounced in participants with low Th/Treg at baseline. | (Lissoni et al., 2008) |
| 6 | Biobran MGN-3 (2g/day for 3 months) | RCT. MM patients (n=48, RBAC=32, placebo=12) | Significant increases in NKC activity of the RBAC group compared to the baseline in the first (p=0.045) and second (p=0.029) months. The circulating mDC percentage (p=0.036) and mDC/pDC ratio (p=0.030) increased substantially after 3 months. | (Cholujova et al., 2013) |
| 7 | Biobran MGN-3 (3g/day for 4 weeks) | RCT. Cervical cancer patients (n=14, RBAC=7, placebo=7) | Both groups experienced declines in NKC activity after chemoradiotherapy compared to the baseline values, with no significant difference between the two groups. | (Itoh et al., 2015) |
| 8 | Biobran MGN-3 (40mg/kg BW p.o. every other day for 8 months) | Wistar rats + carcinogen (MNNG) | MNNG caused significant lymphocyte depletion (↓23.3%, p<0.01) after 8 months compared to healthy controls. RBAC+MNNG promoted lymphocyte recovery to normal levels (p<0.05). | (Badr El-Din, Abdel Fattah, et al., 2016) |
| 9 | Biobran MGN-3 (2g/day for 6 months) | Before & after study. MGUS/SMM patients (n=10) | Half of the patients had neutropenia at baseline. After consuming RBAC, eight participants showed an increased neutrophil count between 10% and 90%. | (Golombick et al., 2016) |
| Abbreviations: BW, body weight; CD, cluster of differentiation; mDC, myeloid dendritic cells; MM, multiple myeloma; MNNG, methylnitronitrosoguanidine; NKC, natural killer cells; p.o., per oral; pDC, plasmacytoid dendritic cells; RBAC, rice bran arabinoxylan compound; RCT, randomised controlled trial; Th, T helper cells; Treg, Regulatory T cells; MGUS/SMM, monoclonal gammopathy of undetermined significance/smoldering multiple myeloma. | ||||
| # | RBAC (dose) | Model | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (1.5mg/day p.o. for 23 days) | ICR mice + S-180 cells | TW ↓66.2% (0.51 ±0.34g vs 3.40+1.46g, p<0.01) compared to the control. Better than PSP with TW ↓49.0% (p<0.05). | (Bae et al., 2004) |
| 2 | RBEP (50mg/kg BW p.o. & i.p., 250mg/kg BW p.o. for 14 days) | (1) ICR mice + S-180; (2) C57/Bl6 mice + B16/Bl6 melanoma | (1) RBEP prolonged survival by 14.6% (31.4 days), 30.3% (35.7 days), and 38.0% (37.8 days) with 50 mg/kg, 250 mg/kg p.o., and 50 mg/kg i.p., respectively. (2) TW ↓35.6% with 50 mg/kg p.o., ↓41.7% (2.155g) 250 mg/kg p.o., and ↓55.1% (1.66g) with 50 mg/kg i.p. |
(Kim et al., 2007) |
| 3 | Biobran MGN-3 (40mg/kg BW i.p. over 3 weeks & i.t. over 5 weeks) | Swiss albino mice + SEC | TV (i.p.) was significantly lower than control starting from day 14 (p<0.05) and ↓63.27% (p<0.01) at day 35; TW (i.p.) was also significantly lower (3.63±0.45 vs 6.62±0.38, p<0.01) compared to the control at day 35. Significant TV (i.t.) reduction from observed from day 28, reaching a ↓44.83% (p<0.01) difference with control on day 45. | (Badr El-Din et al., 2008) |
| 4 | Biobran MGN-3 (25mg/kg BW i.p. over 25 days) | Swiss albino mice + SEC | Early treatment (from day 4) TV ↓54% & TW ↓34% (p<0.01) relative to the control. Late treatment (from day 11) TV ↓24% & TW ↓12% (p<0.05). | (Noaman et al., 2008) |
| 5 | RBEP (250mg/kg p.o. or i.p. for > 2 weeks | ICR mice + S-180 cells | Survival rate: p.o. 5.3% higher (19.9 vs 18.9 days), and i.p. 23.2% higher (23 vs 18.7 days), compared to controls. RBEP-treated mice have significantly lower BW than controls starting from day 13 (p.o.) and day 10 (i.p.) | (An, 2011) |
| 6 | C3G-F (250mg/kg p.o. for 2 or 3 weeks | (1) ICR mice + S-180; (2) C57/Bl6 mice + B16/Bl6 melanoma | (1) BW: significant difference (p<0.05) since day 8. C3G-F prevented BW gain (6.5 g vs 11.8 g, ↓60%) on day 15. (2) TW: ↓19.4% in C3G-F group vs control (0.514±0.129 g vs 0.635±0.241 g, p<0.05) at 3 weeks. |
(Kim et al., 2011) |
| 7 | NKC activated with 100mg/mL Biobran MGN-3 i.v. 2x/week for 4 weeks | NOD-scid IL-2Rgnull mice + NB-1691luc | RBAC-activated NKC treatment significantly lowered TV (p<0.05) under bioluminescence imaging and extended the survival time of the mice than fresh NKC therapy or no treatment. | (Pérez-Martínez et al., 2015) |
| 8 | Biobran MGN-3 (40mg/kg BW i.p. 3x/weeks for 3 weeks) | Swiss albino mice + SEC | RBAC significantly reduced BW loss in SEC-bearing mice (↓4.1% vs 18%, p<0.01) and TW (↓46.3%, p<0.01) compared to control by day 30. Continuous suppression of TV was detected (day 14: ↓33.7%, day 30: ↓49.9%, p<0.01). | (Badr El-Din et al., 2019) |
| Abbreviations: BW, body weight; C3G-F, fermented SuperC3GHi bran; i.p., intraperitoneal injection; i.t., intratumoral injection; i.v., intravenous; NKC, natural killer cells; p.o., per oral; RBAC, rice bran arabinoxylan compound; RBEP, rice bran exo-biopolymer; SEC, solid Ehrlich carcinoma; TV, tumour volume in mm3; TW, tumour weight in g. | ||||
| # | RBAC (dose) | Model | Key Findings | Reference |
|---|---|---|---|---|
| A. Promote cancer cell apoptosis | ||||
| 1 | Biobran MGN-3 (concentration not reported) | In vitro. SCC13 cell line. | A 30% decrease in cell numbers after 48 hours and 50% at 72 hours after incubation with RBAC was detected. Untreated SCC13 cells continued to grow over time. Also found was increased secretion of IL-10 and IL-12 of SCC13 cells by RBAC. | (Ghoneum et al., 2000) |
| 2 | Biobran MGN-3 (100, 500 and 1000 mg/ml) | In vitro. MCF-7 cell line. | Cell survival rates were dose-dependent at 75%, 70% and 63% after 3 days of incubation with 100, 500 and 1000 mg/ml of RBAC, respectively. | (Gollapudi & Ghoneum, 2008) |
| 3 | Biobran MGN-3 (100–1000 μg/ml) | In vitro. MCF-7 & 4T1 cell lines. | IC50 (MCF-7) was 800 μg/ml at 24 hours and about 1000 μg/ml at 48 hours. IC50 (4T1) being 700 μg/ml at 24 hours and 580 μg/ml at 48 hours. | (Ghoneum et al., 2014) |
| 4 | Biobran MGN-3 (0–1000 μg/ml) | In vitro. PC3 & LNCaP cell lines. | Significantly decreases (p<0.05) in the cancer cell proliferation in a dose- and time-dependent manner (24, 48, and 72h). | (Brush et al., 2010) |
| 5 | Biobran MGN-3 (100–1000 μg/ml) | In vitro. HUT 78 cell line. Anti-CD95 antibodies. | Treatment of HUT 78 cells with RBAC (for 3 hours) before incubating with anti-CD95 antibodies increased the specific apoptosis significantly (p<0.01) by 35-42%, about double that of Anti-CD95 antibodies alone. The escalation in apoptosis was not associated with the upregulation of death receptor expression but through sensitising the receptor. | (Ghoneum & Gollapudi, 2003) |
| 6 | Biobran MGN-3 (40 mg/kg BW p.o. every other day for 8 months) | Wistar rats + carcinogen (MNNG) | RBAC mitigated the carcinogenesis effects of MNNG by causing a significant increase in cell-cycle arrest in the subG1 phase (p<0.01) compared to the control, with the AI/PrI ratio increased by 1.67-fold. RBAC increased the apoptotic cancer cells in tumour tissues by 63.7% (p<0.01), most prominently in early apoptosis (230.1%, p<0.01). Downregulation of Bcl-2 and upregulation of P53, Bax, Bax/Bcl-2 ratio, and caspase-3 were detected. | (Badr El-Din, Abdel Fattah, et al., 2016) |
| 7 | Biobran MGN-3 (25 mg/kg BW i.p. 5x/week, either for 12 or 22 weeks) | Wistar albino rats + carcinogen (NDEA+CCl4) | Cell-cycle arrest in the sub-G1 phase was markedly increased by 126% and 99% (p<0.01) through pretreatment and posttreatment of RBAC. RBAC treatment (pre, post) significantly reduced (p<0.01) viable cells (↓74.51%, ↓72.54%) and necrosis (↑89%, ↑75.47%) while increased early (↑316%, ↑309%) and late (↑255%, ↑237%) apoptosis, compared to carcinogen-untreated rats. RBAC significantly (p<0.01) upregulated p53, Bax, and caspase-3 while downregulated Bcl-2 gene expression. | (Badr El-Din et al., 2020) |
| 8 | Biobran MGN-3 (40 mg/kg BW i.p. 3x/weeks for 3 weeks) | In vivo. Swiss albino mice + SEC | RBAC markedly increased cell-cycle arrest in the sub-G1 phase was detected by 102% (p<0.01) in the RBAC group compared to the control. RBAC treatment also increased the AI/PrI ratio by 2-fold (p<0.01). The quantitative histochemical analysis also showed reduced viable cells (28.2±1.25% vs 74.5±2.25%) and increased apoptotic cells (53.1 ± 1.21% vs 18.2±1.68%) in the tumour tissues of RBAC-treated mice than control. RBAC significantly (p<0.01) upregulated p53, Bax, and caspase-3 while downregulated Bcl-2 gene expression. | (Badr El-Din et al., 2019) |
| B. Prevent oxidative stress | ||||
| 9 | Biobran MGN-3 (25 mg/kg BW i.p. 6x per weeks for 25 days) | In vivo. Swiss albino mice + SEC | Mice treated with RBAC did not show elevated MDA like untreated mice and had significantly higher GSH levels (p<0.01) in the blood, liver, and tumour. GPx, GST, SOD, and CAT and the related gene expressions in RBAC-treated mice were also significantly higher (p<0.01) than in untreated mice. | (Noaman et al., 2008) |
| C. Modulate cytokine production | ||||
| 10 | RBAC (40 mg/kg BW i.p. over 3 wks & i.t. over 5 wks) | In vivo. Swiss albino mice + SEC | RBAC treatment showed a significantly increased TNF-α (↑15.63%) and IFN-γ (↑154.54%) compared to control. Untreated SEC mice showed elevated IL-10 (↑111.71%), but the increase was dampened in RBAC-treated mice (↑14.75%, p<0.01). | (Badr El-Din et al., 2008) |
| 11 | Biobran MGN-3 (2g/day p.o. for 3 months) | RCT. MM patients (n=48, RBAC=32, placebo=12) | RBAC significantly elevated (p<0.05) both Th1 cytokines (IFN-γ, IL-12, IL-17, TNF-α) and Th2 cytokines (IL-4, IL-6, IL-9, IL-10, and IL-13) over placebo after 3 months. | (Cholujova et al., 2013) |
| 12 | ONS with 0.4g of RBEP p.o. for 8 weeks | NRCT. Various malignancies (n=34, RBAC=10, control=24) | RBAC significantly lowered (p<0.05) IL-1β, IL-6 and IL-8 and increased IL-12p70 (p<0.05) compared to the control group. A marginally significant rise (p=0.056) in the IL-10 level in the RBAC group compared to baseline was also detected. | (Kim et al., 2020) |
| Abbreviations: AI/PrI, the ratio of the apoptotic index over the proliferation index; BW, body weight; CAT, catalase; CCI4, carbon tetrachloride; CD, cluster of differentiation; GPx, glutathione peroxidase; GST, glutathione S-transferases; GSH, glutathione; IC50, half maximal inhibitory concentration; IFN, interferon; IL, interleukin; i.p., intraperitoneal; i.t., intratumoral; MDA, malondialdehyde; MM, multiple myeloma; MNNG, methylnitronitrosoguanidine; NDEA, N-nitrosodiethyamine; NRCT, nonrandomised controlled trial. ONS, oral nutritional supplement; p.o., per oral; RBAC, rice bran arabinoxylan compound; RBEP, rice bran exo-biopolymer; RCT, randomised controlled trial; SEC, solid Ehrlich carcinoma; SOD, superoxide dismutase; TNF, tumour necrosis factor. | ||||
| # | RBAC (dose) | Model | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (40 mg/kg BW p.o. every other day for 8 months) | Wistar rats + carcinogen (MNNG) | Untreated rats developed mild- and high-grade gastric glandular dysplasia (6/10, 60%) and invasive carcinoma (2/10, 20%). RBAC-treated rats had significantly lower incidence (p<0.01) of mild dysplasia, of which were patchy and small (3.5/12, 29.2%) and carcinoma in situ only (1/12, 8.3%). RBAC also significantly lower Ki-67 tumour proliferation marker expression (39.8% vs 50.8%, p<0.001). | (Badr El-Din, Abdel Fattah, et al., 2016) |
| 2 | Biobran MGN-3 (25 mg/kg BW i.p. 5x/week, either for 12 or 22 weeks) | Wistar rats + carcinogen (NDEA+CCl4) | Both RBAC treatment regimes kept the liver weight at the normal range and significantly reduced (p<0.01) weight loss caused by the carcinogens. Among RBAC-pretreated rats, the liver tissues showed minimal changes in hepatocyte morphology and histology with no inflammation. In contrast, moderate liver damage was observed in the posttreatment group but with only a few degenerated hepatocytes. | (Badr El-Din, Ali, & Othman, 2016) |
| Abbreviations: BW, body weight; CCI4, carbon tetrachloride; i.p., intraperitoneal; MNNG, methylnitronitrosoguanidine; NDEA, N-nitrosodiethyamine; p.o., per oral; RBAC, rice bran arabinoxylan compound. | ||||
| # | RBAC (dose) | Study Design | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (100, 500 and 1000 µg/ml) | In vitro. MCF-7 and HCC70 + daunorubicin | RBAC+daunorubicin lowered the IC50 values against MCF-7 cells by 3-, 5- and 5.5-fold at 100, 500 and 1000 µg/ml, respectively. The IC50 of daunorubicin for HCC70 cells was also consistently decreased by 2.5-fold with RBAC. RBAC enhanced drug transport with increased accumulation of daunorubicin in cells. | (Gollapudi & Ghoneum, 2008) |
| 2 | Biobran MGN-3 (1g/day p.o. for 1 year) | RCT. Liver cancer (n=68, RBAC=38, control=30) + TOCE+PEIT | RBAC significantly improved (p<0.01) the treatment response rate (89% vs 80%), lowered the AFP marker (↓38% vs ↑7%), and decreased TV (↓36% vs ↑0.2%). During the 3 years follow-up, the RBAC group showed lower recurrence and higher survival rates. | (Bang et al., 2010) |
| 3 | Biobran MGN-3 (600, 750 and 1000 µg/ml) | In vitro. MCF-7 and 4T1 + paclitaxel | RBAC+paclitaxel lowered the IC50 values against MCF-7 cells by a factor of 100. For 4T1 cells, the IC50 value for paclitaxel at 24 hours decreased by a factor of ~3 at 600 μg/ml of RBAC and up to a factor of ~100 at 1000 μg/ml. | (Ghoneum et al., 2014) |
| 4 | Biobran MGN-3 (40 mg/kg BW for 30 days) | Swiss albino mice + SEC + paclitaxel (2 mg/kg BW) | The combination therapy significantly reduced (p<0.01) TV by 88.3% compared to no treatment. The TV reduction was more pronounced than the effects of either paclitaxel (↓58.9%) or RBAC (↓77.1%) alone. RBAC+paclitaxel also increased inhibition of tumour proliferation, cancer cell apoptosis, and downregulation of Ki-67 expression. | (Badr El-Din, Ali, Alaa El-Dein, et al., 2016) |
| Abbreviations: AFP, alpha-fetoprotein; IC50, half maximal inhibitory concentration; PEIT, percutaneous ethanol injection treatment; p.o., per oral; RBAC, rice bran arabinoxylan compound; RCT, randomised controlled trial; TOCE, transarterial oily chemoembolization; TV, tumour volume. | ||||
| # | RBAC (dose) | Study Design | Key Findings | Reference |
| 1 | Biobran MGN-3 (0, 5, or 50 mg/kg BW p.o. daily for 11 days) | Sprague-Dawley-derived albino rats + cisplatin (9 mg/kg BW) or doxorubicin (10 mg/kg BW) | RBAC prevented weight loss induced by the chemotherapeutic agents (p<0.05). RBAC at 5 mg/kg BW appeared more effective than at the higher dose of 50 mg/kg in preventing the toxicity and side effects of cisplatin and doxorubicin. | (Jacoby et al., 2001) |
| 2 | Biobran MGN-3 (1 mg/day p.o. and i.p. for 28 days) | BALB/c mice + cisplatin (15 mg/kg BW i.p.) | Statistically significant differences (p<0.05) in BW were detected in phases II (weight loss), III (weight gain) and IV (weight stabilising) of both groups of RBAC (i.p. and p.o.) compared to their respective control groups with the RBAC groups showing trends of reduced weight loss and faster weight recovery over time. | (Endo & Kanbayashi, 2003) |
| 3 | Biobran MGN-3 (3g/day p.o. 1 week before and 1 week after chemo cycle for 6 cycles) | RCT. Breast cancer patients (n=50) receiving chemotherapy | The study found significant differences (p<0.001) in the proportions of patients experiencing anorexia/tiredness (RBAC vs control: 20% vs 88%), nausea/vomiting (40% vs 100%), hair loss (28% vs 100%) between the two groups. 84% of the control group experienced weight loss but none in the RBAC group. | (Masood et al., 2013) |
| Abbreviations: BW, body weight; i.p., intraperitoneal injection; p.o., per oral; RBAC, rice bran arabinoxylan compound; RCT, randomised controlled trial. | ||||
| # | RBAC (dose) | Study Design | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (40 mg/kg BW i.p. q.o.d. for 6 weeks) | Swiss albino mice (n=6) with single dose whole-body γ-Rad | Significantly lesser (p<0.05) BW loss at weeks 1 and 4 after Rad than the control group. RBAC reduced (p<0.05) the kidney and liver organ weight loss induced by Rad at week 1 and prevented anaemia, lymphopenia, neutrophilia, and thrombocytopenia caused by Rad damage. | (Ghoneum et al., 2013) |
| 2 | Biobran MGN-3 (40 mg/kg BW i.p. 5x/week for 3 weeks) | Swiss albino mice + SEC with whole-body X-ray Rad (3 doses) | RBAC+Rad significantly prevented Rad-induced BW loss (p<0.01). RBAC+Rad achieved the highest reduction in TV and TW compared to Rad alone (p<0.01) and RBAC alone (p<0.05). RBAC+Rad increased apoptosis in tumour tissues with the highest cell-cycle arrest while maximising the AI/PrI ratio at 2.2-fold (p<0.01) compared to untreated SEC-bearing mice. Increased apoptotic regulators and their corresponding gene expression were also detected. | (Badr El-Din et al., 2019) |
| 3 | Biobran MGN-3 (40 mg/kg BW i.p. q.o.d. for 6 weeks) | C57BL/6 mice (n=6) with single dose abdominal precision irradiation | After irradiation, RBAC prevented the depletion of mitochondrial respiratory chain complexes and intercellular ATP content in mice’s jejunal and colonic tissues by strengthening the endogenous antioxidative activities and total antioxidant capacity. | (Zhao et al., 2020) |
| 4 | Biobran MGN-3 (3g/day 2 weeks before and 2 months after) | RCT. Head & neck cancer patients undergoing chemoradiotherapy (n=65, RBAC=32, placebo=33) | Significant between-group differences (p<0.05) were in Hb, haematocrit, RBC, platelets, neutrophils, and lymphocytes after 2 months, favouring RBAC. The RBAC group reported significantly (p=0.05) better QoL and lower mortality, blood transfusion, hospitalisation, and metastasis. | (Tan & Flores, 2020) |
| Abbreviations: ATP, adenosine triphosphate; BW, body weight; Hb, haemoglobin; i.p., intraperitoneal injection; q.o.d., every other day; QoL, quality of life; Rad, radiation therapy; RBAC, rice bran arabinoxylan compound; RBC, red blood cells; RCT, randomised controlled trial; TV, tumour volume; TW, tumour weight. | ||||
| # | RBAC (dose) | Study Design | Key Findings | Reference |
|---|---|---|---|---|
| 1 | Biobran MGN-3 (100, 500 and 1000 mg/ml) | In vitro. MCF-7 cell line + yeast (1:10 ratio) | RBAC significantly increased yeast attachment (54% vs 27%, p<0.001) and phagocytosis rate (72% vs 23%, p<0.01) than control at 0.5 hour. RBAC caused dose-dependent increases in phagocytosis-induced cell death of 35.4%, 40.1%, and 33.04% at 100, 500, and 1000 µg/ml, respectively. | (Ghoneum & Gollapudi, 2005a) |
| 2 | Biobran MGN-3 (100 mg/ml) | In vitro. Monolayer MCF-7 cells + yeast (1:10 ratio) | RBAC increased the magnitude of phagocytising of yeast by MCF-7 cells by 2- to 3-fold after 1 to 4 hours. Culturing with RBAC, yeast, or yeast+RBAC caused 58%, 85%, and 92% cell death, respectively, compared to only 9.5% in untreated MCF-7 cells. | (Ghoneum & Gollapudi, 2005b) |
| 3 | Biobran MGN-3 (50 and 100 μg/ml) | In vitro. MM U266 cell line + curcumin (2.5-10 μM) | RBAC+curcumin caused a significant decrease (p<0.0005) in cell survival compared to either agent alone and achieved an 87% decrease in cell count at 100 μg/ml RBAC and 10 μM curcumin. Combining RBAC (50 μg/ml) with curcumin significantly increased apoptosis (p<0.05) to 20.0, 22.0, and 24.7% at 2.5, 5, 10 μM, respectively, compared to control. | (Ghoneum & Gollapudi, 2011) |
| 4 | Biobran MGN-3 (12 to 45 mg/kg BW 2x/week) for ≥6 months | Cross-section survey. Patients with advanced cancer (n=35) + mistletoe extract (5 mg 2x/week) | Improvement in physical activity (71%) and appetite (66%) were two of the most important effects reported by the patients. For those concurrently treated with conventional treatment (n=24), 70.8% (17/24) also cited reducing side effects as a benefit. | (Hajtó, Horváth, & Papp, 2016) |
| 5 | Biobran MGN-3 (1 g/day for 24 weeks) | RCT. Cancer patients with CFS (n=48, RBAC=24, control=24) + oncothermia | RBAC+oncothermia significantly lowered the posttreatment mean CFQ score (14.6±2.3 vs 23.9±2.3, p<0.01) from baseline. The control group with no treatment reported no significant change in mean CFQ. The mean PGIC score of the RBAC group was 2.1±0.5 (much improved after treatment) compared to 4.3±0.9 (no change) of the control group. | (Petrovics et al., 2016) |
| Abbreviations: BW, body weight; CFQ, Chalder Fatigue Scale; CFS, chronic fatigue syndrome; MM, multiple myeloma; PGIC, Patient Global Impression of Change Scale; QoL, quality of life; RBAC, rice bran arabinoxylan compound. | ||||
| Authors (Year) | Conditions | N (male/female)age (year) | Interventions | Concomitants | Time points | Outcome measures | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| RBAC | Control | RBAC | Control | ||||||
| Takahara and Sano (2004) | Progressive cancer (multiple types) stage III-IV | 96 (55/41) µ=56.0 |
109 (59/50) µ=53.5 |
Biobran MGN-3 (3g/day p.o., 18 months) | NC | CAT + anticancer drugs with fewer side-effects | 18 months | Survival. QoL: pain, malaise, nausea, appetite. |
SR: T (54.2%, 52/96) > C (33.9%, 19/56, PP), p<0.05 > C (17.4%, 19/109, ITT), p<0.001. QoL (T vs C): pain (-15.9% ≈ -14.0%), malaise (-17.3% ≈ -17.1%), nausea (-13.3% ≈ -14.6%), appetite (+24.2% > +15.2%). |
| Bang et al. (2010) | Liver cancer | 38 (30/8) µ=49±19 |
30 (24/6) µ=51±17 |
Biobran MGN-3 (1g/day, 1 year) | NC | TOCE+PEIT | 12,24,36 months | Survival. | SR (12, 24, 36 months): T (76%, 35%, 11%) > C (63%, 6.7%, 0%), p<0.01 (12 & 24 months) |
| (Masood et al., 2013) | Locally advanced breast cancer | 25 (0/25) |
25 (0/25) |
Biobran MGN-3 (3g/day, before & after each cycle) | NC | CT x 6 cycles | ~18 weeks | QoL: anorexia, nausea, alopecia, weight. | QoL (T vs C): anorexia (20% < 88%), nausea (40% < 100%), alopecia (28% < 100%), weight gain (64% > 0%), weight loss (0% < 84%), p<0.001. |
| Itoh et al. (2015) | Cervical cancer | 7 (0/7) µ=49.9 |
7 (0/7) µ=57 |
Biobran MGN-3 (3g/day, 4 weeks) | Placebo | CT+RT (50.4 Gy in 28 fractions) | 4 weeks | QoL: nausea, diarrhea, diarrhea agent. | QoL: T < C in nausea and diarrhea, diarrhea agent but p>0.05. |
| Petrovics et al. (2016) | Cancer (multiple types) with CFS | 24 | 24 | Biobran MGN-3 (1 g/day, 24 weeks) + Oncothermia | NC | CT and/or RT | 24 weeks | QoL: pain, QLQ-C3 (physical, emotional, general), fatigue (CFQ, PGIC). | QoL: T < C in pain, physical, emotional & general QoL, but no data reported. Fatigue (T vs C): CFQ (14.6±2.3 < 23.2±7.2), PGIC (2.1±0.5 < 4.3±0.9), p<0.001. |
| (20/28) M=66 | |||||||||
| Tan and Flores (2020) | H&N cancer (stage II-IV) | 32 (24/8) M=49 |
33 (29/4) M=54.5 |
Biobran MGN-3 (3g/day 2 weeks before and 2 months after RT) | Placebo | RT or CT+RT (60-70 Gy in 30-35 fractions) | ~18 weeks | Survival. QoL: weight, QLQ-C3 H&N35 (general), radiation toxicity. |
SR: T (0%, 0/32) > C (33.3%, 11/33), p<0.001. QoL: p>0.05 for weight loss and radiation toxicity. General QoL, T (1.53±0.24) < C (1.72±0.33), p<0.019. |
| Abbreviations: C, comparator; CAT, complementary and alternative therapies; CFQ, Chalder Fatigue Scale; CFS, chronic fatigue syndrome; H&N, head and neck; NC, no comparator; CT, chemotherapy; ITT, intention to treat; M, median; µ, mean; PEIT, percutaneous ethanol injection treatment; PGIC, Patient Global Impression of Change Scale; PP, per protocol; QLQ-C3, European Organisation for Research and Treatment of Cancer’s Quality of Life Questionnaire version 3.0; QoL, quality of life; RT, radiation therapy; SR, survival rate; T, treatment; TOCE, transarterial oily chemoembolization. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





