1. Introduction
Li
7La
3Zr
2O
12 (LLZO) is one of the most promising inorganic solid electrolytes for lithium batteries due to its high melting point, ionic conductivity, chemical stability, and wide electrical potential window (0 to 5V) with respect to lithium [
1]. LLZO exists in two different crystalline phases, cubic and tetragonal. The cubic phase (
c-LLZO) with high ionic conductivity (10
-3 S cm
-1) is preferred over the tetragonal (
t-LLZO) phase with its lower conductivity (10
-6 S cm
-1). The starting component powders are oxidized at temperatures exceeding>1000 °C to form LLZO. It is noteworthy that the cubic phase predominates at higher temperatures. However, upon cooling to room temperature, the tetragonal phase becomes more prominent. Stabilizing the cubic phase at room temperature is crucial; this is often achieved through the addition of cations. Hence, the incorporation of different cations to replace the existing ones, such as Li
+, La
3+ and Zr
4+, in the crystal lattice of LLZO [
2,
3] is employed to address this challenge. The second important challenge is producing solid LLZO electrolytes with high density. Achieving low porosity in LLZO is essential to limit undesired lithium dendrite growth, which can lead to short circuiting. To achieve this, high temperatures (>1000 °C) and prolonged dwell times are often utilized. Therefore, various thermal treatments, such as spark plasma sintering [
4], flash sintering [
5], field-assisted sintering [
6], oscillatory pressure sintering [
7], hot-press sintering [
8], and solid-state reaction (SSR) [
9] have been widely investigated to achieve high density LLZO.
Among these methods, SSR stands out as a cost-effective approach, despite its limitations such as lithium loss and undesired formation of the tetragonal phase due to high temperatures and prolonged dwell times. To address this issue, large amounts of mother powder (powder bed) are consumed during the process. The cost of mother powder is high, mainly due to the lithium precursor. To compensate for lithium loss, excess lithium salt (precursor) can be utilized [
10,
11,
12].
In addition to this, the choice of crucibles used during synthesis plays a significant role. Jiang et al. [
13] investigated the influence of sintering crucibles on phase purity of Ta-LLZO (specifically MgO and Al
2O
3 crucibles) when the lid is used. Their study demonstrated that MgO is the most suitable candidate for synthesis of
c-LLZO over Al
2O
3, owing to its enhanced chemical inertness at elevated temperatures [
14,
15,
16,
17].
Furthermore, numerous studies have employed various methodologies to synthesize
c-LLZO [
18,
19,
20]. However, upscaling of LLZO production using these known synthesis methods can be challenging. Therefore, development of a methodology with the necessary process steps to enable repeatable production in large batch sizes is crucial as a first step.
In this study, 250 grams of pre-sintered LLZO (Li7La3Zr1.6Ta0.4O12) powder per batch were synthesized via SSR for use in tape casting and 3D printing processes. This powder was subsequently pressed and sintered at 1150 °C to verify the phase-purity of the Ta-LLZO. The synthesized Ta-LLZO demonstrates a room-temperature ionic conductivity of 1.96 X 10-4 S cm-1. Electrochemical test indicates that Ta-LLZO exhibits reasonable stability with metallic Li. This pre-sintered powder opens new possibilities for use as a source in the bulk tape casting and 3D printing processes, which may hold a significant importance for next-generation battery technology.
2. Materials and Methods
Li
7La
3Zr
1.6Ta
0.4O
12 was synthesized via solid-state reaction using La
2O
3 (95%, Merck, Germany), Li
2CO
3 (99%, Merck, Germany), Ta
2O
5 (99%, Merck, Germany)
, and monoclinic-ZrO
2 (Tosoh, Netherlands) as starting materials. To obtain 250 g of
c-Ta-LLZO, 146.61 g of La
2O
3, 85.34 g of Li
2CO
3, 22.18 g of ZrO
2, and 26.51 g of Ta
2O
5, were planetary ball-milled (PM 400, Retsch GmbH, Germany). Here, the incorporation of Ta (0.4 mol) in the LLZO stabilizes the crystal structure of cubic-LLZO (c-LLZO) at room temperature [
21]. Milling was performed at 300 rpm for 6 hours in ZrO
2 lined jars. The milling media consisted of 260 g ZrO
2 balls (3 mm diameter) and 2-propanol as the solvent. Addition of a 10% excess lithium source could compensate for lithium loss at elevated temperatures [
13]. The slurry obtained from the milling process was dried at room temperature to remove the excess solvent. The mixed powder was die pressed at 100 MPa for one minute into 50 g pellets, which were then calcined at 950 °C for 6 hours in an Al
2O
3 crucible. The calcined pellets were ground manually using a mortar and pestle to achieve a homogeneous powder. The powder was uni-axially pressed into discs at 120 MPa held at this pressure for 5 minutes then removed and sintered at 1150 °C with a ramp rate of 3 °C/min for 1 hour in an MgO crucible with a closed lid, resulting in a high relatively dense, phase pure
c-Ta-LLZO with disc dimensions of 0.7 mm thickness and 12 mm diameter. The schematic representation of the experimental section is shown in
Figure S1.
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were performed on wet-milled powders at a heating rate of 10 K/min in dynamic airflow (70 ml/min) using a STA-449 F3 Jupiter instrument (Netzsch GmbH & Co. KG, Germany). High temperature dilatometer DIL 402/E (Netzsch GmbH & Co. KG, Germany) with a pyrometer was used to measure the shrinkage of the Ta-LLZO disc under Argon atmosphere up to 1200°C. The phase formation of the disc was investigated using a PANalytical X’Pert powder diffractometer (Netherland). Rietveld refinement of X-ray diffraction patterns was performed using the FullProf software package [
22]. To check for impurities in the Ta-LLZO discs, Raman spectra were collected using the confocal Raman spectroscopy device (WITec Alpha 300 R, Germany) with an excitation of 532 nm for thermally treated pellets and discs. Additionally, X-ray photoelectron spectroscopy (XPS) measurements (Phi Quantum 2000) were performed. The densities of the sintered,
c-Ta-LLZO discs were measured using the Archimedes method with 2-propanol. The density measurements were performed on at least two separate samples and the average value of density was reported. Scanning electron microscopy (SEM, Tascan Vega 3, Czech Republic) was performed on the wet-polished cross-section of both pellets and discs after sputtering with a thickness of 10 nm of Au/Pd alloy using a Leica EM ACE 200 sputter coater (Lecia, Germany). Symmetrical cell (Li|
c-Ta-LLZO|Li) assembly and electrochemical characterization were performed under an argon atmosphere in a glovebox. Using a frequency response analyzer (FRA) (Solartron 1260A), the room temperature impedance of the pellets was measured to extract the resistance and conductivity values for the discs (0.7 mm x 12 mm diameter). The ion-conductivity of
c-Ta-LLZO electrolyte was extracted from PEIS data measured at frequencies between 5 Hz and 25 MHz with a 20-mV sinusoidal amplitude. Electronic conductivity was determined by a DC polarization method (Mott–Schottky) at 1 V versus Li/Li
+ after 16 h stabilization using VMP-300 multi potentiostat (BioLogic, France). All electrochemical measurements were carried out in a Swagelok cell.
3. Results
3.1. Thermal analysis
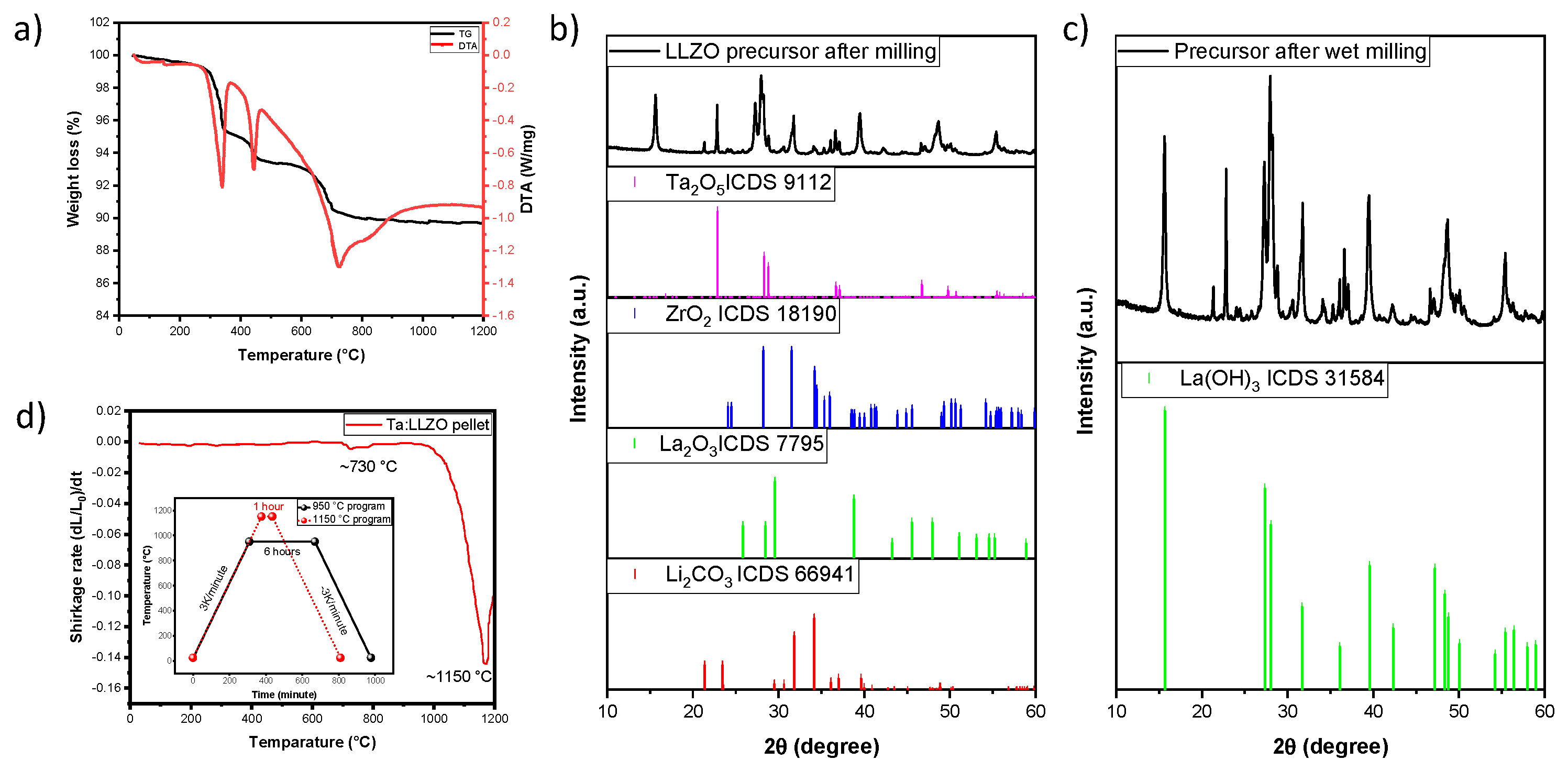
The thermogravimetric analysis and dilatometry analysis were conducted to elucidate the weight loss and shrinkage behavior of Ta-LLZO from room temperature to 1200 °C. In
Figure 1a, TG and DTA curves after wet milling of the Ta-LLZO powder are presented. The TG curve reveals four distinct weight loss steps and the corresponding endothermic peaks. The initial weight loss step (approximately to 300°C) is attributed to the presence of moisture and organic residues in the milled sample. The subsequent weight loss in the temperature range of 250 °C - 550 °C is associated with the transformation of La(OH)
3, formed during wet milling from La
2O
3. Wet milling of the oxide precursor, La
2O
3, leads to the formation of La(OH)
3 due to the topological transformation during the reaction process. This transformation is confirmed by the appearance of new peaks in the XRD spectra shown in
Figure 1b and 1c. Moreover, during the milling process, mechanical activation can enhance the diffusion of dopant Ta into the LLZO lattice and concurrently can reduce the crystallinity of LiCO
3. This process improves the incorporation of Tantalum ions, thereby promoting stabilization of the cubic phase structure.
The TG/DTA curves for each individual starting material after milling are shown in
Figure S2 (a-d). When comparing these TG/DTA curves with the first endothermic peak in the
Figure 1(a), attributed to the formation of La(OH)
3 in the 250 to 550 °C range, it is evident that the next weight loss occurs in the region of 650 °C - 900 °C, corresponding to the decomposition of Li
2CO
3, which is in line with literature findings [
23]. Above 900 °C, there is no weight loss up to 1200°C. Dilatometry measurements on Ta-LLZO pellets in the same temperature range, with a heating rate of 3 K/min (
Figure 1d), reveal a small peak at 730 °C, indicating a slight shrinkage. Subsequently, after 1000°C, the main onset of densification and shrinkage behavior is observed, reaching completion at approximately 1150°C for Ta-LLZO.
3.2. Phase analysis
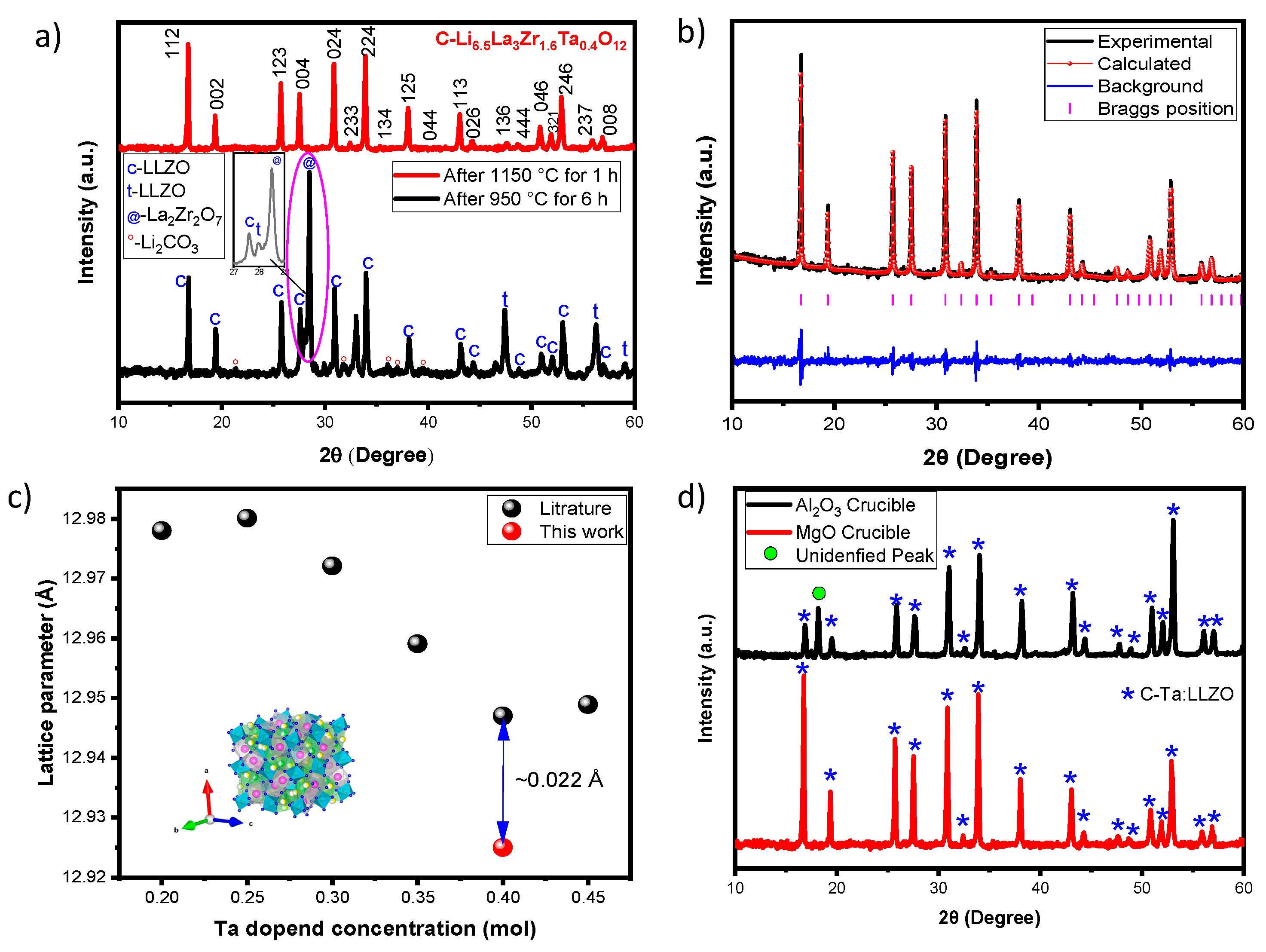
XRD patterns of Ta-LLZO after thermal processes at 950 °C (pellet) and 1150 °C (disc) are shown in
Figure 2a. Following the 950 °C process, the cubic phase (
c-LLZO) is observed in the Ta-LLZO powder, along with some impurity peaks representing tetragonal (
t-LLZO), La
2Zr
2O
7, and Li
2CO
3. A strong diffraction peak at 2θ=28.52°, indicating the formation of La
2Zr
2O
7, is prominently visible. The tetragonal phase is confirmed by peaks at 2θ of 27.97, 47.42, 56.26, and 59.09 °. The presence of lithium carbonate is observed with weak diffraction peaks at 21.40, 31.85, 36.16, 37.05, and 39.49 °. Additionally, unidentified peaks at 29.92, 33.02, and 46.62 were also detected. The pellets achieved 73 % of relative density at 950 °C. Hence, a higher sintering temperature is required to obtain a phase pure and dense Ta-LLZO disc. According to dilatometry measurement, the Ta-LLZO samples were sintered at 1150 °C (
Figure 1d). The peak intensity at 224 increases slightly with the 024 plane indicating the presence of Ta
5+ ions in the LLZO crystal lattice. The ratio of 024/224 plane at 950 °C and 1150 °C is determined as 0.84 and 0.97, respectively. Here, the value of 0.97 that approaches to 1 indicates the presence of the cubic LLZO. Well-defined diffraction peaks perfectly match with cubic Ta-LLZO, indexed with the corresponding hkl planes without any impurity. According to Zhang et al. [
24], a high intense peak was obtained at 33.83° (224) plane for cubic Ta-LLZO samples, and its intensity varies with respect to the concentration of Ta. In the presented XRD pattern (
Figure 2a), a high-intensity peak was observed at 16.80° (112). Rangasamy et al. [
2] reported that Al-LLZO shows a similar XRD pattern with a high-intensity peak at 16.80°. Additionally, alterations in lithium concentration, whether increased or decreased, could influence the phase of
c-Ta-LLZO.
Rietveld refinement was used to examine the lattice parameters of the aforementioned samples. The results of pattern fitting are shown in
Figure 2b. The relative density after sintering the disc at 1150 °C is 96 % of the theoretical density, which is advantageous for electrochemical performance and will be discussed later. A comparison graph, plotting the cell parameter data extracted from the literature for different Ta-concentrations (Ta=0.2, 0.25, 0.30, 0.35, 0.40 and 0.45), is presented in
Figure 2c. It is observed that an increase in Ta-content leads to a decrease in the lattice parameter [
25,
26]. The lattice parameter in this study is 12.944 Å similar to previous works with comparable Ta compositions [
27,
28,
29].
Figure 2d illustrates the impact of the Al
2O
3 crucible in the high temperature sintering of Ta-LLZO discs. Notably, compared to discs sintered in an MgO crucible, those treated in an Al
2O
3 crucible exhibit a peak at 18.18°, which cannot be attributed to any other impurities of LLZO. Therefore, we designate this peak as an unidentified one, likely resulting from Al ions diffusing into LLZO structure at elevated temperatures exceeding 1000 °C. MgO crucibles were identified as suitable crucibles for obtaining dense phase pure cubic Ta-LLZO discs.
3.2. Vibration and elemental analysis
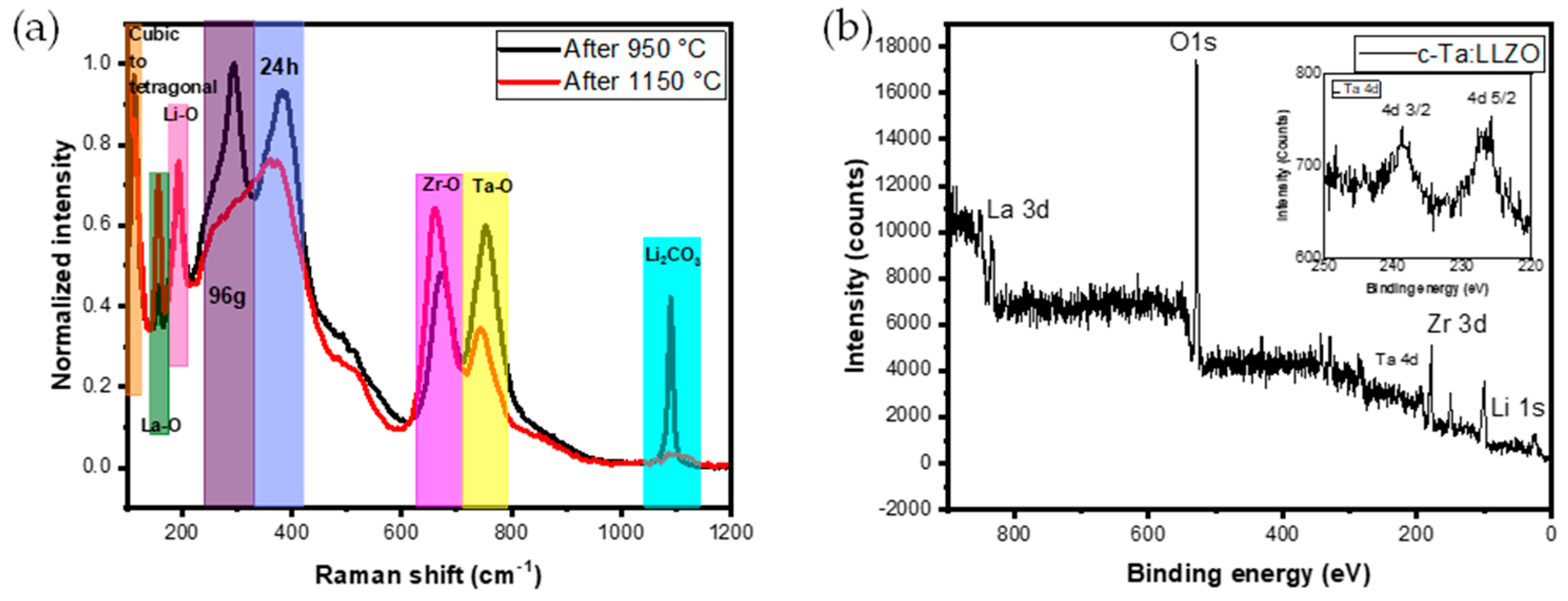
Raman spectroscopy was used to investigate the vibrational spectrum of the Ta-LLZO pellet (950 °C) and disc (1150 °C). In
Figure 3a, all the peaks are indexed based on their vibrations in accordance with the literature [
27,
28,
29,
30,
31,
32]. The presence of the cubic phase has been confirmed by the existence of Li accommodating sites 24d (tetrahedral), and 96h (octahedral). In
Figure 3a, in that specific region, the isolation of the peak is observed for the pellets (950 °C) whereas the discs (1150 °C) show a merged peak without any separation. This indicates the linkage of tetrahedral and octahedral regions contributing to Li movement in the crystal structure. This is a major reason why the cubic phase exhibits a higher ionic conductivity than the tetragonal phase [
33].
Raman spectroscopy was employed to investigate the aforementioned lithium accommodating site linkage. In line with this, the vibrations of Ta, Zr, Li, and La combined with oxygen vibrations were observed after heating cycles at 950°C (pellet) and 1150°C (disc). However, for pellets, the peaks appearing at 1090 cm
-1 correspond to lithium carbonate. This lithium carbonate peak is consistent with the XRD pattern in
Figure 2a. During the phase evaluation, no impurity peaks are identified for the dopant Ta. The presence of Ta is confirmed with the XPS analysis. XPS spectrum of
c-Ta-LLZO is presented in
Figure 3b, showing the peaks corresponding to the presence of La, O, Ta, Zr, and Li [
34]. The inset in
Figure 3b further depicts the presence of Ta.
3.4. Microstructural analysis
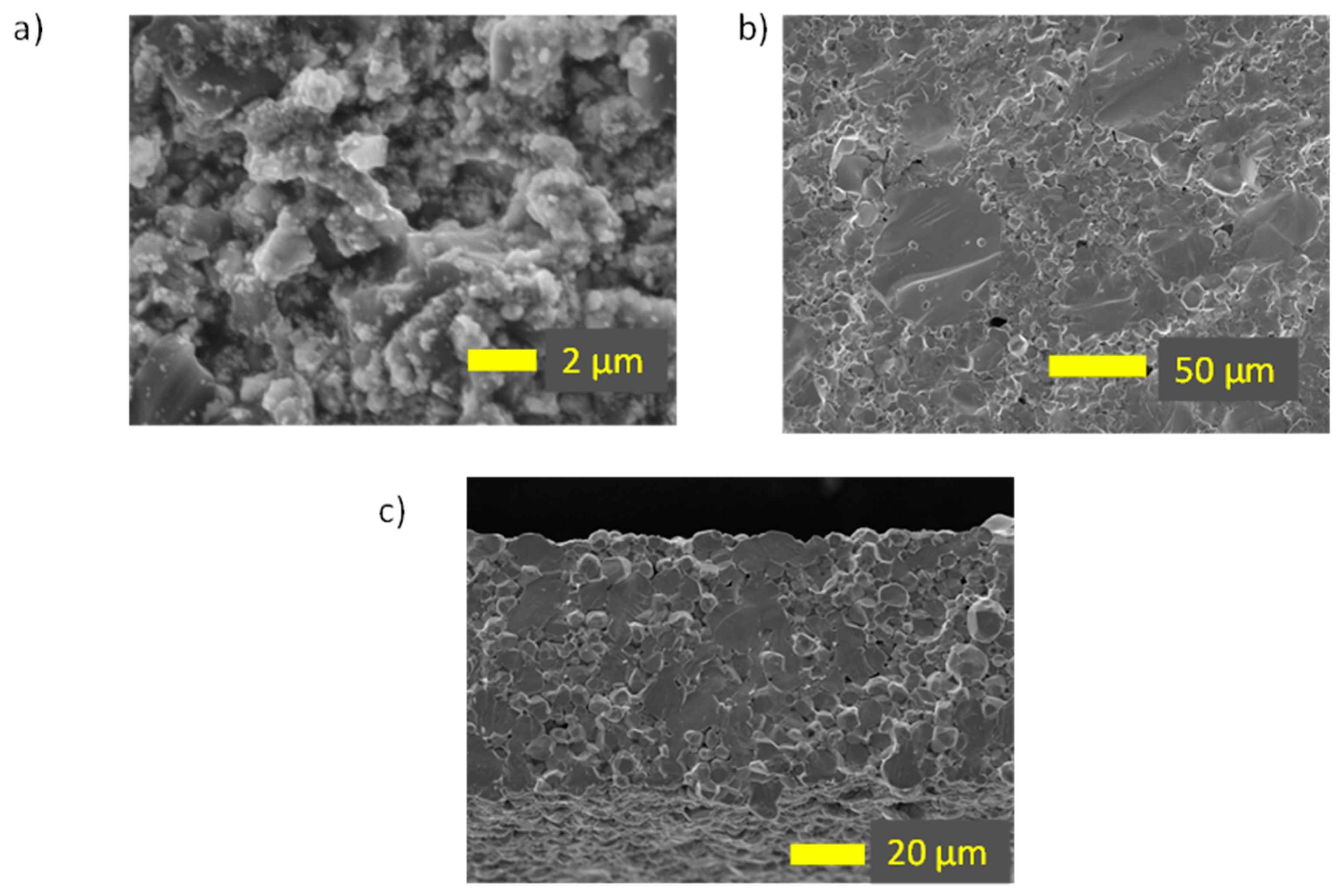
Microstructural analysis was performed to investigate the particle formation after heating to 950 °C (pellets) and 1150 °C (discs) using SEM. The morphology of the particles after 950 °C is observed to be agglomerated, as shown in
Figure 4a. Notably, the morphology of the disc (1150 °C) reveals the absence of large holes and pores on the surface, with well interconnected grains, indicating good densification, as shown in
Figure 4b. Additionally, the cross-section image of the disc in
Figure 4c shows only a few small internal pores in the materials [
35]. These characteristics are crucial for testing the samples for electrochemical measurements.
3.5. Electrochemical analysis
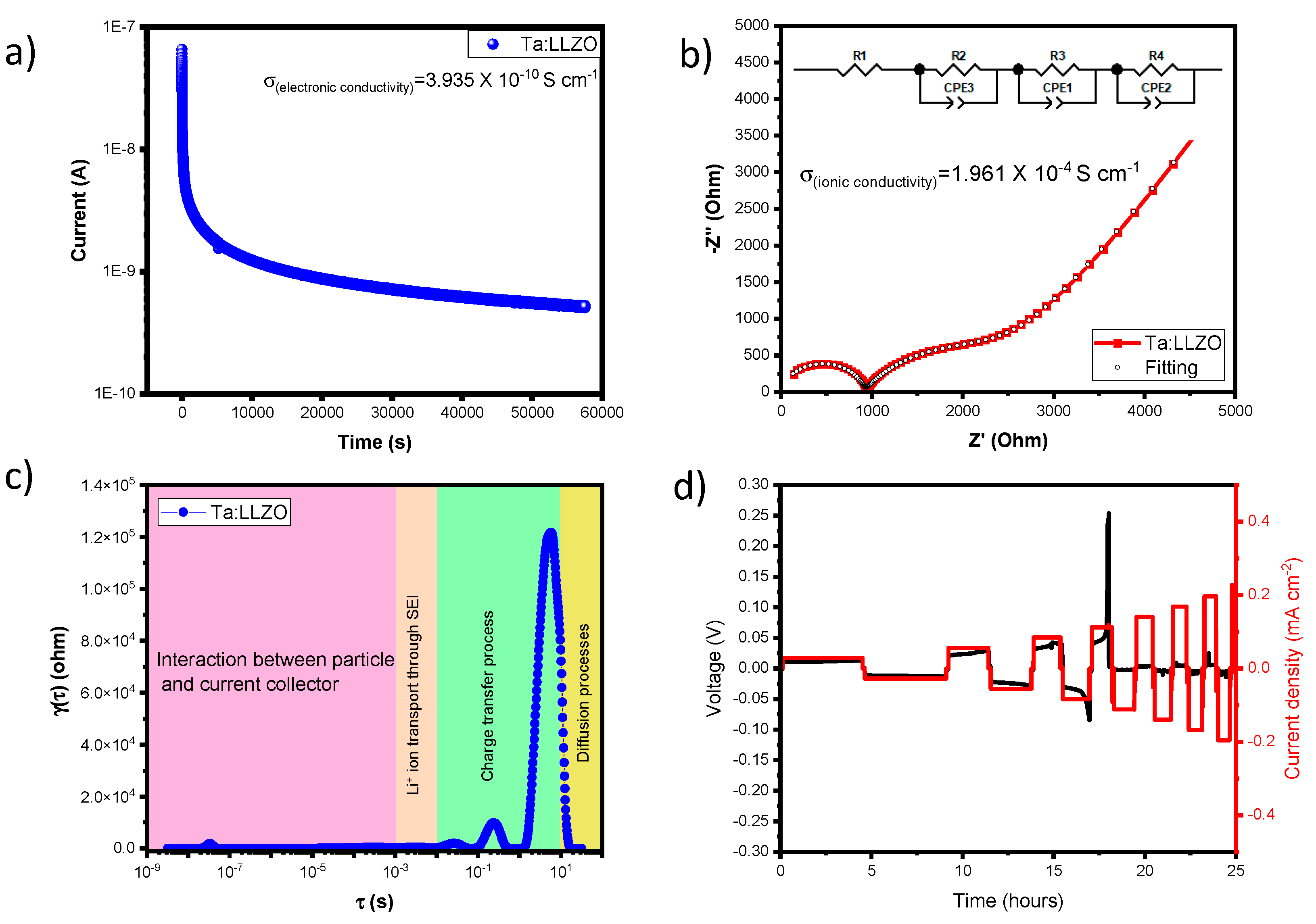
A solid electrolyte should exhibit ionic conductivity while remaining electronically insulating. In this study, the electronic and ionic conduction behaviors of
c-Ta-LLZO discs were assessed. Electronic conductivity of the
c-Ta-LLZO solid solution (Ta-doping) was investigated by using chronoamperometry measurements, applying a constant potential of 1V between Li||
c-Ta-LLZO||Li configuration.
Figure 5a shows the current as a function of time. Initially, the current exhibits a negative slope due to the polarization of the Ta-doped solid solution LLZO. The electronic conductivity was measured as 3.94 x 10
-10 S cm
-1 indicating insulating behavior of the solid electrolyte.
Simultaneously, ionic conductivity was measured through electrochemical impedance spectroscopy (EIS) in the frequency range 24 MHz to 5 Hz.
Figure 5b shows a spectrum with two semi-circles in the high and mid-frequency region and an inclined line in the low-frequency region in the Nyquist plot. The first semicircle represents the bulk boundary and the second represents the grain boundary [
36]. The real axis in the Nyquist plot contributes to the total resistance. The observed two semi-circles and the addition of their individual resistance aligns with the real axis responsible for the total resistance. Fitting the spectrum using Z-view software provided the values for resistance, capacitance, and constant phase elements of the equivalent circuit (
Figure 5b) and the ionic conductivity of the system is calculated as 1.96X10
-4 S cm
-1. The Distribution of Relaxation Times (DRT) was employed for deconvoluting the EIS data to better understand the interactions of the different interfaces. [
37,
38,
39].
Figure 5c shows peaks at different relaxation times, aiding in identification of interactions for charge transfer and diffusion process.
Finally, the critical current density measurement for
c-Ta-LLZO is illustrated in
Figure 5d. This measurement is crucial for identifying lithium dendrite formation during cell cycling at different current densities. It was reported that 0.3 to 0.7 mA cm
-2 is the maximum limit for obtaining a good cyclability without polarization (lithium dendrite) [
40,
41]. However,
c-Ta-LLZO shows better results at a current density of 0.09 mA cm
-2 due to the absence of large voids. However, at 0.12 mA cm
-2, polarization is observed as shown in
Figure 5d due to lithium penetration through the grain boundaries.
4. Conclusions
A cubic Ta-doped (Li7La3Zr1.6Ta0.4O12) (c-Ta-LLZO) inorganic solid electrolyte was synthesized through a solid-state reaction. TG and DTA, and dilatometry analyses were employed to identify weight loss and densification of the material. The discs (sintered at 1150 °C) of c-Ta-LLZO exhibited a relative density of 96% without any large open pores on the surface or inside the structure.
The optimized synthesis process can be theoretically scaled up for larger industrial production, without alterations. Cells prepared from the sintered discs demonstrated promising electronic (3.94 X 10-10 S cm-1) and ionic conductivity (1.96 X 10-4 S cm-1) values, making them suitable as solid electrolytes for next generation solid state Li-ion batteries. Critical current density measurements indicated that c-Ta-LLZO performed well up to 0.09 mA cm-2, but polarization was observed at a current density of 0.12 mA cm-2, attributed to the growth of lithium dendrites via grain boundaries.
The synthesis process was optimized for laboratory-scale production, up to ~250 g of pre-sintered LLZO per batch. This powder is strongly recommended as a source for producing phase-pure c-Ta-LLZO using tape casting and 3D printing processes. Although, 250 g batch sizes were produced in this study, scaling up to several kg batches is feasible.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, G.B., T.G., F.C.; methodology, Y.L., D.Ka., D.Ko., A.H.; validation, N.S.Y, A.H.; formal analysis, D.Ka, D.Ko, N.S.Y., investigation, D.Ka, Y.L., P. V. W. S.; resources, G.B, T.G.; data curation, D.Ka, P.V. W.S.; writing—original draft preparation, D. Ka; writing—review and editing, G.B., N.S.Y.; visualization, N.S.Y., P.V.W. S.; supervision, G.B, F.C.; project administration, G.B.; funding acquisition, G.B., T.G.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by Innosuisse the Swiss Innovation Agency under project title “CAMELOT” funding agreement number 31236.1 IP-EE.
Acknowledgments
The authors would like to acknowledge the Swiss Innovation Agency-Innosuisse for funding this work as part of project 31236.1 IP-EE and Empa Internal Project Funding sources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, F.; Guo, W.; Zeng, D.; Sun, Z.; Gao, J.; Li, J.; Zhao, B.; He, B.; Han, X. A Simple and Highly Efficient Method toward High-Density Garnet-Type LLZTO Solid-State Electrolyte. ACS Applied Materials & Interfaces 2020, 12, 30313–30319. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Buannic, L.; Orayech, B.; López Del Amo, J.-M.; Carrasco, J.; Katcho, N.A.; Aguesse, F.; Manalastas, W.; Zhang, W.; Kilner, J.; Llordés, A. Dual Substitution Strategy to Enhance Li+ Ionic Conductivity in Li7La3Zr2O12 Solid Electrolyte. Chemistry of Materials 2017, 29, 1769–1778. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, K.; Chen, D.; Zeng, J.; Luo, B. Spark plasma sintering plus heat-treatment of Ta-doped Li7La3Zr2O12 solid electrolyte and its ionic conductivity. Materials Research Express 2020, 7, 025518. [Google Scholar] [CrossRef]
- Clemenceau, T.; Andriamady, N.; Kumar, M. K, P.; Badran, A.; Avila, V.; Dahl, K.; Hopkins, M.; Vendrell, X.; Marshall, D.; Raj, R. Flash sintering of Li-ion conducting ceramic in a few seconds at 850 °C. Scripta Materialia 2019, 172, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Tu, R.; Shen, Q.; Zhang, L. Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. Journal of Power Sources 2014, 268, 960–964. [Google Scholar] [CrossRef]
- Li, H.-Y.; Huang, B.; Huang, Z.; Wang, C.-A. Enhanced mechanical strength and ionic conductivity of LLZO solid electrolytes by oscillatory pressure sintering. Ceramics International 2019, 45, 18115–18118. [Google Scholar] [CrossRef]
- Yang, L.; Dai, Q.; Liu, L.; Shao, D.; Luo, K.; Jamil, S.; Liu, H.; Luo, Z.; Chang, B.; Wang, X. Rapid sintering method for highly conductive Li7La3Zr2O12 ceramic electrolyte. Ceramics International 2020, 46, 10917–10924. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Karabay, D.T.; Kovalenko, M.V. On the feasibility of all-solid-state batteries with LLZO as a single electrolyte. Scientific Reports 2022, 12, 1177. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, F.; Shen, Q.; Zhang, L.; Chen, C. Effect of the lithium ion concentration on the lithium ion conductivity of Ga-doped LLZO. Materials Research Express 2019, 6, 085546. [Google Scholar] [CrossRef]
- Yi, E.; Wang, W.; Kieffer, J.; Laine, R.M. Flame made nanoparticles permit processing of dense, flexible, Li+ conducting ceramic electrolyte thin films of cubic-Li7La3Zr2O12 (c-LLZO). Journal of Materials Chemistry A 2016, 4, 12947–12954. [Google Scholar] [CrossRef]
- Badami, P.; Smetaczek, S.; Limbeck, A.; Rettenwander, D.; Chan, C.K.; Kannan, A.N.M. Facile synthesis of Al-stabilized lithium garnets by a solution-combustion technique for all solid-state batteries. Materials Advances 2021, 2, 5181–5188. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Hu, Z.; Huang, Y.; Zhu, X. MgO or Al2O3 crucible: Which is better for the preparation of LLZ-based solid electrolytes? Ceramics International 2020, 46, 3367–3373. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.-Q.; Guo, X.-X. Densification and lithium ion conductivity of garnet-type Li7La3Zr2-xTaxO12(x= 0.25) solid electrolytes. Chinese Physics B 2013, 22, 078201. [Google Scholar] [CrossRef]
- Weller, J.M.; Whetten, J.A.; Chan, C.K. Synthesis of fine cubic Li7La3Zr2O12 powders in molten LiCl–KCl eutectic and facile densification by reversal of Li+/H+ exchange. ACS Applied Energy Materials 2018, 1, 552–560. [Google Scholar] [CrossRef]
- Xia, W.; Xu, B.; Duan, H.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Ionic conductivity and air stability of Al-doped Li7La3Zr2O12 sintered in alumina and Pt crucibles. ACS Applied Materials & Interfaces 2016, 8, 5335–5342. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Guo, H.; Song, Z.; Xiu, T.; Badding, M.E.; Wen, Z. None-mother-powder method to prepare dense Li-garnet solid electrolytes with high critical current density. ACS Applied Energy Materials 2018, 1, 5355–5365. [Google Scholar] [CrossRef]
- Sato, M.; Garcia-Mendez, R.; Sakamoto, J. Mapping hot-pressed Li6.25Al0.25La3Zr2O12 (LLZO) grains and grain boundaries through a simple thermal grooving technique. Journal of Asian Ceramic Societies 2020, 8, 793–803. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, C.H.; Jarry, A.; Chen, W.; Ye, Y.; Zhu, J.; Kostecki, R.; Persson, K.; Guo, J.; Salmeron, M.; et al. Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12 solid electrolytes. ACS Applied Materials & Interfaces 2015, 7, 17649–17655. [Google Scholar] [CrossRef]
- Heo, T.W.; Grieder, A.; Wang, B.; Wood, M.; Hsu, T.; Akhade, S.A.; Wan, L.F.; Chen, L.-Q.; Adelstein, N.; Wood, B.C. Microstructural impacts on ionic conductivity of oxide solid electrolytes from a combined atomistic-mesoscale approach. npj Computational Materials 2021, 7, 214. [Google Scholar] [CrossRef]
- Inada, R.; Takeda, A.; Yamazaki, Y.; Miyake, S.; Sakurai, Y.; Thangadurai, V. Effect of postannealing on the properties of a Ta-doped Li7La3Zr2O12 solid electrolyte degraded by Li dendrite penetration. ACS Applied Energy Materials 2020, 3, 12517–12524. [Google Scholar] [CrossRef]
- Fritsch, C.; Zinkevich, T.; Indris, S.; Etter, M.; Baran, V.; Bergfeldt, T.; Knapp, M.; Ehrenberg, H.; Hansen, A.-L. Garnet to hydrogarnet: effect of post synthesis treatment on cation substituted LLZO solid electrolyte and its effect on Li ion conductivity. RSC Advances 2021, 11, 30283–30294. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, K.; Chen, L.; Lu, Y.; Li, Y.; Wang, C.-A. Sintering behavior of garnet-type Li6.4La3Zr1.4Ta0.6O12 in Li2CO3 atmosphere and its electrochemical property. International Journal of Applied Ceramic Technology 2017, 14, 921–927. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Hu, D.; Chen, F.; Shen, Q.; Zhang, L.; Dong, S. Synergistic regulation of garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte by Li+ concentration and Li+ transport channel size. Electrochimica Acta 2019, 296, 823–829. [Google Scholar] [CrossRef]
- Yi, M.; Liu, T.; Wang, X.; Li, J.; Wang, C.; Mo, Y. High densification and Li-ion conductivity of Al-free Li7-xLa3Zr2-xTaxO12 garnet solid electrolyte prepared by using ultrafine powders. Ceramics International 2019, 45, 786–792. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Progress in Materials Science 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Gajraj, V.; Kumar, A.; Indris, S.; Ehrenberg, H.; Kumar, N.; Mariappan, C.R. Influence of Al on the structure and ion transport in garnet-type Li7La3-xAlxZr2O12 solid electrolytes for Li-ion batteries. Ceramics International 2022. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. Journal of Advanced Ceramics 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: the role of hydration. Journal of Materials Chemistry A 2013, 1, 11419–11428. [Google Scholar] [CrossRef]
- Hirose, E.; Niwa, K.; Kataoka, K.; Akimoto, J.; Hasegawa, M. Structural stability of the Li-ion conductor Li7La3Zr2O12 investigated by high-pressure in-situ X-ray diffraction and Raman spectroscopy. Materials Research Bulletin 2018, 107, 361–365. [Google Scholar] [CrossRef]
- Rettenwander, D.; Welzl, A.; Cheng, L.; Fleig, J.; Musso, M.; Suard, E.; Doeff, M.M.; Redhammer, G.J.; Amthauer, G. Synthesis, crystal chemistry, and electrochemical properties of Li7–2xLa3Zr2–xMoxO12 (x=0.1–0.4): stabilization of the cubic garnet polymorph via substitution of Zr4+ by Mo6+. Inorganic Chemistry 2015, 54, 10440–10449. [Google Scholar] [CrossRef]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. Journal of Materials Chemistry A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Rustad, J.R. The effect of tetrahedral versus octahedral network-blocking atom substitutions on lithium ion conduction in LLZO garnet. arXiv preprint, 2016; arXiv:1605.08598. [Google Scholar]
- Zhu, Y.; Connell, J.G.; Tepavcevic, S.; Zapol, P.; Garcia-Mendez, R.; Taylor, N.J.; Sakamoto, J.; Ingram, B.J.; Curtiss, L.A.; Freeland, J.W.; et al. Dopant-dependent stability of garnet solid electrolyte interfaces with lithium metal. Advanced Energy Materials 2019, 9, 1803440. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Song, Z.; Rui, K.; Wang, Q.; Xiu, T.; Badding, M.E.; Wen, Z. Manipulating Li2O atmosphere for sintering dense Li7La3Zr2O12 solid electrolyte. Energy Storage Materials 2019, 22, 207–217. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhao, S.-X.; Xu, Y.-H.; Nan, C.-W. Effect of the morphology of Li–La–Zr–O solid electrolyte coating on the electrochemical performance of spinel LiMn1.95Ni0.05O3.98F0.02 cathode materials. Journal of Materials Chemistry A 2014, 2, 18889–18897. [Google Scholar] [CrossRef]
- Hahn, M.; Rosenbach, D.; Krimalowski, A.; Nazarenus, T.; Moos, R.; Thelakkat, M.; Danzer, M.A. Investigating solid polymer and ceramic electrolytes for lithium-ion batteries by means of an extended Distribution of Relaxation Times analysis. Electrochimica Acta 2020, 344, 136060. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, M.; Huang, T.; Yu, A. Detection of lithium plating in lithium-ion batteries by distribution of relaxation times. Journal of Power Sources 2021, 496, 229867. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Carrillo, R.E.; Wood, V. EIS2MOD: A DRT-based modeling framework for li-ion cells. IEEE Transactions on Industry Applications 2021, 58, 1429–1439. [Google Scholar] [CrossRef]
- Flatscher, F.; Philipp, M.; Ganschow, S.; Wilkening, H.M.R.; Rettenwander, D. The natural critical current density limit for Li7La3Zr2O12 garnets. Journal of Materials Chemistry A 2020, 8, 15782–15788. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the interface between the lithium anode and a garnet-type solid electrolyte of lithium batteries using an aluminum-nitride layer. Nanomaterials 2022, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).