1. Introduction
Extracorporeal membrane oxygenation is used in ICU for the management of severe respiratory and cardiac failure. Despite recent advances in circuit and membranes technology, the reported morbidity and mortality remains high (1). Mortality is attributed to unsuccessful cardiopulmonary recovery, while some serious complications are usually encountered. ECMO associated coagulopathy (EAC) is a frequent complication leading to high rates of thrombosis or severe haemorrhage (2). Interestingly, bleeding complications are strongly associated with poor outcome and mortality (3) (4). Elucidation and monitoring of the underlying factors of EAC is substantial for effectively managing patients on ECMO. The aim of the current article is to analyse the pathomechanism of EAC, to highlight coagulation monitoring requirements and to conclude to current recommendations.
2. Interactions between coagulation cascade and biosurfaces leading to EAC
2.1. Thrombotic and hemorrhagic complications
EAC might be associated with clinical thrombosis, with reported rates of 15-85% or haemorrhage reported in 15-21% of patients (5). Thrombosis of the oxygenator membrane is observed in 10-15% of ECMO patients (6). The prothrombotic effect of interaction of blood with biosurfaces is described by the three components of Virchov΄s triad: Blood exposure to the artificial endothelium consisted of the biosurface of the oxygenator membrane, the abnormal blood flow through the ECMO circuit and the hypercoagulability caused by the release of free hemoglobulin into the circulation during hemolysis effect (5). Prevention of thrombosis during ECMO is commonly achieved by the continuous infusion of unfractionated heparin, although there is no consensus on the appropriate anticoagulation strategy.
At the same time, haemorrhagic complications might be caused by the administration of unfractionated heparin, thrombocytopenia, platelet dysfunction, acquired Von Willebrand syndrome and consumption of coagulation factors (7). According to a review of Extracorporeal Life Support Organization (ELSO) registry, risk factors associated with haemorrhage in patients receiving veno-venous (VV) ECMO support for respiratory failure, are cardiac arrest, precannulation hypoxemia, metabolic acidosis and shock (8). A prediction model including these risk factors was successfully used in order to identify the ECMO patients with increased probability for haemorrhage. ECMO circuit thrombosis might be accompanied with haemorrhage rendering patient’s subsequent management rather challenging. Transfusion of blood products, administration of coagulation factors or fibrinolysis inhibitors might aggravate ECMO circuit failure. The subsequent formation of thrombi in ECMO circuit will deteriorate the coagulopathy, leading to a vicious cycle, which might require the exchange of the circuit and oxygenator (9).
2.2. Role of underlying disease in EAC pathophysiology
Moreover, patient’s underlying disease might be associated with prothrombotic or bleeding effects, in the context of trauma induced coagulopathy, disseminated intravascular coagulation (DIC) or COVID-19 associated coagulopathy. Treatment algorithm of COVID-19 patients with ARDS, frequently includes ECMO support. The hyperinflammatory state produced by COVID-19 might interact with the effect of exposure of blood to biosurfaces, producing bleeding or thrombotic complications (10). Indeed, according to several reports, bleeding rate in COVID-19 patients managed with ECMO is 14-42%, while reported intracranial haemorrhage rate is about 4% (11) (12). Interestingly this rate is higher than the respective central nervous system bleeding rate (2%) reported in EOLIA (ECMO to rescue Lung Injury in severe ARDS) study (13). COVID-19 associated endotheliopathy and higher doses of anticoagulants might explain the above difference. Moreover, thrombosis is common in hospitalized COVID-19 patients, ranging between 20-30%, while pulmonary embolism is the commonest thrombotic manifestation (14,15). Thrombotic complications are commonly reported in COVID-19 patients managed with ECMO support, despite the higher dose anticoagulant regimens compared to non-COVID-19 ECMO patients (16).
2.3. Activation of coagulation pathway
EAC is initially triggered by exposure of blood to extracorporeal surfaces, which consequently leads to the activation of coagulation factors, complement system, platelets and Von Willebrand factor (VWf) and fibrinolysis (
Figure 1). Hemodilution caused by the priming volume of ECMO circuit, results in a decrease of hematocrit and coagulation factors (17). Moreover, the interaction of blood with the artificial surfaces of circuit, leads to absorption of fibrinogen and proteins to the surfaces, known as «Vroman effect» (18). Coagulation factors, as high molecular weight kininogen (HMWK), factor XII, albumin, immunoglobulins and complement components (C3) are absorbed by fibrinogen layer, undergoing modification which renders their molecules thrombogenic (19). The protein and fibrinogen layer which is formed on the artificial «endothelium» of ECMO circuit, triggers the activation of coagulation, which in turn regulates the inflammatory process induced by the innate host response. The major pathological role of coagulation in the innate host response has recently been elucidated and defined as immunothrombosis, a phenomenon playing an important role in the pathophysiology of sepsis and ARDS (20). Therefore, platelets accumulate at the site of artificial «endothelium» of biosurface. Their activation leads to the release of the content of their granules, consisting of immunomodulatory mediators, antimicrobial peptides (AMPs) and microparticles (MPs). These molecules contribute to the migration of neutrophils and activation of complement. Platelet-neutrophil complex shows different properties than platelets or neutrophils alone, in terms of increased adhesion molecule expression, greater phagocytotic activity, production of toxic oxygen radicals and expression of pro-inflammatory cytokines, as interleukins – 1β, -6 and -8, tumor necrosis factor (TNF-α) which amplifies the inflammatory response and generates the immunothrombotic procedure (figure 1) (21). Indeed, levels of proinflammatory cytokines increase rapidly after initiation of ECMO circulation, contributing to endothelial damage (22).
2.3. Role of platelets
Platelets adhesion to the artificial «endothelium» of surfaces of ECMO circuit, plays an orchestrate role in immunothrombosis procedure. Severe thrombocytopenia (<50x109/L) is common in patients on ECMO, requiring frequent transfusions, independently of the duration of the extracorporeal circulation (23). Additionally, shear stress caused by blood passing through the ECMO circuit, results on impaired platelets’ aggregation and loss of VWF multimers (24). This phenomenon has been described 15 minutes after extracorporeal circuit initiation and can last during the whole procedure. Reduced glycoprotein (GP) Ibα and GPVI levels, which are receptors for VWF and collagen respectively, are implicated in the platelets dysfunction (25).
Platelets’ dysfunction is confirmed by lower aggregation demonstrated by light transmission aggregometry, and consequently results in haemorrhagic complications (26). Additionally, microthrombi might be produced within surfaces of the ECMO circuit, and subsequently be embolized in vital organs. Neurologic complications have been reported in patients during extracorporeal circulation (27).
Platelet derived microparticles (MPs) might also be implicated in EAC. MPs are shed from precursor cells after several triggering factors, like inflammation or shear stress caused by extracorporeal blood circulation (28).They preserve the lipid bilayers of parent cells, while they contain proteins, ribosomal RNA, messenger RNA and microRNA (29). These mediators are secreted by MPs towards various target cells, generating intercellular information exchange. MPs’ membranes contain large quantities of phosphatidyloserine, providing a suitable environment for the activation of the coagulation cascade(30).
2.3. Tissue factor (TF)
TF plays a constitutional role in the activation of coagulation, which is tightly correlated with the inflammatory process (31).It is a membrane protein expressed in the fibroblasts of the adventitia of vessels, but it is also distributed by epithelial cells, endothelial cells, platelets and monocytes, particularly under inflammatory conditions. According to the cell-based model of coagulation, TF is released into the bloodstream and interacts with the proteases of the coagulation cascade. Therefore, TF binds to Factor VII, while the TF: Factor VIIa complex consequently activates Factor X. Factor Xa and Factor Va constitute the prothrombinase complex, in the presence of calcium and phospholipid surface provided by activated platelets. The prothrombinase complex activates prothrombin to thrombin. Thrombin leads to the ample formation of fibrin and microthrombi. There are several reports shown an increased expression of TF in monocytes in biosurfaces of ECMO circuits in vitro, resulting in a procoagulant effect (32,33).
2.4. Contact pathway and Von Willebrand factor
Contact pathway activation might also contribute to EAC, as it has been shown in neonates on ECMO, in whom, kallikrein inhibitory capacity was decreased, while thrombin-antithrombin complexes were present (34). Reduced levels of FXII and increased levels of FXIIa and precallikreine have been demonstrated in patients on ECMO (35,36). Other coagulation factors might be compromised during ECMO, contributing to bleeding or thrombotic diathesis: Level of FVIII were decreased on animal models in ECMO (37), in parallel with a decrease in fibrinogen and VWF. Decreased thrombin generation associated with decreased levels of factor X and prothrombin has been described in patients on extracorporeal circulation (38), while in ECMO patients with severe hemorrhagic complications, decreased levels of factors VII and X were measured (39).
Acquired Von Willebrand disease is also implicated in the pathophysiology of EAC. High molecular weight polymers of VWF are cleaved by metalloproteinases, due to shear stress caused by ECMO circulation. Decreased levels of large polymers of vWF have been demonstrated in patients of venoarterial or venovenous ECMO, within one day of extracorporeal circulation (40). Therefore, platelet adhesion, a process promoted by vWF, is compromised, resulting in bleeding complications, especially from respiratory system, puncture sites and mucosa (41).vWF levels normalize, in 24 hours after the end of ECMO treatment(42).
2.5. Complement system
Activation of the complement system contributes to the development of EAC. Complement pathway is activated after binding of immunoglobulin on the artificial «endothelium» of ECMO circuit. The excessive complement cascade activation which cannot be supressed, as the normal endothelial regulatory mechanisms are absent, causes capillary leak syndrome and inflammatory response (17,39). Complement degradation leads to the production of anaphylatoxins, which contribute to the subsequent inflammatory reaction. Moreover, anaphylatoxins might bind to ECMO circuit, participating in the immunothrombosis procedure (FR). Complement degradation products contribute to the adhesion of granulocytes and monocytes to ECMO surfaces, as a result of activation of respective receptors (46). Moreover, anaphylatoxins pass in the systemic circulation, interacting with proteases of the coagulation cascade, platelets and endothelial cells. Consequently, activation of platelets and endothelial cells, increased expression of TF and further vWF production, play important role in coagulation disorders (48,49).
2.6. Fibrinolysis
Derangement of fibrinolysis might also be involved in EAC, despite the lack of sufficient data. Thrombin generation and several other situations as hypoxia, trauma or sepsis, trigger the release of tissue plasminogen activator (t-PA) which activates plasminogen to plasmin, leading to fibrin degradation. In ECMO patients, a decrease on t-PA and plasminogen activator inhibitor (PAI-1) levels is observed, followed by a respective increase of both (43). Hyperfibrinolysis and hypofibrinaemia has been observed in patients on extracorporeal circulation, while the administration of antifibrinolytic agents might reduce the subsequent hemorrhagic complications (44). Moreover, hypofibrinogenaemia and increased levels of fibrin degradation products in ECMO patients, might be indicative of oxygenator induced hyperfibrinolysis, requiring circuit exchange.
2.7. Hemolysis
Finally, haemolysis is also implicated in EAC. Increased levels of free plasma heamoglobulin have been measured in 67% of ECMO patients, while levels higher than 50 mg/dL are associated with increased mortality (45). The presence of free haemoglobulin contributes to prothrombotic state, by binding nitric oxide (NO). Decreased levels of NO result to vasoconstriction and further platelet activation and adhesion (46). Moreover, hemolysis leads to the release of red blood cells derived microparticles, which also exert prothrombotic effects (47).
3. Monitoring of coagulation profile of ECMO patients
There are no current guidelines defining the optimal method of monitoring the overall hemostatic profile of patients on extracorporeal circulation. Moreover, as monitoring of heparin effect is performed in vitro, it does not take into consideration the in vivo interaction between endothelium, coagulation cascade and oxygen membrane that leads to EAC. The methods used for assessing EAC and heparin effect are plasma based or whole blood based. Plasma based tests do not take into consideration platelets΄ dysfunction or impairment of clot formation. Viscoelastic tests as thromboelastography and thromboelastometry are whole blood tests that dynamically estimate clot formation, although not broadly available and not easily interpreted. The advantages and disadvantages of monitoring technics for EAC and anticoagulation therapy, are depicted in table 1.
3.1. Activated clotting time (ACT)
ACT is a whole blood, point - of - care assay, traditionally used for assessment of anticoagulant effect of heparin, in patients on cardiopulmonary bypass (48). However, many confounding factors especially in the critical care setting, might affect the accuracy of ACT as an assay for monitoring heparin effect on ECMO patients. These include hypothermia, inflammation, liver failure, thrombocytopenia (49). In a recent retrospective analysis of 604 children on ECMO, ACT values did not show significant correlation with heparin dose (50). Therefore, monitoring heparin effectiveness by ACT might lead to suboptimal coagulation assessment and subsequent adjustment of anticoagulants dose, in critically ill patients.
3.2. Activated Partial Thromboplastin Time (aPTT) and anti-Factor Xa level
aPTT is a plasma based assay, measured by adding calcium to plasma, after exposure to a contact activator (51). There are no randomized controlled studies on target levels of aPTT in ECMO patients, despite the fact that aPTT 1.5 to 2.5 times patient’s baseline, has historically been correlated with a decreased risk of thromboembolism (52). Therefore, therapeutic aPTT range is set to 1.5-2.5 times patient’s baseline aPTT, although this was not validated in randomized controlled studies, in ECMO patients. Moreover, in critically ill patients, acute phase proteins and elevated fibrinogen might diminish aPTT value, reflecting coagulation abnormalities rather than the clear effect of heparin. A retrospective study in 149 patients on ECMO, demonstrated the significant correlation between higher aPTT values and the risk of bleeding (53). Given the variability of aPTT measurements, monitoring of anticoagulant effect of heparin is often performed by the anti-Factor Xa assay.
Anti-Xa is a plasma based test that measures heparin effect, by evaluating the ability of heparin to catalyse antithrombin’s inhibition of factor Xa activity. Therefore, anti-Xa directly measures heparin effect, without evaluating the overall coagulation profile of ECMO patients, in the pathophysiology of which, several other procoagulant factors are implicated. Thus, as aPTT is affected by heparin dose and other coagulation factors, it may better predict bleeding risk due to EAC, as compared to anti-Xa. This was well indicated in a retrospective study including 34 ECMO patients, in whom anti-Xa and/or aPTT was measured. High aPTT values were predictive of bleeding, while low anti-Xa values were associated with thrombosis (54). Moreover, a better correlation between heparin dose and anti-Xa was observed, as compared to aPTT. Therefore, an accurate evaluation of bleeding and thrombotic risk should be based on a combination of aPTT and anti-Xa measurement. Moreover, anti-Xa levels are not influenced by the presence of lupus anticoagulant, liver disease and consumptive coagulation disorders (55).
3.3. Viscoelastic hemostatic assays
Thromboelastography (TEG) and thromboelastometry (ROTEM, ClotPro®) are whole blood, point of care assays, providing a global assessment of the viscoelastic properties of the clot, including clot initiation (evaluating coagulation factors and heparin effect), amplitude (evaluating platelets and fibrinogen) and stability (fibrinolysis). Multiple assays are available for each device, to evaluate the extrinsic and intrinsic coagulation pathway, the contribution of fibrinogen in clot formation and heparinase assays to inhibit heparin effect. ROTEM and TEG are recommended in surgical or trauma patients, for the achievement of goal directed transfusion therapy during bleeding (56).However, there are less data regarding the use of viscoelastic assays in ECMO patients. According to recent studies, TEG or ROTEM showing hypercoagulability, might predict thrombotic complications (57), while a reduced maximum clot firmness (MCF) has been associated with bleeding (58). Moreover, in case studies, ROTEM has been used for the differential diagnosis of EAC and the documentation of hyperfibrinolysis, a phenomenon that might impose ECMO circuit exchange (59). In a study including 57 patients, it was shown that V-V ECMO therapy led to a progressive increase of clotting time (CT) and decrease of MCF, while ROTEM assays did not help to the prediction of haemorrhage (60). A randomized controlled study comparing a TEG-based protocol to a aPTT-based one to assess heparin anticoagulation, demonstrated that TEG can be safely used for monitoring coagulation on ECMO patients (61). ClotPro® has been also used for the assessment of EAC in COVID-19 patients on ECMO support (62). More studies are required to determine the exact role of the viscoelastic assays to the monitoring of EAC and to the consequent therapeutic interventions.
3.4. D-Dimers, platelets and antithrombin
Fibrin degradation products, including D-Dimers, are formed after fibrin formation and degradation. Although D-Dimers’ specificity is low, daily measurement is recommended in ECMO patients for the early diagnosis of thrombosis of membrane oxygenator. Low fibrinogen levels, rising D-Dimers and elevated free hemoglobulin might indicate possible thrombosis of circuit, requiring prompt oxygenator change.
Platelets should also be measured on daily routine, as a gradual thrombocytopenia might be heparin induced (HIT). HIT has been described in 4-7% of ECMO patients (63). In COVID-19 patients under ECMO support, the reported incidence of HIT is higher (10.52%), contributing to the thrombotic complications (64). In case of suspected HIT, heparin administration should be stopped. A non-heparin anticoagulant should be administered and enzyme-linked immunosorbent assay for antiplatelet factor 4 antibodies may be performed, to confirm HIT.
Light transmission aggregometry (LTA) is the gold standard assay for the assessment of platelet aggregation. Multiple electrode aggregometry (MEA) has been used as a point-of-care technique for the evaluation of platelets aggregation in patients undergoing cardiopulmonary by pass (65). Platelet aggregation is usually impaired in patients on ECMO, as it has been shown in experimental models of artificial circuit and in small studies in humans (66). However, ECMO-related thrombocytopenia might influence the accurate assessment of platelet function. Moreover, hemolysis might also compound platelet aggregation evaluation (67). Bleeding complications in ECMO patients might be caused by impaired platelet aggregation, confirmed by LTA (68). Despite the normal count of platelets, transfusions and administration of tranexamic acid should be considered.
Antithrombin (AT) deficiency may result in «heparin resistance», as AT is required as a cofactor for heparin to achieve the antithrombotic effect. The role of AT in coagulation procedure consists to the inhibition of thrombin and activation of factor X, after forming a complex with thrombin. Interestingly, AT administration to patients on V-V ECMO was associated with a more rapid decrease of pro-inflammatory cytokines compared to placebo, demonstrating the anti-inflammatory effect of AT, which might be independent of its anticoagulative results (69). The anti-inflammatory effect of AT is contributed to prostacyclin’s release, to the reduction of the activity of P-selectin and to the prevention of activation of leukocytes, resulting to decrease of pro-inflammatory cytokines. However, there is not adequate evidence for recommending routine AT monitoring and subsequent supplementation in patients on ECMO.
4. Recommendations
There is paucity of evidence on optimal anticoagulation management in ECMO patients. Current recommendations are based on retrospective studies, demonstrating the safety of conservative anticoagulation strategies or even no anticoagulation, especially in V-V ECMO patients (70). Several studies have shown that heparin based anticoagulation targeting over 1.5 times patients’ baseline aPTT, resulted to more bleeding events, without reducing mortality and thrombotic complications (71) (72). According to a recent randomized study, low heparin dose (aiming at aPTT <45s) compared to the usual dose (aPTT 50-70s), was associated with a significant decrease of aPTT and anti-Xa, while the rate of bleeding or thrombotic complications remained the same (73). A recent retrospective analysis of all ECMO patients in a single centre, during eight years, showed that high level of coagulation (aPTT ≥55 sec) was significantly associated with fatal bleeding events, without reducing the rate of thrombotic complications (74). A meta-analysis of 7 studies comparing the safety and effectiveness of low dose anticoagulation strategy to standard dose in ECMO patients, confirmed that low dose was associated with less haemorrhagic gastrointestinal and surgical site complications, while the mortality and the rate of thrombotic events and intracranial haemorrhage was similar between the two groups of patients (75).
Another recent meta -analysis of 17 studies regarding the use of aPTT for anticoagulation monitoring in ECMO patients, demonstrated that bleeding complications were observed in patients with longer aPTT, longer ECMO support and higher mortality. However, the study did not confirm any association between aPTT threshold and risk of haemorrhage (76). Moreover, the complex mechanism of EAC resulting in impaired hemostasis, renders the concept of ‘anticoagulation free’ ECMO support, feasible and reasonable. Indeed, there are several retrospective studies demonstrating the safety of no use of anticoagulation (77). A recent systemic review on ECMO support without therapeutic anticoagulation, demonstrated that the incidence of thrombosis was comparable to ECMO patients who traditionally received systemic anticoagulation, while the adverse events profile remained similar (78). Given the paucity of robust data on ideal anticoagulation strategy, ongoing studies (RATE, SAFE-ECMO, A FREE ECMO) aim to confer individualized recommendations for adult patients under ECMO support.
Regarding blood products transfusion strategy, there is still lack of evidence. According to the current ELSO recommendations, hemoglobulin concentration should be 14-15 g/dL, while platelets’ transfusion should be considered, in order to maintain platelet levels > 100000 x 109/L in a bleeding patient and >50000 x 109/L in a non bleeding patient (79). However, more conservative transfusion strategies have been proposed, given the risk of fluid overload, which is strongly associated with mortality. According to a recent survey, an hemoglobulin transfusion threshold of 8-10g/dL has been used in most centres, while platelets transfusion threshold was 50000 x 109/L and fibrinogen’s threshold was 2 g/L (80). Lower thresholds are commonly considered in non-bleeding patients.
Severe hemolysis is strongly associated with circuit thrombosis, and can be easily recognized by measuring free hemoglobulin plasma concentration, which is implicated in acute renal failure and mortality (81). Other markers of hemolysis and subsequent requirement of circuit exchange, are D-Dimers and platelets’ count (82). Practice current recommendations on aPTT and anti-Xa target levels and on transfusion strategies are depicted in table 2.
5. Future perspectives
There are limited data on other point of care tests to assess EAC and monitor heparin activity. PFA-100 is a quite sensitive diagnostic test for von Willebrand disease, although it has not been validated in ECMO patients (83). Moreover, a mass spectrometry method has recently been used for the measurement of vWF cleavage, in patients with left ventricular assist devices (84). Global thrombosis procedure has also been assessed by a point of care test, which identifies prothrombotic risk caused by impaired fibrinolysis. The test has been used in acute coronary syndromes and might also be useful in ECMO patients, as it evaluates the whole coagulation procedure under conditions of shear stress caused by extracorporeal circulation (85,86). Finally, thrombin generation test is based on the stimulation of coagulation by exogenous tissue factor. The value of subsequent endogenous thrombin potential (ETP) in ECMO patients, might be associated with the probability of venous thrombosis or haemorrhage according to small studies (87,88). Interestingly, there are several reports on heparin free V-V ECMO especially as a bridge to lung transplantation in order to avoid perioperative bleeding caused by coagulopathy (89). This assessment is based on the administration of AT during the perioperative period, the use of heparinized ECMO circuits and the control of fibrinolysis with tranexamic acid. More clinical studies are required, in order to elucidate the role of these tests in the early identification of bleeding or prothrombotic risk in ECMO patients and in the subsequent management of anticoagulant therapy.
6. Conclusions
EAC is a multifactorial syndrome associated with thrombotic or bleeding complications, contributing to morbidity and mortality. Despite the evolution of circuit and oxygenator technology, extracorporeal circulation of blood is associated with activation of haemostasis, while the shear stress caused by pumps, inevitably leads to haemolysis and damage of large protein molecules. Activation of intrinsic and extrinsic coagulation pathway, immunothrombosis and haemolysis are associated with circuit thrombosis and systemic thromboembolism. Fibrinolysis, decrease of fibrinogen and acquired Von Willebrand syndrome combined with the administration of therapeutic anticoagulation, might lead to haemorrhagic complications. Point of care viscoelastic analyses including thromboelastometry, as well as conventional coagulations tests, help to the identification of coagulation disorders and to subsequent goal directed transfusion therapy. More studies are required for the further understanding of the pathophysiology of EAC and for the development of treatment algorithms, in order to improve ECMO outcomes in patients with refractory hypoxemia or end-stage lung disease.
Figure 1.
ECMO associated coagulopathy: The exposure of blood to the artificial endothelium consisted of the biosurface of the oxygenator membrane, consequentl leads to the activation of coagulation factors, complement system, platelets and Von Willebrand factor (VWf) and fibrinolysis. TF is released into the bloodstream and interacts with the proteases of the coagulation cascade, while the prothrombinase complex activates prothrombin to thrombin. Thrombin leads to the ample formation of fibrin and microthrombi. The major pathological role of coagulation in the innate host response is defined as immunothrombosis. Therefore, platelets accumulate at the site of artificial «endothelium» of biosurface. Their activation leads to the release of the content of their granules, consisting of immunomodulatory mediators, antimicrobial peptides (AMPs) and microparticles (MPs). These molecules contribute to the migration of neutrophils and activation of complement.Derangement of fibrinolysis and acquired Von Willebrand disease might also be involved in EAC.High molecular weight polymers of VWF are cleaved by metalloproteinases, due to shear stress caused by ECMO circulation.Haemolysis is also implicated in EAC, as free hemoglobulin contributes to the prothrombotic state.
Figure 1.
ECMO associated coagulopathy: The exposure of blood to the artificial endothelium consisted of the biosurface of the oxygenator membrane, consequentl leads to the activation of coagulation factors, complement system, platelets and Von Willebrand factor (VWf) and fibrinolysis. TF is released into the bloodstream and interacts with the proteases of the coagulation cascade, while the prothrombinase complex activates prothrombin to thrombin. Thrombin leads to the ample formation of fibrin and microthrombi. The major pathological role of coagulation in the innate host response is defined as immunothrombosis. Therefore, platelets accumulate at the site of artificial «endothelium» of biosurface. Their activation leads to the release of the content of their granules, consisting of immunomodulatory mediators, antimicrobial peptides (AMPs) and microparticles (MPs). These molecules contribute to the migration of neutrophils and activation of complement.Derangement of fibrinolysis and acquired Von Willebrand disease might also be involved in EAC.High molecular weight polymers of VWF are cleaved by metalloproteinases, due to shear stress caused by ECMO circulation.Haemolysis is also implicated in EAC, as free hemoglobulin contributes to the prothrombotic state.
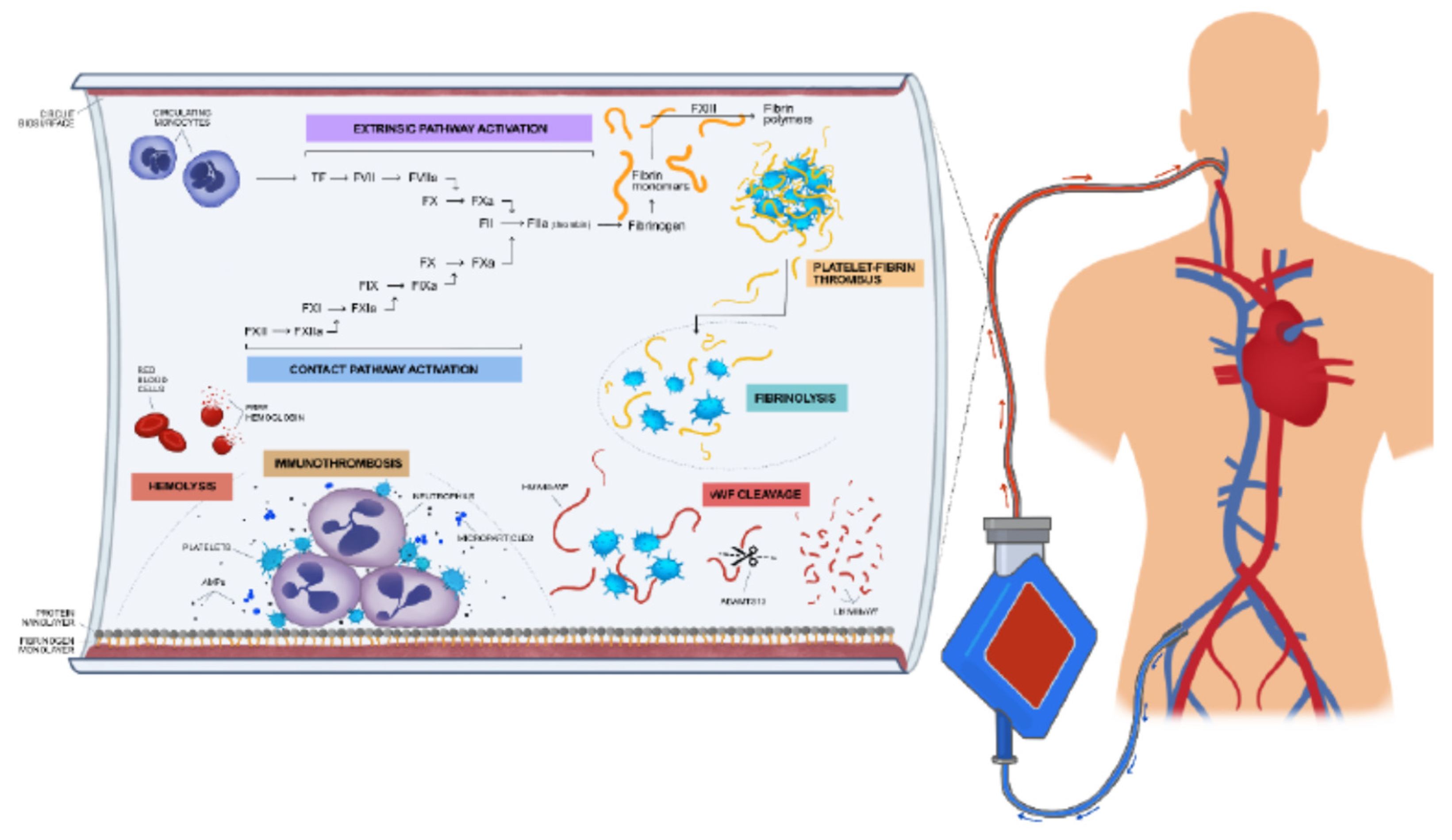
Table 1.
Tests for coagulation monitoring in ECMO patients.
Table 1.
Tests for coagulation monitoring in ECMO patients.
| TEST |
ADVANTAGES |
DISADVANTAGES |
| ACT |
-Point of care
-Whole blood
-Useful in CABG |
-Confounding factors: hypothermia, inflammation, liver failure, thrombocytopenia
-Suboptimal coagulation assessment in critically ill |
| aPTT |
-Plasma based
-High levels predict bleeding risk |
-Confounding factors in critically ill: fibrinogen levels, acute phase proteins
-Variability between laboratories
-Time delay |
| Anti-Xa |
-Plasma based
-Direct measurement of heparin effect
-Low levels predict thrombosis risk
-Not influenced by liver disease, consumptive coagulopathy, lupus anticoagulant |
-Expensive
-Time delay |
| Viscoelastic assays |
-Point of care
-Whole blood
-Information about hypercoagulability, hyperfibrinolysis
-Differential diagnosis of EAC |
-Expensive
-Not widely available
-Limited data on ECMO patients |
Table 2.
Anticoagulation and blood product goals during ECMO support.
Table 2.
Anticoagulation and blood product goals during ECMO support.
| aPTT |
1.5 to 2 patient’s baseline |
| Anti-Xa |
0.3-0.7 U/mL |
| Hemoglobin |
>7-9 g/dL |
| Fibrinogen |
>1.5-2 g/L |
| Platelets |
≥100000 x 109/L in a bleeding patient
≥50000 x 109/L in a bleeding patient
|
Author Contributions
FF and IT are responsible for the paper conception and writing. AT performed the laboratory tests of coagulation. FF, IT, DK, MR, NK, VK, GP, VK, IK and DA contributed to the treatment of the two patients. All the authors reviewed, revised the manuscript and approved the final version.
Funding
“This research received no external funding” .
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Mr. Antonis Makriyannis for his excellent artwork (figure 1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazzeffi M, Greenwood J, Tanaka K, Menaker J, Rector R, Herr D; et al. Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg. 2016 Feb;101(2):682–9. [CrossRef]
- Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J; et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014 Feb;97(2):610–6. [CrossRef]
- Treml B, Breitkopf R, Bukumirić Z, Bachler M, Boesch J, Rajsic S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J Clin Med. 2022 Feb 24;11(5). [CrossRef]
- Nguyen TP, Phan XT, Nguyen TH, Huynh DQ, Tran LT, Pham HM; et al. Major Bleeding in Adults Undergoing Peripheral Extracorporeal Membrane Oxygenation (ECMO): Prognosis and Predictors. Crit Care Res Pract. 2022;2022:5348835. [CrossRef]
- Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front Med. 2018;5:352. [CrossRef]
- Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J Am Soc Artif Intern Organs 1992. 2013 Jun;59(3):202–10. [CrossRef]
- Kanji R, Vandenbriele C, Arachchillage DRJ, Price S, Gorog DA. Optimal Tests to Minimise Bleeding and Ischaemic Complications in Patients on Extracorporeal Membrane Oxygenation. Thromb Haemost. 2022 Apr;122(4):480–91. [CrossRef]
- Willers A, Swol J, van Kuijk SMJ, Buscher H, McQuilten Z, Ten Cate H; et al. HEROES V-V-HEmorRhagic cOmplications in Veno-Venous Extracorporeal life Support-Development and internal validation of multivariable prediction model in adult patients. Artif Organs. 2022 May;46(5):932–52. [CrossRef]
- Zakhary B, Vercaemst L, Mason P, Lorusso R, Brodie D. How I manage drainage insufficiency on extracorporeal membrane oxygenation. Crit Care Lond Engl. 2020 Apr 15;24(1):151. [CrossRef]
- Yusuff H, Zochios V, Brodie D. Thrombosis and coagulopathy in COVID-19 patients rceiving ECMO: A narrative review of current literature. J Cardiothorac Vasc Anesth. 2022 Aug;36(8 Pt B):3312–7. [CrossRef]
- Yang X, Cai S, Luo Y, Zhu F, Hu M, Zhao Y; et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome: A Multicenter Descriptive Study. Crit Care Med. 2020 Sep;48(9):1289–95. [CrossRef]
- Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir Med. 2020 Nov;8(11):1121–31. [CrossRef]
- Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018 May 24;378(21):1965–75. [CrossRef]
- Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F; et al. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020 Jul 14;142(2):184–6. [CrossRef]
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–7. [CrossRef]
- Nunez JI, Gosling AF, O’Gara B, Kennedy KF, Rycus P, Abrams D; et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: An ELSO registry analysis. Intensive Care Med. 2022 Feb;48(2):213–24. [CrossRef]
- Schmid E, Krajewski S, Bachmann D, Kurz J, Wendel HP, Rosenberger P; et al. The volatile anesthetic sevoflurane inhibits activation of neutrophil granulocytes during simulated extracorporeal circulation. Int Immunopharmacol. 2012 Oct;14(2):202–8. [CrossRef]
- Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980 Jan;55(1):156–9.
- Turbill P, Beugeling T, Poot AA. Proteins involved in the Vroman effect during exposure of human blood plasma to glass and polyethylene. Biomaterials. 1996 Jul;17(13):1279–87. [CrossRef]
- Frantzeskaki F, Armaganidis A, Orfanos SE. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respir Int Rev Thorac Dis. 2017;93(3):212–25. [CrossRef]
- Wilm J, Philipp A, Müller T, Bredthauer A, Gleich O, Schmid C; et al. Leukocyte Adhesion as an Indicator of Oxygenator Thrombosis During Extracorporeal Membrane Oxygenation Therapy? ASAIO J Am Soc Artif Intern Organs 1992. 2018 Feb;64(1):24–30. [CrossRef]
- Fortenberry JD, Bhardwaj V, Niemer P, Cornish JD, Wright JA, Bland L. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J Pediatr. 1996 May;128(5 Pt 1):670–8. [CrossRef]
- Abrams D, Baldwin MR, Champion M, Agerstrand C, Eisenberger A, Bacchetta M; et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: A cohort study. Intensive Care Med. 2016 May;42(5):844–52. [CrossRef]
- Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA; et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost JTH. 2016 Nov;14(11):2253–60. [CrossRef]
- Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA; et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost JTH. 2016 Nov;14(11):2253–60. [CrossRef]
- Kalbhenn J, Schlagenhauf A, Rosenfelder S, Schmutz A, Zieger B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2018 Aug;37(8):985–91. [CrossRef]
- Harrison MJ, Pugsley W, Newman S, Paschalis C, Klinger L, Treasure T; et al. Detection of middle cerebral emboli during coronary artery bypass surgery using transcranial Doppler sonography. Stroke. 1990 Oct;21(10):1512. [CrossRef]
- Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol Baltim Md 1950. 2011 Aug 15;187(4):1856–65. [CrossRef]
- Lim MY, Ataga KI, Key NS. Hemostatic abnormalities in sickle cell disease. Curr Opin Hematol. 2013 Sep;20(5):472–7. [CrossRef]
- Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006 Dec;116(12):3211–9. [CrossRef]
- Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost. 2006 Feb;32(1):33–9. [CrossRef]
- Kappelmayer J, Bernabei A, Edmunds LHJ, Edgington TS, Colman RW. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ Res. 1993 May;72(5):1075–81. [CrossRef]
- Fischer M, Sperling C, Tengvall P, Werner C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials. 2010 Mar;31(9):2498–507. [CrossRef]
- Plötz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993 May;105(5):823–32. [CrossRef]
- Boisclair MD, Lane DA, Philippou H, Esnouf MP, Sheikh S, Hunt B; et al. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood. 1993 Dec 1;82(11):3350–7.
- Wendel HP, Jones DW, Gallimore MJ. FXII levels, FXIIa-like activities and kallikrein activities in normal subjects and patients undergoing cardiac surgery. Immunopharmacology. 1999 Dec;45(1–3):141–4. [CrossRef]
- Passmore MR, Fung YL, Simonova G, Foley SR, Diab SD, Dunster KR; et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit Care Lond Engl. 2017 Jul 29;21(1):191. [CrossRef]
- Davidson SJ, Burman JF, Philips SM, Onis SJ, Kelleher AA, De Souza AC; et al. Correlation between thrombin potential and bleeding after cardiac surgery in adults. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2003 Feb;14(2):175–9. [CrossRef]
- Kreyer S, Muders T, Theuerkauf N, Spitzhüttl J, Schellhaas T, Schewe JC; et al. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: A retrospective data analysis. J Thorac Dis. 2017 Dec;9(12):5017–29. [CrossRef]
- Granja T, Hohenstein K, Schüssel P, Fischer C, Prüfer T, Schibilsky D; et al. Multi-Modal Characterization of the Coagulopathy Associated With Extracorporeal Membrane Oxygenation. Crit Care Med. 2020 May;48(5):e400–8. [CrossRef]
- Kalbhenn J, Schmidt R, Nakamura L, Schelling J, Rosenfelder S, Zieger B. Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J Atheroscler Thromb. 2015;22(3):265–71. [CrossRef]
- Kalbhenn J, Schlagenhauf A, Rosenfelder S, Schmutz A, Zieger B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2018 Aug;37(8):985–91. [CrossRef]
- McVeen RV, Lorch V, Carroll RC, Goldberg L, Keszler M, Podlasek S; et al. Changes in fibrinolytic factors in newborns during extracorporeal membrane oxygenation (ECMO). Am J Hematol. 1991 Nov;38(3):254–5. [CrossRef]
- Hunt BJ, Parratt RN, Segal HC, Sheikh S, Kallis P, Yacoub M. Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg. 1998 Mar;65(3):712–8. [CrossRef]
- Omar HR, Mirsaeidi M, Socias S, Sprenker C, Caldeira C, Camporesi EM; et al. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE. 2015;10(4):e0124034. [CrossRef]
- Da Q, Teruya M, Guchhait P, Teruya J, Olson JS, Cruz MA. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: Implications for circulatory devices. Blood. 2015 Nov 12;126(20):2338–41. [CrossRef]
- Van Der Meijden PEJ, Van Schilfgaarde M, Van Oerle R, Renné T, ten Cate H, Spronk HMH. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost JTH. 2012 Jul;10(7):1355–62. [CrossRef]
- Gorog DA, Price S, Sibbing D, Baumbach A, Capodanno D, Gigante B; et al. Antithrombotic therapy in patients with acute coronary syndrome complicated by cardiogenic shock or out-of-hospital cardiac arrest: A joint position paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J Cardiovasc Pharmacother. 2021 Mar 15;7(2):125–40. [CrossRef]
- Vandenbriele C, Vanassche T, Price S. Why we need safer anticoagulant strategies for patients on short-term percutaneous mechanical circulatory support. Intensive Care Med. 2020 Apr;46(4):771–4. [CrossRef]
- Baird CW, Zurakowski D, Robinson B, Gandhi S, Burdis-Koch L, Tamblyn J; et al. Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007 Mar;83(3):912–9; discussion 919-920. [CrossRef]
- McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J Am Soc Artif Intern Organs 1992. 2022 Mar 1;68(3):303–10. [CrossRef]
- Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972 Aug 17;287(7):324–7. [CrossRef]
- Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016 Dec;6(1):97. [CrossRef]
- Arnouk S, Altshuler D, Lewis TC, Merchan C, Smith DE 3rd, Toy B; et al. Evaluation of Anti-Xa and Activated Partial Thromboplastin Time Monitoring of Heparin in Adult Patients Receiving Extracorporeal Membrane Oxygenation Support. ASAIO J Am Soc Artif Intern Organs 1992. 2020 Mar;66(3):300–6. [CrossRef]
- Raghunathan V, Liu P, Kohs TCL, Amirsoltani R, Oakes M, McCarty OJT; et al. Heparin Resistance Is Common in Patients Undergoing Extracorporeal Membrane Oxygenation but Is Not Associated with Worse Clinical Outcomes. ASAIO J Am Soc Artif Intern Organs 1992. 2021 Aug 1;67(8):899–906. [CrossRef]
- Boer C, Meesters MI, Milojevic M, Benedetto U, Bolliger D, von Heymann C; et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018 Feb;32(1):88–120. [CrossRef]
- McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J Am Soc Artif Intern Organs 1992. 2022 Mar 1;68(3):303–10. [CrossRef]
- Nair P, Hoechter DJ, Buscher H, Venkatesh K, Whittam S, Joseph J; et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. 2015 Apr;29(2):288–96. [CrossRef]
- Durila M, Smetak T, Hedvicak P, Berousek J. Extracorporeal membrane oxygenation-induced fibrinolysis detected by rotational thromboelastometry and treated by oxygenator exchange. Perfusion. 2019 May;34(4):330–3. [CrossRef]
- Hellmann C, Schmutz A, Kalbhenn J. Bleeding during veno-venous ECMO cannot reliably be predicted by rotational thrombelastometry (ROTEMTM). Perfusion. 2018 May;33(4):289–96. [CrossRef]
- Panigada M, E Iapichino G, Brioni M, Panarello G, Protti A, Grasselli G; et al. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: A safety and feasibility pilot study. Ann Intensive Care. 2018 Jan 16;8(1):7. [CrossRef]
- Bareille M, Hardy M, Douxfils J, Roullet S, Lasne D, Levy JH; et al. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J Clin Med. 2021 Apr 16;10(8). [CrossRef]
- Arachchillage DRJ, Laffan M, Khanna S, Vandenbriele C, Kamani F, Passariello M; et al. Frequency of Thrombocytopenia and Heparin-Induced Thrombocytopenia in Patients Receiving Extracorporeal Membrane Oxygenation Compared With Cardiopulmonary Bypass and the Limited Sensitivity of Pretest Probability Score. Crit Care Med. 2020 May;48(5):e371–9. [CrossRef]
- Arachchillage DJ, Rajakaruna I, Scott I, Gaspar M, Odho Z, Banya W; et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br J Haematol. 2022 Feb;196(3):566–76. [CrossRef]
- Ranucci M, Colella D, Baryshnikova E, Di Dedda U. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth. 2014 Dec;113(6):970–6. [CrossRef]
- Van Poucke S, Stevens K, Kicken C, Simons A, Marcus A, Lancé M. Platelet Function During Hypothermia in Experimental Mock Circulation. Artif Organs. 2016 Mar;40(3):288–93. [CrossRef]
- Sniderman J, Monagle P, Annich GM, MacLaren G. Hematologic concerns in extracorporeal membrane oxygenation. Res Pract Thromb Haemost. 2020 May;4(4):455–68. [CrossRef]
- Balle CM, Jeppesen AN, Christensen S, Hvas AM. Platelet Function During Extracorporeal Membrane Oxygenation in Adult Patients: A Systematic Review. Front Cardiovasc Med. 2018;5:157. [CrossRef]
- Panigada M, Spinelli E, De Falco S, Consonni D, Novembrino C, Boscolo Anzoletti M; et al. The relationship between antithrombin administration and inflammation during veno-venous ECMO. Sci Rep. 2022 Aug 22;12(1):14284. [CrossRef]
- Kurihara C, Walter JM, Karim A, Thakkar S, Saine M, Odell DD; et al. Feasibility of Venovenous Extracorporeal Membrane Oxygenation Without Systemic Anticoagulation. Ann Thorac Surg. 2020 Oct;110(4):1209–15. [CrossRef]
- Wood KL, Ayers B, Gosev I, Kumar N, Melvin AL, Barrus B; et al. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann Thorac Surg. 2020 May;109(5):1458–66. [CrossRef]
- Byun JH, Jang IS, Kim JW, Koh EH. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016 Sep;51(3):171–4. [CrossRef]
- Aubron C, McQuilten Z, Bailey M, Board J, Buhr H, Cartwright B; et al. Low-Dose Versus Therapeutic Anticoagulation in Patients on Extracorporeal Membrane Oxygenation: A Pilot Randomized Trial. Crit Care Med. 2019 Jul;47(7):e563–71. [CrossRef]
- Song K, Kim JB. Safety of low-dose anticoagulation in extracorporeal membrane oxygenation using the Permanent Life Support System: A retrospective observational study. J Yeungnam Med Sci. 2023 Jul;40(3):276–82. [CrossRef]
- Lv X, Deng M, Wang L, Dong Y, Chen L, Dai X. Low vs standardized dose anticoagulation regimens for extracorporeal membrane oxygenation: A meta-analysis. PLoS ONE. 2021;16(4):e0249854. [CrossRef]
- Rajsic S, Treml B, Jadzic D, Breitkopf R, Oberleitner C, Bachler M; et al. aPTT-guided anticoagulation monitoring during ECMO support: A systematic review and meta-analysis. J Crit Care. 2023 Oct;77:154332. [CrossRef]
- Wood KL, Ayers B, Gosev I, Kumar N, Melvin AL, Barrus B; et al. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann Thorac Surg. 2020 May;109(5):1458–66. [CrossRef]
- Olson SR, Murphree CR, Zonies D, Meyer AD, Mccarty OJT, Deloughery TG; et al. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J Am Soc Artif Intern Organs 1992. 2021 Mar 1;67(3):290–6. [CrossRef]
- McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J Am Soc Artif Intern Organs 1992. 2022 Mar 1;68(3):303–10. [CrossRef]
- Esper SA, Welsby IJ, Subramaniam K, John Wallisch W, Levy JH, Waters JH; et al. Adult extracorporeal membrane oxygenation: An international survey of transfusion and anticoagulation techniques. Vox Sang. 2017 Jul;112(5):443–52. [CrossRef]
- Omar HR, Mirsaeidi M, Socias S, Sprenker C, Caldeira C, Camporesi EM; et al. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE. 2015;10(4):e0124034. [CrossRef]
- Dornia C, Philipp A, Bauer S, Stroszczynski C, Schreyer AG, Müller T; et al. D-dimers Are a Predictor of Clot Volume Inside Membrane Oxygenators During Extracorporeal Membrane Oxygenation. Artif Organs. 2015 Sep;39(9):782–7. [CrossRef]
- Favaloro EJ. Utility of the platelet function analyser (PFA-100/200) for exclusion or detection of von Willebrand disease: A study 22 years in the making. Thromb Res. 2020 Apr;188:17–24. [CrossRef]
- Zhou Y, Qin S, Hilton T, Tang L, Cruz M, Hernandez R; et al. Quantification of Von Willebrand Factor Cleavage by adamts-13 in Patients Supported by Left Ventricular Assist Devices. ASAIO J Am Soc Artif Intern Organs 1992. 2017 Dec;63(6):849–53. [CrossRef]
- Gorog DA, Lip GYH. Impaired Spontaneous/Endogenous Fibrinolytic Status as New Cardiovascular Risk Factor?: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Sep 10;74(10):1366–75.
- Spinthakis N, Gue Y, Farag M, Ren G, Srinivasan M, Baydoun A; et al. Impaired endogenous fibrinolysis at high shear using a point-of-care test in STEMI is associated with alterations in clot architecture. J Thromb Thrombolysis. 2019 Apr;47(3):392–5. [CrossRef]
- Wexels F, Dahl OE, Pripp AH, Seljeflot I. Thrombin Generation in Patients With Suspected Venous Thromboembolism. Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2017 Jul;23(5):416–21. [CrossRef]
- Mazzeffi M, Strauss E, Meyer M, Hasan S, Judd M, Abuelkasem E; et al. Coagulation Factor Levels and Underlying Thrombin Generation Patterns in Adult Extracorporeal Membrane Oxygenation Patients. Anesth Analg. 2019 Sep;129(3):659–66. [CrossRef]
- Scaravilli V, Fumagalli J, Rosso L, Polli F, Panigada M, Abbruzzese C; et al. Heparin-Free Lung Transplantation on Venovenous Extracorporeal Membrane Oxygenation Bridge. ASAIO J Am Soc Artif Intern Organs 1992. 2021 Nov 1;67(11):e191–7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






