Submitted:
17 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
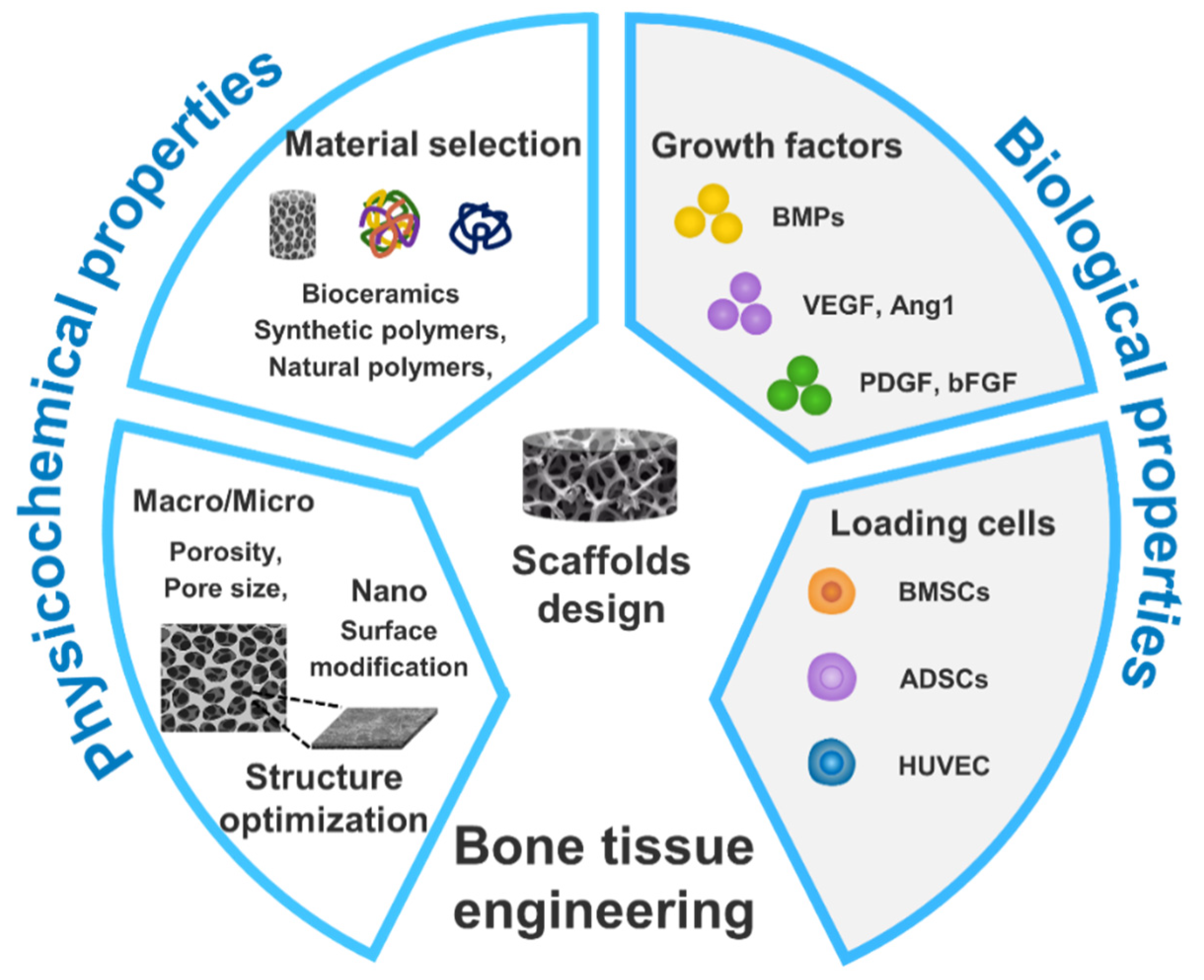
1. Introduction
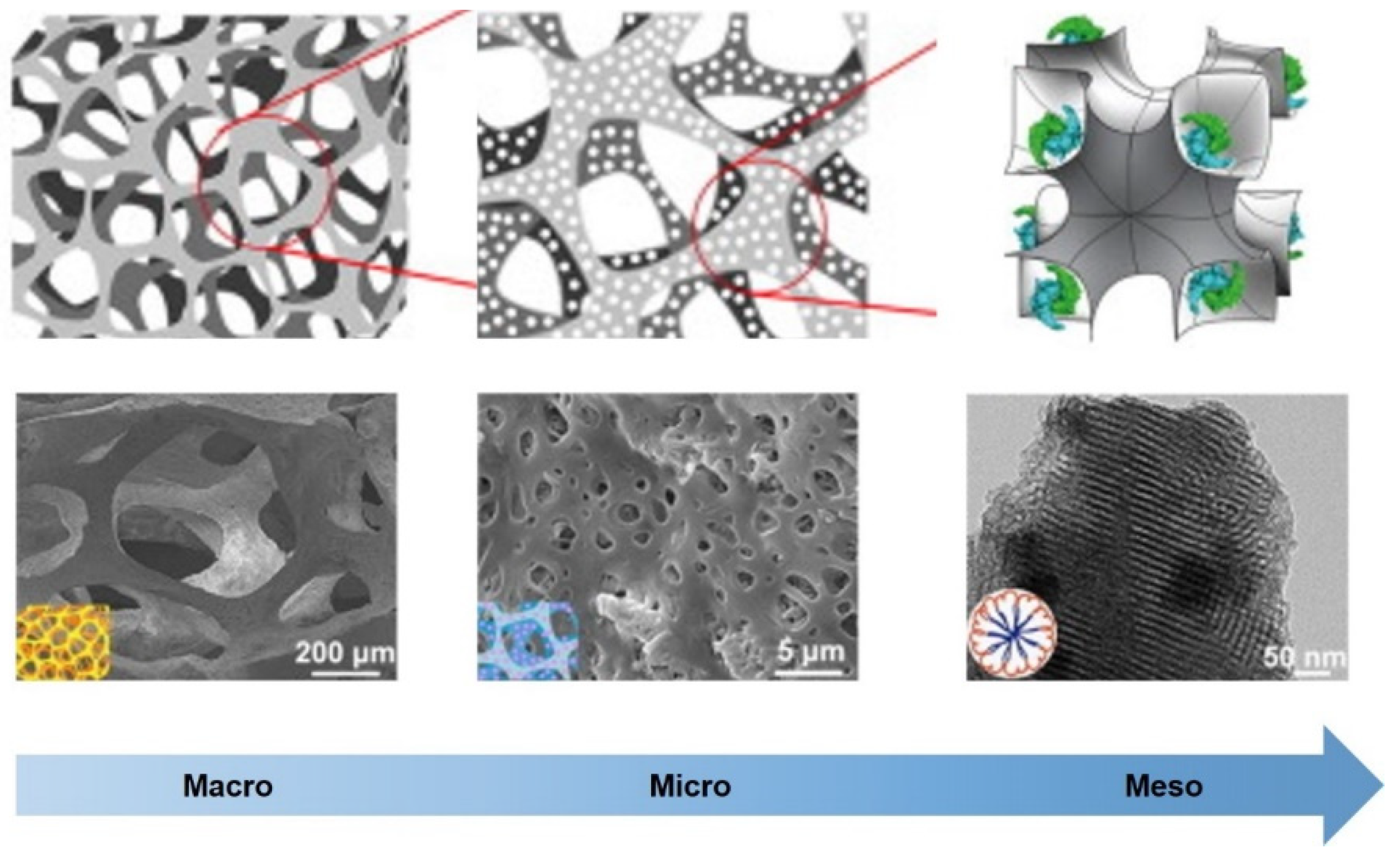
2. Multi-scale hierarchical scaffolds and functions
| Materials | Multiscale pores | Process | Characteristic | Ref |
|---|---|---|---|---|
| Geltin, alginate, PVA | ~800μm, ~40μm | 3-D printing, electrospinning, vacuum freeze-drying | Enhance initial cells attachment; enhence the mechanical interlocking between the scaffold and the tissue; promote the substances transportation and cell infiltration; facilitate vascularization. | [100] |
| Silk fibroin | 100-390μm, 1-30μm, | Paraffin sphere leaching, phase separation | Provide certain mechanical property, promote cell attachment and proliferation. | [101] |
| Poly (butylene succinate), cellulose nanocrystals | ~68.9 μm, ~11 μm | Supercritical carbon dioxide foaming process | Strengthen, increase hydrophilicity, and optimize the degradation rate. | [102] |
| Poly (ε-caprolactone) (PCL), HA, β-TCP | ~350 μm, ~105 μm | Freeze casting, sacrificial templating | Accelerate osteogenic differentiations, provide biomechanical support. | [103] |
| PCL, mesoporous bioactive glass (MBG) | 5.6nm, <40μm | 3D printing, porogen leaching | The early mineralization in the MBG led to an increased surface hardness, while the presence of micro-pores enhanced cell activity and stimulated the osteogenic differentiation. | [104] |
| PCL | 500μm, 100μm | High-resolution EHD 3D printing | Promote cell orientation; exceptional permeability for cell infiltration, substances transportation. | [105] |
| PCL | >100μm, <30μm | 3D printing based a multi-scale direct writing system | Provide sufficient strength for mechanical support; offer a cell-appropriate microenvironment. | [106] |
| PCL | 300μm, 75μm, 50μm | 3D printing integrated with FDM, SE, and MEW system | Exhibited optimal biocompatibility, facilitating cell adhesion, and demonstrating the potential to promote cell alignment. | [107] |
| PCL | ~315μm, ~325μm, ~ 8μm | Emulsion templating, 3D printing | Enhance bioactivity, promote differentiation of precursors into mature bone cells, and induce angiogenesis. | [108] |
| PCL | 300μm, ~20.2 μm | 3D printing, electrospinning | Possess osteoinductive properties, stimulating the expression of osteogenic markers in MC-3T3 osteoblasts. | [109] |
| PCL | ~400μm, ~10μm | 3D plotting systems, non-solvent-induced phase separation | Demonstrated mechanical characteristics similar to cancellous bone, enhance cell attachment, proliferation, and differentiation. | [110] |
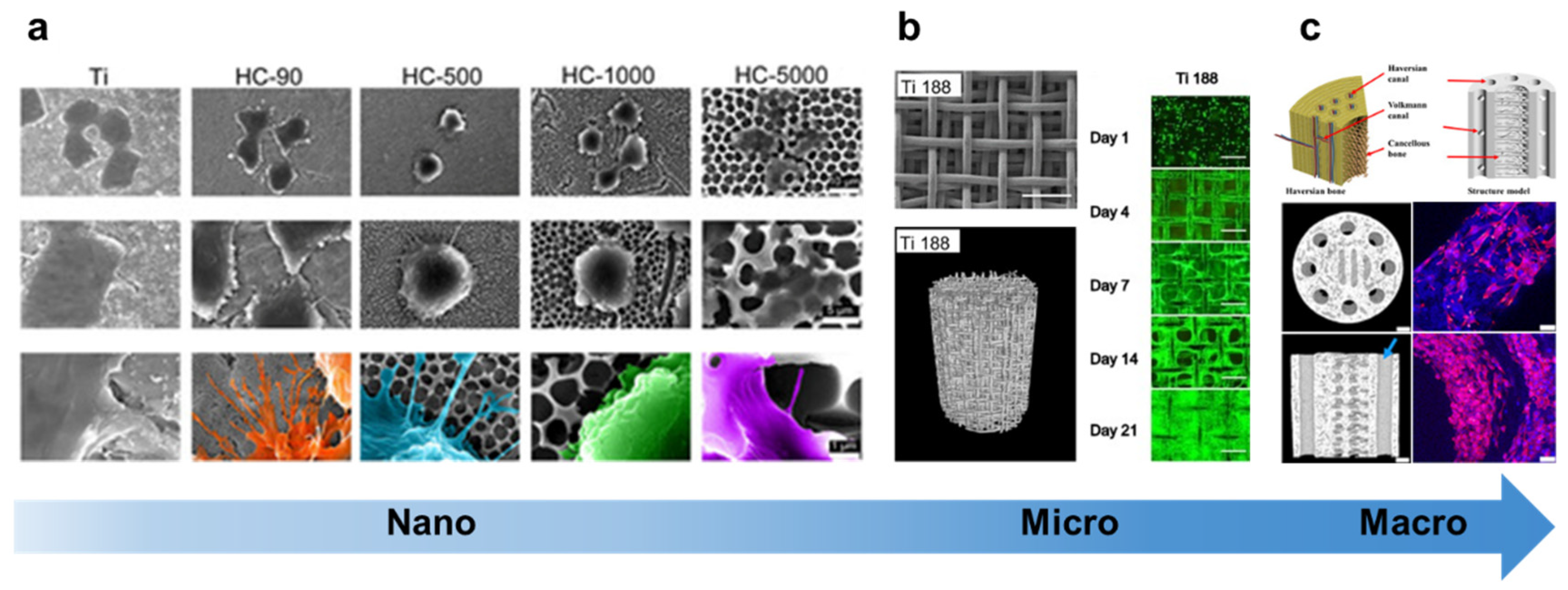
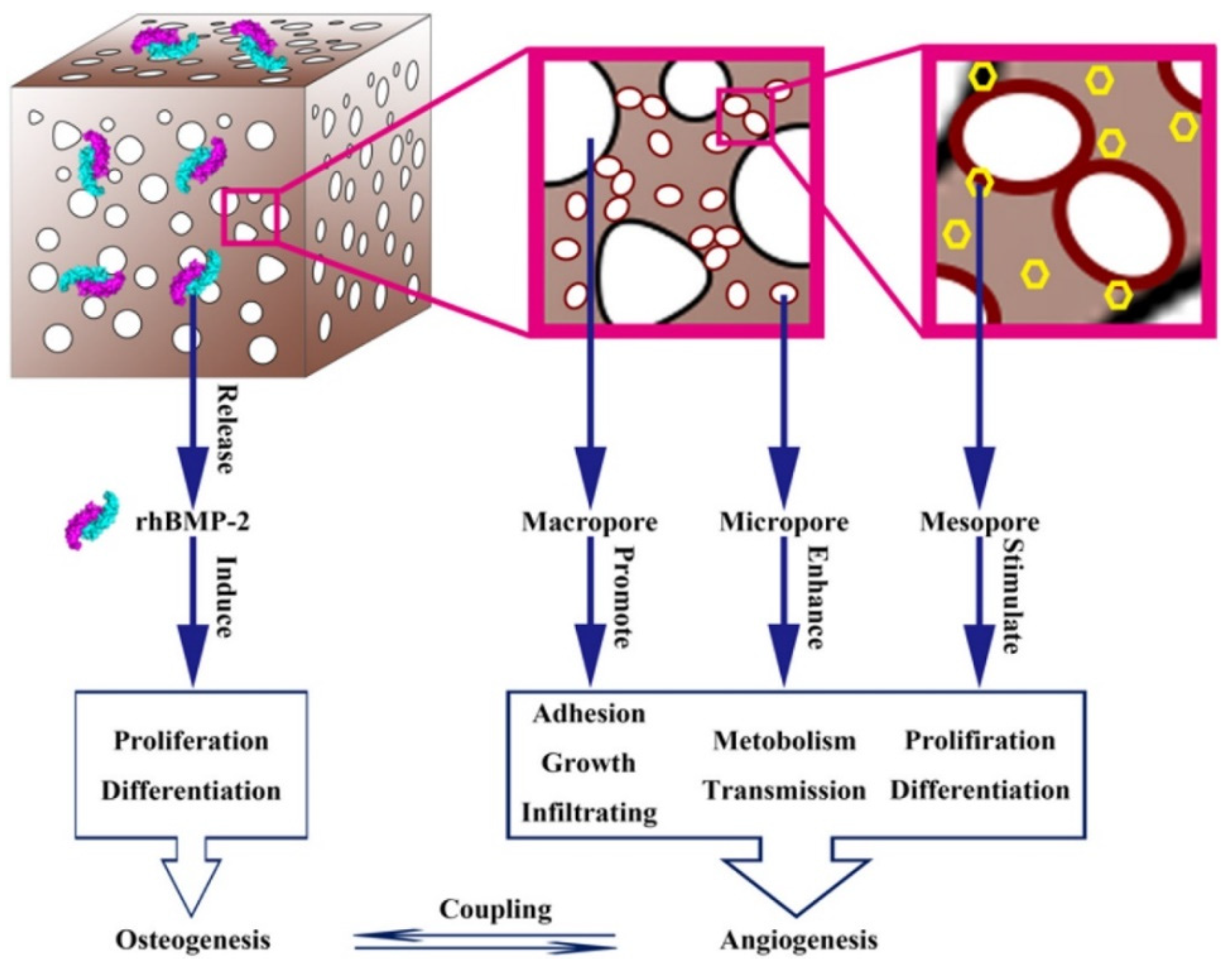
3. The structures of living organisms can serve as biomimetic hierarchical structure design models for BTE scaffolds.
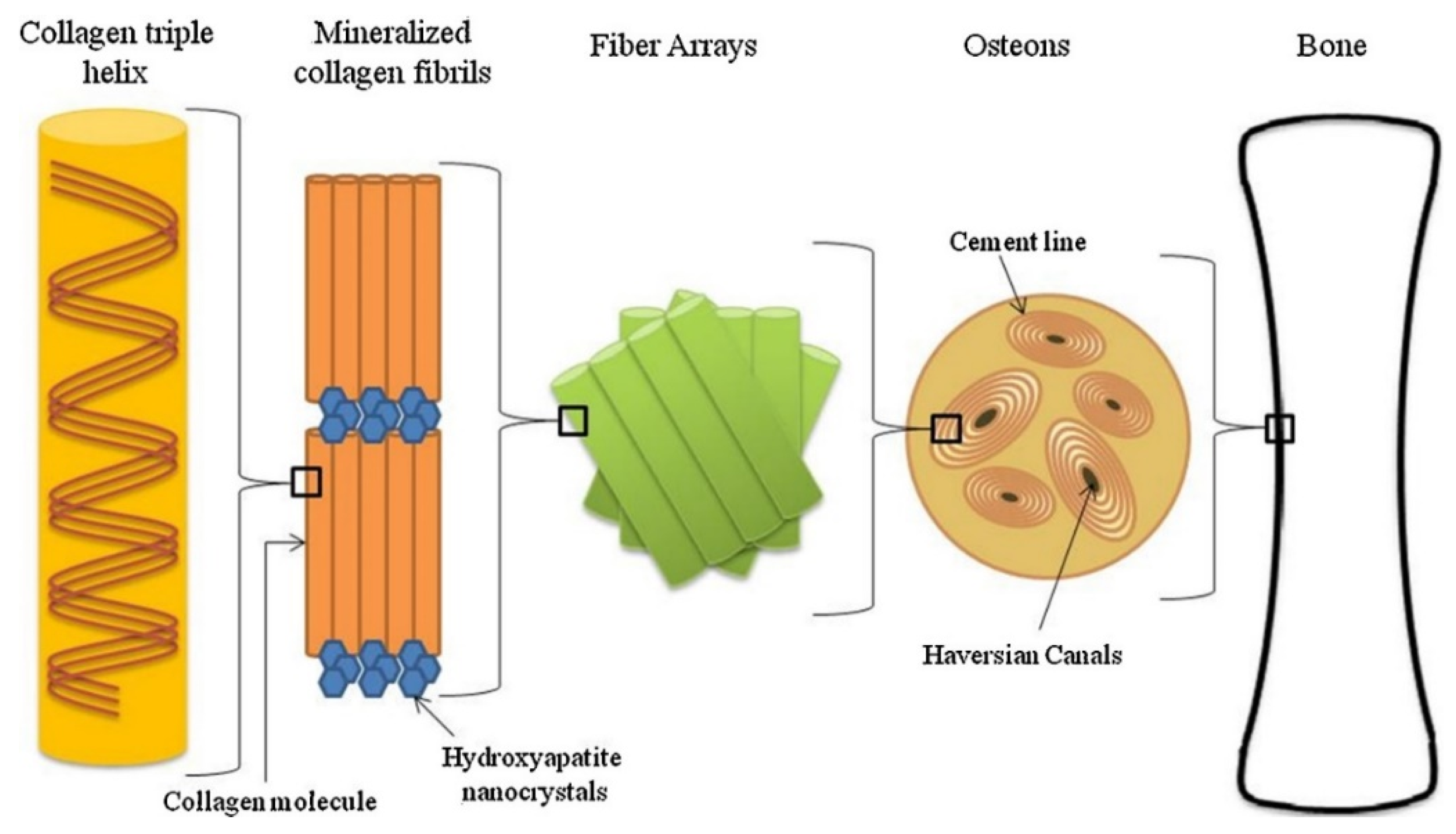
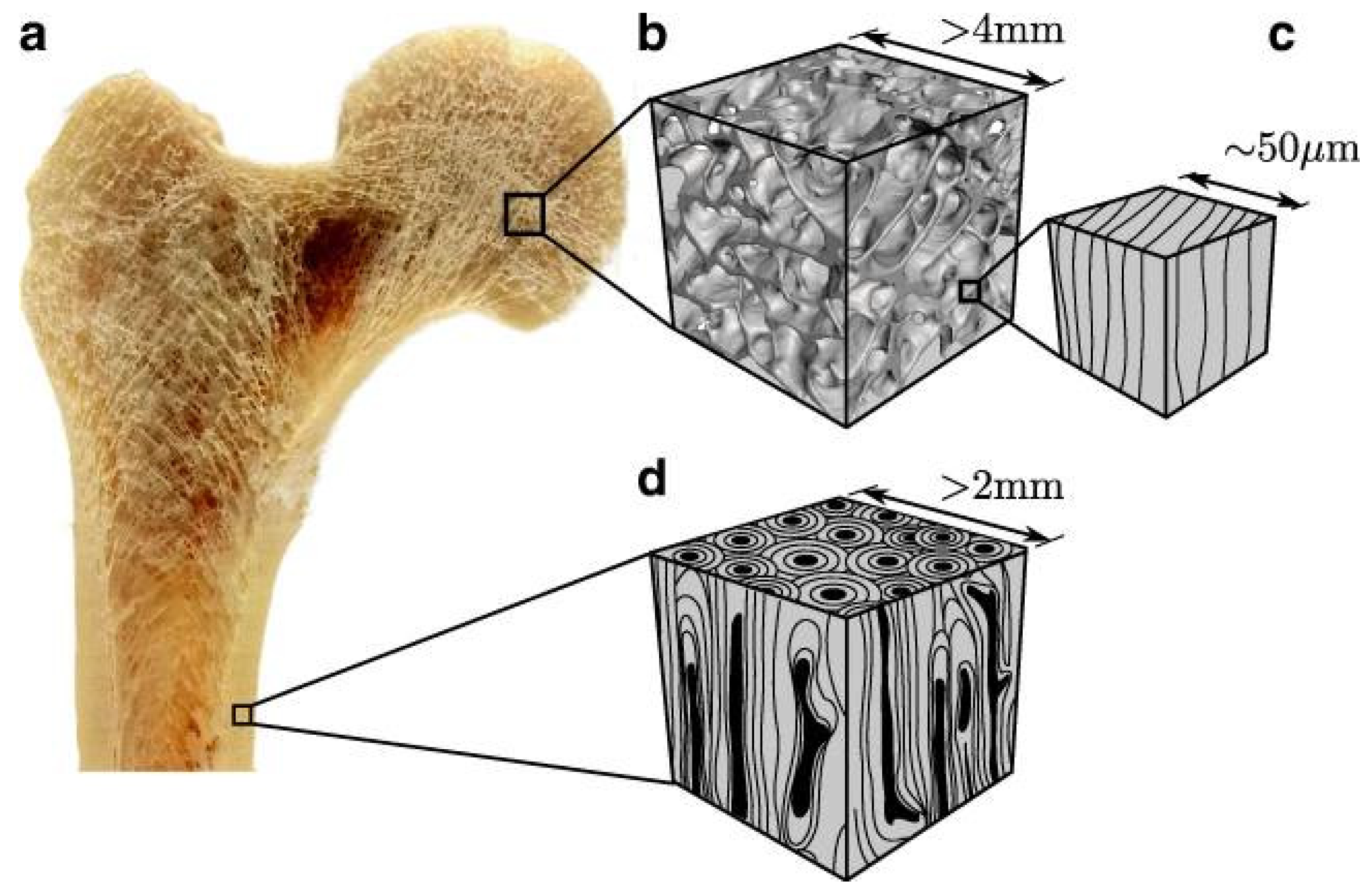
3.1. Natural bone as a multi-scale structural model
3.2. Bamboo and wood as multi-scale structural models
3.3. Nacre as multi-scale structural models
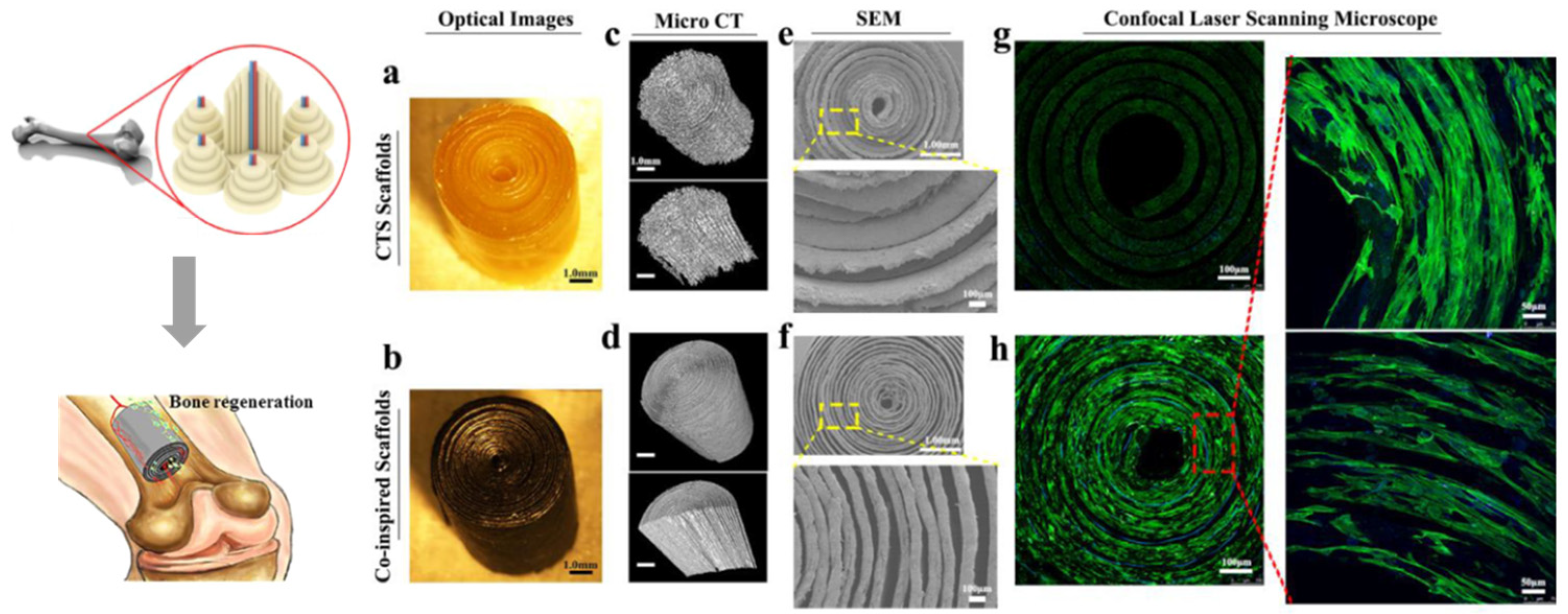
3.3. Other multi-scale structural models
4. The manufacturing technology of multi-scale hierarchical scaffolds
4.1. Traditional manufacturing techniques
4.2. Advanced manufacturing techniques
4.3. Combination of advanced and traditional manufacturing techniques
5. Conclusion and outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Science translational medicine 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! Journal of orthopaedic trauma 2017, 31 Suppl 5, S20–s22. [Google Scholar] [CrossRef]
- Xie, C.; Ye, J.C.; Liang, R.J.; Yao, X.D.; Wu, X.Y.; Koh, Y.W.; Wei, W.; Zhang, X.Z.; Ouyang, H.W. Advanced Strategies of Biomimetic Tissue-Engineered Grafts for Bone Regeneration. Advanced healthcare materials 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive materials 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-engineered autologous grafts for facial bone reconstruction. Science translational medicine 2016, 8, 343ra383. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: which is the ideal biomaterial? Journal of clinical periodontology 2019, 46 Suppl 21, 92–102. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2017, 4, 1700401. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Chu, M.; Sun, H.; Jin, J.; Yu, K.; Wang, Q.; Zhou, Q.; Chen, Y. Electrically assisted 3D printing of nacre-inspired structures with self-sensing capability. Science advances 2019, 5, eaau9490. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Tan, Y.; Wang, J.; Liu, H.; Wang, Y.; Yang, S.; Shi, M.; Zhao, S.; Zhang, Y.; et al. A Difunctional Regeneration Scaffold for Knee Repair based on Aptamer-Directed Cell Recruitment. Advanced materials (Deerfield Beach, Fla.) 2017, 29. [Google Scholar] [CrossRef]
- Yu, L.; Dawson, L.A.; Yan, M.; Zimmel, K.; Lin, Y.L.; Dolan, C.P.; Han, M.; Muneoka, K. BMP9 stimulates joint regeneration at digit amputation wounds in mice. Nature communications 2019, 10, 424. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Gordon, R.; Zhang, L.; Bergan, R.; Keene, D.R.; Jones, J.M.; Xie, H.; Chen, Z.; Tao, J.; et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nature communications 2019, 10, 3520. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.; Xie, C.; Wu, X.; Ren, Q.; Wang, F.; Shen, X.; Hong, Y.; Wu, H.; Liao, Y.; et al. Msx1(+) stem cells recruited by bioactive tissue engineering graft for bone regeneration. Nature communications 2022, 13, 5211. [Google Scholar] [CrossRef] [PubMed]
- Hunter, N.L.; Sherman, R.E. Combination products: modernizing the regulatory paradigm. Nature reviews. Drug discovery 2017, 16, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gorman, J.H., 3rd; Gorman, R.C. Acellular biomaterials: an evolving alternative to cell-based therapies. Science translational medicine 2013, 5, 176ps174. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent approaches towards bone tissue engineering. Bone 2022, 154, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioactive materials 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Du, Y.; Guo, J.L.; Wang, J.; Mikos, A.G.; Zhang, S. Hierarchically designed bone scaffolds: From internal cues to external stimuli. Biomaterials 2019, 218, 119334. [Google Scholar] [CrossRef]
- Stuckensen, K.; Schwab, A.; Knauer, M.; Muiños-López, E.; Ehlicke, F.; Reboredo, J.; Granero-Moltó, F.; Gbureck, U.; Prósper, F.; Walles, H.; et al. Tissue Mimicry in Morphology and Composition Promotes Hierarchical Matrix Remodeling of Invading Stem Cells in Osteochondral and Meniscus Scaffolds. Advanced materials (Deerfield Beach, Fla.) 2018, 30, e1706754. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nature materials 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Nepal, D.; Kang, S.; Adstedt, K.M.; Kanhaiya, K.; Bockstaller, M.R.; Brinson, L.C.; Buehler, M.J.; Coveney, P.V.; Dayal, K.; El-Awady, J.A.; et al. Hierarchically structured bioinspired nanocomposites. Nature materials 2023, 22, 18–35. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nature materials 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Zhao, H.W.; Liu, S.J.; Wei, Y.; Yue, Y.H.; Gao, M.R.; Li, Y.B.; Zeng, X.L.; Deng, X.L.; Kotov, N.A.; Guo, L.; et al. Multiscale engineered artificial tooth enamel. Science (New York, N.Y.) 2022, 375, 551. [Google Scholar] [CrossRef]
- Meng, X.-S.; Zhou, L.-C.; Liu, L.; Zhu, Y.-B.; Meng, Y.-F.; Zheng, D.-C.; Yang, B.; Rao, Q.-Z.; Mao, L.-B.; Wu, H.-A.; et al. Deformable hard tissue with high fatigue resistance in the hinge of bivalve Cristaria plicata. Science (New York, N.Y.) 2023, 380, 1252–1257. [Google Scholar] [CrossRef]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Colfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science (New York, N.Y.) 2016, 354, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.K.; Samaei, A.T.; Gheshlaghi, B.; Lu, J.; Lu, Y. Asymmetric flexural behavior from bamboo’s functionally graded hierarchical structure: Underlying mechanisms. Acta Biomaterialia 2015, 16, 178–186. [Google Scholar] [CrossRef]
- Wegst, U.G.K. Bending efficiency through property gradients in bamboo, palm, and wood-based composites. Journal of the Mechanical Behavior of Biomedical Materials 2011, 4, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.W.; Fu, H.Y.; Han, Z.Y.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Advanced healthcare materials 19. [CrossRef] [PubMed]
- Lin, X.H.; Xing, X.; Li, S.S.; Wu, X.Y.; Jia, Q.Q.; Tu, H.; Bian, H.L.; Lu, A.; Zhang, L.N.; Yang, H.Y.; et al. Anisotropic Hybrid Hydrogels Constructed via the Noncovalent Assembly for Biomimetic Tissue Scaffold. Adv. Funct. Mater. 2022, 32, 15. [Google Scholar] [CrossRef]
- Ha, Y.J.; Ma, X.J.; Li, S.K.; Li, T.; Li, Z.H.; Qian, Y.H.; Shafiq, M.; Wang, J.W.; Zhou, X.J.; He, C.L. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 14. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2016, 3, 1600058. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M.; et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Advanced materials (Deerfield Beach, Fla.) 2015, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Brazill, J.M.; Beeve, A.T.; Craft, C.S.; Ivanusic, J.J.; Scheller, E.L. Nerves in Bone: Evolving Concepts in Pain and Anabolism. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2019, 34, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, M.; Li, X.; Hou, J.; Chen, S.; Yang, G.; Liu, X.; Zhou, S. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Advanced healthcare materials 2022, 11, e2102236. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zheng, J.C.; Xiong, Y.; Wang, H.T.; Yang, S.H.; Sun, X.D.; Zhao, L.Y.; Mikos, A.G.; Wang, X.M. Hierarchically Engineered Artificial Lamellar Bone with High Strength and Toughness. Small Struct. 9. [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering - A mini review. International journal of biological macromolecules 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Pahr, D.H.; Reisinger, A.G. A Review on Recent Advances in the Constitutive Modeling of Bone Tissue. Current osteoporosis reports 2020, 18, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Wang, M.H.; Wang, Z.Y.; Gao, H.L.; Chen, S.M.; Cong, Y.H.; Yang, L.; Wen, S.M.; Cheng, D.D.; He, J.C.; et al. Radially Porous Nanocomposite Scaffolds with Enhanced Capability for Guiding Bone Regeneration In Vivo. Adv. Funct. Mater. 2022, 32, 12. [Google Scholar] [CrossRef]
- Feng, Y.H.Z.; Gao, H.L.; Wu, D.; Weng, Y.T.; Wang, Z.Y.; Yu, S.H.; Wang, Z.L. Biomimetic Lamellar Chitosan Scaffold for Soft Gingival Tissue Regeneration. Adv. Funct. Mater. 2021, 31, 12. [Google Scholar] [CrossRef]
- Guan, Q.F.; Han, Z.M.; Zhu, Y.B.; Xu, W.L.; Yang, H.B.; Ling, Z.C.; Yan, B.B.; Yang, K.P.; Yin, C.H.; Wu, H.A.; et al. Bio-Inspired Lotus-Fiber-like Spiral Hydrogel Bacterial Cellulose Fibers. Nano Lett. 2021, 21, 952–958. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, Y.N.; Ma, B.J.; Shao, J.L.; Liu, H.R.; Ge, S.H. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. Acs Applied Materials & Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef]
- Lv, Z.H.; Hu, T.T.; Bian, Y.X.; Wang, G.Y.; Wu, Z.K.; Li, H.; Liu, X.Y.; Yang, S.Q.; Tan, C.L.; Liang, R.Z.; et al. A MgFe-LDH Nanosheet-Incorporated Smart Thermo-Responsive Hydrogel with Controllable Growth Factor Releasing Capability for Bone Regeneration. Adv. Mater. 2023, 35, 12. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Li, F.; Jiang, P.; Cai, F.Y.; Lin, Q.; Zhou, M.J.; Liu, H.M.; Yan, F. Remote control of the recruitment and capture of endogenous stem cells by ultrasound for in situ repair of bone defects. Bioactive Materials 2023, 21, 223–238. [Google Scholar] [CrossRef]
- Zhou, X.J.; Qian, Y.H.; Chen, L.; Li, T.; Sun, X.; Ma, X.J.; Wang, J.W.; He, C.L. Flowerbed-Inspired Biomimetic Scaffold with Rapid Internal Tissue Infiltration and Vascularization Capacity for Bone Repair. ACS Nano 2023, 17, 5140–5156. [Google Scholar] [CrossRef]
- He, Y.; Tian, M.; Li, X.L.; Hou, J.W.; Chen, S.; Yang, G.; Liu, X.; Zhou, S.B. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Advanced healthcare materials 2022, 11, 16. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Moroni, L. Janus 3D printed dynamic scaffolds for nanovibration-driven bone regeneration. Nature communications 2021, 12, 12. [Google Scholar] [CrossRef]
- Arora, A.; Kothari, A.; Katti, D.S. Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design. J Mech Behav Biomed Mater 2015, 51, 169–183. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.Y.; Ding, L.; Zhang, P.; Liu, C.; Chen, D.F.; Zhao, F.J.; Wang, G.; Chen, X.F. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. Acs Applied Materials & Interfaces 2022, 14, 36395–36410. [Google Scholar] [CrossRef]
- Lu, Q.J.; Diao, J.J.; Wang, Y.Q.; Feng, J.L.; Zeng, F.S.; Yang, Y.; Kuang, Y.D.; Zhao, N.R.; Wang, Y.J. 3D printed pore morphology mediates bone marrow stem cell behaviors via RhoA/ROCK2 signaling pathway for accelerating bone regeneration. Bioactive Materials 2023, 26, 413–424. [Google Scholar] [CrossRef]
- Gu, P.Y.; Xu, Y.; Liu, Q.Y.; Wang, Y.X.; Li, Z.L.; Chen, M.Y.; Mao, R.Q.; Liang, J.; Zhang, X.D.; Fan, Y.J.; et al. Tailorable 3DP Flexible Scaffolds with Porosification of Filaments Facilitate Cell Ingrowth and Biomineralized Deposition. Acs Applied Materials & Interfaces 2022, 14, 32914–32926. [Google Scholar] [CrossRef]
- Li, Y.W.; Zha, Y.; Hu, W.K.; Chen, J.; Liu, S.B.; Zhang, S.M.; Wang, J.L. Monoporous Microsphere as a Dynamically Movable Drug Carrier for Osteoporotic Bone Remodeling. Advanced healthcare materials 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wang, D.Y.; Wang, S.; Fan, W.Z.; Yang, Y.L.; Gao, P.F.; Chen, M.W.; Yang, W.H.; Cai, K.Y. Promoting osseointegration by in situ biosynthesis of metal ion-loaded bacterial cellulose coating on titanium surface. Carbohydr. Polym. 2022, 297, 10. [Google Scholar] [CrossRef]
- Mao, M.T.; Zhu, S.B.; Zhang, L.; Liu, F.W.; Kong, L.; Xue, Y.; Rotello, V.M.; Han, Y. An Extracellular Matrix-like Surface for Zn Alloy to Enhance Bone Regeneration. Acs Applied Materials & Interfaces 2022, 10. [Google Scholar] [CrossRef]
- Hao, J.X.; Bai, B.S.; Ci, Z.; Tang, J.C.; Hu, G.H.; Dai, C.X.; Yu, M.Y.; Li, M.; Zhang, W.; Zhang, Y.X.; et al. Large-sized bone defect repair by combining a decalcified bone matrix framework and bone regeneration units based on photo-crosslinkable osteogenic microgels. Bioactive Materials 2022, 14, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, J.H.; Jeong, J.; Kim, S.H.L.; Koh, R.H.; Kim, I.; Bae, S.; Lee, H.; Hwang, N.S. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials 2020, 257, 13. [Google Scholar] [CrossRef]
- Lai, J.H.; Wang, C.; Liu, J.; Chen, S.S.; Liu, C.Y.; Huang, X.X.; Wu, J.; Pan, Y.; Xie, Y.C.; Wang, M. Low temperature hybrid 3D printing of hierarchically porous bone tissue engineering scaffolds with in situ delivery of osteogenic peptide and mesenchymal stem cells. Biofabrication 2022, 14, 18. [Google Scholar] [CrossRef]
- Abu Awwad, H.A.M.; Thiagarajan, L.; Kanczler, J.M.; Amer, M.H.; Bruce, G.; Lanham, S.; Rumney, R.M.H.; Oreffo, R.O.C.; Dixon, J.E. Genetically-programmed, mesenchymal stromal cell-laden & mechanically strong 3D bioprinted scaffolds for bone repair. Journal of controlled release : official journal of the Controlled Release Society 2020, 325, 335–346. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials (Basel, Switzerland) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190-191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater 2021, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Yilmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Gupta, D.; Vashisth, P.; Bellare, J. Multiscale porosity in a 3D printed gellan-gelatin composite for bone tissue engineering. Biomed. Mater. 2021, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, S.; Yang, Y.; Zhang, J.; Zhang, Z. Multiscale regeneration scaffold in vitro and in vivo. Journal of biomedical materials research. Part B, Applied biomaterials 2018, 106, 1218–1225. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, A.; Rani, S.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. 3D Printed Hierarchical Porous Poly(ε-caprolactone) Scaffolds from Pickering High Internal Phase Emulsion Templating. Langmuir : the ACS journal of surfaces and colloids 2023, 39, 1927–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, S.K.; He, J.K.; Lei, Q.; Wu, C.; Song, A.P.; Zhang, C. Electrohydrodynamic printing of submicron-microscale hybrid scaffolds with improved cellular adhesion and proliferation behaviors. Nanotechnology 2023, 34, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, P.; Liu, Y.; Yang, J.; Li, S. Three-Dimensional-Bioprinted Bioactive Glass/Cellulose Composite Scaffolds with Porous Structure towards Bone Tissue Engineering. Polymers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Advanced healthcare materials 2022, e2202766. [Google Scholar] [CrossRef]
- Wang, Z.; Florczyk, S.J. Freeze-FRESH: A 3D Printing Technique to Produce Biomaterial Scaffolds with Hierarchical Porosity. Materials (Basel, Switzerland) 2020, 13. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nature materials 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Tan, J.; Saltzman, W.M. Biomaterials with hierarchically defined micro- and nanoscale structure. Biomaterials 2004, 25, 3593–3601. [Google Scholar] [CrossRef]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res A 2009, 89, 1040–1048. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Mainardi, V.L.; Talò, G.; McCarthy, A.; John, J.V.; Teusink, M.J.; Hong, L.; Xie, J. Biomaterials with structural hierarchy and controlled 3D nanotopography guide endogenous bone regeneration. Science advances 2021, 7. [Google Scholar] [CrossRef]
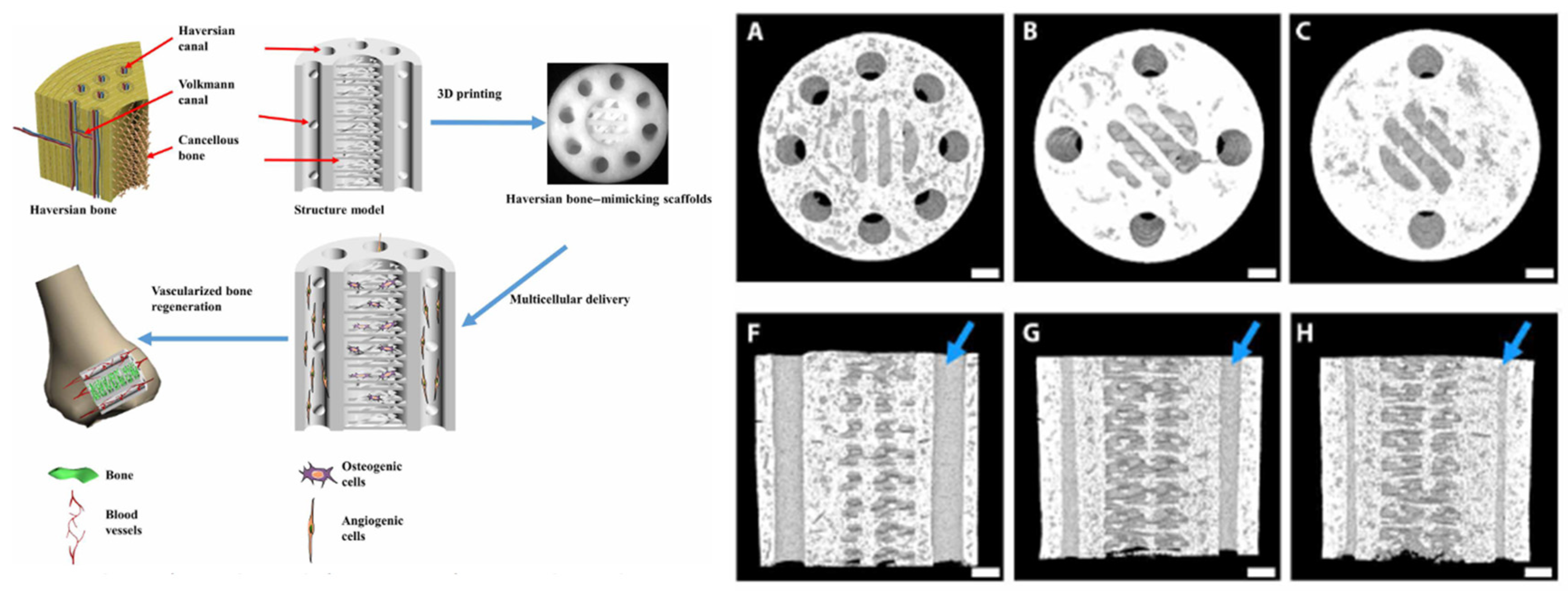
- Zhang, M.; Lin, R.; Wang, X.; Xue, J.; Deng, C.; Feng, C.; Zhuang, H.; Ma, J.; Qin, C.; Wan, L.; et al. 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Science advances 2020, 6, eaaz6725. [Google Scholar] [CrossRef]
- Wang, S.F.; Kempen, D.H.R.; de Ruiter, G.C.W.; Cai, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J.; Lu, L.C. Molecularly Engineered Biodegradable Polymer Networks with a Wide Range of Stiffness for Bone and Peripheral Nerve Regeneration. Adv. Funct. Mater. 2015, 25, 2715–2724. [Google Scholar] [CrossRef]
- Kim, W.; Gwon, Y.; Kim, Y.K.; Park, S.; Kang, S.J.; Park, H.K.; Kim, M.S.; Kim, J. Plasma-assisted multiscale topographic scaffolds for soft and hard tissue regeneration. npj Regen. Med. 2021, 6, 13. [Google Scholar] [CrossRef]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta biomaterialia 2017, 60, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Garoli, D.; Lovato, L.; Della Giustina, G.; Oliverio, M.; Francardi, M.; Zanchetta, E.; Brusatin, G.; De Angelis, F. Directly nanopatternable nanoporous titania - Application to cell growth engineering. Microelectron. Eng. 2016, 155, 102–106. [Google Scholar] [CrossRef]
- Bidgoli, M.R.; Alemzadeh, I.; Tamjid, E.; Khafaji, M.; Vossoughi, M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior. Materials science & engineering. C, Materials for biological applications 2019, 103, 109688. [Google Scholar] [CrossRef]
- Sgarminato, V.; Tonda-Turo, C.; Ciardelli, G. Reviewing recently developed technologies to direct cell activity through the control of pore size: From the macro- to the nanoscale. Journal of biomedical materials research. Part B, Applied biomaterials 2020, 108, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, R.Y.; Tang, W.M.; Yang, F.; Tian, H.F.; Peng, S.P.; Pan, H.; Shuai, C.J. Structural and Functional Adaptive Artificial Bone: Materials, Fabrications, and Properties. Adv. Funct. Mater. 2023, 33, 29. [Google Scholar] [CrossRef]
- Dang, H.P.; Vaquette, C.; Shabab, T.; Perez, R.A.; Yang, Y.; Dargaville, T.R.; Shafiee, A.; Tran, P.A. Porous 3D Printed Scaffolds For Guided Bone Regeneration In a Rat Calvarial Defect Model. Appl. Mater. Today 2020, 20, 16. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Zhu, X.; Fan, H.; Fan, Y.; Zhang, X. Dynamic competitive adsorption of bone-related proteins on calcium phosphate ceramic particles with different phase composition and microstructure. Journal of biomedical materials research. Part B, Applied biomaterials 2013, 101, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Clauder, F.; Bellmann-Sickert, K.; Beck-Sickinger, A.G. Improvement of wound healing by the development of ECM-inspired biomaterial coatings and controlled protein release. Biological chemistry 2021, 402, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen Biomater 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Polak, S.J.; Rustom, L.E.; Genin, G.M.; Talcott, M.; Wagoner Johnson, A.J. A mechanism for effective cell-seeding in rigid, microporous substrates. Acta biomaterialia 2013, 9, 7977–7986. [Google Scholar] [CrossRef]
- Bertsch, C.; Maréchal, H.; Gribova, V.; Lévy, B.; Debry, C.; Lavalle, P.; Fath, L. Biomimetic Bilayered Scaffolds for Tissue Engineering: From Current Design Strategies to Medical Applications. Advanced healthcare materials 2023, e2203115. [Google Scholar] [CrossRef]
- Xu, M.; Li, H.; Zhai, D.; Chang, J.; Chen, S.; Wu, C. Hierarchically porous nagelschmidtite bioceramic-silk scaffolds for bone tissue engineering. Journal of materials chemistry. B 2015, 3, 3799–3809. [Google Scholar] [CrossRef]
- Greiner, J.F.; Gottschalk, M.; Fokin, N.; Büker, B.; Kaltschmidt, B.P.; Dreyer, A.; Vordemvenne, T.; Kaltschmidt, C.; Hütten, A.; Kaltschmidt, B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomedicine : nanotechnology, biology, and medicine 2019, 17, 319–328. [Google Scholar] [CrossRef]
- Jin, S.S.; He, D.Q.; Luo, D.; Wang, Y.; Yu, M.; Guan, B.; Fu, Y.; Li, Z.X.; Zhang, T.; Zhou, Y.H.; et al. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment To Promote Endogenous Bone Regeneration. ACS Nano 2019, 13, 6581–6595. [Google Scholar] [CrossRef]
- Chen, H.P.; Xie, S.K.; Yang, Y.M.; Zhang, J.; Zhang, Z.Y. Multiscale regeneration scaffold in vitro and in vivo. J. Biomed. Mater. Res. Part B 2018, 106, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Li, W.; Jiang, Z.Y.; Wang, L.Y.; Kong, D.L.; Xu, B.S.; Zhu, M.F. Hierarchical macro/micro-porous silk fibroin scaffolds for tissue engineering. Mater. Lett. 2019, 236, 1–4. [Google Scholar] [CrossRef]
- Ju, J.; Gu, Z.; Liu, X.; Zhang, S.; Peng, X.; Kuang, T. Fabrication of bimodal open-porous poly (butylene succinate)/cellulose nanocrystals composite scaffolds for tissue engineering application. International journal of biological macromolecules 2020, 147, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Montesi, M.; Hautcoeur, D.; Dozio, S.M.; Chamary, S.; De Barra, E.; Tampieri, A.; Leriche, A. Bone-like ceramic scaffolds designed with bioinspired porosity induce a different stem cell response. Journal of materials science. Materials in medicine 2021, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, M.N.; Peña, J.; Ivanovski, S.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. Multiscale porosity in mesoporous bioglass 3D-printed scaffolds for bone regeneration. Materials science & engineering. C, Materials for biological applications 2021, 120, 111706. [Google Scholar] [CrossRef]
- Li, Y.; Lv, S.; Yuan, H.P.; Ye, G.; Mu, W.B.; Fu, Y.; Zhang, X.; Feng, Z.Y.; He, Y.; Chen, W. Peripheral Nerve Regeneration with 3D Printed Bionic Scaffolds Loading Neural Crest Stem Cell Derived Schwann Cell Progenitors. Adv. Funct. Mater. 2021, 31, 16. [Google Scholar] [CrossRef]
- Gao, Q.; Xie, C.Q.; Wang, P.; Xie, M.J.; Li, H.B.; Sun, A.Y.; Fu, J.Z.; He, Y. 3D printed multi-scale scaffolds with ultrafine fibers for providing excellent biocompatibility. Materials Science and Engineering C-Materials for Biological Applications 2020, 107, 9. [Google Scholar] [CrossRef]
- Wang, C.J.; Xu, Y.Y.; Xia, J.J.; Zhou, Z.Z.; Fang, Y.C.; Zhang, L.; Sun, W. Multi-scale hierarchical scaffolds with aligned micro-fibers for promoting cell alignment. Biomed. Mater. 2021, 16, 13. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Reilly, G.C.; Claeyssens, F. Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration. ACS Appl Mater Interfaces 2020, 12, 12510–12524. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Carranza, T.; Santos-Vizcaino, E.; Igartua, M.; Guerrero, P.; Hernandez, R.M.; de la Caba, K. Hybrid 3D Printed and Electrospun Multi-Scale Hierarchical Polycaprolactone Scaffolds to Induce Bone Differentiation. Pharmaceutics 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.M.; Rajangam, T.; Jeong, J.E.; Cheong, S.; Joo, S.M.; Oh, S.J.; Shin, H.; Kim, S.H.; Park, S.A. Fabrication of 3D plotted scaffold with microporous strands for bone tissue engineering. J. Mat. Chem. B 2020, 8, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Science advances 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta biomaterialia 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.; Cao, L.; Zhang, X.; Wang, J.; Liu, C. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Materials science & engineering. C, Materials for biological applications 2020, 110, 110622. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Guarnieri, D.; Iannone, M.; Zeppetelli, S.; Netti, P.A. Effect of micro- and macroporosity of bone tissue three-dimensional-poly(epsilon-caprolactone) scaffold on human mesenchymal stem cells invasion, proliferation, and differentiation in vitro. Tissue engineering. Part A 2010, 16, 2661–2673. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta biomaterialia 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lin, D.; Yu, Y.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta biomaterialia 2016, 32, 309–323. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G. Three-Dimensional Hierarchical Nanofibrous Collagen Scaffold Fabricated Using Fibrillated Collagen and Pluronic F-127 for Regenerating Bone Tissue. ACS Appl Mater Interfaces 2018, 10, 35801–35811. [Google Scholar] [CrossRef]
- Wen, S.M.; Chen, S.M.; Gao, W.; Zheng, Z.; Bao, J.Z.; Cui, C.; Liu, S.; Gao, H.L.; Yu, S.H. Biomimetic Gradient Bouligand Structure Enhances Impact Resistance of Ceramic-Polymer Composites. Advanced materials (Deerfield Beach, Fla.) 2023, 35, e2211175. [Google Scholar] [CrossRef]
- Meng, Y.F.; Zhu, Y.B.; Zhou, L.C.; Meng, X.S.; Yang, Y.L.; Zhao, R.; Xia, J.; Yang, B.; Lu, Y.J.; Wu, H.A.; et al. Artificial Nacre with High Toughness Amplification Factor: Residual Stress-Engineering Sparks Enhanced Extrinsic Toughening Mechanisms. Advanced materials (Deerfield Beach, Fla.) 2022, 34, e2108267. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Guille, M.M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nature materials 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Liang, K.Y.; Zhao, C.C.; Song, C.X.; Zhao, L.; Qiu, P.C.; Wang, S.Y.; Zhu, J.J.; Gong, Z.; Liu, Z.M.; Tang, R.K.; et al. In Situ Biomimetic Mineralization of Bone-Like Hydroxyapatite in Hydrogel for the Acceleration of Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 292–308. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Jiang, Y.; Liu, S.; Zheng, J.; Li, N.; Wang, G.; Dong, F.; Chen, J.; Xie, Y.; et al. A 3D biomimetic optoelectronic scaffold repairs cranial defects. Science advances 2023, 9, eabq7750. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, J.; Sun, Y.; Liu, Q.Q.; Zhang, C.; Shao, C.Y.; Yu, K.; Ge, M.J.; Mi, R.; Gu, J.Y.; et al. A Hierarchical 3D Graft Printed with Nanoink for Functional Craniofacial Bone Restoration. Adv. Funct. Mater. 2023, 20. [Google Scholar] [CrossRef]
- Nepal, D.; Kang, S.; Adstedt, K.M.; Kanhaiya, K.; Bockstaller, M.R.; Brinson, L.C.; Buehler, M.J.; Coveney, P.V.; Dayal, K.; El-Awady, J.A.; et al. Hierarchically structured bioinspired nanocomposites. Nature materials 2023, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Zhang, S.C.; Gao, H.L.; Wang, Q.; Zhou, L.C.; Zhao, H.Y.; Li, X.Y.; Gong, M.; Pan, X.F.; Cui, C.; et al. Mechanically robust bamboo node and its hierarchically fibrous structural design. Natl. Sci. Rev. 2023, 10, 13. [Google Scholar] [CrossRef]
- Xue, J.M.; Ma, H.S.; Song, E.H.; Han, F.; Li, T.; Zhang, M.; Zhu, Y.F.; Liu, J.J.; Wu, C.T. Bamboo-Based Biomaterials for Cell Transportation and Bone Integration. Advanced healthcare materials 2022, 11, 11. [Google Scholar] [CrossRef]
- Wang, X.F.; Fang, J.; Zhu, W.W.; Zhong, C.X.; Ye, D.D.; Zhu, M.Y.; Lu, X.; Zhao, Y.S.; Ren, F.Z. Bioinspired Highly Anisotropic, Ultrastrong and Stiff, and Osteoconductive Mineralized Wood Hydrogel Composites for Bone Repair. Adv. Funct. Mater. 2021, 31, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and bioinspired materials: Structure leading to functional and mechanical performance. Bioactive materials 2020, 5, 745–757. [Google Scholar] [CrossRef]
- Wu, M.H.; Chen, F.X.; Wu, P.; Yang, Z.Q.; Zhang, S.; Xiao, L.F.; Deng, Z.M.; Zhang, C.; Chen, Y.; Cai, L. Bioinspired Redwood-Like Scaffolds Coordinated by In Situ-Generated Silica-Containing Hybrid Nanocoatings Promote Angiogenesis and Osteogenesis both In Vitro and In Vivo. Advanced healthcare materials 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.L.; Chen, S.M.; Mao, L.B.; Song, Z.Q.; Yao, H.B.; Colfen, H.; Luo, X.S.; Zhang, F.; Pan, Z.; Meng, Y.F.; et al. Mass production of bulk artificial nacre with excellent mechanical properties. Nat. Commun. 2017, 8, 8. [Google Scholar] [CrossRef]
- Naleway, S.E.; Porter, M.M.; McKittrick, J.; Meyers, M.A. Structural Design Elements in Biological Materials: Application to Bioinspiration. Adv. Mater. 2015, 27, 5455–5476. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xue, J.M.; Yu, X.P.; Zhai, D.; Lin, R.C.; Zhang, M.; Xia, L.G.; Wang, X.Y.; Yao, Q.Q.; Chang, J.; et al. Co-inspired hydroxyapatite-based scaffolds for vascularized bone regeneration. Acta Biomaterialia 2021, 119, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ma, B.; Xue, J.M.; Zhai, D.; Zhao, P.Y.; Chang, J.; Wu, C.T. Bioinspired Biomaterials with a Brick-and-Mortar Microstructure Combining Mechanical and Biological Performance. Advanced healthcare materials 2020, 9, 9. [Google Scholar] [CrossRef]
- Sgarminato, V.; Tonda-Turo, C.; Ciardelli, G. Reviewing recently developed technologies to direct cell activity through the control of pore size: From the macro- to the nanoscale. J. Biomed. Mater. Res. Part B 2020, 108, 1176–1185. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.J.; Deng, C.J.; Li, G.L.; Chang, J.; Zhang, Z.Y.; Jiang, X.Q.; Wu, C.T. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv. Sci. 2017, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.; Sharma, N.S.; Holubeck, P.A.; Brown, D.; Shah, R.; McGoldrick, D.; John, J.V.; Shahriar, S.M.S.; Xie, J.W. Extracellular Matrix Secretion Mechanically Reinforces Interlocking Interfaces. Adv. Mater. 2023, 35, 9. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nature materials 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta biomaterialia 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta biomaterialia 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Science translational medicine 2020, 12. [Google Scholar] [CrossRef]
- Gu, P.; Xu, Y.; Liu, Q.; Wang, Y.; Li, Z.; Chen, M.; Mao, R.; Liang, J.; Zhang, X.; Fan, Y.; et al. Tailorable 3DP Flexible Scaffolds with Porosification of Filaments Facilitate Cell Ingrowth and Biomineralized Deposition. ACS Appl Mater Interfaces 2022. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications - A review. J. Drug Deliv. Sci. Technol. 2020, 55, 18. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Advanced healthcare materials 2023, 12, e2202766. [Google Scholar] [CrossRef]
- Chikelu, C.W.; Berns, M.; Conover, D.; Habas, R.; Han, L.; Street, R.M.; Schauer, C.L. Collagen Nanoyarns: Hierarchical Three-Dimensional Biomaterial Constructs. Biomacromolecules 2023, 24, 1155–1163. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, C.; Tomsia, A.P. Freeze Casting for Assembling Bioinspired Structural Materials. Advanced materials (Deerfield Beach, Fla.) 2017, 29. [Google Scholar] [CrossRef]
- Chen, X.; Ergun, A.; Gevgilili, H.; Ozkan, S.; Kalyon, D.M.; Wang, H. Shell-core bi-layered scaffolds for engineering of vascularized osteon-like structures. Biomaterials 2013, 34, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Piard, C.; Baker, H.; Kamalitdinov, T.; Fisher, J. Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication 2019, 11, 025013. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Luan, S.; Zhou, G. Low-Temperature Printed Hierarchically Porous Induced-Biomineralization Polyaryletherketone Scaffold for Bone Tissue Engineering. Advanced healthcare materials 2022, 11, e2200977. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, X.; Sun, Z.; Fang, Q.; Geng, X.; Zhang, H.; Wang, G.; Dou, Y.; Hu, P.; Zhu, K.; et al. Biomimetic porous silk fibroin/biphasic calcium phosphate scaffold for bone tissue regeneration. Journal of materials science. Materials in medicine 2018, 30, 4. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Lee, Y.K.; Shin, J.S.; Lim, K.J. Preparation of interconnected porous chitosan scaffolds by sodium acetate particulate leaching. Journal of biomaterials science. Polymer edition 2011, 22, 1319–1329. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.; Evans, R.; Reilly, G.C.; Claeyssens, F. Combined Porogen Leaching and Emulsion Templating to produce Bone Tissue Engineering Scaffolds. International journal of bioprinting 2020, 6, 265. [Google Scholar] [CrossRef]
- Wang, X.F.; Salick, M.R.; Gao, Y.H.; Jiang, J.; Li, X.Y.; Liu, F.F.; Cordie, T.; Li, Q.; Turng, L.S. Interconnected porous poly(-caprolactone) tissue engineering scaffolds fabricated by microcellular injection molding. J. Cell. Plast. 2018, 54, 379–397. [Google Scholar] [CrossRef]
- Sainitya, R.; Sriram, M.; Kalyanaraman, V.; Dhivya, S.; Saravanan, S.; Vairamani, M.; Sastry, T.P.; Selvamurugan, N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int J Biol Macromol 2015, 80, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids and surfaces. B, Biointerfaces 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Nethala, S.; Tripathi, A.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. Int J Biol Macromol 2011, 49, 1167–1172. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhu, Y.; Wang, X.; Li, M.; Lin, Z.; Hu, X.; Zhang, Y.; Yin, Q.; Xia, H.; et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl Mater Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah, A.A.A.; Iftikhar, F.J.; Bano, B.; Khan, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, K.; Wu, X.; Xiao, X. Preparation of nanofibrous poly (L-lactic acid) scaffolds using the thermally induced phase separation technique in dioxane/polyethylene glycol solution. Designed monomers and polymers 2023, 26, 77–89. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Wang, Z.L.; Gao, Z.T.; Zhang, P.B.; Chen, X.S. PREPARATION OF POROUS NANOCOMPOSITE SCAFFOLDS WITH HONEYCOMB MONOLITH STRUCTURE BY ONE PHASE SOLUTION FREEZE-DRYING METHOD. Chin. J. Polym. Sci. 2011, 29, 215–224. [Google Scholar] [CrossRef]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater 2018, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Wang, X.; Song, G.; Gu, Z.; Yang, Z. Fabrication of PLLA/β-TCP nanocomposite scaffolds with hierarchical porosity for bone tissue engineering. Int J Biol Macromol 2014, 69, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating. Biotechnology and bioengineering 2011, 108, 963–976. [Google Scholar] [CrossRef]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H.D. Multi-scale porous Ti6Al4V scaffolds with enhanced strength and biocompatibility formed via dynamic freeze-casting coupled with micro-arc oxidation. Mater. Lett. 2016, 185, 21–24. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, J.W.; Koh, Y.H.; Kim, H.E. Dual-Scale Porosity Alumina Structures Using Ceramic/Camphene Suspensions Containing Polymer Microspheres. Materials (Basel, Switzerland) 2022, 15. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Pan, Y.; Miszuk, J.M.; Sun, H. One-pot porogen free method fabricated porous microsphere-aggregated 3D PCL scaffolds for bone tissue engineering. Journal of biomedical materials research. Part B, Applied biomaterials 2020, 108, 2699–2710. [Google Scholar] [CrossRef]
- Li, S.; Kuddannaya, S.; Chuah, Y.J.; Bao, J.; Zhang, Y.; Wang, D. Combined effects of multi-scale topographical cues on stable cell sheet formation and differentiation of mesenchymal stem cells. Biomaterials science 2017, 5, 2056–2067. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.J.; Lv, J.; Yin, C.; Cai, H.; Song, C.L.; Leng, H.J.; Zheng, Y.F.; Liu, Z.J.; Cheng, Y. Hierarchical Micropore/Nanorod Apatite Hybrids In-Situ Grown from 3-D Printed Macroporous Ti6Al4V Implants with Improved Bioactivity and Osseointegration. J. Mater. Sci. Technol. 2017, 33, 179–186. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Carranza, T.; Santos-Vizcaino, E.; Igartua, M.; Guerrero, P.; Hernandez, R.M.; de la Caba, K. Hybrid 3D Printed and Electrospun Multi-Scale Hierarchical Polycaprolactone Scaffolds to Induce Bone Differentiation. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Feng, J.Y.; Xu, R.X.; Zhao, J.M.; Zhang, L.X. Multi-scale Nano/Micro Fiber Scaffolds with Different Topological Morphologies. Fiber. Polym. 2022, 23, 935–943. [Google Scholar] [CrossRef]
- Yan, F.F.; Liu, Y.Y.; Chen, H.P.; Zhang, F.H.; Zheng, L.L.; Hu, Q.X. A multi-scale controlled tissue engineering scaffold prepared by 3D printing and NFES technology. AIP Adv. 2014, 4, 8. [Google Scholar] [CrossRef]
- Gao, Q.; Xie, C.; Wang, P.; Xie, M.; Li, H.; Sun, A.; Fu, J.; He, Y. 3D printed multi-scale scaffolds with ultrafine fibers for providing excellent biocompatibility. Materials science & engineering. C, Materials for biological applications 2020, 107, 110269. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Xia, J.; Zhou, Z.; Fang, Y.; Zhang, L.; Sun, W. Multi-scale hierarchical scaffolds with aligned micro-fibers for promoting cell alignment. Biomedical materials (Bristol, England) 2021, 16. [Google Scholar] [CrossRef]
- Lin, H.; Shi, S.; Lan, X.; Quan, X.; Xu, Q.; Yao, G.; Liu, J.; Shuai, X.; Wang, C.; Li, X.; et al. Scaffold 3D-Printed from Metallic Nanoparticles-Containing Ink Simultaneously Eradicates Tumor and Repairs Tumor-Associated Bone Defects. Small Methods 2021, 5, e2100536. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, J.; Ma, Z.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.; Pei, X.; Wang, J.; Wan, Q. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng Regen Med 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Electrospun Nanomaterials Based on Cellulose and Its Derivatives for Cell Cultures: Recent Developments and Challenges. Polymers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jang, J.; Kim, K.; Kim, J.; Park, C.B. “Tree to Bone”: Lignin/Polycaprolactone Nanofibers for Hydroxyapatite Biomineralization. Biomacromolecules 2019, 20, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, J.; Gao, X.; Yuan, D.; Gu, Z.; Xu, Y. Electrospun nanofibers for bone regeneration: from biomimetic composition, structure to function. Journal of materials chemistry. B 2022, 10, 6078–6106. [Google Scholar] [CrossRef]
- Xu, T.; Yang, H.; Yang, D.; Yu, Z.Z. Polylactic Acid Nanofiber Scaffold Decorated with Chitosan Islandlike Topography for Bone Tissue Engineering. ACS Appl Mater Interfaces 2017, 9, 21094–21104. [Google Scholar] [CrossRef] [PubMed]
- Abadi, F.J.H.; Tehran, M.A.; Zamani, F.; Nematollahi, M.; Mobarakeh, L.G.; Nasr-Esfahani, M.H. Effect of Nanoporous Fibers on Growth and Proliferation of Cells on Electrospun Poly (epsilon-caprolactone) Scaffolds. Int. J. Polym. Mater. Polym. Biomat. 2014, 63, 57–64. [Google Scholar] [CrossRef]
- Hejazi, F.; Mirzadeh, H.; Shojaei, S. PCL-based 3D nanofibrous structure with well-designed morphology and enhanced specific surface area for tissue engineering application. Progress in biomaterials 2023, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Moroni, L. Janus 3D printed dynamic scaffolds for nanovibration-driven bone regeneration. Nature communications 2021, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact Mater 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, D.R.; Tian, H.M.; Huang, Q.Y.; Wang, C.H.; Chen, X.L.; Gao, Y.; Li, X.M.; Chen, X.M.; Zheng, Z.J.; et al. Bioinspired Hierarchical Structures for Contact-Sensible Adhesives. Adv. Funct. Mater. 2022, 32, 9. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Materials science & engineering. C, Materials for biological applications 2019, 104, 109960. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. International journal of oral science 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Elsayed, H.; Franchin, G.; Colombo, P. 3D printing of polymer-derived SiOC with hierarchical and tunable porosity. Addit. Manuf. 2020, 36, 10. [Google Scholar] [CrossRef]
- Kim, J.A.; Lim, J.; Naren, R.; Yun, H.S.; Park, E.K. Effect of the biodegradation rate controlled by pore structures in magnesium phosphate ceramic scaffolds on bone tissue regeneration in vivo. Acta Biomater 2016, 44, 155–167. [Google Scholar] [CrossRef]
- Zhou, C.C.; Yang, K.; Wang, K.F.; Pei, X.; Dong, Z.H.; Hong, Y.L.; Zhang, X.D. Combination of fused deposition modeling and gas foaming technique to fabricated hierarchical macro/microporous polymer scaffolds. Mater. Des. 2016, 109, 415–424. [Google Scholar] [CrossRef]
- Shabab, T.; Bas, O.; Dargaville, B.L.; Ravichandran, A.; Tran, P.A.; Hutmacher, D.W. Microporous/Macroporous Polycaprolactone Scaffolds for Dental Applications. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Men, N.T.H.; Jeong, T.H.; Kim, S.Y.; Kim, K.B.; Ha, T.H.; Ahn, S.J.; Kim, Y.H. Porous structures prepared by a novel route: Combination of digital light processing 3D printing and leaching method. J. Manuf. Process. 2021, 67, 46–51. [Google Scholar] [CrossRef]
- Montelongo, S.A.; Chiou, G.; Ong, J.L.; Bizios, R.; Guda, T. Development of bioinks for 3D printing microporous, sintered calcium phosphate scaffolds. Journal of materials science. Materials in medicine 2021, 32, 94. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Pei, X.; Song, P.; Sun, H.; Li, H.Y.; Fan, Y.J.; Jiang, Q.; Zhou, C.C.; Zhang, X.D. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. Pt. B-Eng. 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Snyder, J.E.; Hunger, P.M.; Wang, C.; Hamid, Q.; Wegst, U.G.; Sun, W. Combined multi-nozzle deposition and freeze casting process to superimpose two porous networks for hierarchical three-dimensional microenvironment. Biofabrication 2014, 6, 015007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Zhou, J.; Liu, C.; Zhang, J.M.; Shibata, Y.; Kong, N.; Corbo, C.; Harris, M.B.; Tao, W. Emerging biomimetic nanotechnology in orthopedic diseases: progress, challenges, and opportunities. Trends Chem. 2022, 4, 420–436. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomedical materials (Bristol, England) 2016, 11, 014102. [Google Scholar] [CrossRef] [PubMed]

| Technique | Advantages | Disadvantages |
|---|---|---|
| Solvent casting/particle leaching | low-cost; effective control and tunable of appropriate structures | organic solvents residues; time consuming; limited bulk volume |
| Freeze-drying | high porosity and interconnection; low-cost | poor control of the location of pore space |
| Phase separation | high degree of adjustability; easy to combine with other manufacturing techniques | utilization of organic solvents; Inadequate regulation of pore interconnection |
| additive manufacturing | creating scaffolds with precise design | high-cost; time-consuming |
| electrospinning | produce nano-scale fibers to mimic nano features | high-cost; time-consuming |
| Assembly process | Manufactrure of multi-scale structure | Characteristics of multi-scale structure | Ref |
|---|---|---|---|
| Gas foaming + porogen leaching | The size and concentration of the pore-foaming agent were utilized to control the formation and size of macro-pores. The micro-pores were controlled by adjusting the gas foaming parameters. | The multi-scale porosity was beneficial to cell adhesion, proliferation and differentiation. | [163] |
| Dynamic freeze casting (DFC) + micro-arc oxidation (MAO) | DFC was used to produce a porous structure with relatively large pores and high porosity, MAO was used to form microporous surface. | The compressive strength and elastic modulus were controlled by manipulating the porosity. The multi-scale porous scaffolds showed better biological response. | [164] |
| Freeze casting + porogen leaching | Freeze casting was used to produce relatively large dendritic pores with several tens of microns size, porogen leaching was used to form micron-sized small spherical pores. | The two methods are combined to readily tailored the porosity and compressive strength. | [165] |
| Thermally induced phase separation (TIPS) + porogen leaching | The size of the nanofibers could be regulated by adjusting the concentration of the polymer and phase separation temperature. The large pore size can also be customized according to the template sphere size. | The hierarchical structure of the scaffolds provided a large surface area, enhancing both the bioactivity and the potential as a drug delivery depot. | [166] |
| Photolithography + chemical modification | The multi-scale features of the hole/column/groove/ridge based on 3D were prepared on the PDMS carrier by photolithography, and the nano surface were obtained by chemical modification. | The multi-scale structures synergically affected the cell behaviors. | [167] |
| 3D printing + MAO | The macroporous titanium implants prepared by 3D printing were treated with MAO to obtain nano-morphology. | Such scaffolds with micro-nano morphology were beneficial to enhance apatite induction ability in vitro and bone integration ability in vivo. | [168] |
| 3D printing + electrospinning | The macro- /micro-scale porous scaffolds with multi-scale (bimodal) pore diamerter distribution of 300μm and 20μm were prepared by combining 3D printing and electrospinning techniques. | The scaffolds could promote the expression of osteogenic markers in MC3T3 cells. | [169] |
| 3D printing + electrospinning | Polyester warp knitted scaffolds were utilized to mimic the collagen fiber layer of the natural ECM with a pore size range of 1-5 µm. Electrospinning were used to prepare nano-scale morphology and 3D printing were employed to replicate micro-scale morphology. | The multi-scale scaffold can provide structural and mechanical support. | [170] |
| 3D printing + near-field electro-spinning (NFES) | 3D printing were used to prepare the macro-scaffold, the NFES were used to build tissue micro-morphology . | The macro- and micro-structures have high controllability, which meet the needs of mechanical properties in tissue engineering. | [171] |
| Melt deposition modeling (FDM)+ electro-hydrodynamic (EHD)printing | Through the manipulation of modules to regulate the electric field and extrusion, it is possible to convert between FMD and EHD modes during the printing process. This enables the creation of a multi-scale direct writing system wherein both coarse and fine fibers can be effortlessly printed using a single device. | Within this multi-scale composite scaffold, the coarse fibers offer adequate strength, while the fine fibers create a microenvironment that is conducive to cell adhesion | [172] |
| Melt deposition modeling (FDM)+ Melt electrospinning (MEW)+ solution electrospinning (SE) | The meso-, micro-, and nano-fibers fabricated via FDM, MEW, and SE can provide structural support, promote cell alignment, and create a biomimetic microenvironment to facilitate cell function. | Multi-scale hierarchical scaffolds can improve cell adhesion and proliferation, as well as facilitate cell alignment. | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




