Submitted:
15 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
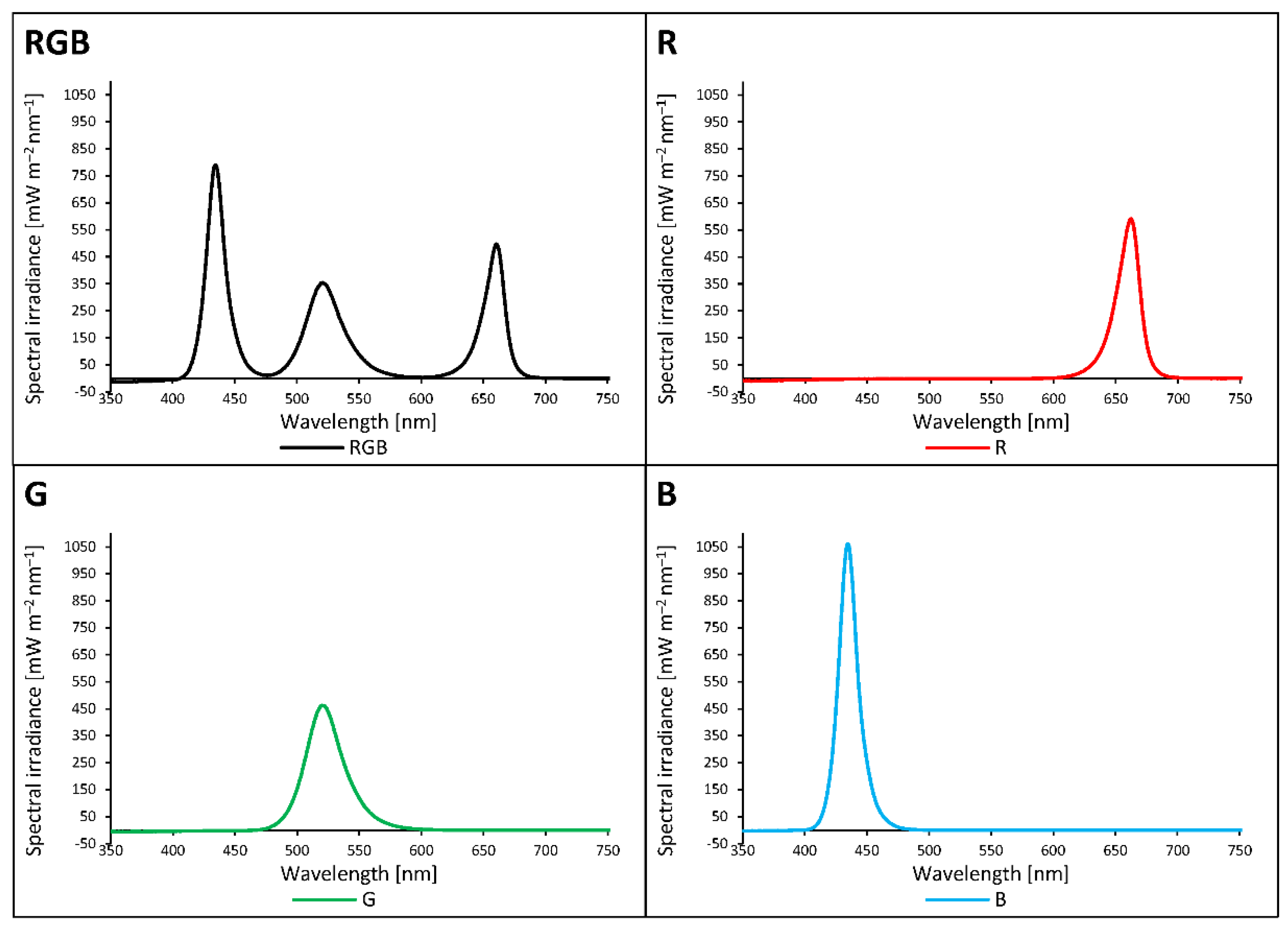
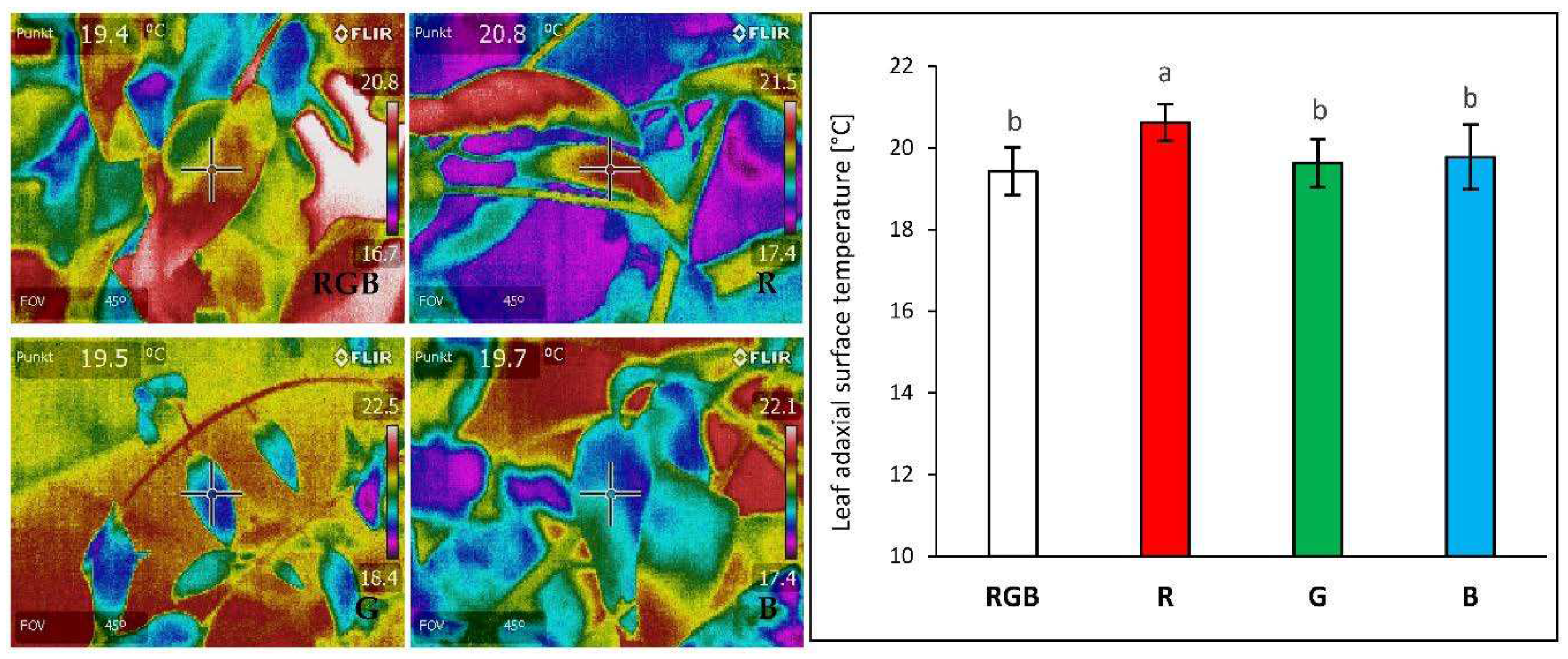
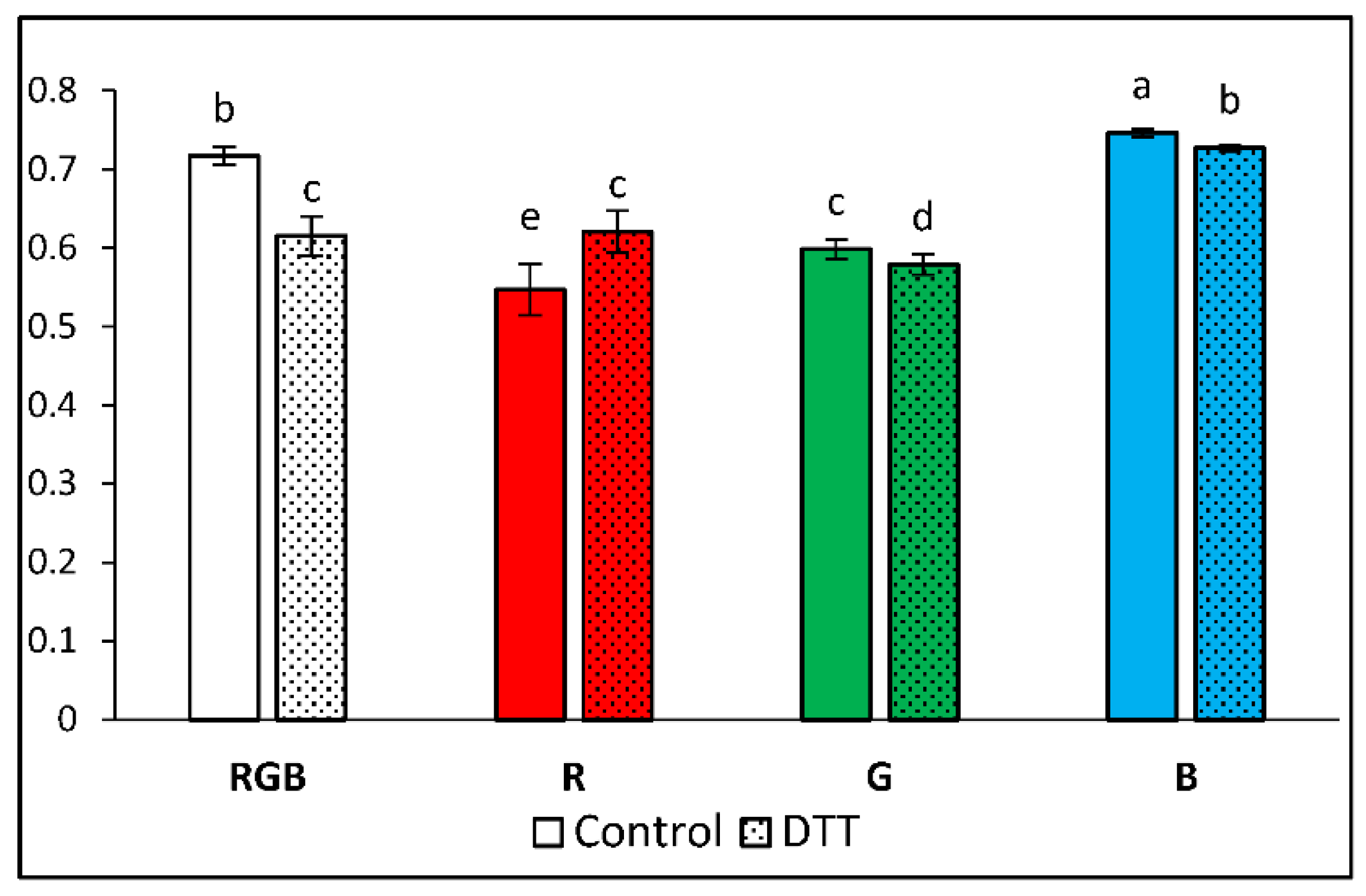
2.1. Influence of grow light spectra on the energy quenching and adaxial leaf surface temperature
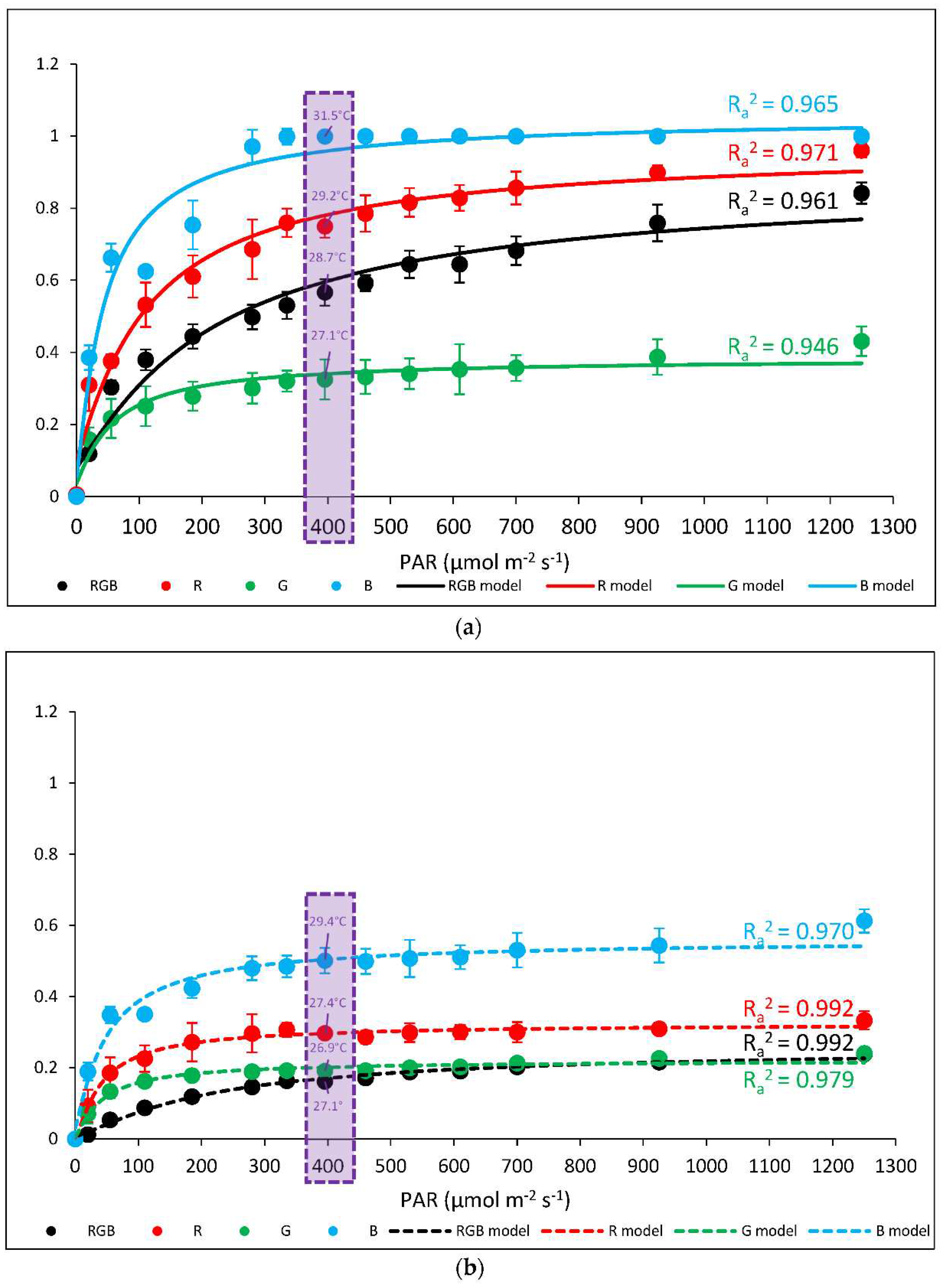
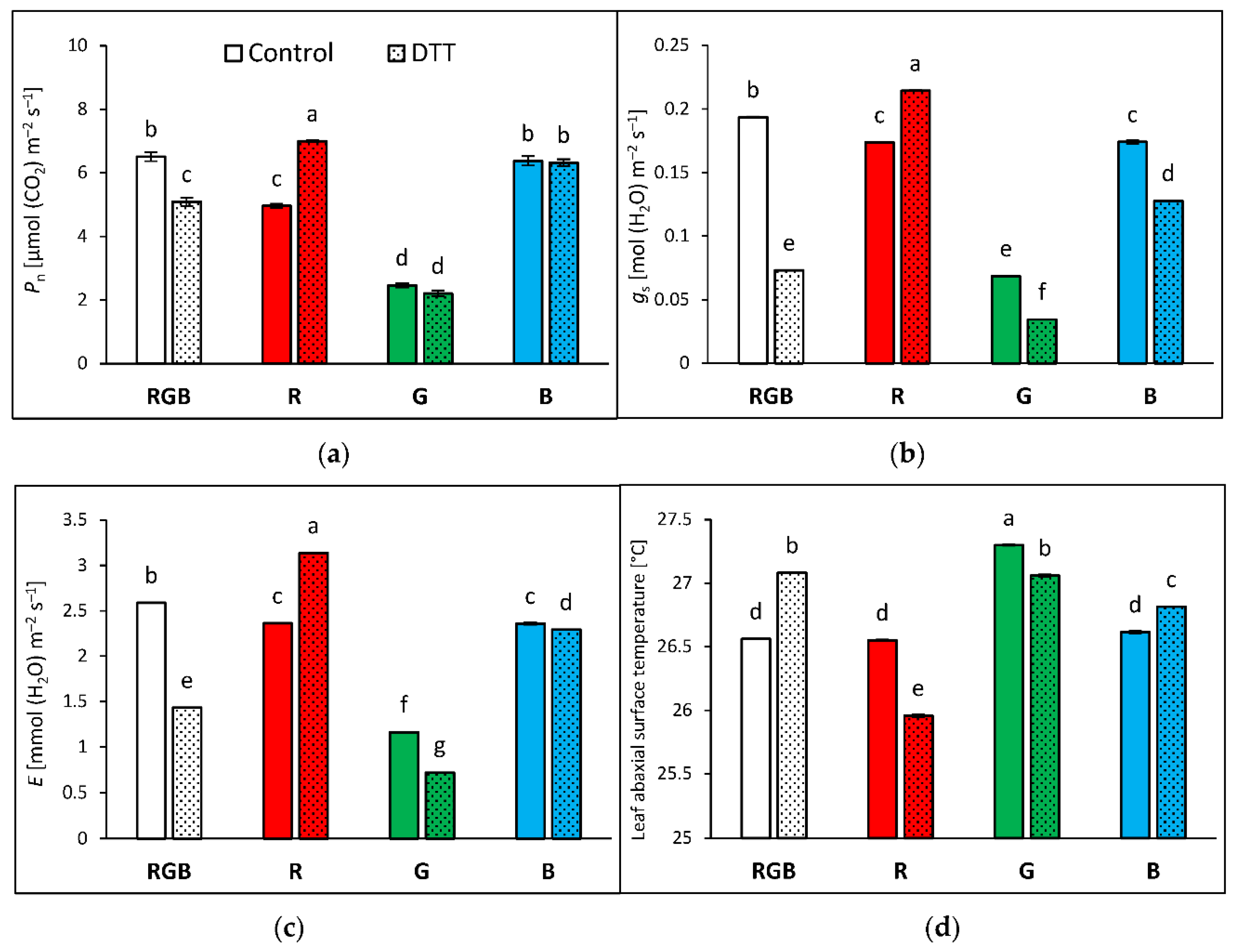
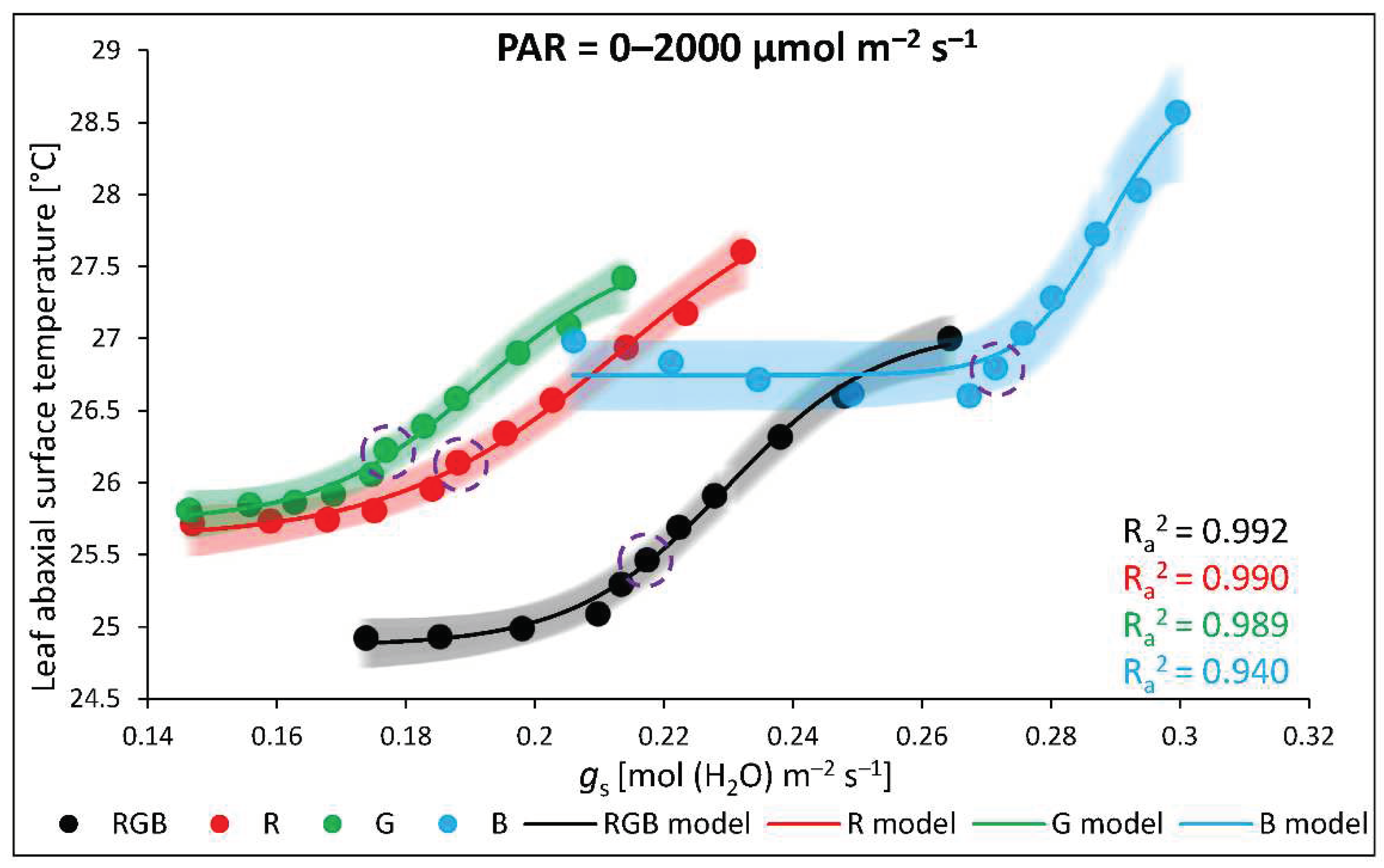
2.2. Influence of grow light spectra on stomatal traits, gas exchange and abaxial leaf surface temperature
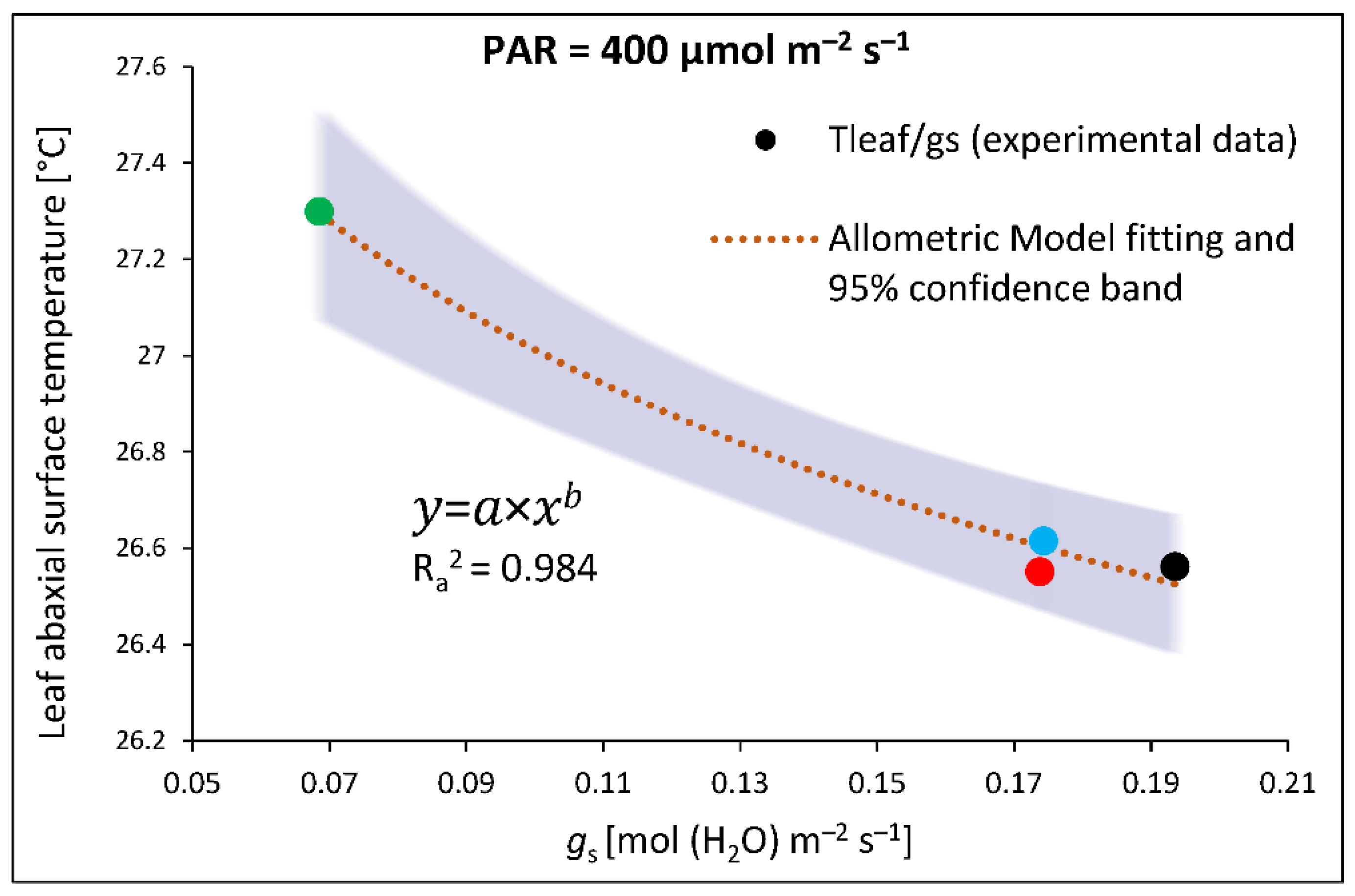
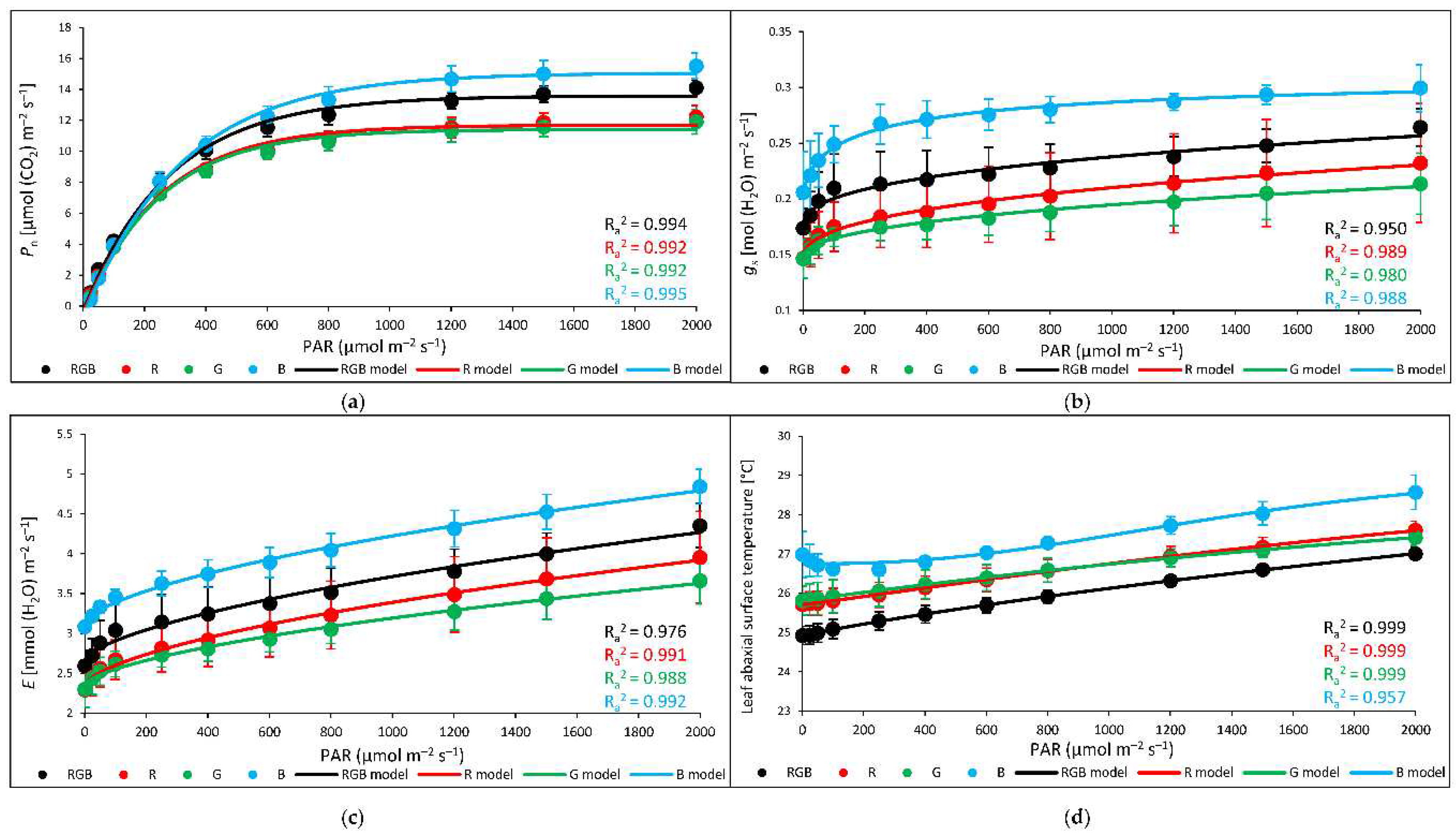
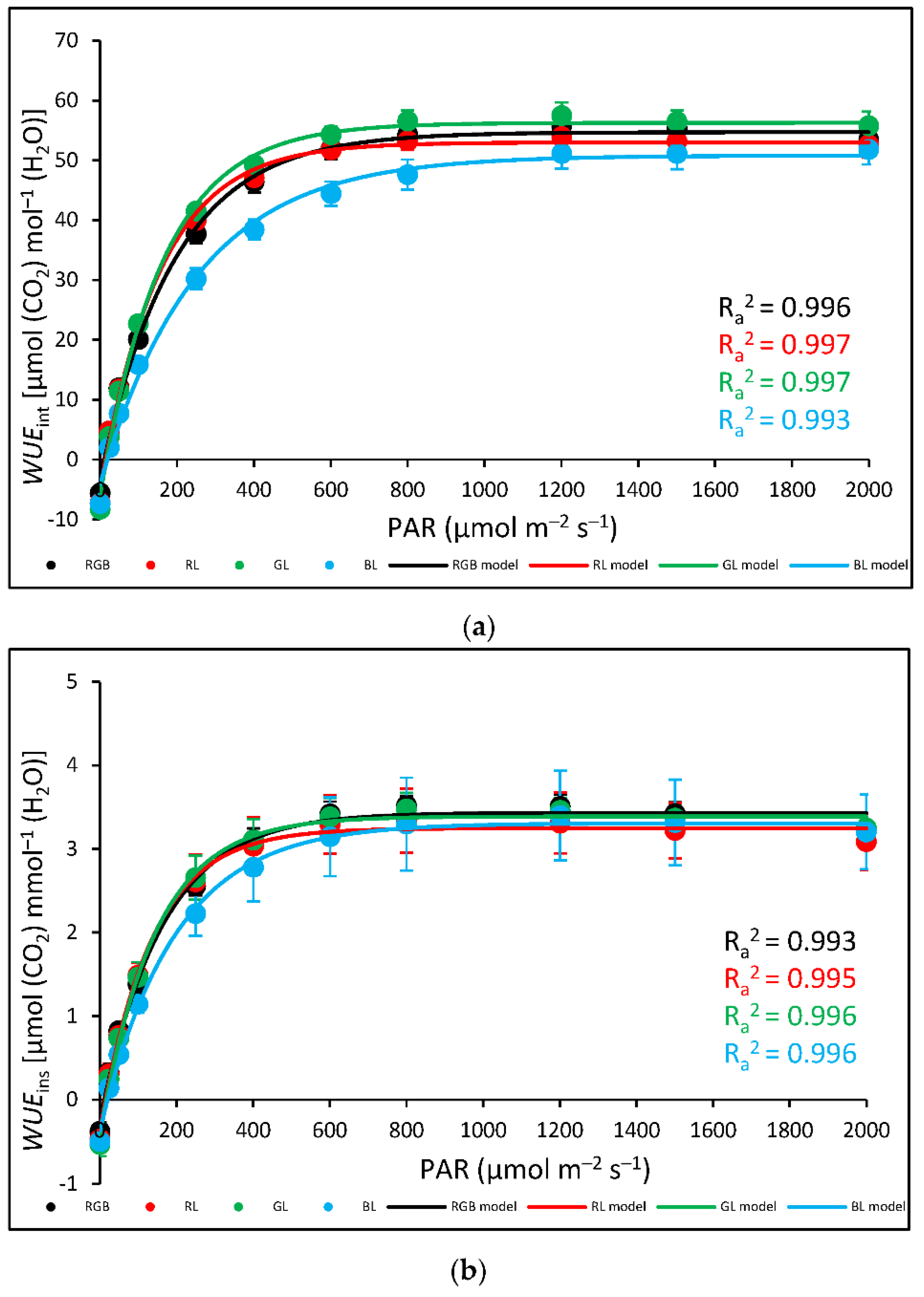
2.3. Influence of grow light spectra on gas exchange and abaxial leaf surface temperature in response to increasing light intensity (LC)
3. Discussion
3.1. Effect of long-term exposition of tomato plants to different light compositions on maximum PSII photochemical efficiency
3.2. Effect of long-term exposition of tomato plants to different light compositions on energy quenching and adaxial leaf surface temperature
3.3. Effect of long-term exposition of tomato plants to different light compositions on gas exchange parameters, stomatal traits and abaxial leaf surface temperature
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Pre-Illumination of Dithiothreitol-Infiltrated Leaf Samples
4.3. Rapid Light Curve (RLC) of Chlorophyll Fluorescence (ChF) Analyses
4.4. Determination of Leaf Temperature with FLIR
4.5. Stomatal Traits in the Various Lighting Spectra
4.6. Leaf Gas Exchange and Temperature Determination of Plants Grown in the Various Lighting Spectra
4.7. Models for Fitting of Experimental Data of Tleaf and NPQ or gs
- Linear regression fitting model (1) and double logarithmic reciprocal function of Bradley regression model (nonlinear, 2) for fitting data (x) of light-induced leaf temperature increase (ΔT) and the efficiency of light conversion to heat with qZ component of non-photochemical quenching (ΔNPQ) at 396 µmol m–2 s–1 B (450 nm) light:
- Allometric model (nonlinear, 3) for fitting data (x) of leaf abaxial surface temperature (Tabaxial) and the stomatal conductance (gs) at 400 µmol m–2 s–1 RGB (1:1:1) light:
- Log-logistic equation with variable Hill slope (p) model (nonlinear, 4) for fitting data (x) of leaf abaxial surface temperature (Tabaxial) and the stomatal conductance (gs) at 0–2000 µmol m–2 s–1 RB (R:B 10:1) light:
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iseki, K.; Olaleye, O. A new indicator of leaf stomatal conductance based on thermal imaging for field-grown cowpea. Plant Prod. Sci. 2020, 23, 136–147. [Google Scholar] [CrossRef]
- Gerhards, M.; Rock, G.; Schlerf, M.; Udelhoven, T. Water stress detection in potato plants using leaf temperature, emissivity, and reflectance. Int. J. Appl. Earth Obs. Geoinf. 2016, 53, 27–39. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E. Light quality-dependent regulation of non-photochemical quenching in tomato plants. Biology 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Stangl, Z.R.; Salmon, Y.; Burgess, A.J.; Murchie, E.H.; Robson, T.M. Sunflecks in the upper canopy: dynamics of light-use efficiency in sun and shade leaves of Fagus sylvatica. New Phytol. 2022, 235, 1365–1378. [Google Scholar] [CrossRef]
- Sukhova, E.; Khlopkov, A.; Vodeneev, V.; Sukhov, V. Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim. Biophys. Acta - Bioenerg. 2020, 1861, 148138. [Google Scholar] [CrossRef]
- Chang, C.Y.Y.; Bräutigam, K.; Hüner, N.P.; Ensminger, I. Champions of winter survival: cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef]
- Omasa, K.; Takayama, K. Simultaneous measurement of stomatal conductance, non-photochemical quenching, and photochemical yield of photosystem II in intact leaves by thermal and chlorophyll fluorescence imaging. Plant Cell Physiol. 2003, 44, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Kaňa, R.; Vass, I. Thermoimaging as a tool for studying light-induced heating of leaves: Correlation of heat dissipation with the efficiency of photosystem II photochemistry and non-photochemical quenching. Environ. Exp. Bot. 2008, 64, 90–96. [Google Scholar] [CrossRef]
- Alonso, L.; Van Wittenberghe, S.; Amorós-López, J.; Vila-Francés, J.; Gómez-Chova, L.; Moreno, J. Diurnal cycle relationships between passive fluorescence, PRI and NPQ of vegetation in a controlled stress experiment. Remote Sens. 2017, 9, 770. [Google Scholar] [CrossRef]
- Kang, H.X.; Zhu, X.G.; Yamori, W.; Tang, Y.H. Concurrent increases in leaf temperature with light accelerate photosynthetic induction in tropical tree seedlings. Front. Plant Sci. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Kulasek, M.; Bernacki, M.J.; Ciszak, K.; Witoń, D.; Karpiński, S. Contribution of PsbS function and stomatal conductance to foliar temperature in higher plants. Plant Cell Physiol. 2016, 57, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Trojak, M.; Skowron, E.; Sobala, T.; Kocurek, M.; Pałyga, J. Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynth. Res. 2022, 151, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Ahmed, M.A.; Ali, W.M.; Kalaji, H.M.; Elsheery, N.; Wróbel, J.; Xu, Y.; Chen, F. Effects of light spectrum on morpho-physiological traits of grafted tomato seedlings. PloS One 2021, 16, e0250210. [Google Scholar] [CrossRef]
- Ziegler-Jöns, A.; Knoppik, D.; Selinger, H. The calibration of thermocouples for leaf temperature measurements in gas exchange cuvettes. Oecologia 1986, 68, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2007, 58, 773–784. [Google Scholar] [CrossRef]
- Welc, R.; Luchowski, R.; Kluczyk, D.; Zubik-Duda, M.; Grudzinski, W.; Maksim, M.; Reszczynska, E.; Sowinski, K.; Mazur, R.; Nosalewicz, A.; Gruszecki, W.I. Mechanisms shaping the synergism of zeaxanthin and PsbS in photoprotective energy dissipation in the photosynthetic apparatus of plants. Plant J. 2021, 107, 418–433. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Winter, K.; Kruger, A.; Czygan, F.C. Zeaxanthin and the induction and relaxation kinetics of the dissipation of excess excitation energy in leaves in 2% O2, 0% CO2. Plant Physiol. 1989, 90, 887–893. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams III, W.W.; Heber, U.; Neimanis, S.; Winter, K.; Krüger, A.; Czygan, F.C.; Bilger, W.; Björkman, O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990, 92, 293–301. [Google Scholar] [CrossRef]
- Srivastava, A.; Zeiger, E. The inhibitor of zeaxanthin formation, dithiothreitol, inhibits blue-light-stimulated stomatal opening in Vicia faba. Planta 1995, 196, 450–457. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Dold, C. Water-use efficiency: advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X. G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Yang, Y.N.; Le, T.T.L.; Hwang, J.H.; Zulfugarov, I.S.; Kim, E.H.; Kim, H.U.; Jeon, J.S.; Lee, D.H.; Lee, C.H. High Light Acclimation Mechanisms Deficient in a PsbS-Knockout Arabidopsis Mutant. Int. J. Mol. Sci. 2022, 23, 2695. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M. Sager, J.C. Stomatal conductance of lettuce grown under or exposed to different light qualities. Ann. Bot. 2004, 94, 691–697. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J. Light Quality Affects Water Use of Sweet Basil by Changing Its Stomatal Development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Lin, A.; Yang, Y.; Zhang, G.; Xu, P.; Wu, Y.; Yang, Z. Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves. Agronomy 2023, 13, 1941. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.M.; Szymańska, R.; Niewiadomska, E.; Rozpądek, P.; Wątor, K. Thermal analysis of stomatal response under salinity and high light. Int. J. Mol. Sci. 2021, 22, 4663. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bueno, M.L.; Illescas-Miranda, J.; Martín-Forero, A.F.; de Marcos, A.; Barón, M.; Fenoll, C.; Mena, M. An extremely low stomatal density mutant overcomes cooling limitations at supra-optimal temperature by adjusting stomatal size and leaf thickness. Front. Plant Sci. 2022, 13, 919299. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wang, H.Y.; Gong, X.C.; Zeng, Z.H.; Xue, X.Z.; Hu, Y.G. Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J. Integr. Agric. 2021, 20, 2914–2931. [Google Scholar] [CrossRef]
- Nie, W.F.; Li, Y. , Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B, Biol. 2023, 241, 112673. [Google Scholar] [CrossRef]
- Su, P.; Ding, S.; Wang, D.; Kan, W.; Yuan, M.; Chen, X.; Tang, C.; Hou, J.; Wu, L. Plant morphology, secondary metabolites and chlorophyll fluorescence of Artemisia argyi under different LED environments. Photosynth. Res. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef]
- Jiang, C.D.; Gao, H.Y.; Zou, Q.; Jiang, G.M. Inhibition of photosynthesis by shift in the balance of excitation energy distribution between photosystems in dithiothreitol treated soybean leaves. Photosynthetica 2004, 42, 409–415. [Google Scholar] [CrossRef]
- Ferroni, L.; Colpo, A.; Baldisserotto, C.; Pancaldi, S. In an ancient vascular plant the intermediate relaxing component of NPQ depends on a reduced stroma: Evidence from dithiothreitol treatment. J. Photochem. Photobiol. B, Biol. 2021, 215, 112114. [Google Scholar] [CrossRef]
- Laisk, A.; Oja, V.; Eichelmann, H.; Dall’Osto, L. Action spectra of photosystems II and I and quantum yield of photosynthesis in leaves in State 1. Biochim. Biophys. Acta - Bioenerg. 2014, 1837, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, E.; van Amerongen, H.; Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta - Bioenerg. 2013, 1827, 420–426. [Google Scholar] [CrossRef]
- Materová, Z.; Sobotka, R.; Zdvihalová, B.; Oravec, M.; Nezval, J.; Karlický, V.; Vrábl, D.; Štroch, M.; Špunda, V. Monochromatic green light induces an aberrant accumulation of geranylgeranyled chlorophylls in plants. Plant Physiol. Biochem. 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Nelson, J. A.; Bugbee, B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PloS One 2015, 10, e0138930. [Google Scholar] [CrossRef]
- Kume, A. Importance of the green color, absorption gradient, and spectral absorption of chloroplasts for the radiative energy balance of leaves. J. Plant Res. 2017, 130, 501–514. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- Holzwarth, A.R.; Miloslavina, Y.; Nilkens, M.; Jahns, P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 2009, 483, 262–267. [Google Scholar] [CrossRef]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta - Bioenerg. 2010, 1797, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Belgio, E.; Ruban, A.V. Comparison of the protective effectiveness of NPQ in Arabidopsis plants deficient in PsbS protein and zeaxanthin. J. Exp. Bot. 2015, 66, 1259–1270. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Okoń, K.; Skorupka, M. Non-Photochemical Quenching under Drought and Fluctuating Light. Int. J. Mol. Sci. 2022, 23, 5182. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, J.N.; Bahamonde, H.A.; Mühling, K.H.; Fernández, V. Tomato and Pepper Leaf Parts Contribute Differently to the Absorption of Foliar-Applied Potassium Dihydrogen Phosphate. Plants 2023, 12, 2152. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhang, X.; Wang, Y.; Lu, C. Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L.) seedlings under drought conditions. Environ. Exp. Bot. 2019, 167, 103844. [Google Scholar] [CrossRef]
- Busch, B.L.; Schmitz, G.; Rossmann, S.; Piron, F.; Ding, J.; Bendahmane, A.; Theres, K. Shoot branching and leaf dissection in tomatoes are regulated by homologous gene modules. Plant Cell 2011, 23, 3595–3609. [Google Scholar] [CrossRef]
- Pinnola, A.; Dall’Osto, L.; Gerotto, C.; Morosinotto, T.; Bassi, R.; Alboresi, A. Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell 2013, 25, 3519–3534. [Google Scholar] [CrossRef]
- Kalituho, L.; Beran, K.C.; Jahns, P. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol. 2007, 143, 1861–1870. [Google Scholar] [CrossRef]
- Herlory, O.; Richard, P.; Blanchard, G.F. Methodology of light response curves: application of chlorophyll fluorescence to microphytobenthic biofilms. Mar. Biol. 2007, 153, 91–101. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Wang, F.F.; Lian, H.L.; Kang, C.Y.; Yang, H.Q. Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol. Plant 2010, 3, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Rui, Y.; Kandemir, B.; Wang, J.Z.; Anderson, C.T.; Puri, V.M. Mechanical effects of cellulose, xyloglucan, and pectins on stomatal guard cells of Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]

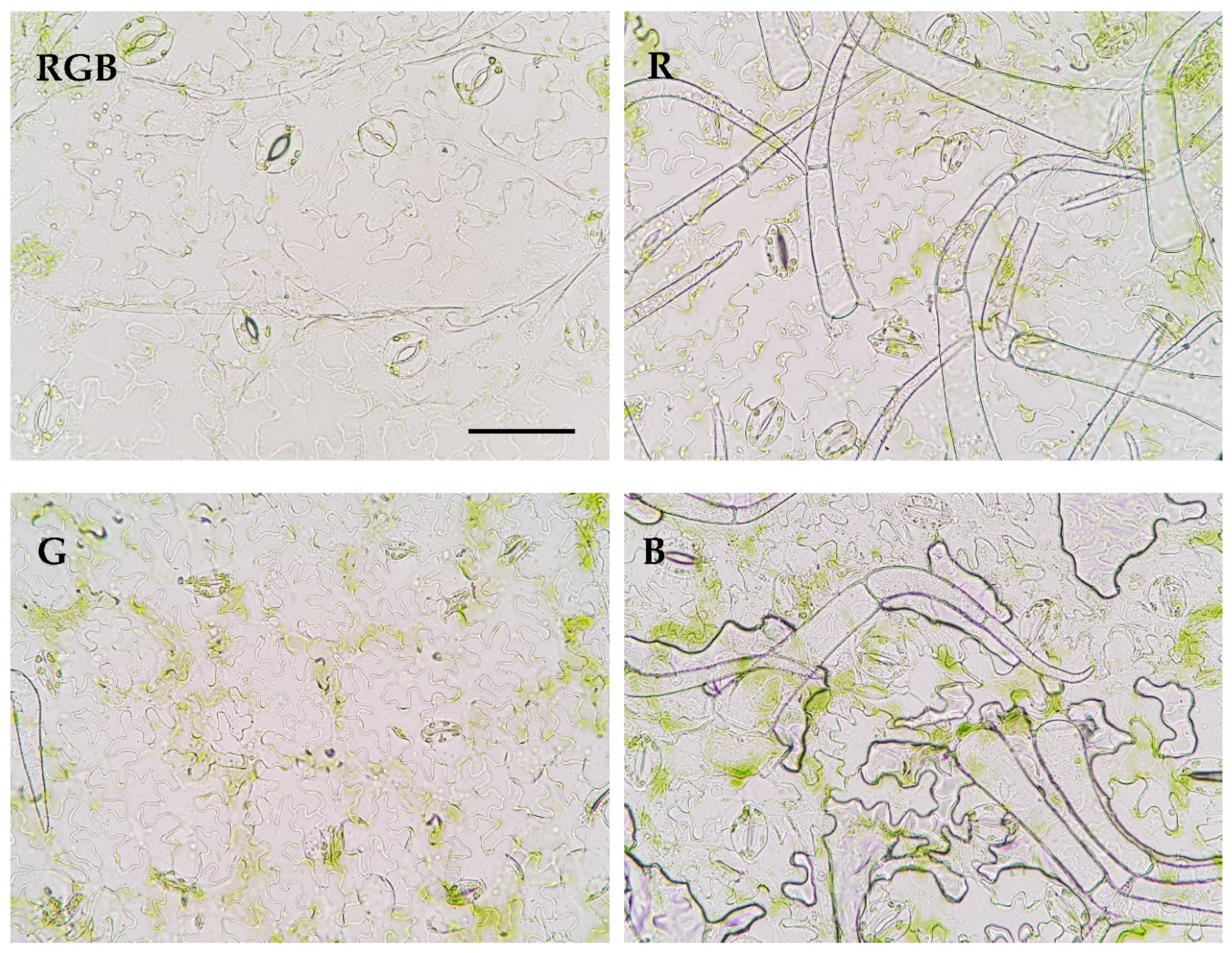
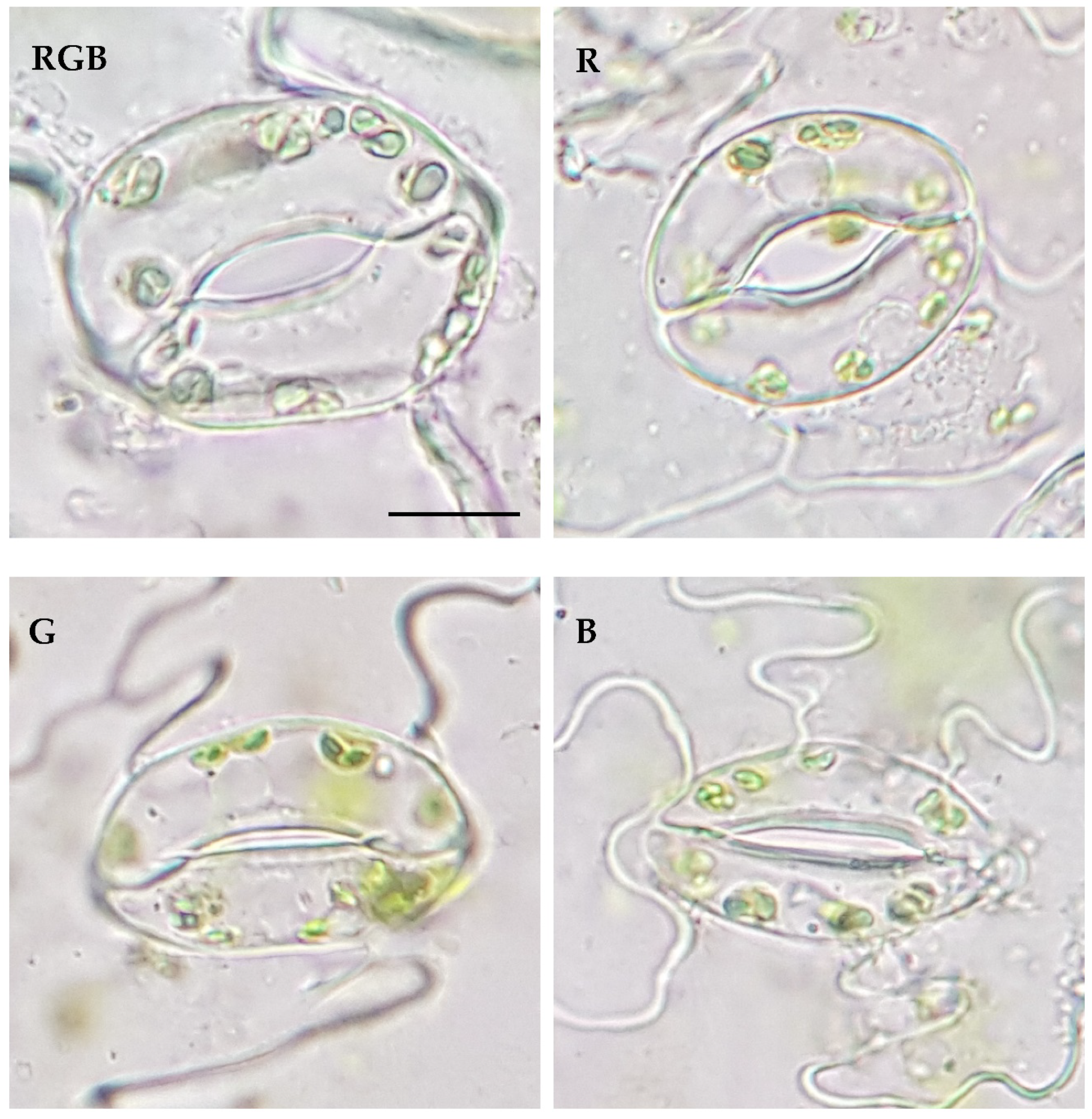
| Parameter | Treatment | |||
|---|---|---|---|---|
| Stomatal traits (abaxial leaf surface) | RGB | R | G | B |
| Stomatal complex width (µm) | 24.00±3.49a | 19.39±1.87b | 17.84±0.81b | 17.62±2.14b |
| Stomatal complex length (µm) | 29.82±3.19a | 24.24±2.17b | 25.06±3.42b | 26.07±1.86b |
| Stomatal width/length ratio | 0.80±0.06a | 0.80±0.04a | 0.72±0.1b | 0.68±0.08b |
| Stomatal complex area (µm2) | 568.44±139.38a | 373.63±59.72b | 349.19±61.03b | 347.07±60.38b |
| Pore width (µm) | 4.04±0.88ab | 4.34±0.91a | 1.90±0.67c | 3.65±0.99b |
| Pore length (µm) | 12.32±1.59b | 12.02±1.22b | 12.26±2.18b | 13.78±1.62a |
| Aspect ratio of pores (width/length) | 0.33±0.06a | 0.36±0.07a | 0.16±0.05c | 0.26±0.06b |
| Stomatal pore area (µm2) | 34.14±9.12a | 35.27±8.69a | 17.15±6.00b | 29.90±8.89a |
| Stomatal density Sd (no. mm–2) | 117.22±5.08b | 140.66±8.79a | 90.84±13.43c | 137.73±18.30a |
| Pore area per leaf area (µm2 mm–2) | 4002.09±1069.27b | 4960.61±1222.53a | 1557.91±545.44c | 4100.42±1219.19b |
| Total pore area per total leaf surface (µm2 cm–2) | 18019.50±4814.41a | 11952.84±2945.76b | 4321.61±1513.05c | 19751.74±5872.84a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





