Introduction
Coral reefs are degrading through the past few decades, reducing aquatic species diversity around the world (DeVantier, Turak and Szava-Kovats, 2020). Over this time, coral live cover has been heavily impacted by anthropogenic activities (Eddy et al., 2021), in turn affecting the local economy and international human welfare significantly (Costanza et al., 2014). These reefs majorly contribute to the Philippine economy through fishing yields and sustaining local communities around the region, with small-scale fishers alone contributing more than $1 billion annually to its economy (White et al, 2000). However, they have been subject to major reef decline since the 1970s, with only 30% of the coral reefs with greater than 50% coral cover in them, a number that will only decline faster as the area becomes affected humanity (Gomez et al., 1994). <- need newer ref.
Palawan is one of the last frontiers for marine life in the Philippines. The area has seen a significant decline in both animal biomass and reef health, induced by warmer temperatures and human activity (Bernert et al., 2014). There have been many immigrants seeking to reap the benefits of the high fishing yields since the popularization of the province, as well as increasing tourism, transport and agricultural activity within the country before the COVID-19 pandemic occurred (Pestano et al, 2014). Development continually rises in this coastal region, with the high coral cover and biodiversity of species which the area is known for, decreasing at a fascinating rate (Aludia, 2022). Through various means of destructive fishing (Hodgson and Dixon, 1988), as well as increasing numbers of commercial-scale fishing boats throughout the area, Marine Protected Areas (MPAs) are not shown to be a sole solution towards overfishing and reef harm in the country with no significant differences between those protected and unprotected (Panga et al., 2021). Overfishing continues to affect reefs greater than most other non-human-induced sources (Jackson et al., 2001), and there has been significantly less herbivores, such as the Chaetodontidae fish species, which clear reef substrate of algae for coral recruitment (Cole, Pratchett and Jones, 2008). With both anthropogenic and other natural events affecting the reef, permanent damage to them could affect the local ecosystem and economy as a whole (Hoegh-Guldberg et al., 2017).
Popular methods people use to attempt and reverse this issue are through protection of the reefs. With reef protection methods becoming more effective as more attention is brought to their significance, there are many pathways used to attempt to prevent the damage. For example, the implementation of MPAs to degrading coral reefs (DR-MPAs) is an important step in the right direction, as protecting degraded coral reefs is not a waste of resources but instead allows degraded reefs a chance to slowly recover. (Abelson et al., 2016). However, protection itself cannot solely bring the reef back to its original state; it needs to be combined with active rehabilitation of the area (Anthony et al., 2020). Without a boost in rehabilitation, reefs would be left to recover from destructive anthropogenic actions which hamper their ability to survive within normal conditions (Prada et al., 2019).
For a reef to recover properly, coral reef rehabilitation must also be focused on the specific conditions which the area faces (Boström-Einarsson et al., 2020). Many rehabilitation efforts are misguided in this manner, and may not see influential reef rehabilitation, or even face detrimental consequences towards the reef. A commonly used technique of rehabilitation is coral transplantation, which brings more healthy coral into a damaged area of the reef (Rodgers et al., 2017). If implemented correctly and in the right conditions, this allows for greater coral recruitment, letting the reef resist and return to its original state after a major disturbance (Martinez and Abelson, 2013). Therefore, reef transplantations must be done cautiously and with accurate understanding regarding the reef’s stressors.
With the reduction of coral cover and fish biomass, as well as a lack of Crown of Thorns Starfish predators, settlement values are a large concern for the development of reefs. If coral settlement decreases alongside high mortality rates, reefs are less likely to maintain their current healthy state (Fadlallah, 1983). Healthy coral reefs create sizable volumes of coral recruits, contributing to reef diversity; the reliance on recruitment for a reef in its developing and rehabilitation stages is vital (Martinez and Abelson, 2013). Sections of reefs which see low larval supply are problematic, as less coral larvae choosing to settle in these areas will affect the reef’s natural future rehabilitation and resilience. While fish biomass records, species diversity and structural complexity of the reefs are important factors of reef health, recruitment is crucial to the resilience and future of the reefs (Robinson et al., 2018).
This is where Pre-Launch Assessments prior to coral transplantation are useful. These recruitment surveys can be applied before coral transplantation to ensure the need of any intervention to the reef, as altering the structure of already healthy reefs may still cause detrimental effects. These assessments can be impactful in many areas of the reefs and their surroundings, and are easily measured. They can be taken by citizen scientists, as methods for measuring recruitment are particularly straightforward. Limited expert knowledge is needed, as coral species do not need to be defined, due to transplantation interventions mainly focusing on the limited frequency of species in the area. Particularly areas without this expert knowledge can highly benefit from this to determine vital reef stressors and locations of transplantation.
This study is used as an example assessment prior to rehabilitation efforts within the reef of Dimakya Island, in order to help decision-making of the approach of restoration. To ensure the proper implementation and need of rehabilitation effects such as coral transplantation, we will be measuring the varying levels of initial recruitment and comparing them to the frequency of older reef-building coral in the area. This will be synthesized with the impact of many external factors on the environment of the reef from both past events as well as observations during data collection, in order to identify the reef’s needs in rehabilitation and possibly further protection.
Materials and Methods
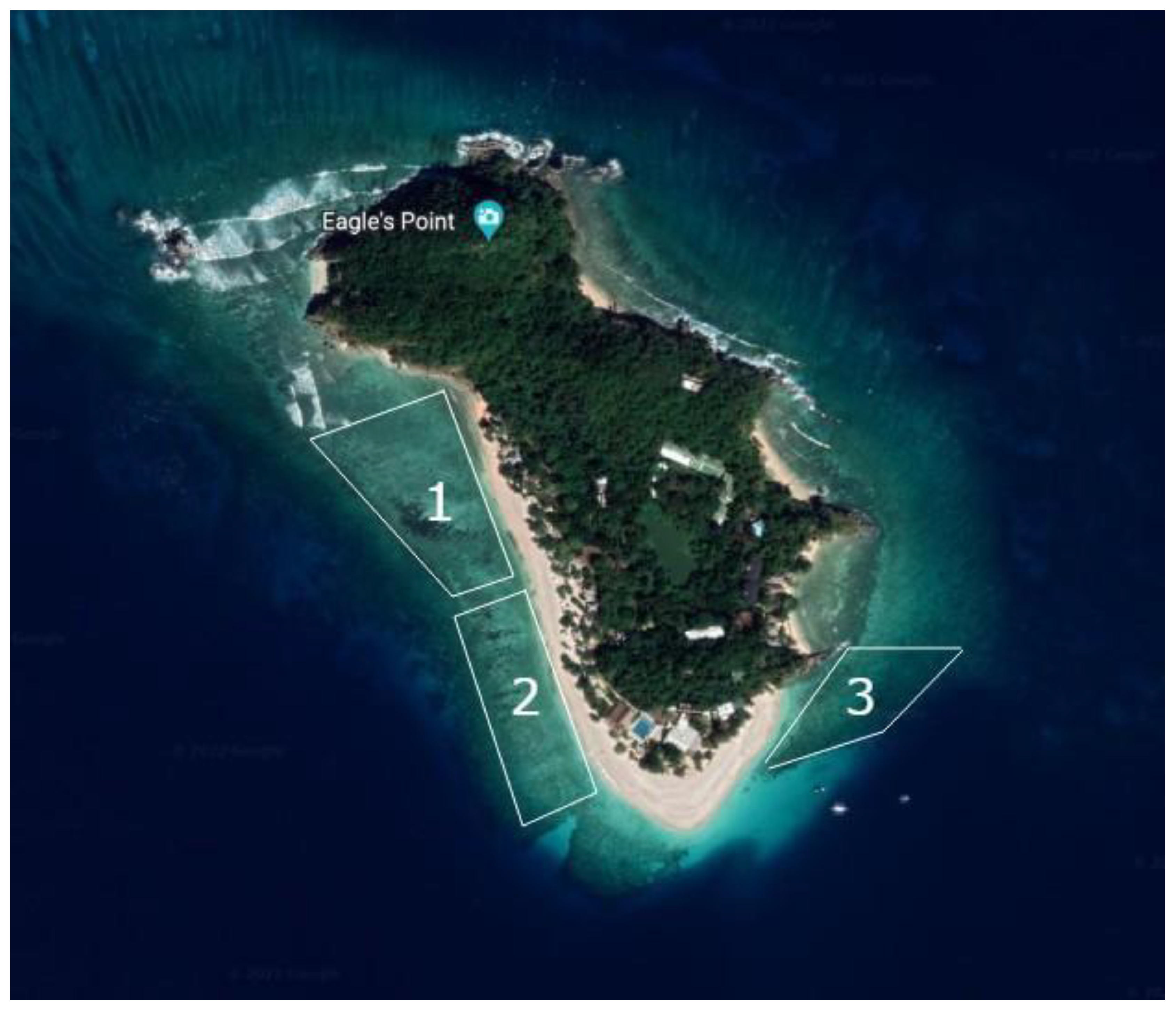
A survey of young corals, including newly settled coral recruits has been conducted in the reef of Dimakya Island, Palawan Province, Philippines (Fig.1) (12.2344°N, 120.0889°E). The studied reef is divided into three major sections, with sections 1 and 2 two facing westward and section 3 facing southeast. The sampling was conducted using a 1x1 meter quadrat with a 0.1m mesh grid placed haphazardly on the reef. During the survey we sampled coral recruitment at ca. 10m depth of the sloping reef. A flat and parallel plane was ensured between the surface and the quadrat. Survey dives were conducted during dusk hours (mostly from 6pm and 7pm GMT+8), between early July to August of 2022, to enable the identification of coral recruits using the UV light (Shick et al., 1996), and yellow-tinted ultraviolet goggles (model Blue Star and BlueBlock; NightSea, Bedford, MA, USA). Many coral emit fluorescent color when they settle, making it easy to distinguish from other types of unilluminated marine life in conditions with low light (Baird et al., 2006).
Figure 1.
- the three sections of the studied coral reef on Dimakya Island.
Figure 1.
- the three sections of the studied coral reef on Dimakya Island.
Four different diameter categories of corals were recorded within each measured quadrat: <1 cm, 1-3 cm, 3-5 cm, >5 cm. Videos were also taken using a GoPro and white light, as well as ultraviolet light, to verify the validity of the larger diameter of coral and distinguish it from other types of substrates post-dive in the lab.
Results
A total of 44 quadrats were recorded, 16 in section 1, 13 in section 2, and 14 in section 3 of the reef, amassing a total of 2247 corals recorded throughout the sampling process. There was a significant difference in the frequency of coral sizes recorded in all three of the areas, as well as a large variation in result patterns between the sections of the reef.
Section 1: (one-way ANOVA, p < 0.001)
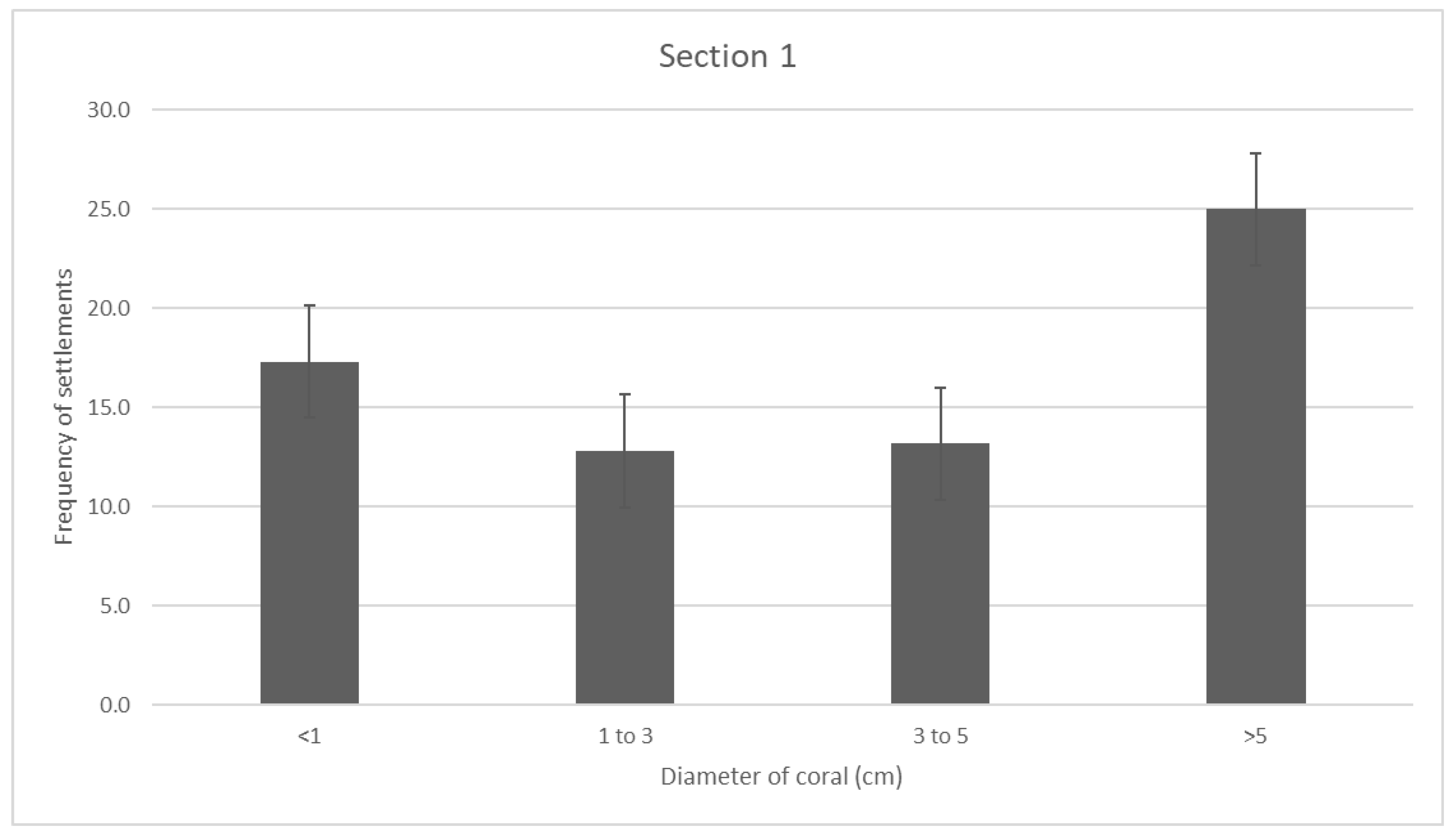
Data trends were similar in the section 1 reef, with d > 5cm having a much greater frequency of coral at 25.0. Unlike the section 2 reef, this reef also has a greater frequency in newly settled recruits, at 17.3, in comparison to 1 < d < 3 and 3 < d < 5, with a mean frequency of 12.8 and 13.2 respectively. Even with these different proportions of data, frequency in all diameters is significantly higher than the respective diameters recorded in the section 1 reef. The average recruits/sqm measured in section 1 is (INSERT).
Figure 2.
- section 1 reef coral distribution through the four different diameters measured. Error bars are standard error.
Figure 2.
- section 1 reef coral distribution through the four different diameters measured. Error bars are standard error.
Section 2: (one-way ANOVA, p < 0.001)
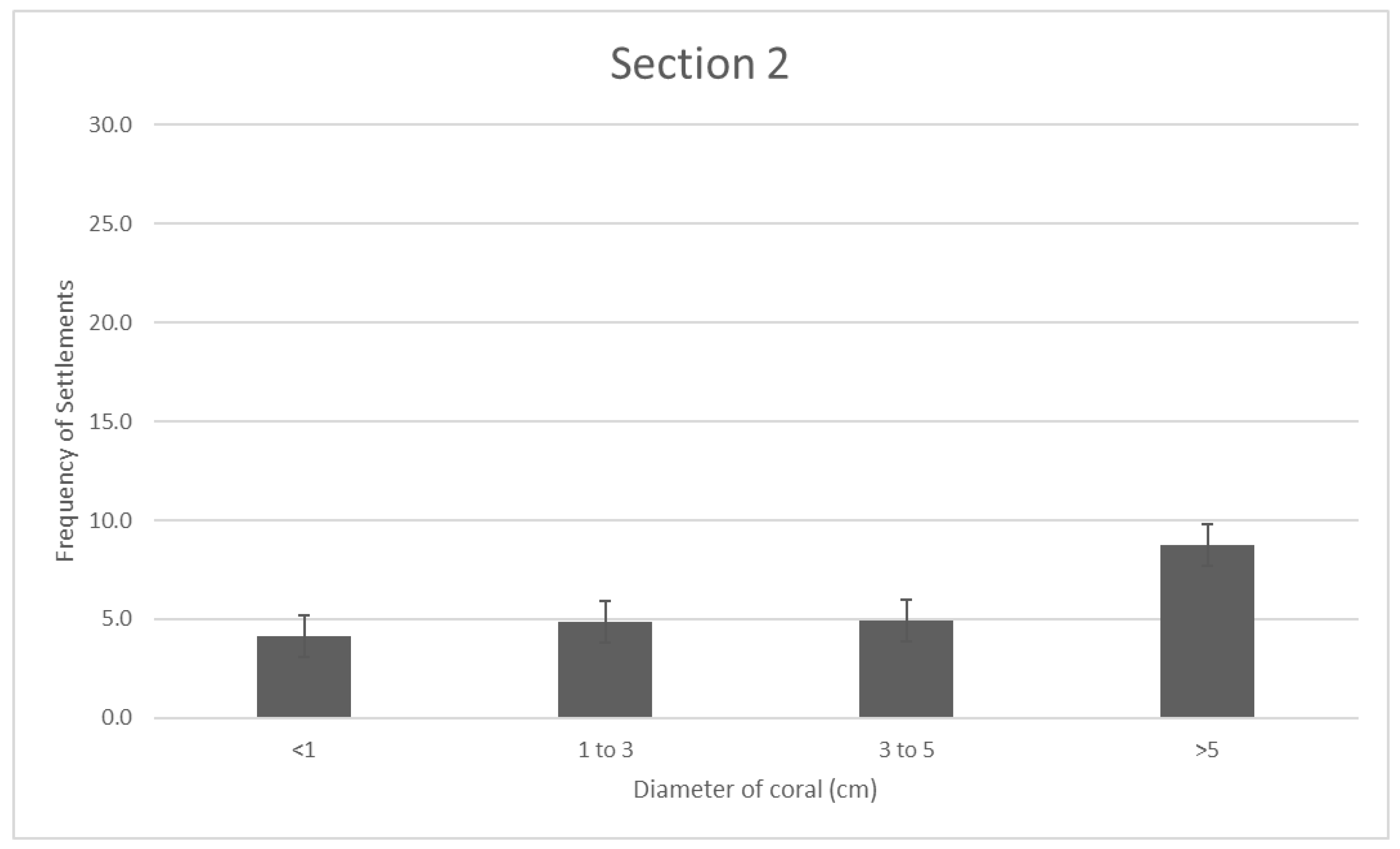
In section 2, there was a significant increase in corals with d > 5cm in comparison to the other three diameters collected, with a mean frequency of 8.8cm for d > 5. The other diameters have frequencies between 4 and 5, much lower than the larger diameter coral, and this section has the most even coral spread out of all three sites. Frequencies across all diameters gathered is visibly shown to be much lower than the other two sites. The average recruits/sqm measured in section 2 is (INSERT).
Figure 3.
- Section 2 reef coral distribution. Error bars are standard error.
Figure 3.
- Section 2 reef coral distribution. Error bars are standard error.
Section 3: (two-way ANOVA, p < 0.05)
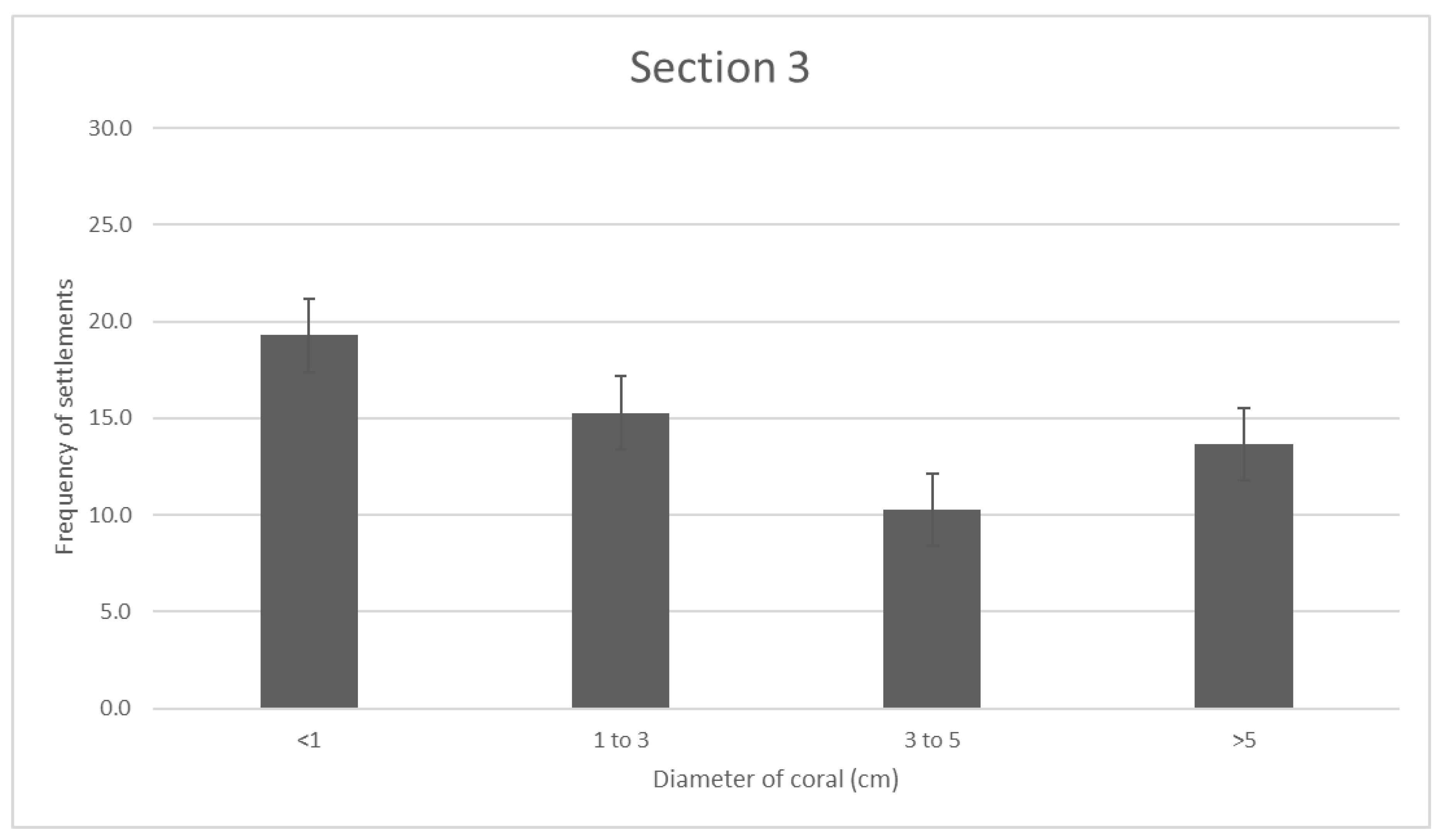
Section 3 shows very different results, as the frequency for new recruits is the greatest at a mean value of 19.3, while the frequencies of 3 < d < 5 and d > 5cm are proportionately lower, with mean frequency values of 10.3 and 13.6. This is the only section of coral reef where the smallest recruitment diameter has the highest frequency. The average recruits/sqm measured in section 1 is (INSERT).
Figure 4.
- Section 3 reef coral distribution. Error bars are standard error.
Figure 4.
- Section 3 reef coral distribution. Error bars are standard error.
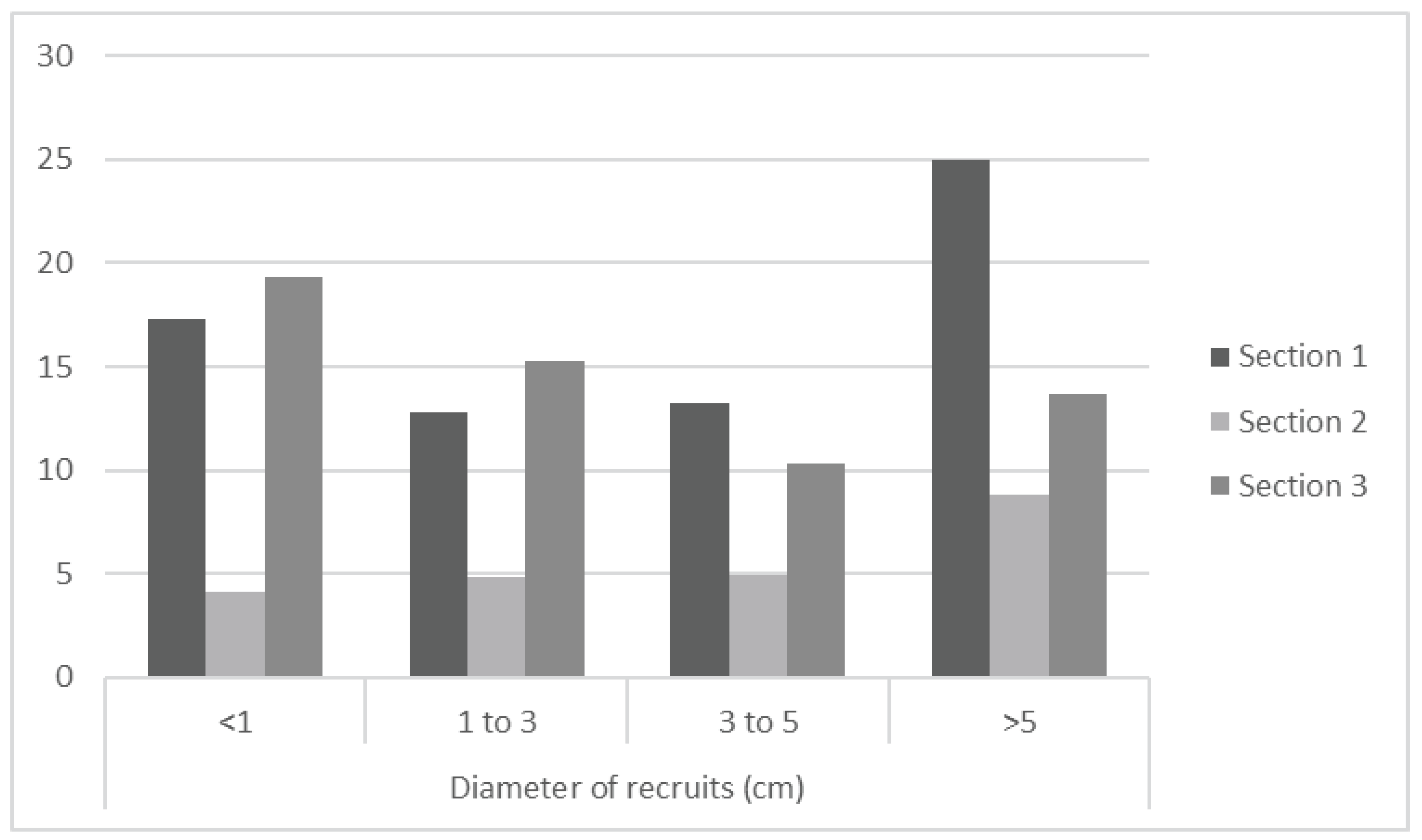
A comparison of all three sites indicates that section 3 has the greatest frequency of recently settled recruits, section 1 for larger diameter corals, and section 2 to be significantly smaller in coral cover and recruitment in comparison to the other sites.
Figure 5.
- all three sections plotted onto one graph, using a clustered column chart to represent and compare different frequencies.
Figure 5.
- all three sections plotted onto one graph, using a clustered column chart to represent and compare different frequencies.
Discussion
The results indicate significant differences between the three reef sections, with section 2 showing significantly less recruitment relative to the other two sections. While there is no significant correlation between the size of the coral and frequency, it is important to note the slight increase in frequency for s > 5cm for section 2, as well as the significantly larger frequency for section 3 in regards to s > 5cm. These may be attributed to recent impacts towards the coral reef, causing recruitment for newer coral to be less viable within the area as conditions change. We also must consider the greater potential range which corals in size class s > 5cm have in comparison to the other size classifications, as corals could range anywhere from recent larger settlements to massive old-growth corals.
Comparing each section of the reef to an average wide range of coral measures of 30 corals/sqm (Dietzel et al., 2021), section 2 has less than this average, with it being 21.07 corals/sqm. Sections 1 and 3 are well above the average, with the average corals/sqm calculated to be 70.07 for section 1, and 58.5 for section 3. Another study of coral recruits after a mass mortality event records the corals/sqm value to be 21.4 recruits (Tamelander, 2002): this value is around that of section 2, and much lower than both section 1 and 3. Comparing these recruitment numbers to another study after a mass coral bleaching event (Stobart et al., 2005), with the average recruitment at 10m to be 7-9 recruits, we can see recruitment numbers across all sites in our study to be much higher. This suggests the reef measured on average has relatively high values of coral cover relative to those amongst other foreign colonies.
This pre-launch assessment has many important factors; gaining the larval supply and settlement patterns for reef-building coral within the different areas gives insight into the main points of potential rehabilitation. Transplanting coral into section 2 may prove beneficial to reef and organism health in that area, as compared to the relatively healthier sections 1 and 3. Since data between different sizes of recruits was also gathered, we can find patterns in the survival rate of recruits as they mature within different areas. This survival rate and location allows us to determine potential harmful factors to certain sections of the reef (Martinez and Abelson, 2013). As we can see a significantly larger portion of mature coral (>5 cm) as compared to newer settlers in sections 1 and 2, we can hypothesize that these areas have been subject to a decline in optimal conditions for recruitment, and can link this to anthropogenic factors based on qualitative observations within the island.
Human impacts are arguably the most pressing factor of coral endangerment (Hoegh-Guldberg et al., 2017). Firstly, points of agricultural and animal culture are located at the mainland of the reef, specifically a cove facing the southeast side of the island (section 3), as well as a few rivers which all open into the ocean located at a nearby cove on the mainland, which collect agricultural runoff from the nearby rice paddies and farms. Some reefs around the area show a very murky upwelling of fresh water, with a depth around 1-2m above the reefs, and a significant greenish pigment. Coral reefs within this cove seem to be significantly more damaged and affected (Carlson et al, 2019), with healthy hard coral rarely present. However, despite the negative impacts of river runoff onto the coral reefs within the cove, recruitment levels are actually most promising in section 3 of the island, with recruitment frequency values of 19.3 and 15.1 for coral sizes <1 cm and 1-3 cm respectively. Resort boats are also frequently used section 2 to dock before, with previous management allowing anchors and boat speeding within the section, as there is a significantly large sandbed east of section 2 at the southern tip of the island, which has frequent dugong and sea turtles grazing. Anchors damaging all sizes of coral and scaring away fish may have been a contribution to the low frequency of coral across the board. Oil and fuel waste omitted by the boats may be a reason for lower frequency of coral in the area (Nordborg, Brinkman and Negri, 2022), and direct human activity is a probable reason as to the overall lower recruitment and coral cover values in this section. There is a mean frequency of only 4.1 corals with <1cm diameter in the area, and 8.8 corals with >5cm diameter, the lowest in any section by a noticeable proportion.
There are also many factors affecting coral recruitment and cover overall based on impacts created by non-anthropogenic means, initially through the analysis of the section 2 reef. Results show significantly lower coral recruitment in section 2. Though observations suggest section 2’s fish biomass to be the most plentiful in the area (Ditzel et al., 2022), there was a lack of smaller critters during the nighttime compared to the other two sections. The clicking of snapping shrimp was significantly more noticeable in areas of section 1 as well as the eastern side of section 3, but shown to be lacking in section 2 during night dives. Sections 1 and 3 had an abundance of micro critters, such as the Polychaete, which would be revealed during night dives. The frequency of these sounds seems to have a relationship with the values of coral cover in the areas, with sections 1 and 3 having a much higher frequency of coral recruits (avg. 15 and 20 <1 cm frequency, respectively) as compared to section 1 (4.1 corals with <1cm). This is reflected in the >5cm coral in each of the areas, as section 1 (avg. 25) and section 3 (avg. 13.8) compared to section 2 (avg. 9). The correlation between snapping rates and cover of coral habitat of snapping shrimp (Lillis and Mooney, 2018) are most likely attributed to this, however it does not explain the reason for a lack of fish biomass in comparison to the other two sites. Being located near the equator and exposed to the Pacific Ocean, the Philippines has experienced many typhoons in recent history due to its geographic location (Ribera, 2008). Typhoons can play an important role as a pressing factor of reef health, for instance the devastating impact of Typhoon Caloy on Apo Reef (Duquil, 2016), close to the site of Dimakya Island. Strong waves and currents created, even by small typhoons or heavy winds, may cause damage to corals within the surface of the reef (Heron et al., 2008). This is a major consideration when making decisions around the restoration of the reef, in order to strategically locate places to maintain reef health.
By synthesizing the results and qualitative observations throughout the reefs’ surroundings, we can assume the areas with lower coral recruitment survival rates which are not glaringly affected by constant anthropogenic stressors are potentially the best areas for coral transplantation. The reef of section 2 serves to be one of these areas, with significantly lower coral recruitment values in comparison to the other two, yet qualitative observations hinting at a denser volume of critters, which are indicators of a potentially healthy reef. Transplanting healthy corals into the area would enable hastened recovery of this section of the reef. The reef of section 3 has a positive outlook, with levels of early coral recruitment much higher than the larger sizes in the area, suggesting protective measures need to be implemented into this area to continue this volume of recruitment and maintain reef health.
References
- Abelson, A. et al. (2015) “Upgrading Marine Ecosystem Restoration Using Ecological-Social Concepts,” BioScience, 66(2), pp. 156–163. https://doi.org/10.1093/biosci/biv171. [CrossRef]
- Aludia, G. (2022) Status of coral reefs, butterflyfishes, and benthic macro-invertebrates in Araceli and Dumaran, Palawan, Philippines. https://aquadocs.org/handle/1834/42501.
- Anthony, K.R.N. et al. (2020) “Interventions to help coral reefs under global change—A complex decision challenge,” PLOS ONE, 15(8), p. e0236399. https://doi.org/10.1371/journal.pone.0236399. [CrossRef]
- Baird, A.H., Salih, A. and Trevor-Jones, A. (2006) “Fluorescence census techniques for the early detection of coral recruits,” Coral Reefs, 25(1), pp. 73–76. https://doi.org/10.1007/s00338-005-0072-7. [CrossRef]
- Bernert, K., [Karina Bernert] et al. (2014) Impact Assessment of Climate Change of Coral Reefs in Busuanga, Palawan. https://www.dlsu.edu.ph/wp-content/uploads/pdf/conferences/research-congress-proceedings/2014/SEE/SEE-IV-036-FT.pdf (Accessed: April 1, 2023).
- Boström-Einarsson, L. et al. (2020) “Coral restoration – A systematic review of current methods, successes, failures and future directions,” PLOS ONE, 15(1), p. e0226631. https://doi.org/10.1371/journal.pone.0226631. [CrossRef]
- Carlson, R., Foo, S.A. and Asner, G.P. (2019) “Land Use Impacts on Coral Reef Health: A Ridge-to-Reef Perspective,” Frontiers in Marine Science, 6. https://doi.org/10.3389/fmars.2019.00562. [CrossRef]
- Cole, A.J., Pratchett, M.S. and Jones, G. (2008) “Diversity and functional importance of coral-feeding fishes on tropical coral reefs,” Fish and Fisheries, 9(3), pp. 286–307. https://doi.org/10.1111/j.1467-2979.2008.00290.x. [CrossRef]
- Costanza, R. et al. (2014) “Changes in the global value of ecosystem services,” Global Environmental Change-human and Policy Dimensions, 26, pp. 152–158. https://doi.org/10.1016/j.gloenvcha.2014.04.002. [CrossRef]
- DeVantier, L., Turak, E. and Szava-Kovats, R. (2020) “Species Richness and Abundance of Reef-Building Corals in the Indo-West Pacific: The Local–Regional Relation Revisited,” Frontiers in Marine Science, 7. https://doi.org/10.3389/fmars.2020.00487. [CrossRef]
-
Tonan Ajia Kenkyu, Torikai, Y. (1993). [[Development of the Philippine frontier--labor absorption and internal migration to Palawan province]]. Tonan Ajia Kenkyu, [online] 31(3), pp.255–284.
- Ditzel, P. et al. (2022) “Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef,” Oceans, 3(1), pp. 1–14. https://doi.org/10.3390/oceans3010001. [CrossRef]
-
Nature Ecology & Evolution, Dietzel, A. et al (2021). The population sizes and global extinction risk of reef-building coral species at biogeographic scales. Nature Ecology & Evolution, [online] pp.1–7. doi:https://doi.org/10.1038/s41559-021-01393-4. [CrossRef]
- Duquil, R. (2016) “MANAGEMENT STRATEGIES AND PROGRAMS FOR THE PROTECTED AREA: A REVIEW FOR THE MANAGEMENT PLAN OF APO REEF NATURAL PARK. Management Plan Review,” www.academia.edu [Preprint]. https://www.academia.edu/21476257/MANAGEMENT_STRATEGIES_AND_PROGRAMS_FOR_THE_PROTECTED_AREA_A_REVIEW_FOR_THE_MANAGEMENT_PLAN_OF_APO_REEF_NATURAL_PARK_Management_Plan_Review.
- Eddy, T.D. et al. (2021) “Global decline in capacity of coral reefs to provide ecosystem services,” One Earth, 4(9), pp. 1278–1285. https://doi.org/10.1016/j.oneear.2021.08.016. [CrossRef]
- El-Naggar, H.A. (2020) “Human Impacts on Coral Reef Ecosystem,” IntechOpen eBooks [Preprint]. https://doi.org/10.5772/intechopen.88841. [CrossRef]
- Fadlallah, Y.H. (1983) “Sexual reproduction, development and larval biology in scleractinian corals,” Coral Reefs, 2(3), pp. 129–150. https://doi.org/10.1007/bf00336720. [CrossRef]
- Gomez, E.D. et al. (1994) “A review of the status of Philippine reefs,” Marine Pollution Bulletin, 29(1–3), pp. 62–68. https://doi.org/10.1016/0025-326x(94)90427-8. [CrossRef]
- Heron, S.F. et al. (2008) “Hurricanes and their effects on coral reefs,” ResearchGate [Preprint]. https://www.researchgate.net/publication/266558093_Hurricanes_and_their_effects_on_coral_reefs.
- Hodgson, G. and Dixon, J.A. (1988) Logging Versus Fisheries and Tourism in Palawan: An Environmental and Economic Analysis.
- Hoegh-Guldberg, O. et al. (2017) “Coral Reef Ecosystems under Climate Change and Ocean Acidification,” Frontiers in Marine Science, 4. https://doi.org/10.3389/fmars.2017.00158. [CrossRef]
- Jackson, J.B.C. et al. (2001) “Historical Overfishing and the Recent Collapse of Coastal Ecosystems,” Science, 293(5530), pp. 629–637. https://doi.org/10.1126/science.1059199. [CrossRef]
- Lillis, A. and Mooney, T.A. (2018) “Snapping shrimp sound production patterns on Caribbean coral reefs: relationships with celestial cycles and environmental variables,” Coral Reefs, 37(2), pp. 597–607. https://doi.org/10.1007/s00338-018-1684-z. [CrossRef]
- Martinez, S. and Abelson, A. (2013) “Coral recruitment: the critical role of early post-settlement survival,” Ices Journal of Marine Science, 70(7), pp. 1294–1298. https://doi.org/10.1093/icesjms/fst035. [CrossRef]
- Nordborg, F.M., Brinkman, D.L. and Negri, A.P. (2022) “Coral recruits are highly sensitive to heavy fuel oil exposure both in the presence and absence of UV light,” Environmental Pollution, 309, p. 119799. https://doi.org/10.1016/j.envpol.2022.119799. [CrossRef]
- O’Leary, J.K. and McClanahan, T.R. (2010) “Trophic cascades result in large-scale coralline algae loss through differential grazer effects,” Ecology, 91(12), pp. 3584–3597. https://doi.org/10.1890/09-2059.1. [CrossRef]
- Panga, F. et al. (2021) “Through the Boundaries: Environmental Factors Affecting Reef Benthic Cover in Marine Protected Areas in the Philippines,” Frontiers in Marine Science, 8. https://doi.org/10.3389/fmars.2021.702071. [CrossRef]
- Pestano, Z., Rivera, Ma.A.T. and Belandres, R.L. (2014) Urban Markets Adviser: Beyond Tourism: A Closer Look to Palawan. https://www.researchgate.net/publication/333981964_Urban_Markets_Adviser_Beyond_Tourism_A_Closer_Look_to_Palawan.
- Prada, F. et al. (2019) “Anthropogenic impact is negatively related to coral health in Sicily (Mediterranean Sea),” Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-49713-w. [CrossRef]
- West, J.M. and Salm, R.V. (2003). Resistance and Resilience to Coral Bleaching: Implications for Coral Reef Conservation and Management. Conservation Biology, 17(4), pp.956–967. [CrossRef]
- Ribera, P. (2008) Historical deadly typhoons in the Philippines. https://www.semanticscholar.org/paper/Historical-deadly-typhoons-in-the-Philippines-Ribera-Garcia-Herrera/3b10698ebcb5ad48562d24dbf3d10b5f46422ada.
- Robinson, J.A. et al. (2018) “Environmental conditions and herbivore biomass determine coral reef benthic community composition: implications for quantitative baselines,” Coral Reefs, 37(4), pp. 1157–1168. https://doi.org/10.1007/s00338-018-01737-w. [CrossRef]
- Rodgers, K.B. et al. (2017) “Effectiveness of coral relocation as a mitigation strategy in Kāne‘ohe Bay, Hawai‘i,” PeerJ [Preprint]. https://doi.org/10.7717/peerj.3346. [CrossRef]
- Shick, J.M., Lesser, M.P. and Jokiel, P.L. (1996) “Effects of ultraviolet radiation on corals and other coral reef organisms,” Global Change Biology, 2(6), pp. 527–545. https://doi.org/10.1111/j.1365-2486.1996.tb00065. [CrossRef]
- Stobart, Y.B. et al. (2005) “Coral recovery at Aldabra Atoll, Seychelles: five years after the 1998 bleaching event,” Philosophical Transactions of the Royal Society A, 363(1826), pp. 251–255. https://doi.org/10.1098/rsta.2004.1490. [CrossRef]
- Tamelander, J. (2002) “Coral recruitment following a mass mortality event,” AMBIO: A Journal of the Human Environment, 31(7), pp. 551–557. https://doi.org/10.1579/0044-7447-31.7.551. [CrossRef]
- White, A., Vogt, H. and Arin, T. (2000) “Philippine Coral Reefs Under Threat: The Economic Losses Caused by Reef Destruction,” Marine Pollution Bulletin, 40(7), pp. 598–605. https://doi.org/10.1016/s0025-326x(00)00022-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).