Submitted:
14 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
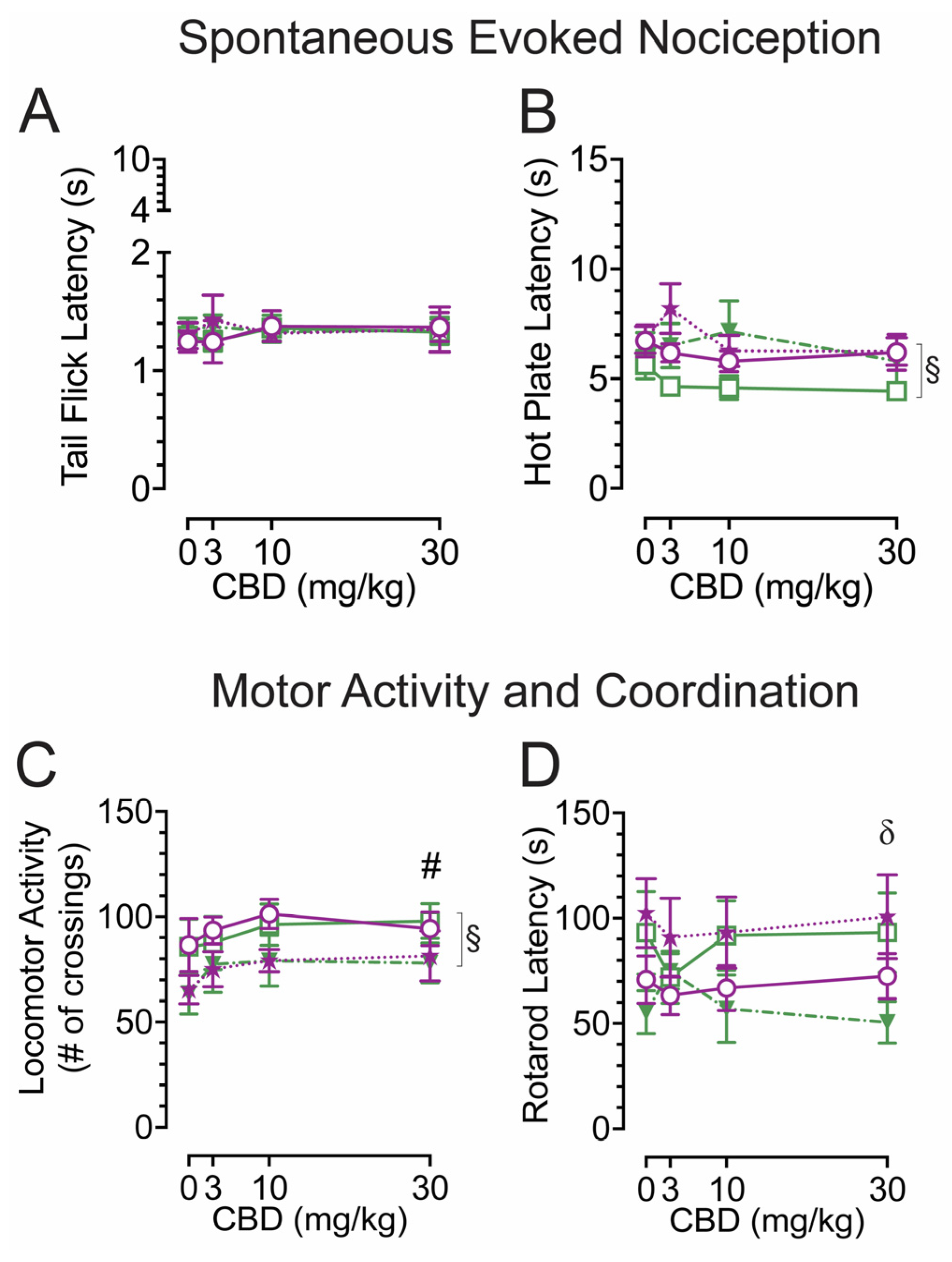
2.1. Spontaneous heat-evoked nociception
2.2. Locomotor activity and rotarod coordination
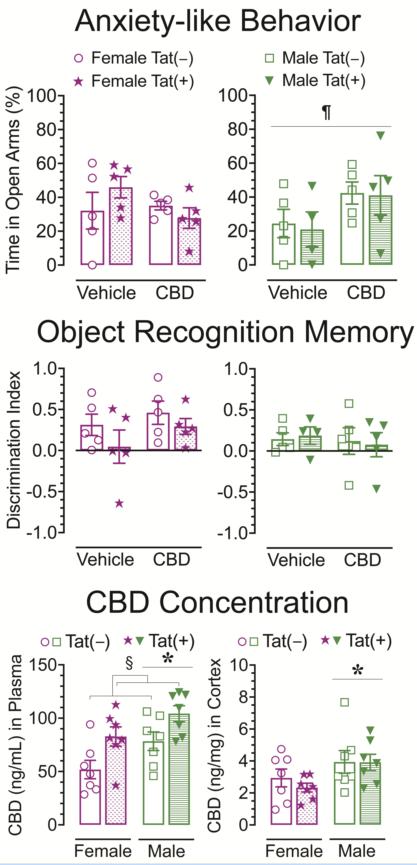
2.3. Anxiety-like behavior
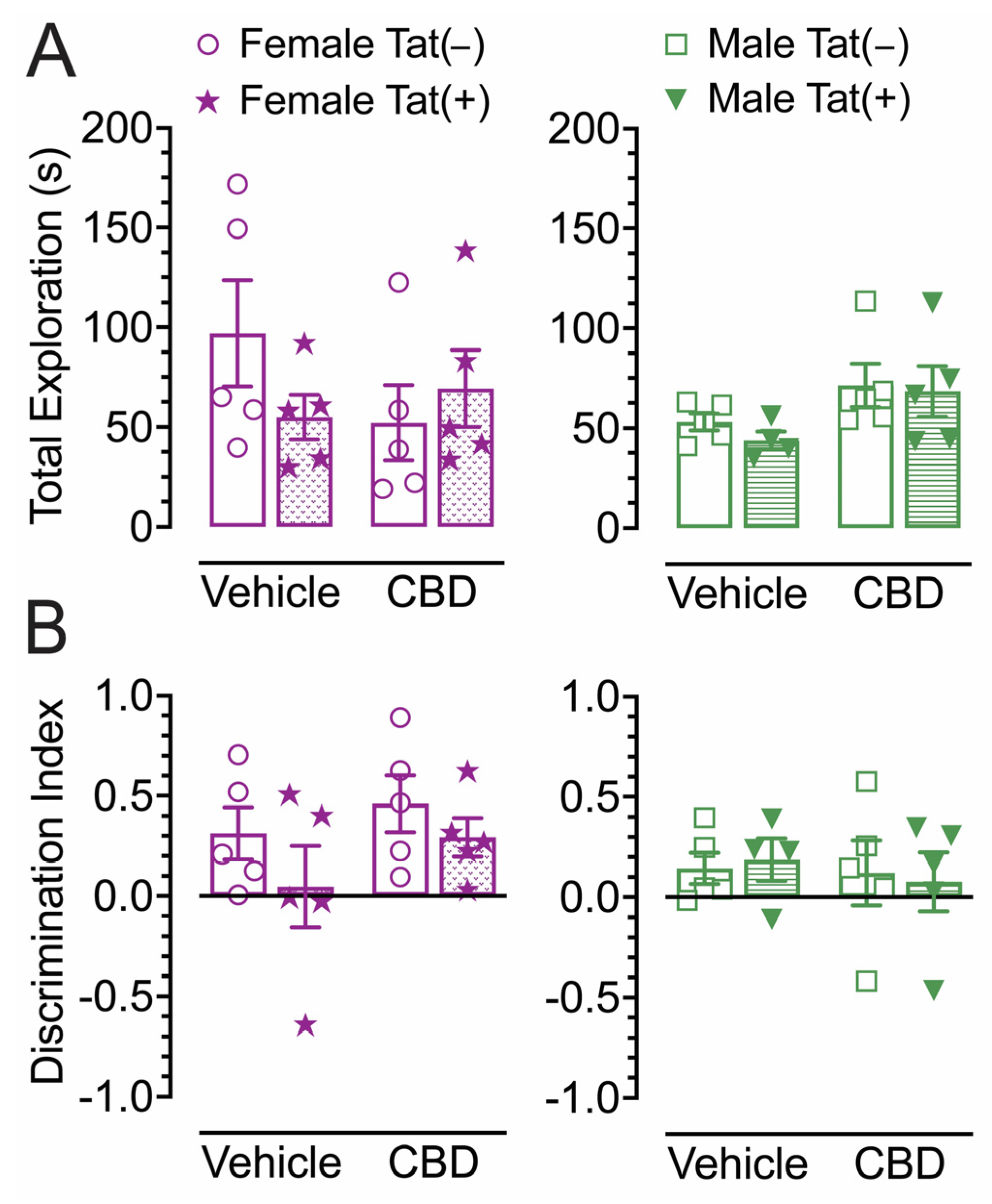
2.4. Novel object recognition
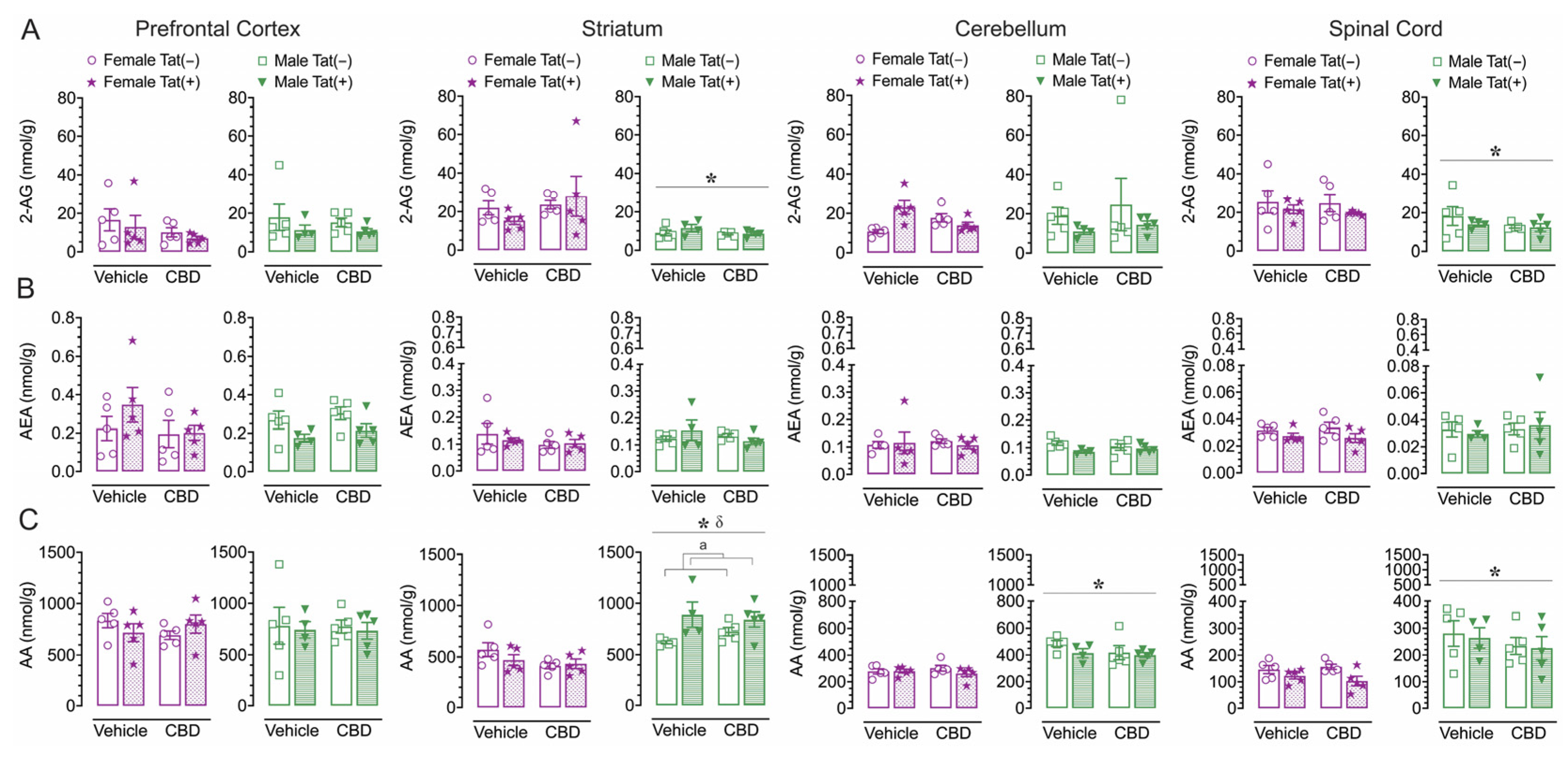
2.5. CNS levels of endocannabinoids and related lipids
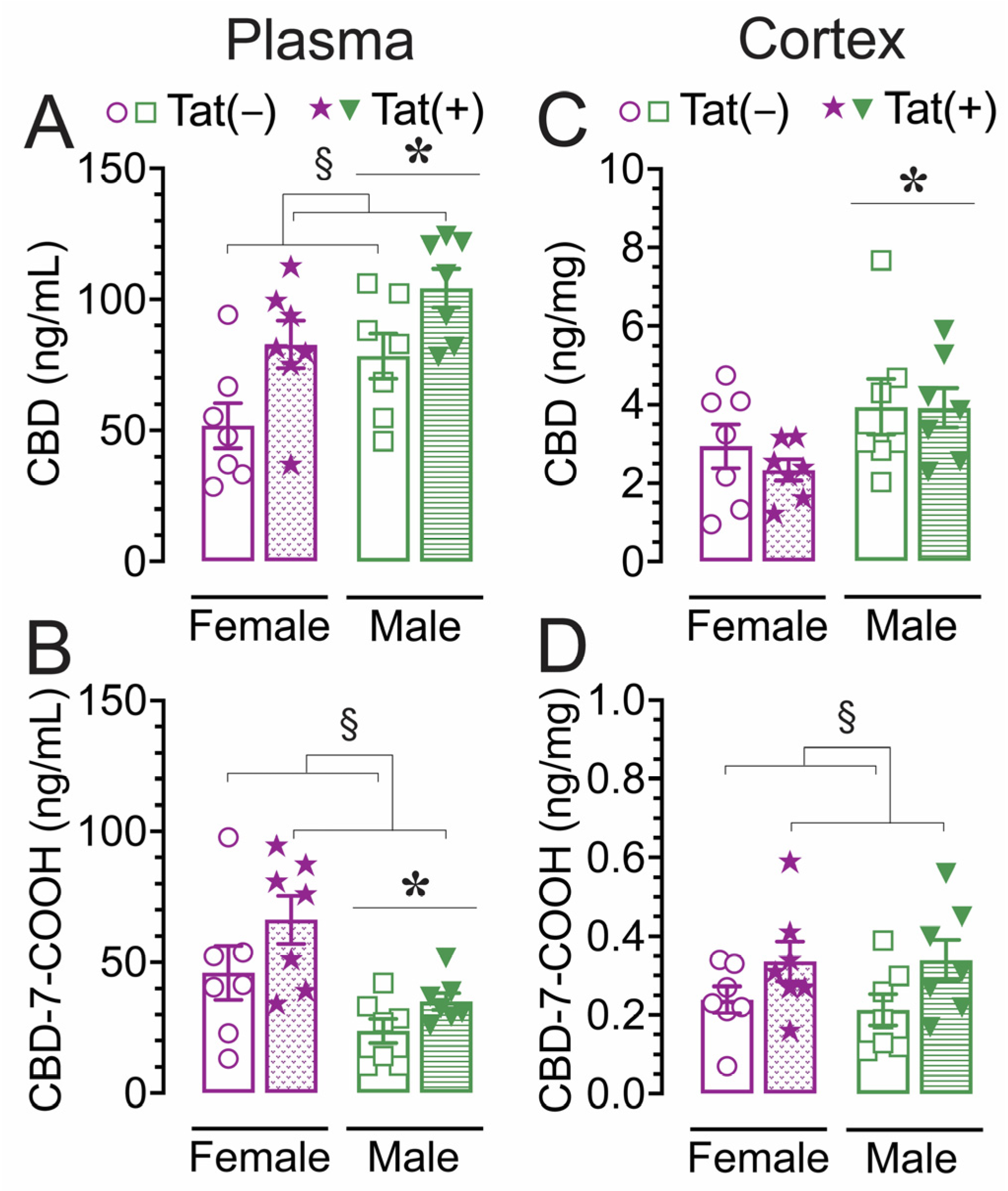
2.6. Concentration of CBD and its metabolite CBD-7-COOH in plasma and cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental design
4.4. Behavioral procedure
4.4.1. Spontaneous heat-evoked nociception
4.4.2. Locomotor activity
4.4.3. Rotarod
4.4.4. Elevated plus maze (EPM)
4.4.5. Novel object recognition (NOR)
4.5. Analysis of endocannabinoids and related lipids
4.6. Analysis of CBD and CBD-7-COOH levels
4.7. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS statistics - Fact Sheet. Available online: https://home.liebertpub.com/publications/cannabis-and-cannabinoid-research/633/for-authors (accessed on October 13, 2023).
- Harrison, K.M.; Song, R.; Zhang, X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J. Acquir. Immune. Defic. Syndr. 2010, 53, 124–130. [Google Scholar] [CrossRef]
- May, M.T.; Sterne, J.A.; Costagliola, D.; Sabin, C.A.; Phillips, A.N.; Justice, A.C.; Dabis, F.; Gill, J.; Lundgren, J.; Hogg, R.S.; et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet 2006, 368, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.L.; Chao, C.R.; Leyden, W.A.; Xu, L.; Quesenberry, C.P., Jr.; Klein, D.B.; Towner, W.J.; Horberg, M.A.; Silverberg, M.J. Narrowing the Gap in Life Expectancy Between HIV-Infected and HIV-Uninfected Individuals With Access to Care. J. Acquir. Immune. Defic. Syndr. 2016, 73, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Sacktor, N.; Skolasky, R.L.; Seaberg, E.; Munro, C.; Becker, J.T.; Martin, E.; Ragin, A.; Levine, A.; Miller, E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016, 86, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell. Infect. Microbiol. 2020, 10, 61. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Thames, A.D.; Mahmood, Z.; Burggren, A.C.; Karimian, A.; Kuhn, T.P. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS care 2016, 28, 628–632. [Google Scholar] [CrossRef]
- Thames, A.D.; Kuhn, T.P.; Williamson, T.J.; Jones, J.D.; Mahmood, Z.; Hammond, A. Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV- adults. Drug Alcohol Depend. 2017, 170, 120–127. [Google Scholar] [CrossRef]
- Heaton, R.K.; Grant, I.; Butters, N.; White, D.A.; Kirson, D.; Atkinson, J.H.; McCutchan, J.A.; Taylor, M.J.; Kelly, M.D.; Ellis, R.J.; et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1995, 1, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Marcotte, T.D.; Mindt, M.R.; Sadek, J.; Moore, D.J.; Bentley, H.; McCutchan, J.A.; Reicks, C.; Grant, I.; Group, H. The impact of HIV-associated neuropsychological impairment on everyday functioning. J. Int. Neuropsychol. Soc. 2004, 10, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, T.D.; Wolfson, T.; Rosenthal, T.J.; Heaton, R.K.; Gonzalez, R.; Ellis, R.J.; Grant, I.; Group, H.I.V.N.R.C. A multimodal assessment of driving performance in HIV infection. Neurology 2004, 63, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.M.; Marder, K.; Dooneief, G.; Bell, K.; Sano, M.; Todak, G.; Stern, Y. Neuropsychologic impairment in early HIV infection. A risk factor for work disability. Arch. Neurol. 1995, 52, 525–530. [Google Scholar] [CrossRef] [PubMed]
- van Gorp, W.G.; Baerwald, J.P.; Ferrando, S.J.; McElhiney, M.C.; Rabkin, J.G. The relationship between employment and neuropsychological impairment in HIV infection. J. Int. Neuropsychol. Soc. 1999, 5, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Velin, R.A.; McCutchan, J.A.; Gulevich, S.J.; Atkinson, J.H.; Wallace, M.R.; Godfrey, H.P.; Kirson, D.A.; Grant, I. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom. Med. 1994, 56, 8–17. [Google Scholar] [CrossRef]
- Martin, C.; Solders, G.; Sonnerborg, A.; Hansson, P. Painful and non-painful neuropathy in HIV-infected patients: an analysis of somatosensory nerve function. Eur. J. Pain 2003, 7, 23–31. [Google Scholar] [CrossRef]
- Mitra, P.; Sharman, T. HIV Neurocognitive Disorders. In StatPearls; Treasure Island (FL), 2022.
- Whetten, K.; Reif, S.; Whetten, R.; Murphy-McMillan, L.K. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom. Med. 2008, 70, 531–538. [Google Scholar] [CrossRef]
- Mediouni, S.; Darque, A.; Baillat, G.; Ravaux, I.; Dhiver, C.; Tissot-Dupont, H.; Mokhtari, M.; Moreau, H.; Tamalet, C.; Brunet, C.; et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infect. Disord. Drug. Targets 2012, 12, 81–86. [Google Scholar] [CrossRef]
- King, J.E.; Eugenin, E.A.; Buckner, C.M.; Berman, J.W. HIV tat and neurotoxicity. Microbes Infect. 2006, 8, 1347–1357. [Google Scholar] [CrossRef]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef]
- Hudson, L.; Liu, J.; Nath, A.; Jones, M.; Raghavan, R.; Narayan, O.; Male, D.; Everall, I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000, 6, 145–155. [Google Scholar] [CrossRef]
- Wiley, C.A.; Baldwin, M.; Achim, C.L. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS 1996, 10, 843–847. [Google Scholar] [CrossRef]
- Bertrand, S.J.; Aksenova, M.V.; Mactutus, C.F.; Booze, R.M. HIV-1 Tat protein variants: critical role for the cysteine region in synaptodendritic injury. Exp. Neurol. 2013, 248, 228–235. [Google Scholar] [CrossRef]
- Carey, A.N.; Sypek, E.I.; Singh, H.D.; Kaufman, M.J.; McLaughlin, J.P. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav. Brain Res. 2012, 229, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Fitting, S.; Ignatowska-Jankowska, B.M.; Bull, C.; Skoff, R.P.; Lichtman, A.H.; Wise, L.E.; Fox, M.A.; Su, J.; Medina, A.E.; Krahe, T.E.; et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol. Psychiatry 2013, 73, 443–453. [Google Scholar] [CrossRef] [PubMed]
- League, A.F.; Gorman, B.L.; Hermes, D.J.; Johnson, C.T.; Jacobs, I.R.; Yadav-Samudrala, B.J.; Poklis, J.L.; Niphakis, M.J.; Cravatt, B.F.; Lichtman, A.H.; et al. Monoacylglycerol Lipase Inhibitor MJN110 Reduces Neuronal Hyperexcitability, Restores Dendritic Arborization Complexity, and Regulates Reward-Related Behavior in Presence of HIV-1 Tat. Front. Neurol. 2021, 12, 651272. [Google Scholar] [CrossRef]
- Hahn, Y.K.; Masvekar, R.R.; Xu, R.; Hauser, K.F.; Knapp, P.E. Chronic HIV-1 Tat and HIV reduce Rbfox3/NeuN: evidence for sex-related effects. Curr. HIV Res. 2015, 13, 10–20. [Google Scholar] [CrossRef]
- Wodarski, R.; Bagdas, D.; Paris, J.J.; Pheby, T.; Toma, W.; Xu, R.; Damaj, M.I.; Knapp, P.E.; Rice, A.S.C.; Hauser, K.F. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep. 2018, 3, e654. [Google Scholar] [CrossRef]
- Marks, W.D.; Paris, J.J.; Barbour, A.J.; Moon, J.; Carpenter, V.J.; McLane, V.D.; Lark, A.R.S.; Nass, S.R.; Zhang, J.; Yarotskyy, V.; et al. HIV-1 Tat and Morphine Differentially Disrupt Pyramidal Cell Structure and Function and Spatial Learning in Hippocampal Area CA1: Continuous versus Interrupted Morphine Exposure. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fan, Y.; Vann, P.H.; Wong, J.M.; Sumien, N.; He, J.J. Long-term HIV-1 Tat Expression in the Brain Led to Neurobehavioral, Pathological, and Epigenetic Changes Reminiscent of Accelerated Aging. Aging. Dis. 2020, 11, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Barillari, G.; Salahuddin, S.Z.; Gallo, R.C.; Wong-Staal, F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature 1990, 345, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, J.M.; Vives, E.; Mabrouk, K.; Benjouad, A.; Rochat, H.; Duval, A.; Hue, B.; Bahraoui, E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J. Virol. 1991, 65, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Conant, K.; Tornatore, C.; Atwood, W.; Meyers, K.; Traub, R.; Major, E.O. In vivo and in vitro infection of the astrocyte by HIV-1. Adv. Neuroimmunol. 1994, 4, 287–289. [Google Scholar] [CrossRef]
- Kannan, M.; Singh, S.; Chemparathy, D.T.; Oladapo, A.A.; Gawande, D.Y.; Dravid, S.M.; Buch, S.; Sil, S. HIV-1 Tat induced microglial EVs leads to neuronal synaptodendritic injury: microglia-neuron cross-talk in NeuroHIV. Extracell. Vesicles Circ. Nucl. Acids 2022, 3, 133–149. [Google Scholar] [CrossRef]
- Fitting, S.; Knapp, P.E.; Zou, S.; Marks, W.D.; Bowers, M.S.; Akbarali, H.I.; Hauser, K.F. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload. J. Neurosci. 2014, 34, 12850–12864. [Google Scholar] [CrossRef]
- Philippon, V.; Vellutini, C.; Gambarelli, D.; Harkiss, G.; Arbuthnott, G.; Metzger, D.; Roubin, R.; Filippi, P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology 1994, 205, 519–529. [Google Scholar] [CrossRef]
- Prendergast, M.A.; Rogers, D.T.; Mulholland, P.J.; Littleton, J.M.; Wilkins, L.H., Jr.; Self, R.L.; Nath, A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002, 954, 300–307. [Google Scholar] [CrossRef]
- Hermes, D.J.; Yadav-Samudrala, B.J.; Xu, C.; Paniccia, J.E.; Meeker, R.B.; Armstrong, M.L.; Reisdorph, N.; Cravatt, B.F.; Mackie, K.; Lichtman, A.H.; et al. GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration. Exp. Neurol. 2021, 341, 113699. [Google Scholar] [CrossRef]
- Bertrand, S.J.; Mactutus, C.F.; Aksenova, M.V.; Espensen-Sturges, T.D.; Booze, R.M. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J. Neurochem. 2014, 128, 140–151. [Google Scholar] [CrossRef]
- Cheng, J.; Nath, A.; Knudsen, B.; Hochman, S.; Geiger, J.D.; Ma, M.; Magnuson, D.S. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience 1998, 82, 97–106. [Google Scholar] [CrossRef]
- Jin, J.; Lam, L.; Sadic, E.; Fernandez, F.; Tan, J.; Giunta, B. HIV-1 Tat-induced microglial activation and neuronal damage is inhibited via CD45 modulation: A potential new treatment target for HAND. Am. J. Transl. Res. 2012, 4, 302–315. [Google Scholar]
- Hahn, Y.K.; Vo, P.; Fitting, S.; Block, M.L.; Hauser, K.F.; Knapp, P.E. β-chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. J. Neurochem. 2010, 114, 97–109. [Google Scholar] [CrossRef]
- Nath, A.; Conant, K.; Chen, P.; Scott, C.; Major, E.O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J. Biol. Chem. 1999, 274, 17098–17102. [Google Scholar] [CrossRef]
- Kesby, J.P.; Markou, A.; Semenova, S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 2016, 109, 205–215. [Google Scholar] [CrossRef]
- Bagdas, D.; Paris, J.J.; Carper, M.; Wodarski, R.; Rice, A.S.C.; Knapp, P.E.; Hauser, K.F.; Damaj, M.I. Conditional expression of HIV-1 Tat in the mouse alters the onset and progression of tonic, inflammatory, and neuropathic hypersensitivity in a sex-dependent manner. Eur. J. Pain 2020. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Amet, T.; Byrd, D.; Chang, K.H.; Shah, K.; Hu, N.; Grantham, A.; Hu, S.; Duan, J.; Tao, F.; et al. Direct effects of HIV-1 Tat on excitability and survival of primary dorsal root ganglion neurons: possible contribution to HIV-1-associated pain. Plos One 2011, 6, e24412. [Google Scholar] [CrossRef] [PubMed]
- Cirino, T.J.; Alleyne, A.R.; Duarte, V.; Figueroa, A.; Simons, C.A.; Anceaume, E.M.; Kendrick, J.; Wallman, O.; Eans, S.O.; Stacy, H.M.; et al. Expression of Human Immunodeficiency Virus Transactivator of Transcription (HIV-Tat1-86) Protein Alters Nociceptive Processing that is Sensitive to Anti-Oxidant and Anti-Inflammatory Interventions. J. Neuroimmune. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.F.; Mahdi, F.; Paris, J.J. HIV-1 Tat Dysregulates the Hypothalamic-Pituitary-Adrenal Stress Axis and Potentiates Oxycodone-Mediated Psychomotor and Anxiety-Like Behavior of Male Mice. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Qrareya, A.N.; Mahdi, F.; Kaufman, M.J.; Ashpole, N.M.; Paris, J.J. HIV-1 Tat promotes age-related cognitive, anxiety-like, and antinociceptive impairments in female mice that are moderated by aging and endocrine status. Geroscience 2021, 43, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.K.; Paris, J.J.; Lichtman, A.H.; Hauser, K.F.; Sim-Selley, L.J.; Selley, D.E.; Knapp, P.E. Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiol. Dis. 2016, 92, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.R.; Stacy, S.; Sumien, N.; Ghorpade, A.; Borgmann, K. Astrocyte HIV-1 Tat Differentially Modulates Behavior and Brain MMP/TIMP Balance During Short and Prolonged Induction in Transgenic Mice. Front. Neurol. 2020, 11, 593188. [Google Scholar] [CrossRef]
- Paris, J.J.; Fenwick, J.; McLaughlin, J.P. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm. Behav. 2014, 65, 445–453. [Google Scholar] [CrossRef]
- Kasten, C.R.; Zhang, Y.; Boehm, S.L. , 2nd. Acute and long-term effects of Delta9-tetrahydrocannabinol on object recognition and anxiety-like activity are age- and strain-dependent in mice. Pharmacol. Biochem. Behav. 2017, 163, 9–19. [Google Scholar] [CrossRef]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018, 31, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yadav-Samudrala, B.J.; Fitting, S. Mini-review: The therapeutic role of cannabinoids in neuroHIV. Neurosci. Lett. 2021, 750, 135717. [Google Scholar] [CrossRef]
- Woolridge, E.; Barton, S.; Samuel, J.; Osorio, J.; Dougherty, A.; Holdcroft, A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manage. 2005, 29, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wardell, J.D.; Rueda, S.; Fox, N.; Costiniuk, C.T.; Jenabian, M.A.; Margolese, S.; Mandarino, E.; Shuper, P.; Hendershot, C.S.; Cunningham, J.A.; et al. Disentangling Medicinal and Recreational cannabis Use Among People Living with HIV: An Ecological Momentary Assessment Study. AIDS Behav. 2023, 4, 1350–1363. [Google Scholar] [CrossRef]
- Shiau, S.; Arpadi, S.M.; Yin, M.T.; Martins, S.S. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict. Behav. 2017, 68, 39–44. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Saneei, Z.; Salahuddin, S.; Cox, J.; Routy, J.P.; Rueda, S.; Abdallah, S.J.; Jensen, D.; Lebouche, B.; Brouillette, M.J.; et al. Cannabis Consumption in People Living with HIV: Reasons for Use, Secondary Effects, and Opportunities for Health Education. Cannabis Cannabinoid Res. 2019, 4, 204–213. [Google Scholar] [CrossRef]
- Whitfield, R.M.; Bechtel, L.M.; Starich, G.H. The impact of ethanol and Marinol/marijuana usage on HIV+/AIDS patients undergoing azidothymidine, azidothymidine/dideoxycytidine, or dideoxyinosine therapy. Alcohol Clin. Exp. Res. 1997, 21, 122–127. [Google Scholar] [CrossRef]
- Cristiani, S.A.; Pukay-Martin, N.D.; Bornstein, R.A. Marijuana use and cognitive function in HIV-infected people. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 330–335. [Google Scholar] [CrossRef]
- Pacek, L.R.; Towe, S.L.; Hobkirk, A.L.; Nash, D.; Goodwin, R.D. Frequency of Cannabis Use and Medical Cannabis Use Among Persons Living With HIV in the United States: Findings From a Nationally Representative Sample. AIDS Educ. Prev. 2018, 30, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Wilson, N.; Peterson, S. Cannabis and Inflammation in HIV: A Review of Human and Animal Studies. Viruses 2021, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Herron, C.E. Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer’s Disease. Neurochem. Res. 2019, 44, 703–713. [Google Scholar] [CrossRef]
- Garcia-Baos, A.; Puig-Reyne, X.; Garcia-Algar, O.; Valverde, O. Cannabidiol attenuates cognitive deficits and neuroinflammation induced by early alcohol exposure in a mice model. Biomed. Pharmacother. 2021, 141, 111813. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Weston-Green, K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci. Biobehav. Rev. 2017, 72, 310–324. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Babic, I.; Huang, X.F.; Weston-Green, K. Improved Social Interaction, Recognition and Working Memory with Cannabidiol Treatment in a Prenatal Infection (poly I:C) Rat Model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1E9 mice. Psychopharmacology (Berl.) 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants (Basel) 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. B.r J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Pazos, M.R.; Satta, V.; Ramos, J.A.; Pertwee, R.G.; Fernandez-Ruiz, J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J. Neurosci. Res. 2011, 89, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Galaj, E.; Bi, G.H.; Yang, H.J.; Xi, Z.X. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology 2020, 167, 107740. [Google Scholar] [CrossRef]
- Vilela, L.R.; Lima, I.V.; Kunsch, E.B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, E.L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy & behavior: E&B 2017, 75, 29–35. [Google Scholar] [CrossRef]
- Fogaca, M.V.; Campos, A.C.; Coelho, L.D.; Duman, R.S.; Guimaraes, F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018, 135, 22–33. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimaraes, F.S.; Joca, S.R. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Correa, F.M.; Guimaraes, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratu, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS One 2011, 6, e28668. [Google Scholar] [CrossRef]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol goes nuclear: The role of PPARgamma. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef]
- Sonego, A.B.; Prado, D.D.S.; Guimaraes, F.S. PPARgamma receptors are involved in the effects of cannabidiol on orofacial dyskinesia and cognitive dysfunction induced by typical antipsychotic in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110367. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjogren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaca, M.V.; Aguiar, D.C.; Diaz-Alonso, J.; Ortega-Gutierrez, S.; Vazquez-Villa, H.; Moreira, F.A.; Guzman, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Nguyen, J.D.; Morgenson, D.; Taffe, M.A.; Ranganathan, M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)-Tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Turchan-Cholewo, J.; Smart, E.J.; Geurin, T.; Chauhan, A.; Reid, R.; Xu, R.; Nath, A.; Knapp, P.E.; Hauser, K.F. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 2008, 56, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Turchan, J.; Pocernich, C.B.; Bruce-Keller, A.J.; Roth, S.; Butterfield, D.A.; Major, E.O.; Nath, A. Intracellular human immunodeficiency virus tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J. Biol. Chem. 2003, 278, 13512–13519. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kongara, K.; Harding, D.; Ward, N.; Dukkipati, V.S.R.; Johnson, C.; Chambers, P. Comparison of electroencephalographic changes in response to acute electrical and thermal stimuli with the tail flick and hot plate test in rats administered with opiorphin. BMC Neurol. 2018, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Roberts, D.J. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J. Pharm. Pharmacol. 1968, 20, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Miyakawa, T. Effects of test experience, closed-arm wall color, and illumination level on behavior and plasma corticosterone response in an elevated plus maze in male C57BL/6J mice: a challenge against conventional interpretation of the test. Mol. Brain 2021, 14, 34. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Ennaceur, A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef]
- Miedel, C.J.; Patton, J.M.; Miedel, A.N.; Miedel, E.S.; Levenson, J.M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-beta and Tau Neuropathology. J. Vis. Exp. 2017, 123, 55523. [Google Scholar] [CrossRef]
- Dempsey, S.K.; Gesseck, A.M.; Ahmad, A.; Daneva, Z.; Ritter, J.K.; Poklis, J.L. Formation of HETE-EAs and dihydroxy derivatives in mouse kidney tissue and analysis by high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2019, 1126-1127, 121748. [Google Scholar] [CrossRef]
- Slawek, D.E. People living with HIV and the emerging field of chronic pain-what is known about epidemiology, etiology, and management. Curr. HIV/AIDS Rep. 2021, 18, 436–442. [Google Scholar] [CrossRef]
- Lu, H.J.; Fu, Y.Y.; Wei, Q.Q.; Zhang, Z.J. Neuroinflammation in HIV-Related Neuropathic Pain. Front. Pharmacol. 2021, 12, 653852. [Google Scholar] [CrossRef]
- Namisango, E.; Harding, R.; Atuhaire, L.; Ddungu, H.; Katabira, E.; Muwanika, F.R.; Powell, R.A. Pain among ambulatory HIV/AIDS patients: multicenter study of prevalence, intensity, associated factors, and effect. J. Pain 2012, 13, 704–713. [Google Scholar] [CrossRef]
- Fitting, S.; Scoggins, K.L.; Xu, R.; Dever, S.M.; Knapp, P.E.; Dewey, W.L.; Hauser, K.F. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur. J. Pharmacol. 2012, 689, 96–103. [Google Scholar] [CrossRef]
- Toma, W.; Paris, J.J.; Warncke, U.O.; Nass, S.R.; Caillaud, M.; McKiver, B.; Ondo, O.; Bagdas, D.; Bigbee, J.; Knapp, P.E.; et al. Persistent sensory changes and sex differences in transgenic mice conditionally expressing HIV-1 Tat regulatory protein. Exp. Neurol. 2022, 358, 114226. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef]
- Wanasuntronwong, A.; Kaewsrisung, S.; Rotpenpian, N.; Arayapisit, T.; Pavasant, P.; Supronsinchai, W. Efficacy and mechanism of the antinociceptive effects of cannabidiol on acute orofacial nociception induced by Complete Freund’s Adjuvant in male Mus musculus mice. Arch. Oral Biol. 2022, 144, 105570. [Google Scholar] [CrossRef]
- Genaro, K.; Fabris, D.; Arantes, A.L.F.; Zuardi, A.W.; Crippa, J.A.S.; Prado, W.A. Cannabidiol Is a Potential Therapeutic for the Affective-Motivational Dimension of Incision Pain in Rats. Front. Pharmacol. 2017, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; van den Brande, G.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009, 34, 672–680. [Google Scholar] [CrossRef]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom. Manage. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Robinson-Papp, J.; Gensler, G.; Navis, A.; Sherman, S.; Ellis, R.J.; Gelman, B.B.; Kolson, D.L.; Letendre, S.L.; Singer, E.J.; Valdes-Sueiras, M.; et al. Characteristics of Motor Dysfunction in Longstanding Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 71, 1532–1538. [Google Scholar] [CrossRef]
- Kronemer, S.I.; Mandel, J.A.; Sacktor, N.C.; Marvel, C.L. Impairments of Motor Function While Multitasking in HIV. Front. Hum. Neurosci. 2017, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Mugisha, J.; De Hert, M.; Probst, M.; Firth, J.; Gorczynski, P.; Stubbs, B. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disabil. Rehabil. 2018, 40, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Pinacchio, C.; Santinelli, L.; Adami, P.E.; Borrazzo, C.; Cavallari, E.N.; Vullo, A.; Innocenti, G.P.; Mezzaroma, I.; Mastroianni, C.M.; et al. Physical Activity and HIV: Effects on Fitness Status, Metabolism, Inflammation and Immune-Activation. AIDS Behav. 2020, 24, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Tzeng Yang, A.R.; Bryant, J.L.; Jones, O.; Royal, W. , 3rd. Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat. J. Neurovirol. 2009, 15, 380–389. [Google Scholar] [CrossRef]
- Moran, L.M.; Booze, R.M.; Webb, K.M.; Mactutus, C.F. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp. Neurol. 2013, 239, 139–147. [Google Scholar] [CrossRef]
- Hahn, Y.K.; Podhaizer, E.M.; Farris, S.P.; Miles, M.F.; Hauser, K.F.; Knapp, P.E. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct. Funct. 2015, 220, 605–623. [Google Scholar] [CrossRef]
- Jacobs, I.R.; Xu, C.; Hermes, D.J.; League, A.F.; Xu, C.; Nath, B.; Jiang, W.; Niphakis, M.J.; Cravatt, B.F.; Mackie, K.; et al. Inhibitory Control Deficits Associated with Upregulation of CB1R in the HIV-1 Tat Transgenic Mouse Model of Hand. J. Neuroimmune Pharmacol. 2019, 14, 661–678. [Google Scholar] [CrossRef]
- Poulia, N.; Delis, F.; Brakatselos, C.; Ntoulas, G.; Asprogerakas, M.Z.; Antoniou, K. CBD Effects on Motor Profile and Neurobiological Indices Related to Glutamatergic Function Induced by Repeated Ketamine Pre-Administration. Front. Pharmacol. 2021, 12, 746935. [Google Scholar] [CrossRef]
- Viudez-Martinez, A.; Garcia-Gutierrez, M.S.; Medrano-Relinque, J.; Navarron, C.M.; Navarrete, F.; Manzanares, J. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacol. Sin. 2019, 40, 358–364. [Google Scholar] [CrossRef]
- Zieba, J.; Sinclair, D.; Sebree, T.; Bonn-Miller, M.; Gutterman, D.; Siegel, S.; Karl, T. Cannabidiol (CBD) reduces anxiety-related behavior in mice via an FMRP-independent mechanism. Pharmacol. Biochem. Behav. 2019, 181, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.; Watt, G.; Kreilaus, F.; Karl, T. Medium-Dose Chronic Cannabidiol Treatment Reverses Object Recognition Memory Deficits of APP (Swe)/PS1DeltaE9 Transgenic Female Mice. Front. Pharmacol. 2020, 11, 587604. [Google Scholar] [CrossRef]
- Florensa-Zanuy, E.; Garro-Martinez, E.; Adell, A.; Castro, E.; Diaz, A.; Pazos, A.; Mac-Dowell, K.S.; Martin-Hernandez, D.; Pilar-Cuellar, F. Cannabidiol antidepressant-like effect in the lipopolysaccharide model in mice: Modulation of inflammatory pathways. Biochem. Pharmacol. 2021, 185, 114433. [Google Scholar] [CrossRef]
- Schleicher, E.M.; Ott, F.W.; Muller, M.; Silcher, B.; Sichler, M.E.; Low, M.J.; Wagner, J.M.; Bouter, Y. Prolonged Cannabidiol Treatment Lacks on Detrimental Effects on Memory, Motor Performance and Anxiety in C57BL/6J Mice. Front. Behav. Neurosci. 2019, 13, 94. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Calapai, G.; Di Mauro, D.; Trimarchi, F.; Ammendolia, I.; Mannucci, C. Effects of Cannabidiol on Locomotor Activity. Life (Basel) 2022, 5, 652. [Google Scholar] [CrossRef]
- Magen, I.; Avraham, Y.; Ackerman, Z.; Vorobiev, L.; Mechoulam, R.; Berry, E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010, 159, 950–957. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Bonaccorso, S.; Ricciardi, A.; Zangani, C.; Chiappini, S.; Schifano, F. Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology 2019, 74, 282–298. [Google Scholar] [CrossRef]
- Huffstetler, C.M.; Cochran, B.; May, C.A.; Maykut, N.; Silver, C.R.; Cedeno, C.; Franck, E.; Cox, A.; Fadool, D.A. Single cannabidiol administration affects anxiety-, obsessive compulsive-, object memory-, and attention-like behaviors in mice in a sex and concentration dependent manner. Pharmacol. Biochem. Behav. 2023, 222, 173498. [Google Scholar] [CrossRef]
- Wright, M.; Di Ciano, P.; Brands, B. Use of Cannabidiol for the Treatment of Anxiety: A Short Synthesis of Pre-Clinical and Clinical Evidence. Cannabis Cannabinoid Res. 2020, 5, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Carvalho, M.C.; Brandao, M.L.; Prado, W.A.; Zuardi, A.W.; Crippa, J.A.; de Oliveira, A.R.; Lovick, T.A.; Genaro, K. Sex-dependent differences in the anxiolytic-like effect of cannabidiol in the elevated plus-maze. J. Psychopharmacol. 2022, 36, 1371–1383. [Google Scholar] [CrossRef]
- Salviato, B.Z.; Raymundi, A.M.; Rodrigues da Silva, T.; Salemme, B.W.; Batista Sohn, J.M.; Araujo, F.S.; Guimaraes, F.S.; Bertoglio, L.J.; Stern, C.A. Female but not male rats show biphasic effects of low doses of Delta(9)-tetrahydrocannabinol on anxiety: can cannabidiol interfere with these effects? Neuropharmacology 2021, 196, 108684. [Google Scholar] [CrossRef]
- Cryan, J.F.; Holmes, A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef]
- Marks, W.D.; Paris, J.J.; Schier, C.J.; Denton, M.D.; Fitting, S.; McQuiston, A.R.; Knapp, P.E.; Hauser, K.F. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J. Neurovirol. 2016. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel) 2012, 5, 529–552. [Google Scholar] [CrossRef]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Schroder, N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berl) 2012, 219, 1133–1140. [Google Scholar] [CrossRef]
- Razavi, Y.; Shabani, R.; Mehdizadeh, M.; Haghparast, A. Neuroprotective effect of chronic administration of cannabidiol during the abstinence period on methamphetamine-induced impairment of recognition memory in the rats. Behav. Pharmacol. 2020, 31, 385–396. [Google Scholar] [CrossRef]
- Stark, T.; Ruda-Kucerova, J.; Iannotti, F.A.; D’Addario, C.; Di Marco, R.; Pekarik, V.; Drazanova, E.; Piscitelli, F.; Bari, M.; Babinska, Z.; et al. Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology 2019, 146, 212–221. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Poklis, J.L.; Karin, K.N.; Elmer, J.A.; Porter, J.H.; Parra, V.; Lu, D.; Schlosburg, J.E.; Lichtman, A.H. Investigation of Cannabidiol in the Mouse Drug Discrimination Paradigm. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yadav-Samudrala, B.J.; Xu, C.; Nath, B.; Mistry, T.; Jiang, W.; Niphakis, M.J.; Cravatt, B.F.; Mukhopadhyay, S.; Lichtman, A.H.; et al. Inhibitory Neurotransmission Is Sex-Dependently Affected by Tat Expression in Transgenic Mice and Suppressed by the Fatty Acid Amide Hydrolase Enzyme Inhibitor PF3845 via Cannabinoid Type-1 Receptor Mechanisms. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Hermes, D.J.; Xu, C.; Poklis, J.L.; Niphakis, M.J.; Cravatt, B.F.; Mackie, K.; Lichtman, A.H.; Ignatowska-Jankowska, B.M.; Fitting, S. Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS. Neuropharmacology 2018, 141, 55–65. [Google Scholar] [CrossRef]
- Swinton, M.K.; Sundermann, E.E.; Pedersen, L.; J. D., N.; Grelotti, D.J.; Taffe, M.A.; Iudicello, J.E.; Fields, J.A. Alterations in Brain Cannabinoid Receptor Levels Are Associated with HIV-Associated Neurocognitive Disorders in the ART Era: Implications for Therapeutic Strategies Targeting the Endocannabinoid System. Viruses 2021, 13, 1742. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.A.; Bauman, A.; Zhao, M.L.; Morgello, S.; Suh, H.S.; Lee, S.C. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 2011, 37, 464–483. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits - A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Bosetti, F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J. Neurochem. 2007, 102, 577–586. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Hauser, K.F.; El-Hage, N.; Stiene-Martin, A.; Maragos, W.F.; Nath, A.; Persidsky, Y.; Volsky, D.J.; Knapp, P.E. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J. Neurochem. 2007, 100, 567–586. [Google Scholar] [CrossRef]
- Chen, N.C.; Partridge, A.T.; Sell, C.; Torres, C.; Martin-Garcia, J. Fate of microglia during HIV-1 infection: From activation to senescence? Glia 2017, 65, 431–446. [Google Scholar] [CrossRef]
- Duarte, E.A.C.; Benevides, M.L.; Martins, A.L.P.; Duarte, E.P.; Weller, A.B.S.; de Azevedo, L.O.C.; de Oliveira Thais, M.E.R.; Nunes, J.C. Female sex is strongly associated with cognitive impairment in HIV infection. Neurol. Sci. 2021, 42, 1853–1860. [Google Scholar] [CrossRef]
- Rubin, L.H.; Neigh, G.N.; Sundermann, E.E.; Xu, Y.; Scully, E.P.; Maki, P.M. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 94. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Heaton, R.K.; Pasipanodya, E.; Moore, R.C.; Paolillo, E.W.; Rubin, L.H.; Ellis, R.; Moore, D.J.; Group, H. Sex differences in HIV-associated cognitive impairment. Aids 2018, 32, 2719–2726. [Google Scholar] [CrossRef]
- Maki, P.M.; Martin-Thormeyer, E. HIV, cognition and women. Neuropsychol. Rev. 2009, 19, 204–214. [Google Scholar] [CrossRef]
- Maki, P.M.; Rubin, L.H.; Springer, G.; Seaberg, E.C.; Sacktor, N.; Miller, E.N.; Valcour, V.; Young, M.A.; Becker, J.T.; Martin, E.M.; et al. Differences in Cognitive Function Between Women and Men With HIV. J. Acquir. Immune. Defic. Syndr. 2018, 79, 101–107. [Google Scholar] [CrossRef]
- Santinelli, L.; Ceccarelli, G.; Borrazzo, C.; Innocenti, G.P.; Frasca, F.; Cavallari, E.N.; Celani, L.; Nonne, C.; Mastroianni, C.M.; d’Ettorre, G. Sex-related differences in markers of immune activation in virologically suppressed HIV-infected patients. Biol. Sex. Differ. 2020, 11, 23. [Google Scholar] [CrossRef]
- Ziegler, S.; Altfeld, M. Sex differences in HIV-1-mediated immunopathology. Curr. Opin. HIV AIDS 2016, 11, 209–215. [Google Scholar] [CrossRef]
- Yuan, N.Y.; Maung, R.; Xu, Z.; Han, X.; Kaul, M. Arachidonic Acid Cascade and Eicosanoid Production Are Elevated While LTC4 Synthase Modulates the Lipidomics Profile in the Brain of the HIVgp120-Transgenic Mouse Model of NeuroHIV. Cells 2022, 11. [Google Scholar] [CrossRef]
- Levine, A.; Liktor-Busa, E.; Lipinski, A.A.; Couture, S.; Balasubramanian, S.; Aicher, S.A.; Langlais, P.R.; Vanderah, T.W.; Largent-Milnes, T.M. Sex differences in the expression of the endocannabinoid system within V1M cortex and PAG of Sprague Dawley rats. Biol. Sex Differ. 2021, 12, 60. [Google Scholar] [CrossRef]
- Arkell, T.R.; Kevin, R.C.; Vinckenbosch, F.; Lintzeris, N.; Theunissen, E.; Ramaekers, J.G.; McGregor, I.S. Sex differences in acute cannabis effects revisited: Results from two randomized, controlled trials. Addict. Biol. 2022, 27, e13125. [Google Scholar] [CrossRef]
- MacNair, L.; Kulpa, J.; Hill, M.L.; Eglit, G.M.L.; Mosesova, I.; Bonn-Miller, M.O.; Peters, E.N. Sex Differences in the Pharmacokinetics of Cannabidiol and Metabolites Following Oral Administration of a Cannabidiol-Dominant Cannabis Oil in Healthy Adults. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
- Batinic, A.; Sutlovic, D.; Kuret, S.; Burcul, F.; Kalajzic, N.; Matana, A.; Dujic, G.; Vrdoljak, J.; Kumric, M.; Bozic, J.; et al. Differences in Plasma Cannabidiol Concentrations in Women and Men: A Randomized, Placebo-Controlled, Crossover Study. Int. J. Mol. Sci. 2023, 24, 10273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




