Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
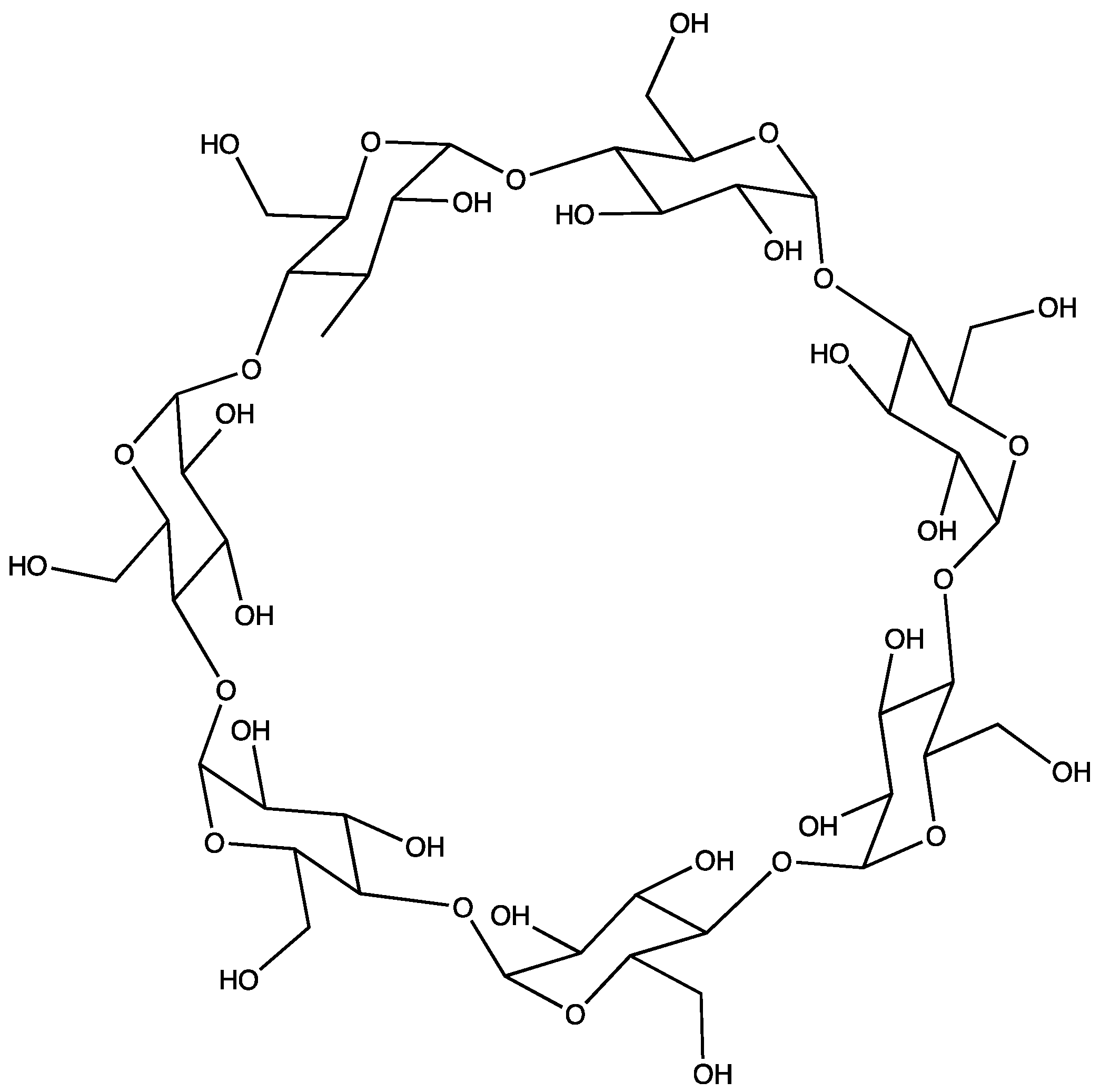
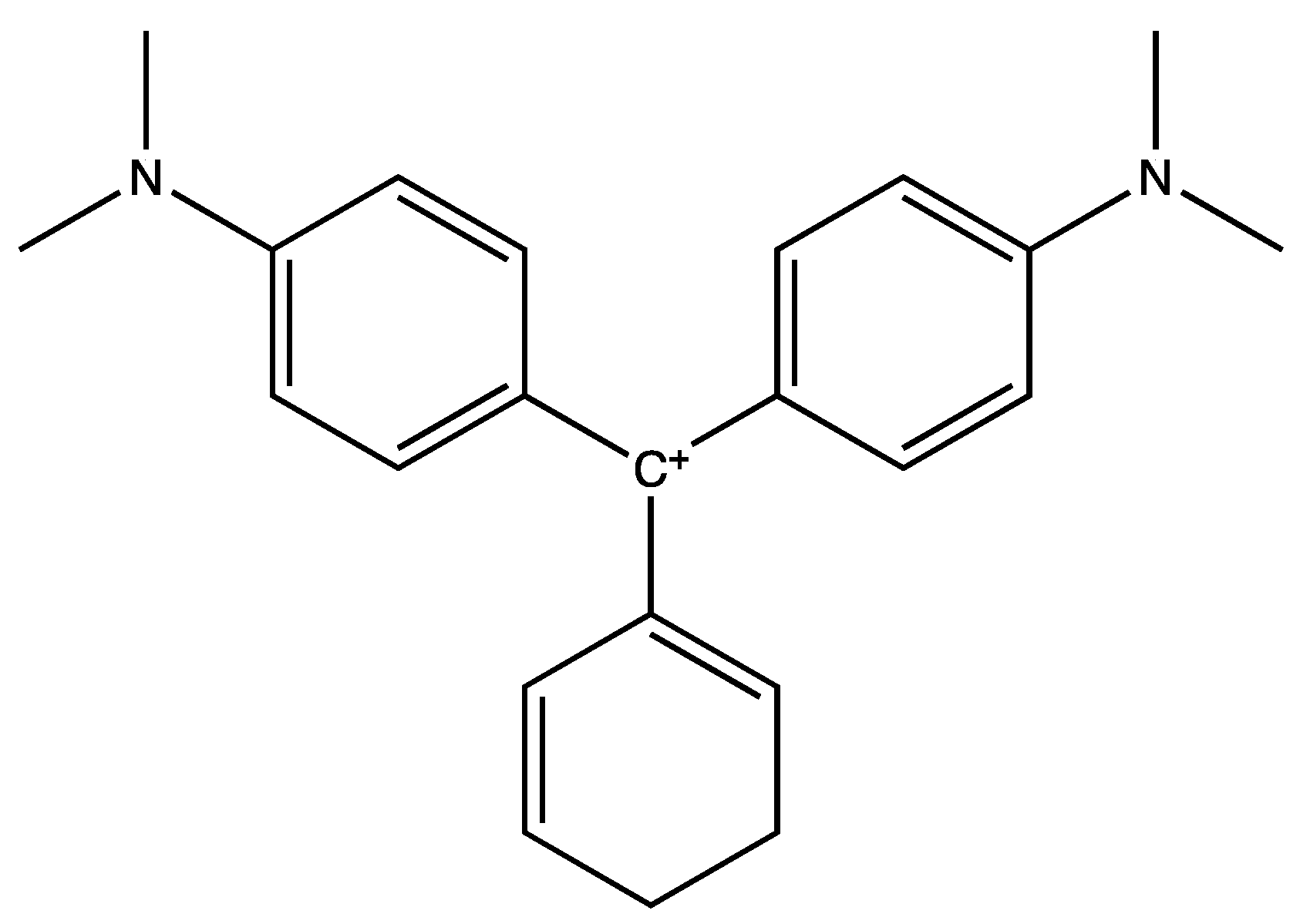
1. Introduction
2. Materials and Methods
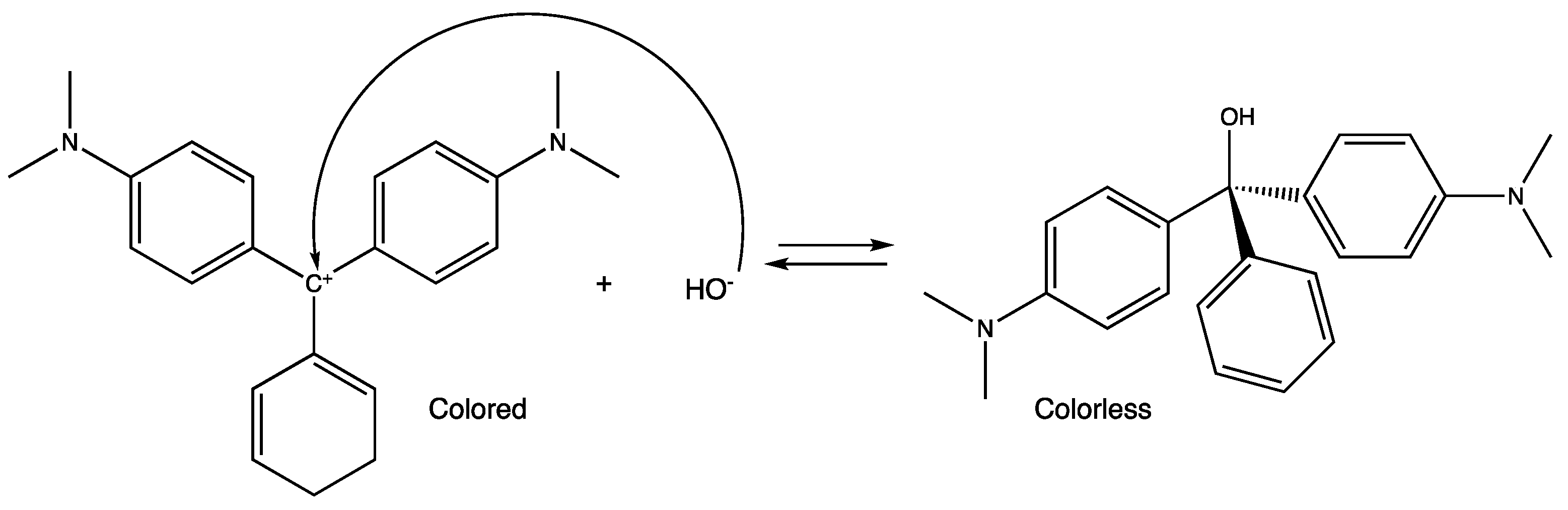
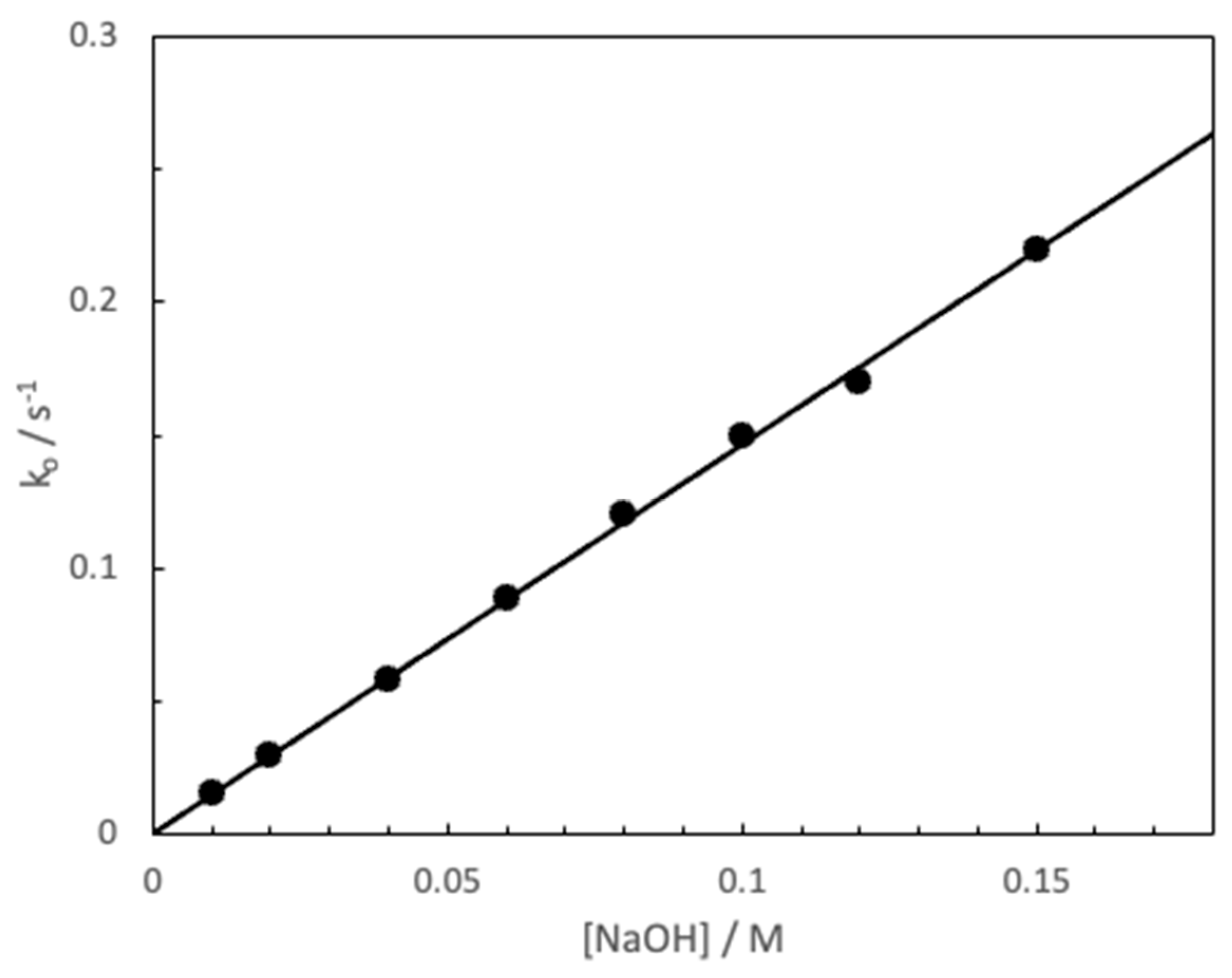
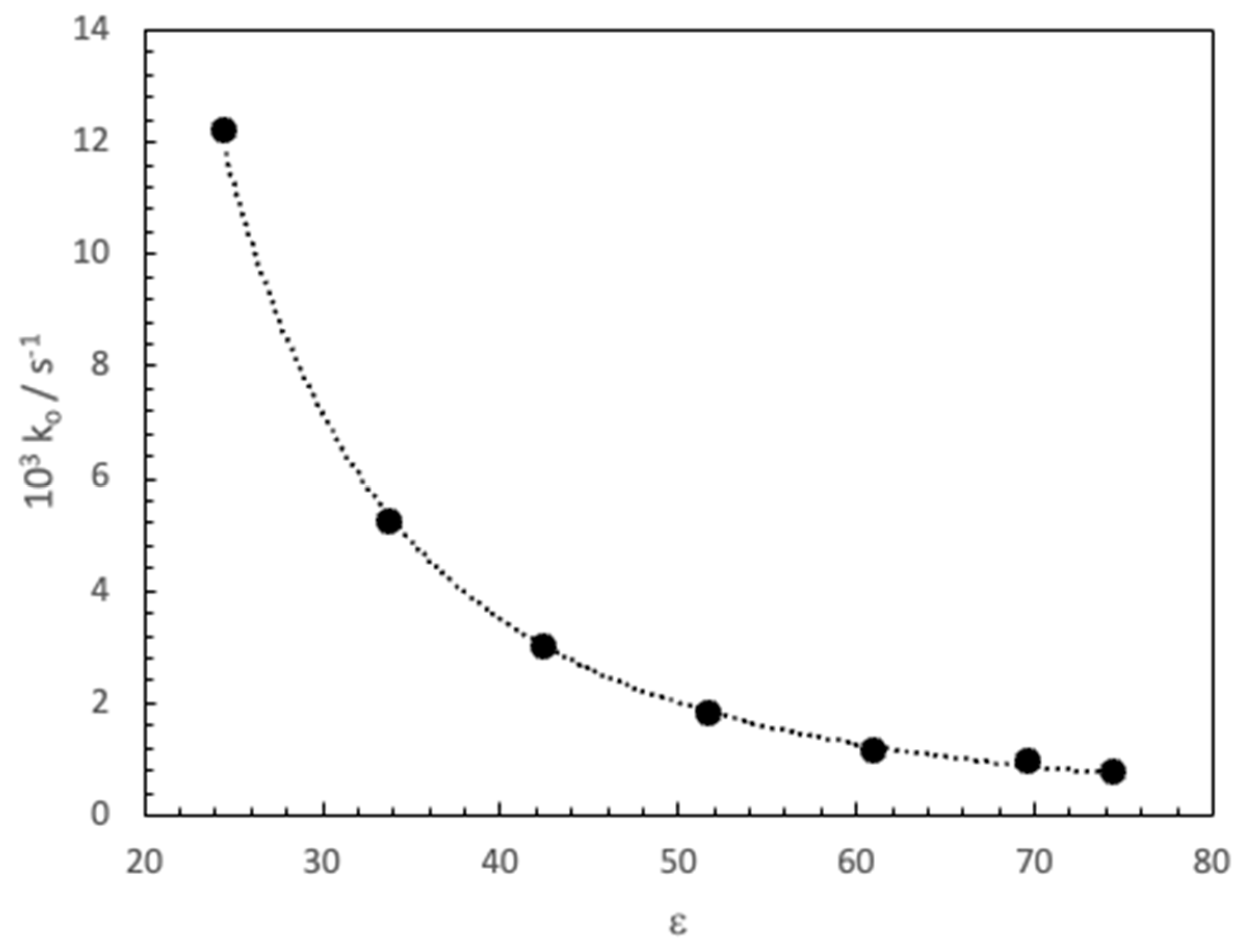
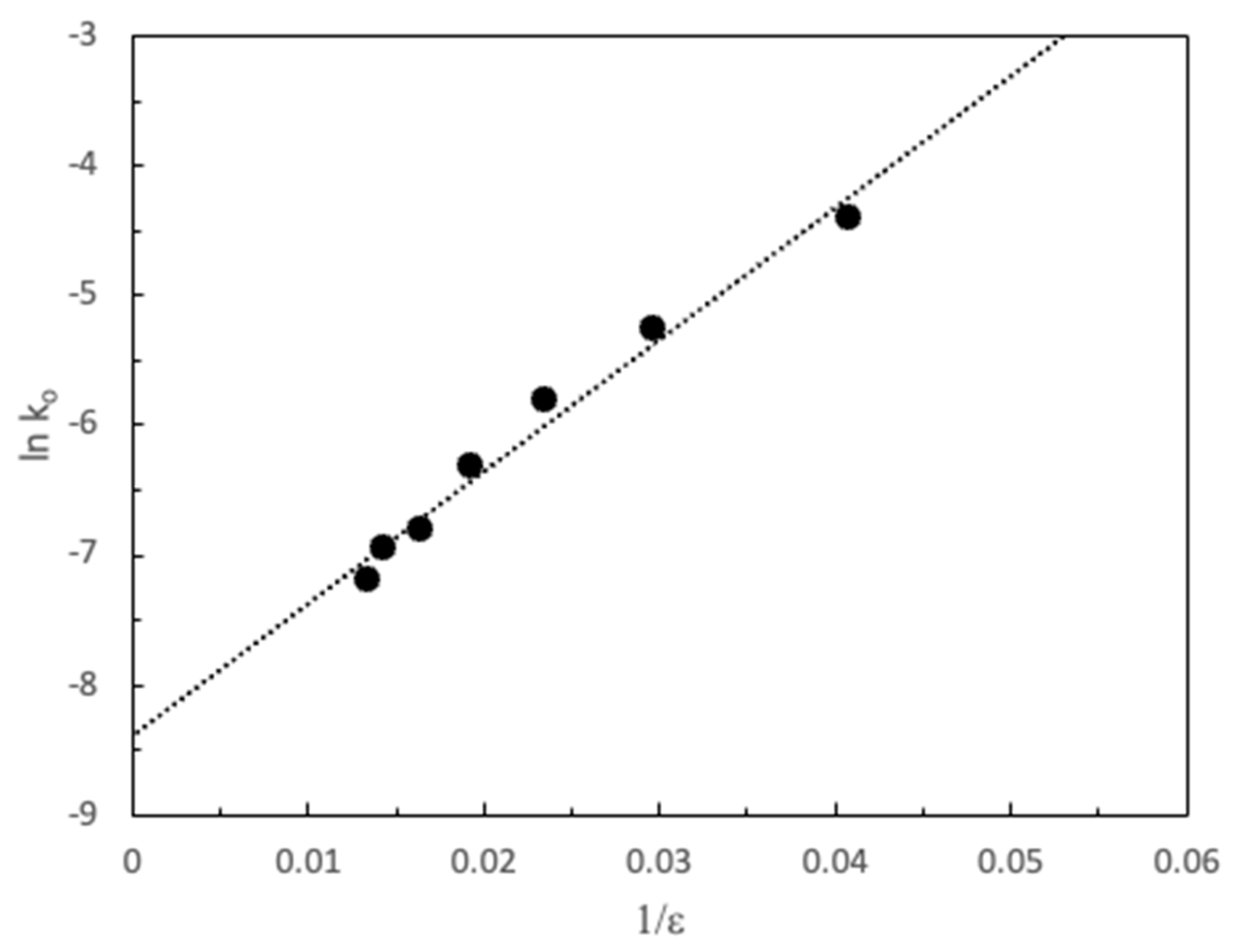
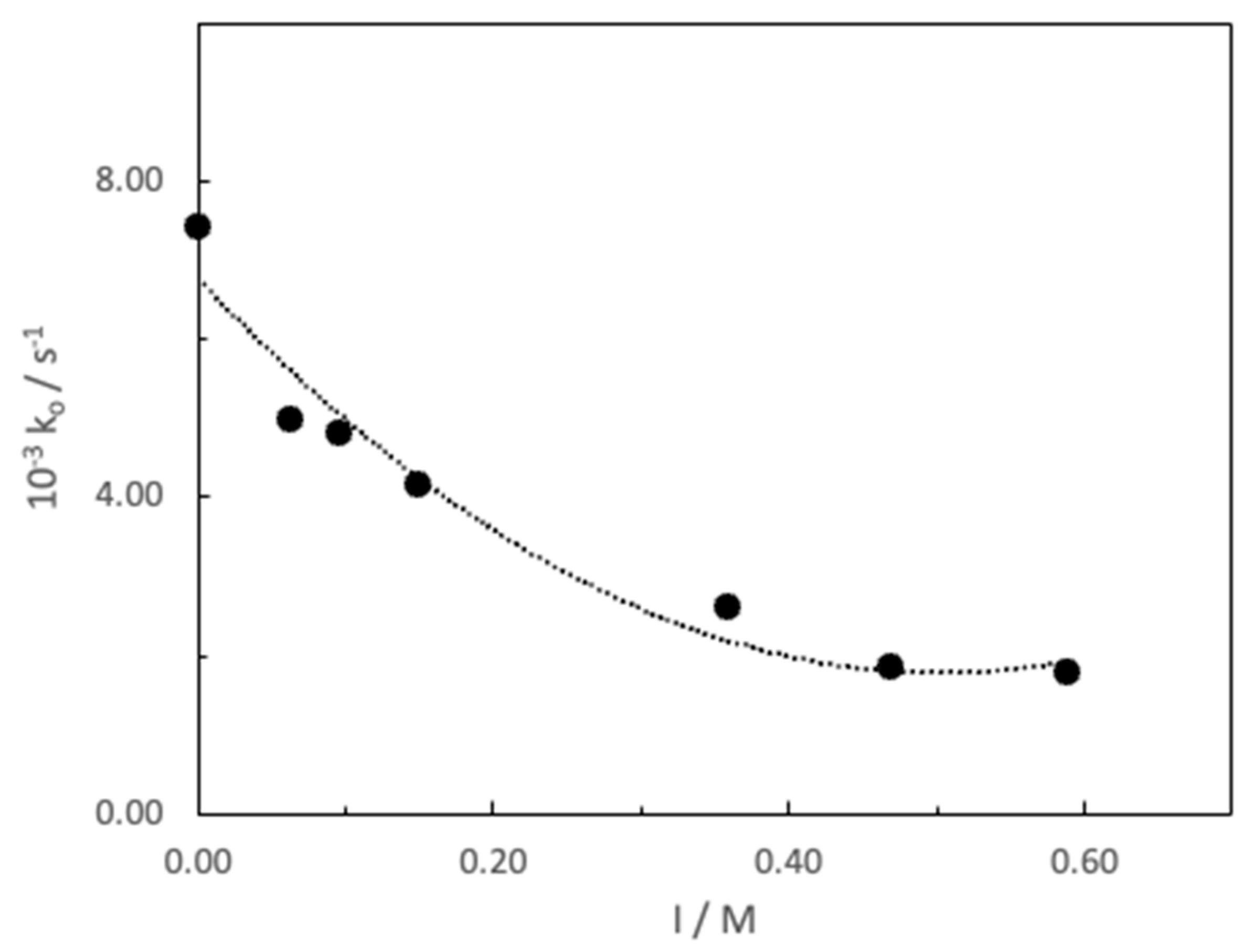
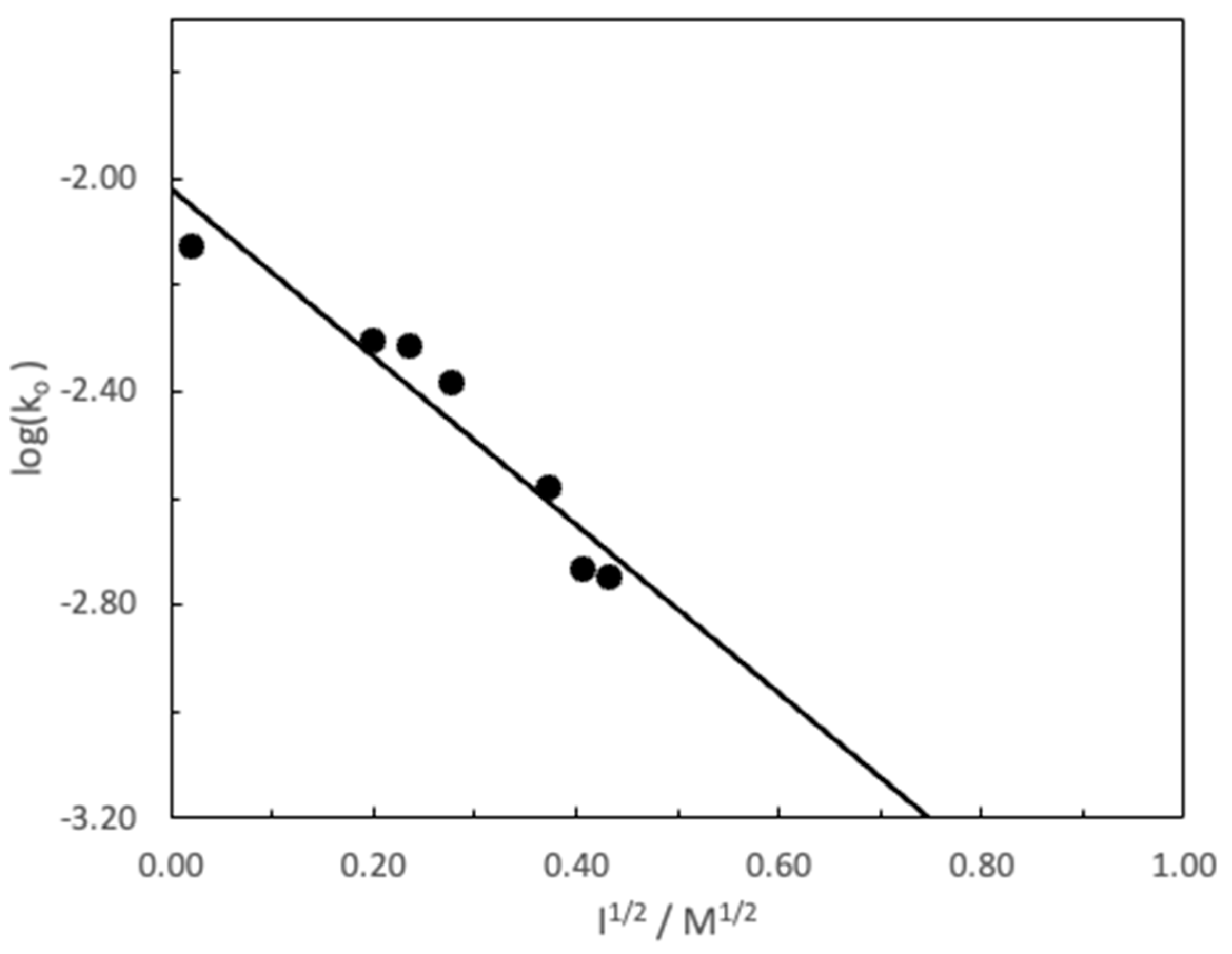
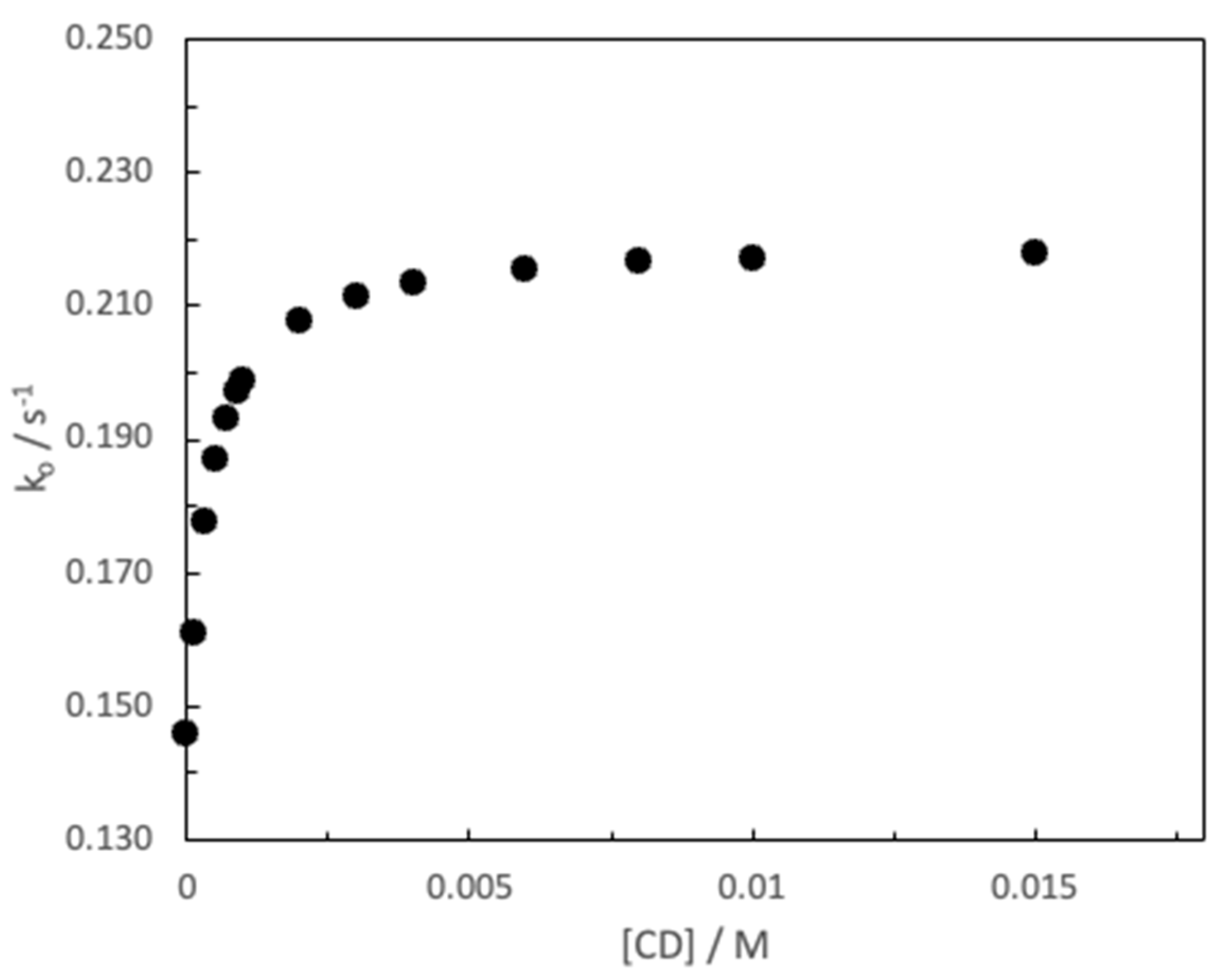
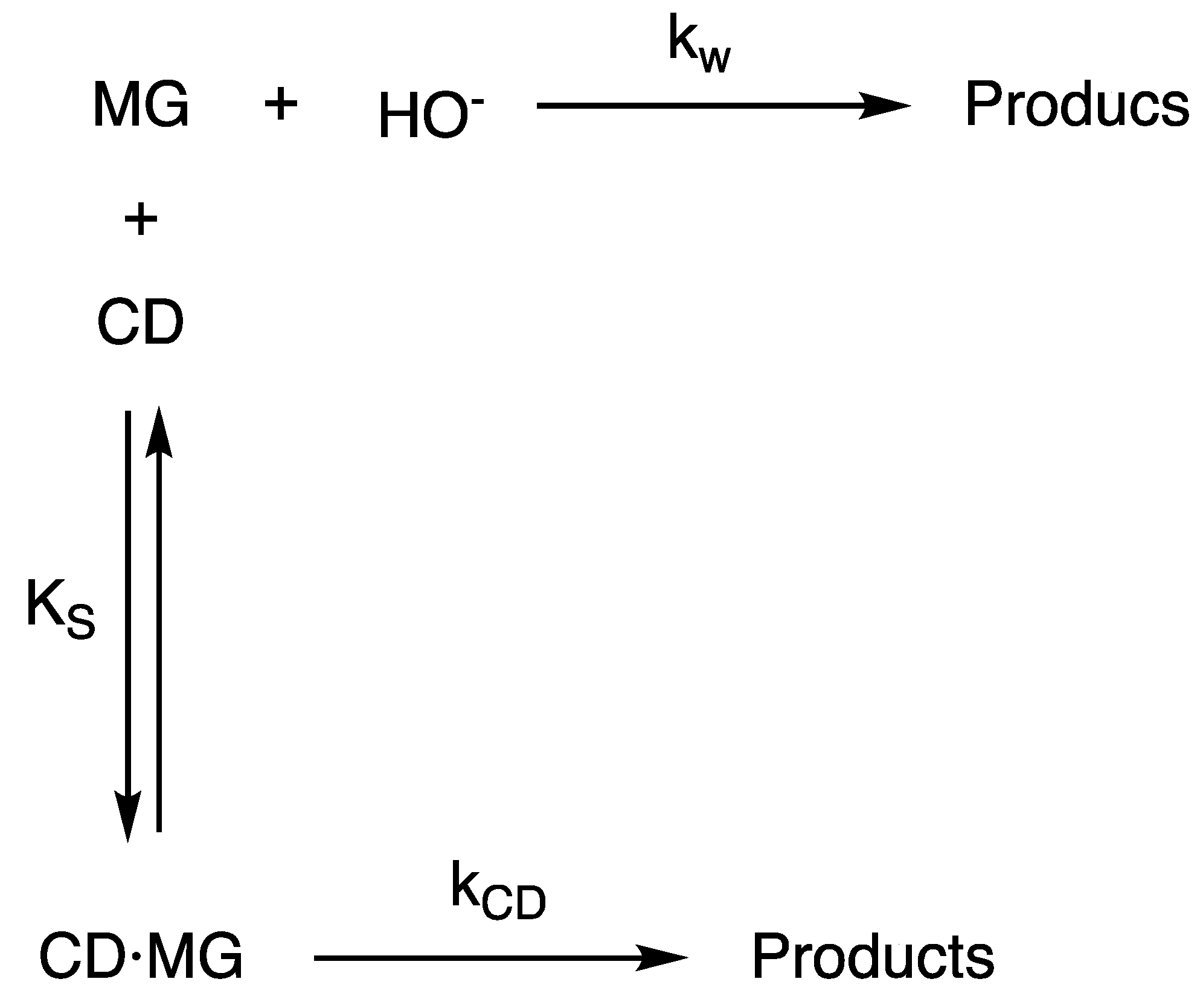
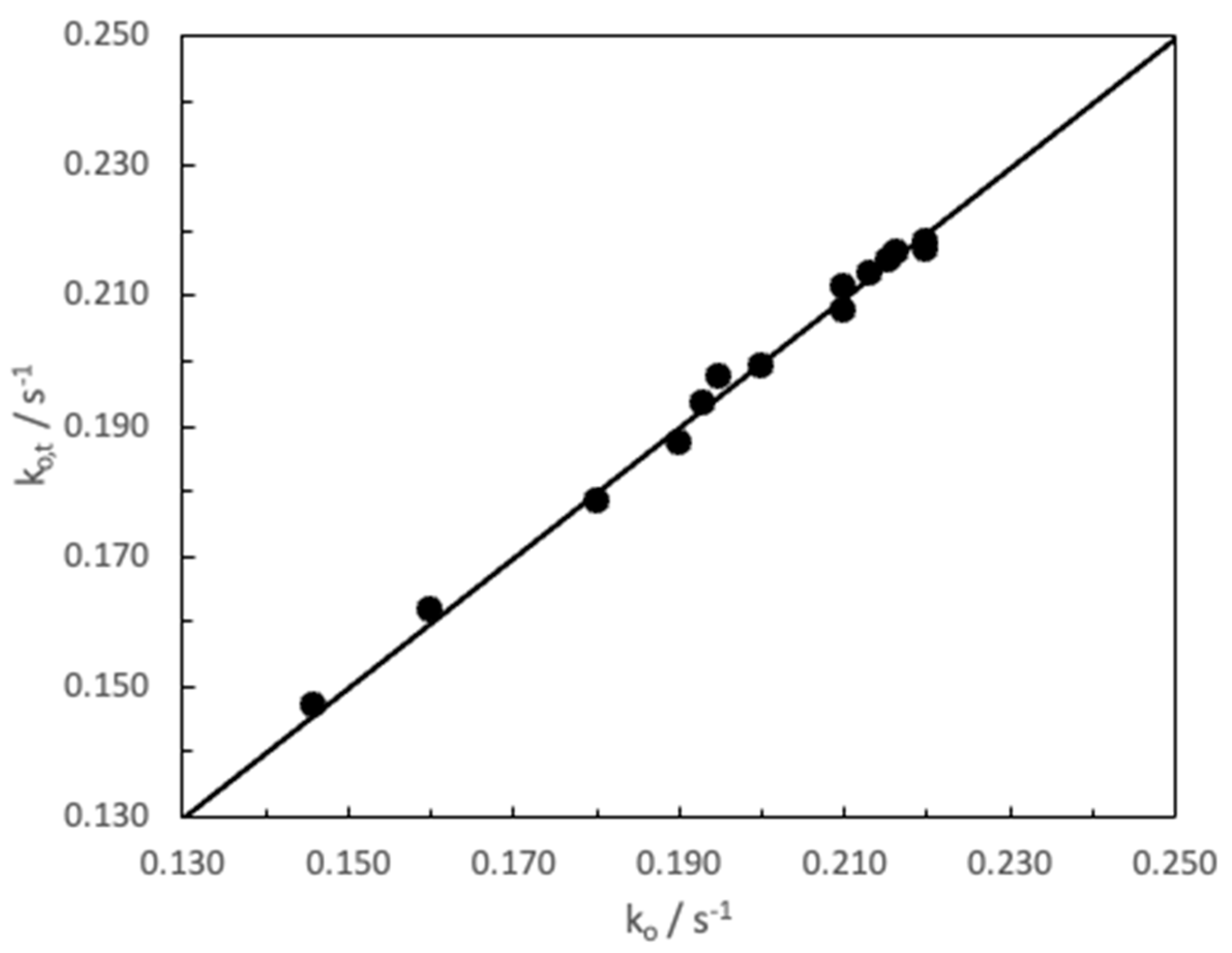
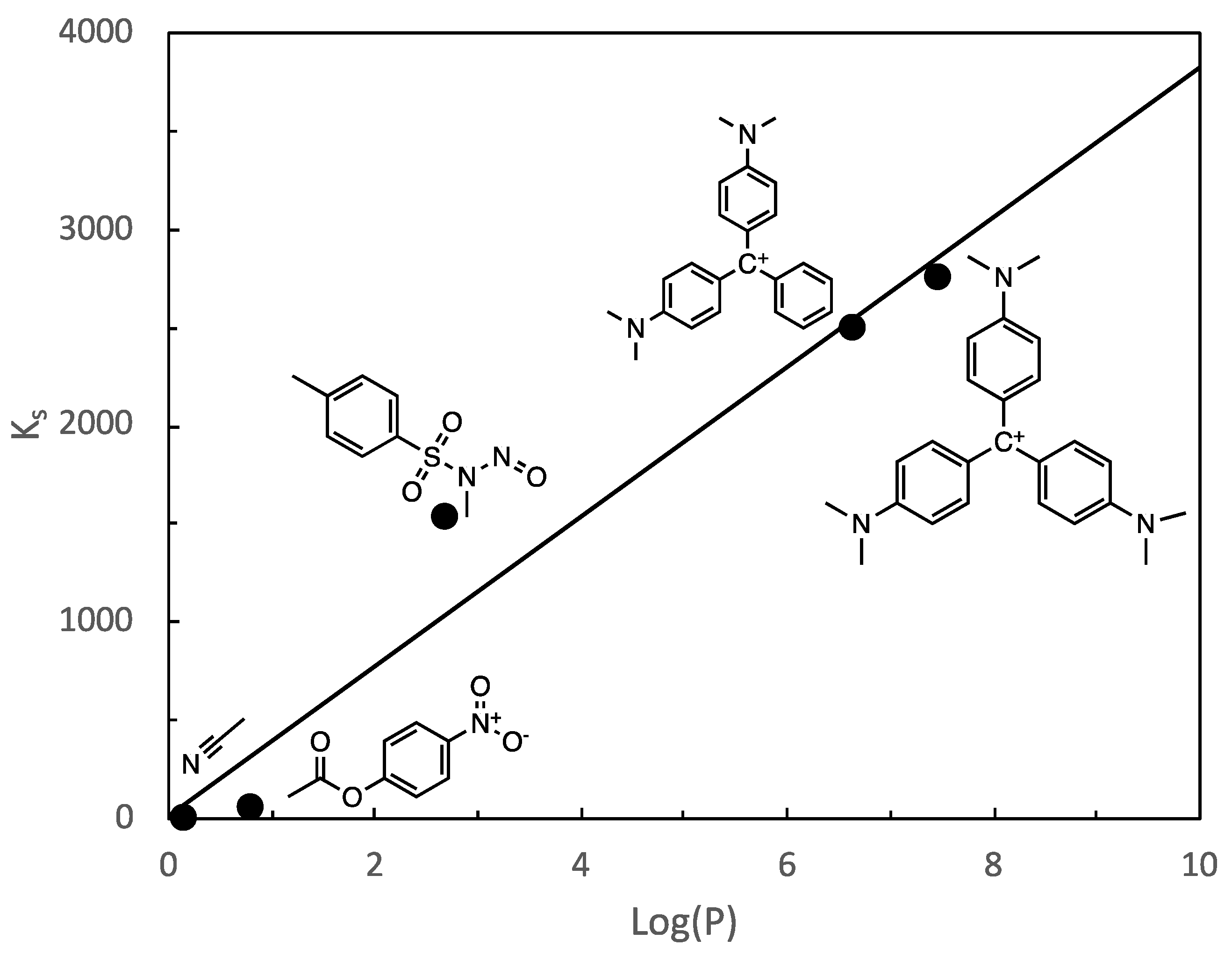
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mejuto, J.C.; Simal-Gandara, J. Host-Guest Complexes. Int. J. Mol. Sci. 2022, 23, 15730. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Mellet, C.O.; Fernández, J.M.G.; Benito, J.M. Cyclodextrin-Based Gene Delivery Systems. Chem. Soc. Rev. 2011, 40, 1586–1608. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Leija, S.; Espinosa-Barrera, L.; Velazquez-Cruz, B.; Cárdenas-Conejo, Y.; Virgen-Ortíz, R.; Valencia-Cruz, G.; Saenz, R.A.; Marín-Tovar, Y.; Gómez-Manzo, S.; Hernández-Ochoa, B.; et al. Mining for Novel Cyclomaltodextrin Glucanotransferases Unravels the Carbohydrate Metabolism Pathway via Cyclodextrins in Thermoanaerobacterales. Sci. Rep. 2022, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Kinart, Z. Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions. Molecules 2023, 28, 292. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Bortolus, P.; Grabner, G.; Köhler, G.; Monti, S. Photochemistry of Cyclodextrin Host-Guest Complexes. Coord. Chem. Rev. 1993, 125, 261–268. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular Chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Second.; Wiley: NJ, USA, 2013; ISBN 978-0-470-51233-3. [Google Scholar]
- Iglesias, E.; Fernández, A. Cyclodextrin Catalysis in the Basic Hydrolysis of Alkyl Nitrites. J. Chem. Soc.{,} Perkin Trans. 2, 1700. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, K.; Arya, V.; Kumar, A. Encapsulation of Tinospora Cordifolia Plant in Ni Doped TiO2 Nanoparticles for the Degradation of Malachite Green Dye. Nanofabrication 2023, 8. [Google Scholar] [CrossRef]
- Yan, J.; Niu, J.; Chen, D.; Chen, Y.; Irbis, C. Screening of Trametes Strains for Efficient Decolorization of Malachite Green at High Temperatures and Ionic Concentrations. Int. Biodeterior. Biodegradation 2014, 87, 109–115. [Google Scholar] [CrossRef]
- Lunestad, B.T.; Samuelsen, O. 4 - Veterinary Drug Use in Aquaculture. In Improving Farmed Fish Quality and Safety; Lie, Ø., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing, 2008; pp. 97–127 ISBN 978-1-84569-299-5.
- Dasmandal, S.; Mandal, H.K.; Kundu, A.; Mahapatra, A. Kinetic Investigations on Alkaline Fading of Malachite Green in the Presence of Micelles and Reverse Micelles. J. Mol. Liq. 2014, 193, 123–131. [Google Scholar] [CrossRef]
- Gessner, T.; Mayer, U. Triarylmethane and Diarylmethane Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd, 2000 ISBN 9783527306732.
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological Effects of Malachite Green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, L.G.; Roberts, R.J. Towards Strategic Use of Fungicides against Saprolegnia Parasitica in Salmonid Fish Hatcheries. J. Fish Dis. 1992, 15, 1–13. [Google Scholar] [CrossRef]
- Diggles, B.K. A Mycosis of Juvenile Spiny Rock Lobster, Jasus Edwardsii (Hutton, 1875) Caused by Haliphthoros Sp., and Possible Methods of Chemical Control. J. Fish Dis. 2001, 24, 99–110. [Google Scholar] [CrossRef]
- Lilley, J.H.; Inglis, V. Comparative Effects of Various Antibiotics, Fungicides and Disinfectants on Aphanomyces Invaderis and Other Saprolegniaceous Fungi. Aquac. Res. 1997, 28, 461–469. [Google Scholar] [CrossRef]
- Deokar, R.; Sabale, A. Biosorption of Methylene Blue and Malachite Green From Binary Solution onto Ulva Lactuca. Int.J.Curr.Microbiol.App.Sci 2014, 3, 295–304. [Google Scholar]
- Hema, M.; Arivoli, S. Adsorption Kinetics and Thermodynamics of Malachite Green Dye unto Acid Activated Low Cost Carbon. J. Appl. Sci. Environ. Manag. 2008, 12, 43–51. [Google Scholar] [CrossRef]
- Samiey, B.; Toosi, A.R. Kinetics Study of M이achite Green Fading in the Presence of TX-100, DTAB and SDS. Bull. Korean Chem. Soc. 2009, 30, 2051–2056. [Google Scholar] [CrossRef]
- Leis, J.R.; Mejuto, J.C.; Pena, M.E. Comparison between the Kinetics of the Alkaline Fading of Carbocation Dyes in Water/Sodium Bis (2-Ethylhexyl) Sulfosuccinate/Isooctane Microemulsions and in Homogeneous Media. Langmuir 1993, 9, 889–893. [Google Scholar] [CrossRef]
- Dasmandal, S.; Mandal, H.K.; Rudra, S.; Kundu, A.; Majumdar, T.; Mahapatra, A. Kinetic Exploration Supplemented by Spectroscopic and Molecular Docking Analysis in Search of the Optimal Conditions for Effective Degradation of Malachite Green. RSC Adv. 2015, 5, 38503–38512. [Google Scholar] [CrossRef]
- Ritchie, C.D. Nucleophilic Reactivities toward Cations. Acc. Chem. Res. 1972, 5, 348–354. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chemie Int. Ed. English 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Felix, L.D.; Adesoji, A. Kinetics and Thermodynamic Study of Alkaline Fading of Malachite Green in Aqueous Solution. J. Appl. Fundam. Sci. 2017, 3, 52–57. [Google Scholar]
- Dalal, M. Ionic Reactions: Single and Double Sphere Models. In A textbook of physical chemistry. Volume I.; Dalal Institute: Rohtak, India, 2018; ISBN 978-81-938720-1-7. [Google Scholar]
- Laider, K.J. Chemical Kinetics, 3rd ed.; Pentice Hall: New York, NY, USA, 1987; ISBN 978-0060438623. [Google Scholar]
- García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C. Kinetic and Spectroscopic Evidence for the Formation of Ion-Pairs between Crystal Violet and Perchlorate Ion. J. Chem. Res. 1997, 326–327. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Basic Hydrolysis of M-Nitrophenyl Acetate in Micellar Media Containing β-Cyclodextrins. J. Phys. Chem. B 1998, 102, 4581–4587. [Google Scholar] [CrossRef]
- Alvarez, A.R.; García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Basic Hydrolysis of Substituted Nitrophenyl Acetates in β-Cyclodextrin/Surfactant Mixed Systems. Evidence of Free Cyclodextrin in Equilibrium with Micellized Surfactant. Langmuir 1999, 15, 8368–8375. [Google Scholar] [CrossRef]
- Astray, G.; Cid, A.; Manso, J.A.; Mejuto, J.C.; Moldes, O.A.; Morales, J. Alkaline Fading of Triarylmethyl Carbocations in Self-Assembly Microheterogeneous Media. Prog. React. Kinet. Mech. 2011, 36, 139–165. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Navarro-Vázquez, A.; Pérez-Juste, J.; Rodriguez-Dafonte, P. Basic Hydrolysis of Crystal Violet in β-Cyclodextrin/Surfactant Mixed Systems. Langmuir 2004, 20, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, M. Synthesis of Cyclodextrin Derivatives. In Cyclodextrin Fundamentals, Reactivity and Analysis.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-76158-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




