Submitted:
17 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
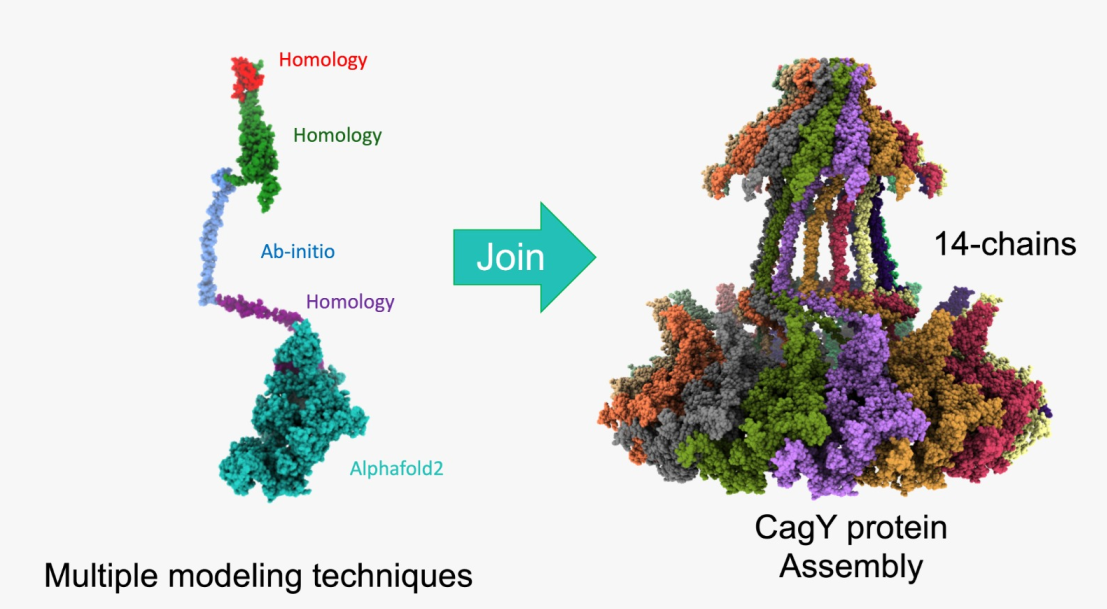
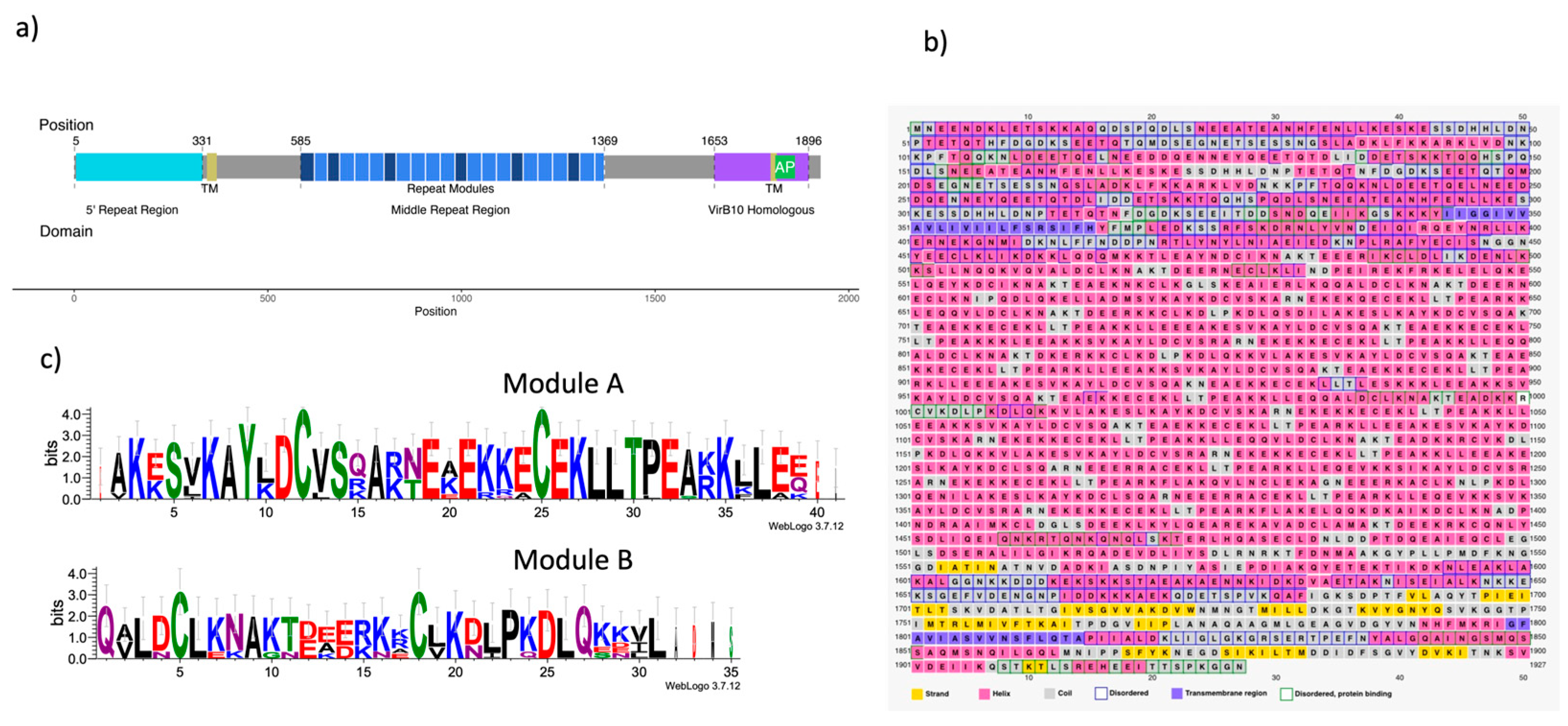
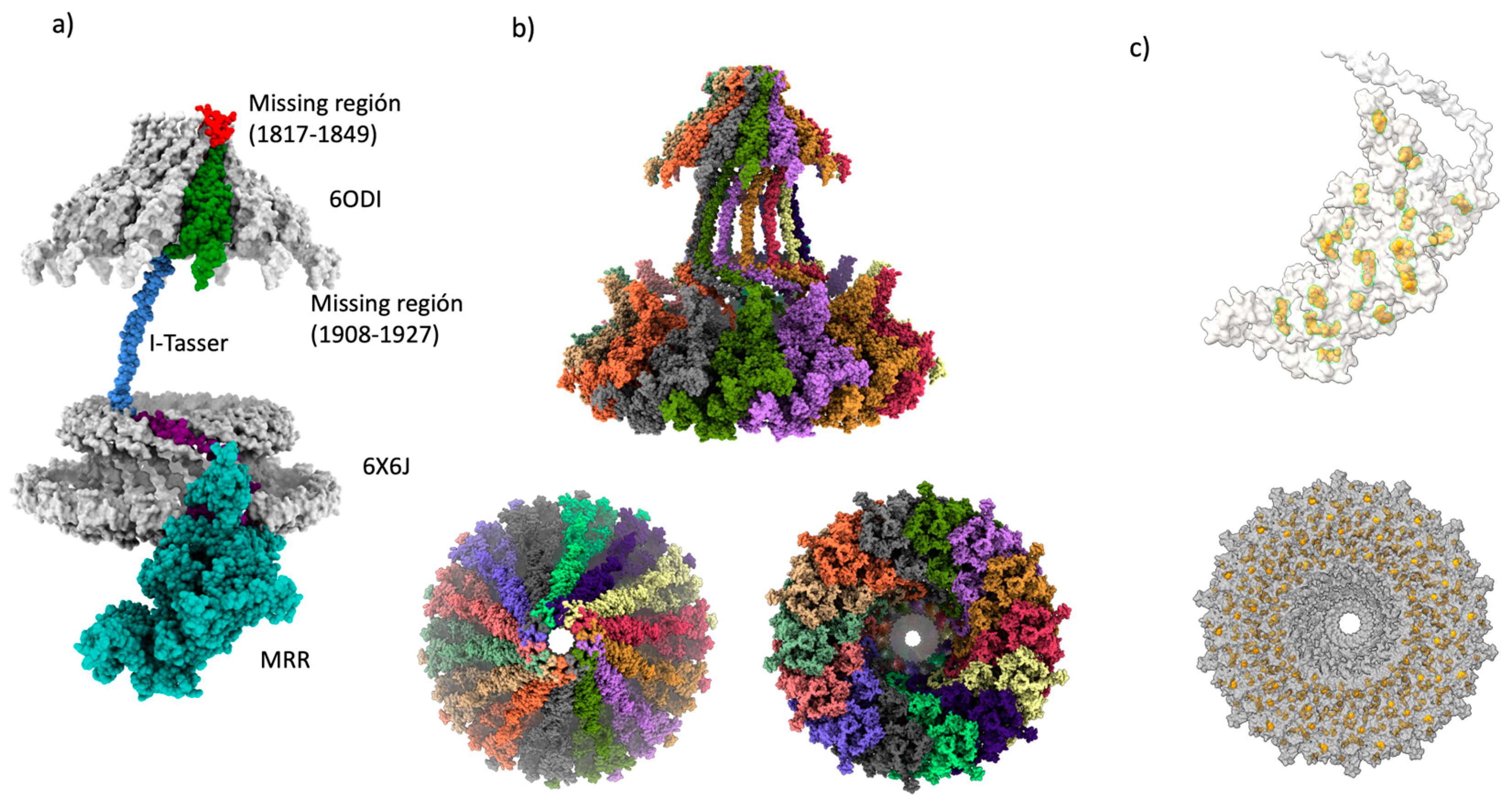
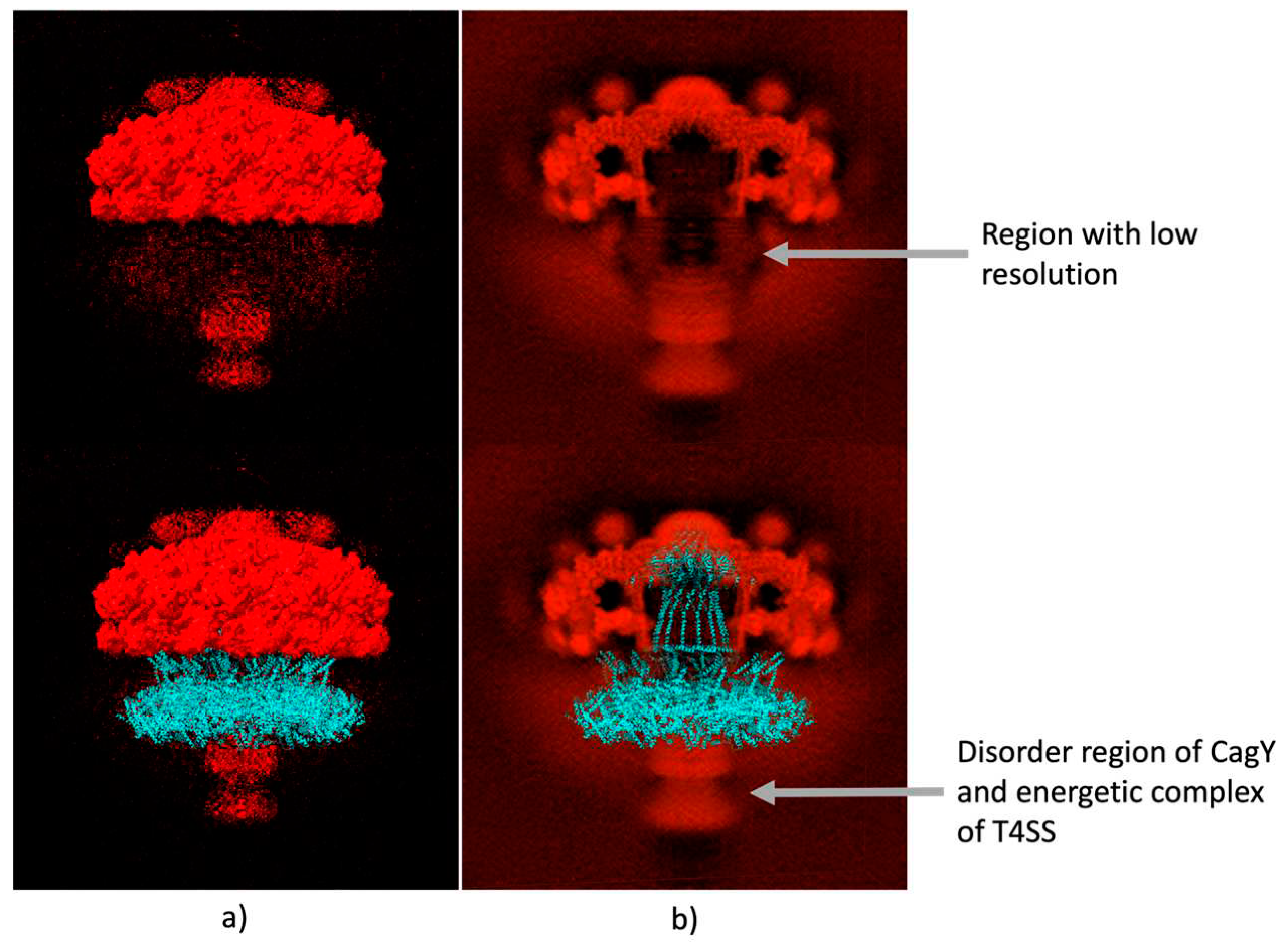
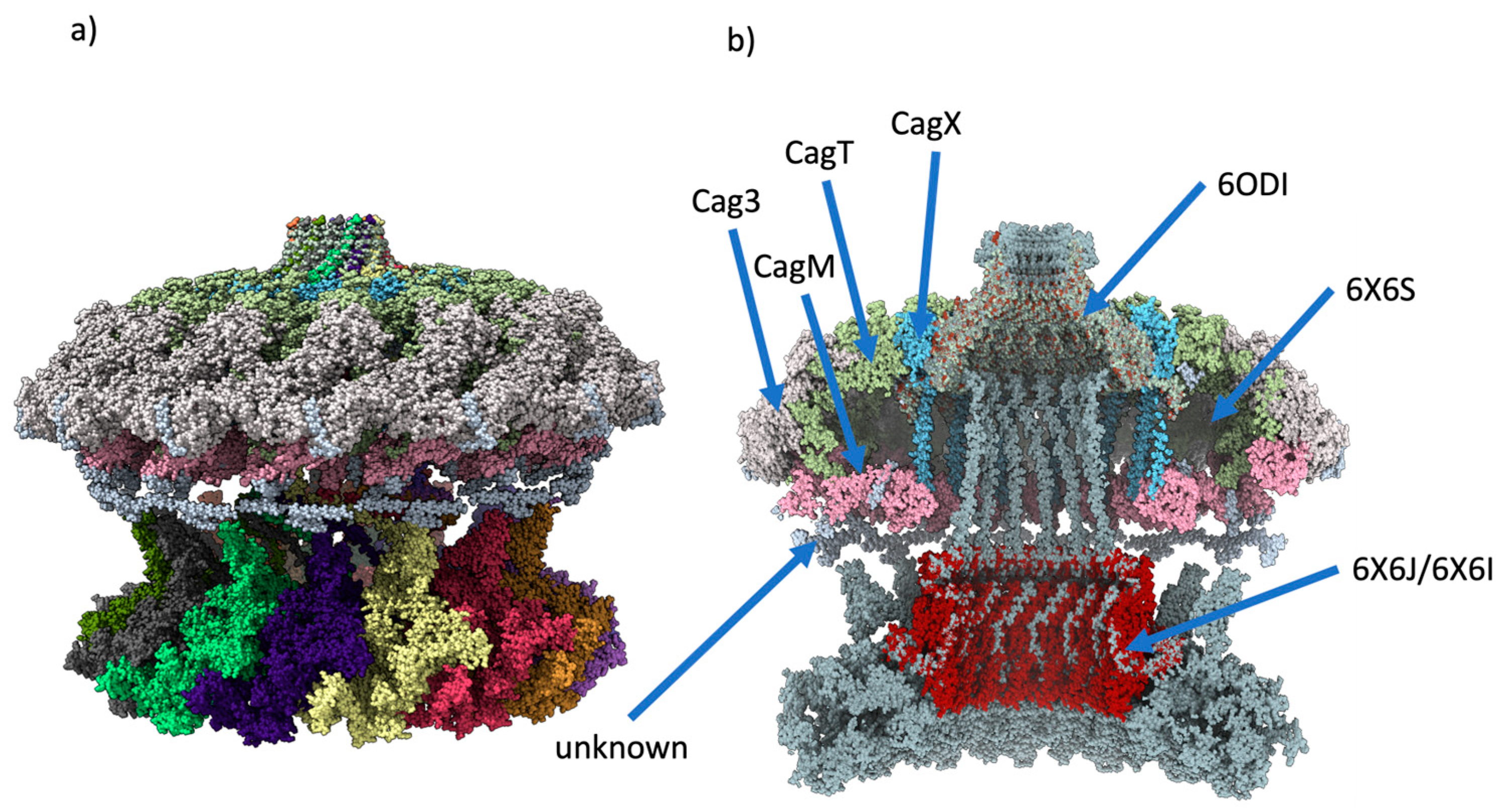
2.1. Building the CagY model.
2.2. Modeling of the homologous VirB10 Region
2.3. Modeling of the Middle Region of CagY
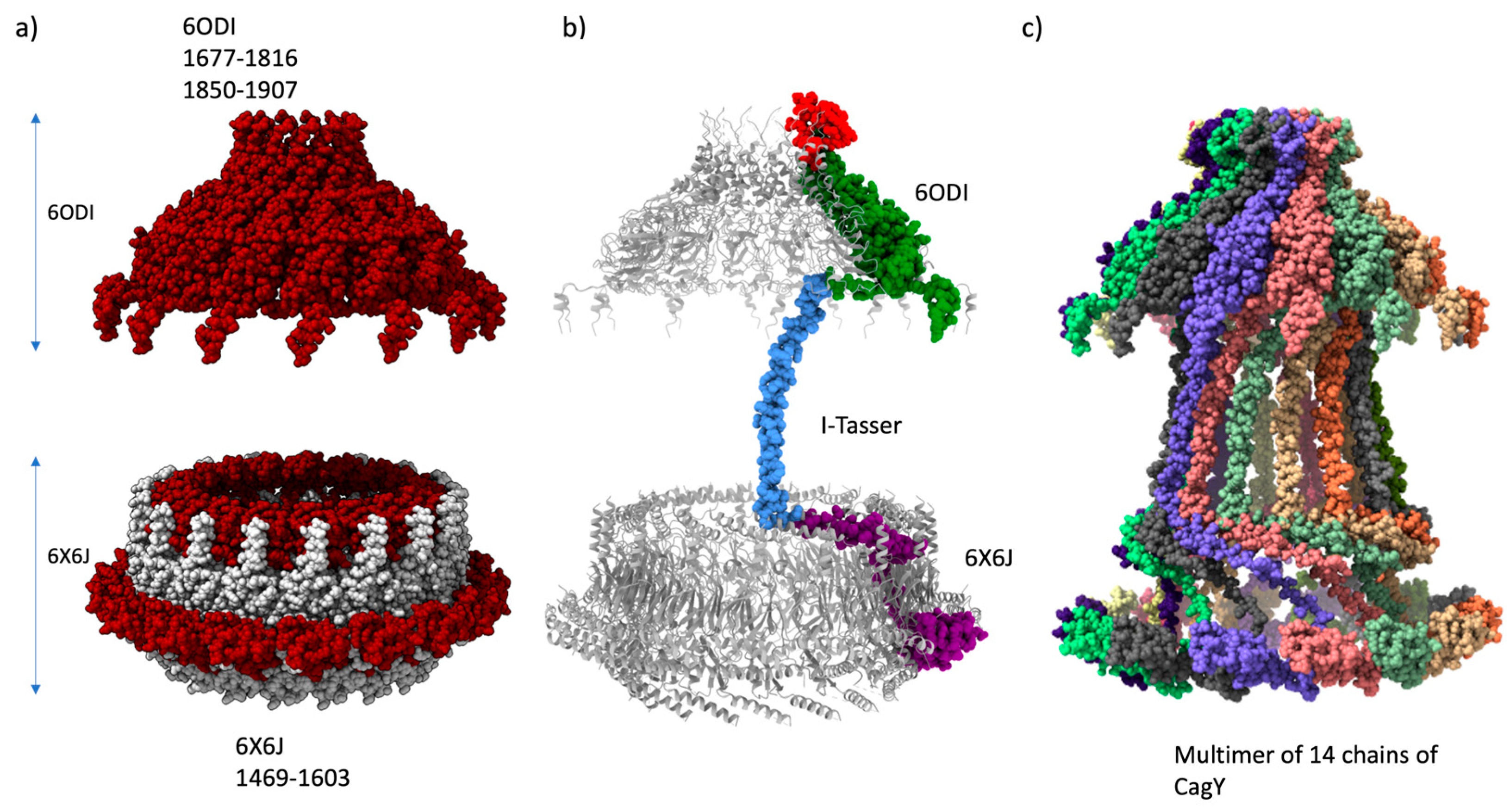
2.4. Final assembly of CagY
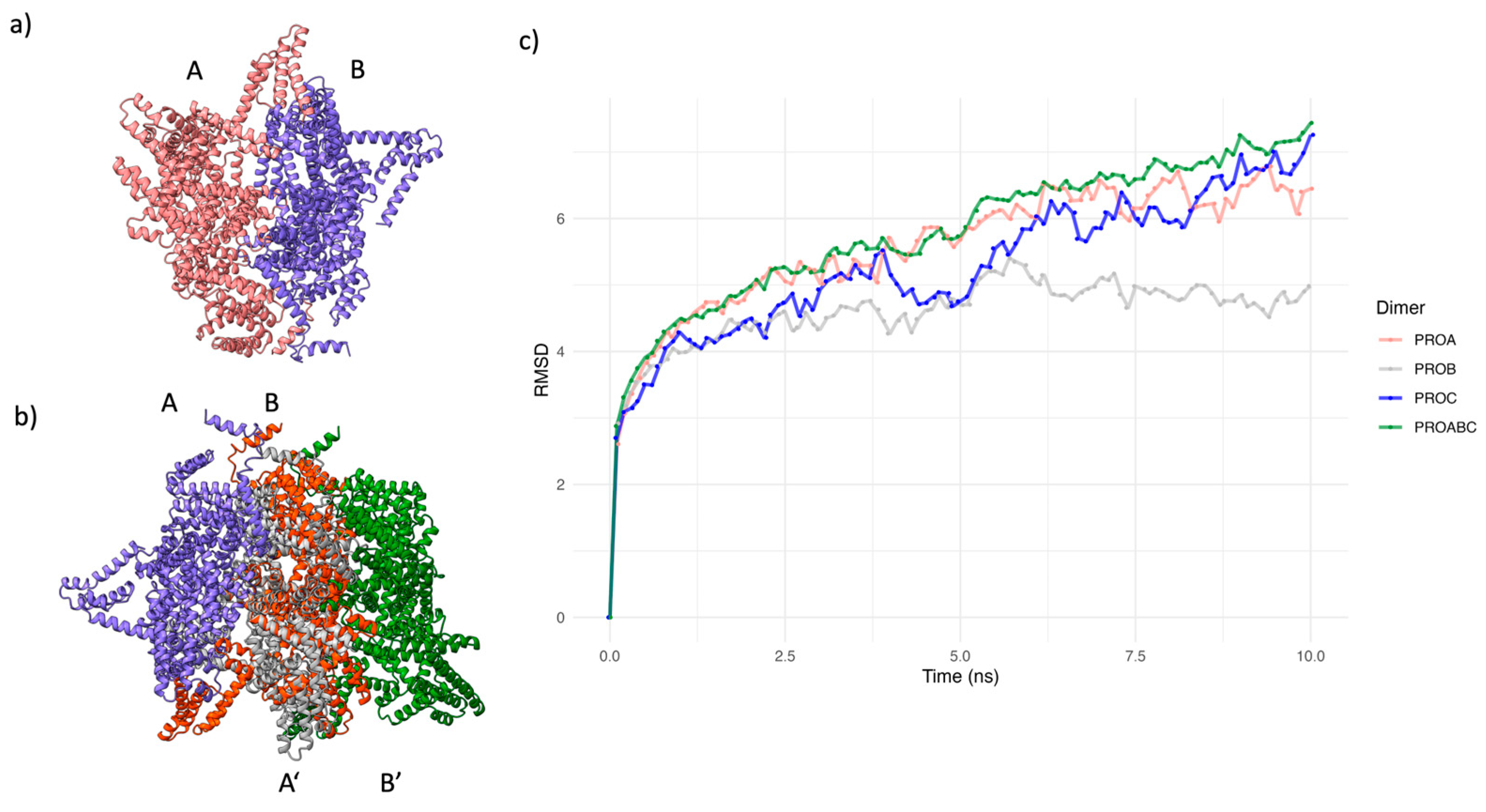
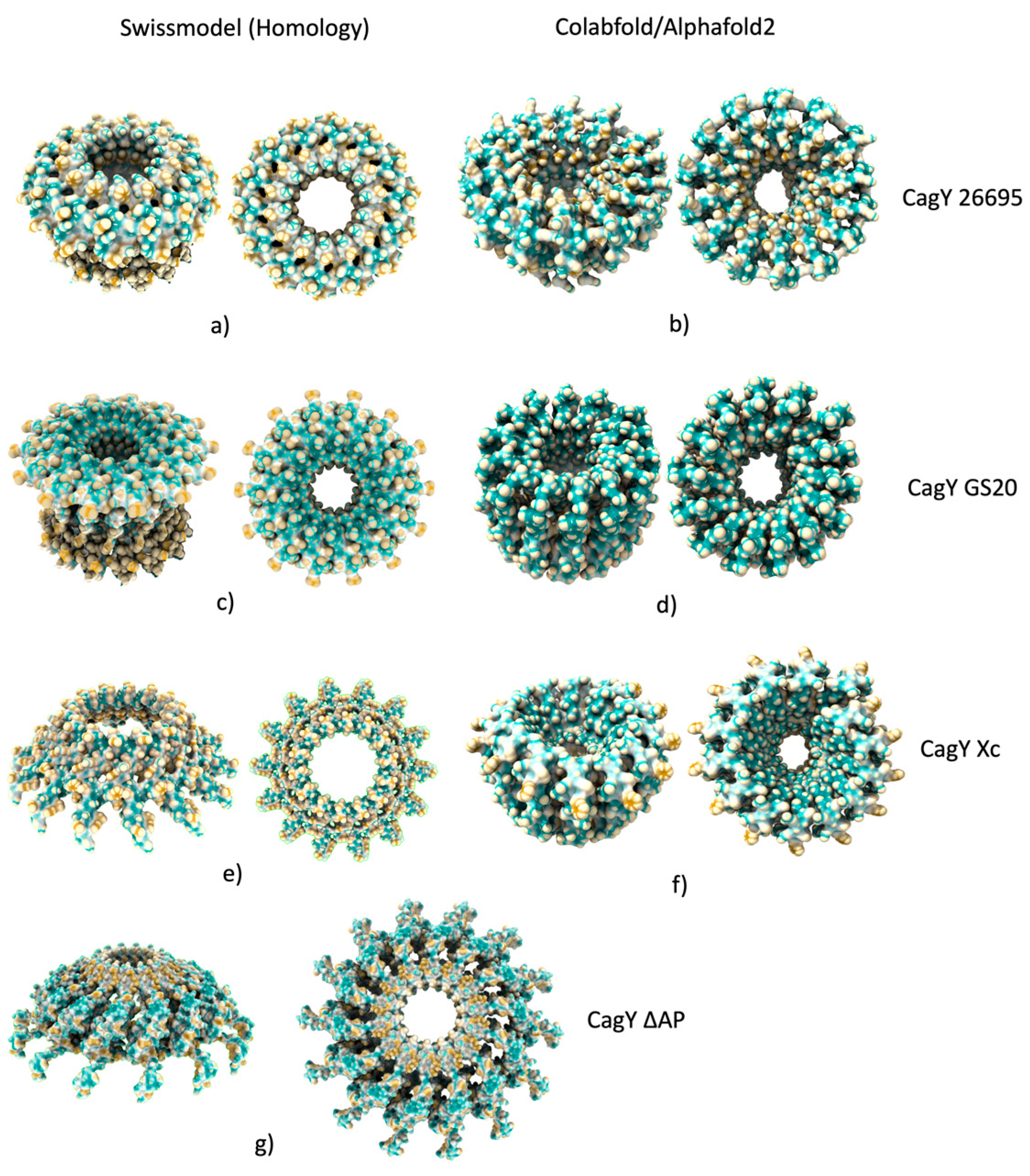
2.5. Changes in the AP region of CagY alters the conformation of the OMC
3. Discussion
4. Materials and Methods
4.1. CagY model sequence and structure models.
4.2. Determination of Secondary Structure and Transmembrane Regions
4.3. Modeling of the Protein Monomer and Construction of the Multimer
4.4. Modeling of the Middle Repeat Region (MRR) of CagY
4.5. Final Assembly of CagY multimer
4.6. Modeling of the Antenna Projections (AP) of CagY
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falush, D.; Wirth, T.; Linz, B.; Pritchard, J.K.; Stephens, M.; Kidd, M.; Blaser, M.J.; Graham, D.Y.; Vacher, S.; Perez-Perez, G.I.; et al. Traces of human migrations in Helicobacter pylori populations. Science 2003, 299, 1582–1585. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Reshetnyak, V.I.; Reshetnyak, T.M. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol 2017, 23, 4867–4878. [Google Scholar] [CrossRef]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’ Angelis, G.L. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Biomed 2018, 89, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Clyne, M.; Tegtmeyer, N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal 2011, 9, 28. [Google Scholar] [CrossRef]
- Odenbreit, S.; Puls, J.; Sedlmaier, B.; Gerland, E.; Fischer, W.; Haas, R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000, 287, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Skoog, E.C.; Morikis, V.A.; Martin, M.E.; Foster, G.A.; Cai, L.P.; Hansen, L.M.; Li, B.; Gaddy, J.A.; Simon, S.I.; Solnick, J.V. CagY-Dependent Regulation of Type IV Secretion in Helicobacter pylori Is Associated with Alterations in Integrin Binding. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Akopyants, N.S.; Clifton, S.W.; Kersulyte, D.; Crabtree, J.E.; Youree, B.E.; Reece, C.A.; Bukanov, N.O.; Drazek, E.S.; Roe, B.A.; Berg, D.E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol 1998, 28, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Banta, L.M.; Bohne, J.; Lovejoy, S.D.; Dostal, K. Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J Bacteriol 1998, 180, 6597–6606. [Google Scholar] [CrossRef]
- Chung, J.M.; Sheedlo, M.J.; Campbell, A.M.; Sawhney, N.; Frick-Cheng, A.E.; Lacy, D.B.; Cover, T.L.; Ohi, M.D. Structure of the Helicobacter pylori Cag type IV secretion system. Elife 2019, 8. [Google Scholar] [CrossRef]
- Sheedlo, M.J.; Chung, J.M.; Sawhney, N.; Durie, C.L.; Cover, T.L.; Ohi, M.D.; Lacy, D.B. Cryo-EM reveals species-specific components within the Helicobacter pylori Cag type IV secretion system core complex. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.M.; Balkwill, G.D.; Bunting, K.A.; Edwards, W.; Atherton, J.C.; Searle, M.S. The highly repetitive region of the Helicobacter pylori CagY protein comprises tandem arrays of an alpha-helical repeat module. J Mol Biol 2008, 377, 956–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; McDaniel, T.K.; Falkow, S.; Karlin, S. Sequence anomalies in the Cag7 gene of the Helicobacter pylori pathogenicity island. Proc Natl Acad Sci U S A 1999, 96, 7011–7016. [Google Scholar] [CrossRef]
- Barrozo, R.M.; Hansen, L.M.; Lam, A.M.; Skoog, E.C.; Martin, M.E.; Cai, L.P.; Lin, Y.; Latoscha, A.; Suerbaum, S.; Canfield, D.R.; et al. CagY Is an Immune-Sensitive Regulator of the Helicobacter pylori Type IV Secretion System. Gastroenterology 2016, 151, 1164–1175 e1163. [Google Scholar] [CrossRef] [PubMed]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A 1996, 93, 14648–14653. [Google Scholar] [CrossRef]
- Grohmann, E.; Christie, P.J.; Waksman, G.; Backert, S. Type IV secretion in Gram-negative and Gram-positive bacteria. Mol Microbiol 2018, 107, 455–471. [Google Scholar] [CrossRef]
- Hayashi, T.; Senda, M.; Morohashi, H.; Higashi, H.; Horio, M.; Kashiba, Y.; Nagase, L.; Sasaya, D.; Shimizu, T.; Venugopalan, N.; et al. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe 2012, 12, 20–33. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Sierra, J.C.; Suarez, G.; Piazuelo, M.B.; Luis, P.B.; Baker, D.R.; Romero-Gallo, J.; Barry, D.P.; Schneider, C.; Morgan, D.R.; Peek, R.M., Jr.; et al. alpha-Difluoromethylornithine reduces gastric carcinogenesis by causing mutations in Helicobacter pylori cagY. Proc Natl Acad Sci U S A 2019, 116, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Rappuoli, R.; Covacci, A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A 2000, 97, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Neddermann, M.; Lind, J.; Pachathundikandi, S.K.; Sharafutdinov, I.; Gutierrez-Escobar, A.J.; Bronstrup, M.; Tegge, W.; Hong, M.; Rohde, M.; et al. Toll-like Receptor 5 Activation by the CagY Repeat Domains of Helicobacter pylori. Cell Rep 2020, 32, 108159. [Google Scholar] [CrossRef]
- Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 2004, 4, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol 2020, 28, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Sheedlo, M.J.; Ohi, M.D.; Lacy, D.B.; Cover, T.L. Molecular architecture of bacterial type IV secretion systems. PLoS Pathog 2022, 18, e1010720. [Google Scholar] [CrossRef]
- Fischer, W.; Tegtmeyer, N.; Stingl, K.; Backert, S. Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function. Front Microbiol 2020, 11, 1592. [Google Scholar] [CrossRef]
- Kondabala, R.; Kumar, V. Computational Intelligence Tools for Protein Modeling. Singapore, 2019; pp. 949-956.
- Coluzza, I. Computational protein design: a review. J Phys Condens Matter 2017, 29, 143001. [Google Scholar] [CrossRef]
- Jisna, V.A.; Jayaraj, P.B. Protein Structure Prediction: Conventional and Deep Learning Perspectives. Protein J 2021, 40, 522–544. [Google Scholar] [CrossRef]
- Chowdhury, R.; Bouatta, N.; Biswas, S.; Floristean, C.; Kharkar, A.; Roy, K.; Rochereau, C.; Ahdritz, G.; Zhang, J.; Church, G.M.; et al. Single-sequence protein structure prediction using a language model and deep learning. Nat Biotechnol 2022, 40, 1617–1623. [Google Scholar] [CrossRef]
- Soding, J.; Remmert, M. Protein sequence comparison and fold recognition: progress and good-practice benchmarking. Curr Opin Struct Biol 2011, 21, 404–411. [Google Scholar] [CrossRef]
- Xiao, X.; Lin, W.Z.; Chou, K.C. Recent advances in predicting protein classification and their applications to drug development. Curr Top Med Chem 2013, 13, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Mahajan, S.P.; Sulam, J.; Gray, J.J. Deep Learning in Protein Structural Modeling and Design. Patterns (N Y) 2020, 1, 100142. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, V.K. Current directions in combining simulation-based macromolecular modeling approaches with deep learning. Expert Opin Drug Discov 2021, 16, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.C.; McClain, M.S.; Cover, T.L. Role of the CagY antenna projection in Helicobacter pylori Cag type IV secretion system activity. Infect Immun 2023, 91, e0015023. [Google Scholar] [CrossRef]
- Barrozo, R.M.; Cooke, C.L.; Hansen, L.M.; Lam, A.M.; Gaddy, J.A.; Johnson, E.M.; Cariaga, T.A.; Suarez, G.; Peek, R.M., Jr.; Cover, T.L.; et al. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog 2013, 9, e1003189. [Google Scholar] [CrossRef]
- Jackson, L.K.; Potter, B.; Schneider, S.; Fitzgibbon, M.; Blair, K.; Farah, H.; Krishna, U.; Bedford, T.; Peek, R.M., Jr.; Salama, N.R. Helicobacter pylori diversification during chronic infection within a single host generates sub-populations with distinct phenotypes. PLoS Pathog 2020, 16, e1008686. [Google Scholar] [CrossRef]
- Della Bella, C.; Soluri, M.F.; Puccio, S.; Benagiano, M.; Grassi, A.; Bitetti, J.; Cianchi, F.; Sblattero, D.; Peano, C.; D’Elios, M.M. The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Chang, Y.W.; Shaffer, C.L.; Rettberg, L.A.; Ghosal, D.; Jensen, G.J. In Vivo Structures of the Helicobacter pylori cag Type IV Secretion System. Cell Rep 2018, 23, 673–681. [Google Scholar] [CrossRef]
- Hu, B.; Khara, P.; Song, L.; Lin, A.S.; Frick-Cheng, A.E.; Harvey, M.L.; Cover, T.L.; Christie, P.J. In Situ Molecular Architecture of the Helicobacter pylori Cag Type IV Secretion System. mBio 2019, 10. [Google Scholar] [CrossRef]
- Frick-Cheng, A.E.; Pyburn, T.M.; Voss, B.J.; McDonald, W.H.; Ohi, M.D.; Cover, T.L. Molecular and Structural Analysis of the Helicobacter pylori cag Type IV Secretion System Core Complex. mBio 2016, 7, e02001–02015. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: making protein folding accessible to all. Nat Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Skolnick, J.; Gao, M.; Zhou, H.; Singh, S. AlphaFold 2: Why It Works and Its Implications for Understanding the Relationships of Protein Sequence, Structure, and Function. J Chem Inf Model 2021, 61, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Aras, R.A.; Fischer, W.; Perez-Perez, G.I.; Crosatti, M.; Ando, T.; Haas, R.; Blaser, M.J. Plasticity of repetitive DNA sequences within a bacterial (Type IV) secretion system component. J Exp Med 2003, 198, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Bassot, C.; Elofsson, A. Accurate contact-based modelling of repeat proteins predicts the structure of new repeats protein families. PLoS Comput Biol 2021, 17, e1008798. [Google Scholar] [CrossRef]
- Hatahet, F.; Boyd, D.; Beckwith, J. Disulfide bond formation in prokaryotes: history, diversity and design. Biochim Biophys Acta 2014, 1844, 1402–1414. [Google Scholar] [CrossRef]
- Dombkowski, A.A. Disulfide by Design: a computational method for the rational design of disulfide bonds in proteins. Bioinformatics 2003, 19, 1852–1853. [Google Scholar] [CrossRef]
- Miseta, A.; Csutora, P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 2000, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Bhopatkar, A.A.; Uversky, V.N.; Rangachari, V. Disorder and cysteines in proteins: A design for orchestration of conformational see-saw and modulatory functions. Prog Mol Biol Transl Sci 2020, 174, 331–373. [Google Scholar] [CrossRef]
- Dumrese, C.; Slomianka, L.; Ziegler, U.; Choi, S.S.; Kalia, A.; Fulurija, A.; Lu, W.; Berg, D.E.; Benghezal, M.; Marshall, B.; et al. The secreted Helicobacter cysteine-rich protein A causes adherence of human monocytes and differentiation into a macrophage-like phenotype. FEBS Lett 2009, 583, 1637–1643. [Google Scholar] [CrossRef]
- Bocian-Ostrzycka, K.M.; Lasica, A.M.; Dunin-Horkawicz, S.; Grzeszczuk, M.J.; Drabik, K.; Dobosz, A.M.; Godlewska, R.; Nowak, E.; Collet, J.F.; Jagusztyn-Krynicka, E.K. Functional and evolutionary analyses of Helicobacter pylori HP0231 (DsbK) protein with strong oxidative and chaperone activity characterized by a highly diverged dimerization domain. Front Microbiol 2015, 6, 1065. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.; Kichler, S.; Oickle, B.; Fairweather, S.; Oberc, A.; Chahal, J.; Ratnayake, D.; Creuzenet, C. Characterization of Helicobacter pylori HP0231 (DsbK): role in disulfide bond formation, redox homeostasis and production of Helicobacter cystein-rich protein HcpE. Mol Microbiol 2015, 96, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Silvers, R.; Sziegat, F.; Tachibana, H.; Segawa, S.; Whittaker, S.; Gunther, U.L.; Gabel, F.; Huang, J.R.; Blackledge, M.; Wirmer-Bartoschek, J.; et al. Modulation of structure and dynamics by disulfide bond formation in unfolded states. J Am Chem Soc 2012, 134, 6846–6854. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, C.; Kumar, A.; Lang, A.; Ohlenschlager, O. Cysteines and Disulfide Bonds as Structure-Forming Units: Insights From Different Domains of Life and the Potential for Characterization by NMR. Front Chem 2020, 8, 280. [Google Scholar] [CrossRef]
- Erdos, G.; Meszaros, B.; Reichmann, D.; Dosztanyi, Z. Large-Scale Analysis of Redox-Sensitive Conditionally Disordered Protein Regions Reveals Their Widespread Nature and Key Roles in High-Level Eukaryotic Processes. Proteomics 2019, 19, e1800070. [Google Scholar] [CrossRef]
- Ryu, S.E. Structural mechanism of disulphide bond-mediated redox switches. J Biochem 2012, 151, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem Rev 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Giganti, D.; Yan, K.; Badilla, C.L.; Fernandez, J.M.; Alegre-Cebollada, J. Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity. Nat Commun 2018, 9, 185. [Google Scholar] [CrossRef]
- Sali, A. Comparative protein modeling by satisfaction of spatial restraints. Mol Med Today 1995, 1, 270–277. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 2015, 43, W174–181. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; DiMaio, F.; Wang, R.Y.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High-resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: a local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Cheng, X.; Lee, J.; Kim, S.; Park, S.J.; Patel, D.S.; Beaven, A.H.; Lee, K.I.; Rui, H.; Park, S.; et al. CHARMM-GUI 10 years for biomolecular modeling and simulation. J Comput Chem 2017, 38, 1114–1124. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmuller, H.; MacKerell, A.D., Jr. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Henin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J Chem Phys 2020, 153, 044130. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. J Mol Graph 1996, 14, 33–38, 27. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Jablonska, J.; Pravda, L.; Varekova, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci 2018, 27, 129–134. [Google Scholar] [CrossRef]
| Region | inicio | fin |
|---|---|---|
| FRR | 1 | 331 |
| TM | 343 | 368 |
| MRR | 585 | 1369 |
| TM | 1798 | 1814 |
| AP | 1820 | 1851 |
| VirB10 | 1653 | 1896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





