1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV2) infection has caused significant morbidity and mortality worldwide [
1]. The SARS-CoV2 outbreak rapidly spread coronavirus disease 2019 (COVID-19) with a lower respiratory syndrome as a severe and often deadly pneumonia, especially dangerous in older patients and/or patients with secondary health problems (reviewed in [
2]). Most young and healthy people were affected by less severe symptoms of fever, chills, sore throat, myalgia, headache, and anosmia or ageusia [
2]. Meanwhile, SARS-CoV2 virus has continuously mutated with deleterious effects for mutants, as most mutants were swiftly purged [
3]. While some “non-purged” mutants remained biologically neutral, a small fraction of mutants transformed COVID-19 outcomes [
3]. Over the last two years, the dangerous “beta” SARS-CoV2 virus mutated into a less deadly but more infectious “omicron” SARS-CoV2 mutant causing cold-like symptoms without pneumonia (reviewed in [
4]). The most recent FDA recommendation (September 2023) revealed that omicron variant XBB1.5 was accounted for 40% infections in United States, and thus undertaking steps to produce a boosting dose. Since, the COVID-19 pathogenesis was dependent on the effective virus clearance by the immune system, maintaining vaccine-based protection is crucial. The balance between the viral elimination after vaccination and the control over immune tissue injuries reflects the COVID19 severity.
SARS-CoV2 is a single-stranded sense RNA virus with an envelope [
5]. The virion includes four membrane proteins, namely spike (S)1, S2, receptor-binding domain (RBD), and nucleocapsid (N) proteins [
6]. Viral entry into cells is mediated by S1 binding to the angiotensin converting enzyme 2 (ACE2) receptors on cell membranes. Such S1/ACE2 association induces a conformational change exposing a cleavage on the S2 subunit for transmembrane protease serine 2 (TMPRSS2) enzymes on cell membranes. The S2 secures a viral/membrane fusion leading to the viral entry into the cell [
6]. Blocking S1/ACE2 association and S2/TMPRSS2 function by IgM/IgG/IgA antibodies have been critical for SARS-CoV2 infection and COVID-19 development [
7].
The COVID-19 pandemic prompted efforts to develop an effective vaccine. Out of multiple offers two mRNA vaccines have been approved by FDA: the mRNA-1273 by Moderna and BNT162b2 mRNA by Pfizer-BioNTech. Following intramuscular injection, each mRNA vaccine is translated into an immunogenic protein, evoking an immune response [
8]. A two-dose regimen of BNT162b2 demonstrated 95% effectiveness in preventing severe COVID-19 [
9]. The BNT162b2 vaccine induced humoral and cellular immune responses [
10,
11]. Based on successful clinical trials, both mRNA vaccines were approved by the FDA [
8]. In September 2023, FDA approved the production of a boosting dose targeting most recent omicron variants including XBB1.5.
Because of continuous immunosuppression, kidney transplant (KT) recipients have an increased risk of COVID-19 [
12,
13], including severe symptoms requiring hospitalization or even mechanical ventilation. Williamson et al. identified a hazard ratio for mortality at 6.0 for organ transplant recipients, which was significantly higher than for the general population [
14]. When matched for age, gender and comorbidities, organ transplant recipients had a statistically significantly higher risk of death and/or the need for mechanical ventilation [
13]. Risk factors contributed to poor outcomes in transplant patients included immunosuppression, obesity, hypertension, cardiovascular disease and diabetes mellitus [
15]. An effective vaccination is needed for these vulnerable patients [
16,
17]. Current recommendations advise vaccination prior to transplantation whenever possible [
18] and a booster vaccination [
19].
Considering the high-risk status, transplant recipients were excluded from clinical trials [
9,
11]. It was concluded from vaccination programs that the vaccination is safe for KT recipients as for other patients [
20,
21]. Concerns regarding possible transplant damage or rejection caused by vaccination has not been substantiated [
21]. Generally, it is assumed that mRNA-1273 Moderna and BMT162b2 Pfizer-BioNTech vaccines are safe for KT recipients. Unfortunately, ample data has demonstrated a relatively low immunogenicity of the BNT162b2 Pfizer-BioNTech vaccine in transplant patients with low, or even absent seroconversion [
22,
23,
24,
25]. Sattler et al. showed a poor cellular response following vaccination of transplant recipients [
24]. There is little information about the quality of immunization to different mRNA vaccines in recipients.
Our study investigated the effectiveness of mRNA-1273 Moderna and BNT162b2 Pfizer-BioNTech in KT recipients. We measured IgM/IgG/IgA antibody response as well as the frequency of IL-2-, IFN-γ-, and/or TNF-α-producing T-cells. Our analysis showed that mRNA-1273 Moderna vaccine was superior to BNT162b2 Pfizer-BioNTech in KT recipients.
2. Materials and Methods
2.1. Study Participants
This study involved volunteers and KT recipients tested at the University of Toledo Transplant Center after vaccination against the SARS-CoV-2, who did not have a record of previous SARS-CoV-2 infection. KT recipients were vaccinated after transplantation. There was a total of 99 KT-recipients and 66 healthy volunteers who were fully vaccinated with the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine. All cohort was tested 12 months after the last dose of vaccination and the subgroup of this cohort, 72 KT recipients and 27 healthy volunteers, was additionally tested 6 months after last dose. Blood samples were collected when participants consented by signing the informed consent form. The study was approved by the University of Toledo Institutional Review Board (IRB# 300931).
2.2. Detection and Quantification of Serum Antibodies
Serum IgG and IgM levels were measured using solid-phase sandwich ELISA assay by Invitrogen (catalog #s BMS2324 and BMS2325, respectively), with detection antibodies targeted against SARS-CoV-2 trimerized protein pre-coated on plate. Optical density values were read at 450 nm wavelength promptly after 5-minute incubation with TMB, and quantitative IgG levels were inferred based on provided standards. For qualitative comparison, we used two definitions of vaccine response. First definition as seropositivity or seronegativity was based on the manufacturer’s instructions: based on the ratio of absorbance of the sample to the absorbance in the calibrator control. Unlike responder definition, which was based on a fixed IgG concentration, the seropositivity definition included intermediate values which were treated as inconclusive and were not included in statistical calculations. Second definition, as responder or non-responder was based on detectable IgG concentration of 2,000 Units/milliliter (U/ml) threshold. Only assays with R2 value of at least 0.9 (≥0.9), indicating good calibration curves fit, were used.
To confirm the potency of the detected serum antibodies to neutralize the virus, we used the GenScript SARS-CoV-2 surrogate virus neutralization kit (catalog #L00847-A). The kit was designed to test for the presence of antibodies that block the interaction between receptor binding domain of the SARS-CoV-2 glycoprotein and human ACE2 receptor.
2.3. ELISpot Assay
Freshly collected whole blood samples were subjected to density gradient spinning to isolate peripheral blood mononuclear cells. The freshly isolated cells were counted using an automatic cell counter and plated at 50,000 cells per well to manufacturer’s ELISpot plates (Immunospot). Cells were incubated for a designated amount of time with or without stimulation. For assaying TNF-α, IL-2, IL-10 and IL-17 secretion, stimulation with LPS at 5 ng/ml was used. After incubation, plates were stained, and spots read using the Immunospot S6 Entry machine.
2.4. Statistical Analyses
Mann-Whitney test was used to evaluate the difference in IgG OD450 levels between groups: for groups <50 participants, single-tailed p-values were reported; for groups ≥50 two-tailed p-values were reported. To assess the relationship between the immune status (controls vs. KT recipients) or vaccine type (Moderna or Pfizer-BioNTech) with binary outcome (seropositive or IgG concentration greater than 2,000 U/ml), Chi-squared test was used when each group’s size was at least 5, and Fisher’s exact test was used for comparisons including one or more groups smaller than 5.
The association between a vaccine type and an immune response was evaluated for Th1 and Tr1 readouts. For Mann-Whitney test, participants were divided by an immune status, or a vaccine type as described above for the IgG concentration. The frequency of cytokine-secreting cells in an ELISpot assay (spots per 50,000 PBMCs, or spots/5×104) was reported after 24-48 hrs of LPS (5 ng/ml) or PHA-P (5 µg/ml) stimulation. For the frequency of Th1 and Tr1 cells, LPS stimulation was used for comparison between KT recipients and controls, and PHA-P was used the comparison of participants vaccinated with Moderna vs. Pfizer-BioNTech.
3. Results
3.1. Cohorts and Patient Characteristics at 6 and 12 Months
For the analysis, we selected KT recipients and healthy controls who were vaccinated against the SARS-CoV-2. Between September 2021 and April 2022, there were 72 kidney KT recipients and 27 healthy controls for testing at 6 months (
Table 1) whereas 99 KT recipients and 66 controls for testing at 12 months. As required by the protocol, all selected participants were fully immunized with either BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine and they never were infected with SARS-CoV-2 virus; their data are presented in
Table 1 for the 6-month group and
Supplemental Table 1 for the 12-month group. The comparison of all participants or KT recipients grouped by the vaccine type showed no significant differences in their social and clinical characteristics. Groups compared by vaccine types at 6 or 12 months had similar distribution of race, gender, clinical immunosuppression, and other variables, thus allowing us to measure their immune metrics related to Moderna vs. Pfizer-BioNTech mRNA vaccine (
Table 1 and
Supplemental Table 1).
3.2. Anti-SARS-CoV-2 Seropositivity Rates at 6 and 12 Months post Vaccination
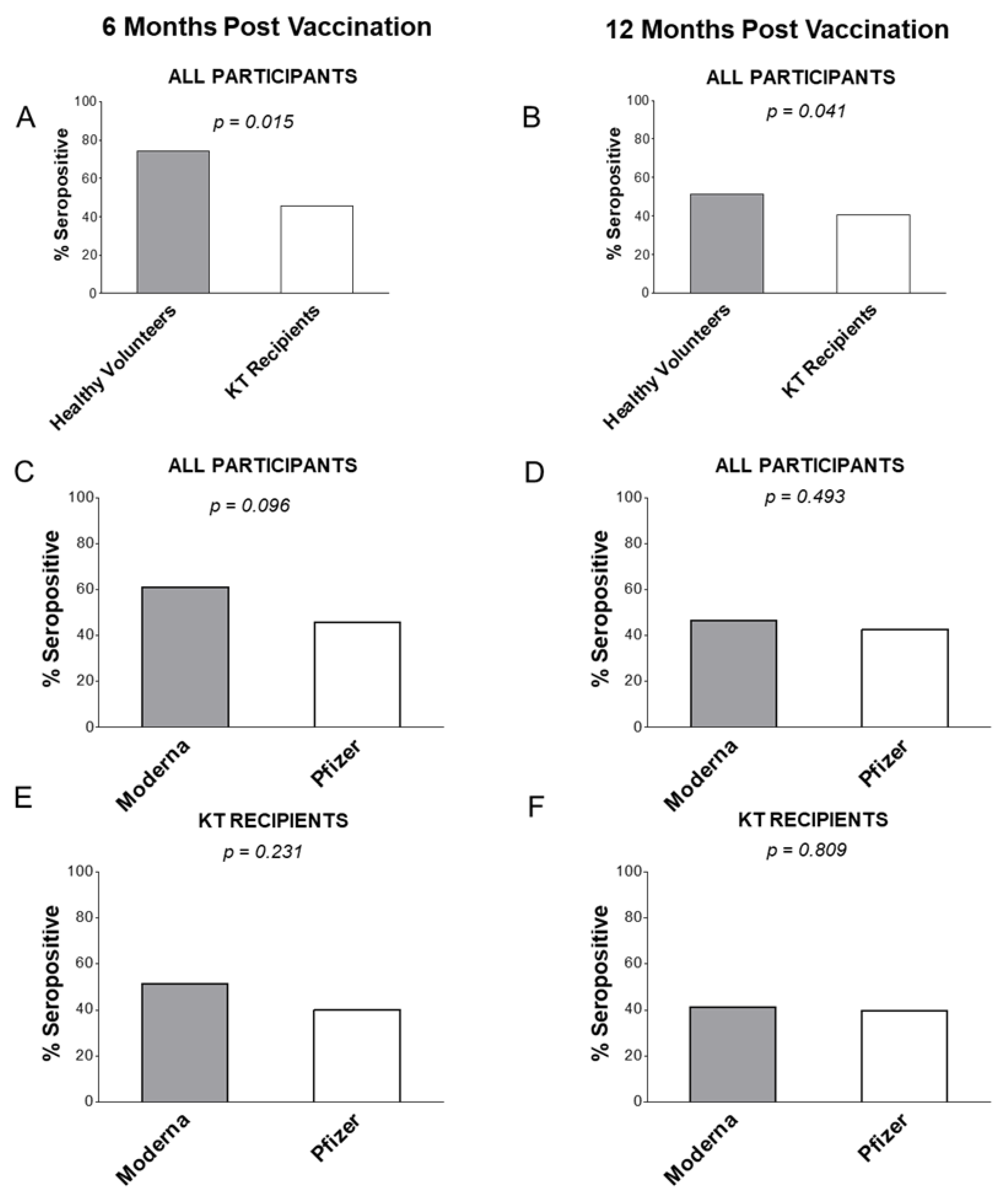
At 6 months, and independently of the mRNA vaccine type, 74.1% of healthy volunteers had anti-SARS-CoV-2 IgG, which was higher than 45.8% among transplant recipients (p=0.015;
Figure 1A;
Supplemental Table 2). This pattern was repeated at 12 months post vaccination: healthy volunteers vaccinated with either mRNA vaccine had 51.5% anti-SARS-CoV-2 IgG seropositivity rate vs. 40.4% in KT patients in the 12 months group (p=0.041,
Figure 1B;
Supplemental Table 2). Overall, mRNA vaccines were more effective in healthy individuals that in KT patients.
By mRNA vaccine type, Moderna vaccine induced IgG response in 63.8% of all participants (healthy controls + KT patients) vs. 46.9% for Pfizer-BioNTech (p=0.096;
Figure 1C;
Supplemental Table 2) in patients tested 6 months after vaccination. The trend for better Moderna efficacy was nearly gone at the 12 months: Moderna vaccine was similarly effective in 50.6% compared to 45.1% participants for Pfizer-BioNTech vaccine (p=0.493;
Figure 1D;
Supplemental Table 2).
When looking only at KT recipients, the differences were not statistically significant between the two vaccines: 54.3% were IgG seropositive for Moderna vaccine compared to 40.0% for Pfizer-BioNTech vaccine (p=0.231;
Figure 1E;
Supplemental Table 2) at 6 months post vaccination. At 12 months post vaccination, IgG was present in 42.8% of KT recipients after immunization with the Moderna vaccine and 40.4% after Pfizer-BioNTech vaccine (p=0.809;
Figure 1F;
Supplemental Table 2). In further comparison, healthy volunteers at 6 months after vaccination with the Moderna vaccine were more frequently seropositive with 91.7% vs. 64.3% for those vaccinated with the Pfizer-BioNTech (NS;
Supplemental Table 2). At 12 months, healthy controls were seropositive in 61.8% when vaccinated with Moderna vaccine vs. 54.2% after Pfizer-BioNTech vaccine (p=0.563;
Supplemental Table 2). Clinical confounders such as age, gender, race, or time post latest vaccination were not associated with seropositivity for IgG (
Table 2).
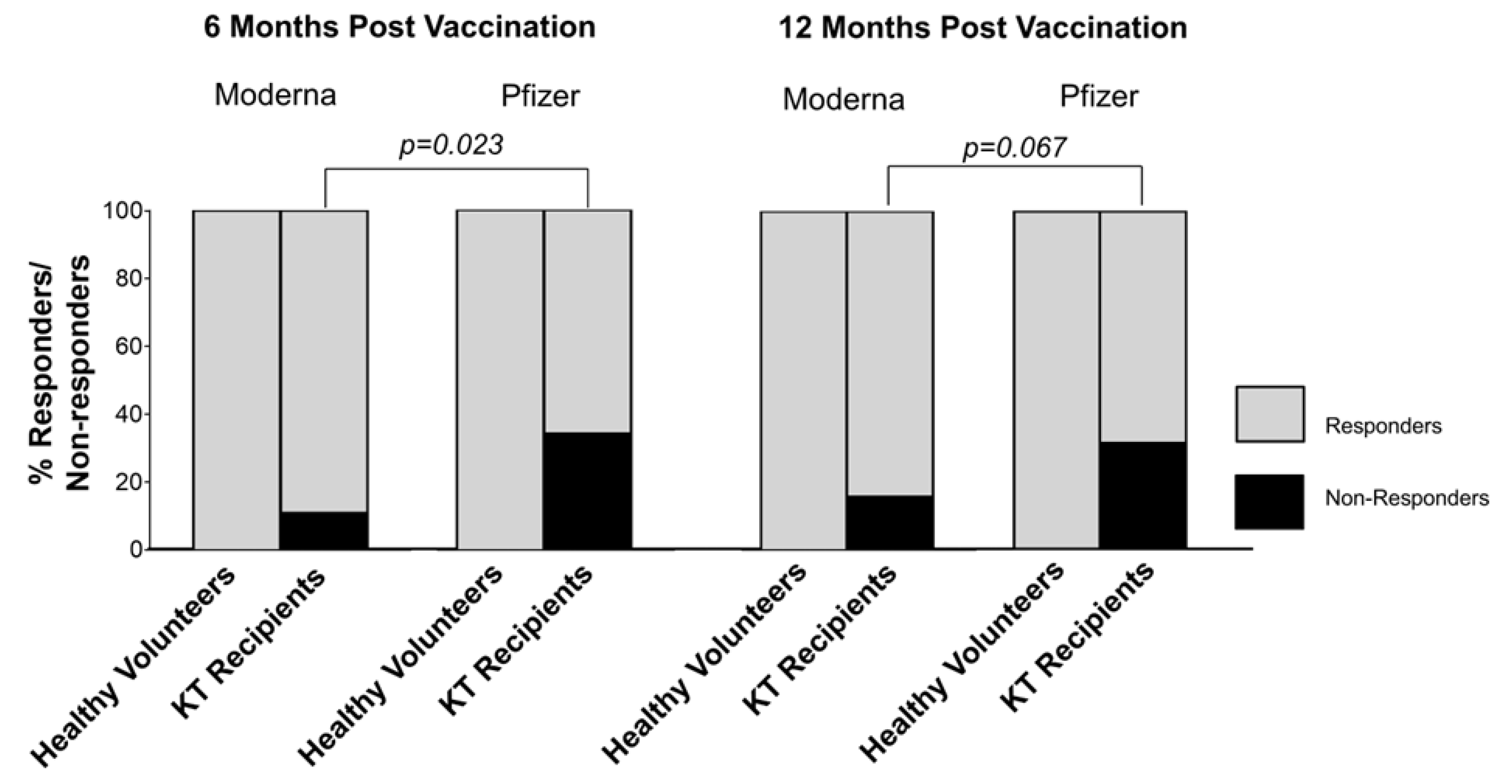
To better describe the difference in efficiency between Moderna and Pfizer-BioNTech vaccines, we defined responders vs. non-responders who were distinguished by the IgG level of detection (IgG ≤ 2,000 U/ml). As shown in
Figure 2, all healthy controls vaccinated with either mRNA vaccine displayed anti-SARS-CoV-2 IgG more than 2000 U/ml. In contrast, KT recipients at 6 months had 10.8% non-responders in Moderna group while 34.3% non-responders for Pfizer-BioNTech group (p=0.023;
Figure 2). Similarly at 12 months and despite nonsignificant value, there were only 15.7% non-responders for Moderna and 31.3% for Pfizer-BioNTech groups (p=0.067;
Figure 2). Thus, all healthy controls were responders while all non-responders were among KT recipients with more of them after Pfizer-BioNTech than Moderna vaccination.
3.3. Quantitative IgG Levels at 6 and 12 Months post Vaccination
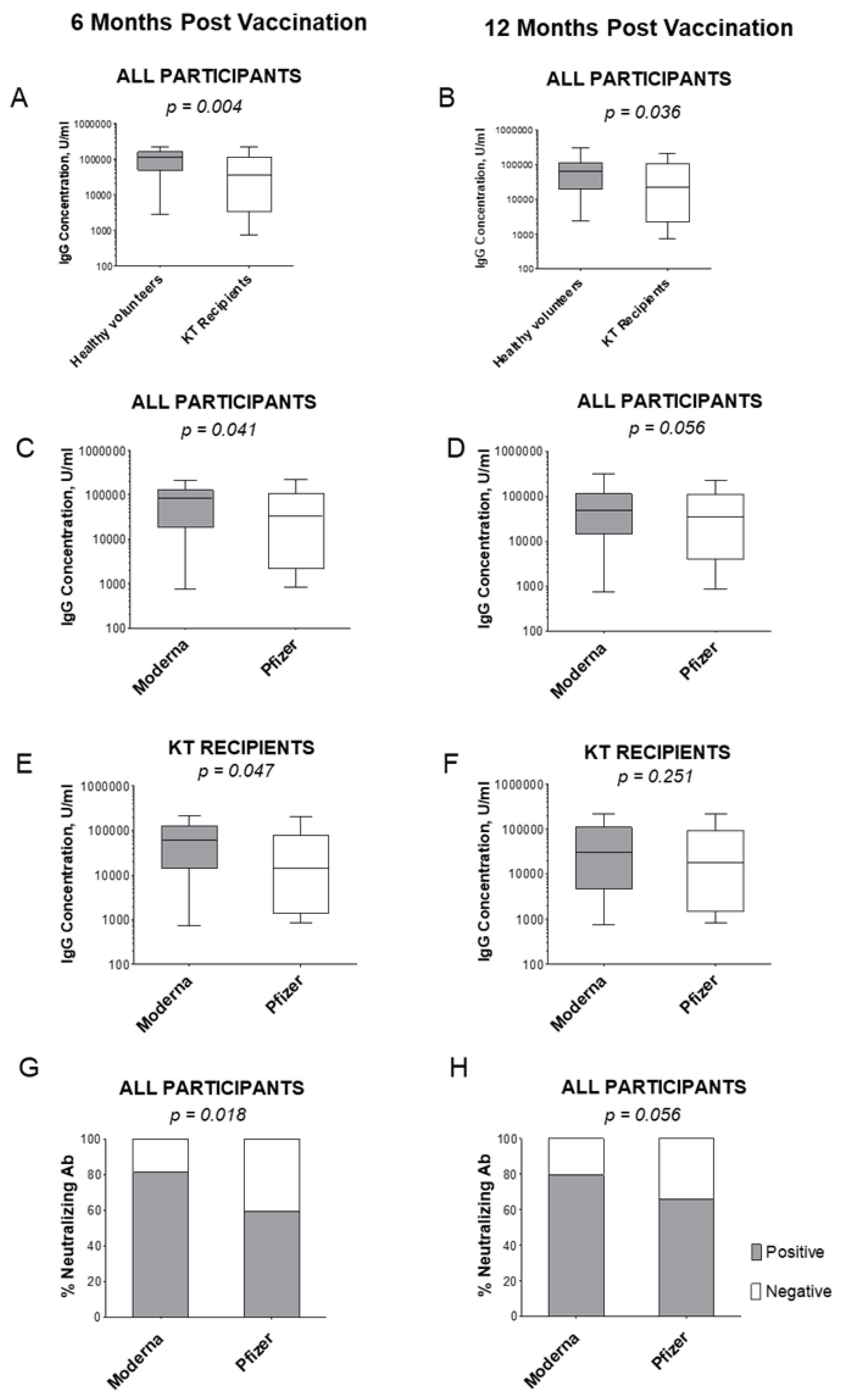
Quantitatively at 6 months, KT recipients had significantly lower IgG levels after either vaccine compared to controls: an average concentration of IgG in healthy volunteers’ sera was 103,244 U/ml while in KT recipients’ sera was 63,975 U/ml (p=0.004;
Figure 3A). Quantitative data at 12 months displayed also reduced IgG concentrations from 76,762 U/ml in healthy volunteers to 57,972 U/ml in patients (p=0.036;
Figure 3B).
There was also a significant difference in IgG levels between different vaccine types in all participants at 6 months (p=0.041,
Figure 3C) and a trend in the 12 months post last vaccination group (p=0.056,
Figure 3D). In solely KT recipients, the average IgG concentration was 86,778 in Moderna’s group vs. 62,829 in Pfizer’s group at 6 months (p=0.047,
Figure 3E), while at 12 months it was 62,352 and 53,314 U/ml for Moderna and Pfizer-BioNTech-vaccinated participants, respectively (p=0.251,
Figure 3F). The Moderna vaccine trended slightly more effective in protection for healthy volunteers (NS; not shown). Unlike IgG, IgM did not show an association with the immune status or vaccine type (not shown).
Interestingly, exclusion of non-responder sera from comparisons resulted in leveling of the average IgG concentrations between participants vaccinated with either vaccine. After removing non-responders from the 6-month cohort, average IgG concentration was 110,761 U/ml in Moderna and 67,277 U/ml in Pfizer-BioNTech groups (p=0.202;
Supplemental Figure 1A and B). At 12 months, removing of IgG sera-negative KT patients produced an average IgG concentration of 76,180 U/ml in Moderna and 76,701 U/ml in Pfizer-BioNTech (p=0.474
Supplemental Figure 1C,D). Thus, lower efficacy for Pfizer-BioNTech stems from the higher number of non-responder patients.
3.4. Neutralizing Antibody Frequency at 6 and 12 Months post Vaccination
We tested all study participants for the presence of virus-specific neutralizing IgM/IgG/IgA antibodies. At 6 months, 81.3% of Moderna vaccinated participants had anti-SARS-CoV-2 neutralizing antibodies, in comparison with 59.2% of Pfizer-BioNTech vaccinated (p=0.018,
Figure 3G). The same pattern was repeated at 12 months: 79.5% of Moderna vaccinated participants were positive for neutralizing antibodies whereas among sera in Pfizer-BioNTech vaccinated participants only 66.2% were positive for neutralizing antibodies (p=0.056,
Figure 3H).
We compared neutralizing antibody rates in KT patients alone. Among KT recipients, 75.0% of the Moderna vaccinated group were positive for neutralizing antibodies. In contrast, 48.6% of the Pfizer-BioNTech group displayed neutralizing antibodies (p=0.022, not shown). Such difference was not observed among healthy volunteers as all except two (vaccinated with the Pfizer-BioNTech vaccine) had detectable neutralizing antibodies.
The difference was further clarified when non-responders (which comprised 11% of participants vaccinated with Moderna and 37% of participants vaccinated with Pfizer-BioNTech, p=0.006) were excluded from comparison. Among the remaining responders, 81% of the Moderna and 73% of the remaining Pfizer-BioNTech participants had neutralizing antibodies (NS; not shown). Our observations suggest that a possible common mechanism may be involved in an increased number of non-responders in Pfizer-BioNTech group.
3.5. T Cell Pro-Inflammatory Response to Vaccination
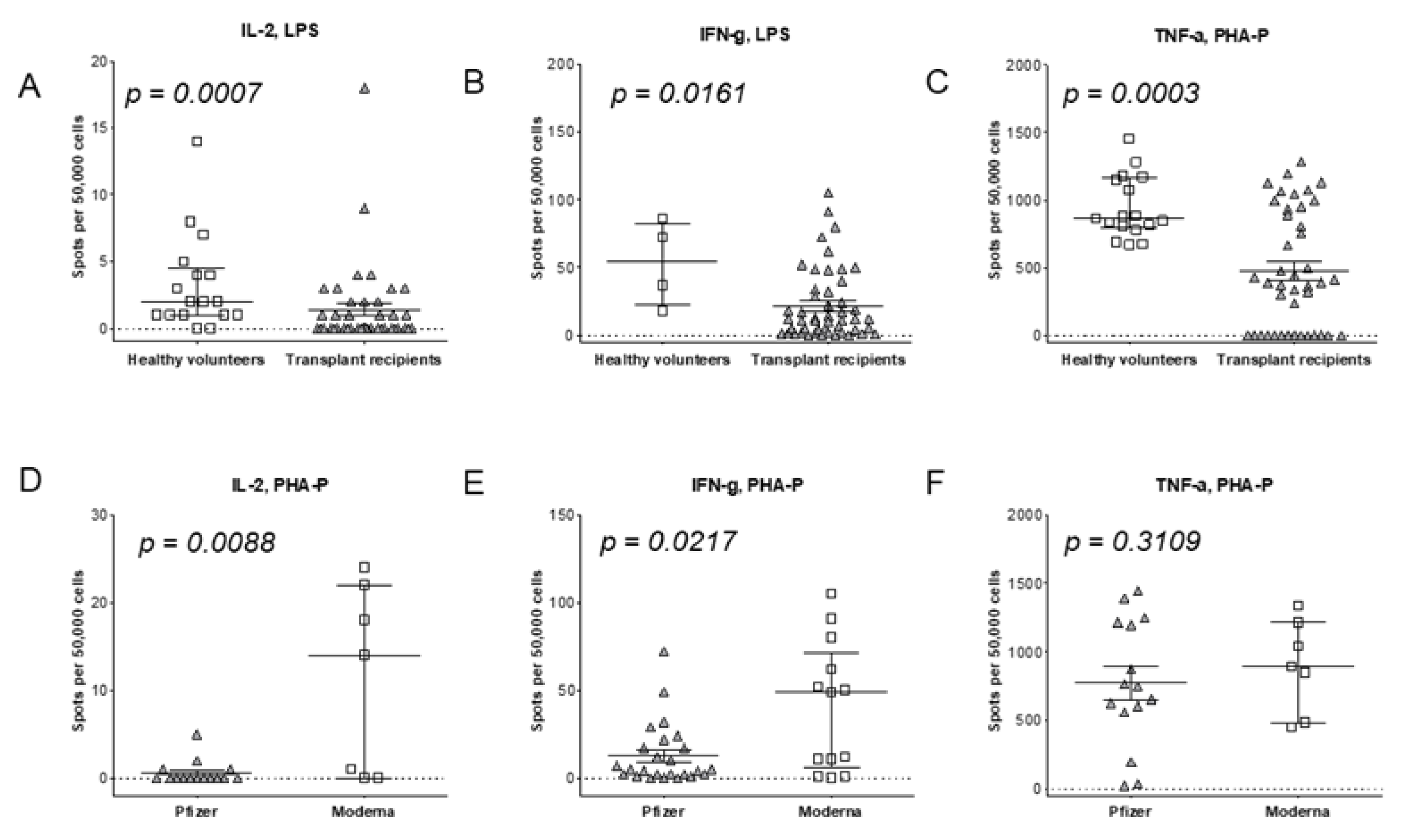
To better explain vaccination efficacy, we evaluated T cell immune responses. PBMCs were explored by an ELISpot assay measuring frequencies of T cells producing IL-2 (fT
IL-2), IFN-γ, (fT
IFN-α), and/or TNF-α, (fT
TNF-α), representing T helper 1 (Th1) cells. The IL-2 response was 2.4-fold lower in KT recipients with 1.4 spots per 50,000 PBMCs (1.4/5×10
4 fT
IL-2) vs. 3.3/5×10
4 fT
IL-2 in controls (p<0.001;
Figure 4A). The production of IFN-γ by Th1 was 3.6-fold decreased in KT recipients: the fT
IFN-γ was 21.7/5×10
4 fTIFN-γ in recipients compared to 79/5×10
4 fTIFN-γ in controls (p=0.016;
Figure 4B). Furthermore, the frequency of TNFα-producing Th1 cells was also 2-fold lower in KT recipients: 473.8/5×10
4 fT
TNF-γ in KT recipients and 945.5/5×10
4 fT
TNF-α in controls (p<0.001;
Figure 4C), showing twice the expansion of Th1 cells in controls versus KT recipients.
Overall, Moderna benefited Th1 response in KT recipients more effectively than did Pfizer-BioNTech: the average 0.6/5×10
4 fTIL-2 spots in Pfizer-BioNTech-vaccinated patients were 19-fold lower compared to 11.3/5×104 fTIL-2 spots in those vaccinated with Moderna vaccine (p=0.009,
Figure 4D). The IFN-γ-producing Th1 spot number was 12.8/5×10
4 fT
IFN-γ for Pfizer-BioNTech vaccine while 3.1-fold higher at 40.4/5×10
4 fTIFN-γ for Moderna vaccine (p=0.022,
Figure 4E). Finally, the TNF-α-producing Th1 cells were lower with 771.1/5×10
4 fT
TNF-α for Pfizer-BioNTech vaccine vs. 895/5×10
4 fT
TNF-α for Moderna vaccine (p=0.311,
Figure 4F). Thus, Th1 responses were consistently more robust in healthy volunteers than in KT recipients and Moderna vaccination generated a stronger Th1 response for KT recipients than did Pfizer-BioNTech vaccination.
3.6. T cell Regulatory Response to Vaccination
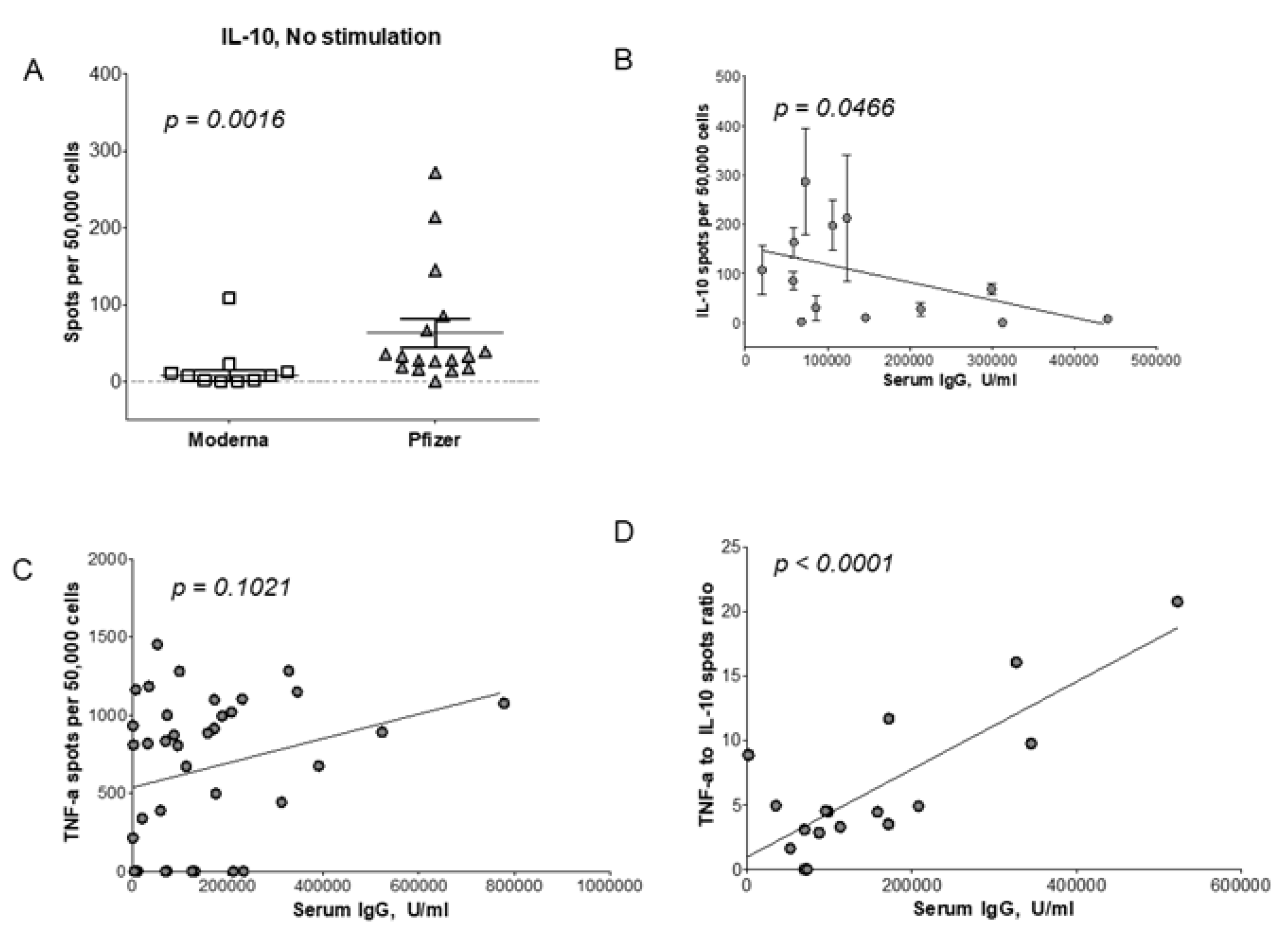
One mechanism affecting more potent humoral and cellular responses is active immune regulation. To explore such a possibility, we measured the frequency of IL-10-producing T regulatory 1 (Tr1) cells (fT
IL-10). In KT recipients and healthy controls, Moderna vaccine had only 16.9/5×10
4 fT
IL-10 and that was 3.7-fold lower than 62.8/5×10
4 fT
IL-10 induced by Pfizer-BioNTech vaccine (p = 0.016;
Figure 5A). These results suggest a regulation by Tr1 cells of Th1 cells resulting in lower IgG level in Pfizer-BioNTech immunized patients. In Chi-squared tests, race, gender, and age of recipients had an impact on the fT
IL-10 cells (
Table 2).
The interdependence comparison indicated that the lower fT
IL-10 correlated with the higher IgG levels (p=0.047;
Figure 5B;). To confirm this observation, we plotted fT
TNF-α and IgG responses: an increased fT
TNF-α correlated with high IgG production (p=0.102;
Figure 5C). Finally, the ratio of fT
TNF-α/fT
IL-10 also correlated with the IgG production (
Figure 5D). An elevated Th1/Tr1 ratio was an indicator for higher IgG levels in vaccinated patients (p<0.001;
Figure 5D). Our analysis showed a reciprocal interaction where Th1 and Tr1 cells influenced IgG production.
4. Discussion
KT recipients are at high risk of severe course and unfavorable outcomes of COVID19, as confirmed by the higher hospitalization and mortality rates than in the general population [
12,
13]. This highlights the importance of an effective vaccination against SARS-CoV-2 in this unique population, which is immunocompromised. Indeed, rates of seroconversion in vaccinated KT recipients had been shown to suffer due to immunosuppression [
22,
24,
25,
26,
27]. In addition, secretion of cytokines, such as Th1-produced IL-2, also has been shown to be lower in transplant recipients compared to the general population [
24].
We propose that the Th1/Tr1 plasticity regulates anti-SARS-CoV-2 IgG response by influencing the rate of responders/non-responders KT patients during a post-transplant vaccination. It looks like immunogenic SARS-CoV-2 antigens induce potent Th1 cells with weak Tr1 cells in some individuals. In contrast, other individuals develop reduced Th1 cells because of dominant Tr1 cells. This Th1/Tr1 immune regulation correlated with higher number of non-responders KT patients vaccinated with Pfizer-BioNTech vaccine.
Recently published work compared two mRNA vaccines for their efficacy [
28,
29]. Out of 1647 health care workers negative for SARS-CoV-2 antibodies who were vaccinated with two doses of SARS-CoV-2 mRNA vaccines, 688 received mRNA-1273 Moderna vaccine and 959 received BNT162b2 Pfizer-BioNTech vaccine [
28]. Higher IgG titers were observed after Moderna than after Pfizer-BioNTech vaccination (p<0.001) [
28]. Participants who were previously infected with SARS-CoV-2 virus and then vaccinated achieved overall higher IgG titers than uninfected participants (p<0.01), but Moderna vaccine produced again better titers than Pfizer-BioNTech vaccine (p<0.001) [
28]. In a different study, naïve KT recipients vaccinated with Moderna vaccine developed IgG seropositivity and had T cell ELISpot positivity in two thirds of KT patients [
29]. In our work Moderna vaccine induced IgG seropositivity in 54.3% of KT patients compared to only 45.7% for Pfizer-BioNTech vaccine (p=0.09;
Supplemental Table 2). Similarly, 65% of Moderna patients were positive for anti-SARS-CoV-2 Th1 cells vs. 36% patients vaccinated with Pfizer-BioNTech.
IgG is essential in COVID-19 defense by fixing complement to destroy infected cells, and by opsonizing viral targets for phagocytosis [
30]. Viral-specific IgG levels rising following vaccinations are maintained in the following months through memory B cells, conferring the long-term immunity [
31]. Efficacy against SARS-CoV-2 relies on IgG as a neutralizing factor, and therefore serum levels of SARS-CoV-2-specific neutralizing IgG antibodies reflect on effectiveness of immunization [
30]. Indeed, vaccination against SARS-CoV-2 virus correlated with a strong IgG response in an effective defense against COVID-19 symptoms [
32]. Our analysis emphasized the generation of IgG in response to anti-COVID-19 vaccination with the neutralizing function correlating with the presence of S1-, S2-, and RBD-specific IgG. Our new observation was that Moderna was better than Pfizer-BioNTech in KT patients by increasing the number of anti-SARS-CoV-2 IgG seropositive KT patients. When analyzed by the vaccine type, Moderna also produced better Th1 response than Pfizer-BioNTech, while Pfizer-BioNTech displayed higher levels of IL-10-producing Tr1 cells than Moderna. The lower Th1/Tr1 ratio reflected both depressed Th1 and IgG responses. It looks that immunosuppression sways the response to mRNA vaccines by the involvement of Tr1 regulation of Th1 influencing the efficacy of vaccination.
During infection, IL-10 inhibits the activity of Th1 cells, NK cells, and macrophages [
33]. On one hand, Th1 cells are required for the optimal pathogen clearance, but on the other hand the same Th1 activity contributes to the tissue damage. Consequently, the best would be for IL-10 not to impede the pathogen clearance but to ameliorate any immunopathology. Similarly, the most effective Th1 response to the mRNA vaccination is necessary to produce an efficient memory response to SARS-CoV-2 infection. Downregulation of Th1 response during immunization by Tr1 cells may impede optimal if not maximal protection against SARS-CoV-2 virus in KT patients. Our results showed that Pfizer vaccine induced a higher Tr1 activity, and this was reflected by lower IgG levels. Our data demonstrate for the first time that active Tr1 regulation is involved in the efficacy of mRNA vaccination in KT recipients.
IL-10, a pleiotropic cytokine with anti-inflammatory functions, acts as a negative regulator of the immune response. In fact, IL-10, including IL-10-produced by Tr1 cells, has been involved in anti-inflammatory function in autoimmunity, viral/bacterial infections, and allograft transplantation [
34,
35,
36,
37,
38,
39,
40]. Alterations in IL-10 producing Tr1 cells was shown to regulate multiple sclerosis, type 1 diabetes, and long-term allograft survival [
41,
42,
43]. Also in our study, Pfizer-BioNTech vaccination induced higher Tr1 levels than Moderna and thus possibly contributing to Th1 down-regulation.
In summary, Moderna and Pfizer-BioNTech vaccines are less effective in inducing anti-SARS-CoV-2 IgG and Th1 responses in immunosuppressed KT recipients than in healthy volunteers. While responders after Moderna or Pfizer-BioNTech vaccine had similar anti-SARS-CoV-2 IgG levels, Moderna vaccine had benefited KT patients with more responder patients than Pfizer-BioNTech vaccine. We propose that IL-10-producing Tr1 cells contributed to the lower number of IgG responder KT patients in Pfizer-BioNTech group.
5. Conclusions
In our study, the measurement of immune response metrics in KT cohort vaccinated after transplantation vs. healthy cohort revealed the following observations: 1) Seroconversion was lower in KT patients than in controls after any mRNA vaccination; 2) Seroconversion was higher in KT patients after Moderna than Pfizer-BioNTech vaccine; 3) Seropositive KT recipients had similar serum anti-SARS-CoV-2 IgG levels after either mRNA vaccine; 4) KT patients had diminished frequencies of SARS-CoV-2-specific Th1 cells (TNF-ɑ, IFN-ɣ, and/or IL-2) compared to controls; 5) Moderna vaccine induced higher Th1 frequencies compared to Pfizer-BioNTech vaccine; 6) Pfizer-BioNTech vaccine induced an increased frequencies of IL-10-producing Tr1 cells than Moderna vaccine; and, 7) Th1/Tr1 ratio influenced anti-SARS-CoV-2 IgG production.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Description of the 12 months cohort; Table S2: Seropositivity for SARS-CoV-2 spike trimer-specific IgG in various groups of study participants in the 6 months cohort.
Author Contributions
Inception and conceptualization, MR and SS; study design, formulating research questions, methodology, all authors; Data acquisition (benchwork), AW, CB, SB, KR, WP; sample and patient data acquisition, MB and TS; Data curation and statistics, SK and FJS; Writing – original draft preparation, BM, SS; Writing – review and editing, DB, DK, SC, RC; Visualization, SB, CB, KR, DK; Supervision – SS; Project administration, DB; Funding acquisition, MR. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of University of Toledo (protocol number 300931-UT, approved on July 6, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
This study was funded by the University of Toledo Research Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiological Genomics. 2020;52:549-57. [CrossRef]
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122-+. [CrossRef]
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nature Reviews Microbiology. 2021;19:409-24. [CrossRef]
- Fan Y, Li X, Zhang L, Wan S, Zhou FF. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduction and Targeted Therapy. 2022;7.
- V'Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology. 2021;19:155-70.
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology. 2022;23:3-20.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-+.
- Szabo GT, Mahiny AJ, Vlatkovic I. COVID-19 mRNA vaccines: Platforms and current developments. Molecular Therapy. 2022;30:1850-68. [CrossRef]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383:2603-15.
- Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572-+. [CrossRef]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021;384:403-16.
- Kremer D, Pieters TT, Verhaar MC, Berger SP, Bakker SJL, van Zuilen AD, et al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. American Journal of Transplantation. 2021;21:3936-45. [CrossRef]
- Nair V, Jandovitz N, Hirsch JS, Nair G, Abate M, Bhaskaran M, et al. COVID-19 in kidney transplant recipients. American Journal of Transplantation. 2020;20:1819-25.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-+. [CrossRef]
- Aziz H, Lashkari N, Yoon YC, Kim J, Sher LS, Genyk Y, et al. Effects of Coronavirus Disease 2019 on Solid Organ Transplantation. Transplantation Proceedings. 2020;52:2642-53. [CrossRef]
- Aslam S, Buggs J, Wyatt K, Kumar A, Rogers E, Watson R. The Impact of Virtual Crossmatch on Cold Ischemic Times and Outcomes Following Kidney Transplantation. American Surgeon. 2021;87:109-13. [CrossRef]
- Chandran S, Stock PG. COVID-19 Vaccination in Kidney Transplant Recipients: An Ounce Pre-Transplant is Worth a Pound Post-Transplant. Journal of the American Society of Nephrology. 2021;32:2977-8. [CrossRef]
- Diseases NCfIaR. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. CDC2022.
- Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021. Mmwr-Morbidity and Mortality Weekly Report. 2021;70:1553-9.
- Danziger-Isakov L, Kumar D, Practice AIC. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clinical Transplantation. 2019;33. [CrossRef]
- Dos Santos G, Haguinet F, Cohet C, Webb D, Logie J, Ferreira GLC, et al. Risk of solid organ transplant rejection following vaccination with seasonal trivalent inactivated influenza vaccines in England: A self-controlled case-series. Vaccine. 2016;34:3598-606. [CrossRef]
- Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. American Journal of Transplantation. 2021;21:2719-26.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. Jama-Journal of the American Medical Association. 2021;325:2204-6.
- Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. Journal of Clinical Investigation. 2021;131.
- Korth J, Jahn M, Dorsch O, Anastasiou OE, Sorge-Hadicke B, Eisenberger U, et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses-Basel. 2021;13.
- Danthu C, Hantz S, Dahlem A, Duval M, Ba B, Guibbert M, et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. Journal of the American Society of Nephrology. 2021;32:2154-9.
- Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney International. 2021;99:1487-9.
- Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. Jama-Journal of the American Medical Association. 2021;326:1533-5.
- Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. American Journal of Transplantation. 2021;21:2727-39.
- Jordan SC. Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses. Clinical and Experimental Immunology. 2021;204:310-20.
- Wisnewski AV, Luna JC, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. Plos One. 2021;16. [CrossRef]
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. European Review for Medical and Pharmacological Sciences. 2021;25:1663-9. [CrossRef]
- Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. Journal of Immunology. 2008;180:5771-7. [CrossRef]
- Gregori S, Amodio G, Passerini L, de Sio FRS. Alteration of interleukin-10-producing Type 1 regulatory cells in autoimmune diseases. Current Opinion in Hematology. 2022;29:218-24. [CrossRef]
- Bahabayi A, Zeng XY, Tuerhanbayi B, Zhang YY, Hasimu A, Guo SY, et al. Changes in circulating TCF1-and GARP-associated regulatory T cell subsets reflect the clinical status of patients with chronic HBV infection. Medical Microbiology and Immunology. [CrossRef]
- Luke E, Swafford K, Shirazi G, Venketaraman V. TB and COVID-19: An Exploration of the Characteristics and Resulting Complications of Co-infection. Frontiers in Bioscience (Schol Ed). 2022;14:1-11. [CrossRef]
- Safar HA, Mustafa A, Amoudy HA, El-Hashim A. The effect of adjuvants and delivery systems on Th1, Th2, Th17 and Treg cytokine responses in mice immunized with Mycobacterium tuberculosis-specific proteins. Plos One. 2020;15. [CrossRef]
- Elizondo DM, Andargie TE, Haddock NL, da Silva RLL, de Moura TR, Lipscomb MW. IL-10 producing CD8(+) CD122(+) PD-1(+) regulatory T cells are expanded by dendritic cells silenced for Allograft Inflammatory Factor-1. Journal of Leukocyte Biology. 2019;105:123-30. [CrossRef]
- Degner KR, Wilson NA, Reese SR, Parajuli S, Aziz F, Garg N, et al. Short-term Immunopathological Changes Associated with Pulse Steroids/IVIG/Rituximab Therapy in Late Kidney Allograft Antibody Mediated Rejection. Kidney360. 2020;1:389-98.
- Chen W, Bai J, Huang HY, Bi LL, Kong XR, Gao Y, et al. Low proportion of follicular regulatory T cell in renal transplant patients with chronic antibody-mediated rejection. Scientific Reports. 2017;7. [CrossRef]
- Shao CY, Chen YH, Nakao T, Amouzegar A, Yin J, Tahvildari M, et al. Local Delivery of Regulatory T Cells Promotes Corneal Allograft Survival. Transplantation. 2019;103:182-90. [CrossRef]
- Dai HL, Peng FH, Lin MJ, Xia JJ, Yu SJ, Lan GB, et al. Anti-OX40L monoclonal antibody prolongs secondary heart allograft survival based on CD40/CD40L and LFA-1/ICAM-1 blockade. Transplant Immunology. 2015;32:84-91.
- McCallion O, Bilici M, Hester J, Issa F. Regulatory T-cell therapy approaches. Clinical and Experimental Immunology. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).