Submitted:
14 November 2023
Posted:
15 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
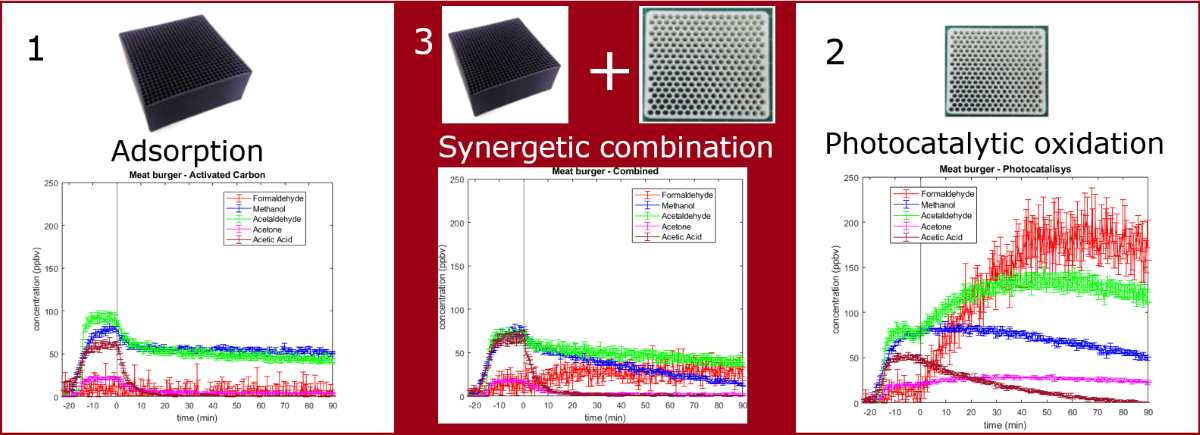
2. Results and Discussion
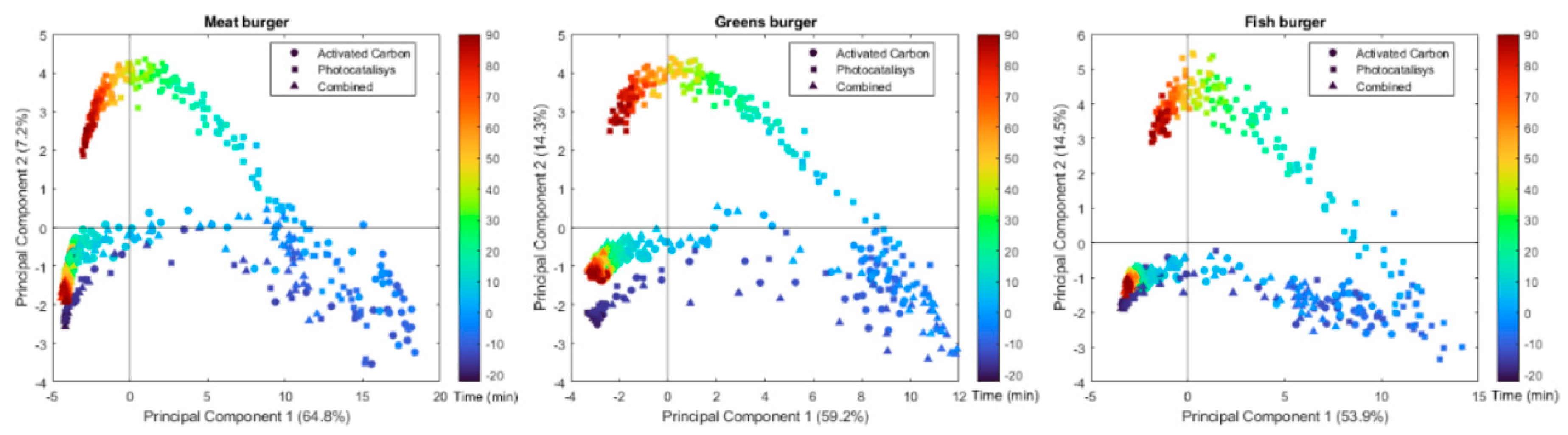
2.1. Principal Component Analysis
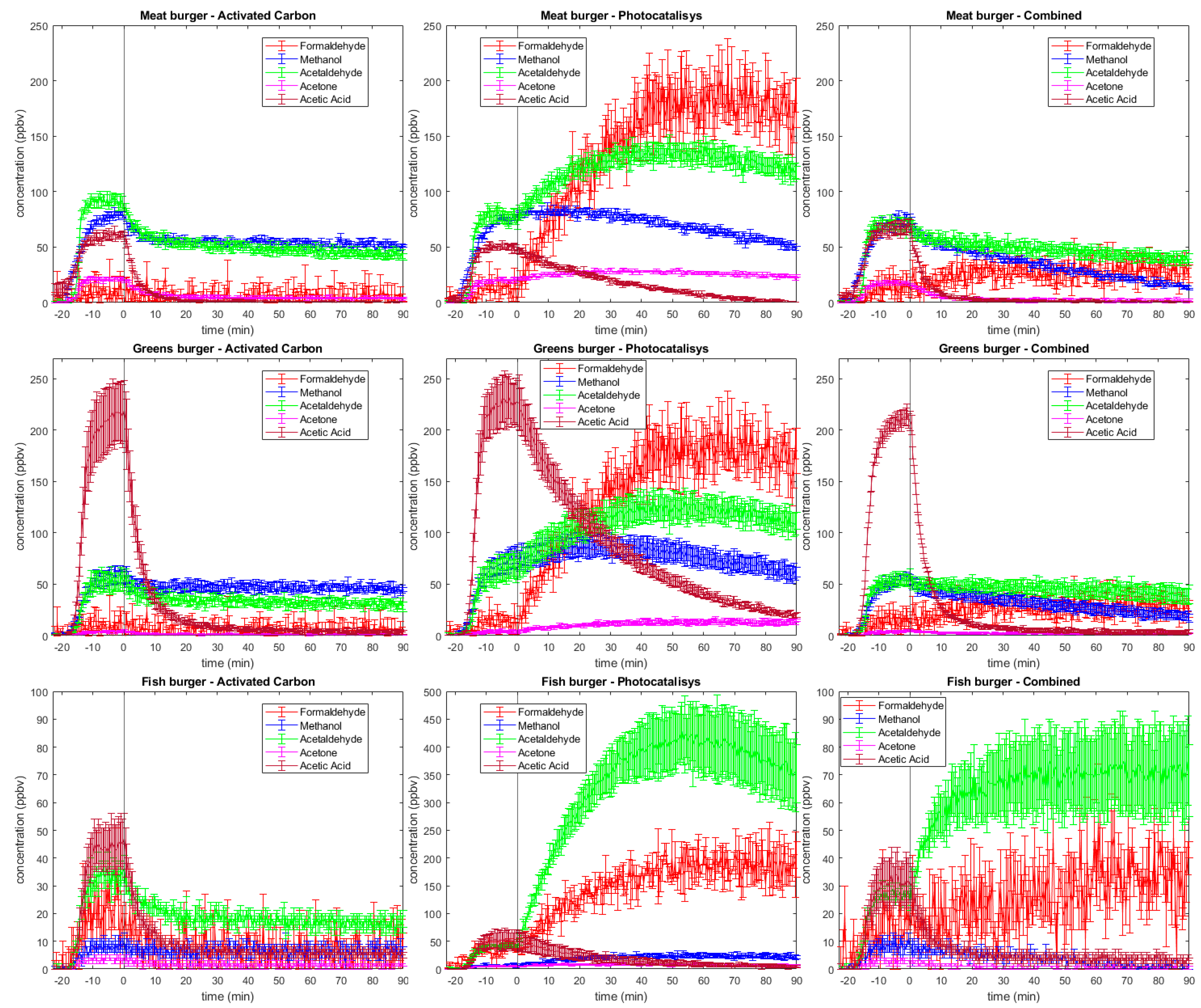
2.2. Time trends
2.2.1. Formaldehyde
2.2.2. Methanol
2.2.3. Acetaldehyde
2.2.4. Acetone
2.2.5. Acetic acid
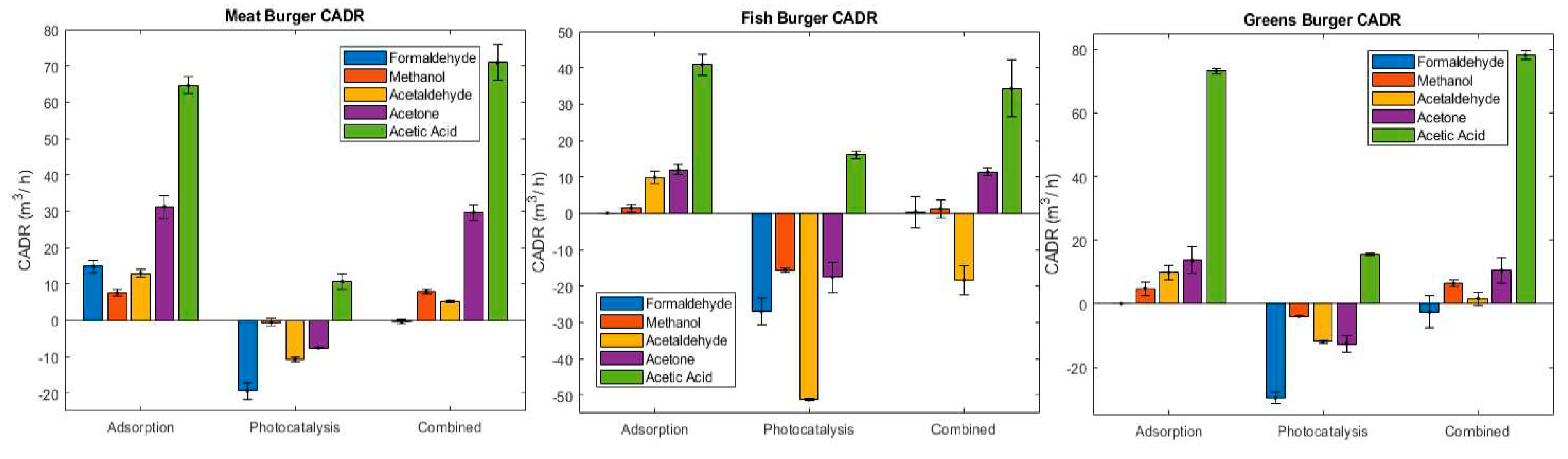
2.2.6. Clean Air Delivery Rate
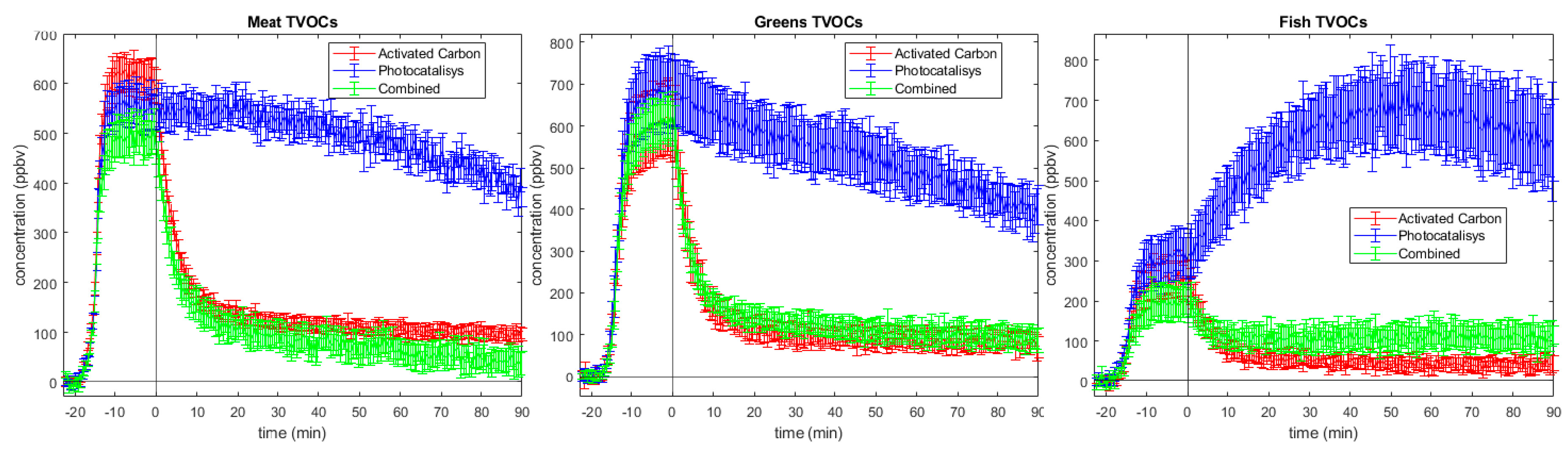
2.3. Total Volatile Organic Compounds (TVOCs)
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ni, Q.; Khomenko, I.; Gallo, L.; Biasioli, F.; Bittante, G. Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat. Foods 2020, 9, 1776. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Samaranayaka, A.G.P.; Pegg, R.B. Malliard Reaction and Browning. Encyclopedia of Meat Sciences 2014, 1, 391–403. [Google Scholar]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.K.; Nawar, W.W. Thermal Interaction of Linoleic Acid and Its Esters with Valine. J Am Oil Chem Soc 1981, 58, 632–635. [Google Scholar] [CrossRef]
- Mansur, A.R.; Lee, H.J.; Choi, H.; Lim, T.; Yoo, M.; Jang, H.W.; Nam, T.G. Comparison of Two Commercial Solid-phase Microextraction Fibers for the Headspace Analysis of Volatile Compounds in Different Pork and Beef Cuts. J Food Process Preserv 2018, 42, jfpp–13746. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated Adsorption and Photocatalytic Degradation of Volatile Organic Compounds (VOCs) Using Carbon-Based Nanocomposites: A Critical Review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors for VOCs Adsorption Process: A Review. Sep Purif Technol 2020, 235, 116213. [Google Scholar] [CrossRef]
- Pui, W.K.; Yusoff, R.; Aroua, M.K. A Review on Activated Carbon Adsorption for Volatile Organic Compounds (VOCs). Reviews in Chemical Engineering 2019, 35, 649–668. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, Y.; Li, Y.; Jin, R.; Geng, Q.; Chen, S. Management of Typical VOCs in Air with Adsorbents: Status and Challenges. Dalton Transactions 2023, 52, 12169–12184. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Harper, M. Sorbent Trapping of Volatile Organic Compounds from Air. J Chromatogr A 2000, 885, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Yang, C.H. Granular-Activated Carbon Adsorption Followed by Annular-Type Photocatalytic System for Control of Indoor Aromatic Compounds. Sep Purif Technol 2009, 66, 438–442. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile Organic Compounds (VOCs) Removal by Photocatalysts: A Review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environmental Science and Pollution Research 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent Advances in VOC Elimination by Catalytic Oxidation Technology onto Various Nanoparticles Catalysts: A Critical Review. Appl Catal B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Hanif, Md.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Kim, Y.S. Photocatalytic VOCs Degradation Efficiency of Polypropylene Membranes by Incorporation of TiO2 Nanoparticles. Membranes (Basel) 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Jaison, A.; Mohan, A.; Lee, Y.-C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of Different Plasmonic Metals on Photocatalytic Degradation of Volatile Organic Compounds (VOCs) by Bentonite/M-TiO2 Nanocomposites under UV/Visible Light. Appl Clay Sci 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical Activated Carbon as an Enhanced Support for TiO2/AC Photocatalysts. Carbon N Y 2014, 67, 104–118. [Google Scholar] [CrossRef]
- Oliver, K.D.; Adams, J.R.; Daughtrey, E.H.; McClenny, W.A.; Yoong, M.J.; Pardee, M.A.; Almasi, E.B.; Kirshen, N.A. Technique for Monitoring Toxic VOCs in Air: Sorbent Preconcentration, Closed-Cycle Cooler Cryofocusing, and GC/MS Analysis. Environ Sci Technol 1996, 30, 1939–1945. [Google Scholar] [CrossRef]
- Cappellin, L.; Loreto, F.; Aprea, E.; Romano, A.; del Pulgar, J.; Gasperi, F.; Biasioli, F. PTR-MS in Italy: A Multipurpose Sensor with Applications in Environmental, Agri-Food and Health Science. Sensors 2013, 13, 11923–11955. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ Sci Technol 2012, 46, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Hansel, A.; Jordan, A. Mass Spectrometry and Ion Processes On-Line Monitoring of Volatile Organic Compounds at Pptv Levels by Means of Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) Medical Applications, Food Control and Environmental Research; 1998; Vol. 173;
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous Photodegradation of VOC Mixture by TiO2 Powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.B.; Feilberg, A.; Kristensen, K. Removal of Volatile Organic Compounds by Mobile Air Cleaners: Dynamics, Limitations, and Possible Side Effects. Build Environ 2023, 242, 110541. [Google Scholar] [CrossRef]
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Maachi, R.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Indoor Air Treatment of Refrigerated Food Chambers with Synergetic Association between Cold Plasma and Photocatalysis: Process Performance and Photocatalytic Poisoning. Chemical Engineering Journal 2020, 382, 122951. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Assadi, A.A.; Kane, A.; Jung, A.-V.; Le Cann, P.; Gerard, A.; Bazantay, F.; Bouzaza, A.; Wolbert, D. Integrated Process for the Removal of Indoor VOCs from Food Industry Manufacturing: Elimination of Butane-2,3-Dione and Heptan-2-One by Cold Plasma-Photocatalysis Combination. J Photochem Photobiol A Chem 2020, 386, 112071. [Google Scholar] [CrossRef]
- Sirivallop, A.; Escobedo, S.; Areerob, T.; de Lasa, H.; Chiarakorn, S. Photocatalytic Conversion of Organic Pollutants in Air: Quantum Yields Using a Silver/Nitrogen/TiO2 Mesoporous Semiconductor under Visible Light. Catalysts 2021, 11, 529. [Google Scholar] [CrossRef]
- Atamaleki, A.; Motesaddi Zarandi, S.; Massoudinejad, M.; Hesam, G.; Naimi, N.; Esrafili, A.; Fakhri, Y.; Mousavi Khaneghah, A. Emission of Aldehydes from Different Cooking Processes: A Review Study. Air Qual Atmos Health 2022, 15, 1183–1204. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.H.; Yoo, K.C.; Lee, J.H. Changes in Acetaldehyde and Formaldehyde Contents in Foods Depending on the Typical Home Cooking Methods. J Hazard Mater 2021, 414, 125475. [Google Scholar] [CrossRef]
- Cappellin, L.; Loreto, F.; Biasioli, F.; Pastore, P.; McKinney, K. A Mechanism for Biogenic Production and Emission of MEK from MVK Decoupled from Isoprene Biosynthesis. Atmos Chem Phys 2019, 19, 3125–3135. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Lu, B.; Sun, S.; Zhang, S.; Zhang, J. Rapid Determination of Six Low Molecular Carbonyl Compounds in Tobacco Smoke by the APCI-MS/MS Coupled to Data Mining. J Anal Methods Chem 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma Compounds Identified in Cooked Meat: A Review. Food Research International 2022, 157, 111385. [Google Scholar] [CrossRef] [PubMed]
- Howard-Reed, C.; Nabinger, S.J.; Emmerich, S.J. Characterizing Gaseous Air Cleaner Performance in the Field. Build Environ 2008, 43, 368–377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




