1. Introduction
Trigger finger is one of the most common conditions treated by hand surgeons. The prevalence in the general population was reported to be 3% [
1]. There were reported several management approaches for the treatment of trigger finger [
2,
3]. Corticosteroid injection is a common management strategy in the initial treatment of symptomatic trigger digits. It was reported that 40-80% of patients had resolution of symptoms following corticosteroid injection [
4]. If conservative interventions or corticosteroid injections are unsuccessful, open surgery is generally conducted. Though success rates of open surgical release of the A1 pulley were reported to be around 90%, some patients suffer from postoperative adverse outcomes, including finger stiffness or pain [
5,
6]. Everding et al. reported that 4.9% of the patients had remaining pain, swelling, or stiffness and that 2.5% had residual contracture after the open surgery [
5].
Some studies revealed the effectiveness of rehabilitation after some kinds of surgeries, including fixation of extremity fractures and joint replacemet [
7,
8,
9]. Rehabilitation is also effective after hand surgeries [
10,
11,
12]. As for trigger finger, it was reported that patients with open surgical release and rehabilitation therapy achieved good results and a low rate of complications [
13]. However, this study is not a comparative study but a case series, thus there are many biases in it. Additionally, it is not clear which type of patients should undergo rehabilitation after open surgery. Therefore, we conducted a randomized controlled study with a primary objective to examine the effectiveness of the early rehabilitation on hand function and patient-reported outcome (PRO) compared to advice alone. A secondary objective was to identify which patient factors, such as severity of disease, age, duration of symptoms, and type of occupation, have influences on the effect of rehabilitation.
2. Materials and Methods
Design
This study was a prospective, multicenter, randomized, controlled trial (RCT). Approval was obtained from the institutional ethics committee at Okayama University Hospital (UMIN000036137).
The participants were selected from a consecutive group seen at three institutions from April 2019 to March 2021. Inclusion criteria were (1) older than 20 years old, younger than 90 years old; (2) continued subjective symptoms of pain, triggering along the A1 pulley after a few injections into the flexor sheath; and (3) willingness to undergo an operation for trigger finger. We excluded patients who had (1) other fingers with symptoms of trigger finger, (2) an inflammatory or pathologic etiology, including rheumatoid arthritis, of the trigger finger, (3) a finger joint problem by osteoarthritis or joint fracture, or (4) other surgeries during the follow-up period.
Randomization
Participants were assigned to the intervention or control group. An independent investigator (R.N.) made a computer-randomized list for each hospital to conceal treatment allocation. The randomization list was stratified with a block size of four. Based on the lists, the investigator prepared sequentially numbered and sealed envelopes containing the assigned postoperative schedule. After the participants agreed to participation in this study and underwent the surgery for trigger finger, the researcher opened the consecutive envelope to figure out the assigned schedule. Blinding of the participants was precluded because of referral to postoperative rehabilitation.
Treatments
All participants underwent open surgery under local anesthesia by one surgeon. After an incision was made at the level of the A1 pulley, blunt dissection was performed on the A1 pulley. A small dissection scissor was used to make an incision longitudinally and open the A1 pulley. Free movement of the flexor tendon was ensured without triggering. Finally, the skin was closed with interrupted 5-0 nylon sutures.
Intervention
Participants in the rehabilitation group received a referral for postoperative occupational therapy (OT), including scar massage, stretching, and active and passive range of motion exercises within a week after surgery. For 3 months, participants received individual exercise therapy for about 30 minutes twice a week.
The exact type of exercise was left to the hand therapist’s discretion in accordance with the condition of participants’ hands and their preferences. Additionally, therapists gave appropriate individual advice on activities of daily living and lifestyle for each participant.
Participants in the control group were not referred for rehabilitation after surgery. They received only the orientation and advice for a range of motion exercise to be performed by themselves from the primary surgeon during the clinical phase.
Follow-up
Participants were examined and interviewed before and 1, 3, and 6 months after surgery. The follow-up period was determined by referring to other RCTs of treatments for trigger finger [
14,
15]. We evaluated subjective symptoms and physical findings including tenderness at the A1 pulley, grip strength, snapping phenomenon, and whether they gained full range of motion (ROM) of the treated digit. Disability of Arm-Shoulder-Hand (DASH) score, pain-visual analogue scale (VAS) of the treated digit, and complications were also examined.
Statistical Analysis
The primary endpoints were the effect of postoperative rehabilitation on hand function, such as DASH and grip power. Estimation of sample size was performed to determine the number of patients to reach an alpha value of 0.05 and a power of 80%. This sample size was based on the assumed primary outcome (DASH score) differences in population means of 6.0, a within-group standard deviation of 8.0, and an approximately equal number of cases in each group. Finally, the calculated sample size with an anticipated loss to follow-up of 15% was 68 patients.
We performed an intention-to-treat analysis among participants. A participant in the rehabilitation group who converted to the no rehabilitation group was analyzed within the rehabilitation group. Fisher’s exact test and the Student t-test were used to compare dichotomous and continuous data, respectively. The group differences were analyzed by one-way ANOVA followed by Bonferroni post hoc testing. Statistical analyses were performed using R for Windows (
www.r-project.org). The two-sided significance level was set at p < 0.05.
3. Results
Patient Characteristics
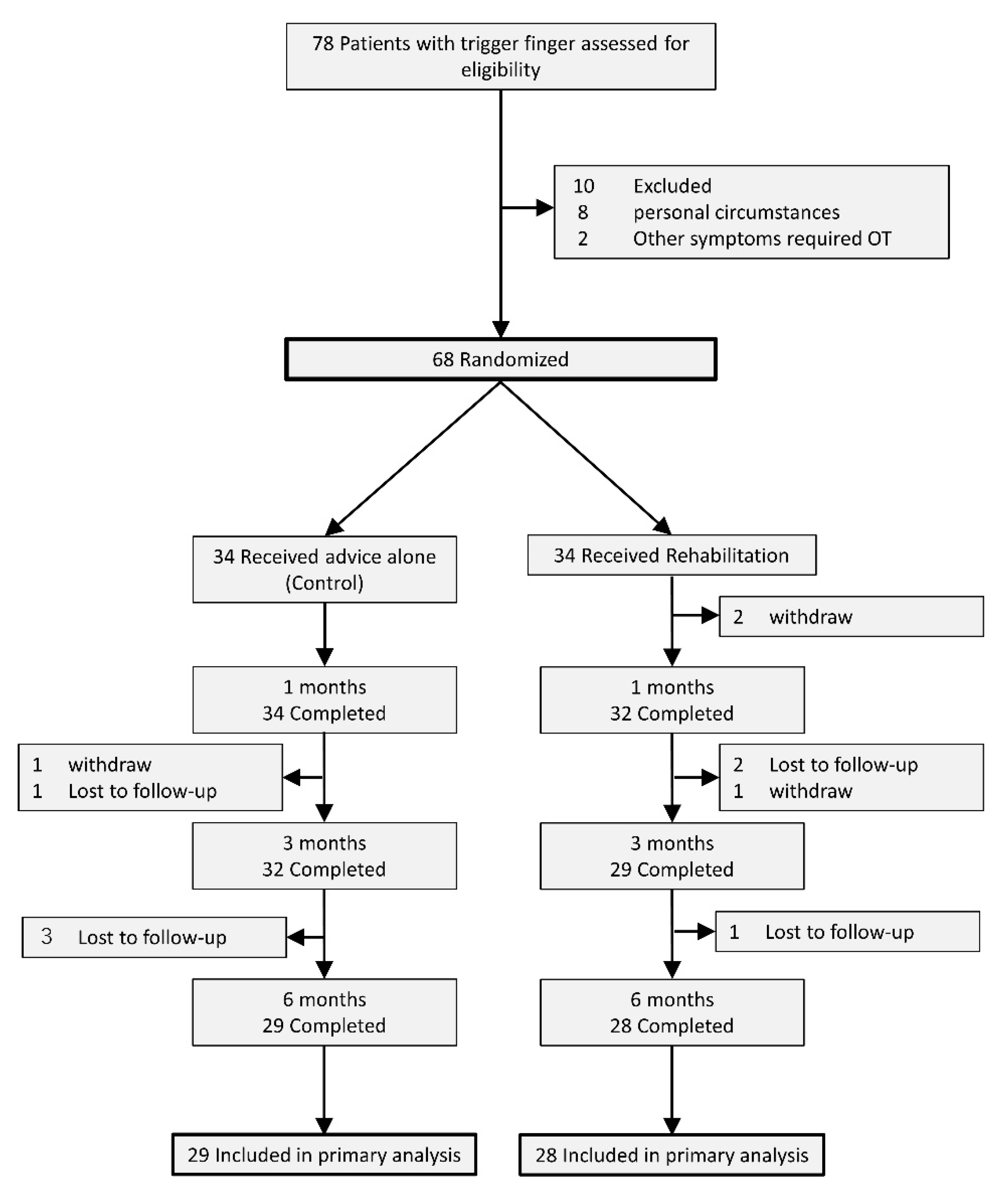
Figure 1 showed the flow of the participants through the trial. Initially, we included 68 patients in this study. Thirty-four of them were randomized into the control and rehabilitation groups, respectively. Four patients in the control group and three patients in the rehabilitation group were lost to follow-up by the final examination. One patient in the control group and three patients in the rehabilitation group withdrew by the final examination.
Patient characteristics are shown in
Table 1. There were no significant differences between the rehabilitation and control groups in relation to demographic variables including gender, age, duration of symptoms, preoperative severity of the trigger finger, dominant hand affected, and affected digits. The preoperative severity was classified using the Quinnell grading [
16]. The prevalence of diabetes mellitus (DM) and restricted range of motion (ROM) also did not differ significantly between these two groups. DM was well-controlled by oral drugs without insulin treatment in both groups.
Effect of postoperative rehabilitation
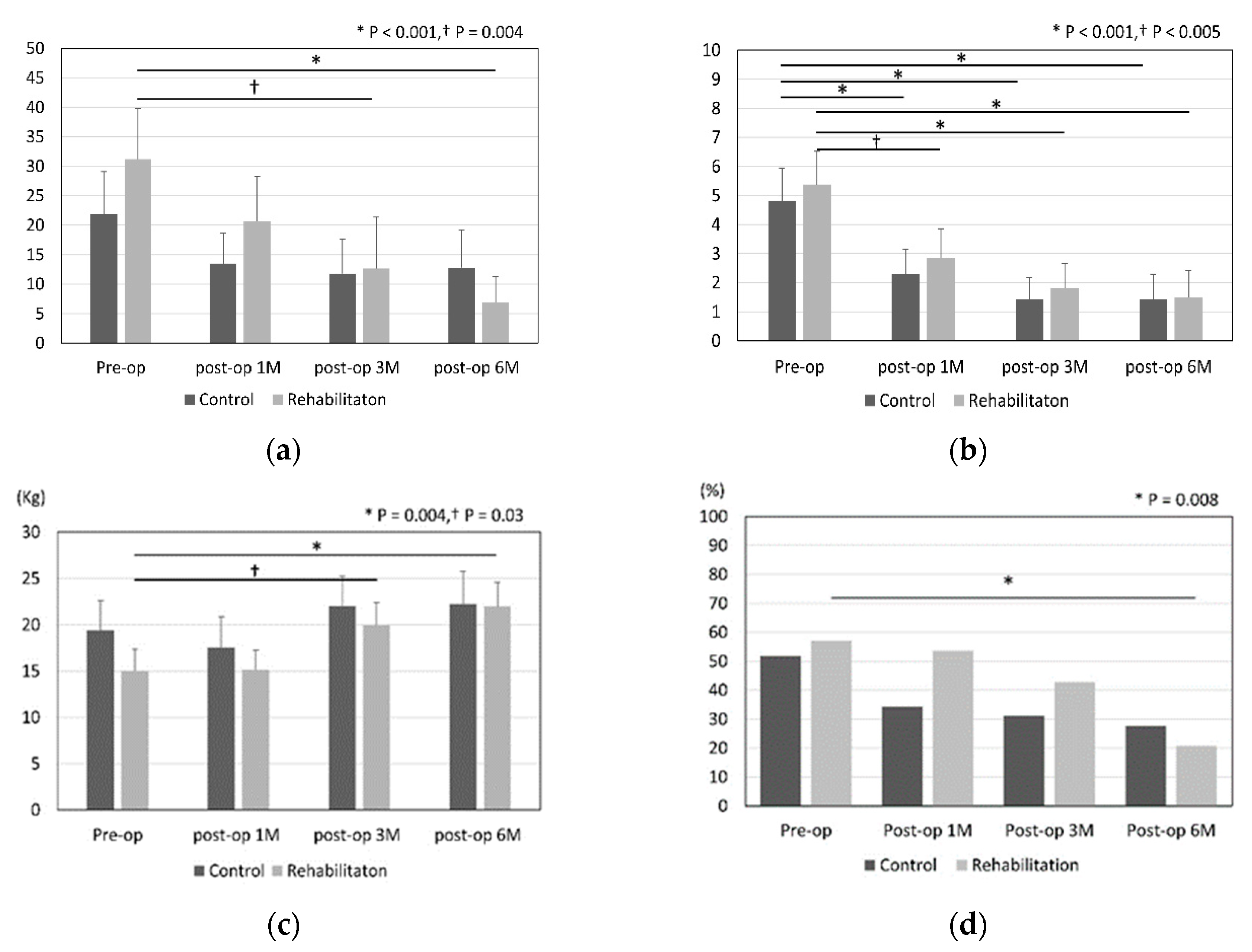
Figure 2 shows the results for DASH score, pain-VAS, grip strength, and range of motion in the control and rehabilitation groups. Improvements of DASH score, grip strength, and ROM were noted significantly in the rehabilitation group at 6 months after surgery (DASH score; p < 0.001, grip strength; p = 0.004, restriction of ROM; p = 0.008).
The pain was significantly improved in both groups between that preoperatively and 6 months postoperatively. We summarised the differences between pre- and 6-month postoperative outcomes in
Table 2. Grip strength and DASH in rehabilitation group were improved significantly than those in control group.
Subanalysis
Rehabilitation made the DASH score in patients performing housework or light work improve significantly in 6 months postoperatively (
Table 3, 14.2 vs 6.1, p = 0.04). In the patients with a duration of symptoms > 12 months, the postoperative DASH score of the rehabilitation group was also significantly better than that of the control group (
Table 4, 19.6 vs 0.7, p = 0.005). There are no statistically significant differences in other factors, such as age, gender, and DM between the rehabilitation and control groups.
Complications
One patient in the rehabilitation group had a superficial incision infection that was treated with debridement and an antibacterial drug. There were no other adverse events during this study.
4. Discussion
This study showed that the outcomes including DASH score, grip power, and restriction of ROM at 6 months were significantly improved after surgery in only the rehabilitation group. Pain-VAS was significantly improved in both groups. In the patients performing housework or light work or the patients with a duration of symptoms over 12 months, there were significant differences in the DASH score 6 months after surgery between the rehabilitation and control groups.
The effect of postoperative rehabilitation was revealed in some kinds of surgeries [
8,
11,
17]. In hip fracture patients, hospital rehabilitation was significantly related with a lower risk of mortality compared to no rehabilitation [
8]. It was also reported that rehabilitation improved mobility for veterans with major lower extremity amputation [
17]. As for hand function, rehabilitation after carpal tunnel release improves hand function one month after surgery and accelerates recovery [
11]. This study revealed that rehabilitation after surgery significantly improved PRO and hand function as with other surgeries.
Early motion after upper limb surgery is effective. Many studies demonstrated benefits of the early motion, including preventing restriction of ROM, faster healing, decreased disability time, and decreased risk of reflex sympathetic dystrophy [
18,
19,
20]. Additionally, the early motion helps edema to decrease, while decreased edema also helps with increased ROM [
21]. The meta-analysis that evaluated the effect of rehabilitation following arthroscopic rotator cuff tear repair revealed that early passive motion results in superior ROM recovery [
22]. In this study, the postoperative rehabilitation was also started within a week, and this early motion by occupational therapists might help improve functional and subjective outcomes.
Some studies revealed the relationship between long-standing symptoms and postoperative outcomes in various kinds of surgeries [
23,
24,
25]. Inderhaug et al. reported that long-standing symptoms (over 12 months) were identified as one of the predictors of inferior long-term outcome after rotator cuff repair [
25]. This study revealed that the patients with long-standing symptoms (over 12 months) tended to show worse postoperative DASH scores. Among such patients, the patients with postoperative rehabilitation could show significantly improved DASH scores compared to the patients without rehabilitation. Degenerative thickening of the flexor tendons causes persistent flexed flexion deformity of the PIP joint [
26]. Therefore, postoperative rehabilitation is more important and recommended especially for patients with long-standing symptoms.
This study also revealed that postoperative rehabilitation for trigger finger was effective for the patients performing housework or light work, while there is no significant difference in postoperative DASH score between those doing heavy manual work with rehabilitation and without it. Postoperative passive and active motion of fingers by doing heavy manual work may act as adequate range of motion exercises for the patients with trigger finger.
DM has an influence on developing trigger finger [
27,
28]. It was reported that the incidence of trigger finger in patients with DM was about four times higher than in the general population [
29] and that approximately 20% of the patients with trigger finger had DM [
30]. This study included 15.8% of patients with DM, consistent with previous reports. As for the impact of DM on surgical outcomes, it was revealed that there were no differences in functional and subjective outcomes between diabetic and nondiabetic patient [
31]. This study also showed no differences between them. Additionally, rehabilitation has no influence on the outcomes for the patients with DM in this study (data not shown). The conditions of all patients with DM were well controlled by oral drugs, which may have had an effect on the outcomes.
This study has some limitations. First, participants could not be blinded due to the nature of the rehabilitation intervention. However, we performed allocation concealment of the participants and minimized the bias in the randomization process. Second, we could not control participants’ uncertainty about the use of their hands after surgery in daily life. These factors may have influenced the outcomes. Third, this was not a long-term follow-up study. However, it was revealed that there were no statistical differences in clinical outcomes, including VAS and Quick DASH, at 6 months and 12 months after percutaneous or open release for trigger finger [
32]. Additionally, several other studies of the treatments for trigger finger also set the follow-up period as under 6 months in the same way as this study [
14,
33,
34,
35].
In conclusion, rehabilitation after open surgery for trigger finger was effective at improving subjective and objective outcomes, especially for those performing housework or light work and patients with long-standing symptoms. The information will help surgeons select the patients to refer to OT postoperatively.
Author Contributions
Study design, T.S., K.N. and T.O.; data acquisition, T.S. and R.N.; analysis and interpretation of data, R.N.; manuscript preparation, T.S., R.N. and K.N.; manuscript review, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Okayama University Hospital (UMIN000036137 and 3/19/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patients’ privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strom, L. Trigger finger in diabetes. J Med Soc N J. 1977, 74, 951–954. [Google Scholar]
- Ryzewicz, M.; Wolf, J.M. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006, 31, 135–146. [Google Scholar] [CrossRef]
- Amirfeyz, R.; McNinch, R.; Watts, A.; Rodrigues, J.; Davis, T.R.C.; Glassey, N.; Bullock, J. Evidence-based management of adult trigger digits. J Hand Surg Eur Vol. 2017, 42, 473–480. [Google Scholar] [CrossRef]
- Leow, M.Q.H.; Hay, A.S.R.; Ng, S.L.; Choudhury, M.M.; Li, H.; McGrouther, D.A.; Tay, S.C. A randomized controlled trial comparing ketorolac and triamcinolone injections in adults with trigger digits. J Hand Surg Eur Vol. 2018, 43, 936–941. [Google Scholar] [CrossRef]
- Everding, N.G.; Bishop, G.B.; Belyea, C.M.; Soong, M.C. Risk factors for complications of open trigger finger release. Hand (N Y). 2015, 10, 297–300. [Google Scholar] [CrossRef]
- Bruijnzeel, H.; Neuhaus, V.; Fostvedt, S.; Jupiter, J.B.; Mudgal, C.S.; Ring, D.C. Adverse events of open A1 pulley release for idiopathic trigger finger. J Hand Surg Am. 2012, 37, 1650–1656. [Google Scholar] [CrossRef]
- Dehghan, N.; Mitchell, S.M.; Schemitsch, E.H. Rehabilitation after plate fixation of upper and lower extremity fractures. Injury. 2018, 49, S72–S77. [Google Scholar] [CrossRef]
- Tedesco, D.; Gibertoni, D.; Rucci, P.; Hernandez-Boussard, T.; Rosa, S.; Bianciardi, L.; Rolli, M.; Fantini, M.P. Impact of rehabilitation on mortality and readmissions after surgery for hip fracture. BMC Health Serv Res. 2018, 18, 701. [Google Scholar] [CrossRef]
- Snell, D.L.; Hipango, J.; Sinnott, K.A.; Dunn, J.A.; Rothwell, A.; Hsieh, C.J.; DeJong, G.; Hooper, G. Rehabilitation after total joint replacement: a scoping study. Disability and Rehabilitation. 2018, 40, 1718–1731. [Google Scholar] [CrossRef]
- Sultana, S.S.; MacDermid, J.C.; Grewal, R.; Rath, S. The effectiveness of early mobilization after tendon transfers in the hand: a systematic review. J Hand Ther. 2013, 26, 1–20; quiz 21. [Google Scholar] [CrossRef]
- Provinciali, L.; Giattini, A.; Splendiani, G.; Logullo, F. Usefulness of hand rehabilitation after carpal tunnel surgery. Muscle Nerve. 2000, 23, 211–216. [Google Scholar] [CrossRef]
- Yoshida, A.; Yamamoto, M.; Li-Tsang, C.W.P.; Iwatsuki, K.; Hirata, H. A systematic review assessing the effectiveness of hand therapy programmes in adults with burns using the International Classification of Functioning, Disability and Health framework. Nagoya J Med Sci. 2022, 84, 689–704. [Google Scholar] [CrossRef]
- Deskur, A.; Deskur, Z. Surgical Treatment and Rehabilitation of Trigger Thumb and Finger. Central European Journal of Sport Sciences and Medicine. 2017, 17, 61–66. [Google Scholar] [CrossRef]
- Sato, E.S.; Gomes Dos Santos, J.B.; Belloti, J.C.; Albertoni, W.M.; Faloppa, F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012, 51, 93–99. [Google Scholar]
- Zyluk, A.; Jagielski, G. Percutaneous A1 pulley release vs steroid injection for trigger digit: the results of a prospective, randomized trial. J Hand Surg Eur Vol. 2011, 36, 53–56. [Google Scholar]
- Quinnell, R.C. Conservative management of trigger finger. Practitioner. 1980, 224, 187–190. [Google Scholar]
- Czerniecki, J.M.; Turner, A.P.; Williams, R.M.; Hakimi, K.N.; Norvell, D.C. The effect of rehabilitation in a comprehensive inpatient rehabilitation unit on mobility outcome after dysvascular lower extremity amputation. Arch Phys Med Rehabil. 2012, 93, 1384–1391. [Google Scholar] [CrossRef]
- Akeson, W.H.; Amiel, D.; Abel, M.F.; Garfin, S.R.; Woo, S.L. Effects of immobilization on joints. Clin Orthop Relat Res. 1987, 28–37. [Google Scholar]
- Namba, R.S.; Kabo, J.M.; Dorey, F.J.; Meals, R.A. Continuous passive motion versus immobilization. The effect on posttraumatic joint stiffness. Clin Orthop Relat Res. 1991, 218–223. [Google Scholar]
- Salter, R.B. The Biological Concept of Continuous Passive Motion of Synovial Joints - the 1st 18 Years of Basic Research and Its Clinical-Application. Bris Myer Z. 1990, 335–353. [Google Scholar]
- Kearney, L.M.; Brown, K.K. The therapist’s management of intra-articular fractures. Hand Clin. 1994, 10, 199–209. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Luo, X.; Wang, K.; Wu, G.; Zhou, J.; Wang, P.; Sun, X. The clinical effect of rehabilitation following arthroscopic rotator cuff repair: A meta-analysis of early versus delayed passive motion. Medicine (Baltimore). 2018, 97, e9625. [Google Scholar]
- DeStefano, F.; Nordstrom, D.L.; Vierkant, R.A. Long-term symptom outcomes of carpal tunnel syndrome and its treatment. J Hand Surg Am. 1997, 22, 200–210. [Google Scholar] [CrossRef]
- Nygaard, O.P.; Kloster, R.; Solberg, T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg. 2000, 92, 131–134. [Google Scholar] [CrossRef]
- Inderhaug, E.; Kollevold, K.H.; Kalsvik, M.; Hegna, J.; Solheim, E. Preoperative NSAIDs, non-acute onset and long-standing symptoms predict inferior outcome at long-term follow-up after rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2017, 25, 2067–2072. [Google Scholar] [CrossRef]
- Le Viet, D.; Tsionos, I.; Boulouednine, M.; Hannouche, D. Trigger finger treatment by ulnar superficialis slip resection (U. S.S.R.). J Hand Surg Br. 2004, 29, 368–373. [Google Scholar] [CrossRef]
- Kuczmarski, A.S.; Harris, A.P.; Gil, J.A.; Weiss, A.C. Management of Diabetic Trigger Finger. J Hand Surg Am. 2019, 44, 150–153. [Google Scholar] [CrossRef]
- Wiwanitkit, S.; Wiwanitkit, V. Trigger digits and diabetes mellitus. N Am J Med Sci. 2012, 4, 117–119. [Google Scholar] [CrossRef]
- Koh, S.; Nakamura, S.; Hattori, T.; Hirata, H. Trigger digits in diabetes: their incidence and characteristics. J Hand Surg Eur Vol. 2010, 35, 302–305. [Google Scholar] [CrossRef]
- Nimigan, A.S.; Ross, D.C.; Gan, B.S. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006, 85, 36–43. [Google Scholar] [CrossRef]
- Stahl, S.; Kanter, Y.; Karnielli, E. Outcome of trigger finger treatment in diabetes. J Diabetes Complicat. 1997, 11, 287–290. [Google Scholar] [CrossRef]
- Grigorios, K.; Pantouvaki, A.; Spyrantis, M.; Christoforidis, C.; Velivasakis, G. Percutaneous or Open Release is the Most Effective Surgical Technique in Diabetic Recurrent Trigger Finger in Short and Long Term Outcomes? A Clinical Review. Acta Scientific Orthopaedics 2020, 3, 33–38. [Google Scholar] [CrossRef]
- Murphy, D.; Failla, J.M.; Koniuch, M.P. Steroid versus placebo injection for trigger finger. J Hand Surg Am. 1995, 20, 628–631. [Google Scholar] [CrossRef]
- Gilberts, E.C.; Beekman, W.H.; Stevens, H.J.; Wereldsma, J.C. Prospective randomized trial of open versus percutaneous surgery for trigger digits. J Hand Surg Am. 2001, 26, 497–500. [Google Scholar] [CrossRef]
- Dierks, U.; Hoffmann, R.; Meek, M.F. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008, 12, 183–187. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).