Introduction
Gastric cancer is the sixth most common cancer worldwide [
1]. It has a high prevalence in East Asia, with approximately 75% of gastric cancer cases worldwide occurring in East Asia. Gastric cancer is the third highest main cause of all cancer deaths in Japan and, the widespread use of endoscopy and the diagnostic advances have allowed early detection of gastric cancer. Endoscopic resection (ER) has been well-known treatment for early gastric cancer. Recently, the rapid widespread adoption of a new ER technique, endoscopic submucosal dissection (ESD), has led to being performed in between 60% and 70% of all gastric cancer cases [
2]. Similar to open surgery, ESD allows en bloc resection regardless of the lesion size if the superficial cancer has a negligible risk for lymph node metastasis. The most important merit of endoscopic treatment, including ESD, is the preservation of the stomach that can offer significantly better quality of life, compared to surgical resection. However, it involves the disadvantage of the remaining gastric mucosa, which has a high risk of carcinogenesis. Specifically, metachronous gastric cancers following ER have an occurrence rate of 2.5%–14% [
3,
4,
5,
6].
A recent randomized controlled trial reported a reduced risk of developing metachronous cancer (included in MSGENs) following eradication of
Helicobacter pylori (
H. pylori) after endoscopic treatment of early gastric cancer [
7]; therefore,
H. pylori eradication is recommended for
H. pylori positive patients who have undergone ER of superficial gastric epithelial neoplasms (SGENs). Contrastingly, other studies have reported that the risk of developing metachronous cancer does not decrease significantly after
H. pylori eradication [
5,
6,
8]. Thus, there is controversy regarding whether
H. pylori eradication is associated with the risk of developing metachronous cancer following ER, particularly ESD which has been widely used in recent days.
This retrospective cohort study aimed to identify the clinicopathological risk factors including H. pylori eradication for developing MSGENs in patients who underwent ESD for SGENs including MSGENs after previous ESD.
Materials and Methods
Patients
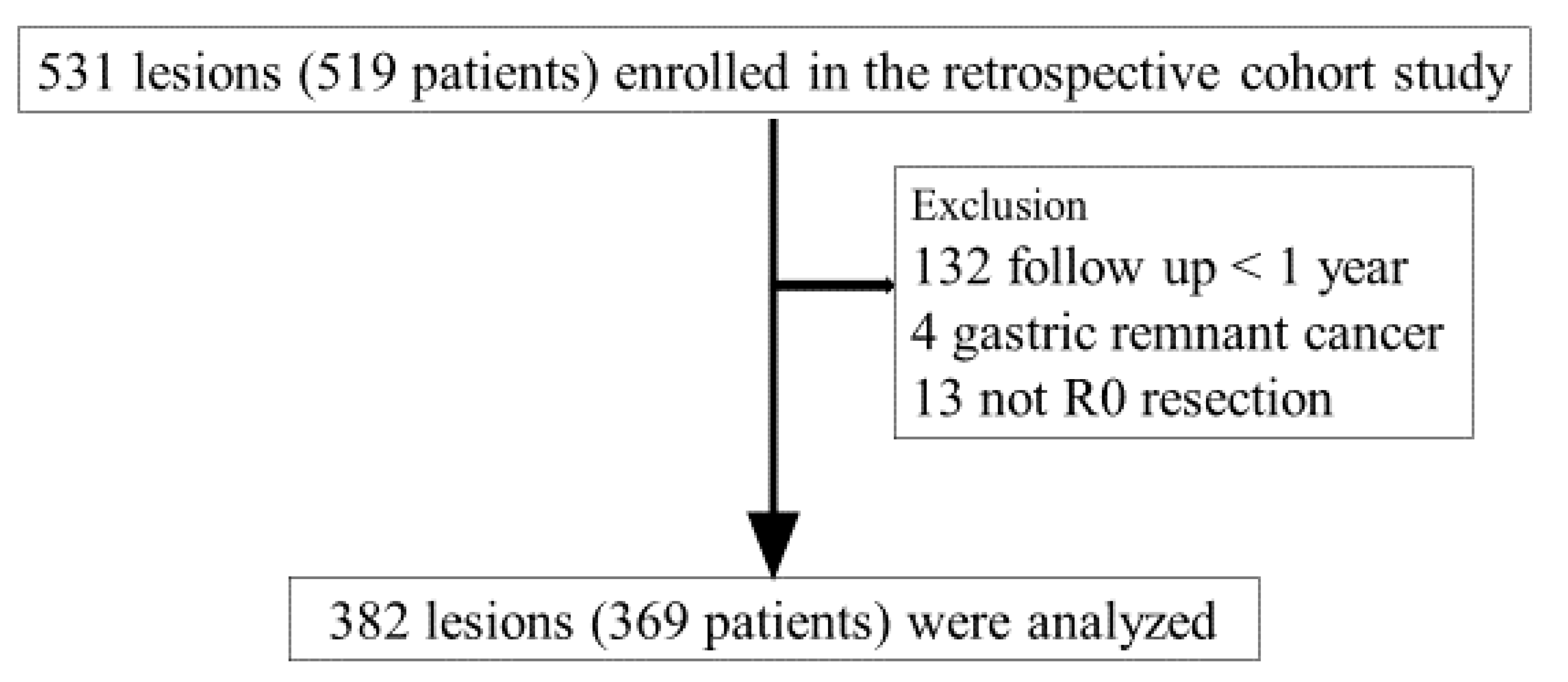
We identified 519 patients (531 lesions) who underwent ESD for SGENs at Dokkyo Medical University Hospital from January 2014 to December 2018. We excluded 132 patients who did not undergo endoscopic follow-up observation within < 1 year after ESD, 4 patients with gastric remnant cancer, and 13 patients who did not undergo R0 resection; finally, we included 369 patients (382 lesions) (
Figure 1). This study protocol was approved by the Institutional Review Board of Dokkyo Medical Universitye, Tochigi, Japan, for clinical research (Registration No. R23-3J). This study was conducted in compliance with the revised Helsinki Declaration (1989).
Methods
This retrospective cohort study extracted the following data from the patients’ electronic medical records and the endoscopy database: clinical findings (age, sex, body mass index, history of alcohol consumption, history of smoking), pre-ESD
H. pylori infection status, endoscopic findings (location of initial neoplasm (U, upper part; M, middle part; L, lower part), presence of atrophic change [yes vs. no] and their scope), and histological findings (intestinal metaplasia, tumor size, histological type, invasion depth, R0 resection, synchronous multicentric cancers [yes vs. no]). Atrophy of gastric mucosa was determined by endoscopy was classified into three groups according to the Kimura & Takemoto classification as follows: no atrophy, closed type (mild), and open type (severe) [
9].
This study included 369 patients with 382 SGEN lesions. 13 patients had two SGENs. A lesion with a larger diameter or deeper invasion was regarded as the initial SGENs for each of the 13 patients.
The primary endpoint was to identify clinicopathological risk factors for the incidence of MSGENs after ESD. The secondary endpoint was to investigate the association of H. pylori eradication with the MSGENs following ESD compared to a control group matched with variables including primary outcomes.
According to a study on MSGEN, secondary SGENs identified within 1 year after endoscopic treatment were defined as synchronous because synchronous multiple tumors might have been overlooked during the initial ESD. Thus, SGENs identified > 1 year after endoscopic treatment was defined as MSGENs [
10].
Spontaneous
H. pylori eradication was defined as having no history of eradication and testing negative for
H. pylori infection but having open-type (severe) mucosal atrophy [
11].
To elucidate the relationship between post-ESD MSGENs and H. pylori eradication therapy, we additionally established a matched not-MSGEN (Mnot-MSGEN) group, which was matched with the MSGEN group according to the clinicopathological risk factors identified in this study, age, and sex. Subsequently, we performed a statistical analysis based on whether H. pylori eradication was performed (yes vs. no) and the age at which H. pylori eradication was performed.
Confirmation of H. pylori Status
Serum antibody testing (Eiken Chemical, Tokyo, Japan) was performed on non-eradication patients before ESD. Moreover, the urea breath test (UBT; Otsuka Pharmaceutical, Tokyo, Japan) was performed on post-eradication patients before ESD to identify infection at the time. A positive result in at least one of these tests was considered evidence of H. pylori infection. Post-ESD eradication was performed on patients who tested positive for H. pylori infection. The primary eradication method was performed using combination therapies using a proton pump inhibitor (PPI; Lansoprazole 60 mg [Takeda Pharmaceutical, Tokyo, Japan]) or a potassium-competitive acid blocker (Vonoprazan 40 mg [Takeda Pharmaceutical]), and the two antibiotics Amoxicillin 1500 mg (Takeda Pharmaceutical) and Clarithromycin 400 mg (Takeda Pharmaceutical). The secondary eradication method was applied in case the primary eradication method had not been successful. The secondary eradication method was performed combination therapies using Lansoprazole 60 mg (Takeda Pharmaceutical) or Vonoprazan 40 mg (Takeda Pharmaceutical), and the two antibiotics, Amoxicillin 1500 mg (Takeda Pharmaceutical) and metronidazole 500 mg (Takeda Pharmaceutical). The eradications were evaluated after 4 to 6 months using UBT or serum antibody testing. We collected data regarding H. pylori status, including whether patients had a history of eradication, from their medical records.
Histopathologic Analysis
We used the Japanese Classification of Gastric Carcinoma, 15th edition [
12], to determine the histopathologic diagnosis of early gastric cancer, location (U; M, L, lower), cancerous histological type (Ditub1 or tub2, por, sig), invasion depth (M, mucosal layer; SM, submucosal layer), ulcer (yes vs. no), and lymphatic/venous invasion. We defined early gastric cancer as the invasion limited to the submucosa (SM). Gastric adenoma was classified into tub1 and mucosal cancer in this study. Histological intestinal metaplasia (yes vs. no) was determined using hematoxylin and eosin staining and periodic acid–Schiff staining of background mucosa in ESD specimens. One pathologist who has expertise in gastrointestinal tumors (H.Y.) diagnosed all pathologic findings .
Statistical Analyses
We performed statistical analysis designed to identify risk factors for the MSGEN group using the t-test/Mann-Whitney U test and the chi-squared test for continuous and categorical variables, respectively. We calculated the cumulative incidence of MSGENs using the Kaplan-Meier method in order to confirm the risks. The follow-up period was calculated from the day of the initial ESD. Regular endoscopic surveillance (every 6 months to 1 year) was performed after ESD; moreover, the follow-up period ended upon MSGENs occurrence. The incidence of MSGENs was analyzed using the person-years method. Univariate analysis was performed using the log-rank test; moreover, we longitudinally analyzed the relation between identified significant factors with significant and MSGEN development. Statistical significance was set at P-value < 0.05. All statistical analyses were performed using SPSS version 20 (SPSS Japan Inc., Tokyo, Japan).
Results
Table 1 shows the clinical findings for the included 369 patients. The median age (IQR) was 73 (67–79) years, and the male-to-female ratio was 2.3: 1. There were 369 SGENs lesions, 66 histological adenoma lesions, and 303 adenocarcinoma lesions (M: 264 lesions, SM: 39 lesions) that underwent ESD. We found 27 patients (27 lesions; 7.0 %: 2 patients with adenomas, 25 patients with carcinoma) with post-ESD MSGENs. Eleven or 2/ 14 lesions of MSGEN were located in U, M/ L parts.
Table 2 shows the clinicopathological findings for 342 and 27 patients in the not-MSGEN and MSGENs groups, respectively.
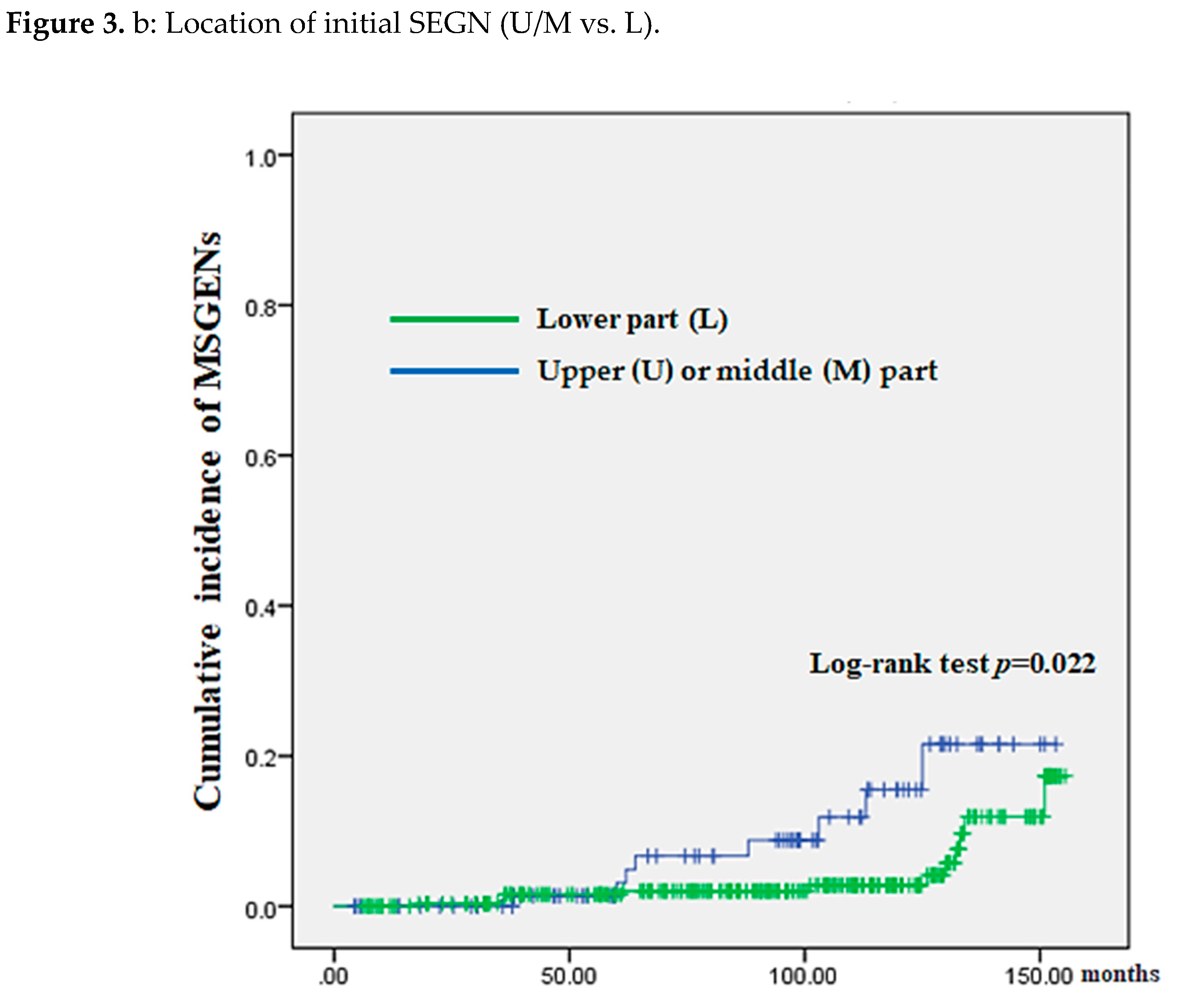
Table 2 demonstrates comparison of clinicopathologic factors between the “not-MSGEN” and “MSGEN” groups. There was a significantly higher incidence of MSGENs in cases of histological intestinal metaplasia (HIM; P = 0.04) and initial neo-plasm location in U or M part (INUM; P = 0.04). A significant difference between the locations (U or M/ L) of initial SGENs and MSGENs (10 or 13/ 4 vs. 11 or 2/ 14: P<0.01).
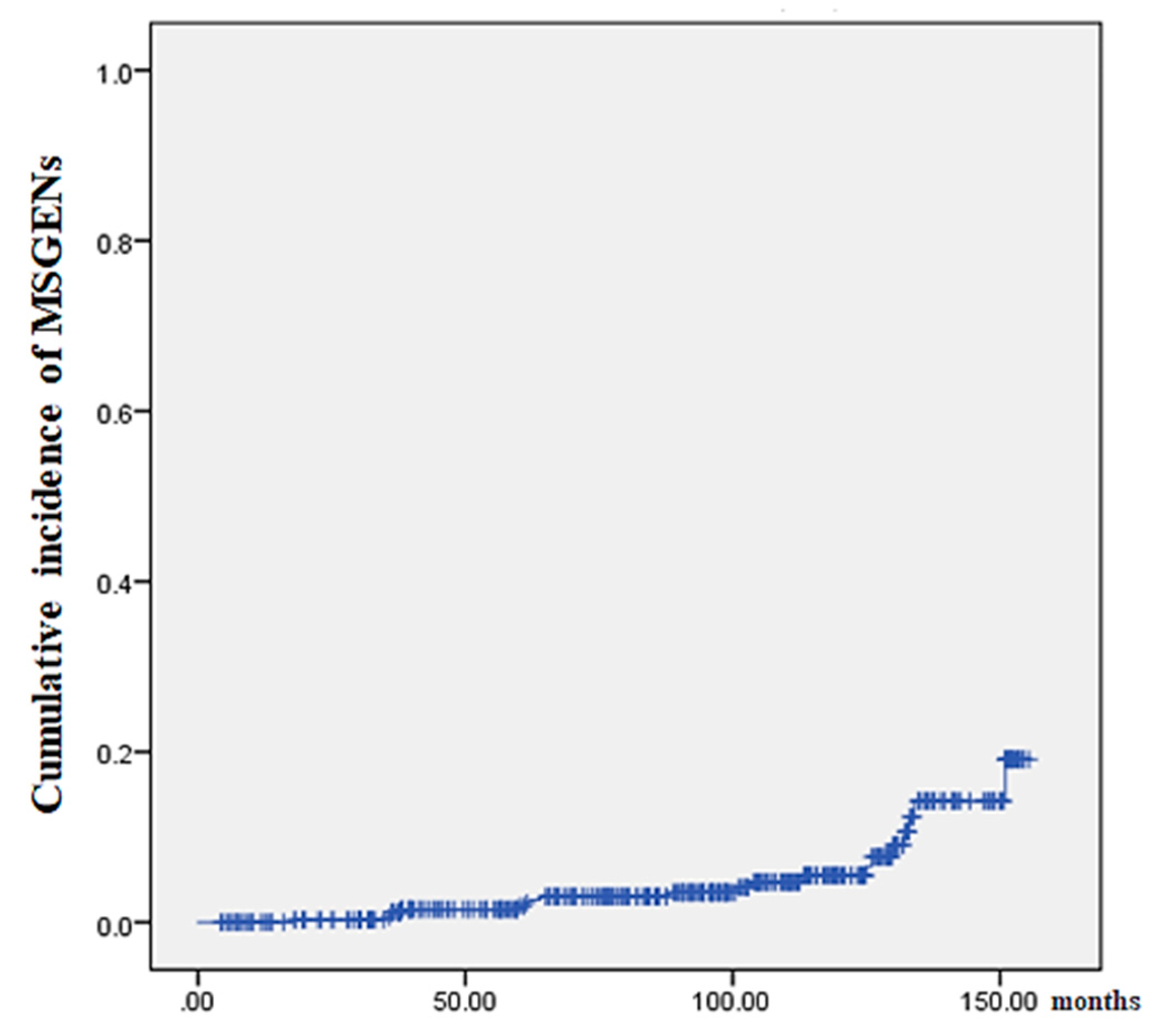
Figure 2 shows the results of our analysis of the cumulative incidence of MSGENs using the Kaplan-Meier methods. The median duration (IQR) from initial ESD to MSGENs was 95 (54–127) months. The shortest and longest durations from ESD to MSGENs detection were 18 months and 151 months, respectively.
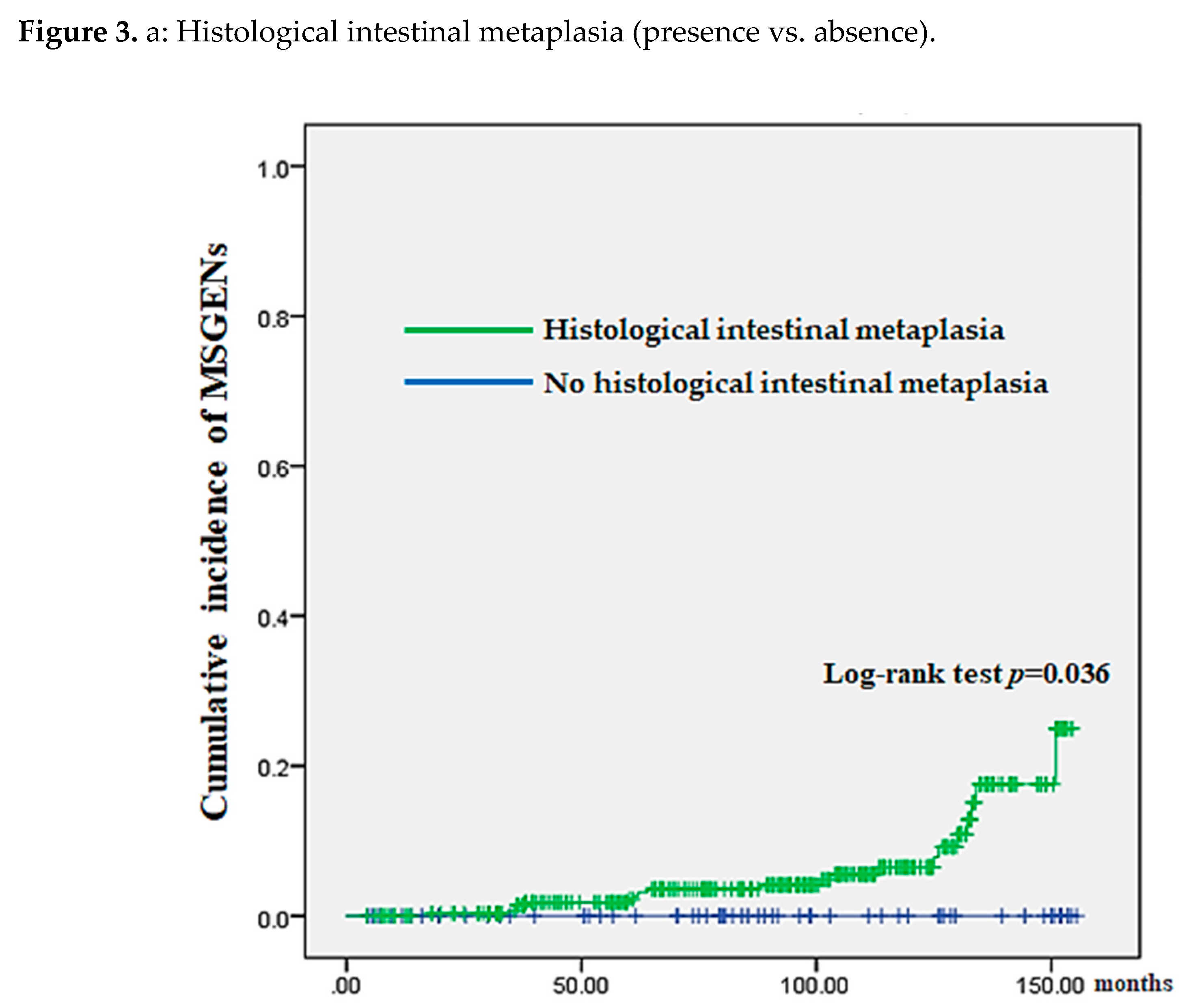
Comparisons after classifying 369 patients into the HIM (yes vs. no) and those into initial neoplasm location (U/M vs. L) revealed a significantly higher cumulative incidence in the HIM (P = 0.036) and INUM groups (P = 0.022) (
Figure 3 (A) and (B), respectively).
We extracted 27 patients in the not-MSGEN group matched to the MSGENs group according to HIM, INUM, age, sex, and histological type (i.e., Mnot-MSGEN group).
Table 3 shows comparison of clinicopathologic factors between Mnot-MSGENs and MSGENs groups. No significant difference between-group difference was found in variables unrelated to
H. pylori eradication. Regarding
H. pylori eradication, there was no significant between-group difference in the age at the time of eradication therapy (P = 0.261). All
H. pylori-positive patients received successful eradication therapy. Compared with the Mnot-MSGENs group, the MSGENs group had a significantly higher number of spontaneous
H. pylori eradication (i.e.,
H. pylori-negative non-eradication patients with severe atrophy) (P = 0.0003).
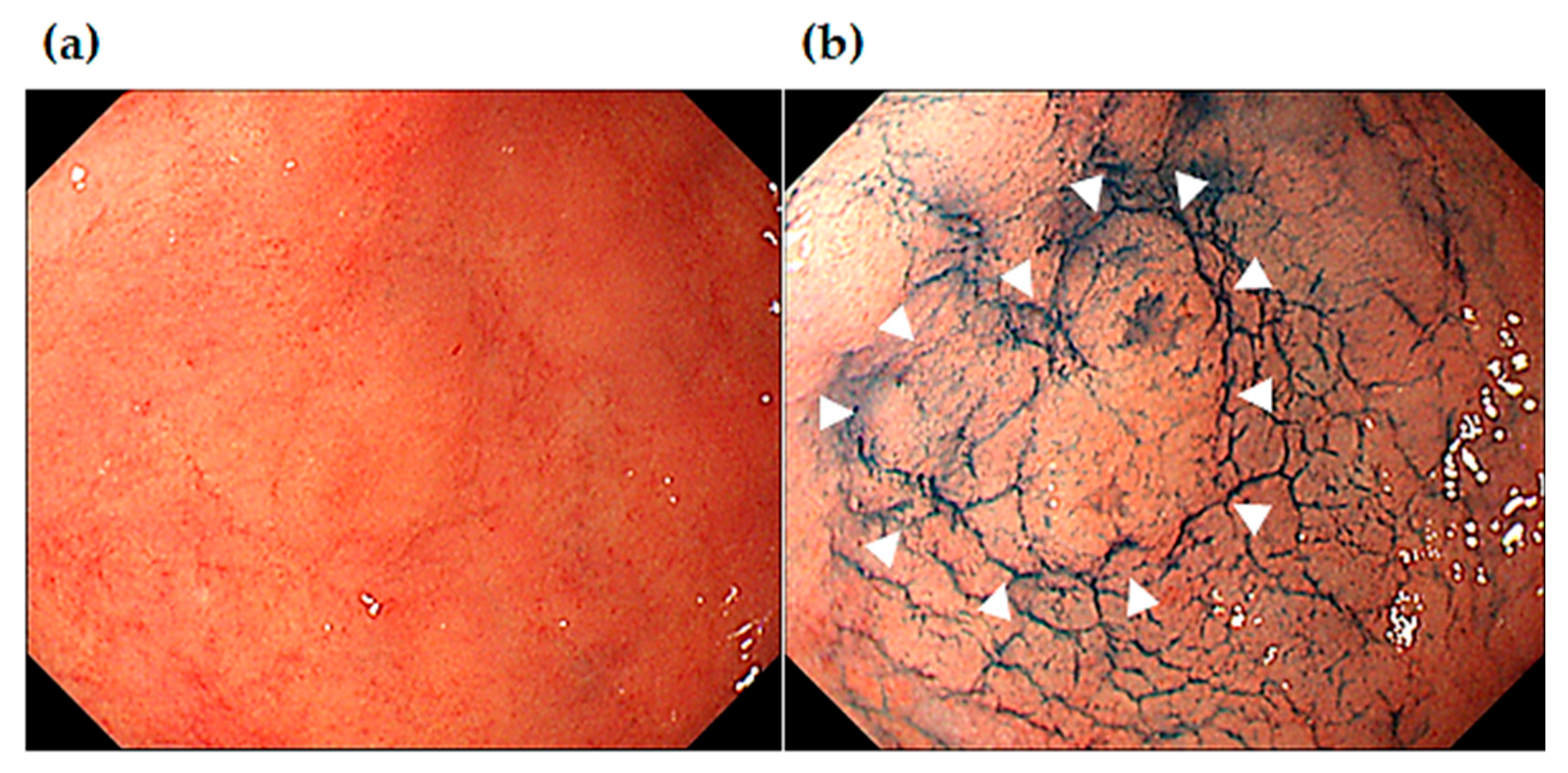
Figure 4,
Figure 5 and
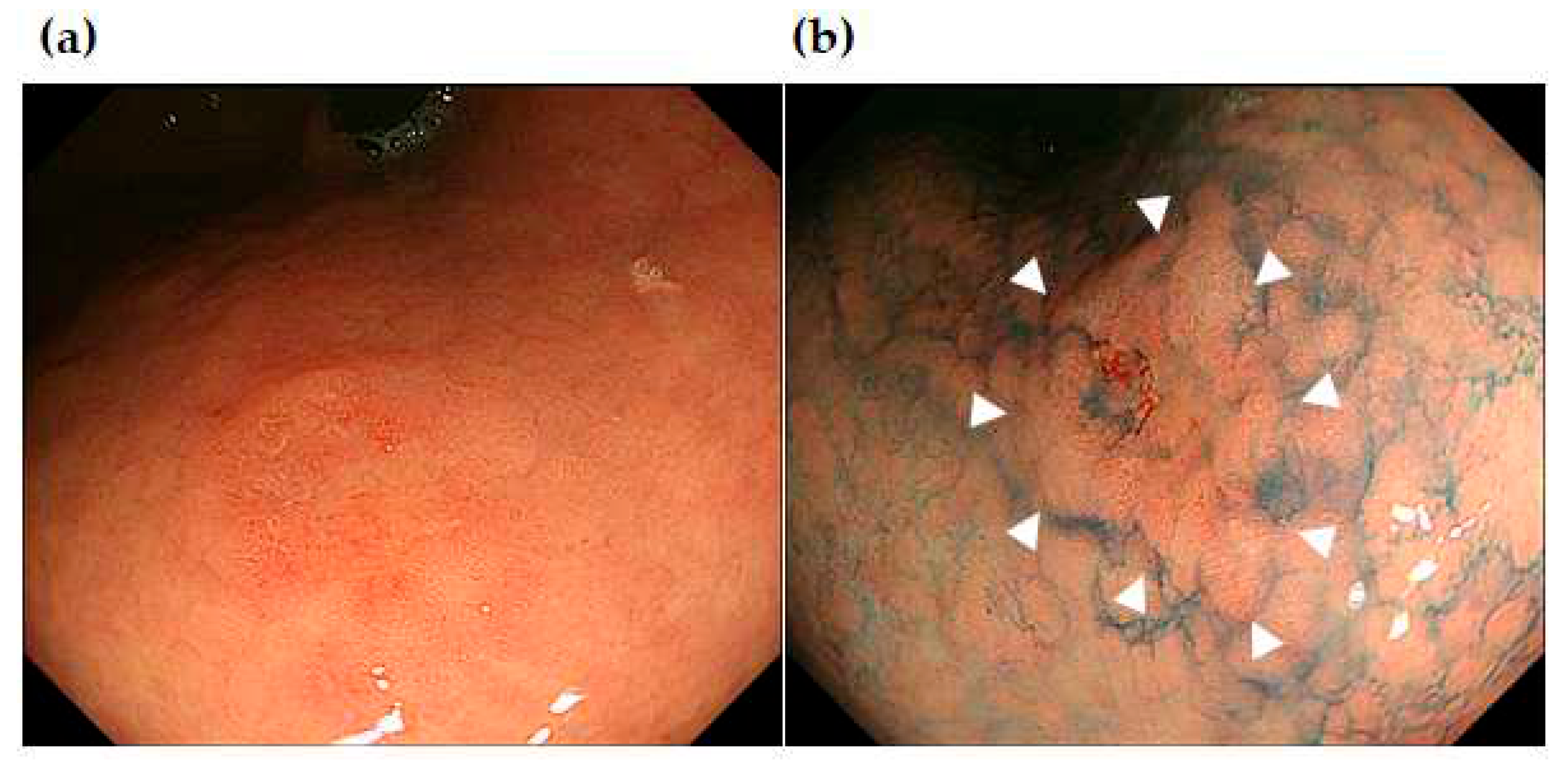
Figure 6 show the representative cases of post-ESD MSGENs. A depressed lesion (0-IIc) measuring 12 mm in diameter and presents a reddish coloration associated with a marginal elevation in the lesser curvature of the upper (U) part (
Figure 4A, B). In the region surrounding the depressed lesion, there are granular mucosal changes (indicated by dished line in
Figure 5A) and atrophic changes over a wide area that is associated with a visible vascular pattern, which are consistent with endoscopic appearance of intestinal metaplasia (
Figure 5A). Histological findings identified in the ESD specimens indicated differentiated adenocarcinoma (tub1) localized within the mucosa; moreover, both vascular invasion and the resection stump were negative, which indicated complete curative resection (
Figure 5B).
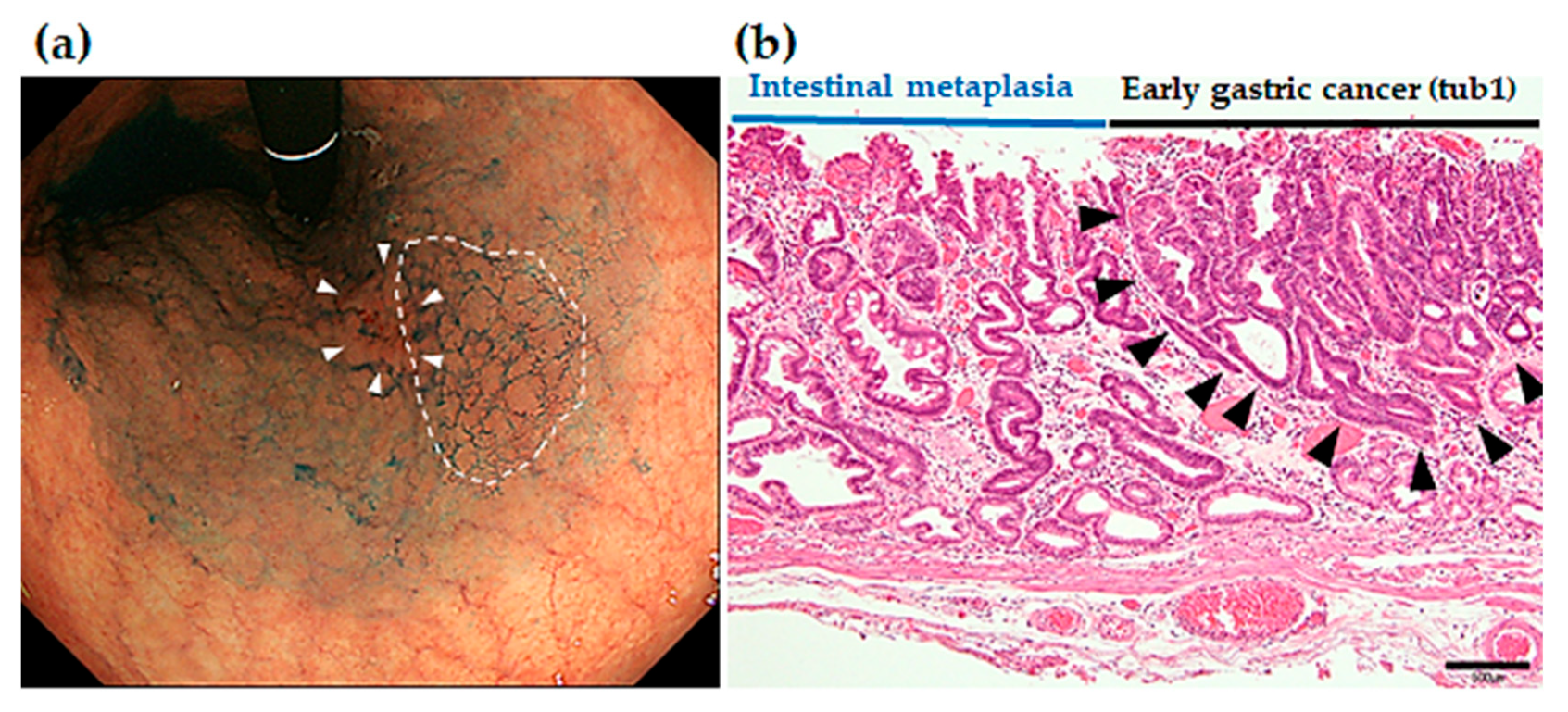
Figure 6 shows endoscopic images of MSGEN developed at 37 months after ESD in the same patient of
Figure 4 and
Figure 5. Endoscopic images shows a slightly elevated (0-IIa) 7-mm lesion in the angle of the lesser curvature of the stomach. Under white light endoscopy observation, it was difficult to identify since it had unclear borders. Indigocarmine chromoendoscopy made the borders clear, which allowed identification of it as a slightly elevated lesion. Histology of the ESD resection sample showed differentiated adenocarcinoma (tub1) confined to the mucosal layer, moreover, it was negative for both vascular invasion and resection margin, which indicated complete curative resection (R0 resection).
Discussion
Our findings suggested that HIM and INUM were independent clinicopathological risk factors for MSGENs in patients who underwent ESD for SGENs. This is because, the patients with the factors have a significantly higher cumulative incidence of MSGENs compared to those without the factors. After group-matching according to these two risk factors, our analysis revealed a significant association between development of MSGENs and the spontaneous eradication.
Our identified risk factors, HIM and INUM, will be influenced by long-term
H. pylori infection [
13]. Because HIM could be caused by decreased gastric motility and in-creased intestinal fluid reflux due to long-term
H. pylori infection [
14]. Although we utilized histological confirmation, intestinal metaplasia can be diagnosed with a high accuracy by using magnification endoscopy or image enhanced endoscopy such as linked color imaging [
15,
16]. These suggest that the degree of risk of developing MSGENs can be estimated by endoscopic inspection.
Regarding the other risk factor of INUM, there will be a strong association with atrophic mucosa, which are the precancerous condition, may develop over a wide area from the middle (M) to the upper (U) part surrounding the initial SGEN lesion. Longer period of
H. pylori infection is related to the greater expansion of mucosal atrophy. Consequently, the greater expansion of atrophic mucosa can bring the higher risk of cancer development [
17]. This suggests that the greater expansion of atrophic mucosa may be associated with the higher risk of MSGENs onset after ESD.
Studies showed that severe atrophic changes (i.e. wide expansion of atrophic mucosa) could also increase the risk of initial gastric cancer onset [
18,
19,
20,
21,
22]. This could be attributed to the chronic inflammation related to
H. pylori infection causing accumulation of various DNA methylation abnormalities, with the degree being correlated to the risk of gastric cancer onset [
23]. Additionally, the mucosa remaining after ESD in patients with gastric cancer who have accumulated DNA methylation abnormalities caused by chronic inflammation could be a high risk of cancer onset. Our finding on HIM/ INUM and previous studies results suggest that severe atrophic changes may be a risk factor for both the initial onset of SGENs and MSGENs.
International research showed that the spontaneous
H. pylori eradication group is at a high risk of initial gastric cancer onset [
24]. This study showed spontaneous
H. pylori eradication was significantly associated with the development of MSGENs after ESD. This is because patients in the spontaneous
H. pylori eradication group have long-term continuous chronic inflammation, which results in highly advanced methylation abnormalities that could contribute to MSGENs onset in this study.
Considering the identified risk factors in this study and accumulation of DNA methylation, H. pylori eradication could contribute to initial gastric cancer as well as reducing a risk for MSGENs onset following ESD. Therefore, H. pylori eradication therapy should be performed in SGEN patients who are proved to positive for H. pylori infection as soon as possible. Earlier eradication in H. pylori-positive patients before the development of atrophic gastritis may facilitate the prevention of post-ESD MSGENs.
According to this study, the median period of onset of metachronous multiple tumors was 95 months; moreover, the longest amount of time was 151 months, which is over 12 years. Since 18 patients underwent ESD for MSGENs during the observation period in this study, MSGENs onset can occur even > 12 years after ESD. This suggests we should perform surveillance endoscopy after ESD over long periods of time 10 years.
We found a significant difference between the locations (U or M/ L) of initial SGENs and MSGENs. Thus, careful observation especially in the L part (the antrum) is needed for detecting MSGENs during the surveillance endoscopy after ESD.
This study has several limitations. First, this was a single-center retrospective study with a relatively small number of patients with MSGENs. Moreover, the number of MSGEN cases were too small to perform matched analysis. Therefore, this study results need to be warranted by further large-scale multicenter study will be needed. Second, since it was difficult to survey the time of H. pylori infection establishment even though this could have been achieved by conducting a follow-up survey; therefore, it was impossible to accurately assess the period of infection. Third, we did not have the biopsy protocol for assessing the extent of atrophic gastritis / HIM in the non-neoplastic gastric mucosa and a pepsinogen test was performed only for part of the patients to estimate the degree of gastric mucosal atrophy. Forth, we did not measure the accumulation of DNA methylation abnormalities. Finally, regarding the secondary endpoint, the statistics by Kaplan-Meier could not be conducted due to the substantial differences in observation periods between Mnot-MSGEN group and MSGEN group.
In conclusion, this study suggested that HIM and INUM were independent clinicopathological risk factors for developing MSGENs after ESD for SGENs. Additionally, spontaneous H. pylori eradication may be a risk factor for MSGENs in the ESD patients who have HIM and INUM. These suggest that we should perform surveillance endoscopy for the ESD patients with the risk factors more strictly than those with no risk factor.
Author Contributions
conception and design; Kenichi Goda; analysis and interpretation of the data; Tsunehiro Suzuki, Akira Kanamori, Manabu Ishikawa, Shintaro Yamaguchi, Tomonori Yoshinaga, Masayuki Kondo, Mimari Kanazawa, Yasuhito Kunogi, Takanao Tanaka, and Keiichiro Abe.;drafting of the article; Tsunehiro Suzuki, Kenichi Goda, and Hidetsugu Yamagishi; critical revision of the article for important intellectual content; Kenichi Goda, Akira Yamamiya, Takeshi Sugaya, Keiichi Tominaga, and Hironori Masuyama; final approval of the article; Kenichi Goda and Atsushi Irisawa.
Acknowledgments
We wish to thank Ishida M, Iizuka S, and Nishimoto M (Department of Gastroenterology, Dokkyo Medical University) for their technical assistance.
Conflicts of Interest
All authors declare that they have no conflicts of interest and received no financial support for the research and authorship.
References
- Freddie B, Jacques F, Isabelle S, Rebbeca L, Lindsey A, Ahmedin J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 2021, 33, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Shiotani A, Haruma K, Graham DY. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J Gastroenterol 2014, 20, 11552–11559. [Google Scholar] [CrossRef] [PubMed]
- Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomized controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc 2012, 75, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol 2014, 12, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Il Ju Choi, Myeong-Cherl Kook, Young-Il Kim, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018, 378, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicenter retrospective cohort study by Osaka University ESD study group. Gut 2013, 62, 1425–1432. [Google Scholar] [CrossRef]
- Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–96. [Google Scholar] [CrossRef]
- Mori G, Nakajima T, Asada K, et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer 2016, 19, 911–918. [Google Scholar] [CrossRef]
- Yamaji Y, Mitsushima T, Ikuma H, et al. Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastriccancer: analysis of 5732 Japanese subjects. Gut 2001, 49, 335–340. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma (the 15th edition); Kanehara-shuppan: Tokyo, 2017. [Google Scholar]
- Kodama M, Murakami K, Okimoto T, et al. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand J Gastroenterol 2013, 48, 1249–1256. [Google Scholar] [CrossRef]
- Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol 2018, 24, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrowband imaging with magnifying endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Ono S, Kato M, Tsuda M, et al. Lavender Color in Linked Color Imaging Enables Noninvasive Detection of Gastric Intestinal Metaplasia. Digestion 2018, 98, 222–230. [Google Scholar] [CrossRef]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995, 19, S37–S43. [Google Scholar]
- Take S, Mizuno M, Ishiki K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol Hepatol 2007, 42, 21–27. [Google Scholar]
- Sakitani, K, Hirata Y, Watabe H, et al. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J. Gastroenterol. Hepatol 2011, 26, 1570–1575. [Google Scholar] [CrossRef]
- Parisa K, Farhad I, Sharmila A, Neal DF, Frain K. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Asada K, Nakajima T, Shimazu T, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut 2015, 64, 388–396. [Google Scholar] [CrossRef]
- Yamaguchi Y, Nagata Y, Hiratsuka R, et al. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - The ABC method. Digestion 2016, 93, 13–18. [Google Scholar] [CrossRef]
- IARC Helicobacter pylori Working Group (2014). Helicobacter pylori Eradication as a strategy for preventing gastric cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).