1. Introduction
The tremendous expansion and differentiation of the cerebral cortex, hereinafter cortex, in Latin tree cover, constitute two important events in the evolution of the mammalian brain. The increase in brain size and complexity opened the door to a spectacular development of cognitive and mental abilities. This expansion during evolution facilitated the addition of microcircuits with a similar basic structure, which increased the complexity of the human brain and contributed to its uniqueness. However, there are even fundamental differences between different species of mammals.
This paper aims to shed light on the fundamental bases, the state of the art and the main lines of research on the evolution and functions of the cerebral cortex. For the conception of the thesis, books and research articles published in prestigious peer-reviewed journals have been used.
The general objective of this paper is to present and analyze the state of the art and some examples of lines of research on the functions of the cerebral cortex and its evolution. This general objective can be broken down into the following sub-objectives:
• Present the main models, parts, and functions of the cortex
• Illustrate the evolution of the brain and cortex size in vertebrates
• Show the most recent contributions in the field of research, such as the role of neurogenesis in the evolution of the connectivity of the cortex , the connectome and its large-scale brain networks.
2. State of the art review
In this section, the layers, areas and functions of the cerebral cortex , the evolution of the cortex in vertebrates, and the evolutionary changes in the size of the cortex in mammals will be reviewed.
2.1. Layers, areas and functions of the cerebral cortex
The cortex is the external part of the brain full of ridges and folds that allows the integration of motor and sensory information . The cortex has an area of about 2,500 cm 2 in a thickness of 1.5-3 mm and presents a large number of folds, which are a natural solution to the enormous cortical structure within a skull small enough to pass through the birth canal (Kolb and Wishaw , 2006).
In general, primates have a cortex more developed than the rest of the animals. Thanks to the cortex , humans can perceive the outside world, imagine, think, rationalize, and make decisions, among other functions. The human cerebral cortex contains between 14 and 16 billion neurons arranged in horizontal layers or sheets. These are organized in horizontal cortical layers and radially in cortical columns and minicolumns. Although cortical neurons vary enormously in size and shape, there are two main types: pyramidal and granular cells (Purves et al, 2012).
The cortex can be divided into two large areas: the allocortex. and the neocortex. The allocortex only occupies 5-10% of the human brain, compared to the neocortex, which occupies 90-95%. The latter appeared with the evolution of the mammalian brain and is therefore called neocortex, from the Greek neos, meaning new.
The allocortex encompasses the olfactory structures, such as the olfactory bulbs or the piriform cortex (palecortex), and the cortex of the hippocampal formation (archicortex), see
Figure 1. The neocortex underwent great development with the appearance of mammals, while the allocortex is older and predominant in lower vertebrates. Higher mental functions are associated with the activity of the cortex and its influence on other structures. It is accepted that the neocortex evolved from two more primitive forms: the achicortex (hippocampus) and the paleocortex (olfactory bulbs). The pattern of connectivity and cytoarchitecture of the different neocortical regions is based on these two ancestral forms. According to these concepts, any sector of the neocortex has a dual origin, on the one hand, archicortical (hippocampal or the “where?” pathway) and, on the other, paleocortical (olfactory or the “what?” pathway) (Pimenta, 2004).
The types of cortexes according to a structural and phylogenetic perspective can be divided into the following four classes, the first three corresponding to the allocortex:
-
1.
Archicortex, archipalium or limbic lobe cortex: it is evolutionarily the oldest part of the cortex and its function is related to primitive aspects such as emotions or memory. In more primitive species, such as fish, the archicortex makes up most of the brain. In humans, the archicortex forms the three cortical layers of the hippocampus (Purves et al, 2012).
-
2.
Paleocortex, piriform cortex or lateral pallium: it is the cortex of the olfactory bulb, corresponding to the termination areas of the olfactory pathways and which phylogenetically occupies an intermediate position between the archicortex and the neocortex. In humans, it has the function of facing situations in a pre-rational, intuitive way, shaped by the environment in the brain structures throughout evolution.
-
3.
Perialocortex forms in transitional areas where either of the other two subtypes of allocortex border the neocortex. The perialocortex is thus subdivided into the peripaleocortex periarchicocortex . It should be noted that the perialocortex It transitions to the isocortex through the proisocortex , calling the sum of both mesocortexes.
-
4.
Isocortex or neocortex (dorsal pallium or neopallium): more evolved areas of the cortex that constitute the neuronal mantle or pallium, which covers the lobes. It is developed in primates and highly developed in the genus homo. It is a set of layers of the mammalian cortex involved in higher brain functions, such as sensory perception, cognition, generation of motor commands, reasoning, and language.
Indeed, the neocortex is thought to be the ratio of the size of the neocortex to the rest of the brain. A high proportion of neocortex is correlated with a few social variables, such as group size and the complexity of social mating behaviors (Semendeferi et al, 2002).
On the other hand,
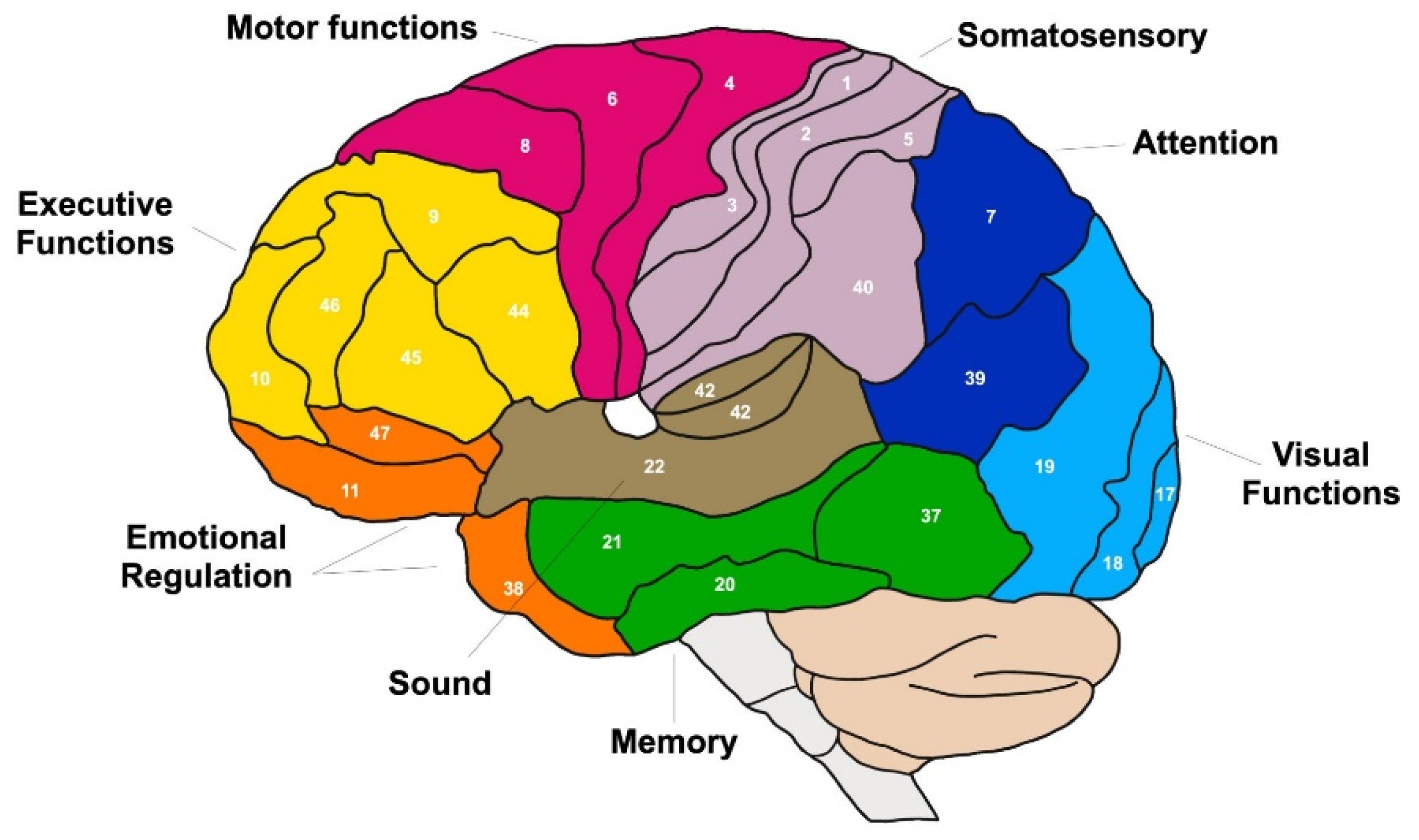
Figure 2 shows the Brodmann areas and their relationship with the functions of the cortex based on the cytoarchitectonic organization of the neurons of the cortex. In 1909, Brodmann published maps of the cortical areas of humans, primates, and others, along with many other discoveries about cell types and the laminar organization of the cortex in mammals. The same Brodmann area number in different species does not necessarily indicate homologous areas (Kolb and Wishaw , 2006).
Brodmann areas have been debated, refined, and renamed for nearly a century and remain the most well-known and cited cytoarchitectural organization of the human cortex. Since then, many of the Brodmann areas that were defined based on neuronal organization alone have been linked to different cortical functions. For example, areas 1, 2, and 3 make up the primary somatosensory cortex; area 4, the primary motor cortex; area 17, the primary visual cortex; and areas 41 and 42 correspond to the primary auditory cortex. Higher-order functions of cortical association areas are also localized to the same Brodmann areas using neurophysiology, functional imaging, and other methods, for example, localization of Broca's areas, speech, and language areas on the left. and 45. However, functional imaging only approximately identifies the localization of brain activations in Brodmann's areas, since the actual boundaries of an individual brain require its histological examination (Kolb and Wishaw, 2006).
The neocortex is formed by a thin layer of nervous tissue made up of neurons and neuronal connections, which surrounds the hemispheres, and is made up of six horizontal layers of cells, called gray matter (Purves et al, 2012). Today we know that the different areas of the cortex have different functionalities, and three large areas of cortical regions can be divided:
Sensory areas: they receive sensory information from nuclei of the thalamus from the different senses, such as sight, smell, smell, taste, etc. These areas can be divided into the primary sensory area (has direct connections with peripheral sensory receptors) and the secondary sensory area (receives sensory information from the primary area and from lower areas of the brain). These areas aim to create patterns of recognition and behavior based on the assimilation of sensory information, and can be classified into the following areas: somatosensory, visual, olfactory, auditory and gustatory.
-
Motor areas: in these areas descending motor signals originate from the cortex to the motor neurons of the trunk and spinal cord. The motor areas of the cortex are involved in the regulation and initiation of voluntary movement. These areas are primarily located within the frontal lobes and include the primary motor cortex, premotor cortex, and supplementary cortex:
- o
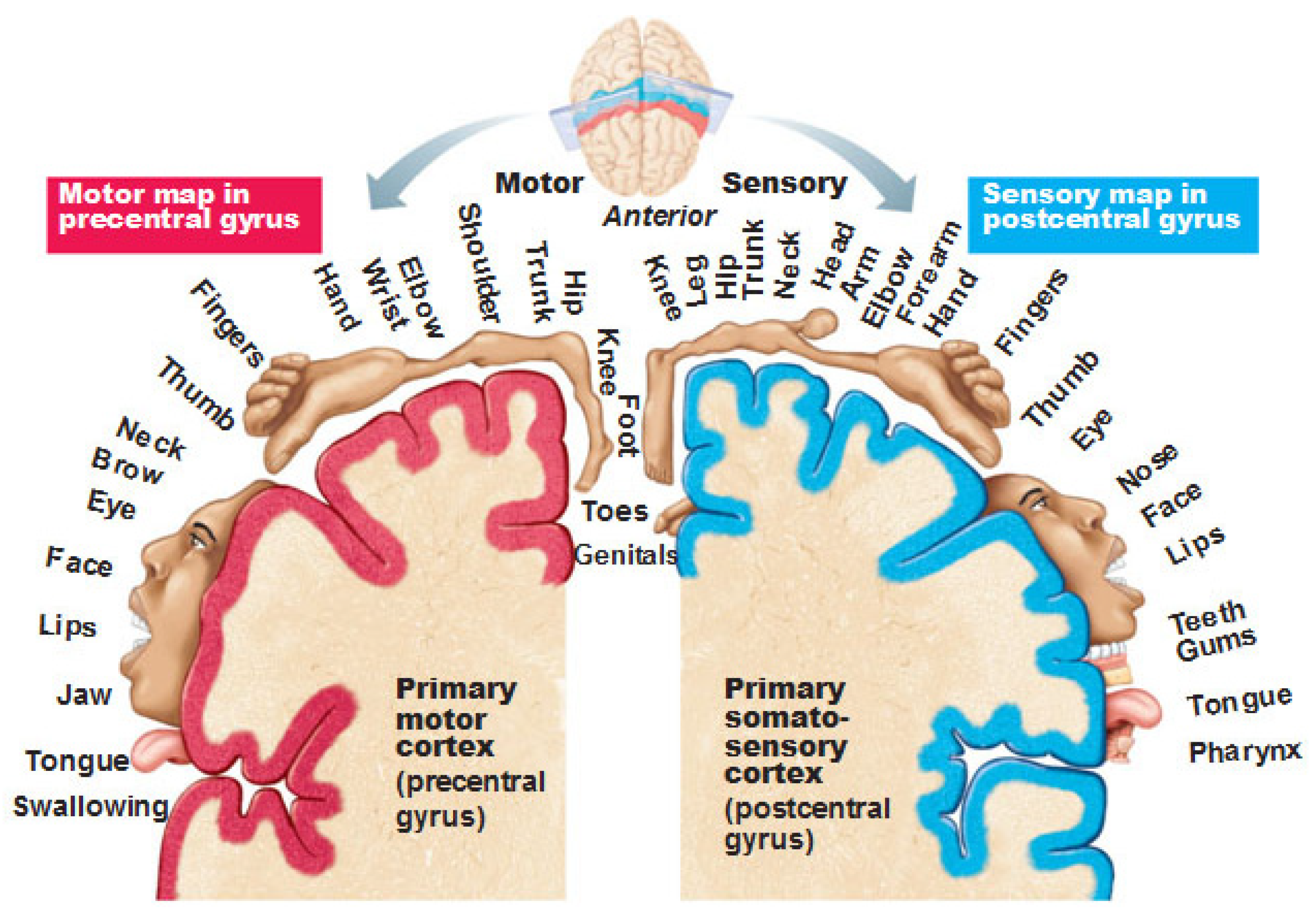
The primary motor cortex is associated with the coordination and initiation of motor movements. Each cerebral hemisphere of the primary motor cortex contains a motor-related representation of the opposite side of the body. There is a representational map of the body with the primary motor cortex, or motor homunculus , see
Figure 3.
- o
The premotor cortex is involved in preparing and executing limb movements, as well as using information from other regions of the cortex to select appropriate movements. The premotor cortex is also necessary for learning, especially by imitation, and social cognition, specifically empathy.
- o
The supplementary cortex is responsible for planning complex movements and contributes to movement control.
-
Areas of association: are those that make complex and abstract mental functions possible, such as memory and cognition mechanisms, emotional mastery, reasoning, will, and development of intelligence and personality. The association areas extend throughout the cerebral cortex in all four lobes. These association areas can also form connections with sensory and motor areas to make meaning and organize information in these areas.
- o
Association areas within the frontal lobes are involved in key processes such as planning, thinking, and feeling. These areas also play a role in personality and control emotional behaviors.
- o
Association areas within the parietal lobe are involved in spatial skills such as spatial awareness and reasoning, as well as being responsible for paying attention to visual stimuli in the environment.
- o
In the temporal lobes, association areas primarily function in memory processes, such as helping to process episodic and procedural memories. These areas also communicate with other lobes of the cortex so that they can complete memory-related processes.
- o
The association areas of the occipital lobe help facilitate the retention of memories associated with images and allow us to think visually. They communicate with other lobes of the cortex to assimilate visual information with memories, sounds and language to understand visual stimuli.
2.2. Evolution of the brain in vertebrates
The dimensions of the brain are different in reptiles, birds and mammals due mainly to the differences in size and complexity of the cortex. The first reptiles to appear in the fossil record, called anapsids, probably gave rise to all modern reptiles, including birds (Rieppel , 1999).
Another group of primitive reptiles, called synapsids, were the ancestors of the first mammals. The relationship of synapsids to stem reptiles is quite uncertain. Interestingly, the cranial cavity of both anapsids and synapsids was small and narrow, suggesting that they had a small brain ( Gauthier et al, 1989) . In both lines, primitive reptiles and mammal-like reptiles, brain expansion was a relatively late event. In the mammalian line, the brain enlarged only when the first true mammals appeared, and it was more or less simultaneous with the origin of the middle ear (Wang et al, 2001).
Therefore, the expansion of the mammalian brain occurred as a very rapid evolutionary step, and relatively late in the history of these animals. This is important because it indicates that brain enlargement may have occurred independently in the line leading to reptiles and in the line leading to mammals. Thus, an important question, from the perspective of comparative neuroanatomy, is to what extent it is correct to consider mammals as derivatives of reptiles as we know them today, since this could result in falling into the misconception of the scala naturae.
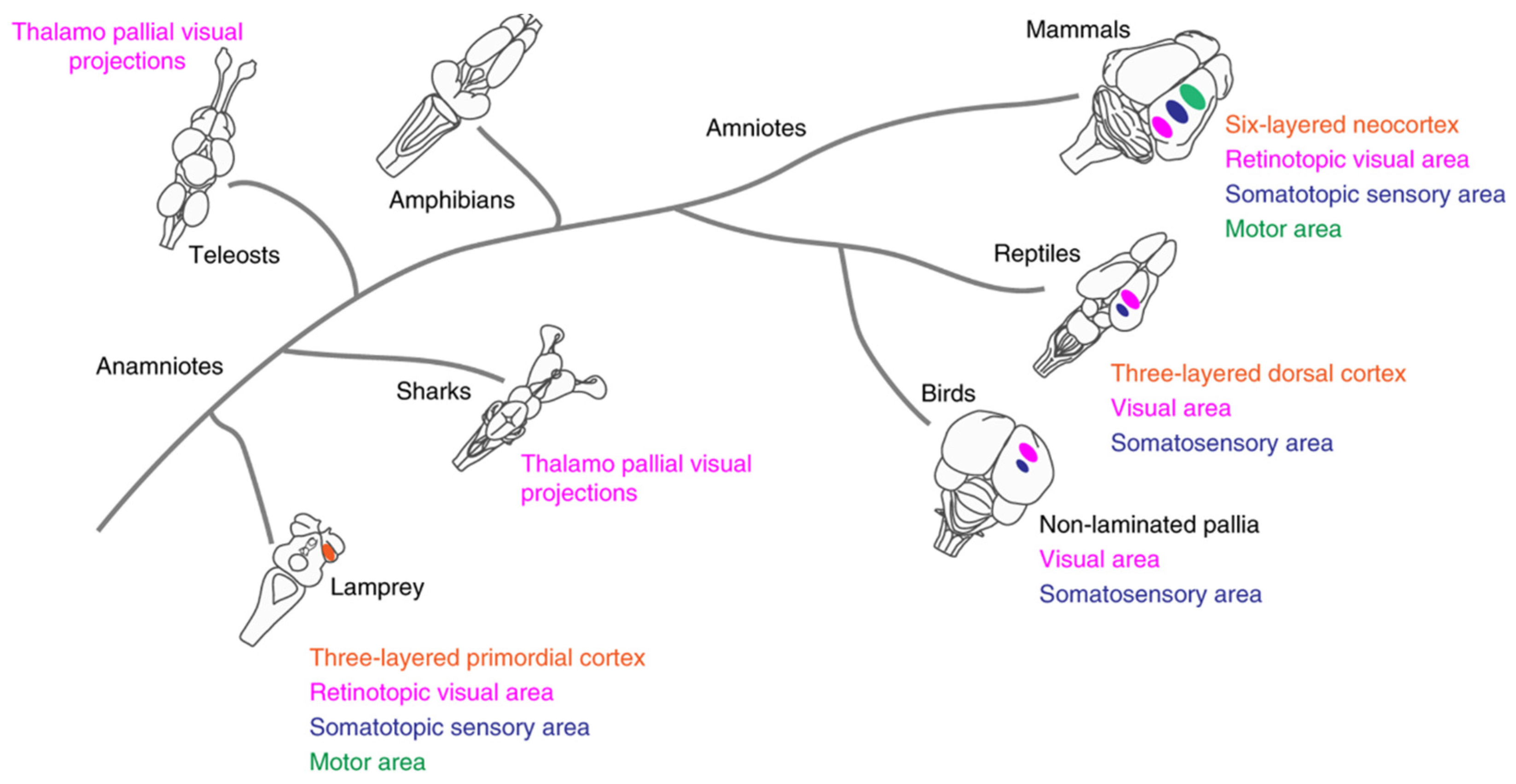
The expansion of the cerebral cortex began with the arrival of amphibians to the mainland, in the Cambrian period 500 million years ago with the appearance of amniotes. A study published by Suryanarayana et al (2020) analyzed the lamprey brain, providing new insights into how the human brain evolved. Research on the lamprey brain has made it possible to delay the birth of the cortex in time from about 300 million years to more than 500 million years ago, thus providing new knowledge about the evolution of the brain. It had long been believed that most of the evolution of the forebrain took place largely in mammals and that the brains of groups of simpler pre-mammalian animals, such as fish and amphibians, lacked a functional cortex.
In the study, the researchers show that even the lamprey, which existed hundreds of millions of years before mammals, has a detailed plan for the development of the cortex , basal ganglia, and dopaminergic system; that is, all the vital ingredients of integrative brain function. The researchers also discovered that the lamprey cortex also has sensory and motor areas, see
Figure 4 (Suryanarayana et al, 2020).
2.3. Evolutionary changes in the size of the cortex in mammals
There are several research questions regarding the origin of the mammalian brain (Aboitiz et al, 2002). The first tries to find out in which region in the brain of a reptile-like ancestor the isocortex originated. Until now there have been two alternative hypotheses, one suggesting that most or all of the isocortex originated from the dorsal pallium, and the other suggesting that part of the isocortex originated from a ventral pallial component . The latter implies that in isocortical development a massive tangential migration of cells from the ventral pallium to the dorsal pallium takes place, something that has not yet been demonstrated (Aboitiz et al, 2002).
The second question concerns the origin of the multi-layered structure of the isocortex from a single-layered structure such as that observed in the cortex of modern reptiles, that is, the origin of the six-layered isocortex from a primitive cortex of three hats. The superficial isocortical layers can be considered an evolutionary acquisition of the mammalian brain because no equivalent structures can be found in the reptilian brain. Furthermore, a characteristic of the isocortex is that it develops according to an inside-out neurogenetic gradient, in which late-producing cells migrate through layers of early-producing cells (Smaers et al, 2021).
Relative brain size has long been considered a reflection of cognitive abilities and has played a critical role in the development of central theories in the life sciences. However, the notion that relative brain size validly represents brain size selection is based on the untested assumptions that brain -body allometry is restricted to a stable scaling relationship between species and that any deviation from this slope is due to brain size selection.
In a recent study, results reveal that mammals with larger brains achieved relatively large brain sizes by highly divergent pathways (Smaers et al, 2021). In the study, five groups of mammals, elephants, great apes, hominids, toothed whales, and delphinids reached their position at the top of the brain-body plane (see
Figure 5) following an unexpectedly diverse range of trajectories. The phylogenetic generalized least squares ( PGLS ) technique was used ( Dechmann and Safi, 2009).
Elephants represent the simplest case, as they evolved directly from the ancestral grade of mammals and achieved large relative brain size by greatly increasing their body size while increasing brain size even more rapidly. In toothed whales and delphinids, the relative size of the brain gradually increased.
The first intercept change occurred in stem fereungulates, which follow a similar, though less pronounced, trajectory to elephants, increasing brain size more than body size. This was followed by a change in intercept in stem-toothed whales, which decreased brain and body size relative to stem cetaceans, with body size decreasing more rapidly than brain size. Delphinids show a third intersecting change driven by decreased body size and increased brain size relative to other toothed whales (Smaers et al, 2021).
The evolutionary trajectory of great apes and hominids was the most complex, beginning with two consecutive downward changes in slope, as brain and body size increased, in apes and great apes, followed by a more marked increase in slope. Delphinids and hominids, which converge at the vertex of brain-body space, are the only two grades with negative brain-body correlations, that is, brain size increased while body size decreased (Smaers et al, 2021).
The five groups of mammals with the largest brain sizes may have originated in the Neogene. This great diversity in evolutionary trajectories and late attainment of maximum relative brain size parallels patterns in birds in which taxa with the largest brains (parrots and corvids) achieved large brain sizes during the Neogene through very different trajectories (Ksepka et al, 2020). These parallel patterns between birds and mammals suggest that, similar to the Paleogene-Neogene transition, they may have created conditions conducive to niche expansions that affected the evolution of brain size.
In summary, in this section the areas and functions of the cerebral cortex, its evolution in vertebrates, and its size evolution in mammals were reviewed. However, these models are sometimes partially biased given the compartmentalized view of different brain areas.
3. Recent contributions
In this section, the most recent contributions in the field of research will be reviewed, including neurogenesis and its role in the evolutionary leap of the neocortex, and the evolution of the neuronal connectivity of the cortex. Finally, the main large-scale brain models of the human connectome are presented, which aims to give a more holistic vision by analyzing the functions of these neural networks.
3.1. Neurogenesis in the neocortex as a key to the evolutionary leap
Cortex size differs dramatically between reptiles, birds, and mammals due to developmental differences in neuron production. In mammals, signaling pathways that regulate neurogenesis have been identified, but the genetic differences behind their evolution between amniotes are still unknown.
Cárdenas et al (2018) showed that direct neurogenesis of radial glia cells, with limited production of neurons, dominates the paleocortex of birds, reptiles, and mammals, while, in the neocortex of recent mammals, most of neurogenesis is indirect through basal progenitors. The study identifies modulation in the activity levels of conserved signaling pathways as a primary mechanism driving the expansion and increase in complexity of the mammalian neocortex during amniote evolution. In this same work it is explained that there was a change that allowed the final number of neurons to multiply, in which a protein called Robo was involved, and this also allowed new types of neurons to appear. Thus, it was possible to go from a relatively simple three-layer cortex, like reptiles, to six layers, like mammals.
In fact, Robo and Dll1 are two genes that have long been known to be involved in brain development. The contribution of this work (Cárdenas et al, 2018) was to discover that Robo collaborates in the development of connections between neurons, guiding nerve fibers within the brain, but that it is not involved in the formation of new neurons. However, the Delta gene is involved in the processes of neurogenesis, which consists of a stem cell dividing and two cells emerging from it: one of them will be a neuron and the other may or may not continue as a stem cell. The Dll1 gene is involved in the decisions that cause a neuron to be born or the two cells to remain stem cells. When the stem cell gives rise to a neuron, thereby consuming itself, this process is called direct neurogenesis. When an intermediate stem cell is formed between them, with the same number of stem cells we will have twice as many neurons, which is called indirect neurogenesis. Thus, at a given moment in evolution, more neurons were generated gradually and there are species that represent different phases of this evolution.
For example, in reptiles, direct neurogenesis is observed, meaning that stem cells generate only neurons and do not increase their number. Consequently, the number of stem cells and neurons is small. However, in birds we begin to see a small number of intermediate stem cells. In mammals, very little direct neurogenesis occurs and almost all that the initial stem cell does is generate intermediate cells that go on to generate many more neurons. This would explain the small brain of reptiles, the larger brain of birds, and the larger brains of mammals. The Dll1 gene participated in the expansion of these brains, while the Robo gene is very important for the development of nerve connections, although it has been discovered to have more functions. Depending on the dose of Robo protein generated by this gene in a stem cell, neurogenesis will be direct or indirect, and if there is little Robo, it will be indirect (Villalba et al, 2021).
Once an animal is born there is no more neurogenesis in the cortex. Different research groups around the world have conclude that there is neurogenesis when we are adults (Abdel- Mannan et al, 2008), but whether there is depends on each species. In previous works it was already observed that birds with a new song each spring have what is called spring neurogenesis (Nottebohm, 1989). In mammals, including humans, and adult reptiles, there is also, although in smaller quantities, neurogenesis in areas other than the cortex.
3.2. Evolution of neuronal connectivity in the cortex
In the neocortex, billions of neurons are interconnected through a massive but highly organized network of axonal and dendritic wiring. This wiring allows nearby and distant neurons to coordinate their responses to external stimulation. Specific patterns of cortical activity generated within this network have been found to correlate with cognitive and perceptual functions (Hofman, 2014), (Chin et al, 2022).
Understanding the organizing principles of cortical wiring, therefore, represents a central goal for explaining human cognition and perception in health and disease. However, despite more than a century of research efforts, the organizing principles and function of cortical connectivity are not well understood (Budd and Kisvárday, 2013).
Studies using diffusion tensor imaging (DTI) have shown that not only are neurons in the neocortex highly organized structurally and functionally, but this is also true for the wiring of the brain (Wedeen et al., 2012). The interconnecting white matter axonal pathways are not a mass of tangled cables, as long thought, but rather form a continuous rectilinear three-dimensional grid with the three major axes of development. Structural connectivity networks, as defined by ITD, have identified a common hub in the medial parietal cortex of humans, chimpanzees, and macaque monkeys. However, the apparent lack of medial prefrontal centers in humans that are present in chimpanzees and macaque monkeys, together with evidence of increased gyrification in the human prefrontal cortex, suggests important evolutionary changes in human prefrontal cortex connectivity. In fact, human brains have relatively more association cortex compared to non-human primate brains (Rilling, 2014).
Although the cortex is not the only brain structure that was selected in evolution to expand, because of increasing environmental pressure for more sophisticated cognitive abilities, it has played a key role in the evolution of information processing in the mammalian brain.
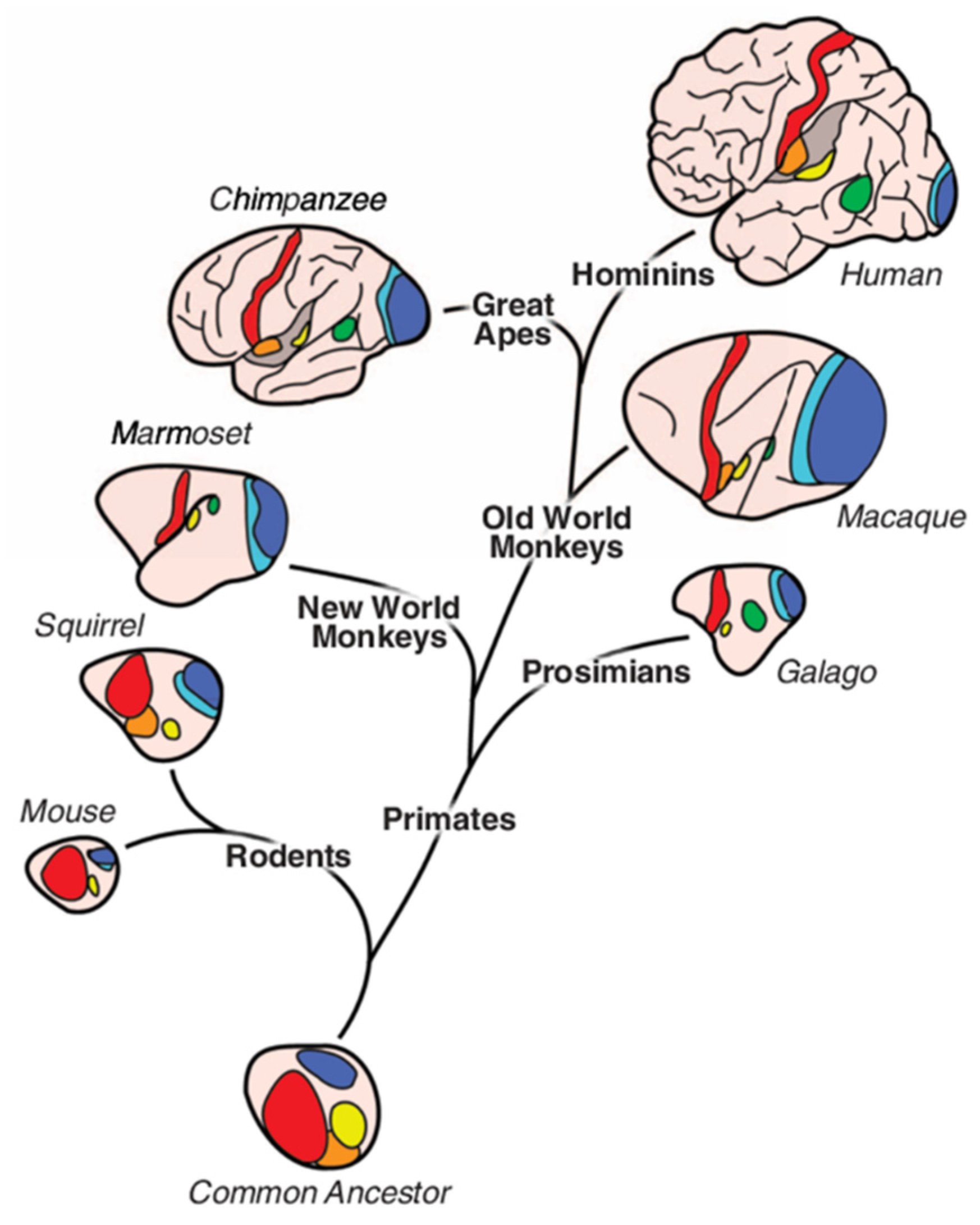
Extant primates possess a greater percentage of the cortical mantle that lies between the primary and secondary sensory systems relative to other placental mammals. In
Figure 6, primates are shown in relation to rodents and the theorized organization of cortical fields in the common ancestor of the placenta (Krubitzer and Kahn, 2003).
The primate cortex, as we have seen, has evolved from a set of underlying structures that restrict its size and the amount of information it can store and process. If an organism's ability to process information about its environment is a driving force behind evolution, then the more information a system, such as the brain, receives, and the faster it can process this information, the more adequately it can respond to environmental challenges. and the better their chances of survival will be (Hofman, 2003). However, the increase in brain and cortical complexity and volume can lead to an increase in sensory processing time.
Therefore, the limit of any intelligent system lies in its ability to process and integrate large amounts of sensory information and compare these signals with as many memory states as possible, and all this in minimal time. It implies that the functional capacity of a neuronal structure is inherently limited by its neuronal architecture and signal processing time (Changizi and Shimojo, 2005).
Processing or transfer of information across cortical regions, rather than within regions, in large-brained primates can only be achieved by reducing the length and number of interconnected axons to set limits on axonal mass. The number of interconnected fibers can be reduced, as we have seen, by compartmentalizing neurons into modular circuits in which each module, containing many neurons, is connected to its neural environment by a small number of axons. The length of interconnected fibers can be reduced by folding the cortical surface and thus shortening the radial and tangential distances between brain regions. Local wiring, preferential connectivity between nearby areas of the cortex, is a simple strategy that helps keep cortical connections short.
In principle, efficient cortical folding could further reduce connection length, which in turn reduces white matter volume and conduction times (Buzsáki et al., 2013). Thus, cortex development appears to coordinate folding with connectivity in a way that could produce smaller, faster brains. It is assumed that an increase in the number of neurons is the basis for the improvement of cognitive abilities in the evolution of the brain. It is then expected that the evolution of human cognition has accompanied a prolongation of the net neural processing time due to the accumulation of processing time of individual neurons over an expanded number of neurons. A hypothesis then arises about the evolution of the human brain: the increase in the number of cortical neurons expanded the time scale of sensory cortical processing, the benefits of which outweighed the disadvantage of slow cognition and reactions (Itoh et al, 2022).
In a study (Hofman , 2014) it was argued that with a brain of about 3,500 cm3, corresponding to a brain volume two or three times larger than that of modern man, the brain would reach maximum processing capacity. According to Hofman (2014), the more the brain grows beyond this critical size, the less efficient it will become, limiting any improvement in cognitive power. This hypothesis, however, assumes of linear growth without considering the possibilities of evolution towards multiscale brain networks (Zheng et al, 2020).
In this latest work we have studied what the multi-scalar spatial organization of the human brain is like, and it has been seen that, just as a geometric network model is described, the layers at different resolutions are self-similar, that is, even if we change scale, the geometric structure and connectivity of the layers remains the same. The results proved that the same principles organize brain connectivity at different scales and lead to efficient decentralized communication (Zheng et al, 2020).
3.3. Large-scale brain networks
Large-scale brain networks, or intrinsic brain networks, are collections of widespread brain regions that show functional connectivity through statistical analysis of the BOLD signal from fMRI and other recording methods such as EEG, PET, and MEG. An emerging paradigm in neuroscience is that cognitive tasks are performed not by individual brain regions working in isolation, but by networks consisting of several discrete brain regions that are said to be functionally connected. Functional connectivity networks can be found using algorithms such as cluster analysis, spatial independent component analysis (ICA), among others (Riedl et al, 2016).
The set of identified brain areas that are linked to each other in a large-scale network varies depending on cognitive function. When the cognitive state is not explicit (i.e., the subject is at rest), the large-scale brain network is a resting-state network. As a physical system with graphical properties, a large-scale brain network has both nodes and edges and cannot be identified simply by the coactivation of brain areas. In recent decades, the analysis of brain networks became feasible thanks to advances in imaging techniques, as well as new tools from graph theory and dynamical systems (Bressler and Menon, 2010). Disruptions in the activity of various networks have been linked to neuropsychiatric disorders such as depression, Alzheimer's, autism spectrum disorder, schizophrenia, attention deficit hyperactivity disorder (ADHD), and bipolar disorder (Menon 2011).
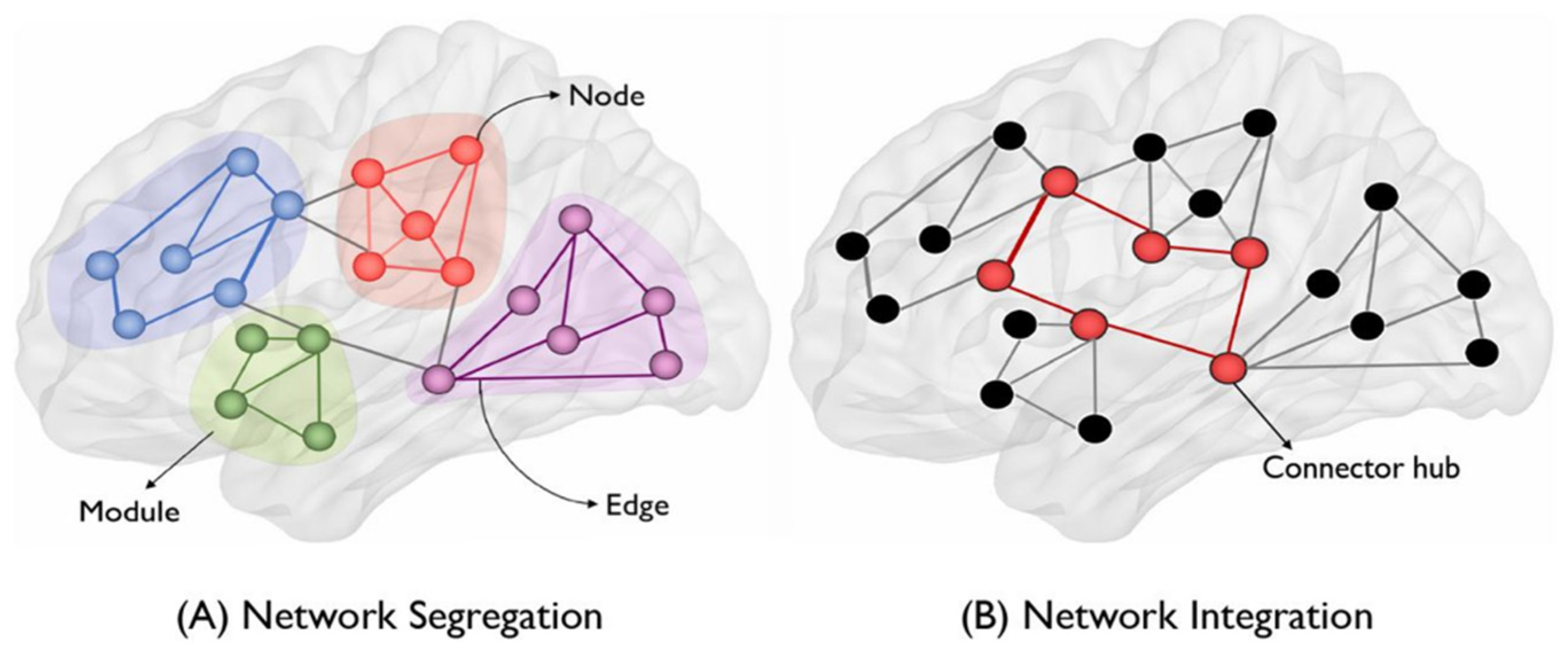
Although the knowledge of the parts of the brain is enormous, the absence of an interpretation or theory that unifies them is also enormous. In this sense, connectomics, using graph theory, identifies brain centers and is dedicated to creating the connectome, that is, a complete map of neuronal connections in the brain that can be considered its “wiring diagram.”
Figure 7 illustrates the basic concepts of graph theory in the context of brain networks, each anatomical region of interest is a node, while the connection between nodes is called an edge. Color shaded regions correspond to modules that are groups of interconnected nodes but have fewer connections to other modules. On the left of
Figure 7 is the concept of network segregation presented as a network of multiple modules (or segregated subnetworks), while on the right is illustrated network integration as a group of interconnected distant regions. by long-range connections through concentrators of connectors called centers or ' hubs ' (nodes with a high degree of connectivity), see Shenoy et al (2021).
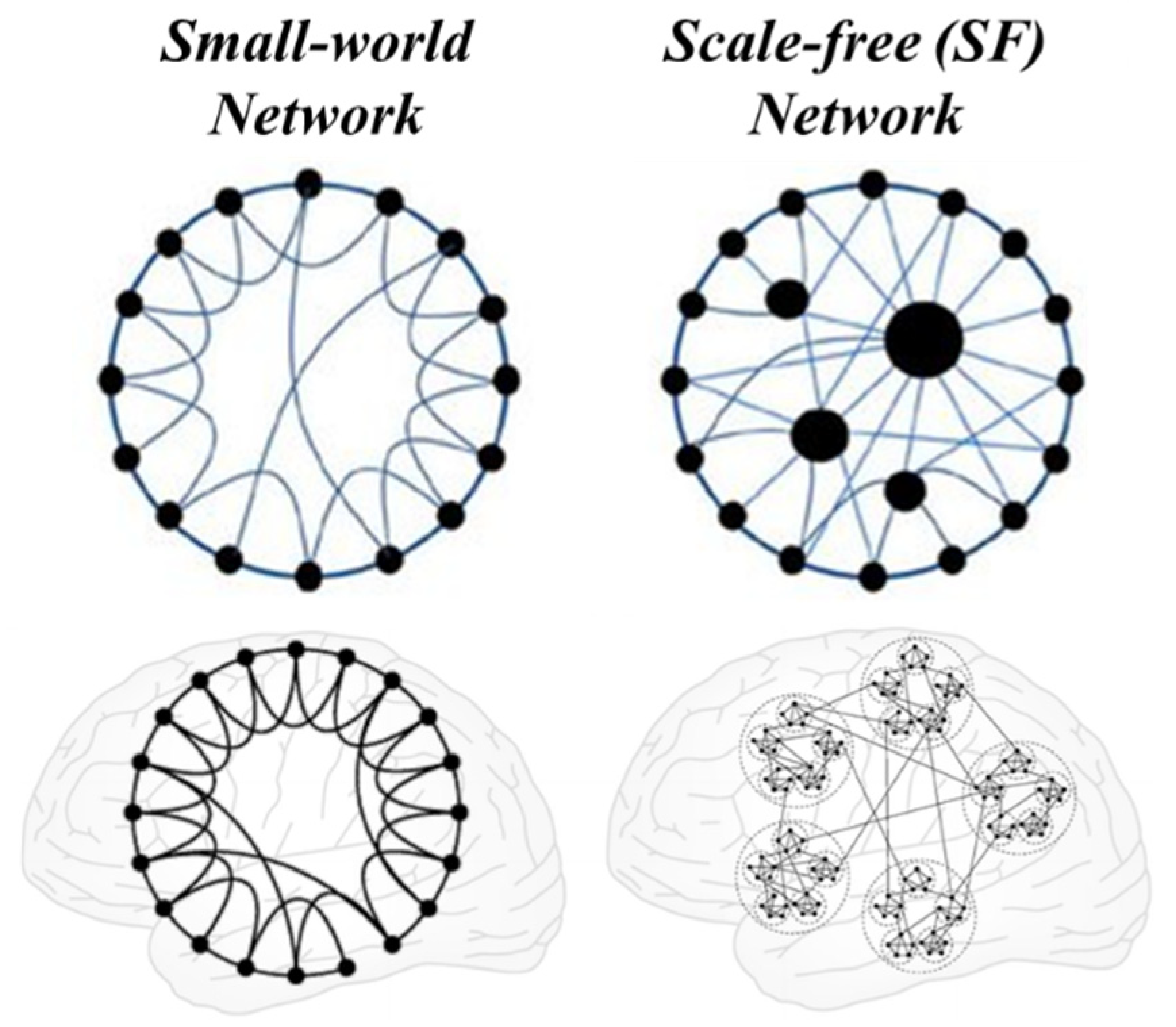
From a theoretical point of view, it is plausible that the brain has evolved to minimize the energetic costs of information processing and therefore maximize efficiency and redirect its function in an adaptive manner, that is, resilience. A brain network with such characteristics, when characterized by graphical analysis, would present small-world and scale-free network properties (Forlim et al, 2019).
Small-world networks can be derived by partially random rewiring of regular networks, resulting in high clustering and relatively short path lengths, see
Figure 8. Small-world is thought to be a crucial aspect of brain organization. efficient that confers significant advantages in signal processing. Consequently, small-world organization is considered essential for healthy brain function. Alterations in small-world characteristics are observed in groups of patients with Alzheimer's disease, autism or schizophrenia (Hilgetag and Goulas, 2016).
In addition to small-world properties, the brain exhibits properties of scale-free networks, which is another type of complex network. In a scale-free network, some nodes, which we will call centers or hubs, are highly connected, that is, they have a large number of links to other nodes, although the degree of connection of almost all nodes is quite low. Random lesions of scale-free networks have negligible effects, but lesions of centers or hubs are catastrophic. Examples in humans are coma and Parkinson's disease caused by small lesions in the brain stem (Freeman and Breakspear, 2007).
Because brain networks can be identified at several different resolutions and with several different neurobiological properties, there is no universal atlas of brain networks that fits all circumstances. While recognizing this issue, Uddin et al (2019) proposed that the following six networks should be defined as core networks based on converging evidence from multiple studies to facilitate communication between researchers:
The default neural network (medial frontoparietal) is the most researched network and is active when a person is awake and at rest. It is preferentially activated when people focus on internally oriented tasks, such as daydreaming, visualizing the future, or retrieving memories. It is negatively correlated with brain systems that focus on external visual cues. Evidence has pointed to disruptions in the RNA of people with Alzheimer's disease and autism spectrum disorder (Buckner et al, 2008).
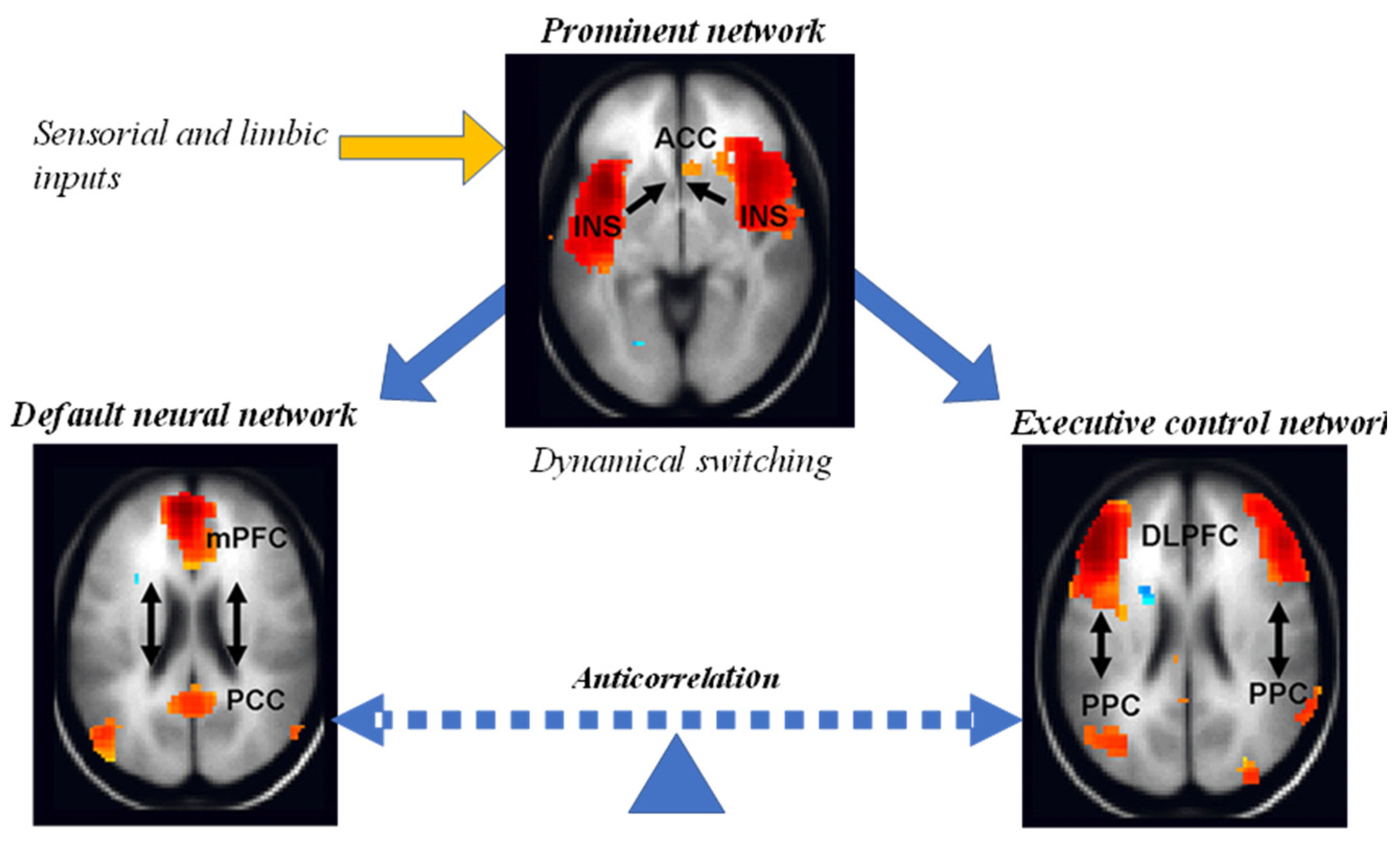
The ventral attention network or prominence (midcingulum-insular) plays the key role of monitoring the salience of external inputs and internal brain events. Specifically, it helps direct attention by identifying important biological and cognitive events. This network includes the temporoparietal junction and ventral frontal cortex of the right hemisphere, which respond when behaviorally relevant stimuli occur unexpectedly. The network is inhibited during focused attention in which top-down processing is used, such as when visually searching for something. This response can prevent goal-directed attention from being distracted by irrelevant stimuli. It becomes active again when the target is found (Menon 2011).
The executive control network (lateral frontoparietal) initiates and modulates cognitive control and comprises 18 subregions of the brain. There is a strong correlation between fluid intelligence and the involvement of the frontoparietal network with other networks, see
Figure 9. Versions of this network have also been called the central executive network or executive control and cognitive control network. Disruption of the nodes of this network has been found in virtually all psychiatric and neurological disorders, from autism, schizophrenia and depression to frontotemporal dementia and Alzheimer's disease (Menon 2011).
The dorsal attention network (dorsal frontoparietal) is involved in the voluntary top-down deployment of attention. Within the dorsal attention network, the intraparietal sulcus and frontal eye fields influence the visual areas of the brain. These influential factors allow the orientation of attention. Reduced connectivity within the dorsal and ventral attention networks has been linked to higher levels of ADHD symptoms. Overactivation of this network was observed in patients with schizophrenia.
The sensorimotor network (pericentral) processes somatosensory information, coordinates movement, and may include the auditory cortex. The network is activated during motor tasks, such as finger tapping, indicating that the network prepares the brain to perform and coordinate motor tasks. Dysfunction in this network has been implicated in several neuropsychiatric disorders, such as bipolar disorder and amyotrophic lateral sclerosis (Menon 2011).
The visual cortex network (occipital) handles the processing of visual information.
Different methods and data have identified other brain networks, many of which largely overlap or are subsets of the best-characterized core networks, namely: limbic, auditory, right/left executive, cerebellar, spatial attention, language, lateral view , temporal, and visual perception/images.
In conclusion, in this section neurogenesis has been shown as the key to the evolutionary leap towards more complex cortices, which require advanced theories of complex systems to analyze the connectome, still in development, and its functions. Beyond a segmented vision based on anatomical brain parts with independent functions, it moves towards a holistic vision of neural networks, which are still subject to research.
4. Analysis and future research trends
The preceding sections highlight select aspects of the vast literature of cortex evolution and functions, demonstrating the presence of species-specific patterns of evolution within both cortical and non-cortical systems. Consistent with this incomplete conceptualization of brain evolution, much modern empirical work in psychology and neuroscience is based on a theoretical scaffolding focused exclusively on the cerebral cortex – ironically called “corticocentric myopia” in (Parvizi, 2009), which suggests that “human-specific” capabilities such as higher-level cognition, consciousness, mentalizing, and morality are limited and entirely dependent on the expansion of the associated area. The problematic effects of this theoretical bias are evident throughout the literature, contributing to the systemic mischaracterization of basic properties of brain functioning across species and the neglect of fundamental aspects of brain evolution in vertebrate mammals. A comprehensive view of brain evolution is therefore necessary (Chin et al, 2022).
Another future line is the evolution of the prefrontal cortex (PFC), which increased it towards new areas in a posterior to anterior trajectory during the evolution of primates. A large expansion of the granular prefrontal cortex occurred in humans along with other association areas, with modifications of cortical connectivity and gene expression, although current evidence does not support the addition of many new human-specific prefrontal cortex areas (Preuss and Wise, 2022).
In this line and despite the differences in methodology that produce different levels of granularity and anatomical limits of the PFC, most research approaches have converged on the six central complex networks mentioned in the previous section, as functional networks anchored in the PFC. An important characteristic of each of these networks is that they span association areas across the frontal, parietal, temporal, and cingulate cortices. Deficits in cognitive control have been observed in many psychiatric and neurological disorders. The cognitive control systems of the brain have been tested in these clinical disorders with multiple brain imaging techniques and as expected, dysfunction is observed in PFC networks. Control theory techniques borrowed from engineering may also offer promising new approaches to testing how PFC networks can be manipulated to facilitate desirable or optimal brain states. This may be relevant to the treatment of psychiatric disorders such as depression, where stimulation of the dorsolateral PFC node is used to alter distal brain targets that influence mood (Menon and D'Esposito, 2022).
Although we still do not have a complete understanding of how the human brain generates unique abilities, recent decades have seen significant progress in the discovery of some of the genetic, cellular, and molecular mechanisms that shape brain development and function. Along these lines, new experimental paradigms are beginning to provide a framework for understanding how the emergence of these human-specific genomic innovations shaped the structure and function of neural circuits in the human brain (Schmidt and Polleux, 2022).