Submitted:
19 October 2023
Posted:
19 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. In Vitro α-Glucosidase Inhibition Assay
2.3. Kinetic Analysis of Enzyme Inhibition
2.4. Fluorescence Spectroscopic Analysis
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results and Discussions
3.1. In Vitro Antidiabetic Effect of Hesperidin on α-Glucosidase Enzyme
3.2. Kinetic Analysis of Enzyme Inhibition
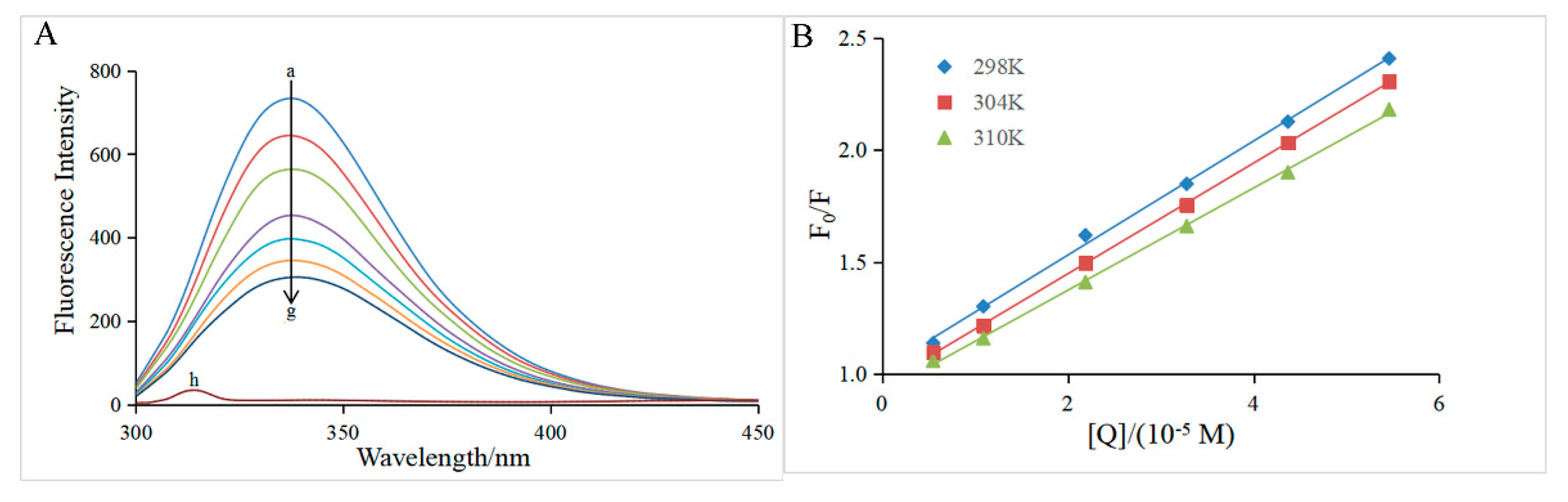
3.3. Fluorescence Quenching Assay
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, F.; Kong, R.; Han, X. Association between globulin and diabetic nephropathy in type2 diabetes mellitus patients: a cross-sectional study. Front. Endocrin. 2022, 13, 890273. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Muggeo, M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia, 2001, 44, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Toeller, M. α-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur. J. Clin. Invest. 1994, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini. Rev. Med.Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tao, Y.; Wang, S.; Wang, M.; Li, L. Inhibitory interaction of narcissoside on α-glucosidase from Aspergillus niger and Saccharomyces cerevisiae by spectral analysis and molecular docking. J. Molec. Struc. 2022, 1264, 133262. [Google Scholar] [CrossRef]
- He, M.; Zhai, Y.; Zhang, Y.; Xu, S.; Yu, S.; Wei, Y.; Song, Y. Inhibition of α-glucosidase by trilobatin and its mechanism: kinetics, interaction mechanism and molecular docking. Food. Func. 2022, 13, 857–866. [Google Scholar] [CrossRef]
- Tian, J.L.; Zhao, M.; Xu, J.Y.; Lv, T.M.; Liu, X.C.; Sun, S.; Hu, P. Inhibitory Mechanism of Prenylated Flavonoids Isolated from Mulberry Leaves on α-Glucosidase by Multi-Spectroscopy and Molecular Dynamics Simulation. J. Agri. Food Chem. 2023, 71, 9135–9147. [Google Scholar] [CrossRef]
- Vergine, M.; Nicolì, F.; Sabella, E.; Aprile, A.; De Bellis, L.; Luvisi, A. Secondary metabolites in Xylella fastidiosa–plant interaction. Pathogens 2020, 9, 675. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Qiu, C. Polyphenols as plant-based nutraceuticals: health effects, encapsulation, nano-delivery, and application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ling, W.; Yan, Z.; Liang, Y.; Guo, C.; Ouyang, Z.; Zhang, J. Effects of storage conditions and heat treatment on the hesperidin concentration in Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) juice. J. Food. Compo. Anal. 2020, 85, 103338. [Google Scholar] [CrossRef]
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Zhang, J. Chemical composition, antioxidant, antibacterial, and tyrosinase inhibition activity of extracts from Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) peel. J. Sci. Food Agri. 2020, 100, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A therapeutic agent for obesity. Drug. Des. Develop. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Falco, T.; Bonesi, M. Ruta chalepensis L. (Rutaceae) leaf extract: chemical composition, antioxidant and hypoglycaemic activities. Nat. Prod. Res. 2018, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Lai, C.; Liang, Y.; Gao, L.; Kaliaperumal, K.; Jiang, Y. Nutraceutical potential of navel orange peel in diabetes management: The chemical profile, antioxidant, α-glucosidase inhibitory and antiglycation effects of its flavonoids. Food Biosci. 2022, 49, 101943. [Google Scholar] [CrossRef]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory effect of corosolic acid on α-glucosidase: kinetics, interaction mechanism, and molecular simulation. J. Sci. Food Agri. 2019, 99, 5881–5889. [Google Scholar] [CrossRef]
- Yang, X.; Du, Z.; Pu, J.; Zhao, H.; Chen, H.; Liu, Y.; Liao, F. Classification of difference between inhibition constants of an inhibitor to facilitate identifying the inhibition type. J. Enz. Inhib. Med. Chem. 2013, 28, 205–213. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens leaf extract and fractions: polyphenol composition, antioxidant, enzymes (α-glucosidase, acetylcholinesterase, and tyrosinase) inhibitory, anticancer, and antidiabetic activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.B. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Xu, Y.; Lu, Y.H. Inhibitory effects of citrus flavonoids on starch digestion and anti-hyperglycemic effects in HepG2 cells. J. Agri. Food Chem. 2012, 26, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Brit. J. Nut. 2019, 121, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ding, H.; Hu, X.; Zhang, G.; Gong, D. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, G.; Lin, S.; Gong, D. Inhibitory mechanism of apigenin on α-glucosidase and synergy analysis of flavonoids. J. Agri. Food Chem. 2016, 64, 6939–6949. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, D.W.; Curtis-Long, M.J.; Park, C.; Son, M.; Kim, J. Y.; Park, K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem. 2016, 114, 201–208. [Google Scholar] [CrossRef]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocoll. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; An, C.; Liu, C.; Zhang, Q.; Ding, H.; Fan, D. Ginsenoside Rg1 alleviates the postprandial blood glucose by inhibiting α-glucosidase. J. Funct. Foods 2023, 107, 105648. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. Why is uncompetitive inhibition so rare?: A possible explanation, with implications for the design of drugs and pesticides. FEBS Lett. 1986, 203, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xue, W.; Xu, X.; Jia, Z.; Yao, X.; Liu, S.; Liu, L. Interaction of erucic acid with bovine serum albumin using a multi-spectroscopic method and molecular docking technique. Food Chem. 2015, 173, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Huang, Z.; Zhao, Z. Phenolics from Sterculia nobilis Smith pericarp by-products delay carbohydrate digestion by uncompetitively inhibiting α-glucosidase and α-amylase. LWT 2023, 173, 114339. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agri. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.; ud din Parray, M.; Maurya, J.K.; Kumar, A.; Patel, R. Interaction of promethazine and adiphenine to human hemoglobin: A comparative spectroscopic and computational analysis. Spectrochim. Acta. Part A: Mol. Biomol. Spectro. 2018, 199, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, H.; Hu, X.; Pan, J.; Liao, Y.; Gong, D.; Zhang, G. Exploring inhibitory mechanism of gallocatechin gallate on α-amylase and α-glucosidase relevant to postprandial hyperglycemia. J. Funct. Foods 2018, 48, 200–209. [Google Scholar] [CrossRef]
- Dohare, N.; Khan, A.B.; Maurya, N.; Thakur, S.; Athar, F.; Singh, P.; Patel, R. An insight into the binding of aceclofenac with bovine serum albumin at physiological condition: a spectroscopic and computational approach. J. Biomol. Struct. Dynam. 2018, 36, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein–polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin–procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Tian, Q.; Zhang, Y.; Zhang, G.; Guan, Y.; Yan, J. Screening and characterization of aldose reductase inhibitors from Traditional Chinese medicine based on ultrafiltration-liquid chromatography mass spectrometry and in silico molecular docking. J. Ethnopharmacol. 2021, 264, 113282. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Ao, J.; Cai, Y.; Xi, M.; Hou, Y.; Li, M.; Luo, A. Inhibitory kinetics and mechanism of active compounds in green walnut husk against α-glucosidase: Spectroscopy and molecular docking analyses. LWT 2022, 172, 114179. [Google Scholar] [CrossRef]
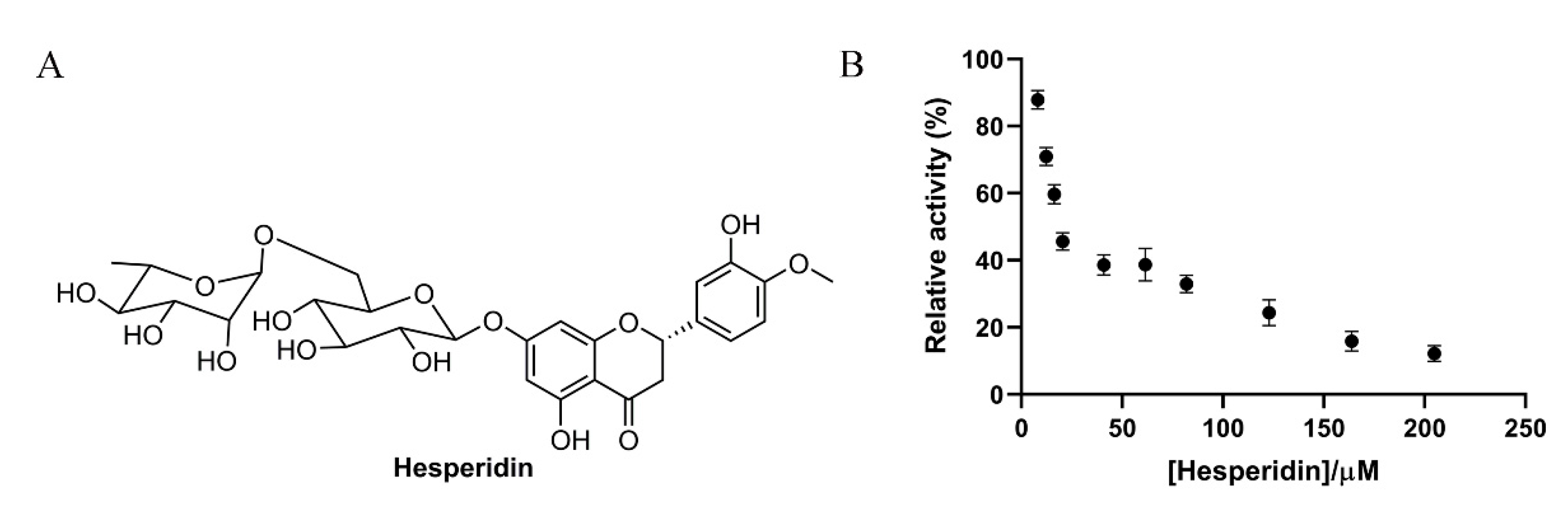


| [I](hesperidin)/µM | Vmax | Km | Kiv/Kik |
|---|---|---|---|
| 0 | 0.29 | 6.70 | // |
| 61.42 | 0.10 | 2.44 | 0.92 |
| 122.84 | 0.04 | 0.91 | 1.02 |
| 163.78 | 0.03 | 0.90 | 0.96 |
| 201.73 | 0.02 | 0.46 | 1.01 |
|
T (K) |
KSV (×105 L mol-1) |
Ka (×104 L mol-1) |
n | ΔHo (kJ mol-1) |
ΔGo (kJ mol-1) |
ΔSo (J mol-1 K-1) |
|---|---|---|---|---|---|---|
| 298 | 2.58±0.01a | 2.97±0.04a | 1.07±0.05a | -22.82±2.06 | -25.51±0.03a | 9.03±0.08 |
| 304 | 2.40±0.05b | 2.44±0.25b | 1.06±0.01a | -25.56±0.02a | ||
| 310 | 2.11±0.18c | 2.08±0.17c | 0.96±0.04a | -25.62±0.02a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





