Submitted:
19 October 2023
Posted:
19 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Apps Currently Used in Anesthesia
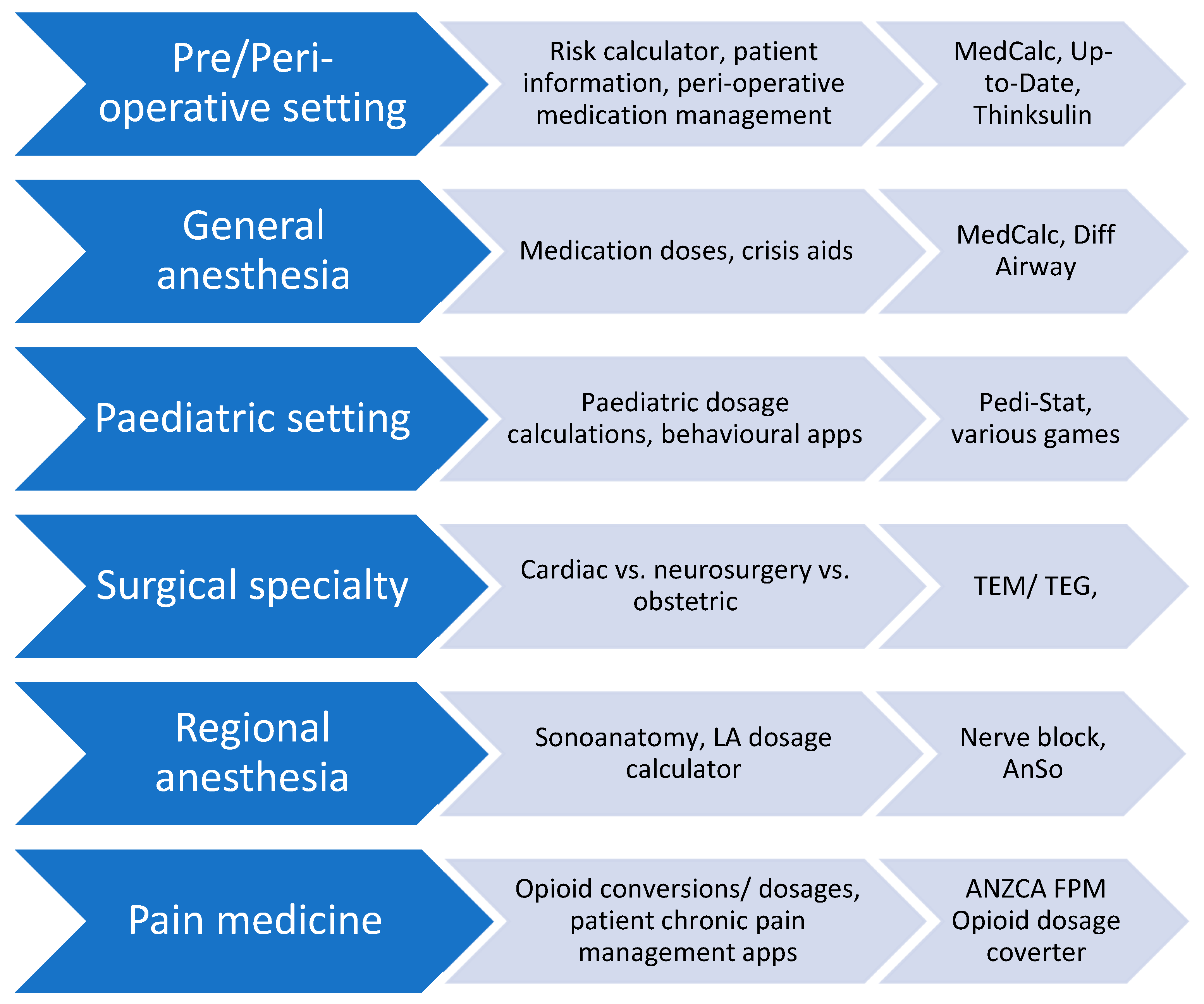
2.1. The definition of an app, and their use in anesthesia
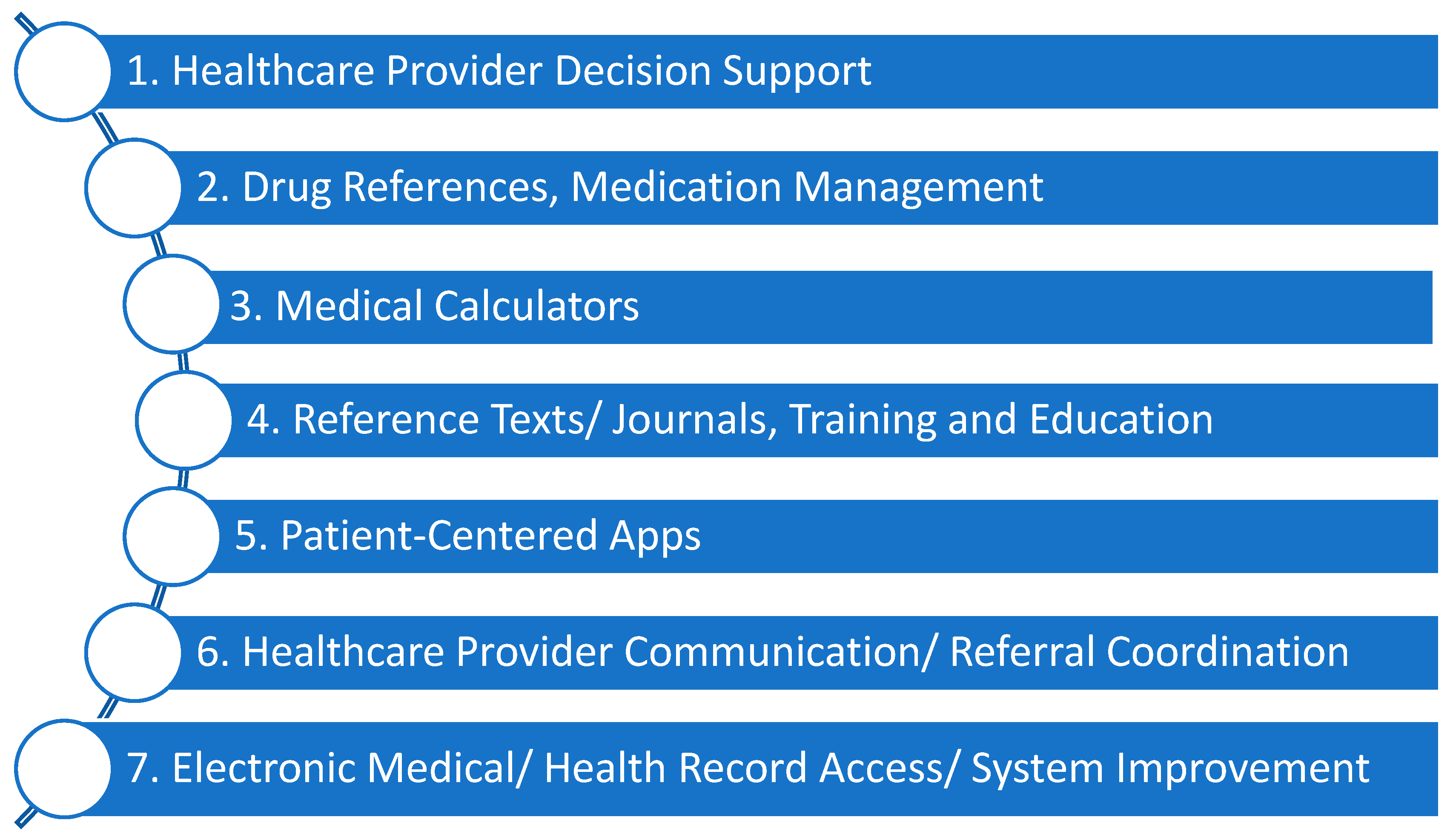
2.2. Point of Care clinical decision support apps
2.3. Drug references
2.4. Medical Calculators
2.5. Reference Texts/ Journals
3. Potential Issues with Medical Application Use in Anesthesia
3.1. Regulation and Medico-legal aspects of apps
3.2. Cyber-security
3.3. Patient Safety
3.4. Barriers to implementation
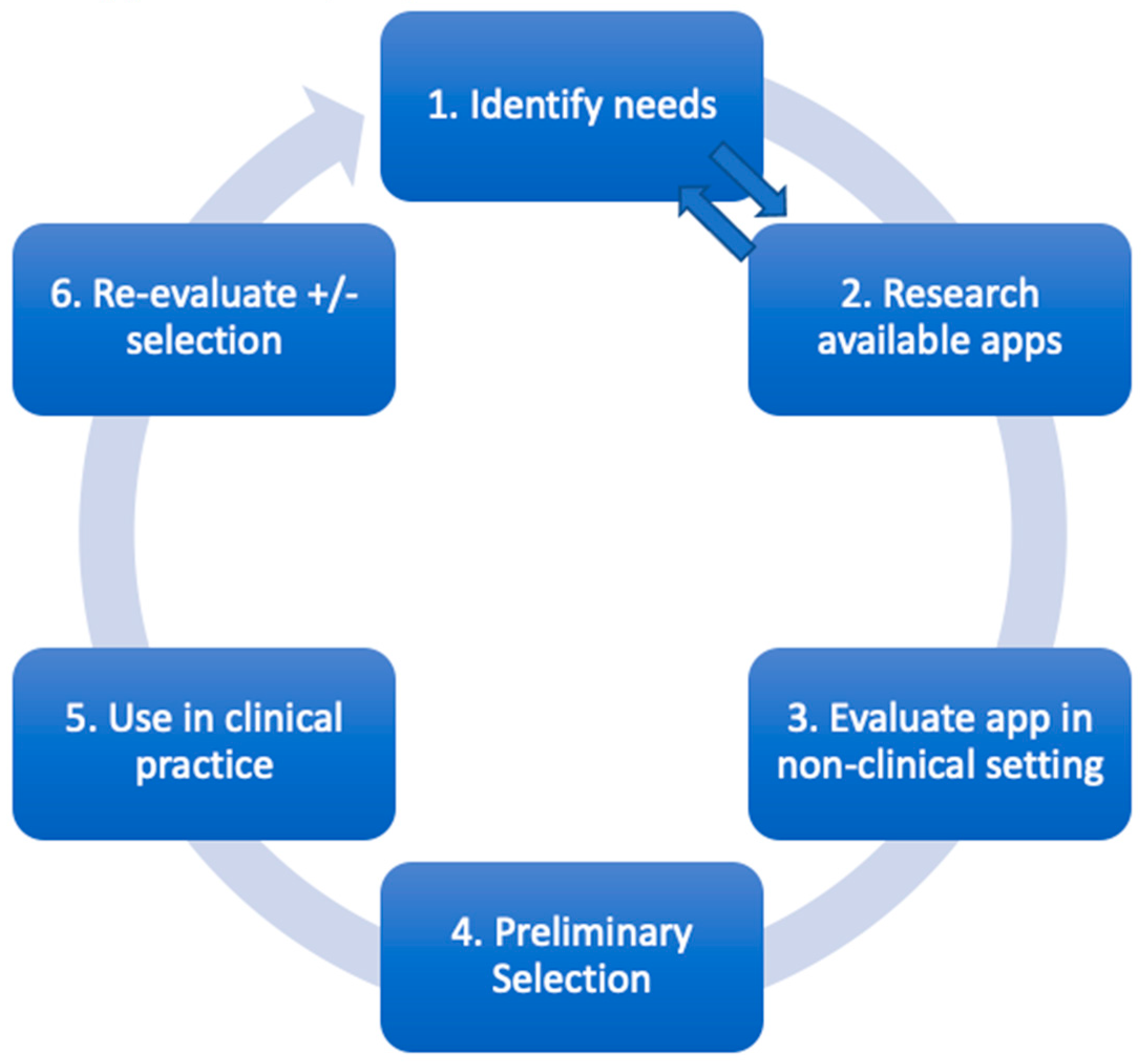
4. Evaluating A Medical App
4.1. Identifying needs
- What aspects of anesthesia could I improve, or do I struggle to keep up-to-date on?;
- What aspects of my practice are currently most time-consuming?;
- Are there emergency algorithms, I could have on my phone for reference?;
- What aspects of anaesthetic practice do I come across infrequently?;
- What aspects of my anaesthetic practice involve mathematics that could be done electronically?;
- I have heard about a new app from others in the department; does this sound like something I need?
4.2. Research available apps
4.3. Evaluate using rubric and cross-reference information
4.4. Preliminary selection
4.5. Use in clinical practice
4.6. Re-evaluate apps
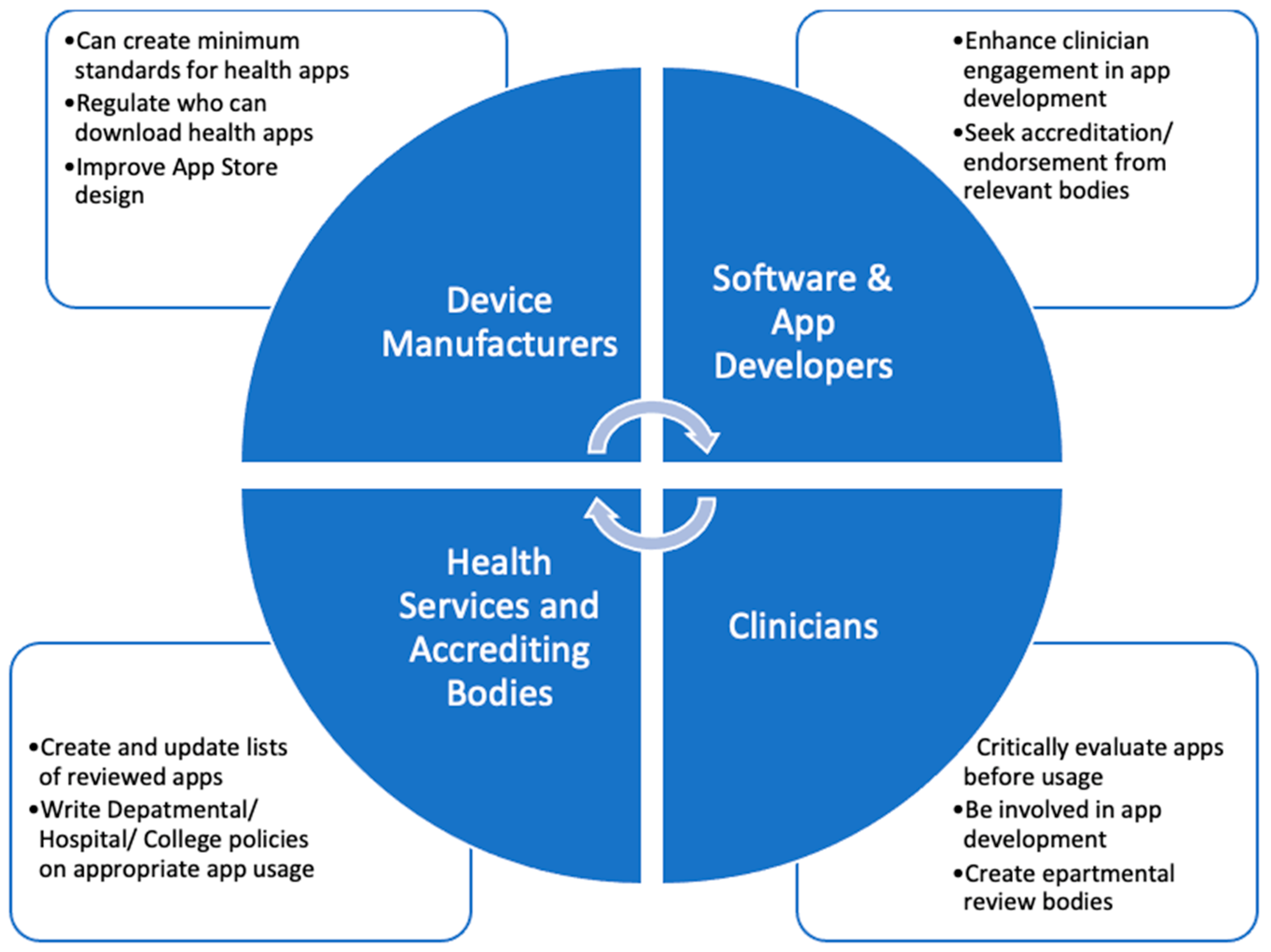
5. Further Steps
5.1. Role of device manufacturers and app developers
5.2. Role of clinicians, health services and regulatory agencies [360]
6. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Author Information
References
- EDN. Available online: https://www.edn.com/arpanet-establishes-1st-computer-to-computer-link-october-29-1969/ (accessed on 4 March 2023).
- Baumann, D.; Dibbern, N.; Sehner, S.; Zöllner, C.; Reip, W.; Kubitz, J.C. Validation of a mobile app for reducing errors of administration of medications in an emergency. JCMC 2019, 33(3), 531–539. [Google Scholar] [CrossRef] [PubMed]
- Karippacheril, J.G.; Mathai, R.T.; Abraham, S.S. Insulin IP Calc: A smartphone application for insulin infusion protocol in Intensive Care Units. Indian J. Anaesth 2015, 59(12), 829–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Thampi, S.; Yap, E.P.; Liu, E.H. Evaluation of a smartphone camera system to enable visualization and image transmission to aid tracheal intubation with the Airtraq(®) laryngoscope. J. Anesth 2016, 30(3), 514–517. [Google Scholar] [CrossRef] [PubMed]
- Lelaidier, R.; Balança, B.; Boet, S.; Faure, A.; Lilot, M.; Lecomte, F.; Lehot, J.J.; Rimmelé, T.; Cejka, J.C. Use of a hand-held digital cognitive aid in simulated crises: The MAX randomized controlled trial. BJA 2017, 119(5), 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.M.T.; Taylor, R. Validation of the mathematics in the anaesthetic impact calculator, a smartphone app for the calculation the CO2 e of inhalational anaesthesia. Anaesth 2020, 75(1), 136–138. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, L.; Pascarella, G.; Grande, S.; Agrò, F.E. Neuromuscular block monitoring by smartphone application (i-TOF© system): An observational pilot study. NPJ Digit. Med. 2020. [Google Scholar] [CrossRef]
- Thomson, A.J.; Nimmo, A.F.; Tiplady, B.; Glen, J.B. Evaluation of a new method of assessing depth of sedation using two-choice visual reaction time testing on a mobile phone. Anaesth 2009, 64(1), 32–38. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Mathew, J.J.; Gundigi Venkatesh, A.; Green, P.; Tariq, R. Utilization of Smartphone Applications by Anesthesia Providers. Anesthesiol. Res. Pract 2018. [CrossRef] [PubMed]
- Perkins, E.J.; Edelman, D.; Brewster, D.J. Smartphone use and perceptions of their benefit and detriment within Australian anaesthetic practice. Anaesth Intensive Care 2020, 48(5), 366–372. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Karliychuk, T.; Gillies, D.; Mintzes, B.; Raven, M.; Grundy, Q. A health app developer’s guide to law and policy: A multi-sector policy analysis. BMC Med. Inform. Decis. Mak 2017. [CrossRef] [PubMed]
- Yang, Y.T.; Silverman, R.D. Mobile health applications: The patchwork of legal and liability issues suggests strategies to improve oversight. Health Aff. (Project Hope). [CrossRef]
- American Society of Health-system Pharmacists. Available online: https://www.ashp.org/-/media/storefiles/mobile-medical-apps.pdf (accessed on 10 March 2023).
- U.S Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-device-software-functions-and-mobile-medical-applications (accessed on 24 April 2023).
- Camindale, J.; von Wartburg, M. The success of third-party apps on the app store. AG 2022. Available from https://www.apple.com/newsroom/pdfs/the-success-of-third-party-apps-on-the-app-store.pdf.
- Gordon, W.J.; Landman, A.; Zhang, H.; Bates, D.W. Beyond validation: Getting health apps into clinical practice. NPJ Digit. Med. [CrossRef]
- Pan., S.; Rong, L.Q. Mobile Applications in Clinical and Perioperative Care for Anesthesia: Narrative Review. JMIR. [CrossRef]
- Alhomary, M.; Kinirons, B. Survey of Smartphone Use among Anaesthetists In Saolta University Health Care Group Midlands Setting. Ir. Med. J. 2018, 111(3), 709. [Google Scholar] [PubMed]
- Bhansali, R.; Armstrong, J. Smartphone applications for pediatric anesthesia. Paediatr. Anaesth. 2012, 22, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.E.; Ginige, J.A. Comparative Study of Medical Reference and Information Mobile Apps for Healthcare Professionals and Students. Stud. Health Technol. Inform. 2018, (254), 43–52. [Google Scholar]
- Seabrook, H.J.; Stromer, J.N.; Shevkenek, C.; Bharwani, A.; de Grood, J.; Ghali, W.A. Medical applications: A database and characterization of apps in Apple iOS and Android platforms. BMC Res. Notes 2014, 7, 573. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Mobile devices and apps for health care professionals: Uses and benefits. P & T, 2014; 39, 356–364. [Google Scholar]
- World Health Organisation. Available online: https://apps.who.int/iris/bitstream/handle/10665/260480/WHO-RHR-18.06-eng.pdf;jsessionid=EF5C7B012765D3D18F62E3B3C71E67DC?sequence=1 (accessed on 18 May 2023).
- Carvalho, H.; Verdonck, M.; Forget, P.; Poelaert, J. Acceptance of mHealth among health professionals: A case study on anesthesia practitioners. BMC anesth. 2020, 20(1), 55. [Google Scholar] [CrossRef] [PubMed]
- UpToDate. Available online: www.uptodate.com (accessed on 2 May 2023).
- Colquhoun, D.A.; Davis, R.P.; Tremper, T.T.; Mace, J.J.; Gombert, J.M.; Sheldon, W.D.; Connolly, J.J.; Adams, J.F.; Tremper, K.K. Design of a novel multifunction decision support/alerting system for in-patient acute care, ICU and floor (AlertWatch AC). BMC anesth. 2021, 21(1), 196. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, P.; Gleibs, F.; Briesenick, L.; Joosten, A.; Saugel, B. Estimation of pulse pressure variation and cardiac output in patients having major abdominal surgery: A comparison between a mobile application for snapshot pulse wave analysis and invasive pulse wave analysis. J. Clin. Monit. Comput. 35(5), 1203–1209. [CrossRef] [PubMed]
- Shin, Y.D.; Bae, J.H.; Kwon, E.J.; Kim, H.T.; Lee, T.S.; Choi, Y.J. Assessment of pupillary light reflex using a smartphone application. Exp. Ther. Med 2016, 12(2), 720–724. [Google Scholar] [CrossRef] [PubMed]
- Berkenstadt, H.; Yusim, Y.; Ziv, A.; Ezri, T.; Perel, A. An assessment of a point-of-care information system for the anesthesia provider in simulated malignant hyperthermia crisis. Anesth. Analg. 2006, 102(2), 530–532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; McEvoy, M.D. Initial Experience of the American Society of Regional Anesthesia and Pain Medicine Coags Regional Smartphone Application: A Novel Report of Global Distribution and Clinical Usage of an Electronic Decision Support Tool to Enhance Guideline Use. Reg. Anesth. Pain Med. 2016, 41(3), 334–338. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, M.D.; Hand, W.R.; Stoll, W.D.; Furse, C.M.; Nietert, P.J. Adherence to guidelines for the management of local anesthetic systemic toxicity is improved by an electronic decision support tool and designated "Reader". Reg. Anesth. Pain Med. 2014, 39(4), 299–305. [Google Scholar] [CrossRef]
- Watson, P.; Watson, D.; Dhesi, A.; New, H.V.; Davidson, A.; Armstrong, R.; Birchall, J. Improving blood-prescribing decisions: Evaluating the efficacy of a blood guidelines app. Transfus. Med. 2020, 30(6), 485–491. [Google Scholar] [CrossRef] [PubMed]
- Batta, A.; Kalra, B.S.; Khirasaria, R. Trends in FDA drug approvals over last 2 decades: An observational study. J. Family Med. Prim. Care 2020, 9(1), 105–114. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Available online: https://www.who.int/publications/i/item/WHO-UHC-SDS-2019.11 (accessed on 12 December 2022).
- Smith, S. Medscape and iPhone apps: The stethoscope of the 21st-century medical student? App review. Med. Stud. J. Aust 2017.
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anaesth. 1999, 91(3), 693–700. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 50–64. [Google Scholar]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137(2), 263–272. [Google Scholar] [CrossRef] [PubMed]
- Suh, E.H.; Lang, K.J.; Zerihun, L.M. Modified PRIEST score for identification of very low-risk COVID patients. Am. J. Emerg. Med. 2021, 47, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Bachu, R.; Adikey, A.; Malik, M.; Shah, M. Factors Related to Physician Burnout and Its Consequences: A Review. Behav. Sci. 2018, 8(11), 98. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J.I.E. Clinical software on personal mobile devices needs regulation. MJA 2012, 196, 437. [Google Scholar] [CrossRef] [PubMed]
- Huckvale, K.; Torous, J.; Larsen, M.E. Assessment of the Data Sharing and Privacy Practices of Smartphone Apps for Depression and Smoking Cessation. JAMA. [CrossRef]
- Soegaard Ballester, J.M.; Bass, G.D.; Urbani, R.; Fala, G.; Patel, R.; Leri, D.; Steinkamp, J.M.; Denson, J.L.; Rosin, R.; Adusumalli, S.; et al. A Mobile, Electronic Health Record-Connected Application for Managing Team Workflows in Inpatient Care. Appl. Clin. Inform. 2021, 12(5), 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Coiera, E.; Magrabi, F. Safety concerns with consumer-facing mobile health applications and their consequences: A scoping review. JAMIA 2020, 27(2), 330–340. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Moreau, J.F.; Akilov, O. Diagnostic Inaccuracy of Smartphone Applications for Melanoma Detection. JAMA Dermatol 2013, 149(4), 422–426. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Azevedo, N.; Carrasquinho, E.; Cardoso de Oliveira, E.; Cavadas, V.; Osório, L.; Fraga, A.; Castelo-Branco, M.; Roobol, M.J. mHealth in Urology: A Review of Experts' Involvement in App Development. PloS one. [CrossRef]
- Monroe, K.S.; Evans, M.A.; Mukkamala, S.G.; Williamson, J.L.; Jabaley, C.S.; Mariano, E.R.; O'Reilly-Shah, V.N. Moving anesthesiology educational resources to the point of care: Experience with a pediatric anesthesia mobile app. Korean J Anesthesiol 2018, 71(3), 192–200. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Reich, C.; Krause, D.; Ruhnke, B.; Daubmann, A.; Weimann, J.; Zöllner, C.; Kubitz, J. For beginners in anaesthesia, self-training with an audiovisual checklist improves safety during anaesthesia induction: A randomised, controlled two-centre study. Eur. J. Anaesthesiol. 2018, 35(7), 527–533. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.Y.A.; Chong, S.Y.; Koh, S.E.A.; Yeo, B.C.; Tan, K.Y.; Ling, M.L. Healthcare workers' beliefs, attitudes and compliance with mobile phone hygiene in a main operating theatre complex. IPIP. [CrossRef]
- Mathews, S.C.; McShea, M.J.; Hanley, C.L.; Ravitz, A.; Labrique, A.B.; Cohen, A.B. Digital health: A path to validation. NPJ Digit. Med. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R. iPhone App ‘Anaesthesia Exams’. BJA 2013, 110(2), 323. [Google Scholar] [CrossRef]
- Alamoodi, A.H.; Garfan, S.; Zaidan, B.B.; Zaidan, A.A.; Shuwandy, M.L.; Alaa, M.; Alsalem, M.A.; Mohammed, A.; Aleesa, A.M.; Albahri, O.S.; et al. A systematic review into the assessment of medical apps: Motivations, challenges, recommendations and methodological aspect. Health Technol. 2020, 10, 1045–1061. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR Mhealth Uhealth. [CrossRef]
- Levine, D.M.; Co, Z.; Newmark, L.P.; Groisser, A.R.; Holmgren, A.J.; Haas, J.S.; Bates, D.W. Design and testing of a mobile health application rating tool. NPJ Digit. Med. [CrossRef]
- LifeBlood. Available online: https://www.lifeblood.com.au/health-professionals/clinical-practice/clinical-indications/anti-coagulation-reversal (accessed on 24 May 2023).
- Sedhom, R.; McShea, M.J.; Cohen, A.B.; Webster, J.A.; Mathews, S.C. Mobile app validation: A digital health scorecard approach. NPJ Digit. Med. [CrossRef]
- Gavali, M.Y.; Khismatrao, D.S.; Gavali, Y.V.; Patil, K.B. Smartphone, the New Learning Aid amongst Medical Students. JCDR. [CrossRef]
- Lo, C.; Yu, J.; Görges, M.; Matava, C. Anesthesia in the modern world of apps and technology: Implications and impact on wellness. Paediatr. Anaesth. 2021, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Aungst, T.; Seed, S.; Gobin, N.; Jung, R. The good, the bad, and the poorly designed: The mobile app stores are not a user-friendly experience for health and medical purposes. Digit. Health 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Horlocker, T.; McEvoy, M.D. Initiative to accelerate guideline distribution using the smartphone app ASRA Coags V.2.0. 2.0. Reg. Anesth. Pain. Med. 2020, 46(4), 354–355. [Google Scholar] [CrossRef] [PubMed]
| Domain | Question | Y/N | Examples of issues | |
|---|---|---|---|---|
| Design & Usability | 1. | Is the app easy to start using? | Does the app:
|
|
| 2. | Is the app’s interface easy to navigate? | Difficult to navigate menus make using an app frustrating and lessens clinical utility, especially in emergency situations. | ||
| 3. | Do the app’s images have sufficient quality? | For example, a picture of an anaphylactic rash to compare to needs to have sufficient resolution and be in colour to be useful clinically. | ||
| 4. | If the app has emergency functions, are these easy to find? | If the app provides emergency information, there should be a one-click function to access these, for example, drug dosages for MH or anaphylaxis, or ALS algorithms. | ||
|
Clinical Content & Evidence Base Clinical Content & Evidence Base Clinical Content & Evidence Base |
5. | Is the information seemingly credible? | Compare information on the app with other references or your own knowledge. | |
| 6. | Is the apps clinical guidance concordant with local anaesthetic practice? | If the information is at least credible, then check if it is applicable locally with:
|
||
| 7. | Does the app give specific enough guidance? | Often to reduce liability, apps may not provide specific guidance (e.g., drug dosages). Assess whether the app gives enough guidance for your practice. | ||
| 8. | Has the app been developed by a reputable organisation?/ has there been clinician input? | Can you see who has developed this app? Can you see there was physician involvement? | ||
| 9. | Does the app provide evidence or references? | There should be referencing of information, with links to original articles. | ||
| Question | Y/N | Examples of issues | ||
| 10. | Has the app been validated in published studies, or had peer review? | Try and search the app using a web browser. Do any clinical studies or reviews come up, or does the app discuss its validation process? | ||
| Technical& Cybersafety | 11. | Is the app compatible with your phone’s OS? | If available on your phone’s app store it will be, however not all apps are usable on all devices, or some functionality may occasionally be lost. | |
| 12. | Does the app have sufficient phone security?1 | If sensitive data is being input, there should be a password-protection process. Carefully check the app’s privacy policy | ||
| 13. | Does the app need an internet connection to function? | If the app does not function at all without internet connection (or does not have an offline option), it may not work in an OT environment.2 | ||
| 14. | How large is the app? | If the apps is large (e.g., >50MB), you may need to make room for the app by deleting files. | ||
| 15. | Does the app give too many/ not enough notifications? | Too many notifications represent an annoyance, whilst urgent updates or breaking medical news may be desirable. | ||
| Ongoing Use | 16. | Are there ongoing subscription costs? | Also consider whether there is an existing hospital/ university licence to use the app. | |
| 17. | Is there a way to contact designers for support? | There should be developer contact details in the event of app malfunction or to submit feedback. | ||
| 18. | Has the app been updated? | Generally, an update should occur at least every two years, if not more frequently [44]. | ||
| 19. | How long will I need this app/ when will I review? | Apps may be downloaded for a specific case or list; if so, then these apps should be deleted after use. If an app is downloaded for longer-term use, then a plan for review is suggested. | ||
| Overall markers of subjective quality | 20. | Would I recommend this app? | Would you suggest to another colleague they use this app? | |
| 21. | Would I pay for this app? | And if so, how much would be appropriate? | ||
| 22. | What is my overall star rating from 1-5 | |||
| 23. | Has this improved anaesthetic practice? | |||
| MH: Malignant hyperthermia; ALS: Advanced life support; OS: operating system; OT: Operating theatre; MB: megabyte. 1If inputting clinical/ personal information, does the app require a password +/- two-step authentication 2This can be tested by placing the mobile device into flight mode and seeing if the app functions. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





