Submitted:
19 October 2023
Posted:
20 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
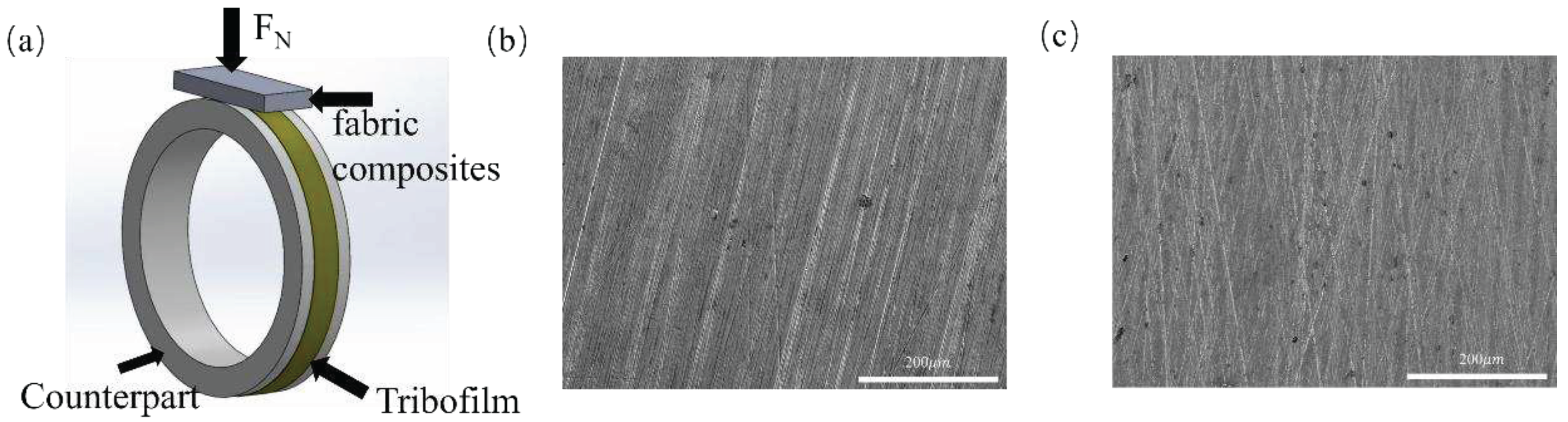
2.3. Tribological Tests
2.4. Testing and Characterization
3. Results and Discussion
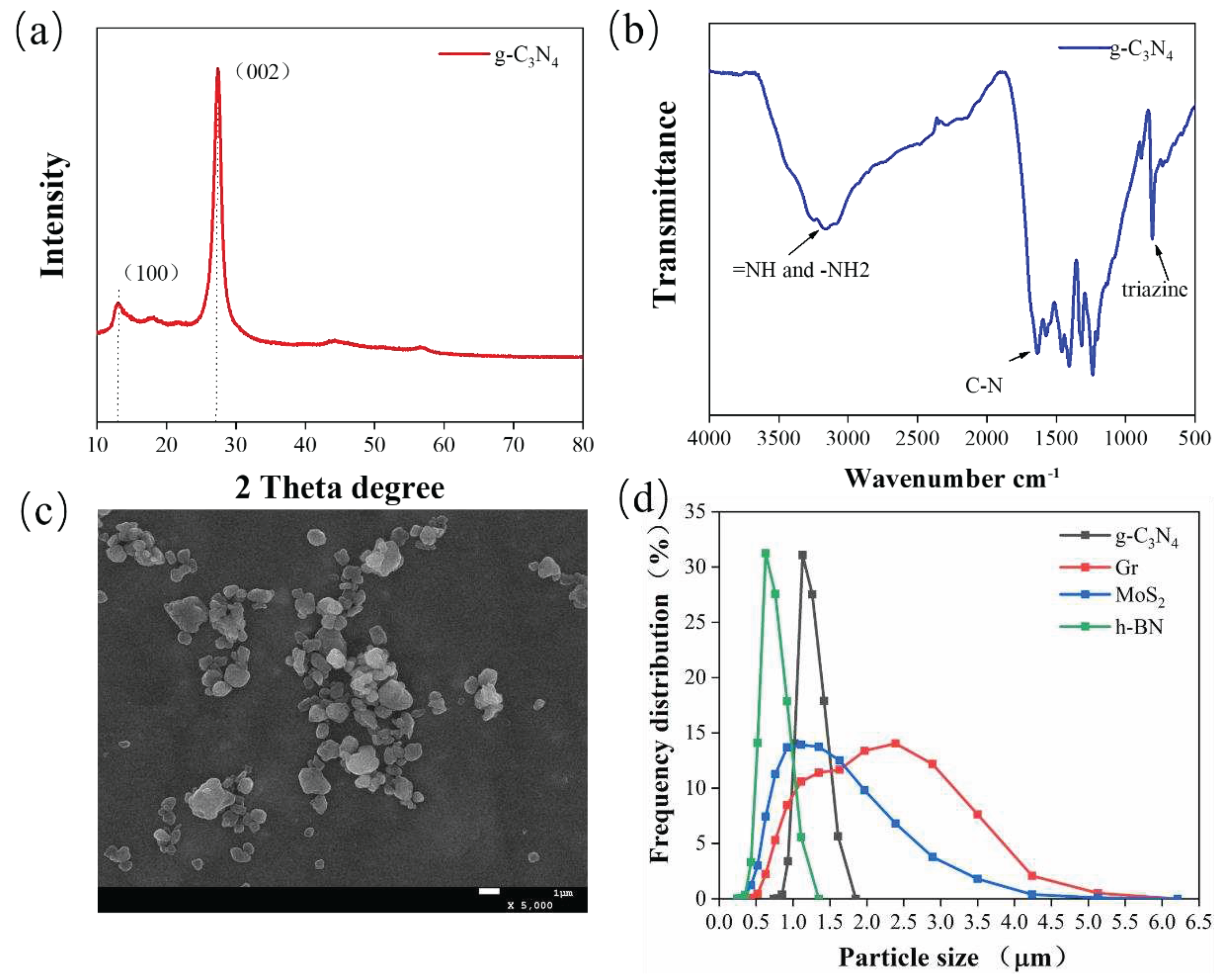
3.1. Characterization of g-C3N4 and Particle Size Distribution of Four Fillers
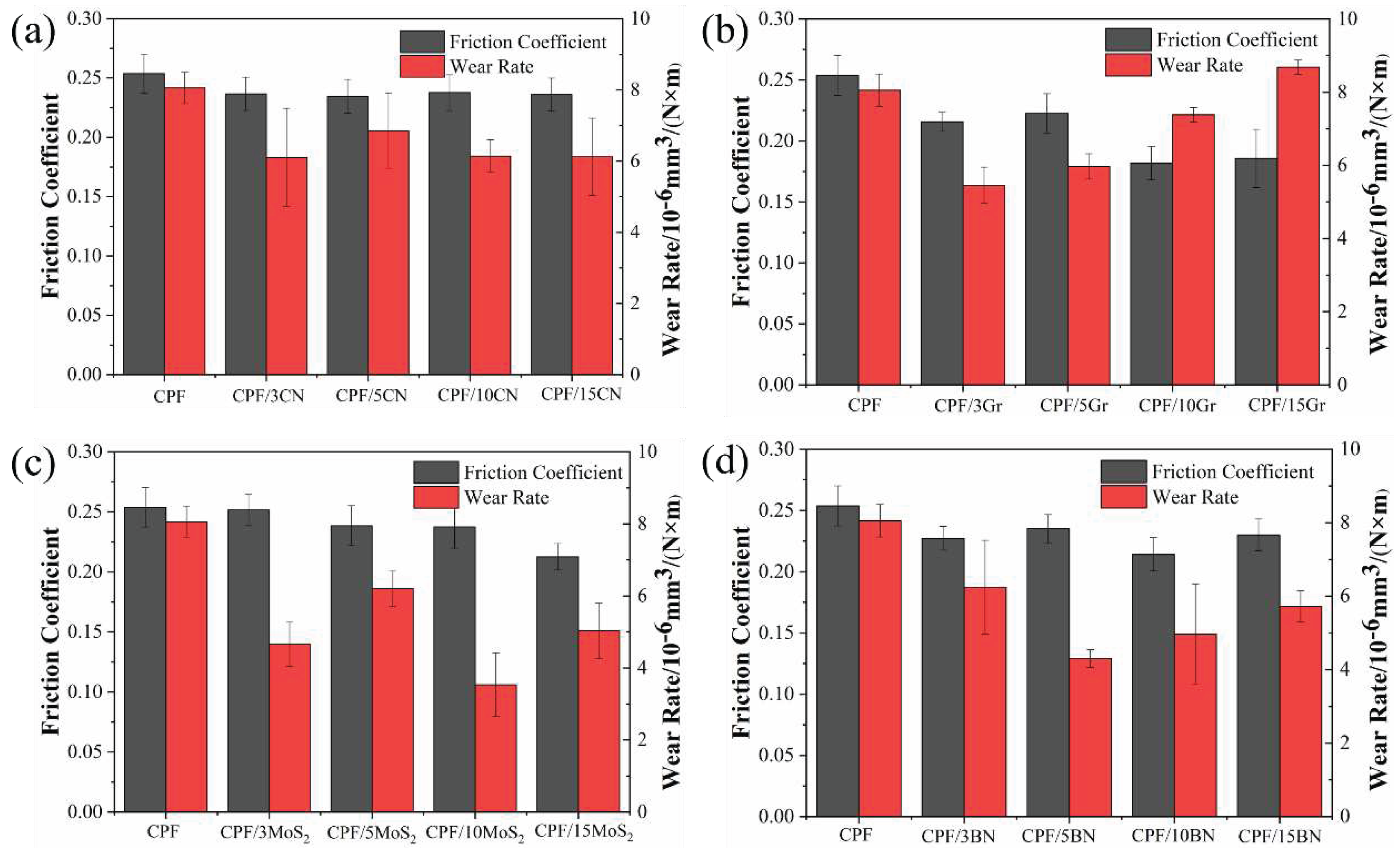
3.2. Tribological Properties
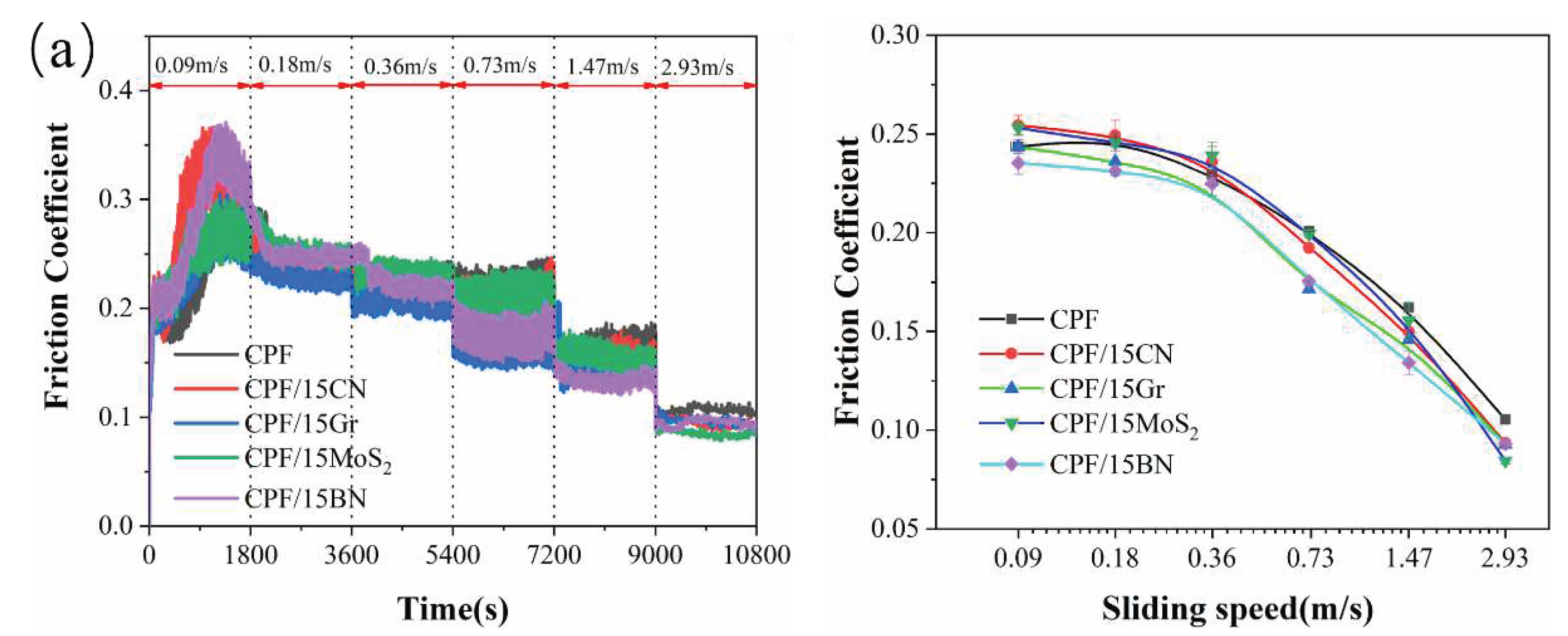
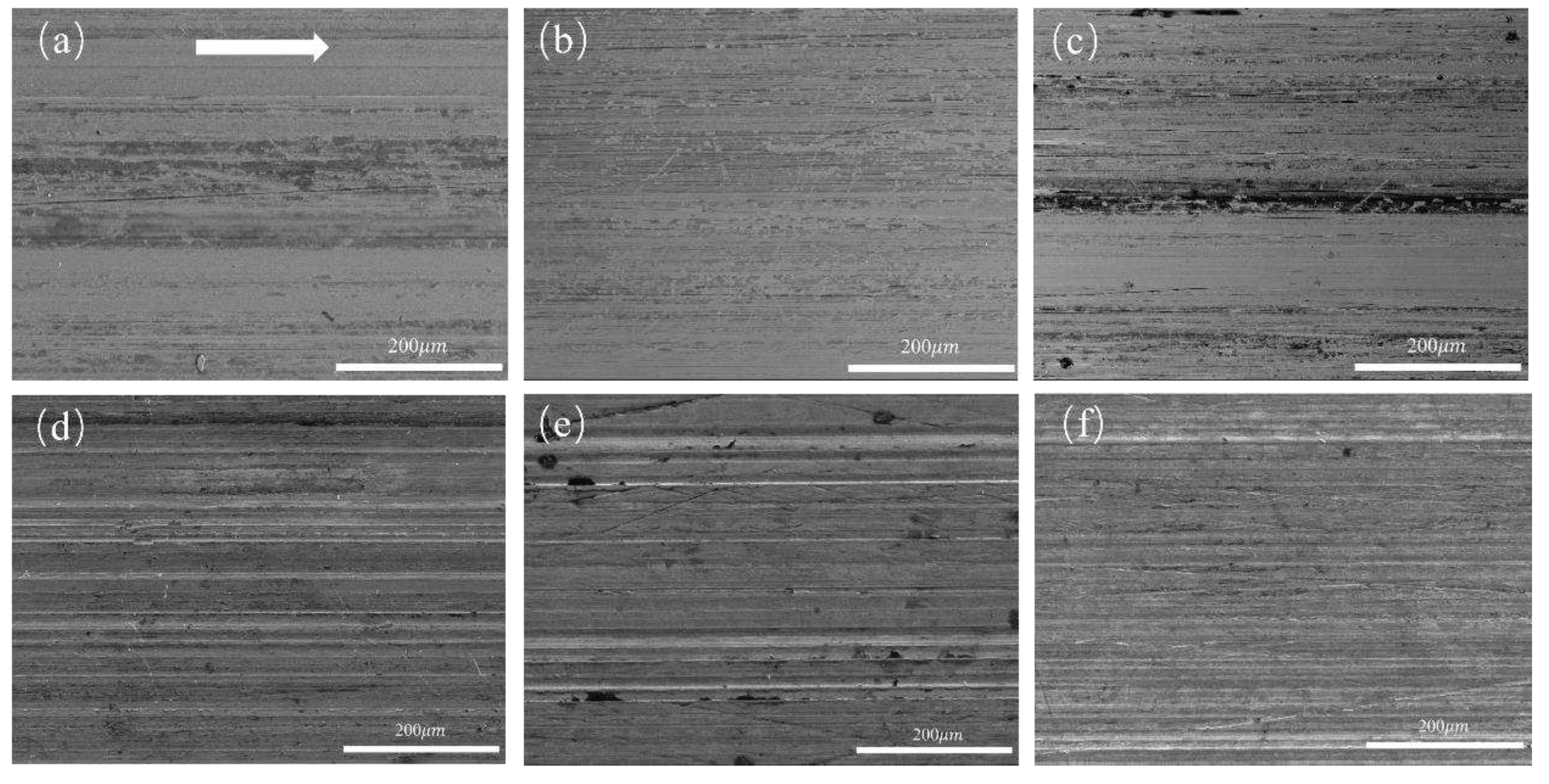
3.2.1. Tribological Behaviors at Varying Sliding Speeds under Water Lubrication
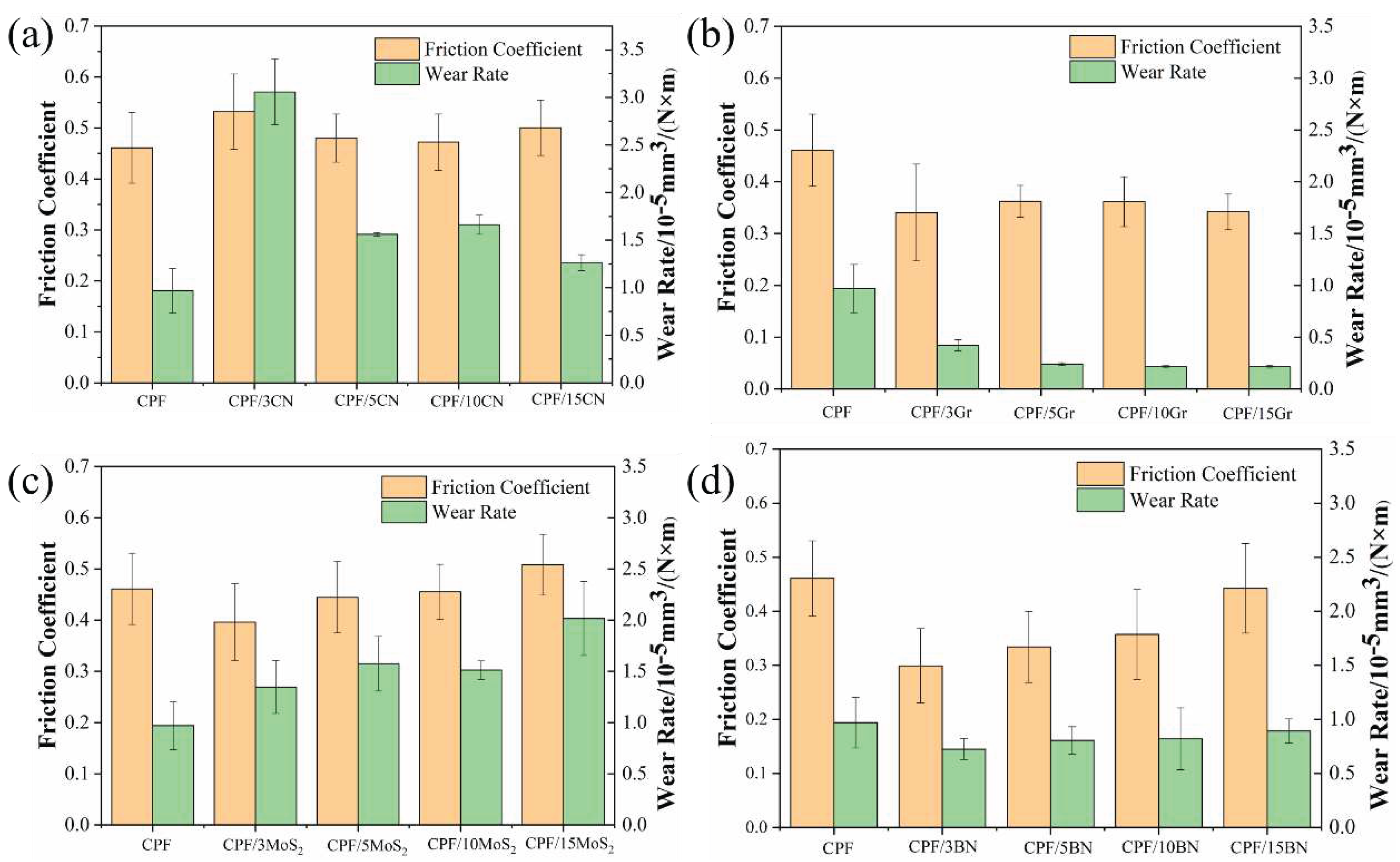
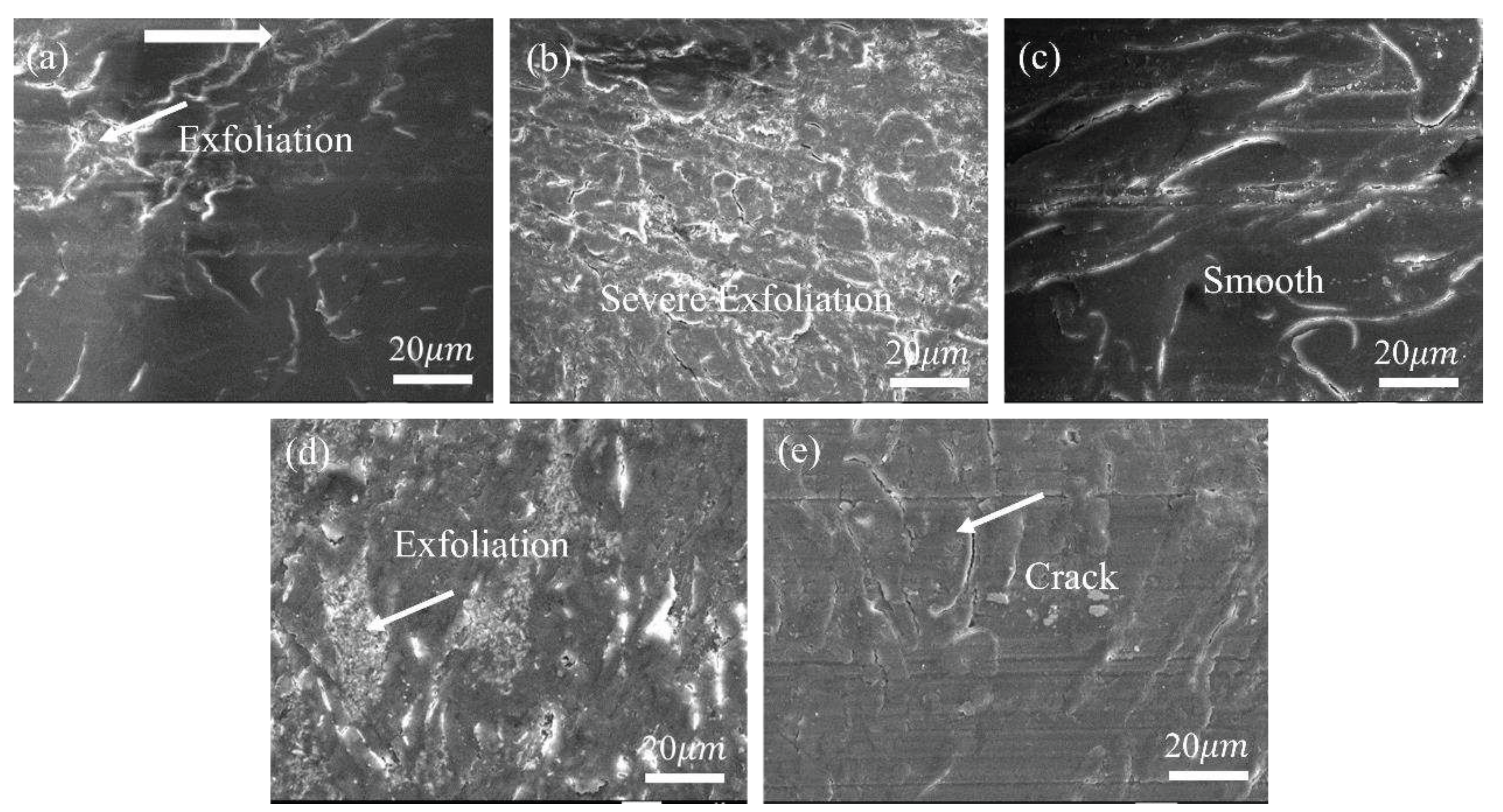
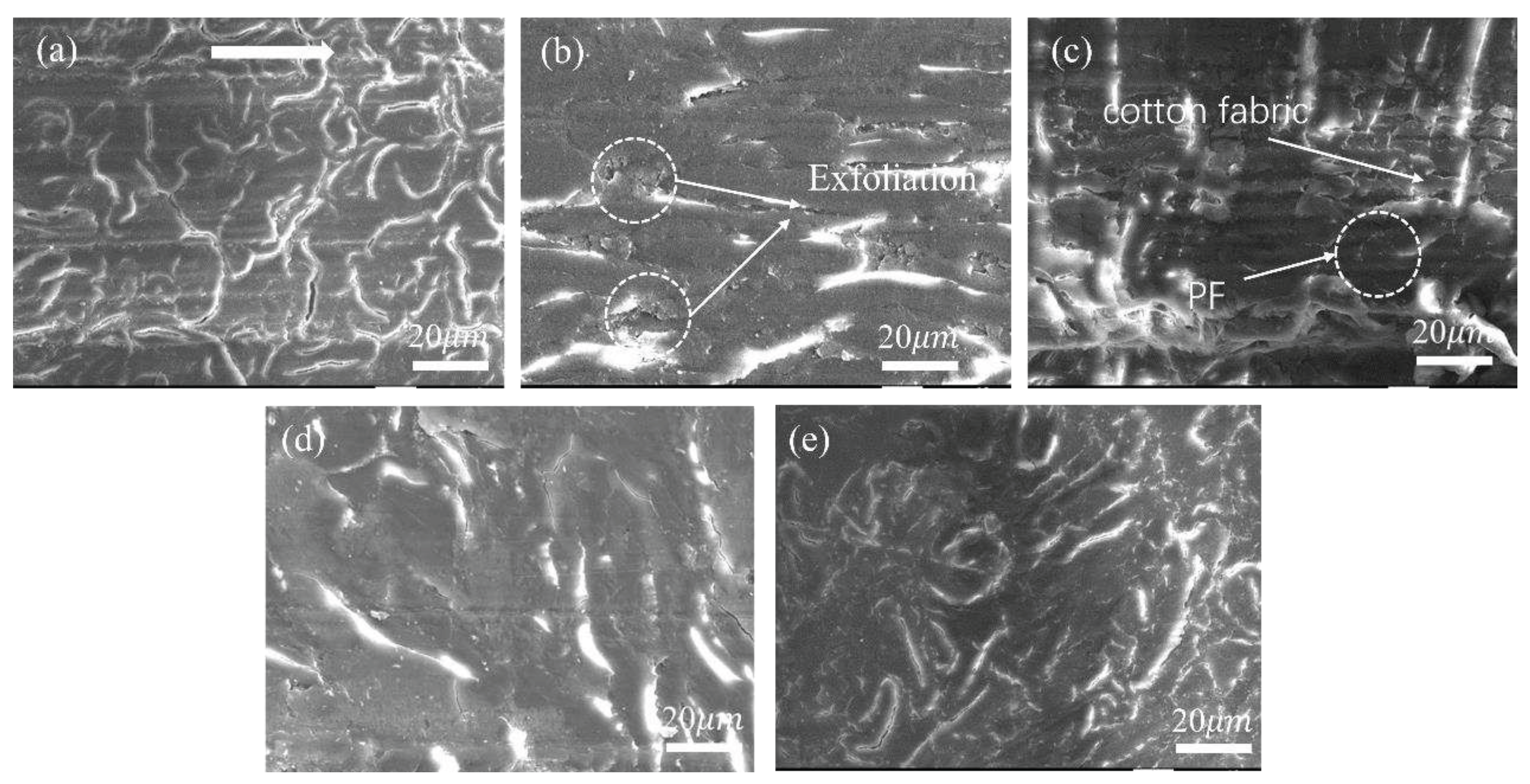
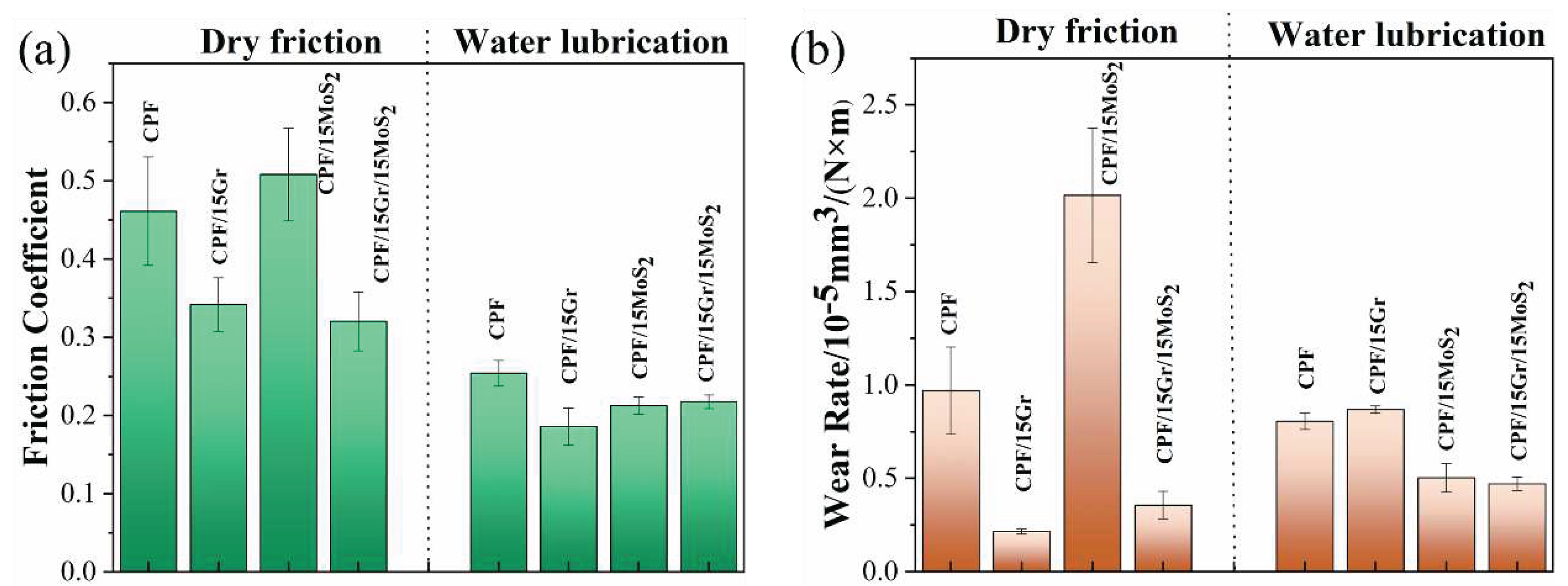
3.2.2. Tribological Behaviors at Constant Sliding Speeds
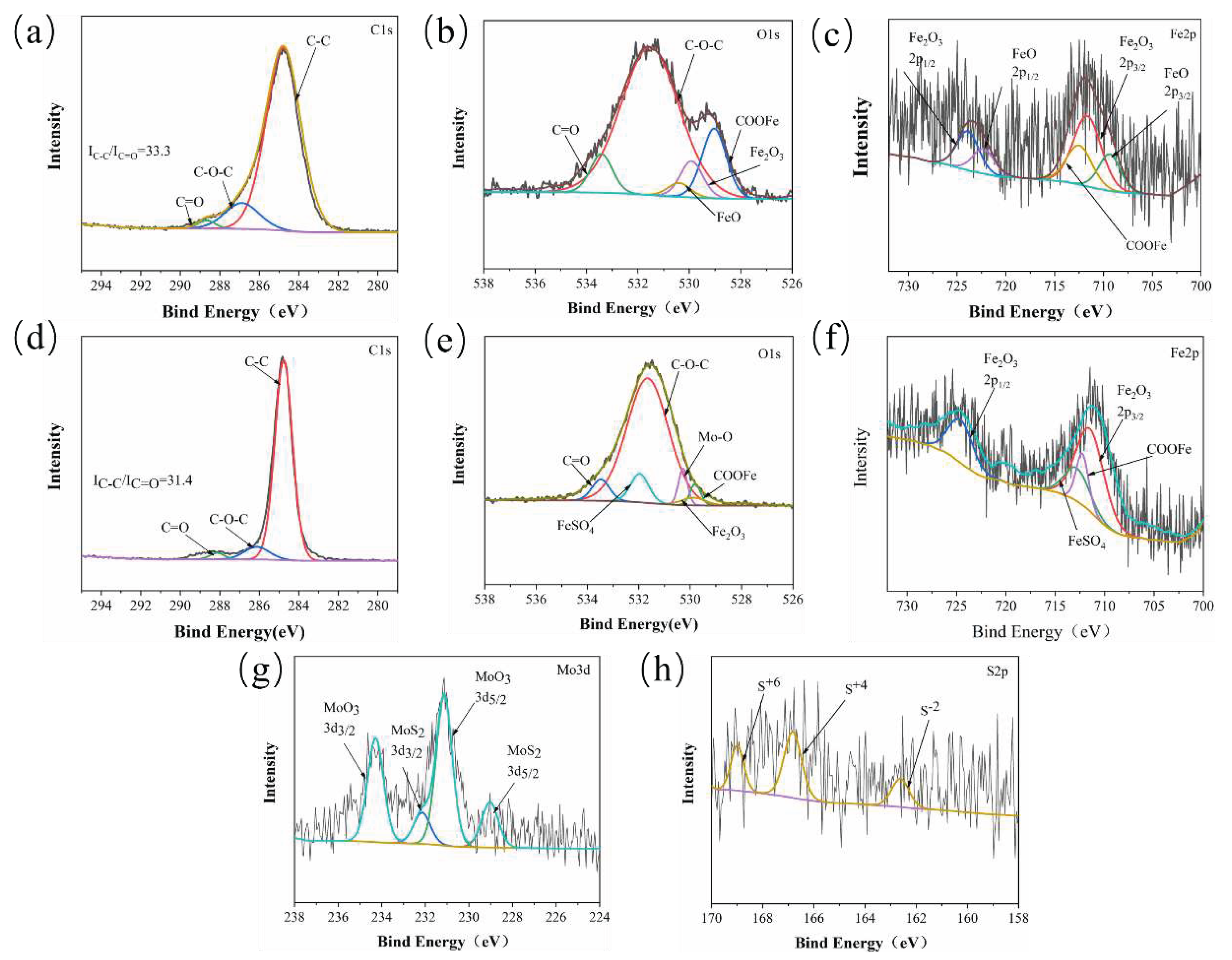
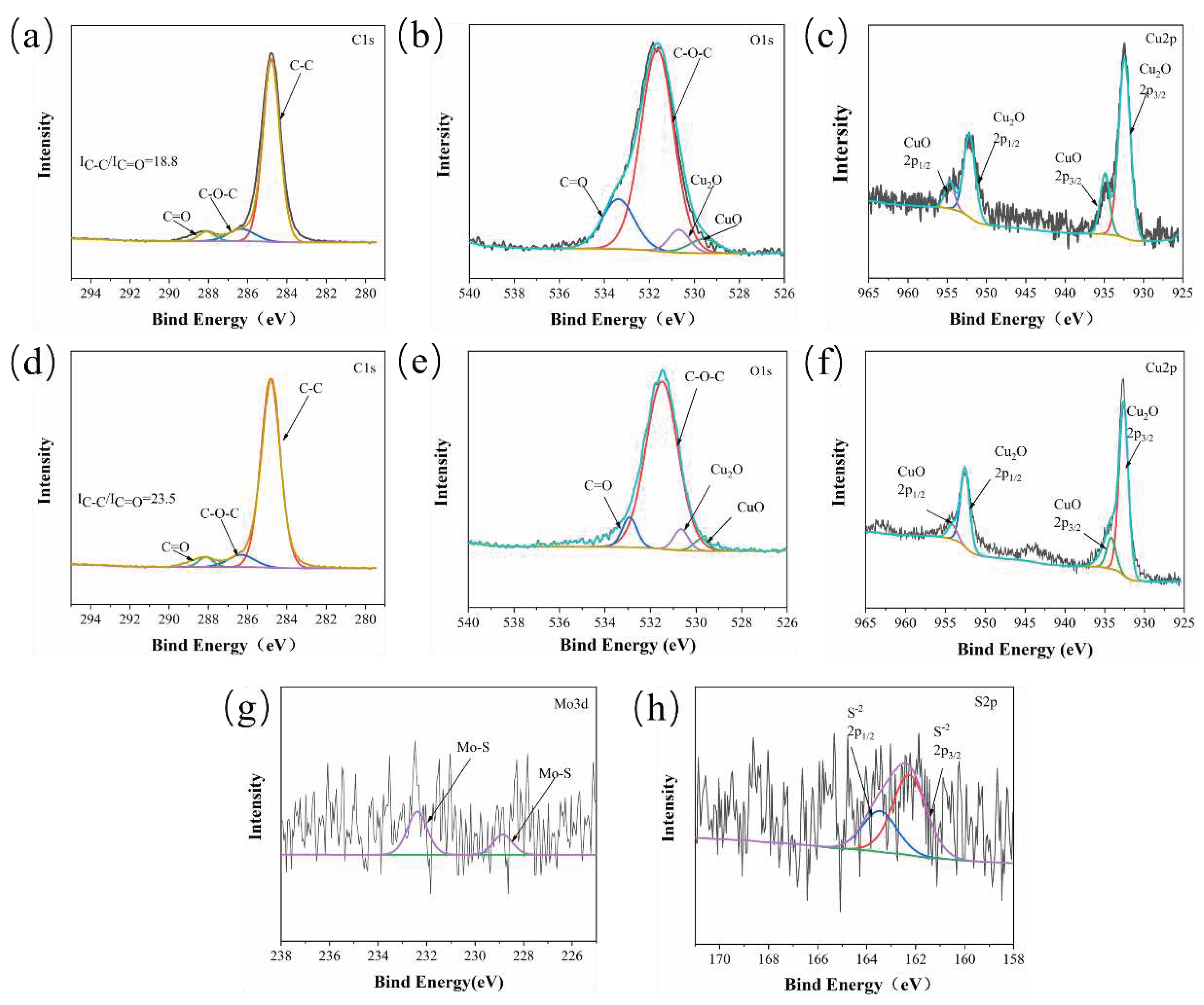
3.2.3. Tribo-Chemistry of Counterpart Surface with Addition of Gr and MoS2
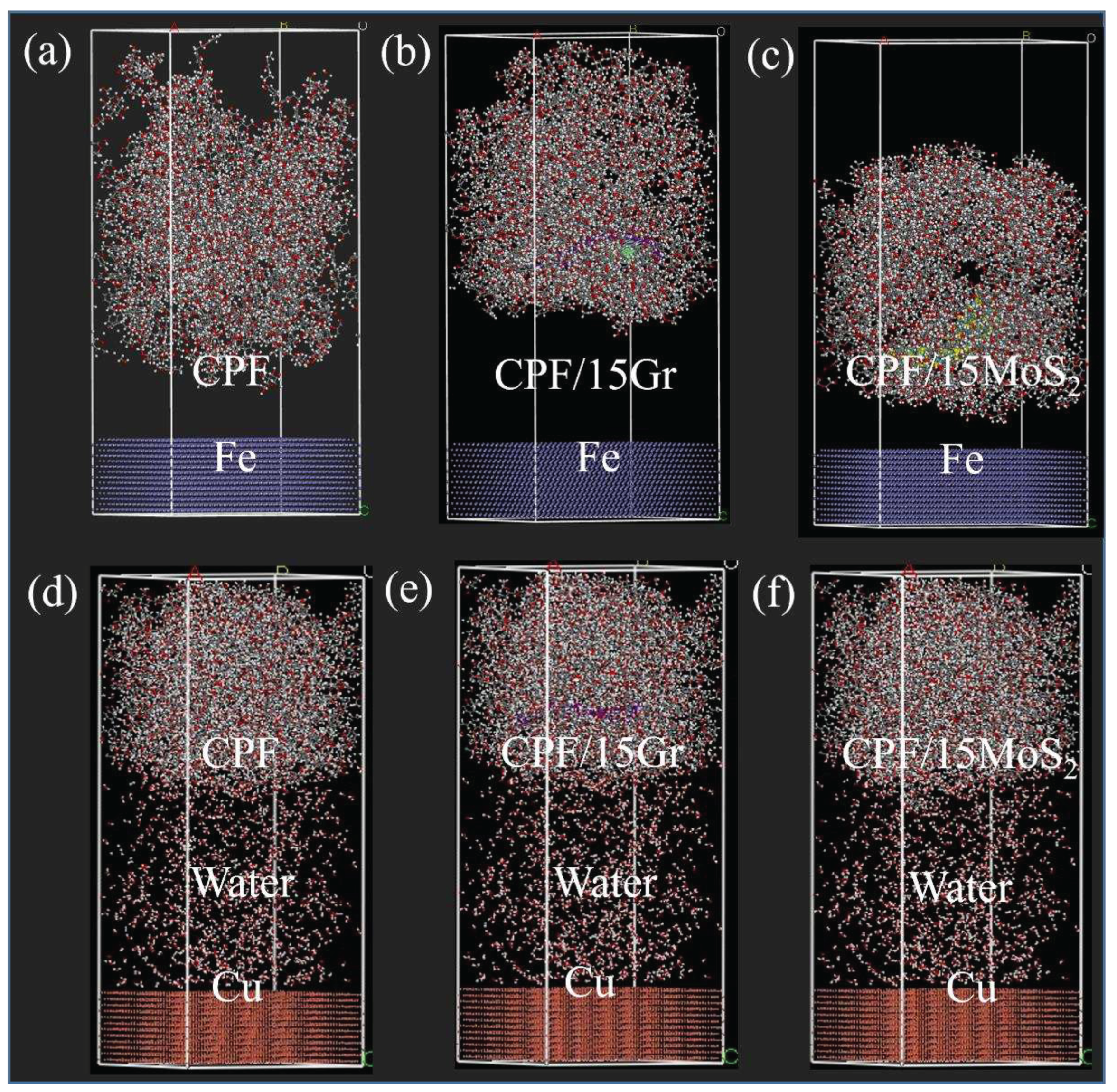
3.2.4. Molecular Dynamics Simulation of CPF Composites Modified by Gr and MoS2
3.2.5. The Synergistic Interaction between MoS2 and Gr
4. Conclusions
- Gr exhibits significantly better tribological performance than the other three fillers under dry friction. When the Gr content increased to 10% and 15%, the friction coefficient decreases by 24%, and the wear rate decreases by 78% compared to unmodified CPF composite.
- Under water lubrication conditions, all four fillers did not significantly alter the friction coefficient of the CPF composites. However, except for an excessive amount of Gr, the other three fillers can reduce the wear rate of the composite material. Particularly, in the case of 5% h-BN and 10% MoS2, the wear rate decreased by 47% and 56%, respectively.
- The adsorption capacity between the composites and the counterpart plays a crucial role in the formation of the transfer film under both dry friction and water lubrication conditions. Calculations of adsorption energy reveal that Gr is well-suited for dry friction, while MoS2 is more suitable for operation in a water environment. Using both MoS2 and Gr as co-modifiers for CPF composite, effective synergistic lubrication can be achieved.
Acknowledgments
Conflicts of Interest
References
- Xie, X.; Guo, Z.; Yuan, C. Investigating the water lubrication characteristics of sisal fiber reinforced ultrahigh-molecular-weight polyethylene material. Polym. Compos. 2020, 41, 5269–5280. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, X.; Wang, J.; et al. Influence of temperature on friction of polymeric materials in water. Wear 2019, 426–427, 868–876. [Google Scholar]
- Zhou, G.; Li, P.; Liao, D.; et al. The Friction-Induced Vibration of Water-Lubricated Rubber Bearings during the Shutdown Process. Materials 2020, 13. [Google Scholar]
- Chen, J.; Guo, Z.; Li, X.; et al. Development of gradient structural composite for improving tribological performance of PU material in water-lubricated bearings. Tribol. Int. 2022, 176. [Google Scholar]
- Zhang, L.; Guo, Y.; Xu, H.; et al. A novel eco-friendly water lubricant based on in situ synthesized water-soluble graphitic carbon nitride. Chem. Eng. J. 2021, 420. [Google Scholar]
- Li, Z.; Ma, S.; Zhang, G.; et al. Soft/Hard-Coupled Amphiphilic Polymer Nanospheres for Water Lubrication. ACS Appl Mater Interfaces 2018, 10, 9178–9187. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dong, S.; Yang, Z.; et al. Tribological Properties of Aramid Fiber-Microcapsule Modified Ultra-high Molecular Weight Polyethylene Composites for Water Lubrication. J. Mater. Eng. Perform. 2022, 31, 6000–6008. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, C.; Wang, L.; et al. Tribological Properties of Water-lubricated Rubber Materials after Modification by MoS2 Nanoparticles. Sci. Rep. 2016, 6, 35023. [Google Scholar] [CrossRef]
- Wang, C.; Bai, X.; Dong, C.; et al. Friction properties of polyacrylamide hydrogel particle/HDPE composite under water lubrication. Polymer 2019, 180. [Google Scholar] [CrossRef]
- Gao, C.; Guo, G.; Zhang, G.; et al. Formation mechanisms and functionality of boundary films derived from water lubricated polyoxymethylene/hexagonal boron nitride nanocomposites. Mater. Des. 2017, 115, 276–286. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Jiang, D.; et al. Enhancement of Underwater Tribological Properties of Hybrid PTFE/Nomex Fabric Laminate Composites by Epoxy Resins. ACS Omega, 2022, 7, 7737–7744. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, Z.; Song, Y.; et al. Effect of MWCNTs-GO hybrids on tribological performance of hybrid PTFE/Nomex fabric/phenolic composite. Compos. Sci. Technol. 2017, 146, 155–160. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Z.; Zhu, X.; et al. Combined effect of air-plasma treatment and lubricant filling on the dry sliding wear behavior of hybrid PTFE/Nomex fabric/phenolic composite. Compos. Sci. Technol. 2014, 100, 204–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Yuan, J.; et al. Friction and wear behaviors of MoS2-multi-walled-carbonnanotube hybrid reinforced polyurethane composite coating. Friction 2018, 7, 316–326. [Google Scholar]
- Liu, Y.; Gao, G.; Jiang, D.; et al. Enhancement of the Water-Lubricated Tribological Properties of Hybrid PTFE/Nomex Fabric Laminate Composite via Epoxy Resin and Graphite Filler. Materials 2021, 15. [Google Scholar]
- He, Y.; Duan, R.; Zhang, Q.; et al. Reinforce the mechanical toughness, heat resistance, and friction and wear resistance of phenolic resin via constructing self-assembled hybrid particles of graphite oxide and zirconia as nano-fillers. Adv. Compos. Hybrid Mater. 2021, 4, 317–323. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Chen, J.; et al. Enhanced mechanical, thermal, and tribological performance of 2D-laminated molybdenum disulfide/RGO nanohybrid filling phenolic resin composites. Adv. Compos. Hybrid Mater. 2022, 5, 1206–1220. [Google Scholar] [CrossRef]
- Renda, C.G.; Bertholdo, R. Study of phenolic resin and their tendency for carbon graphitization. J. Polym. Res. 2018, 25. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Z.; Liu, H.; et al. Improving the strength and oxidation resistance of phenolic resin derived pyrolytic carbons via Cu-catalyzed in-situ formation of SiC@SiO2. Solid State Sci. 2021, 118. [Google Scholar]
- Li, C.; Zhang, J.; Yi, Z.; et al. Preparation and characterization of a novel environmentally friendly phenol–formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Z.; Yang, M.; et al. Coupling hybrid of BN nanosheets and carbon nanotubes to enhance the mechanical and tribological properties of fabric composites. Compos. Part A Appl. Sci. Manuf. 2019, 123, 132–140. [Google Scholar] [CrossRef]
- Zang, C.; Xing, Y.; Yang, T.; et al. The Preparation and Wear Behaviors of Phenol-Formaldehyde Resin/BN Composite Coatings. Polymers 2022, 14. [Google Scholar]
- Zhang, H.-J.; Zhang, Z.-Z.; Guo, F. Studies of the Influence of Graphite and MoS2 on the Tribological Behaviors of Hybrid PTFE/Nomex Fabric Composite. Tribol. Trans. 2011, 54, 417–423. [Google Scholar] [CrossRef]
- Oliver, B.A.; Dong, Q.; Ramezani, M.; et al. Tribological Performance of Bamboo Fabric Reinforced Epoxy Composites. Macromol. Mater. Eng. 2023. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, D.; Zhang, L.; et al. Significance of g-C3N4 nanosheets for enhancing tribological performance of epoxy subjected to starved lubrication. Tribol. Int. 2022, 174, 107762. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, H.; Li, G.; et al. Significantly enhanced wear resistance of PEEK by simply filling with modified graphitic carbon nitride. Mater. Des. 2017, 129, 192–200. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Yang, M.; et al. One-step synthesis of g-C3N4 nanosheets to improve tribological properties of phenolic coating. Tribol. Int. 2019, 132, 221–227. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Z.; Zhu, X.; et al. Influence of lubricant filling on the dry sliding wear behaviors of hybrid PTFE/Nomex fabric composite. J. Mater. Sci. 2014, 49, 3716–3724. [Google Scholar] [CrossRef]
- Wu, H.; Yin, S.; Du, Y.; et al. Alkyl-Functionalized Boron Nitride Nanosheets as Lubricant Additives. ACS Appl. Nano Mater. 2020, 3, 9108–9116. [Google Scholar] [CrossRef]
- Levita, G.; Restuccia, P.; Righi, M.C. Graphene and MoS2 interacting with water: A comparison by ab initio calculations. Carbon 2016, 107, 878–884. [Google Scholar] [CrossRef]
- Hou, X.; Bai, P.; Li, J.; et al. MoS2 reinforced PEEK composite for improved aqueous boundary lubrication. Friction 2023. [Google Scholar]
- Yang, J.; Zhang, H.; Chen, B.; et al. Fabrication of the g-C3N4/Cu nanocomposite and its potential for lubrication applications. RSC Adv. 2015, 5, 64254–64260. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Yan, F. Friction and Wear Behaviors of Several Polymers Sliding Against GCr15 and 316 Steel Under the Lubrication of Sea Water. Tribol. Lett. 2011, 42, 17–25. [Google Scholar] [CrossRef]
- Gao, C.P.; Guo, G.F.; Zhao, F.Y.; et al. Tribological behaviors of epoxy composites under water lubrication conditions. Tribol. Int. 2016, 95, 333–341. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Wang, D.; et al. The development of a Cu@Graphite solid lubricant with excellent anti-friction and wear resistant performances in dry condition. Wear 2022, 488–489, 488–489. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. The effect of hygrothermal history on water sorption and interlaminar shear strength of glass/polyester composites with different interfacial strength. Compos. Part A Appl. Sci. Manuf. 2003, 34, 1117–1124. [Google Scholar] [CrossRef]
- Yu, P.; Li, G.; Zhang, L.; et al. Role of SiC submicron-particles on tribofilm growth at water-lubricated interface of polyurethane/epoxy interpenetrating network (PU/EP IPN) composites and steel. Tribol. Int. 2021, 153. [Google Scholar]
- Xu, Y.; Peng, Y.; Dearn, K.D.; et al. Synergistic lubricating behaviors of graphene and MoS2 dispersed in esterified bio-oil for steel/steel contact. Wear 2015, 342–343, 297–309. [Google Scholar]
- You, Y.-L.; Li, D.-X.; Si, G.-J.; et al. Investigation of the influence of solid lubricants on the tribological properties of polyamide 6 nanocomposite. Wear 2014, 311, 57–64. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, G.; Wang, D.; et al. Significance of combined functional nanoparticles for enhancing tribological performance of PEEK reinforced with carbon fibers. Compos. Part A Appl. Sci. Manuf. 2017, 102, 400–413. [Google Scholar] [CrossRef]
- Longlong, L.N.P.B.Z. Tribological Performance of PTFE/FeOCl Composites under Paraffin-lubricated Conditions. Tribol. Lett. 2023. [Google Scholar]
- Xu, M.; Wang, X.; Wang, T.; et al. Ag nanoparticle decorated graphene for improving tribological properties of fabric/phenolic composites. Tribol. Int. 2022, 176. [Google Scholar]
- Wang, J. Water Lubrication Bearing Technology and Application; Science Press: Beijing, China, 2018. [Google Scholar]

| Composites/wt% | PF | g-C3N4 | Gr | MoS2 | h-BN |
|---|---|---|---|---|---|
| CPF | 100 | / | / | / | / |
| CPF/3CN | 97 | 3 | / | / | / |
| CPF/5CN | 95 | 5 | / | / | / |
| CPF/10CN | 90 | 10 | / | / | / |
| CPF/15CN | 85 | 15 | / | / | / |
| CPF/3Gr | 97 | / | 3 | / | / |
| CPF/5Gr | 95 | / | 5 | / | / |
| CPF/10Gr | 90 | / | 10 | / | / |
| CPF/15Gr | 85 | / | 15 | / | / |
| CPF/3MoS2 | 97 | / | / | 3 | / |
| CPF/5MoS2 | 95 | / | / | 5 | / |
| CPF/10MoS2 | 90 | / | / | 10 | / |
| CPF/15MoS2 | 85 | / | / | 15 | / |
| CPF/3BN | 97 | / | / | / | 3 |
| CPF/5BN | 95 | / | / | / | 5 |
| CPF/10BN | 90 | / | / | / | 10 |
| CPF/15BN | 85 | / | / | / | 15 |
| Etotal | Elayer1 | Elayer2 | E | |
|---|---|---|---|---|
| CPF | 22063.98 | 22129.34 | -42.6215 | 22.7391 |
| CPF/15Gr | 59974.35 | 60044.69 | -42.6215 | 27.7161 |
| CPF/ 15MoS2 | 38583.75 | 38650.52 | -42.6215 | 24.1535 |
| Etotal | Elayer1 | Elayer2 | E | |
|---|---|---|---|---|
| CPF | 24527.14 | 20324.73 | 4227.439 | 25.0309 |
| CPF/15Gr | 52501.46 | 48298.61 | 4227.439 | 24.589 |
| CPF/15MoS2 | 44046.73 | 39845.03 | 4227.439 | 25.7347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





