1. Introduction
Three-dimensional (3D) bioprinting is a promising technology with applications in various fields that were once considered only science fiction [
1,
2]. As research in this area has expanded fast, more specialized devices have been developed to meet the growing demand [
3] and market requirements [
4]. Consequently, a wide range of 3D bioprinters are now available, including devices that have been adapted from plastic 3D printers [
5,
6]. However, due to the diversity of devices, standardization between bioprinters is necessary. The issue of repeatability, which involves producing identical structures with different bioprinters, has not been thoroughly explored but needs to be addressed.
Since bioprinting is a digital manufacturing process, a computer-aided design (CAD) model is developed to represent the structure that will be physically printed. This model, created using software in specific formats, must then be converted into code that can be understood by bioprinters allowing the printing process to occur [
7,
8]. This slicing software processes the model and generates commands configured specifically for each material and bioprinter so that it is possible to actually materialize the digital structure. In summary, actual bioprinting processes is performed by three steps: i) the creation of the CAD model, ii) the selection of the material that will make up the structure in the physical world, and iii) the processing of the CAD model in a slicing software that generates commands that the bioprinter understands [
9].
Bioprinters, although varying in size, price, resolution, and configuration, are based on the same principle: the motion of three axes, which enables the generation of 3D structures. The generation of g-code is essential in the bioprinting workflow, as it provides control over the bioprinting process. Consequently, it is possible to develop universal protocols that allow the replication of bioprinted structures across different bioprinters by adjusting specific parameters. The aim of this study was to investigate the differences between bioprinting devices using a simple and repeatable protocol, facilitating the extrapolation of the production processes of 3D structures through parameter adjustments.
2. Material and Methods
2.1. Bioprinter
Four bioprinters were used: GENESIS and STARTER, two models from 3DBS Biotechnology Solutions; Reb3l from SE3D; and BIOMAKER, a prototype from Due Laser. All bioprinters could be controlled using Pronterface software. While all four bioprinters accommodate 5-mL syringes, only BIOMAKER allowed the use of 10-mL syringes. To compare the bioprinters, 5-mL luer-lock syringes filled with water were connected to each bioprinter, and various extrusion commands were tested. The extruded water was weighed using a small commercial scale, and the extrusion time was recorded. An additional test was performed using BIOMAKER to compare the extrusion of 10 mL and 5 mL syringes.
2.2. G-Code Commands on Different Equipment
The printhead of each device was positioned at the center of the print table using the appropriate command calculated based on the printable area of each bioprinter's table. The command used was G1 Xx Yy, where y = height/2 and x = width/2 (
Table 1). The next step involved setting the printhead's height to approximately 5 cm from the table, allowing for the placement of a 10-mL beaker under the printhead. Subsequently, the extrusion command was sent to the bioprinter using the command line in Pronterface.
3. Results and Discussion
3.1. Extrusion Value
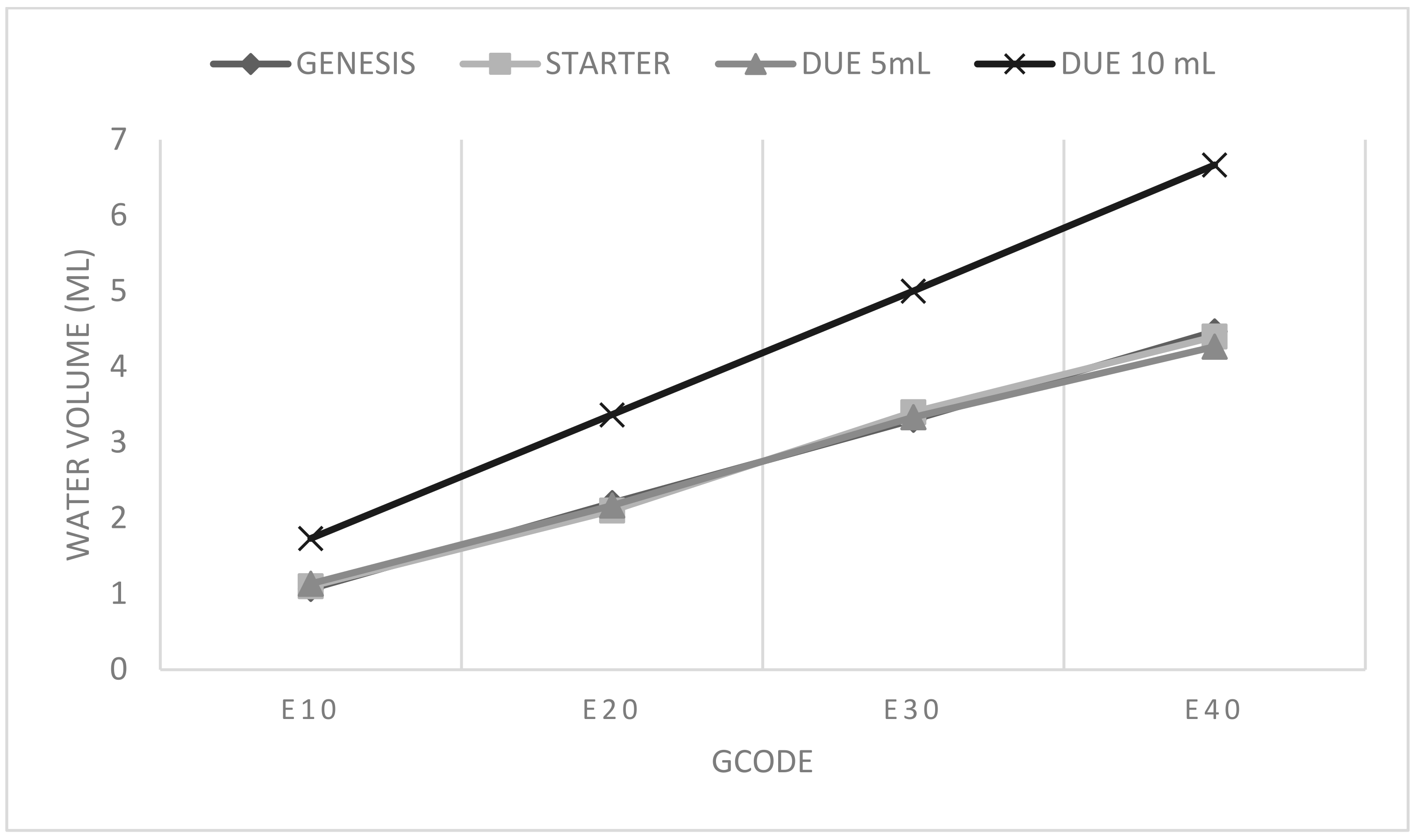
that the study revealed that the same E-value resulted in different volumes of water extruded by different bioprinters. Among all the bioprinters, the Reb3l from SE3D exhibited the greatest variation. For this device, the E10 command in the first test resulted in an extrusion value too low to break the surface tension of the water, resulting in only a small droplet forming at the syringe tip. Consequently, nothing could be weighed by the balance. To obtain usable E-values, they were multiplied by 10, enabling the measurement of extruded values using the established method (water weighing on the balance). The two 3DBS bioprinters exhibited similar behavior, as expected from models produced by the same company. The BIOMAKER prototype bioprinter displayed the corresponding result (
Figure 1).
Since the BIOMAKER bioprinter also accommodated 10-mL syringes, a second test was conducted to compare the extruded volume for the same E-value command (
Figure 1). The results show that once the extruded volume was measured using one syringe volumetric capacity, it could be extrapolated for other sizes. Notably, the inner diameter of the 5-mL syringes was 11 mm, while the 10-mL syringes had an inner diameter of 16 mm. This information could be used instead of performing additional tests for both conditions, providing insights into the behavior of the bioprinter.
Overall, the results suggest a direction for parameterization in 3D bioprinting identical structures on different bioprinters. By establishing a relationship between the same extrusion values on different devices, it becomes possible to target specific parameters in the slicing software. The available software for open-source 3D printers primarily focuses on the Fused Deposition Modeling (FDM) process, which is used for printing plastics, rather than the process of material deposition by extrusion in syringes, as observed in 3D bioprinting [
10]. Consequently, understanding machine behavior allows for software adaptation through precise parameter control.
For the bioprinters tested, knowledge of the relative values of E1 for each bioprinter enables the determination of the ideal extrusion value for a specific machine and extrapolation of this value to other machines using the value ratio (
Table 2). For instance, if the ideal extrusion value for creating a 2 cm line from the center of the table is E0.5 for BIOMAKER (G1 X20 E0.5), the corresponding value for SE3D bioprinter would be E13, calculated based on the ratio between the values. If E0.5 for BIOMAKER corresponds to 0.0549 mL, the command for SE3D bioprinter representing the same volume is E6.4049. Understanding this relationship allows for parameter adjustment, resulting in numerically different E-values that represent the same output on different machines.
3.2. Extrusion Speed
Another variable briefly tested was the extrusion speed, represented by the F-value. The experiments demonstrated an inversely proportional relationship between printing time and speed, as expected. For example, when comparing two speeds where one was five times higher than the other, the extrusion time was five times shorter (
Table 3).
This relationship guarantees a proportional connection between two variables. However, it also raises questions about the relationship between extrusion volume and speed. In this case, the printing time in 3D bioprinting can be a bottleneck for the fabrication of the structures, particularly when using materials derived from biological and organic resources that undergo rapid changes. In tissue regeneration and food applications, for instance, printing time becomes critical factor in maintaining cell viability.
4. Conclusions
Given the diversity of bioprinters, achieving repeatability in structure printing is essential. To accomplish this, it is necessary to understand how the same command corresponds to different machines and how the parameters can be adjusted. Thus, this study presents a method for comparing machines and extrapolating the relationship among them. It is expected that studies of this nature will contribute to achieving process repeatability and, in the future, enable the creation of g-code scripts capable of controlling any 3D bioprinting or food printing device to achieve the consistent results.
Acknowledgments
This research was funded by The Good Food Institute (GFI) and Embrapa, grant number 10.21.00.088.00.00. We also appreciate the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 311825/2021-4). We would like to express our heartfelt gratitude to The Good Food Institute for their support and grant provided for our research project. Their invaluable assistance has greatly contributed to the success and advancement of our work.
References
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Q.; Zourob, M. 3D bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Front. Med. Technol. 2021, 2, 607648. [Google Scholar] [CrossRef]
- Choudhury, D.; Anand, S.; Naing, M.W. The arrival of commercial bioprinters—Towards 3D bioprinting revolution! Int. J. Bioprinting 2018, 4. [Google Scholar] [CrossRef]
- Elemoso, A.; Shalunov, G.; Balakhovsky, Y.M.; Ostrovskiy, A.Y.; Khesuani, Y.D. 3D Bioprinting: The roller coaster ride to commercialization. Int. J. Bioprinting 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Kahl, M.; Gertig, M.; Hoyer, P.; Friedrich, O.; Gilbert, D.F. Ultra-low-cost 3D bioprinting: Modification and application of an off-the-shelf desktop 3D-printer for biofabrication. Front. Bioeng. Biotechnol. 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, B.; Temirel, M.; Knowlton, S.; Lepowsky, E.; Tasoglu, S. Development and characterization of a low-cost 3D bioprinter. Bioprinting 2019, 13, e00044. [Google Scholar] [CrossRef]
- Gulyas, M.; Csiszer, M.; Mehes, E.; Czirok, A. Software tools for cell culture-related 3D printed structures. PLoS ONE 2018, 13, e0203203. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, C.; Popov, D.; Maltsev, E.; Akhatov, I.; Pasko, A. Software for bioprinting. Int. J. Bioprinting 2020, 6. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dávila, J.L.; Manzini, B.M.; da Fonsêca, J.H.L.; Corzo, I.J.M.; Neto, P.I.; de Lima Montalvão, S.A.; ...; da Silva, J.V.L. A parameterized g-code compiler for scaffolds 3D bioprinting. Bioprinting 2022, 27, e00222.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).