1. Introduction
Bone homeostasis is maintained by the balance between the formation of new bone matrix by mononucleated osteoblasts and bone resorption by multinucleated osteoclasts [
1]. Disruption of this homeostasis may result in bone diseases, such as osteoporosis and rheumatoid arthritis [
2]. Increased osteoclast differentiation induced by estrogen deficiency in postmenopausal women augments the risk of bone metabolism disorders owing to bone loss and muscle weakness [
3].
Receptor activator of nuclear factor-κB (NF-κB) (RANK) ligand (RANKL) and macrophage colony-stimulating factors are essential for osteoclast differentiation [
4], as both play an important role in the survival and functional maintenance of osteoclasts, including those expressing tartrate-resistant acid phosphatase (TRAP), cathepsin K, and calcitonin receptor [
5]. RANKL binds to RANK, a receptor expressed by macrophages, which are the precursor cells of osteoclasts, to activate various signaling pathways [
6]. These pathways activate NF-κB through tumor necrosis factor receptor-associated factor 6, inducing the expression of different genes, such as those encoding c-Fos and nuclear factor of activated T cells 1 (NFATc1) [
7,
8,
9]. NFATc1, a master regulator, is an essential factor for osteoclast differentiation, and NFATc1, which is upregulated through this mechanism, induces the expression of TRAPs, cathepsin K, calcitonin receptor, and osteoclast-associated receptors [
5,
10].
The rhizome of
Drynaria fortunei (Kunze) J. Sm., a perennial herb and member of the family
Polypodiaceae, is harvested throughout the year and can be dried, steamed, or smoked [
11]. As per the Donguibogam, the Korean medical encyclopedia,
D. fortunei has a warm property and is bitter and nontoxic; it relieves blood extravasation, stops bleeding, repairs broken bone tissue, treats malignant boils, and kills bacteria [
12]. It is known as “Golsebo” in Korea and as “Gusuibu” in China and is traditionally used for treating bone fractures and related diseases, such as osteoporosis, bone metabolism disorders, and osteoarthritis, thereby preventing hyperlipidemia and promoting bone recovery. Moreover, it has been reported to be clinically beneficial in treating pediatric fractures [
13,
14]. Particularly,
D. fortunei promotes bone health and growth by inducing calcium absorption and increasing alkaline phosphate activity, which plays an important role in proteoglycan synthesis [
15,
16]
The benefits and pharmacological mechanisms of polysaccharides (which are natural biopolymers), including their antiviral, anticancer, antibacterial, anti-inflammatory, blood cholesterol-lowering, and blood pressure-lowering effects, have been reported in multiple studies [
17,
18,
19,
20,
21,
22].
D. fortunei has various biological activities owing to its complex chemical composition that includes flavonoids, proanthocyanidins, triterpenoids, phenolic acids, and lignans [
23]. Recent studies have reported that proanthocyanidins extracted from the rhizome of
D. fortunei can replace plant-derived estrogen for the treatment of postmenopausal osteoporosis and that
D. fortune-derived polysaccharide (DFP) extracts inhibit bone loss following ovariectomy [
24,
25]. However, the molecular mechanism underlying the role of
D. fortunei polysaccharide extracts in improving bone metabolism is unclear. Therefore, we investigated their influenceon the mRNA and protein levels and the activity of signaling proteins in RANKL-induced osteoclast differentiation.
2. Materials and Methods
2.1. Chemicals and Reagents
The chemicals listed in
Table 1 and the reagents (alpha-modified minimum essential medium Eagle and fetal bovine serum) used for cell culture were purchased provided from Thermo Fisher Scientific (Rockford, IL, USA). P-nitrophenyl phosphate was obtained from Sigma-Aldrich (St. Louis, MO, USA). In addition, the antibodies and secondary antibodies (c-Fos, NFATc1, β-actin) used in this study were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Chemical Profiling of DFPs
Drynaria fortunei was purchased from Omniherb (Seoul, Republic of Korea). After the experiment it was deposited labelling #KW-3 at the Korea Institute of Oriental Medicine.
D. fortunei (1.0 kg) was washed by refluxing with distilled water (10 L) for 3 h and dried using Ilsinbiobase-vacuum freeze dryer (Dongduchun, Republic of Korea). A solution mixed the dried DFPs powder (final concentration: 80%, v/v) and cold ethanol was maintained at −20 °C for 12 h. The precipitate was dissolved in water, the protein was prepared by deproteinization using sevage method, ultrafiltration using Vivaspin 20 (Sartorius, Goettingen, Germany; filter cutoff: 3 kDa) and lyophilization (yield: 0.49%). The DFP powders (10 mg/mL) dissolved in distilled water was ultrafiltered (filter cutoff: 10–1,000 kDa) again and stored at -20°C.
To determine the chemical characterization of DFP, it was analysed using formalised methods. Total sugar and uronic acid content [
26], 2-keto-3-deoxy-mannanoic acid content [
27], thiobarbituric acid method [
28] and protein content were analysed according to the Bradford method and and bovine serum albumin, respectively. In addition, the monosaccharide composition and molecular weight patterns of DFP were determined using ultrahigh-performance liquid chromatography–tandem mass spectrometry [
29]. Reference standards and DFP hydrolysates generated using trifluoroacetic acid were derivatized using 1-phenyl-3-methyl-5-pyrazolone. Monosaccharides produced by acid hydrolysis were isolated using the universal column (ACQUITY UPLC BEH C18 column, 150 × 2.1 mm, 1.7 μm) with acetonitrile and 25 mM ammonium acetate in water (pH 8.0, adjusted with ammonia). For identifying the monosaccharides based on their retention times and fragment patterns in the mass spectra, we applicated eleven reference standards. Asahipak GS-620, GS-520, and GS-320 columns (0.76 × 30 cm each; Showa Denko Co., Tokyo, Japan) by injecting 25 μL were rinsed with 50 mM ammonium formate (pH 5.5, adjusted with formic acid) at a flow rate of 0.40 mL/min. Also, the standard curve was showed using a dextran standard set (D-670, 410, 150, 50, 25, 12, and 5 kDa) and below equation:
2.3. Osteoclast Differentiation Assay
Bone marrow-derived macrophages (BMDMs) were procured and cultured as previously described [
30]. It was incubated in 96-well plates (1 × 10
4 cells/well) or 6-well plates (1 × 10
5 cells/well), prepared with different concentrations of DFPs (1–200 μg/mL) for 1 h, followed by RANKL treatment to induce osteoclast differentiation and culturing for 3 days. BMDMs were fixed and permeabilized, and osteoclast formation and maturation were evaluated using TRAP staining, as previous paper [
30]. TRAP-positive multinucleated cells based BMDMs were fixed using a microscope. TRAP activity was determined with p-nitrophenyl phosphate substrate. The viability of BMDMs treated with DFPs (1–200 μg/mL) was determined using the Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Tokyo, Japan).
2.4. Quantitative PCR and Western Blotting
Total RNA was isolated from the cells, using the RNeasy Mini kit (Qiagen, Hilden, Germany) and cDNA kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize from total RNA (1 μg). PCR on an ABI 7500 Real-Time PCR System (Applied Biosystems) was performed using TaqMan primers to amplify region of the specific gene cDNA. The genes were as follows c-Fos (Mm00487425_m1), NFATc1 (Mm00479445_m1), Atp6v0d2 (Mm00656638_m1), DC-STAMP (Mm01168058_m1), cathepsin K (Mm00484036_m1), and 18S ribosomal RNA (rRNA, Hs99999901_s1).
For western blotting data, cell lysates were prepared using lysis buffer (Roche Diagnostics, Indianapolis, IL, USA). The protein concentrations were measured using a bicinchoninic acid kit (Thermo Fisher Scientific) and were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane and blotted using primary (1:1000) and secondary (1:2000) antibodies. The instrument used for visualization was a ChemiDoc imaging system (Bio-Rad Laboratories).
2.5. Statistical Analysis
All data are presented as mean ± standard error (SEM). Differences between groups were assessed by one-way analysis of variance (ANOVA), and p<0.05 was considered statistically significant.
3. Results
3.1. Phytochemical Properties of Polysaccharides
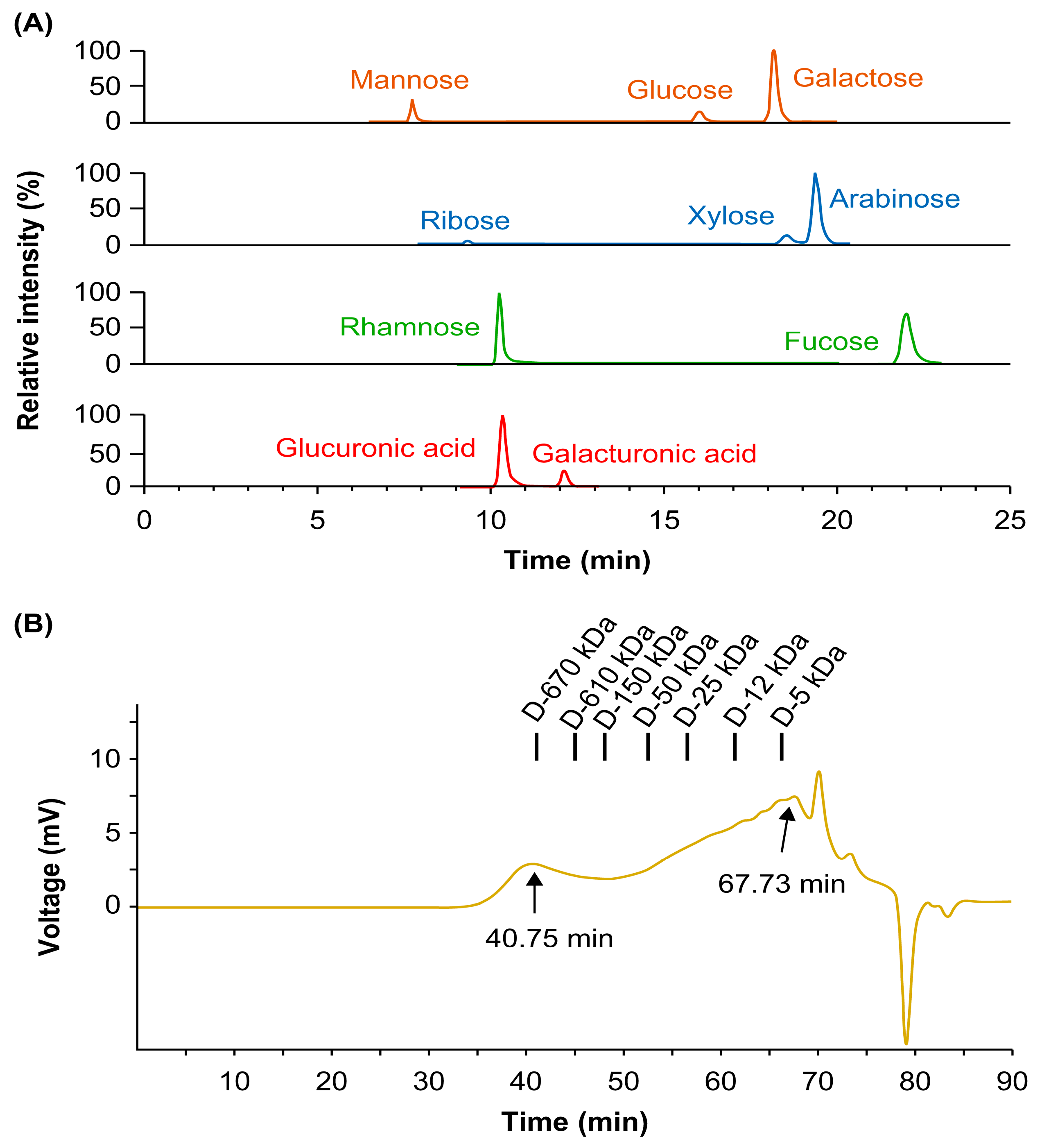
To characterize DFPs, we measured their sugar content, monosaccharide composition, and molecular weight. DFPs were rich in sugars, comprising 65.9% neutral sugar and 23.2% uronic acid (
Table 1). Ultra-high-performance liquid chromatography–tandem mass spectrometry and pre-column derivatization revealed that DFPs were mainly composed of galactose (23.75 mol%), arabinose (15.56 mol%), glucose (14.97 mol%), glucuronic acid (14.84 mol%), mannose (12.93 mol%), rhamnose (5.18 mol%), xylose (3.76 mol%), fucose (3.73 mol%), galacturonic acid (3.45 mol%), and ribose (1.83 mol%) (
Table 1 and
Figure 1a). The molecular weight profile analysis using HPSEC revealed two major peaks at 40.75 and 67.73 min (
Figure 1b).
3.2. DFPs Inhibit Osteoclastogenesis
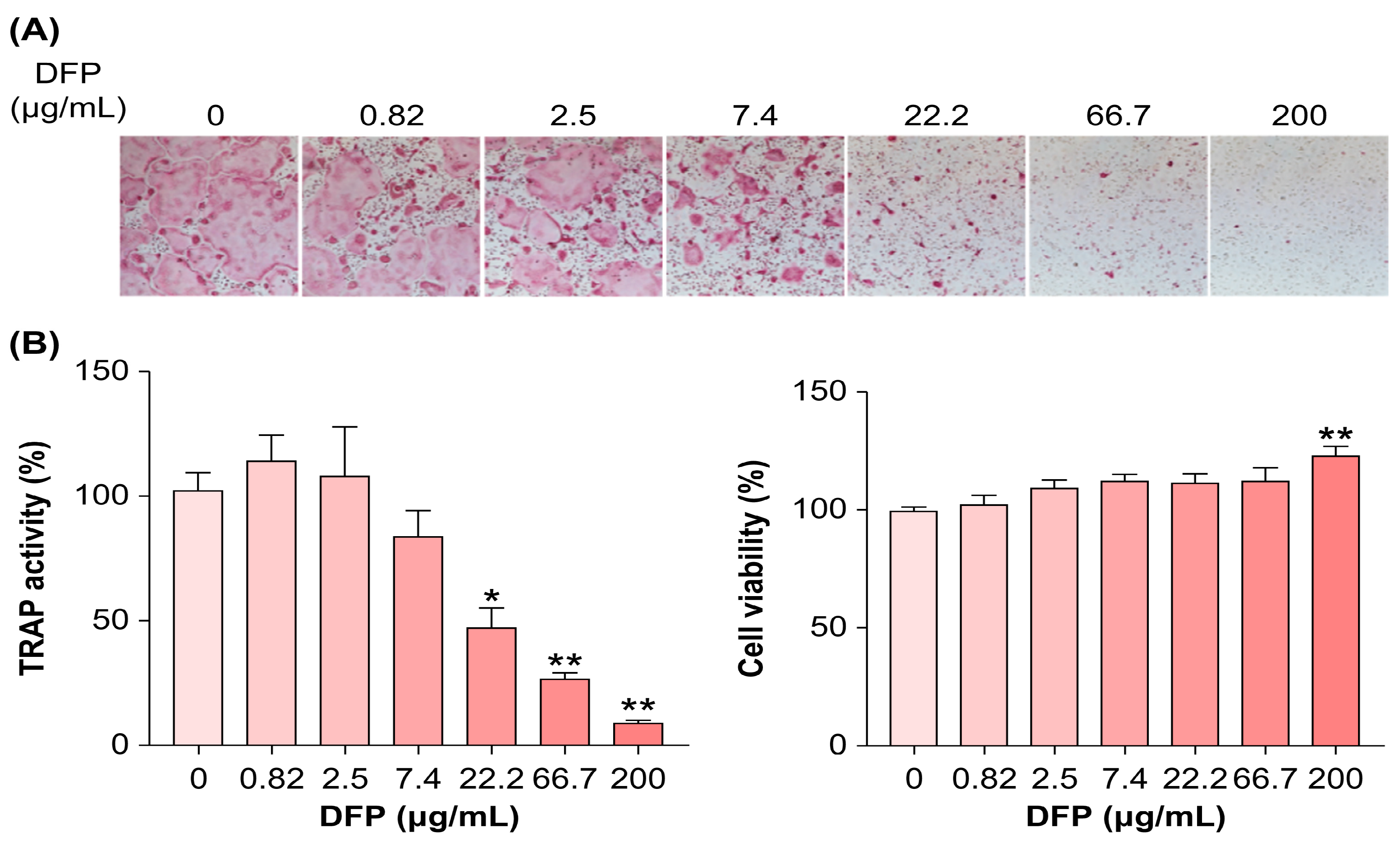
Considering the beneficial effects of DFPs on bone loss
in vivo, we examined whether DFPs and their major constituents modulate osteoclastogenesis in BMDMs. In the presence of macrophage colony-stimulating factors and RANKL, we treated BMDMs with DFPs (0–200 µg/mL) and investigated the formation of TRAP-positive multinucleated cells (
Figure 2a). DFP treatment reduced the differentiation and TRAP activity of osteoclasts in a dose-dependent manner compared to those of control cells (
Figure 2b). Moreover, DFP-related cytotoxicity was not observed for the tested doses, excluding the possibility of osteoclastogenesis inhibition due to cytotoxicity (
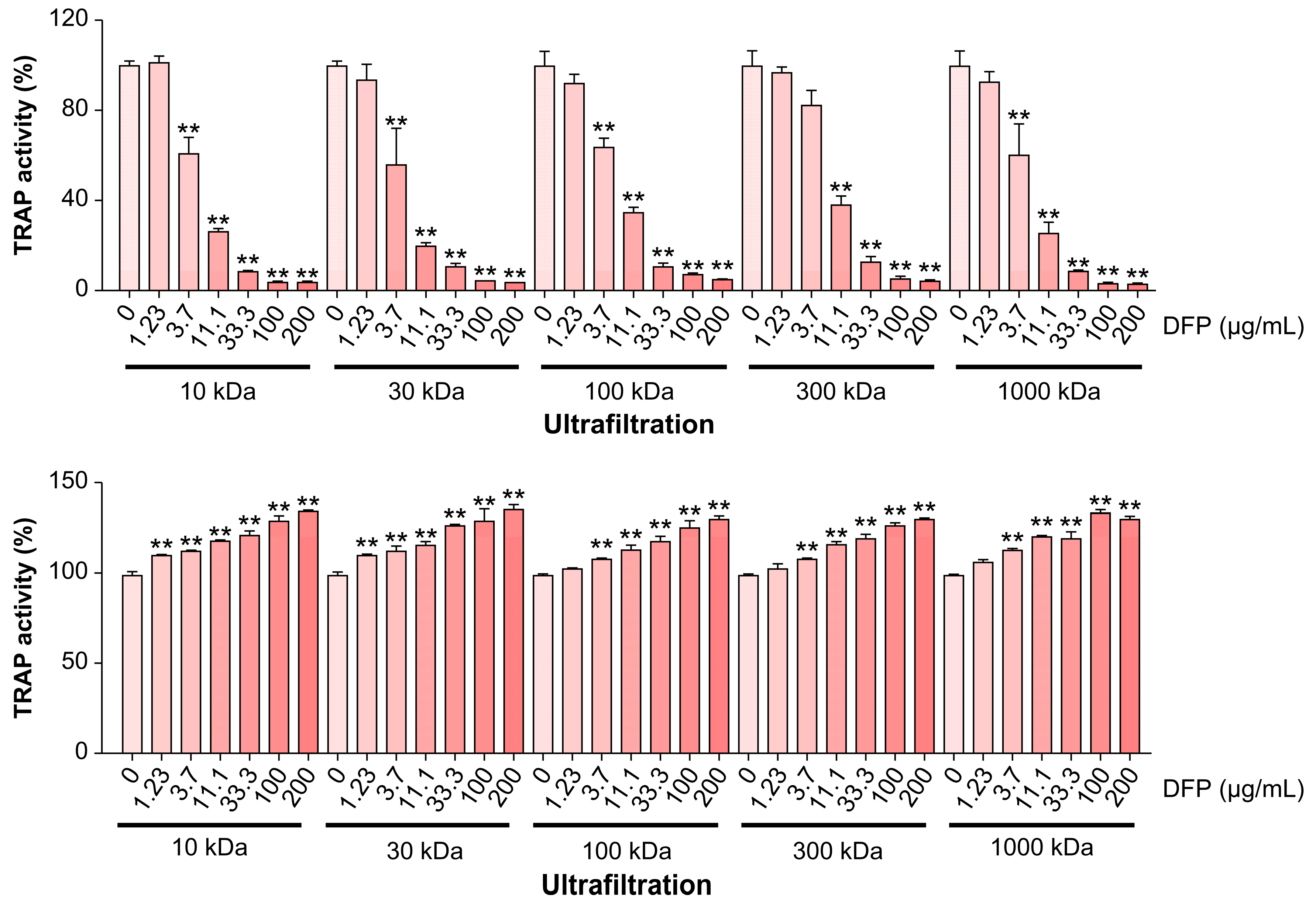
Figure 2b). As shown in
Figure 3, the molecular weights of DFP fragments were 10, 30, 100, 300, and 1,000 kDa, as determined using HPSEC. The molecular weight cutoff of DFPs revealed an impact on TRAP activity at an initial concentration of 3.7 µg/mL (
Figure 3). These findings indicate that DFPs interrupt RANKL-induced osteoclastogenesis.
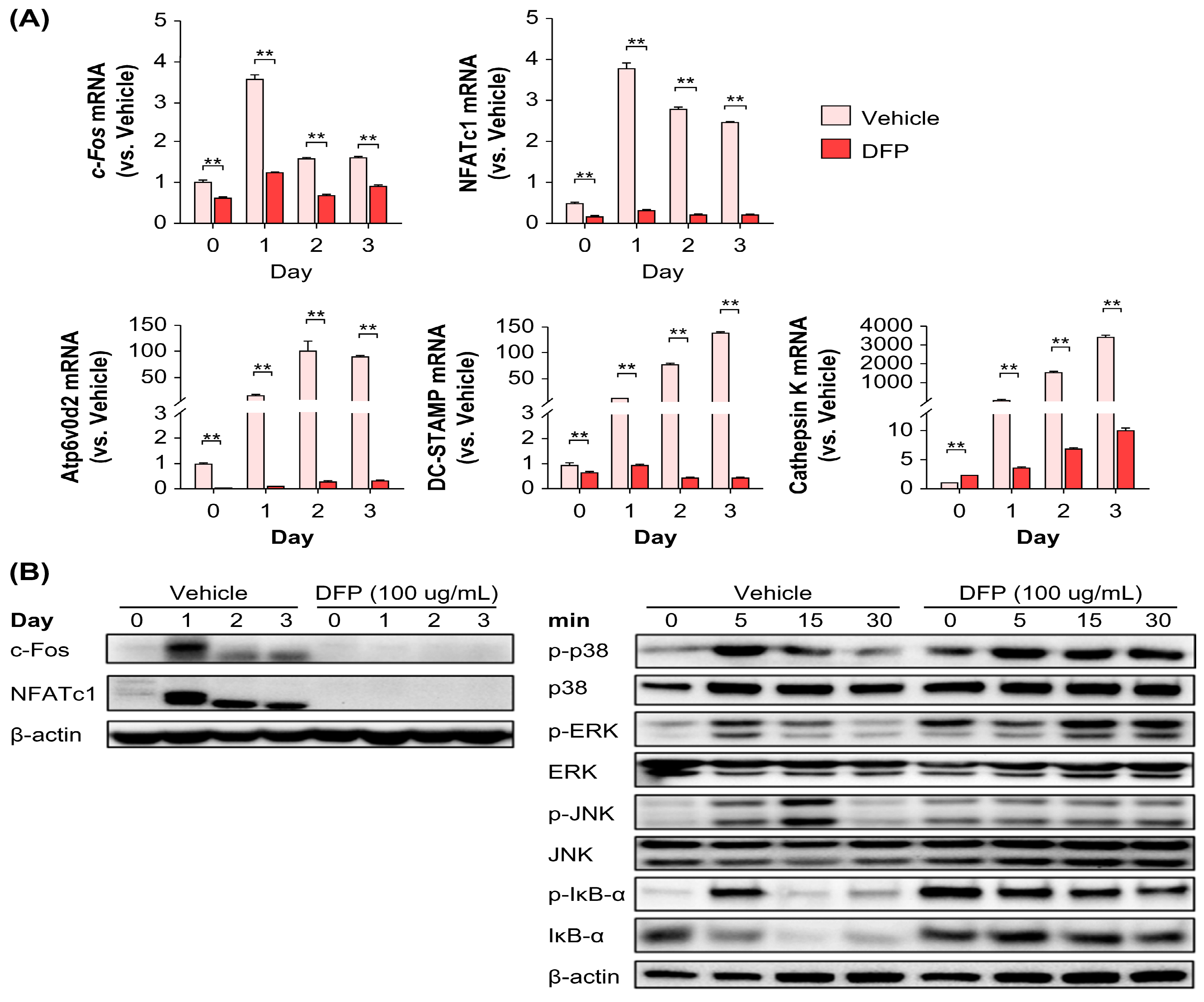
3.3. DFPs Suppress RANKL-Induced Key Osteoclastic Marker Expression
Based on the potent inhibitory effect of DFPs on RANKL-induced osteoclast precursors, we next elucidated their effects on the mRNA and protein levels of key transcription factors and osteoclast-specific genes. DFP treatment (200 µg/mL) reduced the NFATc1 and c-Fos mRNA and protein levels during osteoclast differentiation (
Figure 4a). Furthermore, downregulation of these key transcription factors by DFPs inhibited the expression of DC-STAMP, Atp6v0d2, and cathepsin K genes (
Figure 4a). These results suggest that DFPs suppress early-stage osteoclastogenesis by downregulating osteoclast-specific transcription factors.
To further examine the effect of DFPs on the differentiation mechanism of osteoclasts, BMDMs were pretreated with DFPs and then treated with RANKL each day (0, 1, 2, and 3 days) to determine the levels of specific proteins. c-Fos and NFATc1 protein levels were significantly reduced during osteoclast differentiation in the DFP-treated group (100 µg/mL) (
Figure 4b), which was similar to the mRNA level results. Subsequently, BMDMs pretreated with DFPs were treated with RANKL at different time points within 1 h (0, 5, 15, and 30 min) to examine the levels and phosphorylation of mitogen-activated protein kinases (MAPKs) (
Figure 4b). An increase in the levels of p38, extracellular signal-regulated kinase, and c-Jun N-terminal kinase (JNK) was observed in BMDMs of the vehicle group stimulated by RANKL, but no significant differences were observed in the DFP-treated group, except for JNK. In the vehicle group, the degradation of nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor (IκB)-α was increased, whereas in the DFP-treated group, IκB-α was inhibited (
Figure 4b).
4. Discussion
Previous studies have shown that
D. fortunei is effective in promoting calcium absorption during fracture, preventing pain and hyperlipidemia, as well as being useful for sedation [
31,
32]. The mechanism underlying the effect of DFPs on osteoclast differentiation of RANKL-induced BMDMs appears to be the same as that reported by Kwak et al. (2012). However, in this study, we found that DFPs significantly inhibited osteoclast differentiation. BMDMs treated for 7 days with DFPs from RANKL-induced cells displayed increased TRAP activity, which is important for osteoclast differentiation. Osteoclast formation was suppressed in a dose-dependent manner by 1–200 µg/mL DFPs, and cytotoxicity was not observed. These findings were also obtained for polysaccharides of different molecular weights (10, 30, 100, 300, and 1,000 kDa). These results imply that DFPs suppress osteoclast differentiation, regardless of their size or molecular weight. Furthermore, our findings are similar to those of Sun et al., in which DFPs significantly increased bone mineral density and bone mineral content index in a rat model of postmenopausal osteoporosis compared with those of control rats, thereby exhibiting anti-osteoporotic effects [
25].
RANKL-activated NF-κB is a key transcription factor in osteoclast differentiation [
33]. IκB kinases phosphorylate IκB, which initially inhibits NF-κB expression, and IκB is subsequently degraded by proteolysis through the ubiquitin–proteosome pathway, allowing the translocation of NF-κB to the nucleus [
34]. Therefore, the reduced IκB expression is an important factor in the RANKL-induced differentiation of osteoclasts. We also analyzed the DFP-induced downregulation of the levels of main transcription factors as well as osteoclast-specific factors (at the mRNA and protein levels). NFATc1 and c-Fos mRNA and protein levels were significantly reduced in BMDMs treated with DFPs (100 µg/mL) to induce differentiation into osteoclasts. Additionally, DFP-induced suppression of c-Fos and NFATc1 expression downregulated the transcription and translation of osteoclast-specific genes, including those encoding Atp6v0d2, DC-STAMP, and cathepsin K. IκB degradation was promoted in RANKL-induced BMDMs (vehicle group) but was suppressed in BMDMs treated with DFPs. These results demonstrated that the mechanisms through which DFPs suppress osteoclast differentiation rely on the NF-κB signaling pathway and involve RANKL. Moreover, activating MAPKs is important in RANKL-induced osteoclast differentiation. IκB reduces NF-κB activity to affect osteoclast differentiation. DFP treatment suppresses the activation of NFATc1, an important transcription factor in osteoclast differentiation, by suppressing JNK activation [
35]. These results support the hypothesis that DFP-induced suppression of NF-κB activation reduces the expression of c-Fos and NFATc1 during RANKL-induced osteoclast differentiation, preventing the differentiation of TRAP-positive osteoclasts. Overall, this study confirms that DFPs exert anti-osteoporotic effects; nevertheless, further studies are required to elucidate whether DFPs bind to cognate receptors on the cell surface of progenitor cells and inhibit cell signaling pathways that activate NF-κB and MAPK or whether DFPs freely enter the cell and directly inhibit NF-κB and MAPK activation.
Bisphosphonate and parathyroid hormone differentiate osteoclast precursors into osteoclasts by inducing RANKL expression in osteoblasts co-cultured with osteoclast precursors. One of the most common therapeutics for osteoporosis in clinical settings are bisphosphonates, and more recently, medications containing parathyroid hormone, teriparatide, and abaloparatide have also been used. However, the long-term side effects and high costs of the available osteoporosis medications have necessitated novel medications [
36,
37]. Most polysaccharides, including DFPs, are major components of medicinal plants, and their biosynthesis is mainly influenced by the availability of nutrients and several environmental factors. Recently, dietary polysaccharides have been considered potential modulators of the gut microbiome [
38]. These polysaccharides can serve as energy sources for specific, beneficial gut microbes [
39,
40]. Each plant-derived polysaccharide specifically affects many metabolic diseases, including obesity, inflammatory bowel disease, and immune-mediated conditions [
41]. These useful plant-derived polysaccharides, like DFPs, could be valuable for the preventive management of various diseases. Thus, DFPs can be used to develop cost-effective, natural product-based osteoporosis treatments and alternative drugs.
5. Conclusions
Therefore, we demonstrated that DFPs inhibited RANKL-induced osteoclast differentiation in a dose-dependent method, with no toxicity being observed at 1–200 µg/mL DFPs. Moreover, DFPs inhibited the expression of c-Fos and NFATc1, which are important factors in osteoclast differentiation, implying that their effect is due to increased IκB expression.
Author Contributions
Conceptualization, Y.-H.Y. and H.H.; Methodology, A.L. and T.K.; Investigation, A.L.; Formal Analysis, J.R., A.L., and Y.-H.Y.; Validation, A.L. and Y.-H.Y.; Visualization, J.R. and A.L.; Writing – Original Draft Preparation, J.R. and Y.-H.Y.; Writing – Review & Editing, Y.-H.Y. and H.H.; Supervision, H.H.; Funding Acquisition, T.K.
Funding
This research was funded by the Korea Institute of Oriental Medicine, Ministry of Education, Science and Technology, Republic of Korea, grant number KSN2013330.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aubin, J.E.; Triffitt, J.T. Mesenchymal stem cells and osteoblast differentiation. In Principles of Bone Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 59–81. [Google Scholar] [CrossRef]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoporosis and rheumatic diseases. Reumatismo 2014, 66, 125–135. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, T.; Yan, X.; Liu, Z.; Yan, Y.; Yang, K.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; et al. A novel Rhein derivative modulates bone formation and resorption and ameliorates estrogen-dependent bone loss. J. Bone Miner. Res. 2019, 34, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Kwak, S.-C.; Moon, S.-Y.; Kwack, H.-B.; Jeon, B.-H.; Min, O.-J.; Choi, M.-K.; Kim, J.-J.; Jang, S.-J. Effect of drynariae rhizoma in RANKL-induced osteoclast differentiation. J. Physiol. Pathol. Korean Med. 2012, 26, 506–510. [Google Scholar]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef]
- Ju, Y.S. Ungok's illustrated guide to medicinal materials; Woosuk Press: Jeonju, 2017; Volume 616, p. 632. [Google Scholar]
- Yoon, S.H.; Kim, H.J. Donguibogam; Donguibogam Publishing Company: Seoul, Republic of Korean, 2006; pp. 297–2189. [Google Scholar]
- Ding, Z. Clinical study on TCM orthopedics in treatment of closed pilon fracture in children. ACTA Chinese Medicine 2017, 32, 1799–1801. [Google Scholar]
- Yu, H.-M.; Sun, Y.-J. Clinical study on external fixation by small splint and sequential stages of Chinese medicine treatment on distal epiphyseal fracture of radius of children. Journal of Liaoning University of Traditional Chinese Medicine 2016, 18, 148–150. [Google Scholar]
- Ma, K.-C.; Zhu, T.-Y.; Wang, F.-X. Stimulative effects of gusuibu (Drynaria baronii) injection on chick embryo bone primordium calcification in vitro. Am. J. Chin. Med. 1996, 24, 77–82. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Cai, W.; Wang, Z.-C.; Ding, Y.; Li, X.-Y. [Effect of Drynaria fortunei naringin on the total protein content and ultra-structure of human periodontal ligament cells]. Hua Xi Kou Qiang Yi Xue Za Zhi= West China Journal of Stomatology 2010, 28, 330–333. [Google Scholar]
- Chang, R. Bioactive polysaccharides from traditional Chinese medicine herbs as anticancer adjuvants. J. Altern. Complement. Med. 2002, 8, 559–565. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Zhu, L.; Jiang, J.-G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef]
- Lee, T.-T.; Huang, C.-C.; Shieh, X.-H.; Chen, C.-L.; Chen, L.-J.; Yu, B. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. and its immunostimulant capacity in vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5. [Google Scholar] [CrossRef]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bai, L.; Zhou, Y.; Tong, R.; Zeng, M.; Li, X.; Shi, J. Polysaccharides from Chinese herbal medicine for anti-diabetes recent advances. Int. J. Biol. Macromol. 2019, 121, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Lin, X.-H.; Liang, Y.-H.; Dong, J.; Guo, D.-A.; Ye, M. Comprehensive chemical analysis of the rhizomes of Drynaria fortunei by orthogonal pre-separation and liquid chromatography mass spectrometry. Planta Med. 2014, 80, 330–336. [Google Scholar] [CrossRef]
- Sun, X.; Wei, B.; Peng, Z.; Chen, X.; Fu, Q.; Wang, C.; Zhen, J.; Sun, J. A polysaccharide from the dried rhizome of Drynaria fortunei (Kunze) J. Sm. prevents ovariectomized (OVX)-induced osteoporosis in rats. J. Cell Mol. Med. 2020, 24, 3692–3700. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Lee, A.; Kim, T.; Jang, S.-A.; Ha, H. Anti-osteoporotic effects of Commiphora Myrrha and its poly-saccharide via osteoclastogenesis inhibition. Plants (Basel) 2021, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, T.; Kim, R.; Ha, H. Magnolol inhibits osteoclast differentiation via suppression of RANKL expression. Molecules 2018, 23, 1598. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-C.; Lee, J.-W.; Yoon, C.-H.; Lee, Y.-C.; Chung, K.-H.; Kim, M.-G.; Kim, C.-H. Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 2005, 96, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fernández, H.; Kumar, A.; Revilla, M.A. Working with ferns: issues and applications; Springer Science & Business Media, 2010. [Google Scholar]
- Aoki, K.; Aoki, S.; Saito, H.; Jimi, E.; Shimokawa, H.; Ohya, K. The inhibitory effects of NF-kappa B inhibitor on the osteoclast function. In Proceedings of the Journal of Pharmacological Sciences; Japanese Pharmacological Society; 2004; pp. 188–188. [Google Scholar]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.-W. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- David, J.-P.; Sabapathy, K.; Hoffmann, O.; Idarraga, M.H.; Wagner, E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and-independent mechanisms. J. Cell Sci. 2002, 115, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Christiansen, C. Individualizing osteoporosis therapy. Osteoporos. Int. 2012, 23, 797–809. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; Goulis, D.G. New therapeutic targets for osteoporosis. Maturitas 2019, 120, 1–6. [Google Scholar] [CrossRef]
- Barko, P.; McMichael, M.; Swanson, K.S.; Williams, D.A. The gastrointestinal microbiome: a review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T; et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017, 26, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Mishra, S.K.; Yadav, H. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes. Control Ther. 2017, 4. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).