Conceptual Framework
In this section, we will introduce a few concepts to establish a starting point for our arguments. Electron (
e-) transport is perhaps the most crucial factor that determines a successful battery design. However, certain concepts need to be clarified. For example, a hole (
h+) or positive charge, which is the opposite of an electron, is a theoretical construct because such a particle does not exist. In contrast, a positive ion (cation) is a positively charged particle that does exist. Theorists and experimentalists often develop different definitions to explain physico-chemical phenomena, leading to disagreements between their definitions[
1,
2]. In nature, an electron is considered a fundamental particle and can move on its own, whereas a proton (i.e., hydrogen nucleus), the electron's counterpart, cannot naturally be found and does not move like an electron does. In the context of electrochemistry, the transport of (
e-) and cation (i.e. Li
+) is of interest[
3], proton transport is related to nuclear research.
A positive ion (e.g. Li+) is a positively charged particle that experiences motion under the influence of an external force, such as an electric field, or under the influence of a concentration gradient. These two mechanisms are interrelated, and the best analogy would be heat transport. Heat flows in the presence of a temperature gradient, but the temperature would be the same everywhere in the absence of heat flow - that is, at an equilibrium condition or in the absence of a chemical gradient of concentration.
On the other hand, when an electron moves from one point to another, it generates an electric current flow. The electron moves because of an electric field or to a point of lower electric potential. When an electron leaves the valence cloud of an atom, the atom is oxidized, and the reverse process is called reduction. These two processes are referred to as redox reactions and are the essence of battery designing. An ideal battery would generate a high electric current, and the electrode materials that the battery contains would experience redox reactions with a chemically stable solid electrolyte. The battery would be able to store a high quantity of electrons per unit of mass or volume, and the electrochemical potential difference of the electrodes would be high. This summarizes the essential criteria for a battery from an electrochemical standpoint. Other important characteristics of a battery include the choice of materials for construction (toxicity and earth abundance), cost of manufacturing, safety, and recyclability.
The next concept is the electrochemical potential, also referred to as the chemical potential in photovoltaics. It is the average energy necessary to add or remove an infinitesimally small quantity of electrons to the system and is often called the “Fermi level”. Particular attention must be taken with the strict definition of the Fermi level, which is defined at 0 K and is different from the one used here. In the Electrochemical Community, the term Fermi Level is loosely employed. Electrons move from the higher Fermi level to the lower, which helps to understand which electrode would experience oxidation or reduction. In the context of battery research, it is more accurate to use the term electrochemical potential because there are chemical and electrostatic potential gradients during the operation of a battery. Electrons flow externally of the battery due to an electrostatic potential gradient, whereas the cation diffuses inside the battery due to a concentration gradient and an electric field.
A useful analogy would be that electrons move like a bowling ball from a high to a low height, whereas holes (
h+) move like a helium balloon going to higher heights. Height represents gravitational potential energy from Newtonian physics and is the analogy for electrochemical potential (
μ), which is defined in Eq. 1. The origin of these concepts is explained in detail by Chen[
3] when he describes the band structure of materials, however a brief explanation has been included here as shown in
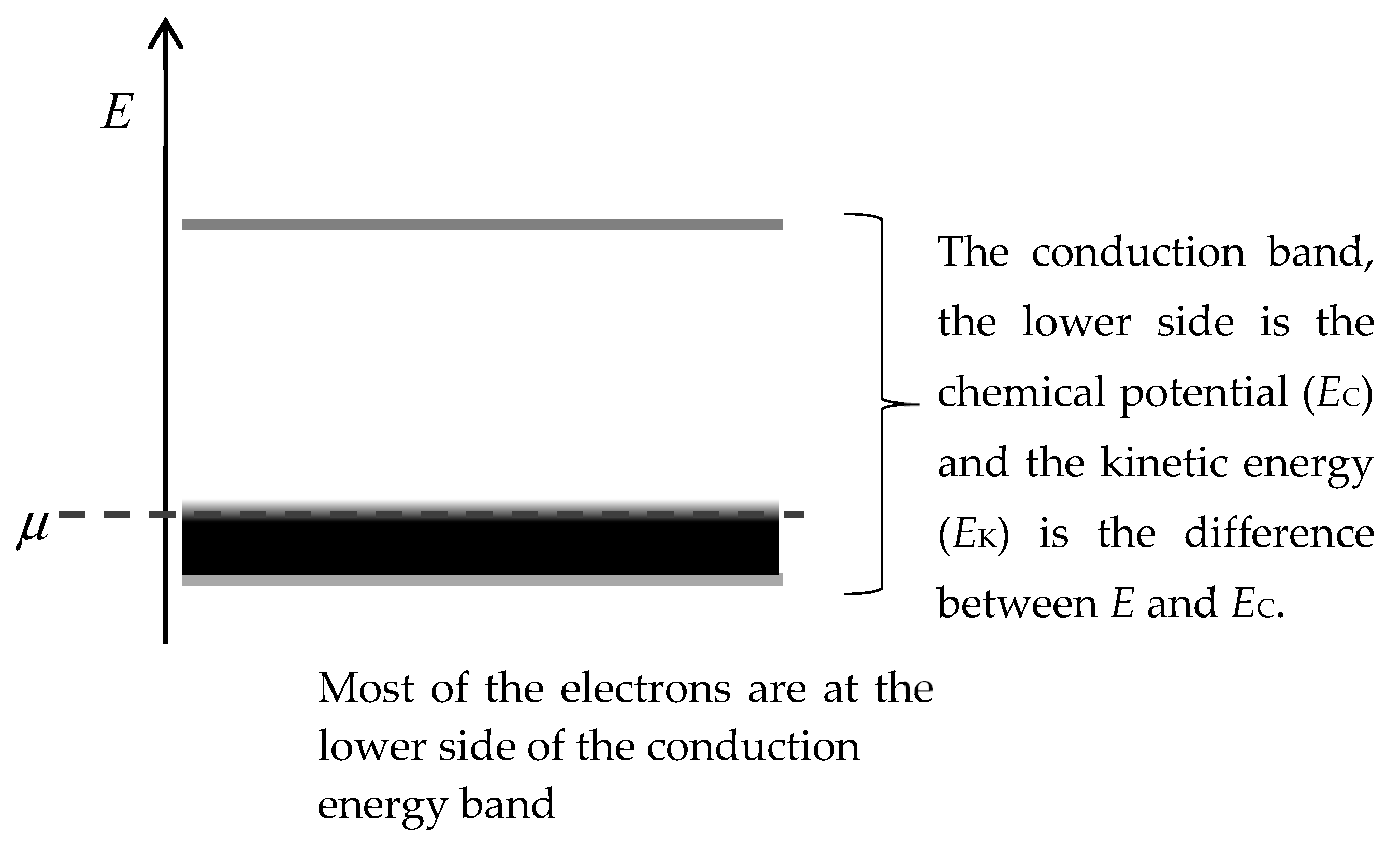
Figure 1. This figure illustrates the conduction band of a conductor, the electrochemical potential, most of the electrons will occupy the lower energy states allowed in the conduction band.
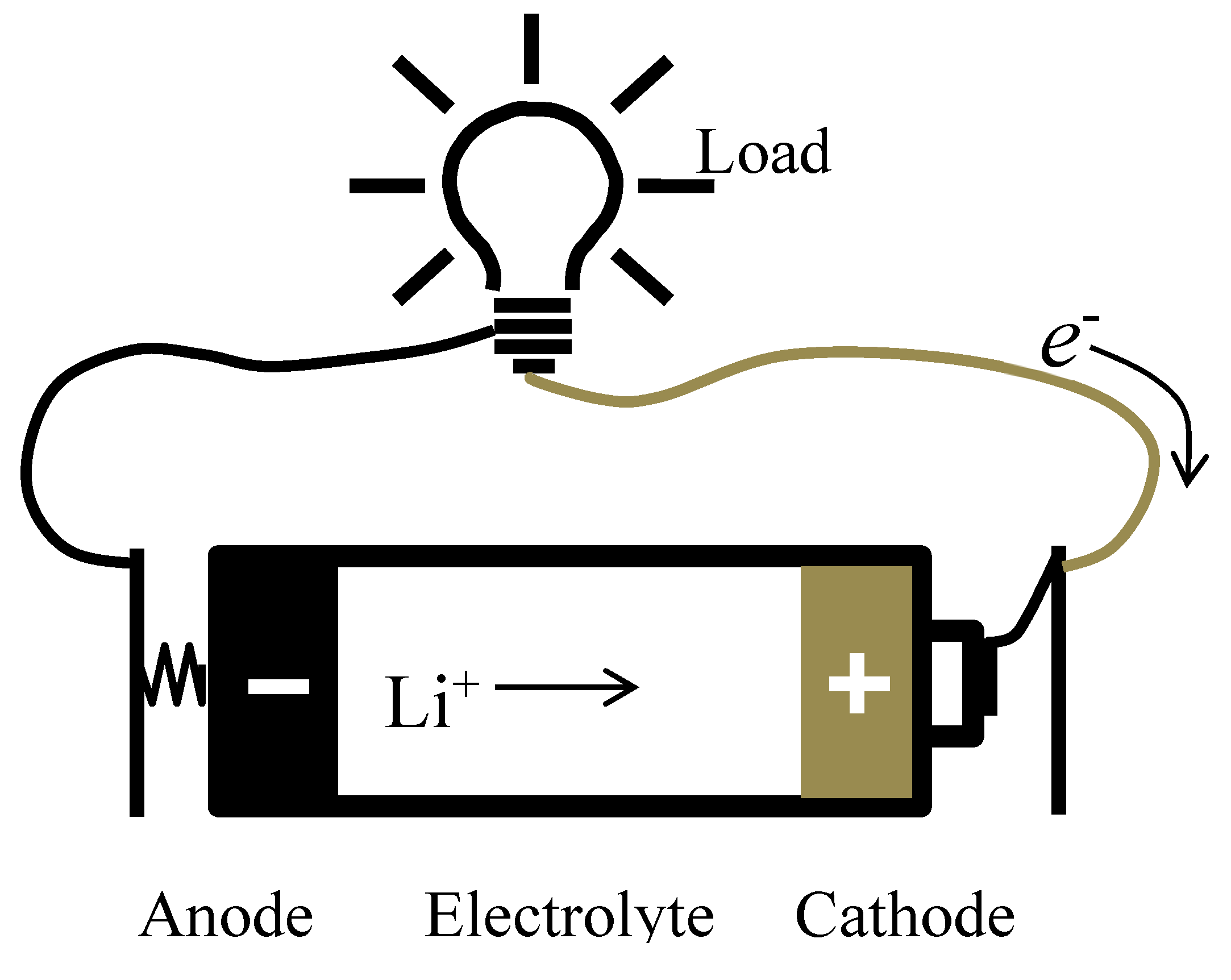
Figure 2 shows the basic components of a battery, where the anode, cathode and electrolyte are assembled to power devices during discharge. Electric current (i.e. holes
h+) flows in the opposite direction of the electrons; and the cations (e.g. lithium ions) flow towards the cathode where they are reduced into solid lithium. In the opposite process when a battery is being charged, the electrons and lithium cations flow towards the anode, cations are reduced again at “home”. The most popular element used in batteries is lithium, and Eq. 2 is the redox process that a lithium cation experiences in a battery in the charge/discharge regime. Common cathode materials for batteries are LiCoO
2, LiMn
2O
4 and LiFePO
4, the electrolyte is an organic liquid and the anode is generally graphite.
The following concept is the redox overpotential (
η), This value emerges by the electrostatic potential that surrounds a cation, for example other molecules that help the ion to dissolve or the opposite when an ion would not dissolve and remain as a precipitate[
1,
4],
Figure 3 illustrates these concepts. The difference between the electrochemical potential of the electrode and the adsorbed surface layer of ions is proportional to the overpotential[
5]. This overpotential dictates the power output of a battery and it is a function of the electric current.
The overpotential is an average property of a reaction. At ideal conditions, as for example, a standard hydrogen electrode (SHE)[
4,
6], the overpotential of the charge transfer reaction is zero, an electron would not distinguish the difference in terms of electrochemical potential between a hydrogen molecule and an adsorbed hydrogen atom on the surface of the catalyst. This is the reason that in any redox potential table there is no overpotential mentioned between a redox pair as for instance Fe
+2/Fe(s), at equilibrium conditions or negligible electric current, the measurement becomes a thermodynamical measurement when “no reaction kinetics” is involved and the overpotential is zero, and it is referred as the electrochemical potential.
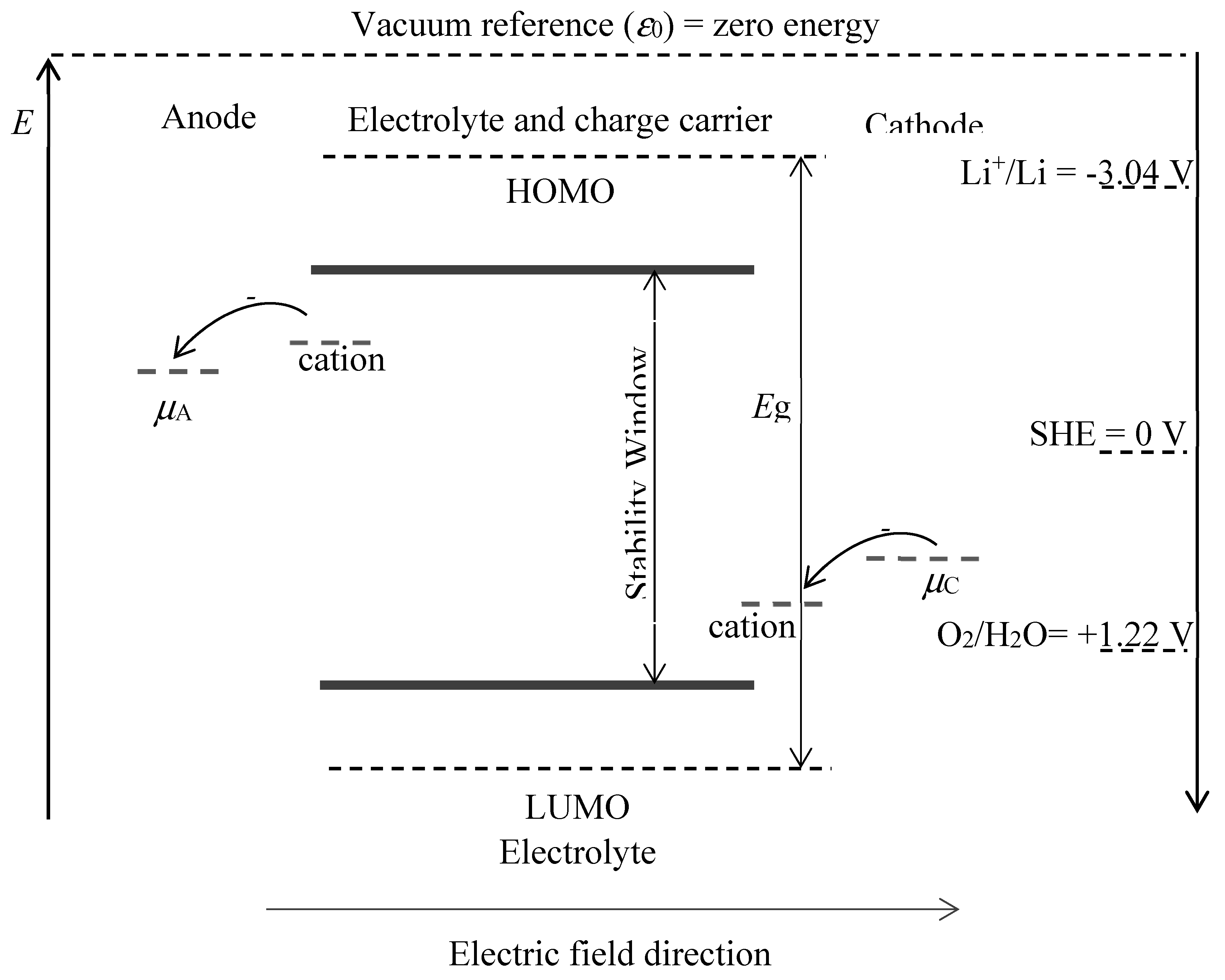
Figure 3 shows the energy diagram of a battery, the electrochemical potential of the electrodes and the stability window of the electrolyte. The first question that arises is the commonly known sign convention,
Figure 2 shows that the sign of the anode as negative and the cathode as positive, this is the convention for batteries. The electric field direction in
Figure 3 is towards the cathode during discharge as the electrons move towards lower potentials. Another question from
Figure 3 is how the cation in the electrolyte has a higher electrochemical potential close to the positive electrode (i.e. anode)?. Imagine a situation where the cation concentration in the electrolyte is uniform, this means that the chemical potential any cation would be the same, inside the electrolyte, however, the electrostatic potential of that cation would be much higher close to the positive electrode because the electron that the cation exchanges at the anode would have to travel against the electric field and gained electrostatic potential energy, increasing its electrochemical potential (refer to Eq. 1).
In
Figure 3 electrons flow from higher to lower electrochemical potentials, the electrochemical potential of these electrons needs to be such that it does not electrochemically react with the electrolyte and this is shown by the stability window. In addition, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of the electrolyte (solvent) are generally used as the redox potentials to determine the electrochemical stability of the electrolyte[
1,
7]. The energy can have two references and scales, the absolute zero as the vacuum (eV) or the SHE electrode (V), the arrows of these two scales point towards the direction of positive values. As an example, if acid water were to be used as the electrolyte, the stability window would be within the SHE and O
2/H
2O region of potential as indicated in
Figure 3, in that case lithium would be outside this region and it would decompose water, defeating the purpose of such a battery in that hypothetical scenario.
The scope of this manuscript is solid-state batteries, specifically those with the potential to be adopted by the automobile industry. Although there are other batteries under research, such as liquid electrolyte, air or CO2 batteries, which could be better suited for the grid. In our opinion, solid-state batteries would be the next generation of batteries for electric vehicles, but we keep an open mind and believe that there will still be a certain diversification where different battery chemistries prove to be more effective. We also consider improving synthesis methods using techniques that have proven to be beneficial in other related disciplines. Additionally, we perform a sustainability analysis of current Li-ion battery technology compared to new battery chemistries.
Why should solid-state batteries be the next step for the automobile industry? Conventional lithium batteries used in vehicles have raised safety concerns, including risks of fires and explosions[
8,
9,
10], leading to large-scale vehicle recalls. The drive to develop solid-state batteries primarily stems from these safety issues. Designing systems that eliminate risks associated with conventional lithium batteries is challenging, and these systems often add significant weight to vehicles due to components such as cooling heat exchangers.
Substantial research has been conducted into potential chemistries[
11,
12,
13,
14,
15,
16], and while it is challenging to pinpoint a specific chemistry, it is more effective to comprehend the potential of selected materials and develop a battery around them. For example, a battery with lithium and sulphur as electrodes, paired with a solid-state electrolyte that is chemically stable and highly ionically conductive. This manuscript therefore focuses on solid-state batteries, particularly those with the potential for implementation, i.e., those using oxides and sulphides as electrolytes. For instance, sulphides are the subject of extensive academic research, and major advancements from reputable commercial companies utilize this chemistry[
17,
18].
Resources for Battery Production
The current CO
2 emissions require aggressive measures from governments[
19,
20,
21]. Electric vehicles in the form of battery electric vehicles (BEVs) or fuel cell electric vehicles (FCEVs) are getting support from governmental policies[
22,
23]. Internal combustion engine vehicles (ICEVs) are being phased out from mayor cities[
22]. Battery electric vehicles have been introduced in the market of transport much earlier than fuel cell electric vehicles, and BEVs have been able to mature and develop a consumer base, despite of their relative higher cost when compared to internal combustion engine vehicles[
23].
There are scenarios where FCEVs will compete with BEVs and the market of passenger cars could potentially consists of these two types of vehicles, however, it is unclear which type of these two would dominate the market in the near future because of similar costs of ownership[
23], the main difference at the moment is that FCEVs can be refuelled in three to five minutes[
24]. What is clear is that in terms of mobility applications, battery powered transportation will be fit for industrial trucks, busses, lorries, light commercial vehicles, motorcycles and passenger cars[
23]. A key metric for BEVs and ICEVs is that at a cost of
$100/kWh for batteries, there would be parity between these two technologies from a cost point of view[
25]. Such a cost is forecast to happen by 2024[
25,
26].
Feltrin[
27] has made a sustainability analysis for photovoltaics (PV), based on energy demand projections up to 2070, when the energy consumption would be in the order of 10 Terawatts and at these levels, technologies would experience limitations based on their materials of construction. In that study[
27], the use of more efficient technologies and alternative materials is concluded, perhaps for electric vehicles it would be necessary to diversify with fuel cells and batteries. For instance silver, indium, platinum, gold, cadmium, etc are limiting for PV and other sectors of the economy that consume these elements were not considered in Feltrin’s study[
27]. In addition, in economics, the law of demand and offer would indicate that when these elements become scarcer, their prices could be such that these technologies would become unaffordable to the society.
Resource forecast could come with conflicting views[
28,
29], one that sees society being unable to make progress and the other, believing that new technologies and economics, balance the progress of the society to move forward.
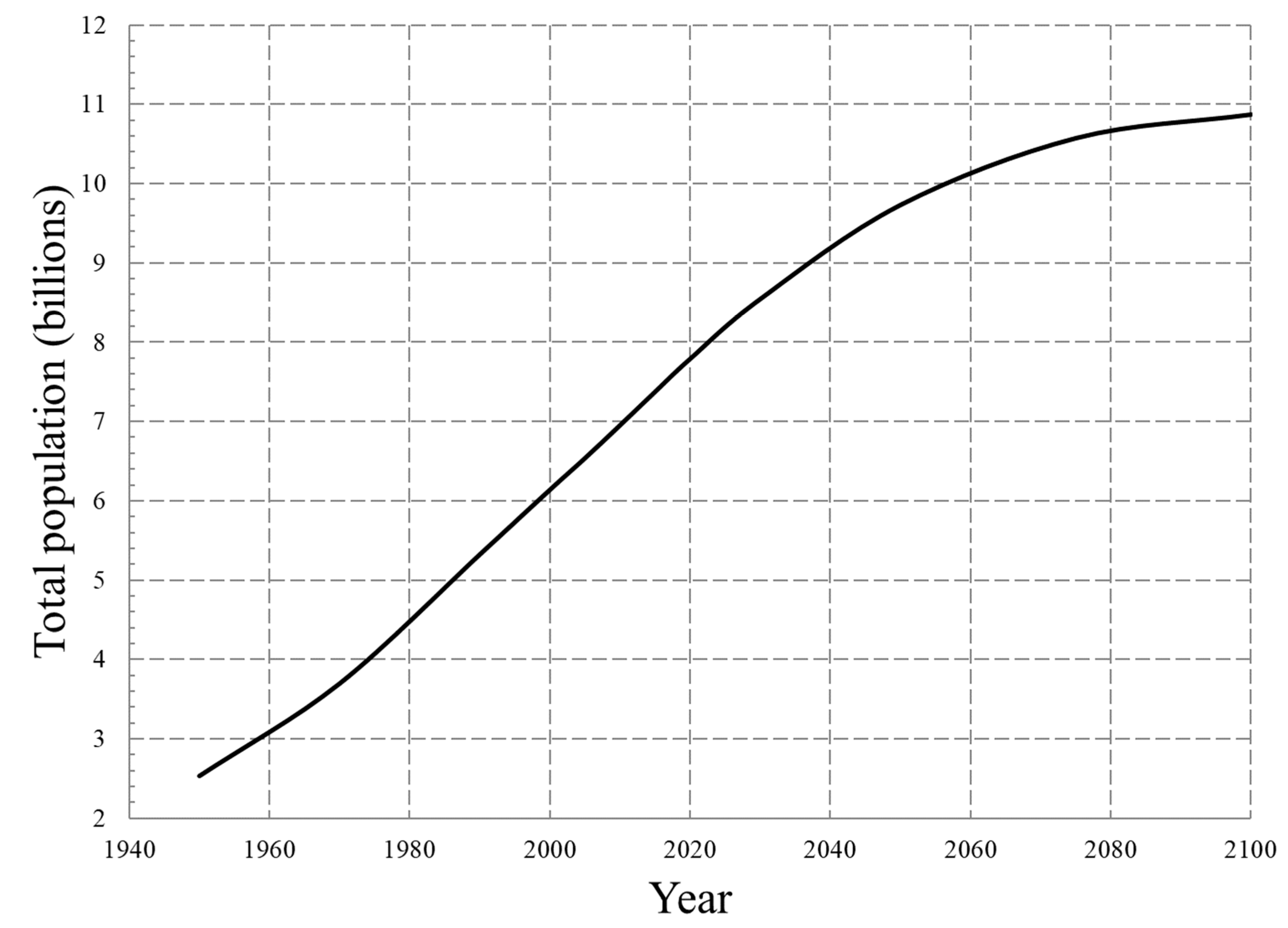
Figure 4 shows the World population forecast[
30] which appears to start to plateau after 2100. In 2070 there would be just over 10 billion people and this will pose a significant challenge to the global supplies when the earth begins to reach the limits of sustainability. It is essential to understand what the options for Li-ion batteries are and how they could be met in the future.
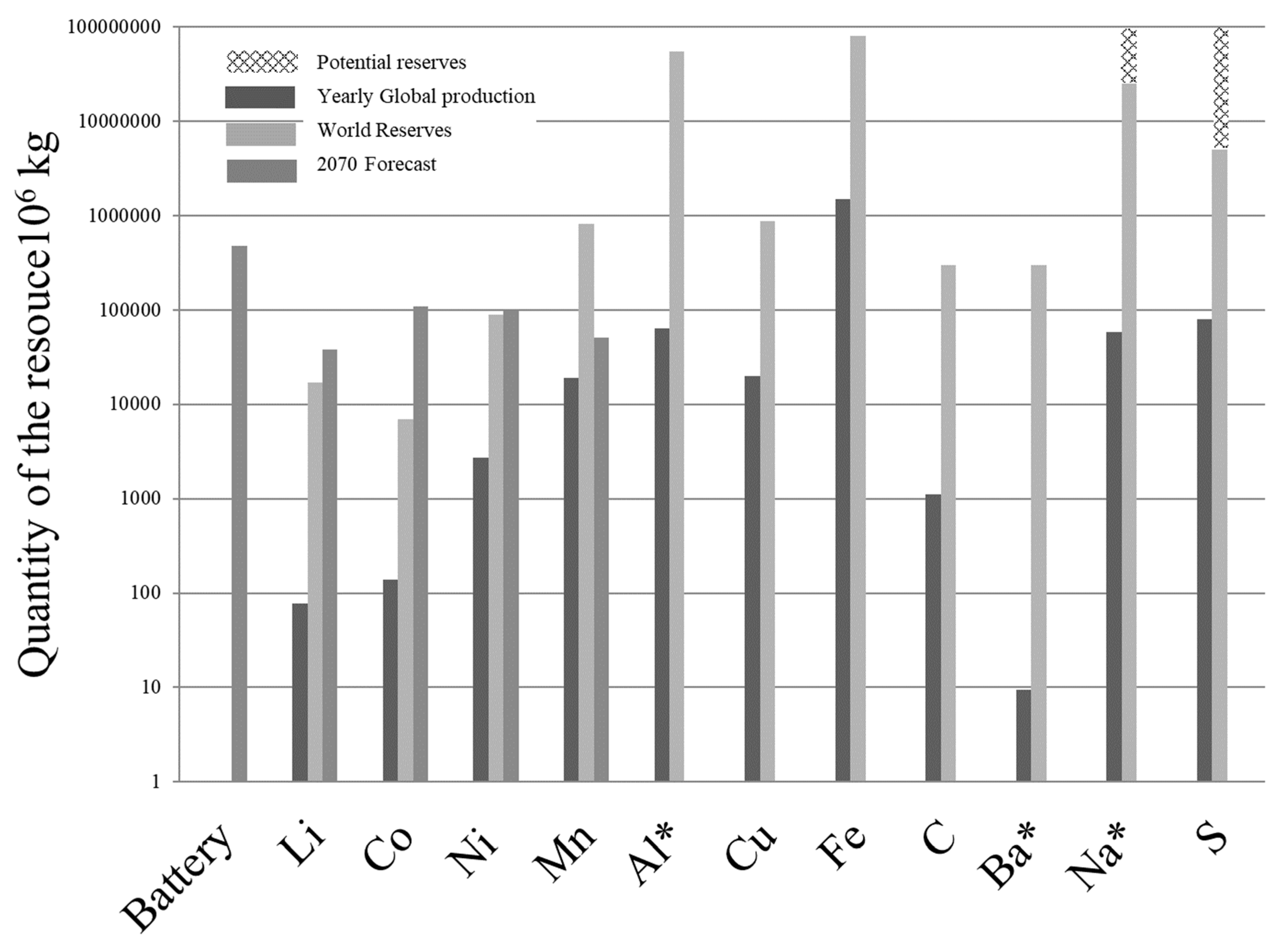
The price of a resource is a reflexion of its scarcity, from
Figure 5, elements such as lithium and cobalt are the scarcest ones for the conventional Li-ion battery technology. Higher prices for this commodities will increase their reserves, for instance lithium from ocean mining could be possible, however it is not included in
Figure 5 because currently there is no mining facilities with such an end, but this could be possible as for instance Uranium mining from seawater has been researched[
31,
32]. To understand the limitations of the Li-ion battery technology a base case scenario has been developed for the 2070 year. This scenario comes with certain simplified assumptions that will be described.
A Li-NMC battery has been considered as representative with a formulation of LiNi
xMn
yCo
zO
2 with x = y = z = 1/3 (i.e. x + y + z =1)[
33,
34]. The specifications of the battery model NCR18650BF[
35] has been taken as representative for most Li-ion batteries. This battery weighs 46.5 g and has a typical gravimetric energy density of 248 Wh kg
-1 , data of its composition has been taken from Vieceli[
36], who shredded samples of Li-ion batteries for recycling and analyzed their chemical composition[
37]. An average passenger vehicle of 85 kWh would have around 7000 of these batteries[
38]. The ratio of global population to cars in 2017, considering the latest available data[
39] for cars and
Figure 4, was at 7.43 people/car, this is assumed to be the same for 2070. These assumptions have been taken to develop the base case scenario for the 2070 forecast.
The sustainability of the Li-ion battery (LiB) can be analyzed from two views, one about the total reserves and the other seeing the economical aspect of it. The total physical weight of the Li-ion batteries that it is forecast for 2070 is just over 0.4 Teragrams (i.e. 1012 g) and this value is a “benchmark” to evaluate what materials are limiting factors for the LiB technology, any material with world reserves higher than this benchmark is not limiting. By 2070 critical materials for LiB are lithium, nickel and cobalt, and options are needed to address this limitation.
What
Figure 5 shows, is that electric vehicles need: diversification into Fuel cell vehicles[
23], Li substitute materials such as sodium (Na)[
7]. LiB material recycling[
36,
37] and to develop ocean Li mining. A 50:50 FCEV/BEV ratio of diversification and LiB recycling will not be sufficient. Ocean mining of Li and Co could prove to be technically challenging as these elements are at extremely low concentrations[
40]. However ocean mining of low concentration elements such as Uranium as a concept has been tested in labs[
31,
32].
Employing sodium as a substitute for lithium is an excellent option and probably the only one to keep BEVs as a sustainable technology[
7,
41]. An increase in the LiB energy density to 1000 Wh/kg for example, does not bring a benefit from a resource management standpoint, to achieve more energy density, more lithium would be packed into the same battery, and this does not translate into less lithium consumption. As transitional methods for sustainability, it is worth to mention efforts done by Braga with her[
42] Li
2.99Ba
0.005O
1+xCl
1-2x solid electrolyte (SE) formulation, this is a step towards sustainability when less lithium is used.
In an ideal ionic conductor, lithium ions would travel along a path with little drag, the dipoles or geometry of the solid electrolyte (eg. glass) constituent particles is such that the Li
+ ion has a lineal motion and “frictionless”. All current solid state electrolytes offer support to a Li ion network with a skeleton framework[
33,
34] and it needs to be electrochemically stable against oxidation and reduction from the cathode and anode. Few publications claim to have developed materials with ionic conductivity comparable to liquid electrolytes[
33,
34] at room temperature. Including Braga’s[
42], there would be only three solid electrolyte chemistries with such a feature, Kamaya’s Li
10GeP
2S
12 crystal[
44] 1.2×10
-2 , Seino’s Li
2S–P
2S
5 glass-ceramic[
45] 1.7×10
-2 and Braga’s glass[
42] > 10
-2 ( all in S cm
-1). As shown in Figure 8, sulphide chemistries for electrolytes are electrochemically unstable and require extra attention to handle. On the other hand Braga’s chemistry of Li
2.99Ba
0.005O
1+xCl
1-2x as an oxide offers better stability. Glass electrolytes that belong to the oxide and sulphate categories[
33,
34] as shown in
Table 1, are promising candidates as solid-state electrolytes. All of them exhibit ionic conductivities higher than 10
-3 S cm
-1.
Most organic liquid electrolytes work up to 4.7 V, when they decompose or combust, and in contrast, solid materials such as glass-based SEs have a high resistance to applied voltage and temperature. However, efforts have been made to improve the safety of liquid electrolytes. Engineering measures, such as introducing coatings between the electrolyte and the electrode, have been applied to prevent the formation of dendrites. One example is the use of ultra-thin glass (σ = 10
-3 S cm
-1)[
54]. In this case, while maintaining the high conductivity of conventional liquid electrolytes, the lithium metal electrode does not form dendrites because the glass impedes their formation. However, most details regarding these approaches remain proprietary[
55]. Nonetheless, this approach should be considered as a starting point.
Goodenough’s patent[
56] about a Li-glass and Na-glass solid electrolytes show promising electrochemical properties, it has just as good ionic conductivity as that of commercial liquid electrolytes which is an important milestone for an electrolyte substitute. Three properties have been highlighted by the battery research community as essential for an electrolyte, 1) high ionic conductivity, 2) low electronic conductivity, and 3) a high dielectric constant. A glass is an amorphous solid with certain properties of liquids, glasses have been a subject of substantial research and no definitive theory has been yet developed to explain their physico-chemical behavior[
57,
58].
In certain glasses, “decoupling” between the translational and rotational motion of their constituent particles has been determined[
58]. In terms of dimension of time, it is possible to measure rotational motion of these particles within relative short elapsed times; this is not the case for translational motion where much longer times are required, unless these are measured above the temperature of transition (Tg). A point of view to describe a glass would be in terms of Entropy. A glass has higher entropy when compared to a crystal, this could be interpreted as, to make the constituent particles of a glass ordered is relatively much easier than in the case of a crystal. Although entropy is a measure of how many possible configurations a particle could have, in a crystal there are less possible allowed arrangements.
A special concept for glasses is ageing. When a glass “ages” because its properties are time-dependent[
58], it could be possible to take advantage of this phenomenon to design materials for specific purposes. Goodenough[
56] claims that the dielectric constant of his developed Li-glass improves when the dipoles realign under the influence of time, pressure or temperature, although to be more accurate below Tg, a glass ages and its properties only change as a function of time[
58]. Crystals do not have the “aging” property that glasses do, a crystal will not change its properties after their synthesis.
What if certain directionality could be given to this developed “Li-glass” electrolyte?. Goodenough[
56] mentions that the ionic conductivity determines the thickness and area of the electrolyte for a desired current output (
I). Goodenough’s material can be improved with Dalal’s research[
59], where he was able to develop an anisotropic glass for a small thickness by vapor deposition, this research could further improve the results obtained in the Li-glass SE[
56]. A glass with preferential ionic conductivity in a certain direction should be grown with a method where this benefit is taken advantage of.
Crystalline materials are “rigid” in terms of what options are available to modify their properties, and glasses offer flexibility, they can be “tuned” to achieve desired properties[
59], it does not come as a surprise all the applications that glasses have in science[
58]. Dalal[
59] has determined by spectroscopic ellipsometry that under 300 nm of glass thickness, it is possible to orient the glass molecules normal to the glass plane or in parallel, depending on the temperature of the substrate in the vapor deposition. This technique of deposition is popular for glass manufacturing[
56,
58,
59]. Further improvement to the results obtained by Goodenough[
56] might be possible if such an approach is considered, this also applies to all glass based solid electrolytes. That patent[
56] does not mention the directionality that the developed Li-glass SE has, it is amorphous and no improvement on its anisotropic properties was done,
this is a gap in that research that it is worth to pursue.
Dalal[
59] reports that glass thin films prepared by his method can have their properties tuned and are highly kinetically stable. It could take 10
4 years of annealing or slow cooling of bulk glass to achieve the properties that a thin glass vapor-deposited has[
59]. Being able to orient the molecules of glass and keeping them in that direction when its kinetic stability is enhanced, could prove to be essential in applications for solid state electrolytes for batteries, fuel cells or electrolyzers. In addition, unexplored methods of glass deposition such as using magnetic fields[
60] during the deposition might prove to be beneficial.
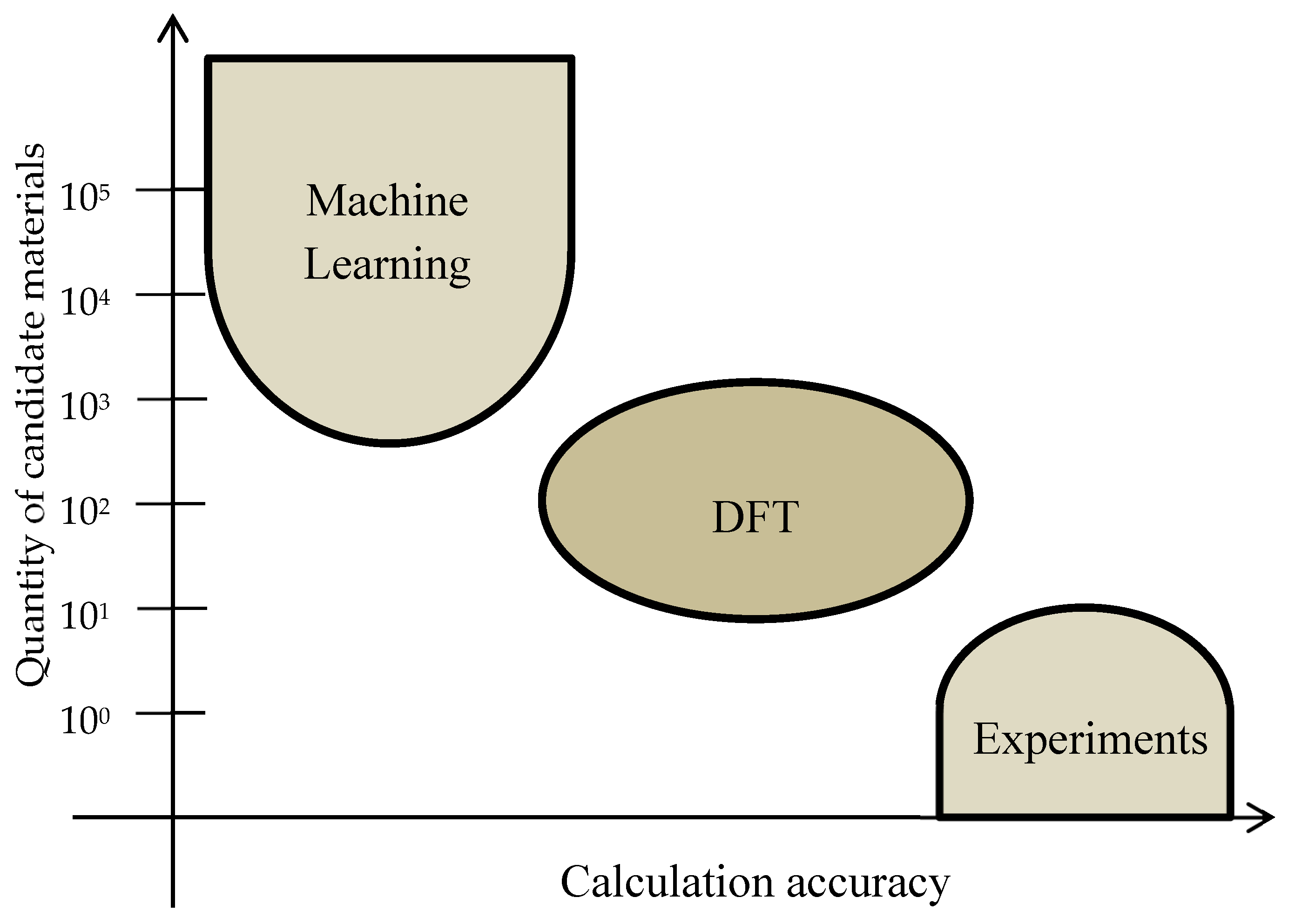
Figure 6 shows a comparison of the computational power and human creativity to develop new materials for science. New candidate materials for superionic conducting materials need to meet certain criteria, and it is difficult for a human brain to develop new chemistries that achieve this. On the other hand, a computer would fail to find a different method of synthesis or accurately forecast the properties of a material. It is a compromise and one should consider both methodologies in the pursue of new materials. Glasses’ properties for instance are hard to predict because there is not a current theoretical framework that accurately models glasses[
58]. Another example, is how new methods of electrode synthesis are developed[
61,
62,
63], where a two stage lithiation process was developed and it can be Co free.
Expanding on
Figure 6, various software applications are available to simulate different components of a battery. Physico-chemical properties can be modelled based on their length scales. For the nano to micron scale, the following tools could be considered: reaction at interfaces can be modelled with ONETEP; diffusion of small molecules through coatings with FORCITE & COMPASS; mobility of atoms in hard materials with GULP and ReaxFF; solvent properties, solubility, and phase behaviour with COSMOtherm; and complex inhomogeneous systems with COSMOplex. On the scale of millimeters to meters, surface growth can be examined with KINETIX; solid phase transformations and dendrite growth with PHASEFIELD; and degradation mechanisms with CANTERA. The application of these tools can accelerate the development of battery chemistries.
Cathode and Anode Materials
What is the cathode and anode?. Most research in batteries report their results vs Li/Li
+ reference electrode. Li redox pair is –3.04 V (vs SHE), it is highly electronegative and more likely to be considered as the cathode or negative electrode. Li metal has a theoretical[
34] 3860 mAh g
-1 of capacity and this is the main reason for a potential selection of it as a cathode material. Current anodes electrodes are made of Graphite (2D) 370 mAh g
-1 and future alternatives for it could be lithium and silicon 4200 mAh g
-1. Current cathodes electrodes: LiCoO
2 (2D) 150 mAh g
-1, LiMn
2O
4 (3D) 150 mAh g
-1 and LiFePO
4 (1D) 170 mAh g
-1 with three-, two- or one-dimensional crystalline channels to store the lithium. A future positive electrode could be sulphur (S
8) with an estimate of 1680 mAh g
-1 of capacity.
Materials in general do not reach their full storage capacity because of mechanical constrains such as breaking apart when they expand beyond their elastic yield. Another example is when their cyclability is improved by the use of additives or a compromise considering electrochemical stability.
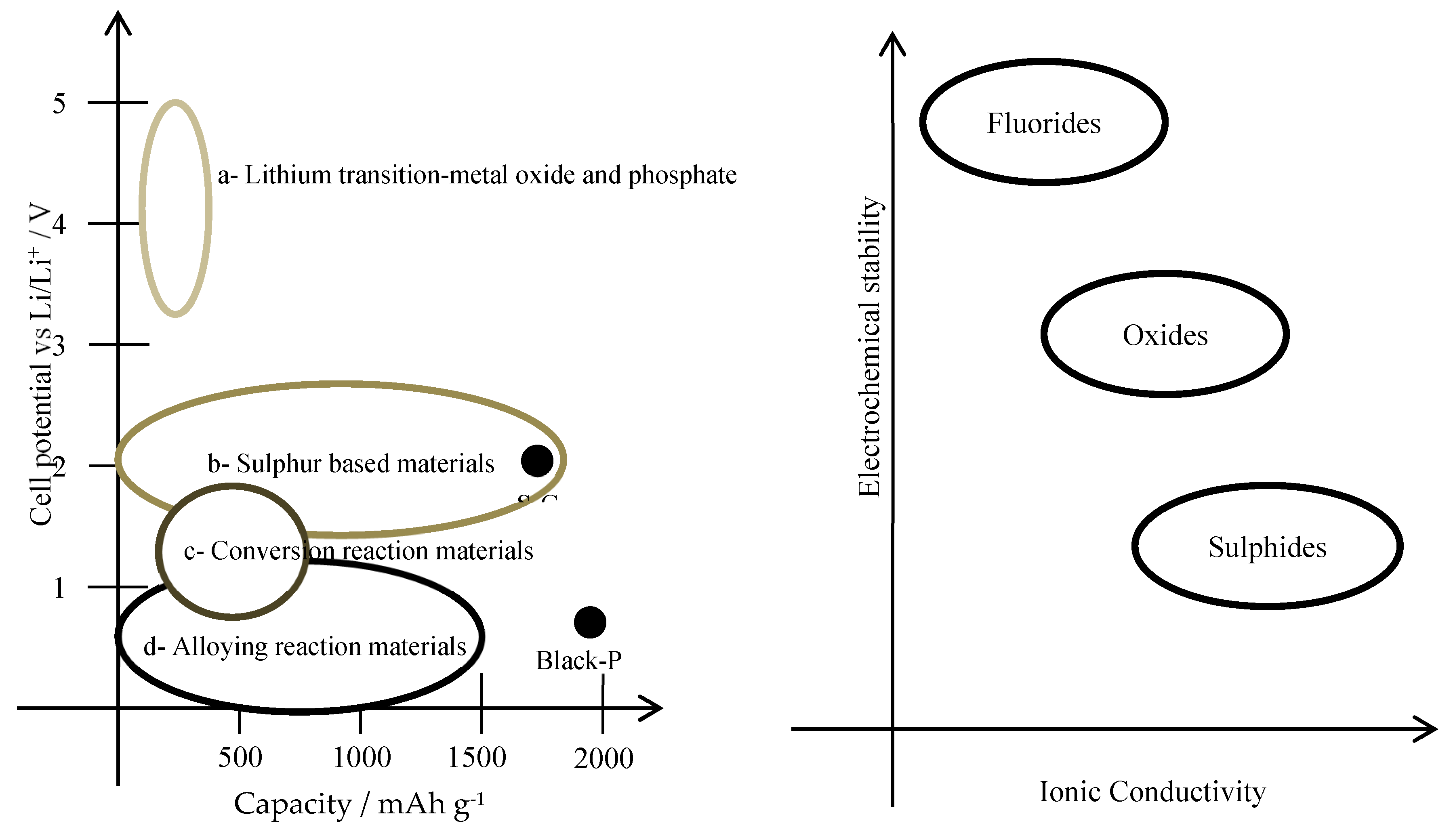
Figure 7 shows tested electrode material combinations and solid electrolytes, it shows what types of compromises have to be done when designing a solid-state battery.
Figure 8 is a forecast of the evolution path of battery design. Metal lithium as the negative electrode is a straight forward selection because it is the most electronegative material for a battery and it has a high energy capacity. What could be a pair for lithium?, electrode materials from the group a) (
Figure 7) will make batteries of high power as these materials are highly electropositive with respect to lithium, however their capacities would be such that commercial electric vehicles will not reach customer needs. Sulphur based electrodes such as S-C is the next best design; it keeps a compromise between power and capacity that would make this electrode material the right one. This reasoning explains the forecast shown in
Figure 8.
A Li/S solid state battery[
42] has already been tested and the cell voltage was in vicinity of 2.5 V. In our opinion this battery once optimized could reach a capacity of about 600 – 800 Wh kg
-1. Such a battery would be a fit for the automobile industry. What is left to do is to improve their cyclability, manufacturing cost, battery life, etc. Thus far no highly electropositive electrode vs Li that has a high capacitance has been achieved. In addition, moving away from nickel and cobalt electrode materials has cost benefits, since their prices in the market are sensitive[
66].
The progression depicted in
Figure 8 has already yielded some results. The transition of the electrode from graphite to silicon has been explored[
67] . It was discovered that the absence of a binder was essential for the sulphide electrolyte to function[
67]. The S-C[
68,
69] chemistry could serve as a starting point in developing the appropriate chemistry for either pure sulphur or a compound thereof. Attempts have already been made with pure sulphur[
41,
68,
70].
With respect to the use of pure lithium as an anode, one study[
17] claims to have improved lithium deposition by incorporating an Ag-C thin film between the solid-state electrolyte and the current collector. This approach could be considered when developing an elemental sulphur cathode. Both cathodes and anodes face similar challenges during cycling; non-uniform deposition or stripping creates mechanical stresses that lead to electrode failure. In the case of lithium, it has been claimed[
17] that this problem has been solved, while other studies[
70] have made improvements using different binder chemistries, or layers that connect the solid-electrolyte with the sulphur cathode. The key to resolving this issue lies in identifying the appropriate chemistry for the binder or interfacial layer, which can enhance the current distribution and achieve uniform deposition.
Another technical difficulty is employing lithium only as an electrode, in contrast to NMC. In that case, how could one guarantee uniform deposition when making a Li electrode?, this is a problem that it is solved by adding additives to the electrolyte in commercial applications[
71,
72]. An electrolyte has what is called a macro- and micro- throwing power, the latter also known as levelling. A macro throwing power makes the electrodeposition of a film to be of uniform thickness on complex geometry surfaces. To achieve a higher level of thickness uniformity, it is preferred to have good levelling. This would have to be addressed when developing batteries where only metal lithium is to be used as an electrode and this is a challenge. Braga[
42] achieved cyclability in a Li-S battery, for a case where the geometry was simple, being a button type battery, this might not be the case for a standard commercial battery.
Lithium metal, as an electrode as depicted in
Figure 8, presents challenges due to the difficulty of achieving uniform deposition. One well-known approach to ensuring uniform deposition involves adding cyanide to the electrodeposition process of many metals[
71], thereby fine-tuning the kinetics. However, in the case of a solid battery, the approach is not as simple as just adjusting the kinetics of deposition. It also necessitates addressing the electrochemical stability of sulphides used as the solid electrolyte. Researchers from SAIT and Samsung[
17] have tackled these two limitations by placing a thin coating between the lithium electrode and the sulphide electrolyte (argyrodite-type). They claim to have achieved uniform deposition and high cyclability, resulting in a chemically stable electrolyte. In addition, they reported no mechanical damage to the lithium electrode and electrolyte due to the low stress experienced during the deposition and stripping of lithium. Although the study by Lee et al.[
17] employs a solid electrolyte of relatively low ionic conductivity (refer to
Table 1 for Boulineau et al.’s chemistry[18), the purpose of their research was to demonstrate the concept that a thin coating can moderate the deposition/stripping of lithium.