1. Introduction
Owing to its high strength-to-weight ratio, excellent corrosion resistance and biocompatibility, titanium alloy have been extensively used in aerospace, marine and biomedical industries[
1]. The TC4 titanium alloy is currently the most widely used α+β titanium alloy, its amount accounts for more than half of the total consumption of titanium alloys[
2]. Components made from titanium alloys are often in tribological contact with different media, under stationary or dynamic loading[
1]. And the poor tribological properties of titanium alloy, such as low surface hardness, high friction coefficient, and poor wear resistance, have limited their scope of application[
3]. Some surface engineering techniques that have been employed for titanium alloy include surface oxidation, PVD/CVD coating, ion implantation etc[
4]. However, these methods are quite expensive and may not provide a thick layer. As the most popular technique for the surface modification of titanium alloy, anodic oxidation generates thin films of amorphous hydrated oxide or crystalline TiO
2 in the anatase form, which can’t provide adequate load support under heavy load[
5,
6].
Micro-arc oxidation (MAO), also referred as plasma electrolytic oxidation (PEO), is an environmentally friendly technology used to produce ceramic coatings with good adhesion on valve metals such as aluminum (Al), titanium (Ti), magnesium (Mg), zirconium (Zr), and their alloys[
7]. This technique is similar to metal anodic oxidation within an electrolytic solution, employing voltages that induce plasma micro-discharges at the electrode's surface. This film is modified through spark micro-discharges initiated at potentials exceeding the breakdown voltage of the growing oxide film and rapidly moving across the anode surface[
8]. At the same time, the local temperature and pressure within the discharge channel can reach 10
3-10
4 Kelvin and 10
2-10
3 MPa[
9]. These conditions are high enough to induce plasma thermochemical interactions between the substrate and the electrolyte[
10]. These interactions result in the formation of melt-quenched high-temperature oxides and complex compounds on the surface. These compounds consist of oxides of both the substrate material and modifying elements carried by the electrolyte[
11]. These coatings typically have high wear resistance due to its high hardness, but exhibit high friction under dry friction condition[
12]. The property and quality of the ceramic coatings can be adjusted by changing the composition of the electrolyte, various electrical parameters and the process temperature[
13].
Researchers have attempted to reduce the friction coefficient and enhance wear resistance of the coatings by synthesizing self-lubricating coatings through a one-step process in lubricant-containing solutions[
14]. Chang et al.[
15] reported that good adhesion and better tribological properties were obtained for the MAO grown coatings by adding 4 g/L MoS
2 particles in the electrolyte. Liu et al.[
16] prepared a composite coating containing graphene nanosheets and TiO
2 on Ti-6Al-4V alloy. The addition of graphene provides higher hardness and smoother surface, resulting in improved wear resistance. Zhang and coworkers[
17] investigated the influence of Graphene oxide (GO) on tribological and corrosion performance of magnesium alloy, showing adding of GO blocked part of the micropores and made the coating more compact and even.
Graphene oxide represents an intermediate byproduct arising during the production of graphene through the oxidation of graphite. It belongs to the class of two-dimensional materials like graphene[
18]. GO is inherently hydrophilic, owing to the presence of oxygenated functional groups, including hydroxyl, epoxide, and carboxyl moieties, which significantly broaden its utility[
19]. Research has already demonstrated that adding GO to the solution can significantly enhance the tribological properties of MAO coatings on magnesium and aluminum alloys[
20,
21]. However, there is limited research regarding the influence of GO concentration on MAO coatings for titanium alloys. In this article, an attempt has been made to explore influence of the amount of GO on MAO ceramic coating by changing the concentration of GO particles in the electrolyte during MAO process. The ceramic coatings were formed on Ti-6Al-4V titanium alloy by micro-arc oxidation with a DC power supply in the aluminate system electrolyte with and without GO. The phase constituent and microstructure of the ceramic coatings were analyzed by X-ray diffraction (XRD), and scanning electron microscopy (SEM). The friction reduction and wear resistance of the coatings prepared in electrolyte containing different concentration of graphene oxide were tested and analyzed. The effects of GO particle concentration on the thickness, hardness and roughness of the coatings were also discussed.
2. Experimental Details
2.1. Material and MAO Procedure
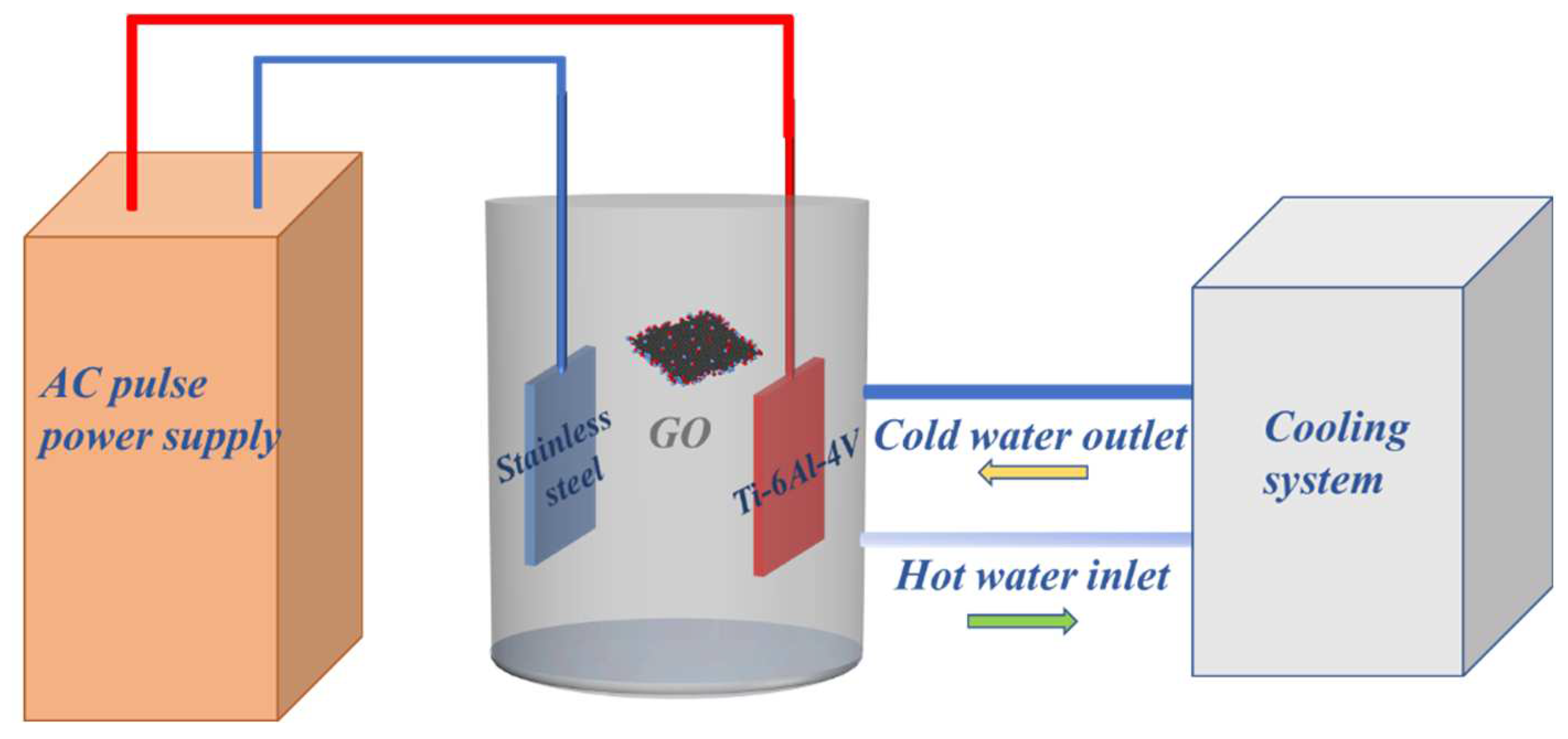
The Ti-6Al-4V alloy (5.5-6.75% Al, 3.5-4.5% V, 0-0.3% Fe, 0-0.2%O, and balance Ti) with a size of 50 × 20 × 1 mm were ground and polished with SiC abrasive paper to obtain an average roughness R
a ≈ 1.0 μm, and then washed in distilled water, ultrasonically degreased in alcohol prior to drying in cool air. A pulse power supply (MAO-WH30A-T) was used to perform MAO treatment at a constant voltage 350 V, frequency of 100 Hz, 10% duty cycle for 20 mins in a water-cooling bath with a stainless steel as cathode. The Ti-6Al-4V alloy sample was connected to the anode. The schematic diagram of the device is shown in
Figure 1. Aqueous solutions of electrolytes were prepared using chemically pure NaAlO
2 and Na
3PO
4, and the concentration is 30 g/L NaAlO
2 and 5 g/L Na
3PO
4. The temperature was maintained between 17-19 ℃. A certain quantity of GO particles (particle size:10-40 μm) was added to the electrolyte to obtain different concentrations of 0,1,3,5,10 g/L with a magnetic stirrer operating to disperse GO particles. The ceramic coatings produced in the electrolyte containing 0, 1, 3, 5, 10 g/L GO were named as S0, S1, S3, S5, S10 respectively. Coated samples were flushed with water after the treatment and dried in warm air.
2.2. Composition and Microstructure Analysis
The composition of coatings was explored by a grazing incidence X-ray diffractometer (Cu Kα radiation,) with the step size of 0.02° and a scan range of 20°-80°. The X-ray generator settings were 30 kV and 40 mA. A SEM was employed to observe the surface and cross-sectional microstructure. The thickness of the ceramic coatings was also observed and measured by a SEM. An energy dispersive spectrometer (EDS) attachment was used for qualitative element chemical analysis. The surface roughness of each coating was analyzed using a profilometer.
2.3. Tribological Evaluation
The microhardness of the coatings was measured by a HVS-1000 Vickers hardness tester at a load of 1 kg. Ten different positions of each coated sample were tested. The tribological performance of MAO films were evaluated using a ball-on-disc tribology tester, which was carried out under a load of 3 N, 100 rpm and a diameter of 3 mm. The counter ball is made of SiC with a diameter of 6.35 mm, and the temperature was controlled to 20±1 ℃. After testing, the morphologies of the wear scars were observed by the scanning electron microscope, and the wear track diameter of the frictional counterparts was measured by an optical microscope.
3. Results and Discussion
3.1. Phase Composition
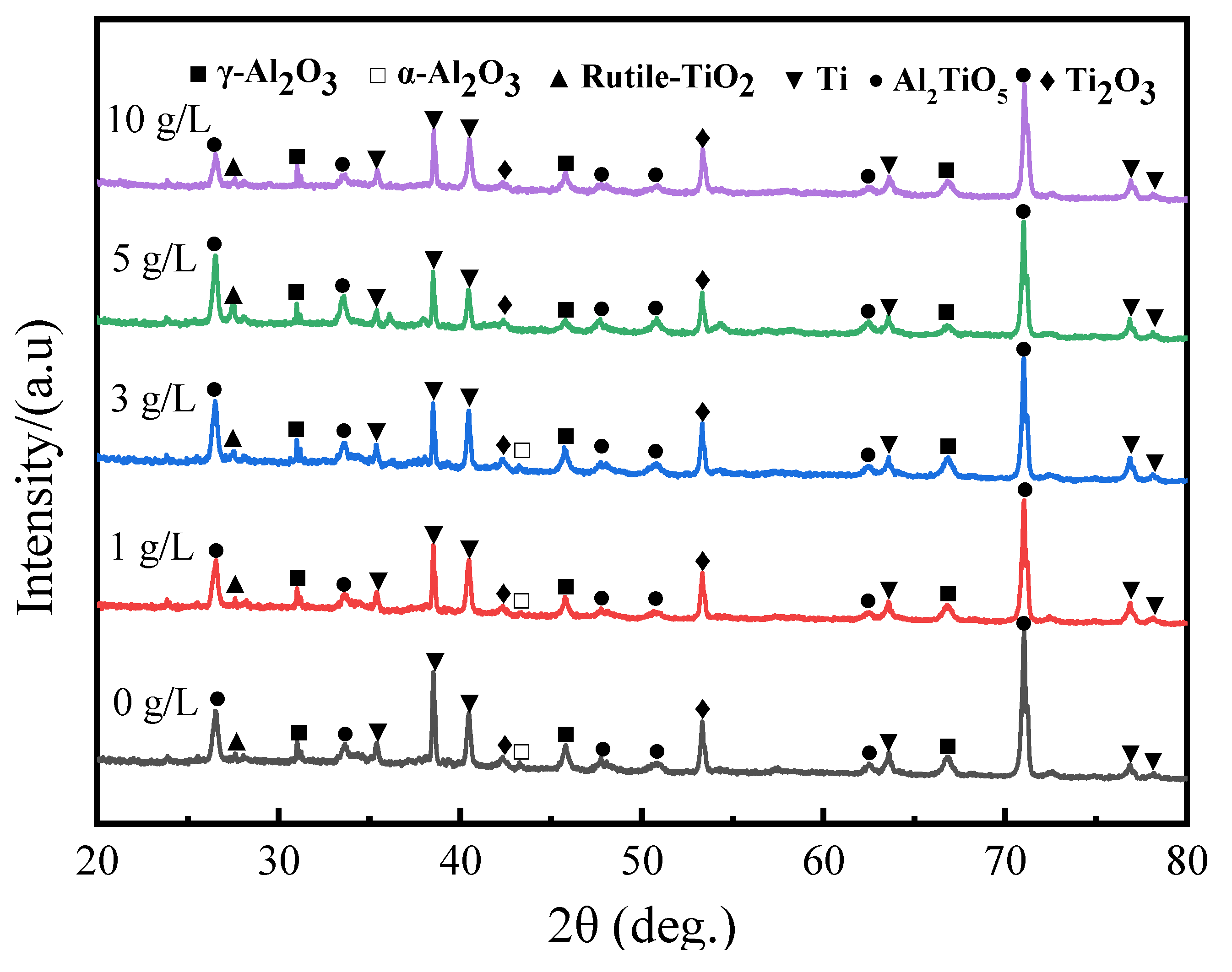
Figure 2 displayed the X-ray diffraction pattern of MAO ceramic coatings fabricated in electrolyte containing different amount of GO. It is clear that the predominant composition of all coatings is Al
2TiO
5, accompanied by a portion of γ-Al
2O
3 phase and minor quantities of low-valence titanium oxide. In addition, a small amount of rutile TiO
2 phase is also detected. Minor amounts of α-Al
2O
3 were detected in S1, S2, and S3, which were prepared in electrolyte with relatively low GO concentration. However, when the GO concentration reached 5 g/L and 10 g/L, α-Al
2O
3 was not detected in the coatings. The peak intensity of Al
2TiO
5 exhibits a decreasing trend with the increase in GO concentration. Furthermore, due to the thickness of the coatings, trace amounts of titanium were detected in all coatings.
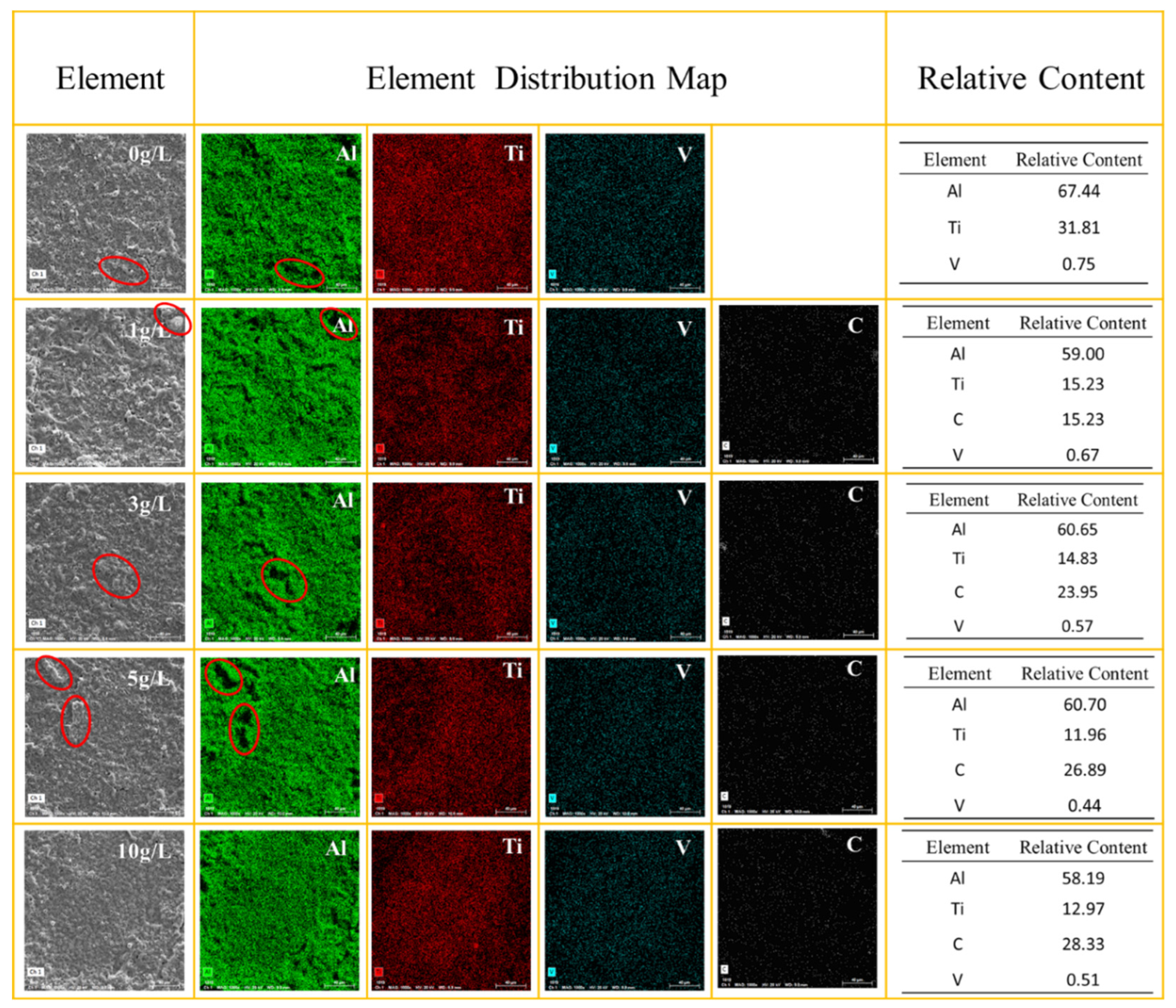
The relative content of the main elements Al, Ti, V and C on the surface of the coating was analyzed by EDS shown in
Figure 3. The results indicate that with the increasing concentration of GO particles in the solution, there is an upward trend in the relative carbon content within the coatings Furthermore, increasing the GO concentration has minimal impact on the Al content, but leads to a decrease in Ti content within the coating. The particle concentration does not exhibit a significant impact on the vanadium element content within the coatings.
Observing the distribution map of Al elements, it can be noticed that the content of Al elements is nearly zero in certain regions. By combining this with SEM images, it becomes apparent that areas with low Al content correspond to the noticeable bumps (as shown by red circles) on the surface. This implies that the bump regions of the coating contain minimal aluminum oxides, and, in turn, suggests that the predominant constituents of these areas are titanium oxides.
3.2. Coating Morphology
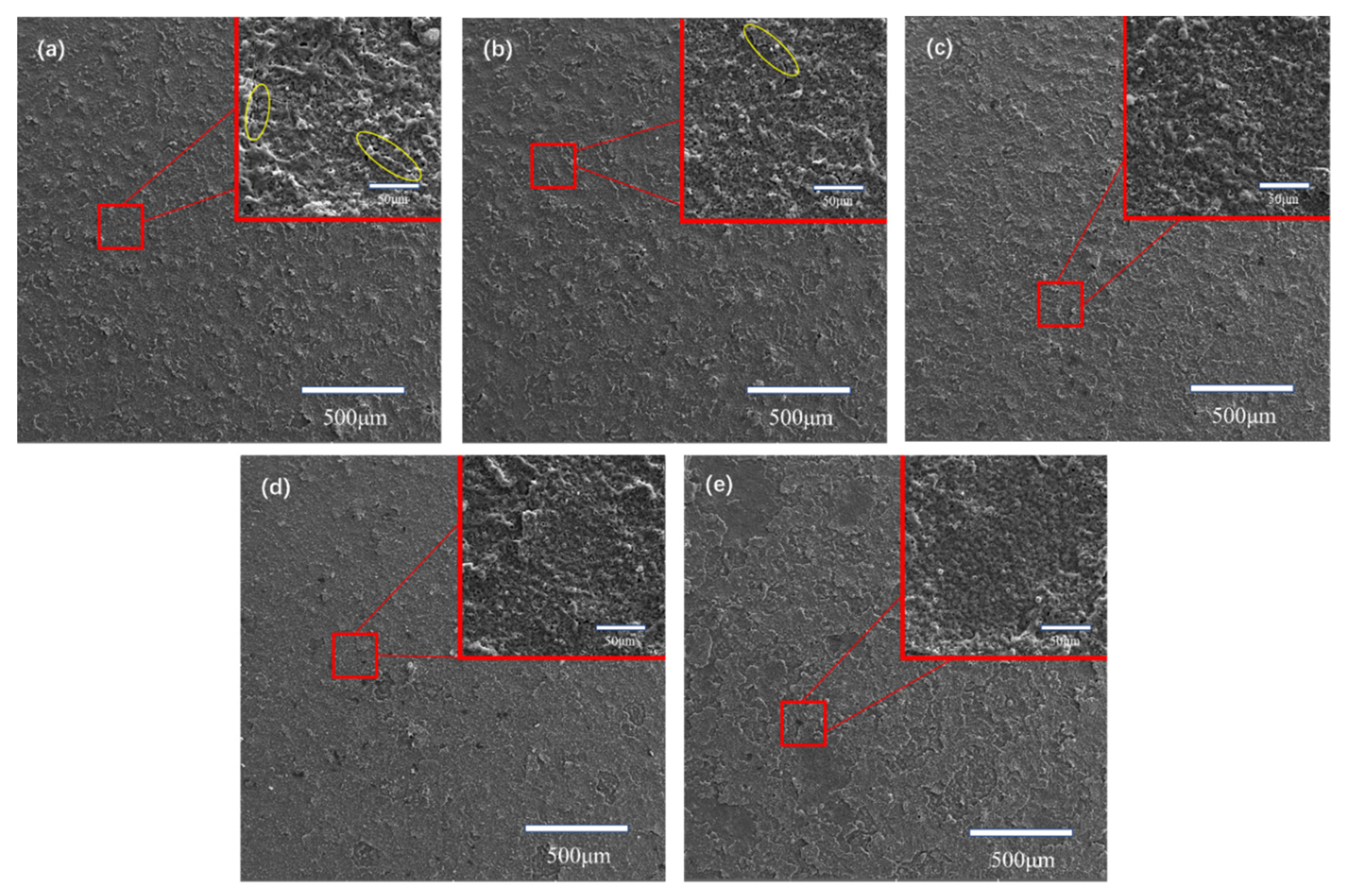
The surface morphologies of MAO coatings with different GO concentration are shown in
Figure 4. From the magnified images, it can be observed that all MAO coatings exhibit a porous microstructure. The pores are typical features of MAO coatings which were mentioned in other papers[
22]. These micro pores were possibly produced by eruption of melted material from inner part of the coating and emission of gas generated by the high-temperature of discharge[
23,
24]. From
Figure 4, it can be observed that the coatings prepared in a solution containing no GO have slightly larger pore sizes than the other coatings, and the pore sizes of coatings containing different concentrations of GO do not show significant differences.
Furthermore, obvious cracks can be observed in
Figure 4(a) and
Figure 4(b) (as shown by yellow circles), the formation of cracks is due to the release of thermal stress generated during the film growth process[
25,
26]. The cracks become smaller and less noticeable on the surface of S3, S4 and S5, which indicates that the addition of GO can decrease thermal stress in the MAO treatment.
The introduction of GO particles into the MAO coatings significantly impacts the surface roughness and coating morphology. The surface of coating S2 and S3 is slightly smoother than that of the coating prepared in particle-free solution. It is clearly evident that the increase in GO concentration results in a smoother and more uniform surface of the coating. However, when the GO concentration reaches 10 g/L, the coating surface exhibits a laminar structure, which is completely different from the other four coatings. This implies relatively weak bonding strength between the layers. The reason is that excessive amount of GO got involved in the coating generation process, which weakened the cohesive strength between layers.
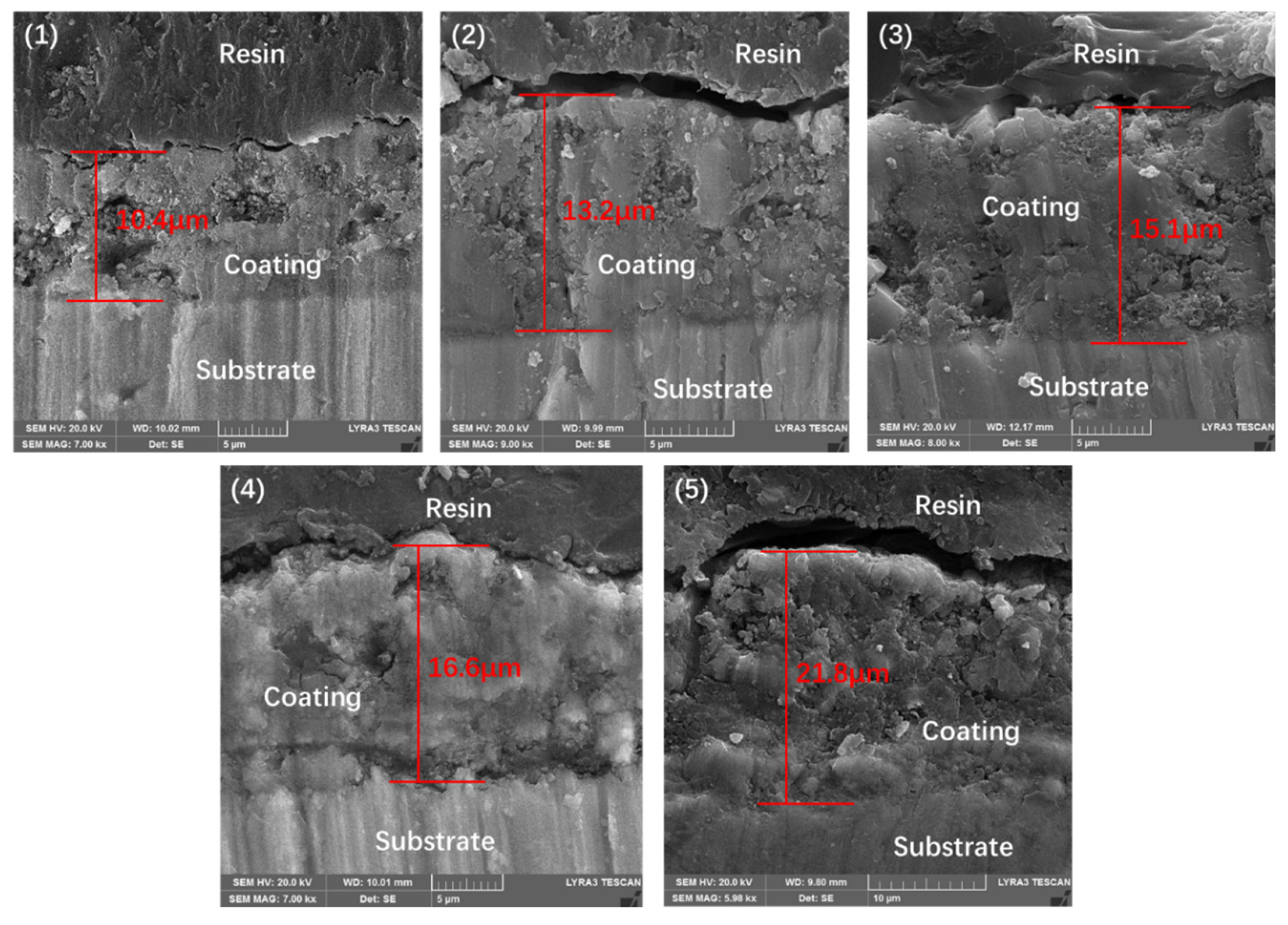
Figure 5 illustrates the cross-sectional microstructure of the ceramic coatings that were generated in the electrolytes with different concentrations of GO particles. As shown in
Figure 5, all coatings exhibited relatively uniform thickness. The addition of GO particles to the electrolyte had a significant effect on the thickness. It can be clearly seen that the coatings become thicker with the increase of GO concentration. As the GO concentration reaches 10 g/L, the thickness rises above 21 μm, which is twice that of the coating prepared in GO-free electrolyte. Several cavities can be clearly seen within the thickness of coating S1, whereas no significant pores were observed in the cross-sectional image of coating prepared in the GO-free electrolyte. This indicates that the addition of GO can reduce the porosity of the coating, thereby increasing its compactness.
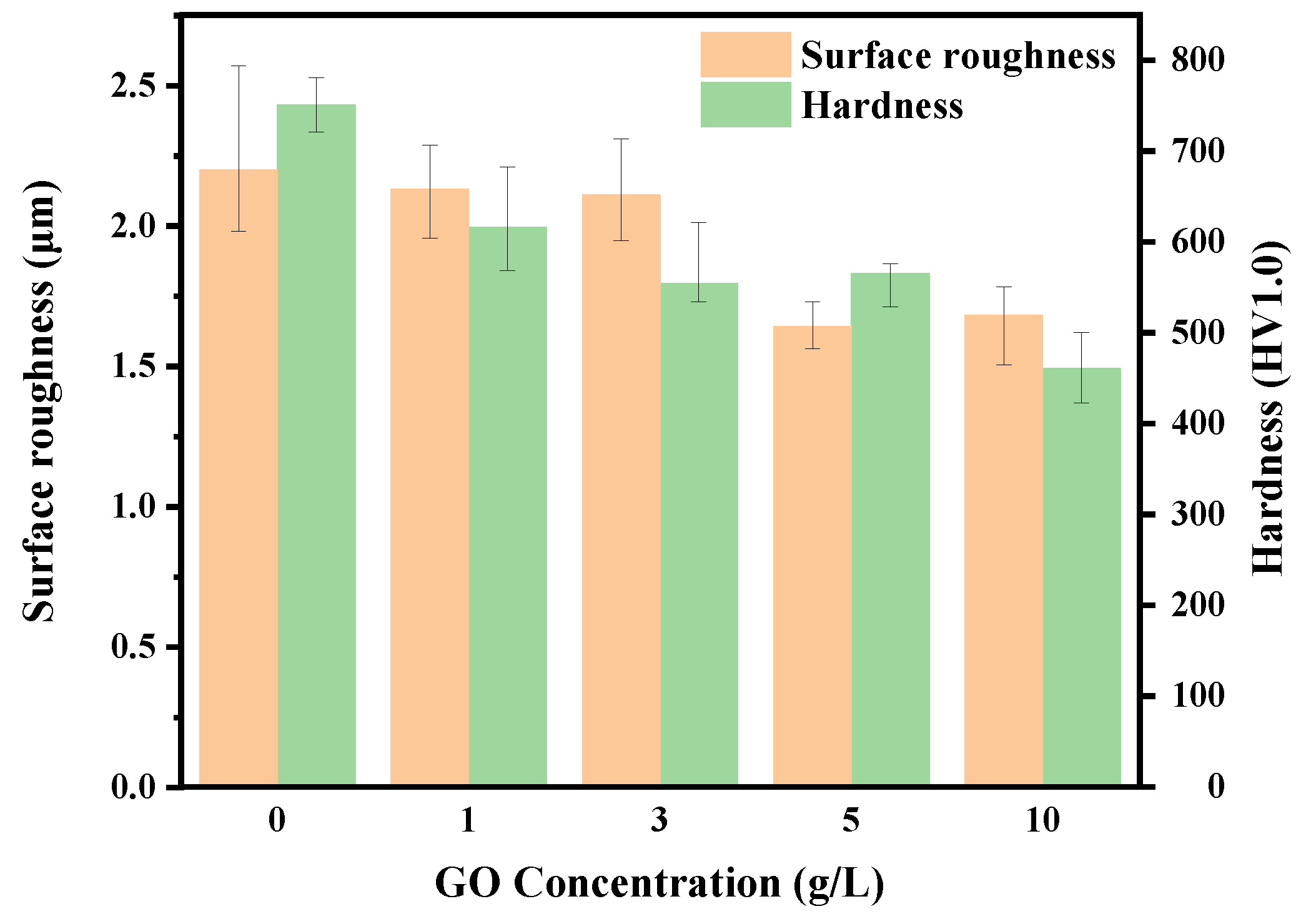
Figure 6 illustrates the surface roughness and hardness of MAO coatings prepared in electrolyte containing different concentrations of GO particles. The microhardness of the MAO coatings was measured by an HV-1000Z hardness tester at a load of 9.8 N applied to a Vickers indenter with a holding time 10 s. Coating S1 exhibits higher hardness compared to the other coatings, and furthermore, with the increasing concentration of GO, the hardness of the coatings shows a decreasing trend. The trend in hardness variations illustrating that the incorporation of GO leads to a decrease in coating hardness, and this decrease is positively correlated with the GO content. With the increase in GO concentration, more particles enter the coating, disrupting continuity of the coating, thereby leading to a decrease in hardness. The roughness of coatings S1, S2, and S3 is quite similar, indicating that there is minimal impact on roughness when the particle content is relatively low. This aligns with the observations by SEM analysis in
Figure 4. The surface roughness of S4 and S5 is nearly identical, indicating that the reduction in coating surface roughness occurs only when the GO concentration surpasses a certain threshold.
3.3. Tribological Performance
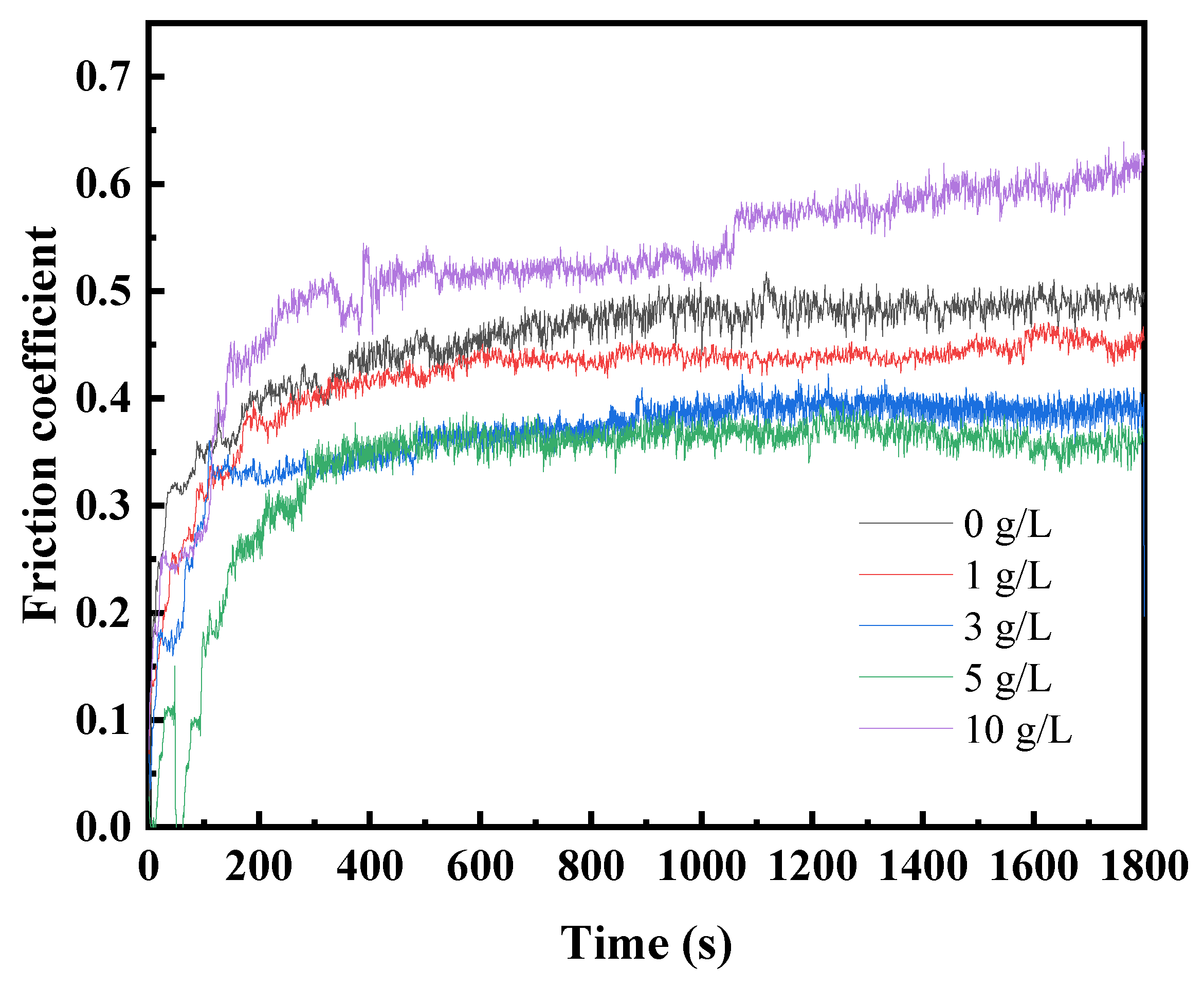
Figure 7 shows the evolution of friction coefficient with sliding time under dry sliding condition for coatings prepared in electrolyte with different GO concentration. All coatings reached a relatively stable level of friction coefficient within five minutes. During the friction process, both the coating and the counterpart ball were continuously worn, resulting in an increasing contact area between them. This led to a gradual rise in the friction coefficient for all coatings over time. The friction coefficients of coating S1, S2, and S3 are all lower and more stable than that of the particle-free coating. As the GO concentration increased from 1 g/L to 5 g/L, the stable friction coefficients show a decreasing trend. However, when the GO concentration reached 10 g/L, the friction coefficient become higher than that of the particle-free coating. The decrease in the friction coefficient with an increase in particle concentration can be attributed to the GO nano-sheet acting as solid lubricants during the friction process. The increase in the friction coefficient of S5 can be attributed to excessively high concentrations causing a reduction in coating hardness. This results in wider wear tracks than those observed in other coatings, which implies a larger contact area during the friction process, subsequently leading to the higher friction coefficient.
Scanning electron micrographs of the wear tracks generated on MAO coatings are exhibited in
Figure 8. The coatings fabricated without the presence of GO in the solution exhibit the narrowest wear scar width. As the concentration of GO increases, the wear scar width become larger. The wear mechanism of MAO coatings can be easily obtained according to the scanning electron micrographs of the wear track. It can be clearly observed that plastic deformations occurred on central area of the wear scar on S1 coating, whereas, in contrast, the GO-composite coatings do not show distinct adhesive damage, which reveals the heavy ploughing because of abrasive wear. As illustrated in
Figure 6, an increase in GO concentration correlates with a decrease in the hardness of the coating. Coatings with lower hardness are more susceptible to wear during the friction process, resulting in wider wear scars[
27]. On the other hand, GO sheets act as lubricants during friction process, thereby reducing adhesive damage in the friction process and lowering the friction coefficient[
28].
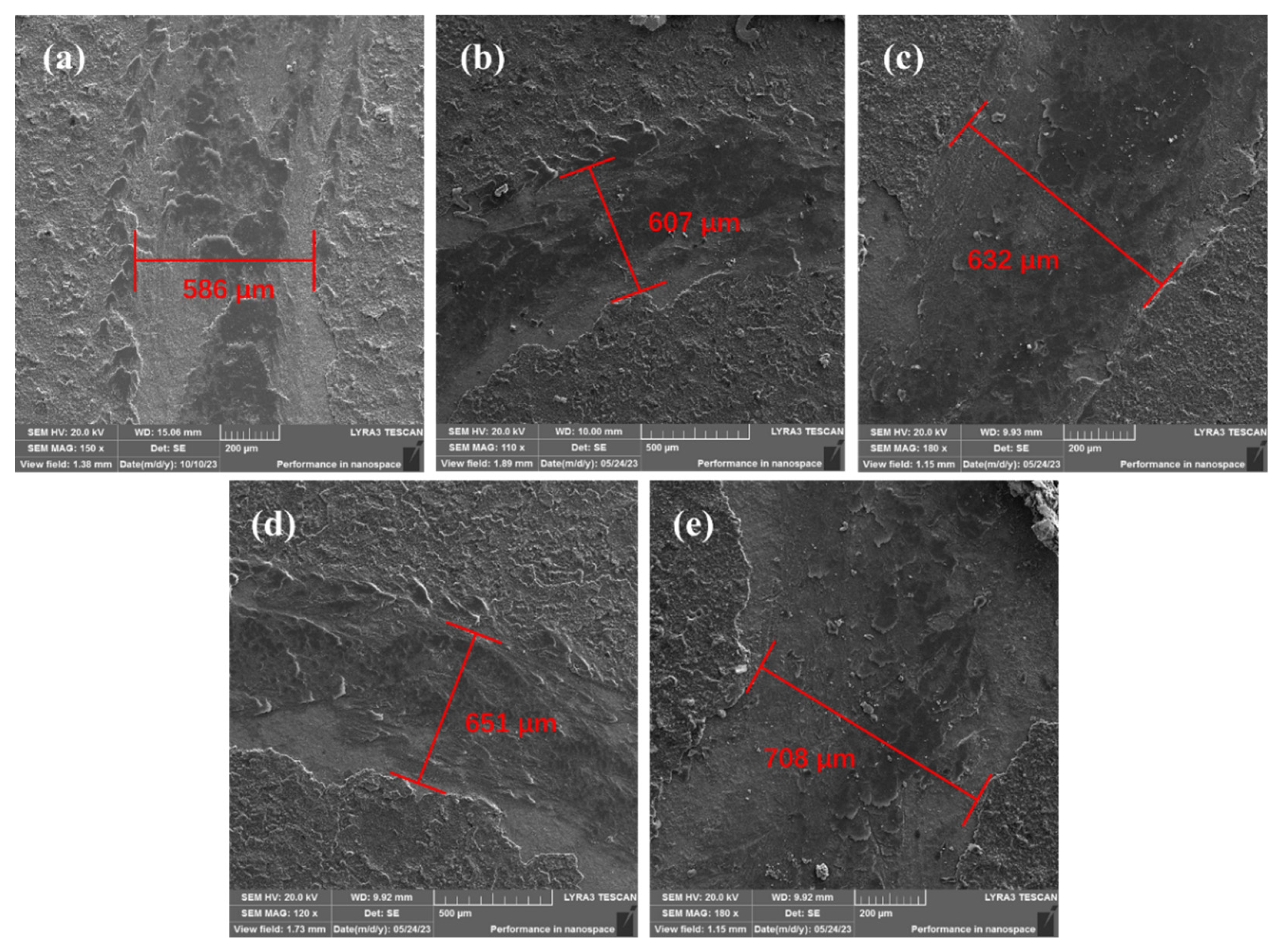
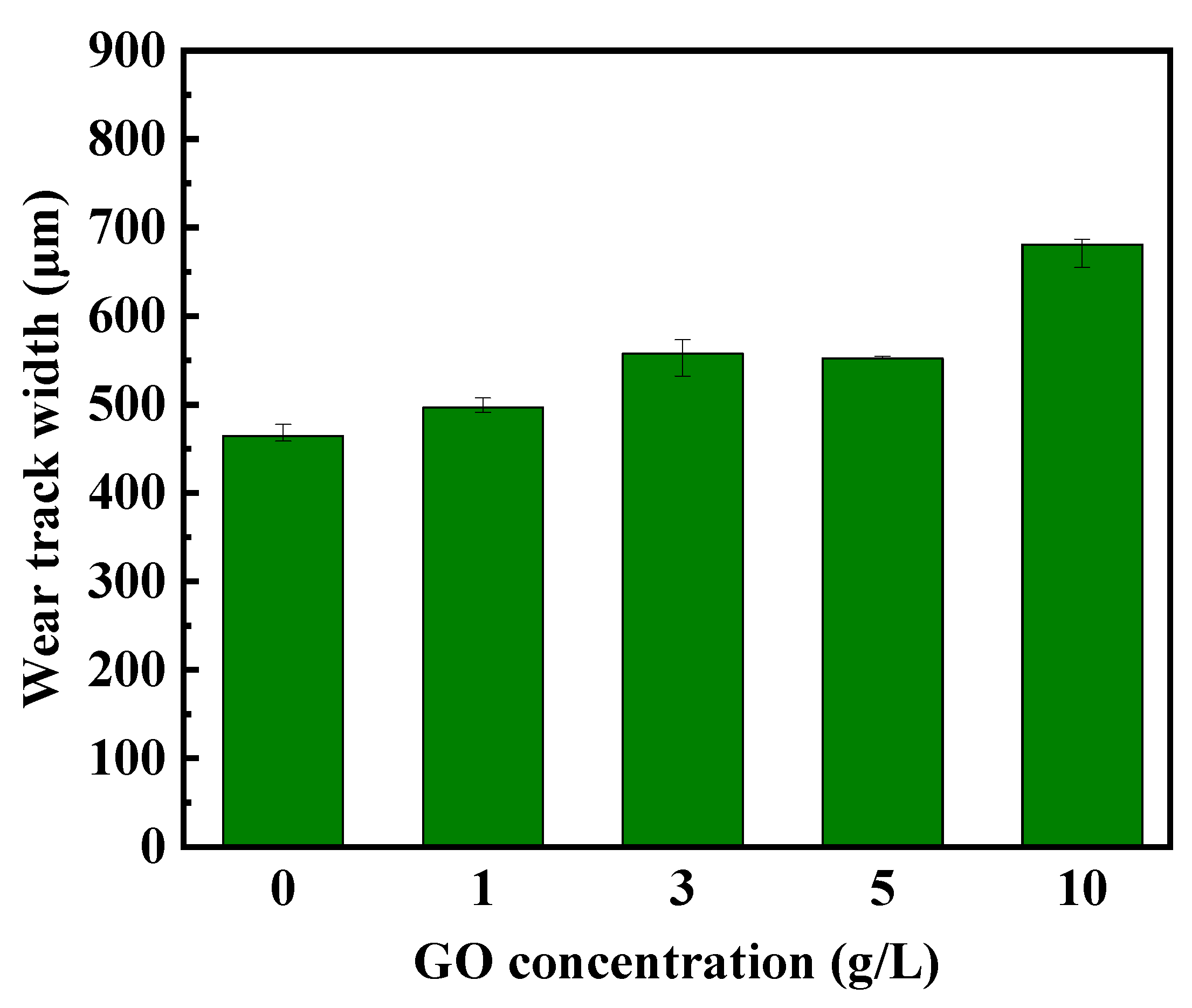
To further validate the impact of particle addition on the wear resistance of the coatings, the variations in wear scar diameter on the counterpart ball against different coatings were studied and shown in
Figure 9. The results indicate that the changes in the diameter of the counter-material wear scars follow a similar pattern to the variations in coating wear scar width. With the increasing concentration of GO, the decrease in coating hardness results in reduced wear resistance, consequently leading to wider wear scars.
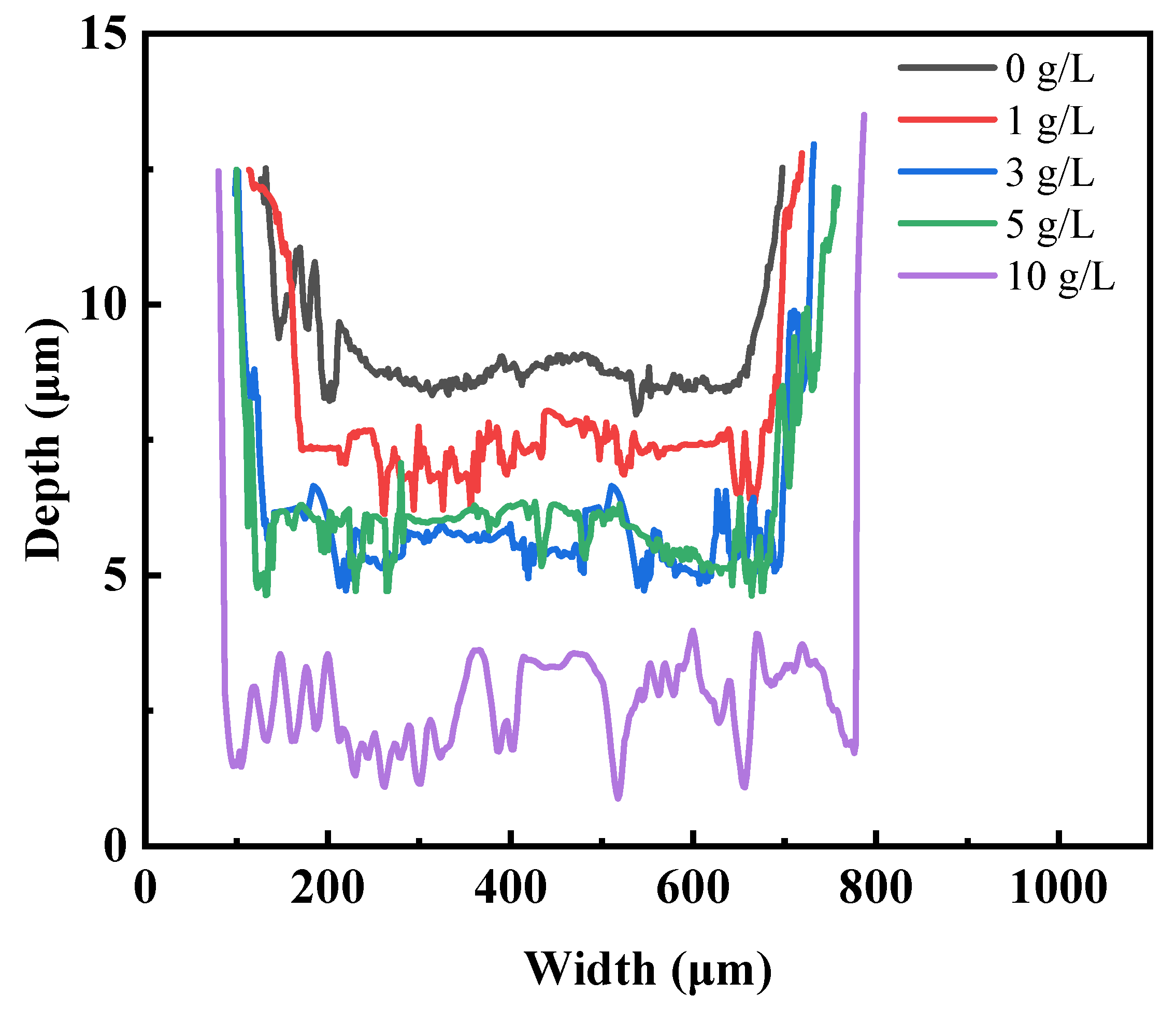
To investigate the influence of GO concentration on the tribological performance of the coatings, the wear track depth profiles of the respective specimens were also measured and shown in
Figure 10. The profile images of the wear scars on the coatings clearly reveal the variations in wear scar depth with changing GO concentrations. The coating without GO exhibits the shallowest wear scar. As the concentration increases, both the depth and width of the wear scar slightly increase, consistent with the SEM results. The wear track depth and width of S3 and S4 show no significant difference, as their hardness is almost the same in
Figure 6. When the GO concentration reaches 10 g/L, the wear scar depth is approximately twice that of S1. The observed differences in wear track depth among the various coatings demonstrate a strong correlation with their respective hardness. With increasing GO concentration, the coating's hardness decreases, resulting in deeper wear scars during the friction process.
4. Conclusions
GO particles were effectively incorporated into MAO coatings on the Ti-6Al-4V alloy surface. The composite coating consisted mainly of Al2TiO5, γ-Al2O3, with minor quantities of low-valence titanium oxide. Particle concentration had little impact on the coating composition. The addition of GO led to a smoother coating surface, with increased smoothness as particle concentration rose. However, at a 10 g/L concentration, excessive particles in the coating disrupted layer cohesion, resulting in a laminar structure on the coating surface.
The incorporation of GO reduced pore size and minimized surface microcracks, resulting in a denser coating structure. Coating hardness decreased with increasing particle concentration due to particle-induced structural disruption. Additionally, coating thickness significantly increased with rising concentration.
Incorporating GO reduced the friction coefficient, with the 5 g/L concentration coating demonstrating optimal anti-friction properties. However, coating hardness and wear resistance were inferior to GO-free coatings. Both wear scar width and depth increased with higher particle content due to the introduced GO disrupting coating structure and reducing cohesion. The addition of GO mitigated adhesive damage during friction, shifting the wear mechanism from adhesive to abrasive.
Author Contributions
Conceptualization, Q.H.; methodology, Q.D.; writing—original draft preparation, Q.H.; investigation, G.W.; formal analysis, X.L.; visualization, Y.R.; writing—review and editing, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by the NSFC (52075247, U2037603) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflict of interest related to this work.
References
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Collier, C.A.; Paillard, J.M.; Curran, J.A. Evaluation of micromechanical behaviour of plasma electrolytic oxidation (PEO) coatings on Ti–6Al–4V. Surf. Coat. Technol. 2010, 204, 3399–3409. [Google Scholar] [CrossRef]
- Zhai, D.; Feng, K.; Yue, H. Growth Kinetics of Microarc Oxidation TiO2 Ceramic Film on Ti6Al4V Alloy in Tetraborate Electrolyte. Metallurgical and Materials Transactions A 2019, 50, 2507–2518. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Liu, C.; Wang, D.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Gladkova, A.A.; Linn, Z.; Strekalina, D.M. The evidence of cathodic micro-discharges during plasma electrolytic oxidation of light metallic alloys and micro-discharge intensity depending on pH of the electrolyte. Surf. Coat. Technol. 2015, 269, 138–144. [Google Scholar] [CrossRef]
- Habazaki, H.; Tsunekawa, S.; Tsuji, E.; Nakayama, T. Formation and characterization of wear-resistant PEO coatings formed on β-titanium alloy at different electrolyte temperatures. Appl. Surf. Sci. 2012, 259, 711–718. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.; Liu, B.; Luo, X.B. Effects of the grain boundary on phase structure and surface morphology of TiO2 films prepared by MAO technology. Surf. Interface Anal. 2012, 44, 276–281. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Matykina, E.; Berkani, A.; Skeldon, P.; Thompson, G.E. Real-time imaging of coating growth during plasma electrolytic oxidation of titanium. Electrochim. Acta 2007, 53, 1987–1994. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, B.; Lei, T.; Guo, L. Dependence of growth features of microarc oxidation coatings of titanium alloy on control modes of alternate pulse. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Suresh, S.; Nagumothu, R.; Sreekanth, D.; Sandhyarani, M. Role of Electric Pulse Duty and Frequency on Properties of Micro-Arc Oxidized Titania Films Developed on Ti-6Al-4V. Mater. Sci. Forum 2013, 765, 688–692. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, J.; Lu, Y.; Du, M.-H.; Han, F.-Z. Effects of single pulse energy on the properties of ceramic coating prepared by micro-arc oxidation on Ti alloy. Appl. Surf. Sci. 2015, 324, 405–413. [Google Scholar] [CrossRef]
- Martin, J.; Melhem, A.; Shchedrina, I.; Duchanoy, T.; Nominé, A.; Henrion, G.; Czerwiec, T.; Belmonte, T. Effects of electrical parameters on plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2013, 221, 70–76. [Google Scholar] [CrossRef]
- Chang, F.-C.; Wang, C.-J.; Lee, J.-W.; Lou, B.-S. Microstructure and mechanical properties evaluation of molybdenum disulfide-titania nanocomposite coatings grown by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 303, 68–77. [Google Scholar] [CrossRef]
- Liu, W.; Blawert, C.; Zheludkevich, M.L.; Lin, Y.; Talha, M.; Shi, Y.; Chen, L. Effects of graphene nanosheets on the ceramic coatings formed on Ti6Al4V alloy drill pipe by plasma electrolytic oxidation. J. Alloys Compd. 2019, 789, 996–1007. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Du, C. Influence of graphene oxide additive on the tribological and electrochemical corrosion properties of a PEO coating prepared on AZ31 magnesium alloy. Tribology International 2020, 146, 106135. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Morozov, A.; Torskaya, E.; Volosova, M.; Yanushevich, O.; Yarygin, N.; Krikheli, N.; et al. Investigation of Tribological Characteristics of PEO Coatings Formed on Ti6Al4V Titanium Alloy in Electrolytes with Graphene Oxide Additives. Materials 2023, 16. [Google Scholar] [CrossRef]
- Shang, W.; Wu, F.; Wang, Y.; Rabiei Baboukani, A.; Wen, Y.; Jiang, J. Corrosion Resistance of Micro-Arc Oxidation/Graphene Oxide Composite Coatings on Magnesium Alloys. ACS Omega 2020, 5, 7262–7270. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.P.; Wang, J.L.; Jiang, B.L. Preparation of MAO coatings doped with graphene oxide. Surf. Eng. 2017, 33, 739–743. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Liu, Z.; Wang, X.; Du, C. Influence of graphene oxide on the antiwear and antifriction performance of MAO coating fabricated on MgLi alloy. Surf. Coat. Technol. 2019, 364, 144–156. [Google Scholar] [CrossRef]
- Chen, X.; Hu, J.; Zhang, D.; Ren, P.; Liao, D.; Cai, L. Study on corrosion resistance of TC4 titanium alloy micro-arc oxidation/(PTFE + graphite) composite coating. Int. J. Appl. Ceram. Technol. 2021, 19, 397–408. [Google Scholar] [CrossRef]
- Ma, K.-J.; Al Bosta, M.M.S.; Wu, W.-T. Preparation of self-lubricating composite coatings through a micro-arc plasma oxidation with graphite in electrolyte solution. Surf. Coat. Technol. 2014, 259, 318–324. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Xue, W.; Wang, C.; Chen, R.; Deng, Z. Structure and properties characterization of ceramic coatings produced on Ti–6Al–4V alloy by microarc oxidation in aluminate solution. Mater. Lett. 2002, 52, 435–441. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Formation mechanism and surface characterization of ceramic composite coatings on pure titanium prepared by micro-arc oxidation in electrolytes containing nanoparticles. Surf. Coat. Technol. 2016, 291, 396–405. [Google Scholar] [CrossRef]
- Martini, C.; Ceschini, L.; Tarterini, F.; Paillard, J.; Curran, J. PEO layers obtained from mixed aluminate–phosphate baths on Ti–6Al–4V: Dry sliding behaviour and influence of a PTFE topcoat. Wear 2010, 269, 747–756. [Google Scholar] [CrossRef]
- Wu, G.; Yin, Y.; Zhang, S.; Wang, Y.; Xiang, Y.; Li, L.; Yao, J. Effect of laser texturing on the antiwear properties of micro-arc oxidation coating formed on Ti-6Al-4V. Surf. Coat. Technol. 2023, 453. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).