Submitted:
20 October 2023
Posted:
20 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Gut microbiota (GM)
Gut Microbiota and nutrition
Gut Microbiota and metabolism
Biotransformation of common Short Chain Fatty Acids
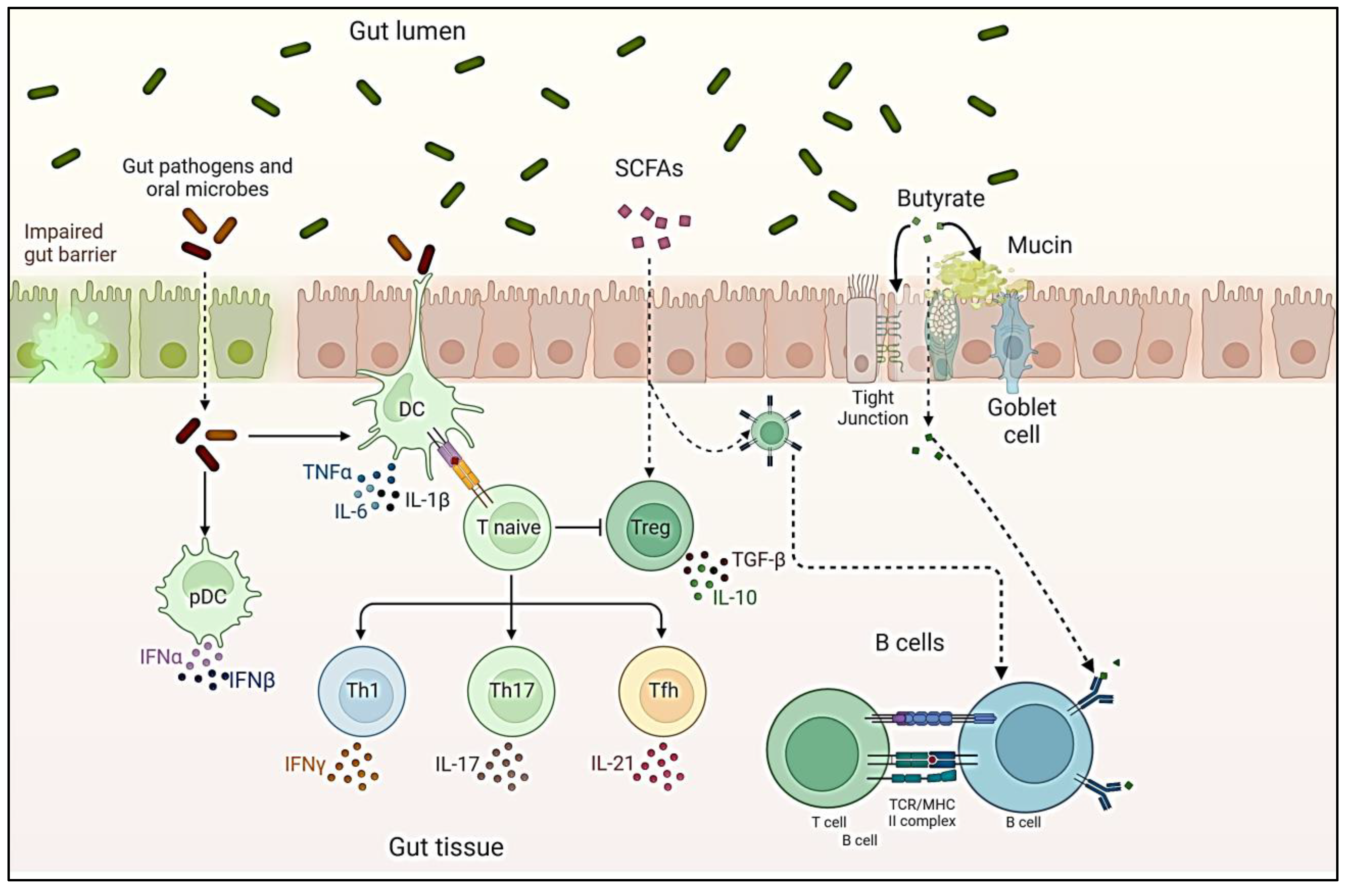
Gut Microbiota and immunomodulation
Gut Microbiota and host interaction
Changes in Gut Microbiota composition.
Aging and Gut Microbiota composition
Nutritional alteration and Gut Microbiota composition
Microbiome composition and pathological bone development and involution
Intervertebral disc (IVD) degeneration (IDD) and gut inflammation
The Gut-spine axis
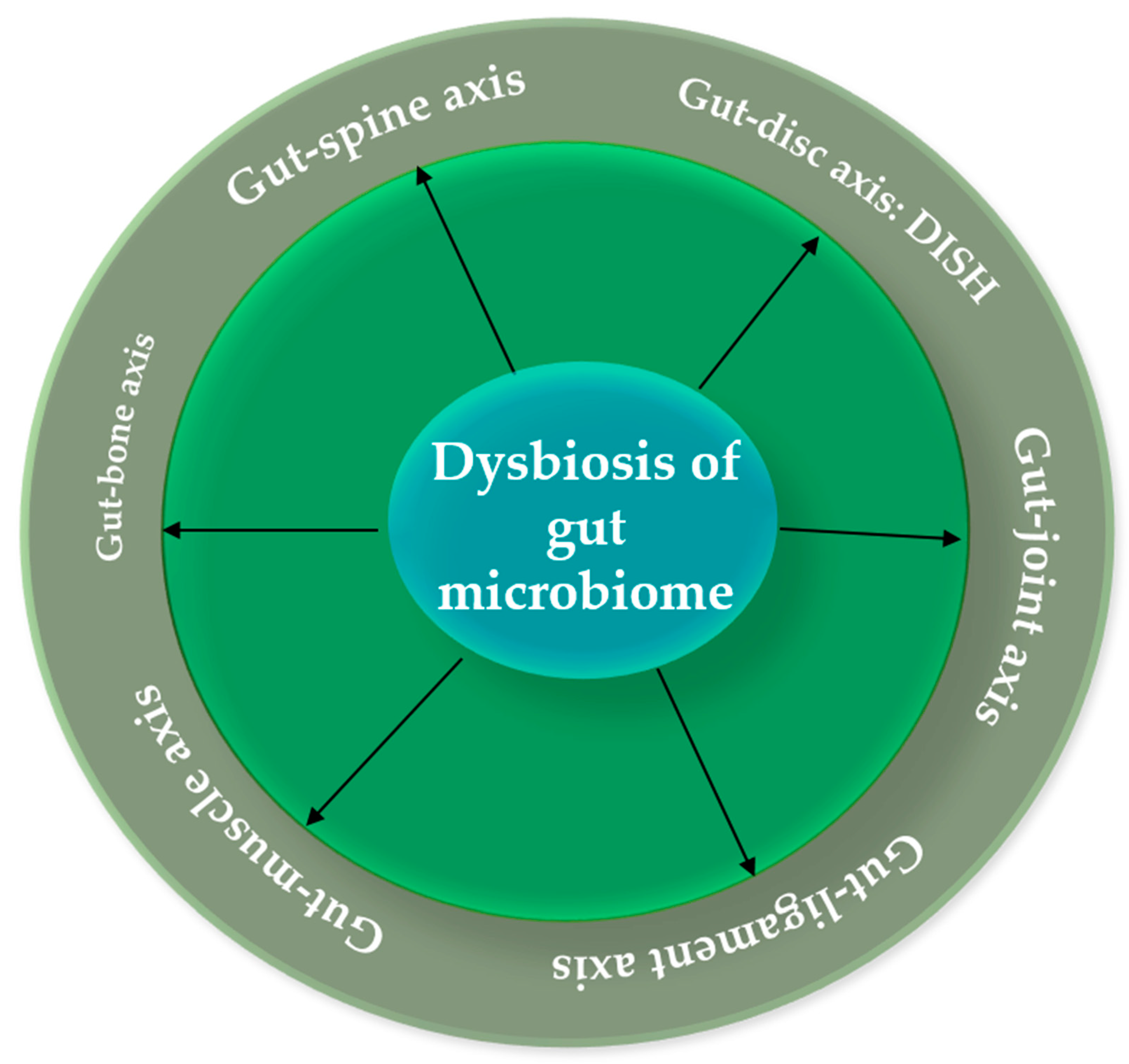
Gut-bone axis: osteoporosis and hyperostotic diseases
Gut-joint axis: Osteoarthritis (OA) and facet joint syndrome
Gut-disc axis: DISH
The Gut-Ligament Axis: Exploring the Link to Lumbar Spinal Stenosis
The Gut-Muscle Axis: Unraveling Its Connection to Spinal Sarcopenia
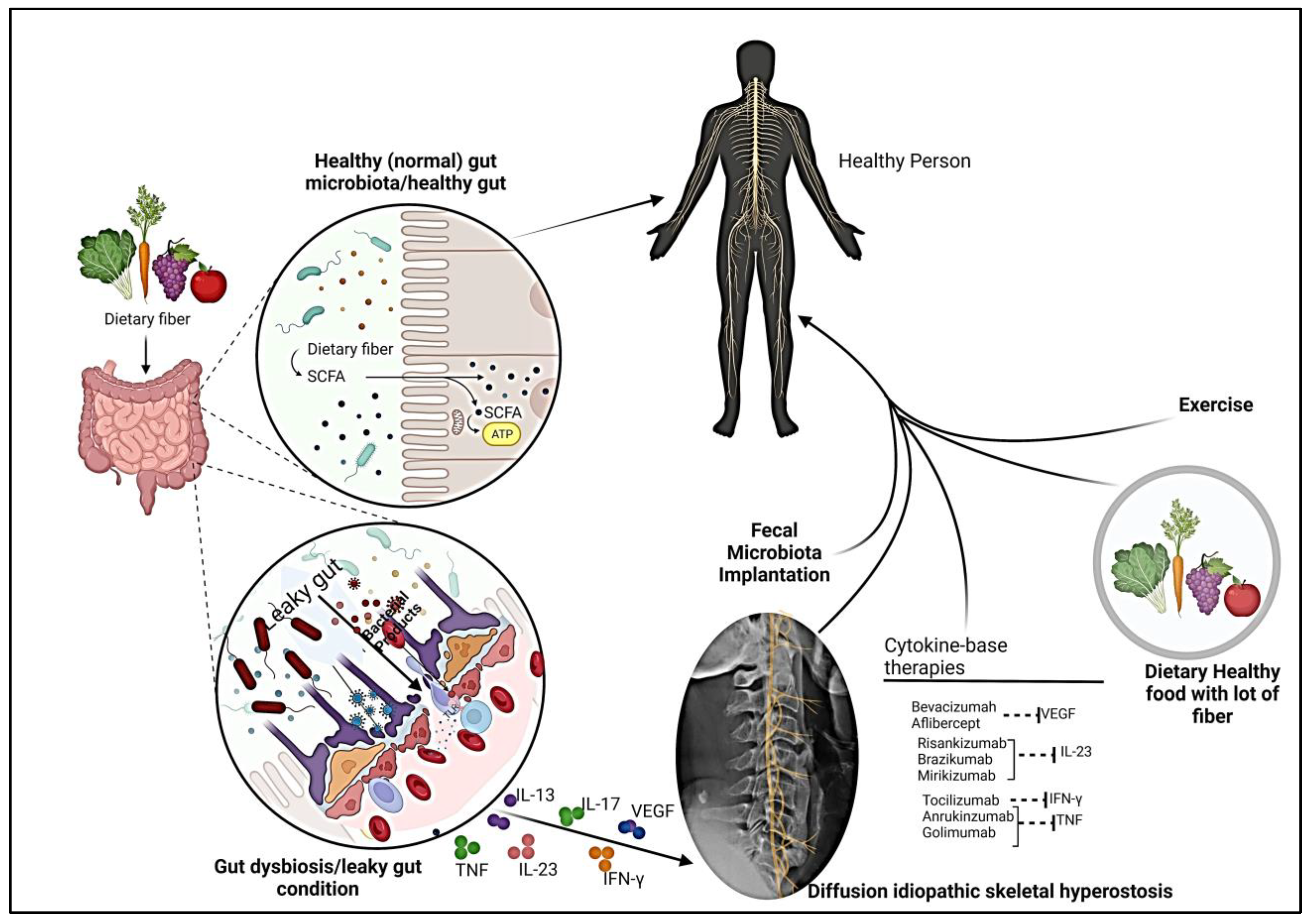
Impact of Gut Microbiota on DISH -derived pain
Fecal microbiome transplant and DISH
Conclusion and perspective
References
- Wójcik, G.; Szulc, A. Diffuse idiopathic skeletal hyperostosis of the spine. Journal of Education, Health and Sport 2018, 8, 593–599. [Google Scholar]
- Srinivasan, W.; Thorell, W.; McCumber, T.L.; Vilburn, M.; Snow, E.L. Hyperostosis cranialis interna and an ectopic ossification on the endosteal dura deep to the trigeminal ganglion: Case analysis and clinical implications. Translational Research in Anatomy 2023, 30, 100239. [Google Scholar]
- Akhtar, A.; Lata, M.; Sunsunwal, S.; Yadav, A.; Lnu, K.; Subramanian, S.; Ramya, T.N.C. New carbohydrate binding domains identified by phage display based functional metagenomic screens of human gut microbiota. Communications Biology 2023, 6, 371. [Google Scholar]
- Wan, X.; Eguchi, A.; Sakamoto, A.; Fujita, Y.; Yang, Y.; Qu, Y.; Hatano, M.; Mori, C.; Hashimoto, K. Impact of broad-spectrum antibiotics on the gut–microbiota–spleen–brain axis. Brain, Behavior, & Immunity-Health 2023, 27, 100573. [Google Scholar]
- Holgate, R.L.; Steyn, M. Diffuse idiopathic skeletal hyperostosis: Diagnostic, clinical, and paleopathological considerations. Clinical anatomy 2016, 29, 870–877. [Google Scholar] [CrossRef]
- Mader, R.; Pappone, N.; Baraliakos, X.; Eshed, I.; Sarzi-Puttini, P.; Atzeni, F.; Bieber, A.; Novofastovski, I.; Kiefer, D.; Verlaan, J.J.; Ambrosino, P. Diffuse idiopathic skeletal hyperostosis (DISH) and a possible inflammatory component. Current Rheumatology Reports 2021, 23, 1–6. [Google Scholar]
- Mader, R. Current therapeutic options in the management of diffuse idiopathic skeletal hyperostosis. Expert opinion on pharmacotherapy 2005, 6, 1313–131. [Google Scholar] [CrossRef]
- Cammisa, M.; De Serio, A.; Guglielmi, G. Diffuse idiopathic skeletal hyperostosis. European Journal of Radiology 1998, 27, S7–S11. [Google Scholar] [CrossRef]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A. Spondyloarthritis and diffuse idiopathic skeletal hyperostosis: two different diseases that continue to intersect. The Journal of Rheumatology 2013, 40, 1251–1253. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F. New developments in our understanding of DISH (diffuse idiopathic skeletal hyperostosis). Current opinion in rheumatology 2004, 16, 287–292. [Google Scholar]
- Mazières, B. Diffuse idiopathic skeletal hyperostosis (Forestier-Rotes-Querol disease): what’s new? Joint Bone Spine 2013, 80, 466–470. [Google Scholar] [CrossRef]
- Van der Merwe, A.E.; Maat, G.J.R.; Watt, I. Diffuse idiopathic skeletal hyperostosis: diagnosis in a palaeopathological context. Homo 2012, 63, 202–215. [Google Scholar] [CrossRef]
- Balling, H.; Weckbach, A. Hyperextension injuries of the thoracolumbar spine in diffuse idiopathic skeletal hyperostosis. Spine 2015, 40, E61–E67. [Google Scholar] [CrossRef]
- Hirasawa, A.; Wakao, N.; Kamiya, M.; Takeuchi, M.; Kawanami, K.; Murotani, K.; Matsuo, T.; Deie, M. The prevalence of diffuse idiopathic skeletal hyperostosis in Japan–the first report of measurement by CT and review of the literature. Journal of Orthopaedic Science 2016, 21, 287–290. [Google Scholar] [CrossRef]
- Ikuma, H.; Hirose, T.; Nakamura, D.; Yamashita, K.; Ueda, M.; Sasaki, K.; Kawasaki, K. The prevalence and characteristics of diffuse idiopathic skeletal hyperostosis (DISH): A cross-sectional study of 1519 Japanese individuals. Diagnostics 2022, 12, 1088. [Google Scholar] [CrossRef]
- Mader, R. Clinical manifestations of diffuse idiopathic skeletal hyperostosis of the cervical spine. In Seminars in arthritis and rheumatism; WB Saunders, October 2002; Volume 32, No. 2, pp. 130–135. [Google Scholar]
- Ibrahim, I.; Syamala, S.; Ayariga, J.A.; Xu, J.; Robertson, B.K.; Meenakshisundaram, S.; Ajayi, O.S. Modulatory effect of gut microbiota on the gut-brain, gut-bone axes, and the impact of cannabinoids. Metabolites 2022, 12, 1247. [Google Scholar]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Frontiers in Endocrinology 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Niu, G.; Jian, T.; Gai, Y.; Chen, J. Microbiome and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Advanced Drug Delivery Reviews 2023, 114774. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Molecular biomedicine 2022, 3, 30. [Google Scholar]
- Ou, J.; Wang, Z.; Liu, X.; Song, B.; Chen, J.; Li, R.; Jia, X.; Huang, R.; Xiang, W.; Zhong, S. Regulatory effects of marine polysaccharides on gut microbiota dysbiosis: A review. Food Chemistry: X 2022, 10044. [Google Scholar] [CrossRef]
- Saranya, G.R.; Viswanathan, P. Gut microbiota dysbiosis in AKI to CKD transition. Biomedicine & Pharmacotherapy 2023, 161, 114447. [Google Scholar]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Molecular psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Fiocchi, A.; Cabana, M.D.; Mennini, M. Current use of probiotics and prebiotics in allergy. The Journal of Allergy and Clinical Immunology: In Practice 2022, 10, 2219–2242. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal microbiota transplantation as new therapeutic avenue for human diseases. Journal of Clinical Medicine 2022, 11, 4119. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Zakerska-Banaszak, O.; Skrzypczak-Zielińska, M.; Łykowska-Szuber, L.; Szymczak-Tomczak, A.; Zawada, A.; Rychter, A.M.; Ratajczak, A.E.; Skoracka, K.; Skrzypczak, D.; Marcinkowska, E. Where Do We Stand in the Behavioral Pathogenesis of Inflammatory Bowel Disease? The Western Dietary Pattern and Microbiota—A Narrative Review. Nutrients 2022, 14, 2520. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Z.; Zhai, T.; Ding, Q.; Yang, H.; Wang, J.; Zhang, L.; Zhao, L. Global research trends on the links between the gut microbiota and diabetes between 2001 and 2021: A bibliometrics and visualized study. Frontiers in Microbiology 2022, 13, 1011050. [Google Scholar] [CrossRef]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Frontiers in Microbiology 2022, 13, 926926. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Markou, E.; Dimitriadis, F.; Sofikitis, N.; Pournaras, S. Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. Journal of Personalized Medicine 2023, 13, 1491. [Google Scholar] [CrossRef]
- Larsen, O.F.; van der Grint, M.; Wiegers, C.; van de Burgwal, L.H. The gut microbiota: Master of puppets connecting the epidemiology of infectious, autoimmune, and metabolic disease. Frontiers in Microbiology 2022, 13, 1604. [Google Scholar]
- He, X.; Sun, J.; Liu, C.; Yu, X.; Li, H.; Zhang, W.; Li, Y.; Geng, Y.; Wang, Z. Compositional alterations of gut microbiota in patients with diabetic kidney disease and type 2 diabetes mellitus. In Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy; 2022; pp. 755–765. [Google Scholar]
- Bresser, L.R.; de Goffau, M.C.; Levin, E.; Nieuwdorp, M. Gut microbiota in nutrition and health with a special focus on specific bacterial clusters. Cells 2022, 11, 3091. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annual Review of Physiology 2023, 85, 449–468. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Frontiers in Nutrition 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yeboah, P.J.; Ayivi, R.D.; Eddin, A.S.; Wijemanna, N.D.; Paidari, S.; Bakhshayesh, R.V. A review and comparative perspective on health benefits of probiotic and fermented foods. International Journal of Food Science & Technology 2023, 58, 4948–4964. [Google Scholar]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; Aadil, R.M. Human gut microbiota in health and disease: Unveiling the relationship. Frontiers in microbiology 2022, 13, 999001. [Google Scholar] [CrossRef]
- Expósito-Almellón, X.; Duque-Soto, C.; López-Salas, L.; Quirantes-Piné, R.; de Menezes, C.R.; Borrás-Linares, I.; Lozano-Sánchez, J. Non-Digestible Carbohydrates: Green Extraction from Food By-Products and Assessment of Their Effect on Microbiota Modulation. Nutrients 2023, 15, 3880. [Google Scholar]
- Ferraz, M.P. An Overview of the Relevance of Human Gut and Skin Microbiome in Disease: The Influence on Atopic Dermatitis. Applied Sciences 2023, 13, 10540. [Google Scholar] [CrossRef]
- Li, S.; Qian, Z.; Yang, J.; Lin, Y.; Li, H.; Chen, L. Seasonal variation in structure and function of gut microbiota in Pomacea canaliculata. Ecology and Evolution 2022, 12, e9162. [Google Scholar] [CrossRef]
- Han, Y.; Shao, D.; Han, C.; Huang, Q.; Zhao, W. Response of human gut microbiota under simulated microgravity. Applied Microbiology and Biotechnology 2022, 106, 5221–5231. [Google Scholar] [CrossRef]
- Ringø, E.; Harikrishnan, R.; Soltani, M.; Ghosh, K. The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 2022, 12, 3016. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the human gut microbiota: From breakdown mechanisms to the impact on metabolic health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Practice & Research Clinical Gastroenterology 2023, 62, 101828. [Google Scholar]
- Hassane, M.; Jouan, Y.; Creusat, F.; Soulard, D.; Boisseau, C.; Gonzalez, L.; Patin, E.C.; Heuzé-Vourc’h, N.; Sirard, J.C.; Faveeuw, C.; Trottein, F. Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response. Mucosal Immunology 2020, 13, 128–139. [Google Scholar] [CrossRef]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin signaling in obesity and colorectal cancer. International journal of molecular sciences 2022, 23, 4713. [Google Scholar] [CrossRef]
- Hamamah, S.; Amin, A.; Al-Kassir, A.L.; Chuang, J.; Covasa, M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients 2023, 15, 3365. [Google Scholar] [CrossRef]
- Pan, S.; Guo, Y.; Hong, F.; Xu, P.; Zhai, Y. Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2022, 1868, 166281. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Sivakumar, A.S.; Lee, C.H.; Kim, S.J. Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical and molecular based treatment strategies via gene and nanotherapy. Biomedicine & Pharmacotherapy 2022, 151, 113134. [Google Scholar]
- Liu, D.; Yang, K.; Gu, H.; Li, Z.; Wang, Y.; Wang, Y. Predictive effect of triglyceride-glucose index on clinical events in patients with acute ischemic stroke and type 2 diabetes mellitus. Cardiovascular Diabetology 2022, 21, 1–14. [Google Scholar] [CrossRef]
- Strikić, D.; Vujević, A.; Perica, D.; Leskovar, D.; Paponja, K.; Pećin, I.; Merćep, I. Importance of Dyslipidaemia Treatment in Patients with Type 2 Diabetes Mellitus. 2023. [Google Scholar]
- Domínguez-Balmaseda, D.; García-Pérez-de-Sevilla, G. The Relationship between the Gut Microbiota and Exercise: A Narrative Review. Hygiene 2022, 2, 152–162. [Google Scholar] [CrossRef]
- Bakhru, A. (Ed.) Nutrition and Integrative Medicine for Clinicians: Volume Two; CRC Press, 2023. [Google Scholar]
- Carloni, S.; Rescigno, M. The gut-brain vascular axis in neuroinflammation. Seminars in Immunology 2023, 69, 101802. [Google Scholar]
- van Deuren, T.; Blaak, E.E.; Canfora, E.E. Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obesity Reviews 2022, 23, e13498. [Google Scholar] [CrossRef]
- Bhatia, Z.; Kumar, S.; Seshadri, S. Exploring the Unexplored Arena: Butyrate as a Dual Communicator in Gut–Brain Axis. In Probiotics, Prebiotics, Synbiotics, and Postbiotics: Human Microbiome and Human Health; Springer Nature Singapore: Singapore, 2023; pp. 153–164. [Google Scholar]
- Guo, T.T.; Zhang, Z.; Sun, Y.; Zhu, R.Y.; Wang, F.X.; Ma, L.J.; Jiang, L.; Liu, H.D. Neuroprotective Effects of Sodium Butyrate by Restoring Gut Microbiota and Inhibiting TLR4 Signaling in Mice with MPTP-Induced Parkinson’s Disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef]
- Xun, Z.; Yao, X.; Ou, Q. Emerging roles of bile acids in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cellular & Molecular Immunology 2023, 1–3. [Google Scholar]
- Lei, Y.; Tang, L.; Chen, Q.; Wu, L.; He, W.; Tu, D.; Wang, S.; Chen, Y.; Liu, S.; Xie, Z.; Wei, H. Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nature Communications 2022, 13, 6862. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, Q.; Zhou, X.; Duan, Y.; Yang, Y.; Gong, S.; Han, M.; Liu, Y.; Yang, Z.; Chen, Q.; Li, F. Role of brain-gut-muscle axis in human health and energy homeostasis. Frontiers in Nutrition 2022, 9, 2250. [Google Scholar]
- Huang, L.; Zheng, J.; Sun, G.; Yang, H.; Sun, X.; Yao, X.; Lin, A.; Liu, H. 5-Aminosalicylic acid ameliorates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota and bile acid metabolism. Cellular and Molecular Life Sciences 2022, 79, 460. [Google Scholar] [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Current gastroenterology reports 2023, 25, 31–44. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; Li, D. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health—A review. Animal Bioscience 2022, 35, 1461. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Translational Research 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Pezzino, S.; Sofia, M.; Greco, L.P.; Litrico, G.; Filippello, G.; Sarvà, I.; La Greca, G.; Latteri, S. Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma. International Journal of Molecular Sciences 2023, 24, 1166. [Google Scholar] [CrossRef]
- Fikree, A.; Byrne, P. Management of functional gastrointestinal disorders. Clinical Medicine 2021, 21, 44. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nature Reviews Microbiology 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Matheson, J.A.T.; Holsinger, R.D. The role of fecal microbiota transplantation in the treatment of neurodegenerative diseases: A review. International Journal of Molecular Sciences 2023, 24, 1001. [Google Scholar] [CrossRef]
- Beyi, A.F.; Wannemuehler, M.; Plummer, P.J. Impacts of gut microbiota on the immune system and fecal microbiota transplantation as a re-emerging therapy for autoimmune diseases. Antibiotics 2022, 11, 1093. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell research 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nature Reviews Immunology 2023, 1–14. [Google Scholar] [CrossRef]
- Costantini, C. The immune system and the microbiota: the two sides of mucosal tolerance. In Translational Autoimmunity; Academic Press, 2022; pp. 297–315. [Google Scholar]
- Yagi, K.; Asai, N.; Huffnagle, G.B.; Lukacs, N.W.; Fonseca, W. Early-life lung and gut microbiota development and respiratory syncytial virus infection. Frontiers in Immunology 2022, 13, 877771. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Vojdani, C. The Immune System: Our Body’s Homeland Security Against Disease. In Integrative and Functional Medical Nutrition Therapy: Principles and Practices; 2020; pp. 285–305. [Google Scholar]
- Edwards, C.; Shah, S.A.; Gebhardt, T.; Jewell, C.M. Exploiting unique features of microneedles to modulate immunity. Advanced Materials 2023, 2302410. [Google Scholar] [CrossRef]
- Ning, X.; Lei, Z.; Rui, B.; Li, Y.; Li, M. Gut microbiota promotes immune tolerance by regulating RORγt+ treg cells in food allergy. Advanced Gut & Microbiome Research 2022. [Google Scholar]
- Schuppan, D.; Gisbert-Schuppan, K.; Schuppan, D.; Gisbert-Schuppan, K. Immunology of the Intestine. In Wheat Syndromes: How Wheat, Gluten and ATI Cause Inflammation, IBS and Autoimmune Diseases; 2019; pp. 11–23. [Google Scholar]
- Samih, M.; Ahami, A.O.T. Consequences of errors in the functioning of the immune system Descriptive review [Running title: Consequences of misstep of the immune system]. International Journal of Innovation and Applied Studies 2023, 40, 444–451. [Google Scholar]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poultry science 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Barnig, C.; Bezema, T.; Calder, P.C.; Charloux, A.; Frossard, N.; Garssen, J.; Haworth, O.; Dilevskaya, K.; Levi-Schaffer, F.; Lonsdorfer, E.; Wauben, M. Activation of resolution pathways to prevent and fight chronic inflammation: Lessons from asthma and inflammatory bowel disease. Frontiers in immunology 2019, 10, 1699. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. In Seminars in immunology; Academic Press, August 2019; Vol. 44, p. 101344. [Google Scholar]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. International journal of molecular sciences 2021, 22, 2719. [Google Scholar] [CrossRef]
- Fang, S.; Ju, D.; Lin, Y.; Chen, W. The role of interleukin-22 in lung health and its therapeutic potential for COVID-19. Frontiers in immunology 2022, 13, 951107. [Google Scholar] [CrossRef]
- Favalli, E.G. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatology and therapy 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Houben, E.; Hellings, N.; Broux, B. Oncostatin M, an underestimated player in the central nervous system. Frontiers in immunology 2019, 10, 1165. [Google Scholar] [CrossRef]
- Dikilitas, A.; Karaaslan, F.; Aydin, E.Ö.; Yigit, U.; Ertugrul, A.S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) in subjects with different stages of periodontitis according to the new classification. Journal of Applied Oral Science 2022, 30. [Google Scholar] [CrossRef]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Archives of pharmacal research 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Lokau, J.; Kespohl, B.; Kirschke, S.; Garbers, C. The role of proteolysis in interleukin-11 signaling. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2022, 1869, 119135. [Google Scholar] [CrossRef]
- Merli, P.; Quintarelli, C.; Strocchio, L.; Locatelli, F. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatric Blood & Cancer 2021, 68, e28900. [Google Scholar]
- Wu, S.; Duan, C.; Kong, L.; Tu, X.; Wang, L.; Guo, Z.; Ye, J. Interleukin-10 (IL-10) participates in host defense against bacterial pathogens and promotes IgM antibody production in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 531, 735829. [Google Scholar] [CrossRef]
- Soyfoo, M.S.; Nicaise, C. Pathophysiologic role of Interleukin-33/ST2 in Sjögren’s syndrome. Autoimmunity Reviews 2021, 20, 102756. [Google Scholar] [CrossRef]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible roles of interleukin-4 and-13 and their receptors in gastric and colon cancer. International Journal of Molecular Sciences 2021, 22, 727. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the pathophysiology of severe asthma. Frontiers in physiology 2019, 10, 1514. [Google Scholar] [CrossRef]
- Kar, S.; Gupta, R.; Malhotra, R.; Sharma, V.; Farooque, K.; Kumar, V.; Chakraborty, S.; Mitra, D.K. Interleukin-9 facilitates osteoclastogenesis in rheumatoid arthritis. International journal of molecular sciences 2021, 22, 10397. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, F.; Tao, H.; Nawaz, W.; Chen, D.; Wu, Z. The potential roles of interleukin-25 in infectious diseases. Frontiers in Immunology 2022, 13, 986118. [Google Scholar] [CrossRef]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nature Reviews Immunology 2023, 23, 24–37. [Google Scholar] [CrossRef]
- Rana, T. Influence and Implications of the Molecular Paradigm of Nitric Oxide Underlying Inflammatory Reactions of the Gastrointestinal Tract of Dog: A Major Hallmark of Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2022, 28, 1280–1288. [Google Scholar]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; Wu, W.K.K. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2022, 71, 2011–2021. [Google Scholar] [CrossRef]
- Brown, J.A.; Sanidad, K.Z.; Lucotti, S.; Lieber, C.M.; Cox, R.M.; Ananthanarayanan, A.; Basu, S.; Chen, J.; Shan, M.; Amir, M.; Schmidt, F. Gut microbiota-derived metabolites confer protection against SARS-CoV-2 infection. Gut Microbes 2022, 14, 2105609. [Google Scholar]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Huynh, U.; Zastrow, M.L. Metallobiology of Lactobacillaceae in the gut microbiome. Journal of Inorganic Biochemistry 2023, 238, 112023. [Google Scholar]
- Abbott, M.; Ustoyev, Y. Cancer and the immune system: the history and background of immunotherapy. In Seminars in oncology nursing; WB Saunders, October 2019; Vol. 35, No. 5, p. 150923. [Google Scholar]
- Lajqi, T.; Köstlin-Gille, N.; Hillmer, S.; Braun, M.; Kranig, S.A.; Dietz, S.; Krause, C.; Rühle, J.; Frommhold, D.; Pöschl, J.; Gille, C. Gut microbiota-derived small extracellular vesicles endorse memory-like inflammatory responses in murine neutrophils. Biomedicines 2022, 10, 442. [Google Scholar]
- Strizova, Z.; Smetanova, J.; Bartunkova, J.; Milota, T. Principles and challenges in anti-COVID-19 vaccine development. International Archives of Allergy and Immunology 2021, 182, 339–349. [Google Scholar] [CrossRef]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J. Immune memory in aging: a wide perspective covering microbiota, brain, metabolism, and epigenetics. Clinical Reviews in Allergy & Immunology 2022, 63, 499–529. [Google Scholar]
- da Silva Soares, N.F.; Quagliariello, A.; Yigitturk, S.; Martino, M.E. Gut microbes predominantly act as living beneficial partners rather than raw nutrients. Scientific Reports 2023, 13, 11981. [Google Scholar] [CrossRef]
- Meng, C.; Feng, S.; Hao, Z.; Dong, C.; Liu, H. Changes in gut microbiota composition with age and correlations with gut inflammation in rats. Plos one 2022, 17, e0265430. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Chen, F.; Yan, X.; Liu, X.; Shao, L.; Jin, G.; Zhou, D.; Jiang, G.; Li, H.; Zhao, L. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: A case-control study. Frontiers in Immunology 2022, 13, 964910. [Google Scholar] [CrossRef]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The relationship between physical activity, physical exercise, and human gut microbiota in healthy and unhealthy subjects: a systematic review. Biology 2022, 11, 479. [Google Scholar] [CrossRef]
- Kiewiet, M.B.; Elderman, M.E.; El Aidy, S.; Burgerhof, J.G.; Visser, H.; Vaughan, E.E.; Faas, M.M.; de Vos, P. Flexibility of gut microbiota in ageing individuals during dietary fiber long-chain inulin intake. Molecular nutrition & food research 2021, 65, 2000390. [Google Scholar]
- Princisval, L.; Rebelo, F.; Williams, B.L.; Coimbra, A.C.; Crovesy, L.; Ferreira, A.L.; Kac, G. Association between the mode of delivery and infant gut microbiota composition up to 6 months of age: a systematic literature review considering the role of breastfeeding. Nutrition Reviews 2022, 80, 113–127. [Google Scholar] [CrossRef]
- Thomas, M.S.; Blesso, C.N.; Calle, M.C.; Chun, O.K.; Puglisi, M.; Fernandez, M.L. Dietary influences on gut microbiota with a focus on metabolic syndrome. Metabolic Syndrome and Related Disorders 2022, 20, 429–439. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Bhatia, U.; Roach, J.; Gunstad, J.; Peril, M.A.A. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clinical Nutrition 2022, 41, 2565–2576. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends in Food Science & Technology 2022, 124, 237–249. [Google Scholar]
- Gasaly, N.; Gotteland, M. Interference of dietary polyphenols with potentially toxic amino acid metabolites derived from the colonic microbiota. Amino acids 2022, 1–14. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Reviews in Endocrine and Metabolic Disorders 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Zhou, X.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Interleukin-2 (IL-2) interacts with IL-2 receptor beta (IL-2Rβ): its potential to enhance the proliferation of CD4+ T lymphocytes in flounder (Paralichthys olivaceus). Frontiers in Immunology 2020, 11, 531785. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Adejuyigbe, B.; Kallini, J.; Chiou, D.; Kallini, J.R. Osteoporosis: Molecular Pathology, Diagnostics, and Therapeutics. International Journal of Molecular Sciences 2023, 24, 14583. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Hai, Y.; Cheng, Y. Regulatory Effect of Inflammatory Mediators in Intervertebral Disc Degeneration. Mediators of Inflammation 2023, 2023. [Google Scholar] [CrossRef]
- Chen, D.Q.; Xu, W.B.; Xiao, K.Y.; Que, Z.Q.; Feng, J.Y.; Sun, N.K.; Cai, D.X.; Rui, G. Gut microbiota and spinal stenosis: a two-sample Mendelian randomization study. 2023. [Google Scholar]
- Moțățăianu, A.; Șerban, G.; Andone, S. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Cross-Talk with a Focus on Amyotrophic Lateral Sclerosis: A Systematic Review. International Journal of Molecular Sciences 2023, 24, 15094. [Google Scholar] [CrossRef]
- Morimoto, T.; Kobayashi, T.; Kakiuchi, T.; Esaki, M.; Tsukamoto, M.; Hirata, H.; Mawatari, M. Gut-spine axis: a possible correlation between gut microbiota and spinal degenerative diseases. Frontiers in Microbiology 14, 1290858. [CrossRef]
- Qin, L.; Li, J.; Guo, K.; Lu, M.; Zhang, Y.; Zhang, X.; Zeng, Y.; Wang, X.; Xia, Q.; Zhao, P.; Zhang, A.B. Insights into the structure and composition of mineralized hard cocoons constructed by the oriental moth, Monema (Cnidocampa) flavescens Walker. Insect Biochemistry and Molecular Biology 2022, 151, 103878. [Google Scholar] [CrossRef]
- Lassmann, Ł.; Pollis, M.; Żółtowska, A.; Manfredini, D. Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines 2022, 10, 1528. [Google Scholar]
- Hinton, J. A radiographic exploration of vitamin D deficiency at the eighteenth-century fortress of Louisbourg, NS. 2019. [Google Scholar]
- Howell, B.M.; Harrod, R.P. Future Directions for an Anthropology of Aging. Perspectives on, 314. [Google Scholar]
- Zi, C.; Wang, D.; Gao, Y.; He, L. The role of Th17 cells in endocrine organs: Involvement of the gut, adipose tissue, liver and bone. Frontiers in Immunology 2023, 13, 1104943. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Abi-Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Proton pump inhibitors and the risk of osseointegrated dental implant failure: a cohort study. Clinical implant dentistry and related research 2017, 19, 222–232. [Google Scholar] [CrossRef]
- Guan, Z.; Jia, J.; Zhang, C.; Sun, T.; Zhang, W.; Yuan, W.; Leng, H.; Song, C. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clinical Science 2020, 134, 3159–3174. [Google Scholar] [CrossRef]
- Ferrillo, M.; Giudice, A.; Migliario, M.; Renó, F.; Lippi, L.; Calafiore, D.; Marotta, N.; de Sire, R.; Fortunato, L.; Ammendolia, A.; Invernizzi, M. Oral–Gut Microbiota, Periodontal Diseases, and Arthritis: Literature Overview on the Role of Probiotics. International Journal of Molecular Sciences 2023, 24, 4626. [Google Scholar] [CrossRef]
- Pariente, E.; Pini, S.F.; Olmos, J.M.; Fierro, P.; Landeras, R.; Ramos, C.; Martínez-Taboada, V.M.; Hernández, J.L. Early stages of diffuse idiopathic skeletal hyperostosis (DISH) and chronic inflammation: the Camargo Cohort Study. Clinical Rheumatology 2023, 1–12. [Google Scholar] [CrossRef]
- Rathour, D.; Shah, S.; Khan, S.; Singh, P.K.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Role of gut microbiota in depression: Understanding molecular pathways, recent research, and future direction. Behavioural Brain Research 2023, 436, 114081. [Google Scholar] [PubMed]
- Fournier, D.E.; Leung, A.E.; Battié, M.C.; Séguin, C.A. Prevalence of diffuse idiopathic skeletal hyperostosis (DISH) and early-phase DISH across the lifespan of an American population. Rheumatology 2023, kead362. [Google Scholar] [CrossRef]
- Shafik, B.M.; Kamel, E.R.; Mamdouh, M.; Elrafaay, S.; Nassan, M.A.; El-Bahy, S.M.; El-Tarabany, M.S.; Manaa, E.A. Performance, blood lipid profile, and the expression of growth hormone receptor (GHR) and insulin-like growth factor-1 (IGF-1) genes in purebred and crossbred quail lines. Animals 2022, 12, 1245. [Google Scholar] [CrossRef]
- Ge, W.; Ding, J. Investigation of the causal relationship between gut microbiota and discitis: A Mendelian randomisation study. 2023. [Google Scholar]
- Rosales-Antequera, C.; Viscor, G.; Araneda, O.F. Inflammation and oxidative stress as common mechanisms of pulmonary, autonomic and musculoskeletal dysfunction after spinal cord injury. Biology 2022, 11, 550. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, Y.H.; Sim, M.; Kim, S.A.; Joung, H.; Shin, D.M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Research in microbiology 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, D.; Ahern, D.P.; Curley, A.E.; Kepler, C.K.; Butler, J.S. Impact of sarcopenia on degenerative lumbar spondylosis. Clinical spine surgery 2021, 34, 43–50. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Yang, Z.; Wang, D.; Li, T.; Yang, F.; Li, Z.; Bai, X.; Wang, Y. Gut-muscle axis and sepsis-induced myopathy: The potential role of gut microbiota. Biomedicine & Pharmacotherapy 2023, 163, 114837. [Google Scholar]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Yamada, K.; Ieguchi, M.; Takahashi, S.; Nakamura, H. Life Expectancy Is Poor in Patients with Diffuse Idiopathic Skeletal Hyperostosis-Related Pyogenic Vertebral Osteomyelitis. Spine Surgery and Related Research 2022, 6, 654–663. [Google Scholar] [CrossRef]
- Tanios, M.; Brickman, B.; Norris, J.; Ravi, S.; Eren, E.; McGarvey, C.; Morris, D.J.; Elgafy, H. Spondyloarthropathies That Mimic Ankylosing Spondylitis: A Narrative Review. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders 2023, 16, 11795441231186822. [Google Scholar] [CrossRef]
- Sethi, A.; Ruby, J.G.; Veras, M.A.; Telis, N.; Melamud, E. Genetics implicates overactive osteogenesis in the development of diffuse idiopathic skeletal hyperostosis. Nature Communications 2023, 14, 2644. [Google Scholar]
- Froeynes, W. My tinnitus story: a symptom medical science has not understood; Tinnire forlag, 2023. [Google Scholar]
| Name | Enzymes | Reaction type | Biotransformation reaction |
|---|---|---|---|
| Methanoic acid | USGBE | beta-Oxidation of carboxylic acid | 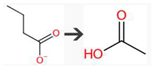 |
| Ethanoic acid | USGBE | beta-Oxidation of carboxylic acid | 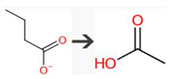 |
| Propanoic acid | ND | ND | ND |
| Butanoic acid | USGBE | beta-Oxidation of carboxylic acid | 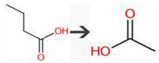 |
| Butanoic acid | Bacterial UDP-glucuronosyltransferase | O-Glucuronidation of aliphatic acid | 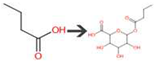 |
| 2-Methylpropanoic acid | SULFOTRANSFERASE | alpha-Amino acid to aldoxime | 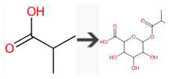 |
| Pentanoic acid | Unspecified gut bacterial enzyme | beta-Oxidation of carboxylic acid | 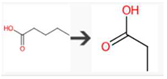 |
| Pentanoic acid | Bacterial UDP-glucuronosyltransferase | O-Glucuronidation of aliphatic acid | 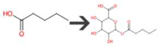 |
| 3-Methylbutanoic acid | Unspecified gut bacterial enzyme | alpha-Oxidation of carboxylic acid | 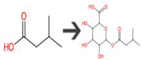 |
| 3-Methylbutanoic acid | Bacterial UDP-glucuronosyltransferase | O-Glucuronidation of aliphatic acid | 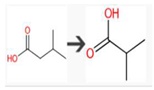 |
| 2-Methylbutanoic acid | Bacterial UDP-glucuronosyltransferase | O-Glucuronidation of aliphatic acid | 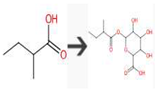 |
| No. | Cytokine | Cell(s) Targeted | Diseases | Function in Intestinal Health and Pathology | References |
|---|---|---|---|---|---|
| 1. | Interleukin-10 (IL-10) | Mφ, T cells, B cells, Dendritic cells, NK cells | Inflammatory bowel diseases, Cancer, Autoimmune diseases, Asthma, Transplant rejection |
|
[81] |
| 2. | Vascular Endothelial Growth Factor (VEGF) |
Nerve Cells, Myocardial Cells, Cancer Cells, Fibroblasts, Mφ | Psoriasis, Cancer, Age-Related Macular Degeneration (AMD), Diabetic Retinopathy, Macular Edema |
|
[59] |
| 3. | Tumor Necrosis Factor-alpha (TNF-α) | Mφ, Endothelial cells, Neutrophils, T cells, B cells, Fibroblast. | Rheumatoid Arthritis, Inflammatory Bowel Disease (IBD), Psoriasis, Ankylosing Spondylitis, Sarcoidosis |
|
[82] |
| 4. | Amphiregulin (Areg) |
Ovarian Cells, Mucosal Cells, Cancer Cells, I mmune cells, Smooth Muscle Cells | Inflammatory Bowel Disease (IBD), Cancer, Lung Diseases, Renal Disease, Arthritis |
|
[83] |
| 5. | Interleukin-6 (IL-6) | T Cells, B cells, Mφ, Neutrophils, CNS cells, Muscle cells, Hepatocytes. | Covid-19, Sepsis, Chronic Obstructive Pulmonary Disease (COPD), Metabolic Disorders, Cardiovascular Disease |
|
[84] |
| 6. | Oncostatin M (OSM) | Hepatocytes, Fibroblasts, Endothelial Cells, Immune Cells, Cartilage Cells, Neurons | Respiratory Diseases, Musculoskeletal Disorders, Cardiovascular Disease, Neurological Diseases, Liver Diseases |
|
[85] |
| 7. | Interleukin-15 (IL-15) |
NK cells, Memory CD8+ T Cells, Tissue-Resident Lymphocytes, Adipocytes, B cells. | HIV/AIDS, Type 1 Diabetes, Celiac Disease, Neurological Disorders, Celiac Disease |
|
[56] |
| 8. | Interleukin-17 (IL-17) | Neutrophils, Mφ, T cells, B cells, Fibroblast, Epithelial cells. | Multiple Sclerosis (MS), Rheumatoid Arthritis, Asthma, Spondyloarthritis, Cytokine Release Syndrome |
|
[87] |
| 9. | Interleukin-11 (IL-11) |
Epithelial cells, Hematopoietic stem cells, Cardiomyocytes, Osteoblasts, Placental Cells. | Multiple Myeloma, Breast Cancer, Ovarian Cancer, Thrombocytosis, Osteolytic Bone Diseases |
|
[88] |
| 10. | Interferon-gamma (IFN-γ) | Mφ, T cells, NK cells, B cells, Dendritic cells, Endothelial cells, Fibroblast | Tuberculosis (TB), Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Chronic Inflammatory Conditions, Cancer |
|
[89] |
| 11. | Interleukin-10 (IL-10) |
Mφ, T cells, B cells, certain epithelial cells, fibroblasts, and endothelial cells. | Multiple Sclerosis (MS), Asthma, HIV/AIDS, Rheumatoid Arthritis (RA), Crohn’s Disease |
|
[90] |
| 12. | Interleukin-22 (IL-22) | Epithelial cells, Keratinocytes, Intestinal epithelial cells, pancreatic islet cells, Respiratory epithelial cells. | Obesity, Psoriasis, Liver Inflammation and Fibrosis, Fungal Infections, Crohn’s Disease |
|
[83] |
| 13. | Interleukin-2 (IL-2) | Tregs, T cells, NK cells, B cells, Dendritic cells, Activated Monocytes and Mφ | Rheumatoid Arthritis (RA), Multiple Sclerosis (MS), Type 1 Diabetes, Graft-versus-Host Disease (GVHD), Cytokine Release Syndrome (CRS) |
|
[84] |
| 14. | Interleukin-23 (IL-23) | Th17, Mφ, NK cells, Dendritic cells | Cancer, Psoriatic Arthritis, Ankylosing Spondylitis, Psoriasis, ulcerative colitis |
|
[85] |
| 15. | Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) | Granulocytes, T cells, Hematopoietic stem cells, Endothelial cells, Mφ | Lung Diseases, Chronic Obstructive Pulmonary Disease (COPD, Asthma, Multiple Sclerosis, Rheumatoid Arthritis |
|
[86] |
| 16. | Transforming Growth Factor-beta (TGF-β) | T cells, B cells, Hematopoietic stem cell, Cancer cells, Fibroblast, Stromal cells, Endothelial cells | Kidney Diseases, Ocular Diseases, Rheumatic Diseases, Cardiovascular Diseases, Fibrosis |
|
[87] |
| 17. | Interleukin-7 (IL-7) |
T cells, B cells, NK cells, Common lymphoid progenitors. | Graft-Versus-Host Disease (GVHD), Bone Marrow Disorders, Lymphopenia, HIV/AIDS, Cancer |
|
[44] |
| 18. | Interleukin-1 (IL-1) | B cells, T cells, Mφ, Monocytes, Nervous system cells, Bone cells, Keratinocytes. | Fever Syndromes, Gout, Osteoarthritis, Rheumatoid Arthritis, Crohn’s disease and ulcerative colitis |
|
[88] |
| 19. | Interleukin-33 (IL-33) |
Mast cells, Th2 cells, ILC2s, Tregs, NK cells, Epithelial cells. | Fibrotic Diseases, Chronic Obstructive Pulmonary Disease (COPD), Neurological Diseases, Rheumatoid Arthritis |
|
[91] |
| 20. | Interleukin-4 (IL-4) | Th2 cells, B cells, Mφ, Mast cells, Skin cells, Hematopoietic stem cells, Fibroblast | Allergic Diseases, Asthma, Eosinophilic Disorders, Atopic Dermatitis, IgG4-Related Disease |
|
[92] |
| 21. | Interleukin-5 (IL-5) |
Eosinophils, B cells, IECs | Eosinophilic Esophagitis (EoE), Asthma, Eosinophilic Bronchitis, Hypereosinophilic Syndrome (HES), Atopic Dermatitis (Eczema) |
|
[93] |
| 22. | Interleukin-9 (IL-9) |
Th9 cells, Mast cells, T cells, B cells, Epithelial cells. | Allergic Rhinitis, Parasitic Infections, Inflammatory Bowel Disease (IBD), Atopic Dermatitis, Asthma |
|
[94] |
| 23. | Interleukin-13 (IL-13) |
B cells, T cells, Mφ, Airway epithelial cells, Smooth muscle cells, Eosinophils | Asthma, Atopic Dermatitis, Allergic Rhinitis, Eosinophilic Esophagitis, Fibrosis |
|
[92] |
| 24. | Interleukin-25 (IL-25) |
Th2 cells, Mast cells, Dendritic cells, Eosinophils, ILC2 cells. | Type 2 Inflammatory Responses, Inflammatory Bowel Disease (IBD), Eosinophilic Esophagitis, Atopic Dermatitis, Allergic Rhinitis |
|
[95] |
| 25. | Thymic Stromal Lymphopoietin (TSLP) |
Dendritic cells, B cells, Epithelial cells, Mast cells, ILCs, T cells | Allergic Conjunctivitis, Psoriasis, Chronic Obstructive Pulmonary Disease (COPD), Eosinophilic Esophagitis, Allergic Rhinitis |
|
[96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





