1. Introduction
Orthotopic heart transplantation (HTX) has been used as a beneficial option for end-stage heart failure (ESHF).[
1,
2,
3] Improvement of the selection criteria, a more accurate determination of urgency, and optimization of preoperative conditions has helped to decrease adverse outcomes. [
4] Nevertheless, HTX still has a considerable complication rate, including primer graft dysfunction (PGD), early graft loss, rejection and mortality. A recent comparison of available risk scores raised concern about their discriminative ability for one-year mortality. [
5]
In contrast, several biomarkers have been used in risk estimation. These diagnostic tests are part of the routine pretransplantation evaluation of factors such as natriuretic peptides, the glomerular filtration rate, bilirubin levels, etc. [
4] Interleukins (IL) and apolipoproteins (Apo) have also been advocated to play important roles in the development and progression of ESHF. [
6,
7] These new biomarkers seem to have promising prognostic roles in the development of diabetes and atherosclerosis. Only sparse data exist regarding the application of these new markers in transplantation medicine. [
8] Based on the known donor shortage, optimization of patient selection, use of aged donors in borderline indications, and adding these new biomarkers to recipient selection might help to improve the risk prediction of the available risk scores.
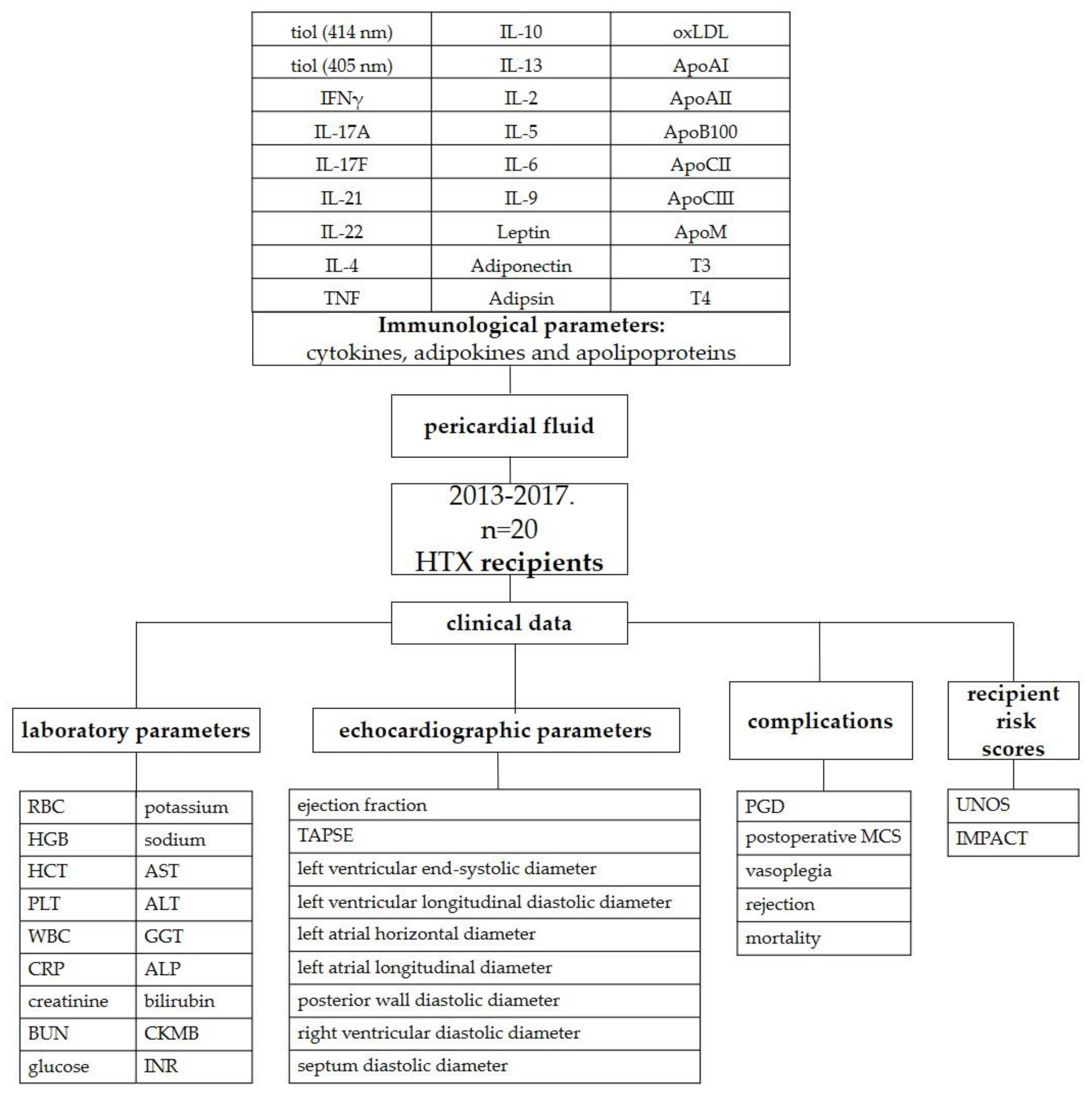
We hypothesized that the extent of immunological injury can be quantified and that we can obtain a closer picture of heart function if aliquots are sampled from pericardial fluid. The aim of this study was to compare the levels of different interleukins and apoproteins in the pericardial fluid with the possible adverse outcomes of recipients after orthotopic heart transplantation. The primary outcome was primary graft dysfunction. Mortality and rejection were also investigated. Additionally, the inotropic score (IS), vasoactive-inotropic score (VIS), United Network for Organ Sharing (UNOS) recipient risk score and The Index for Mortality Prediction After Cardiac Transplantation (IMPACT) score were also correlated with pericardial hormone levels.
2. Results
2.1. Recipient characteristics
The median age of the recipients was 58.5 years, and all recipients were male (n=20). There was gender mismatch in 20% (n=4) of the transplantations, and the medians of the recipients’ and donors’ body mass index (BMI) values were 25.6 and 25.05 kg/m2, respectively. The etiology of heart failure was ischemic in 75% (n=15) and idiopathic in 25% (n=5) of the transplants. Further descriptive characteristics of the recipients are summarized in
Table 1.
2.2. Recipient risk scores and laboratory parameters
The median scores of the patients were 1.00 (IQR: 1.00-2.25) and 5.00 (IQR: 2.00-9.00) for the UNOS recipient score and IMPACT score, respectively. The UNOS-R score showed correlation with the adipsin (p=0.020) and adiponectin (p=0.008) levels. IMPACT scores were not related to the measured panels. ApoAII levels were higher in patients who had bilirubin levels above 1 mg/dL compared to those who had values < 1 mg/dL (p= 0.035). ApoAI showed a correlation with preoperative creatinine, aspartate aminotransferase
(AST) and alanine transaminase (ALT) levels (p=0.040; p=0.004; p=0.005, respectively). Preoperative AST levels were also correlated with ApoCII (p=0.040) and ApoCIII (p=0.040) levels. Postoperative bilirubin levels were correlated with IL-21 (p=0.002), IL-6 (p=0.001), IL-9 (p<0.001) and adiponectin (p=0.001) levels. Further correlations of laboratory parameters are summarized in
Table S7-S8.
2.3. Complications and adverse reactions
After transplantation, five patients needed postoperative MCS, and four patients had PGD. Four patients had ISHLT Grade 2R rejection, and vasoplegia was diagnosed in four cases. The detailed postoperative complications and laboratory parameters are shown in
Table 2.
In the pericardial fluid of the recipients, leptin levels were significantly lower in patients with PGD than in those without it (p= 0.029). Leptin levels were also significantly lower in the MCS group (p = 0.042). The detailed comparisons are shown in
Table 3,
Figure 1, and
Tables S1 and S2.
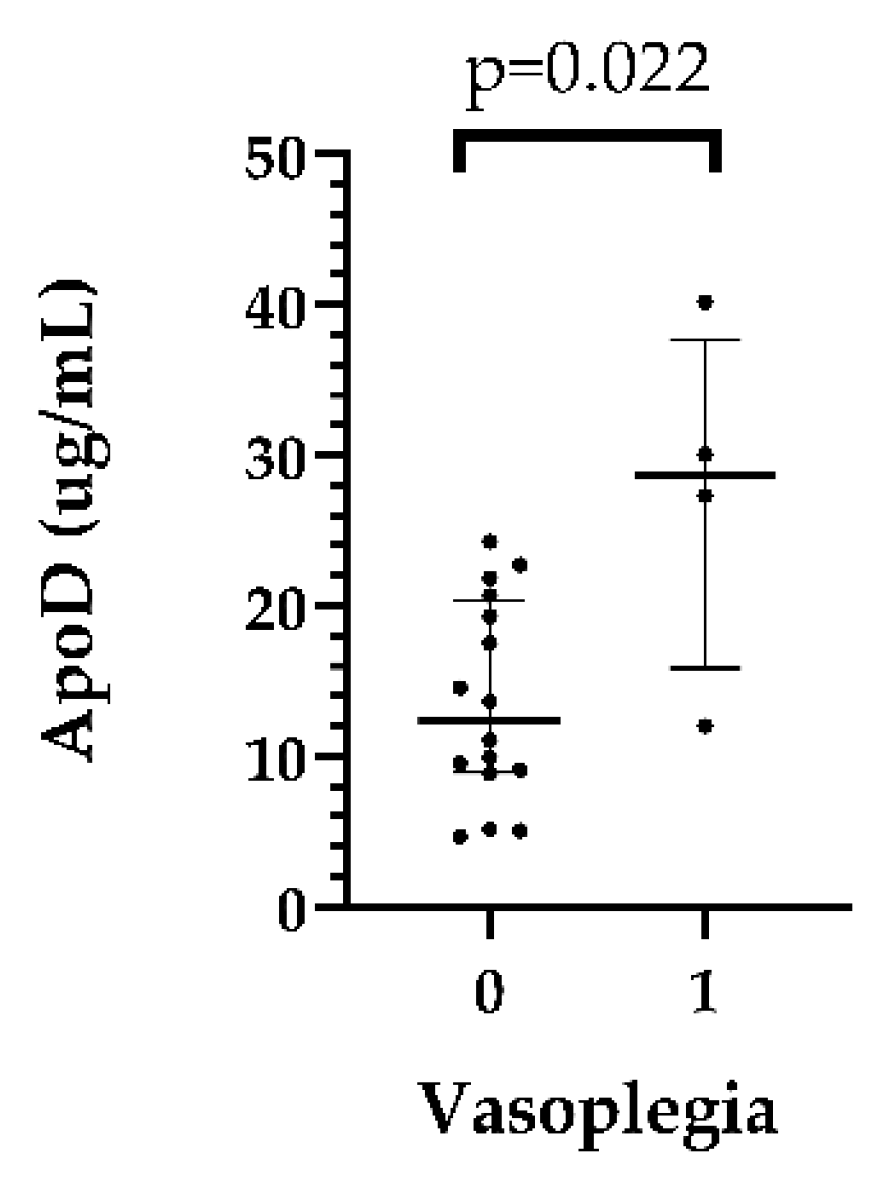
In the recipient pericardial fluids, ApoD levels were significantly higher in patients who had postoperative vasoplegia than in those who had no vasoplegia (median: 28.69 [15.85–37.66] vs. 12.34 [8.92–20.34] µg/mL, p=0,022 in the vasoplegic and nonvasoplegic patients, respectively). (
Table S3,
Figure 2)
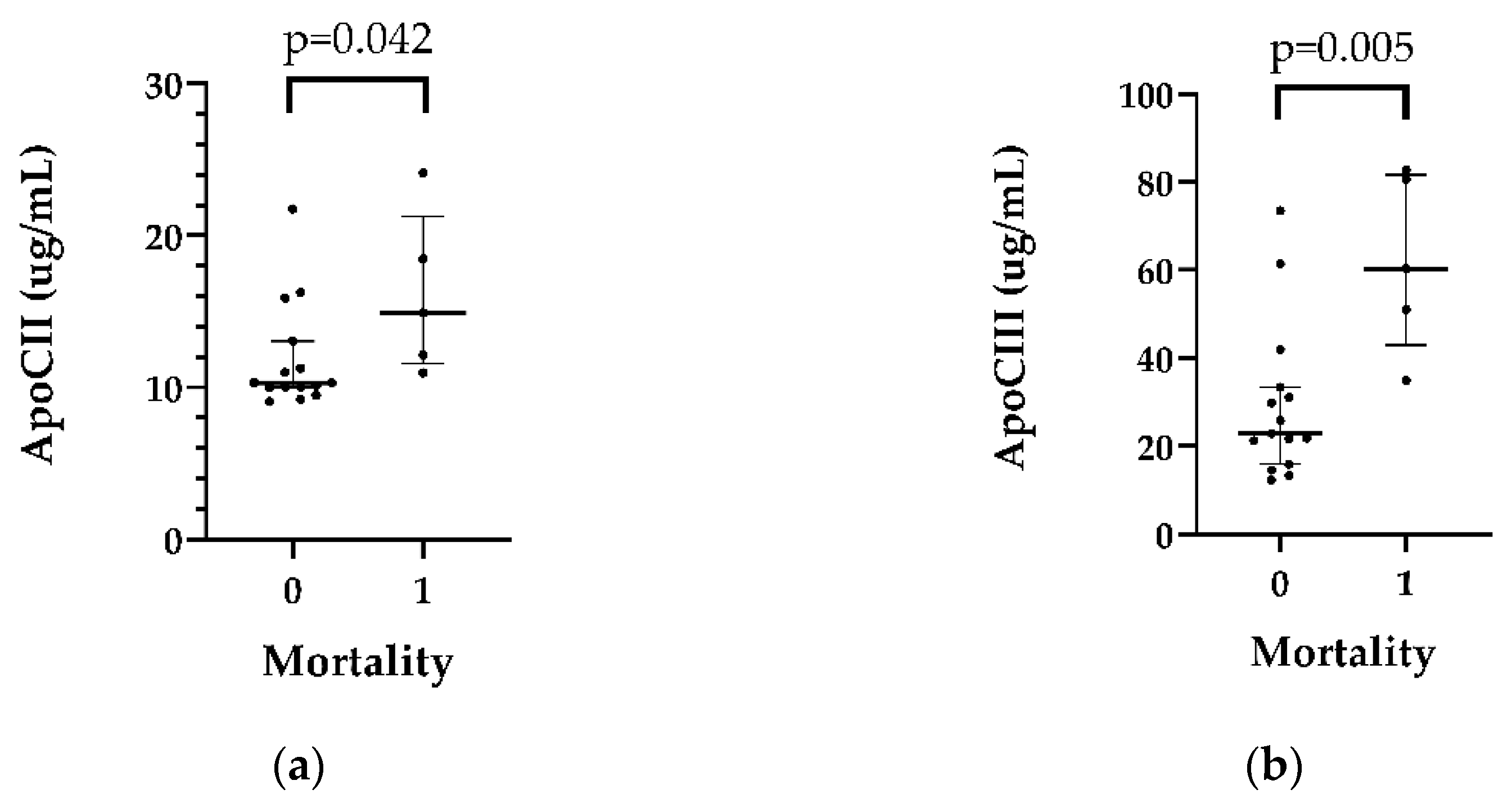
Higher ApoCII (p= 0.042) and ApoCIII (p=0.005) levels were detected in patients (n=5) who died in the first 5 years after HTX (n=5). (
Table 4 and S4,
Figure 3)
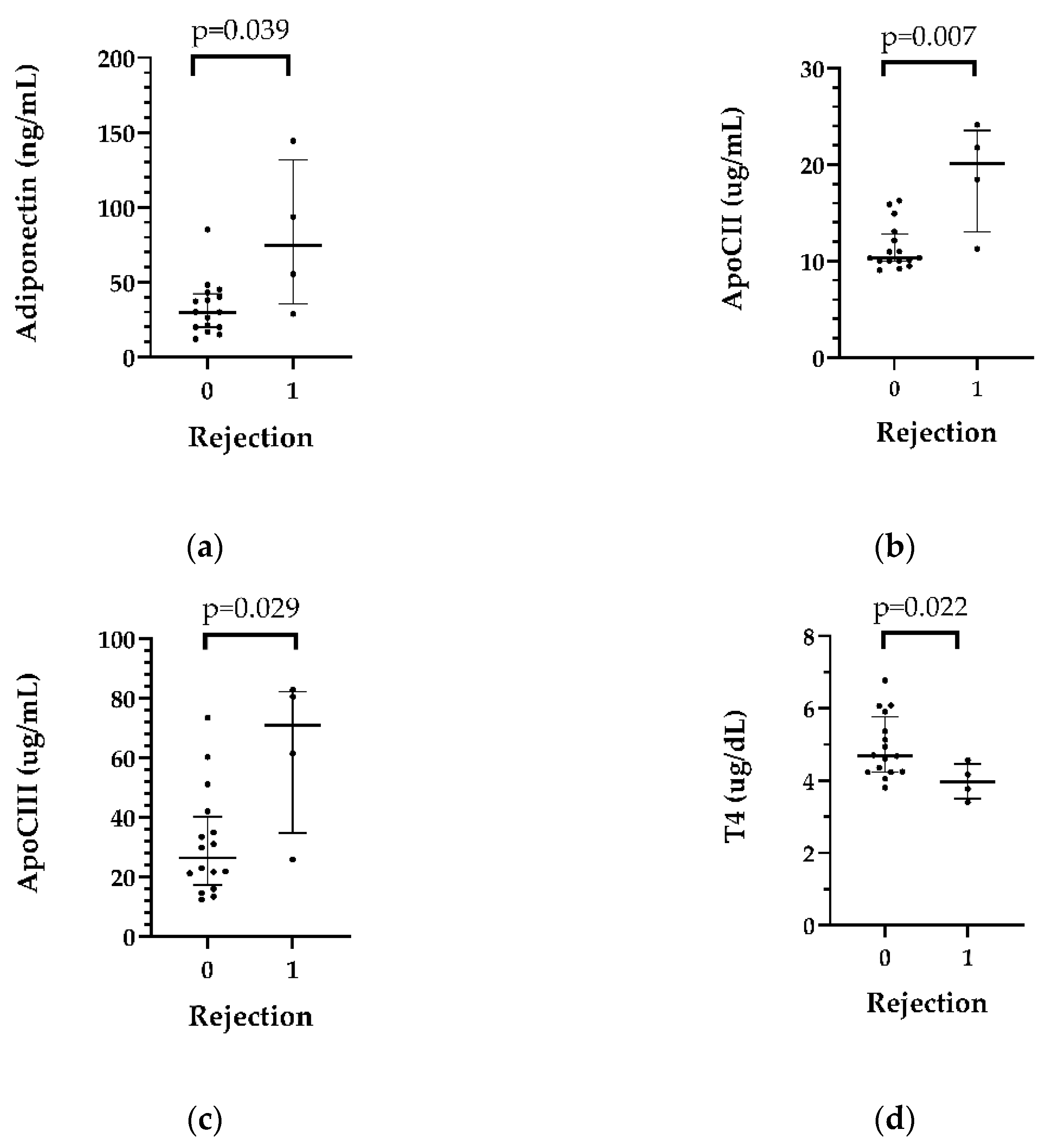
In patients who had rejection (n=4) in the first month after transplantation, adiponectin (p=0.039), ApoCII (p=0.007) and ApoCIII (p=0.029) levels were higher than those in the nonrejection group, while pericardial T4 levels were lower in patients with rejection than in those who did not develop rejection. The data are shown in
Table 5,
Figure 4 and
Table S5.
2.4. Vasoactive-inotropic score and inotropic score
Significant correlations were found between maximum vasoactive-inotropic score, inotropic score and several immunological parameters. The detailed results are shown in
Table S9.
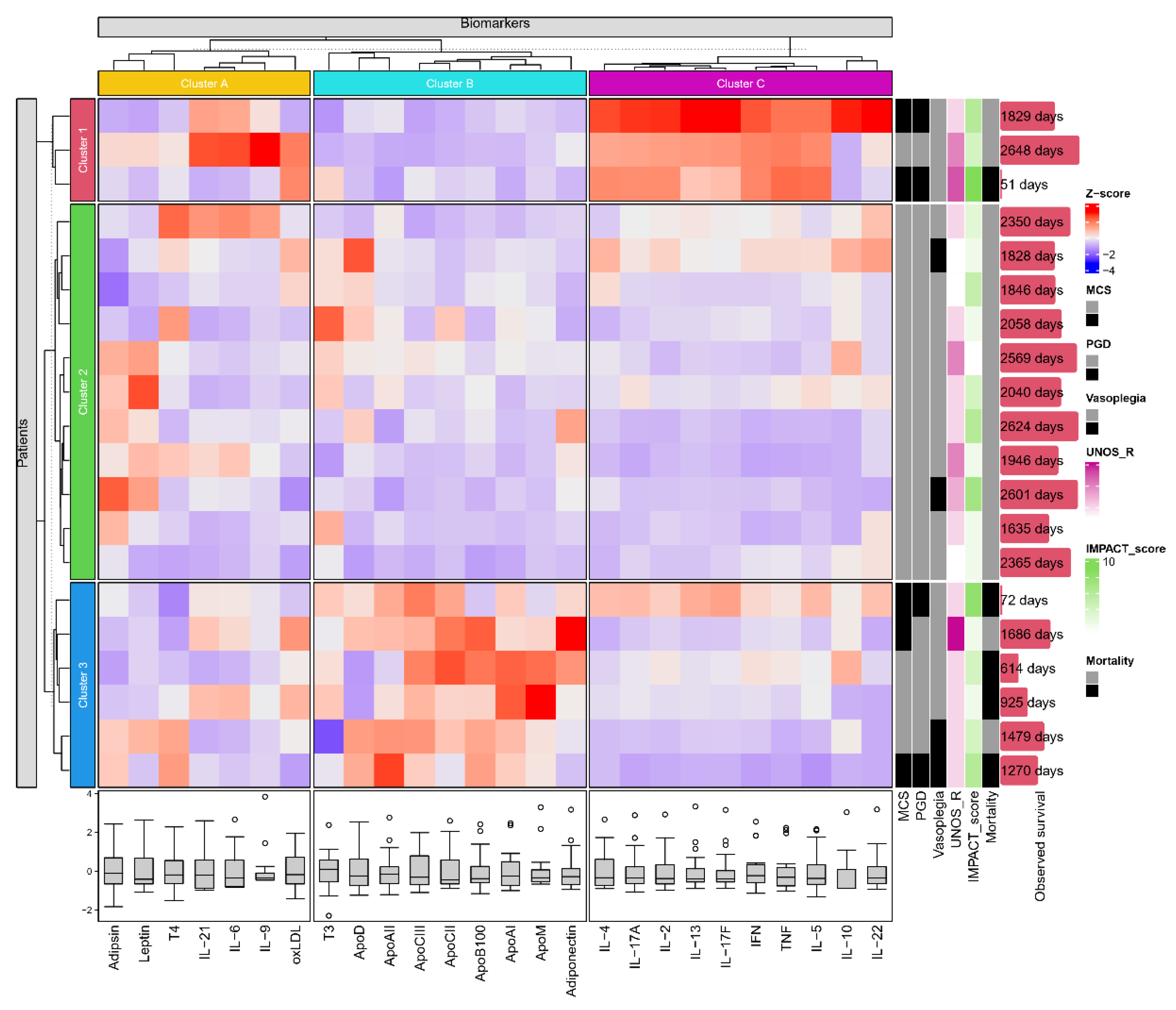
2.5. K-means clustering based on the molecular composition of the pericardial fluid
K-means clustering (
Figure 5) has identified 3 subgroups of immunological parameters (cluster A: Adipsin, Leptin, T4, IL-21, IL-6, IL-9 and oxLDL; cluster B: T3, ApoD, ApoAII, ApoCIII, ApoCII, ApoB100, ApoAI, ApoM and Adiponectin; cluster C: IL-4, IL-17A, IL-2, IL-13, IL-17F, IFN, TNF, IL-5, IL-10 and IL-22) and of patients (clusters 1-3). Patients of cluster 1 have shown overall elevated levels of the interleukin rich cluster C, while cluster 3 was characterized by higher degrees of pericardial apolipoprotein content (cluster B). Furthermore, patient cluster 1 and cluster 3 have demonstrated numerically greater incidences of MCS, PGD and mortality (2/3, 2/3, 1/3 and 3/6, 2/6, 4/6 respectively) compared to patients of cluster 2 (0/11, 0/11, 0/11).
3. Discussion
We found that recipients who subsequently had PGD or required ventricular assist device support had lower pericardial leptin levels than recipients who did not. Higher pericardial ApoD levels were associated with vasoplegia, and higher adiponectin, ApoB100, ApoCII, and ApoCIII levels and lower T4 levels were associated with rejection. Furthermore, higher pericardial ApoCII and ApoCIII levels showed a significant correlation with mortality.
The pathophysiology of ESHF is complex, characterized by volume overload, different extents of end organ dysfunction and neurohormonal changes and inflammation. [
9] Several risk scores have been developed to determine the urgency of heart transplantation or for usage of MCS before transplantation to bridge the unstable state or severe hypoperfusion to candidacy. [
10,
11,
12] Systemic inflammation and cytokine release can further worsen hemodynamic instability and thus can serve as a diagnostic tool in risk estimation. Our results also indicate the strong relationship between the UNOS score and markers of kidney and liver dysfunction.
The current challenges of HTX are the increased age of donors and recipients, the large number of patients who have MCS or are operated on in high urgency status, and increased allosensitization. [
13,
14] The occurrence of early and late complications has also increased. Therefore, the use of biomarkers to detect the ideal matching seems rational. Leptin is a nonglycosylated protein produced mainly by adipose tissue that plays an important role in normal cardiovascular function. [
15] In young, healthy men, there is an inverse correlation between the thickness of the carotid wall and the circulating leptin concentration, which indicates the vascular protective effect of leptin. [
16] In patients with nonischemic cardiomyopathy, lower leptin levels were associated with an increased likelihood of HTX. [
17] ApoD has recently been the subject of research on neurodegenerative diseases and cardiovascular function. Circulating apoD levels have been observed to be increased in heart failure patients, so this apoprotein can even be used as a biomarker. Apolipoprotein D is associated with both developing and mature blood vessels and plays a role in inflammation. [
18,
19] Pericardial recipient levels were found to differ significantly from the normal level in early complications such as PGD, need for MCS and vasoplegia, preceding and forecasting these hemodynamic problems.
Rejection has become more important in the current era; as older age, previous MCS, previous pregnancies, and transfusion frequently lead to allosensitization. ApoCIII is a small 8.8 kDa apolipoprotein produced in the liver and found on the surface of very low density lipoprotein, low density lipoprotein (LDL) and high-density lipoprotein (HDL). ApoCIII has both indirect atherogenic effects (causes hypertriglyceridemia) and direct atherogenic effects (stimulates monocyte adhesion to endothelial cells, promotes the production of inflammatory mediators in these cells and increases LDL retention in the arterial wall). [
20,
21] ApoCII is also mainly produced in the liver and interacts with systemic inflammation. [
22] One piece of Apo B-100 produced by hepatocytes is found on the surface of every atherogenic particle. [
23,
24] The circulating level is an effective measure of cardiovascular risk and coronary artery disease.[
24,
25] A postmortem study revealed that the pericardial fluid of people with severe atherosclerosis contained higher amounts of ApoB than those without atherosclerosis.[
26] Apolipoproteins can be better predictive markers of cardiovascular events than serum lipids used in everyday practice; however, conventional immunoassays used to determine apolipoprotein levels can carry serious limiting factors (for example, interfering antibodies and cross-reactivity). Apolipoproteins have important roles in the maintenance of endothelial function, blood pressure and hemostasis, and adipokines contribute to the regulation of appetite and energy turnover by regulating the secretion of insulin-sensitive tissues and pancreatic β-cells. Our results also indicate that the amount of apolipoproteins is associated with the development of rejection and mortality. On the other hand, monitoring a panel of valid biomarkers may provide patient stratification and better immunosuppression treatment selection. After transplantation, the therapy should be adjusted based on the prediction of rejection episodes (maintained alloreactivity), prognosis of allograft damage progression, and personal drug response. [
27]
Adiponectin is mainly produced by adipose tissue, and low levels of adiponectin are associated with ischemic heart diseases and peripheral arterial diseases. However, a high adiponectin level does not always mean a better outcome because severe cardiovascular patients with liver or kidney disease often have elevated levels due to insufficient clearance as a secondary consequence of cardiovascular disease.[
15] Patients with nonischemic cardiomyopathy plus higher adiponectin levels are more likely to receive a transplant than those with low adiponectin levels.[
17]
3.1. Limitation of the study
The main limitation of this study is that the pericardial fluid samples of the recipients were not available. There are no data about the serum levels. Moreover, the small population size and the heterogeneity of the recipients resulted in lower statistical power.
4. Materials and Methods
4.1. Study design, setting and participants
Well-characterized pseudonymized human pericardial fluid and cell-depleted pericardial fluid samples of 20 randomly chosen recipients from the period between February 2013 and December 2017 were obtained from the Transplantation Biobank of the Heart and Vascular Center at Semmelweis University, Budapest, Hungary. Between January 2013 and April 2017, 206 orthotopic transplantations were performed, and 118 donor pericardial fluid specimens were available (57.3% of the transplantations). Pericardial fluid samples contaminated with blood were an exclusion criterion in the study. The procedure of sample procurement was reviewed and approved by the institutional and national ethics committee (ethical permission numbers: ETT TUKEB 7891/2012/EKU [119/PI/12.] and ETT TUKEB IV/10161-1/2020). Clinical patient data were obtained from the database of the Transplantation Biobank. Our study was conducted in accordance with the Eurotransplant standards for organ sharing and with the Hungarian National Blood Transfusion Service. The last check on the follow-up data was made on January 22th, 2023. [
28]
4.2. Local Protocols
Donor and recipient variables were retrieved from the National and Eurotransplant-based donor data report form and from electronic medical records from our institutional databank. A description of the study is shown in
Figure 6.
4.3. Major study parameters
Due to the relatively small sample size of our study, the UNOS score was calculated for donors, recipients and overall individuals. The donor-specific UNOS (UNOS D) score includes donor age (1 point if aged between 50 and 55 years or 2 points if aged above 55 years), total ischemic time (above 4 hours 2 points), sex mismatch (1 point) and donor diabetes mellitus (1 point). According to these criteria, the UNOS-D score was calculated, and three risk groups were formed: low (score: 0), intermediate (score: 1 or 2) or high (score: ≥3) UNOS-D risk groups. The recipient-specific UNOS score considered the following parameters: age (above 65 years: 1 point), body mass index (30-35 kg/m2: 1 point, >35 2 points), mean pulmonary artery pressure (above 30 mmHg: 1 point), total bilirubin (1.5-1.9 mg/dL: 1 point, >1.9 mg/dL: 2 points), creatinine (1.5-2.0 mg/dL: 1 point; >2 mg/dL: 2 points), previous transplant, previous cancer and pretransplant mechanical ventilation (each 2 points) or mechanical circulatory support (noncontinuous-flow: 2 points).[
29] The total score is the sum of donor- and recipient-specific scores, and this score was used in the multivariable logistic regression analyses for adjustment.
4.3.1. The IMPACT score
The IMPACT score included the following 12 recipient characteristics: age (above 60 years: 1 point), serum bilirubin (0-0.99 mg/dL: 0 point, 1-1.99 mg/dL: 1 point, 2-3.99 mg/dL: 3 points, ≥4 mg/dL: 4 points), creatinine clearance (≥50 mL/min: 0 point, 30-49 mL/min: 2 points, <30 mL/min: 5 points), dialysis between listing and transplant (4 points), female sex (3 points), heart failure etiology (idiopathic 0 point, ischemic 2 points, congenital 5 points, other 1 point), recent infection (3 points), intra-aortic balloon pump (3 points), mechanical ventilation pretransplant (5 points), race (Caucasian: 0 points, African American: 3 points, Hispanic: 0 points, other: 0 points), temporary circulatory support (7 points), and ventricular assist device (older-generation pulsatile: 3 points, newer-generation continuous: 5 points, Heartmate II: 0 point).[
10]
4.4. Sample collection and preparation
After sternal splitting and opening of the pericardium, ACD (trisodium citrate with citric acid and dextrose) vacutainer tubes (BD Vacutainer® System, BD Biosciences, San Jose, California, USA) were used for the collection of pericardial fluid samples. After pelleting cells (300 g, 10 min, 4 °C), the supernatant was centrifuged at 2000 g for 10 min at 4 °C to remove large particles. The cell-free supernatants were collected, divided into three 500 µL aliquots and stored in liquid nitrogen until use.
4.5. Flow cytometric multiplexed bead-based immunoassays
LEGENDplex™ bead-based immunoassays (BioLegend, San Diego, CA, USA) were used for the quantification of pericardial fluid cytokines, adipokines and apolipoproteins according to the manufacturer’s instructions.
4.6. Enzyme-linked immunosorbent assays (ELISA)
Pericardial fluid oxidized low-density lipoprotein (oxLDL), triiodothyronine (T3) and thyroxine (T4) concentrations were determined by ELISA (human OxLDL ELISA® Kit of Cloude Clone; T3 and T4 Human ELISA Kits Abcam, Cambridge, UK) according to the manufacturer’s instructions. Oxidative damage of pericardial fluid proteins was assessed by determining the free SH content expressed in cysteine equivalents via the DTNB (5,5'-dithio-bis-(2-nitrobenzoic acid = Ellman reagent) method (Thermo Scientific Pierce Ellman's Reagent).
4.7. Outcomes
Our primary outcome was primary graft dysfunction (PGD) in recipients. PGD was defined according to the consensus criteria of the International Society of Heart and Lung Transplantation (ISHLT).[
30] The decision regarding mechanical circulatory support (MCS) implantation was made by a team of experts (including a cardiologist, a cardiac surgeon and a cardiac anesthesiologist), according to international guidelines.[
31] Acute rejection was defined as an event that necessitated increased immunosuppression with an ISHLT grade ≥ 2R endomyocardial biopsy result or with non-cellular reactions with hemodynamic compromise. Patients underwent routine surveillance for allograft function via endomyocardial biopsy and echocardiography at 1, 2, 3 and 4 weeks, as well as at 3, 6, 9 and 12 months after transplantation. Posttransplantation vasoplegia was defined according to the ISHLT criteria.[
32] Mortality was also assessed, and survival was checked at April 30, 2023.
The maximum vasoactive-inotropic score (VIS) and the length of their administration were also analyzed. VIS was calculated using vasopressor (norepinephrine, epinephrine, vasopressin) and inotropic (dobutamine, dopamine, levosimendan, milrinone) medication doses. For the calculation of IS, the medication doses of dopamine, dobutamine and epinephrine were used.[
33]
4.8. Statistical analysis
Data are expressed as the median and interquartile range (first–third [IQR]). Differences between the groups were assessed by the nonparametric Mann‒Whitney U test for continuous variables. The association between outcomes and inflammatory variables was tested via Spearman’s correlation. Comparisons between interleukins (IL), apolipoproteins and recipient scores were conducted by performing 1-way analysis of variance on the ranks test. Data were analyzed by using IBM-SPSS 22.0 software (International Business Machines Corporation, Armonk, New York, USA). K-means clustering was performed in R (R Foundation for Statistical Computing, Vienna, Austria) using the ComplexHeatmap package and Z-score normalization [
34], utilizing the algorithm of Hartigan and Wong [
35] with the number of expected clusters defined as 3. Elements of the resulting clusters were further clustered hierarchically. All of the statistical tests were two-sided, and a p value < 0.05 was considered to indicate statistical significance.
5. Conclusions
As the number of patients on the waiting list exceeds the potential of HTX, new prognostic scores or more sensitive biomarkers are needed to determine the optimal candidates for transplantation. The ideal immunesuppression would be a noninvasive strategy that can reliably discriminate the presence and absence of rejection and overimmunosuppression. [
36]
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Relationship between recipient interleukin levels and primary graft dysfunction, Table S2: Relationship between recipient interleukin levels and postoperative mechanical circulatory support, Table S3: Relationship between recipient interleukin levels and vasoplegia, Table S4: Relationship between recipient interleukin levels and morality, Table S5: Relationship between recipient interleukin levels and rejection, Table S6: Postoperative laboratory parameters, Table S7: Correlations with preoperative laboratory parameters, Table S8: Correlations with postoperative laboratory parameters, Table S9: Correlations with maximum inotropic score and maximum vasoactive-inotropic score.
Author Contributions
Conceptualization, É.P.; A.S., E.I.B. and T.R.; methodology, É.P. and A.S.; software, E.T.; M.I. validation, É.P.; A.S.; formal analysis, E.L.; investigation, A.K.; resources, A.K.; data curation, E.T.; M.I., B.S.; E.L., A.K.; writing—original draft preparation, É.P.; A.S.; E.T.; M.I.; writing—review and editing, E.I.B.; I.J.B.; Z.D.; B.A.B.; T.R.; visualization, E.T.; supervision, E.I.B.; T.R.; B.M.; project administration, É.P.; A.S.; funding acquisition, E.I.B., T.R., and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. RRF-2.3.1-21-2022-00003 was implemented with the support provided by the European Union. TKP2021-EGA-23 and TKP2021-NVA-15 have been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA and TKP2021-NVA funding schemes, respectively. The NKFIH-1277-2/2020 project was financed by the Thematic Excellence Programme (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary within the framework of the Bioimaging Thematic Programme of Semmelweis University. This project was supported by grants from the National Research, Development and Innovation Office (NKFIH) of Hungary (K134939 to T.R.). The work was supported by the European Union's Horizon 2020 Research and Innovation Programme under grant agreement no. 739593 and the 2019-2.1.7-ERA-NET-2021-00015 grant. NVKP_16-1-2016-0017 (“National Heart Program”) has been implemented with support provided by the National Research, Development and Innovation Fund of Hungary.
Institutional Review Board Statement
The procedure of sample procurement was reviewed and approved by the institutional and national ethics committee (ethical permission numbers: ETT TUKEB 7891/2012/EKU [119/PI/12.] and ETT TUKEB IV/10161-1/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors acknowledge all patients who were willing to participate in the recent study and the medical staff of the Heart and Vascular Centre of Semmelweis University who helped the authors conduct the present research.
Conflicts of Interest
The authors disclose that EIB is a member of the Advisory Board of Sphere Gene Therapeutics Inc. (Boston, MA, USA) and ReNeuron (UK). The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA Key Data Elements and Definitions for Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circ Cardiovasc Qual Outcomes. 2021, 14, e000102. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro MG, Costanzo MR, Gustafsson F, et al. Heart transplantation: focus on donor recovery strategies, left ventricular assist devices, and novel therapies. Eur Heart J. 2022, 43, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Park SM, Lee SY, Jung MH, et al. Korean Society of Heart Failure Guidelines for the Management of Heart Failure: Management of the Underlying Etiologies and Comorbidities of Heart Failure. Korean Circ J. 2023, 53, 425–451. [Google Scholar] [CrossRef] [PubMed]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; Garcia-Guereta, L.; Jamero, G.; Khush, K. , et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2023, 42, e1–e141. [Google Scholar] [CrossRef]
- Coutance, G.; Kransdorf, E.; Bonnet, G.; Loupy, A.; Kobashigawa, J.; Patel, J.K. Statistical performance of 16 posttransplant risk scores in a contemporary cohort of heart transplant recipients. Am J Transplant 2021, 21, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Tsirebolos, G.; Tsoporis, J.N.; Drosatos, I.A.; Izhar, S.; Gkavogiannakis, N.; Sakadakis, E.; Triantafyllis, A.S.; Parker, T.G.; Rallidis, L.S.; Rizos, I. Emerging markers of inflammation and oxidative stress as potential predictors of coronary artery disease. Int J Cardiol 2023, 376, 127–133. [Google Scholar] [CrossRef]
- Van de Voorde, J.; Pauwels, B.; Boydens, C.; Decaluwe, K. Adipocytokines in relation to cardiovascular disease. Metabolism 2013, 62, 1513–1521. [Google Scholar] [CrossRef]
- Puchinger, J.; Ryz, S.; Nixdorf, L.; Edlinger-Stanger, M.; Lassnigg, A.; Wiedemann, D.; Hiesmayr, M.; Spittler, A.; Bernardi, M.H. Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery-A Prospective Cohort Study. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J. , et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017, 19, 821–836. [Google Scholar]
- Kilic, A.; Allen, J.G.; Weiss, E.S. Validation of the United States-derived Index for Mortality Prediction After Cardiac Transplantation (IMPACT) using international registry data. J Heart Lung Transplant 2013, 32, 492–498. [Google Scholar] [CrossRef]
- Assmann, A.; Beckmann, A.; Schmid, C.; Werdan, K.; Michels, G.; Miera, O.; Schmidt, F.; Klotz, S.; Starck, C.; Pilarczyk, K. , et al. Use of extracorporeal circulation (ECLS/ECMO) for cardiac and circulatory failure -A clinical practice Guideline Level 3. Esc Heart Fail 2022, 9, 506–518. [Google Scholar] [CrossRef]
- Saeed, D.; Feldman, D.; Banayosy, A.E.; Birks, E.; Blume, E.; Cowger, J.; Hayward, C.; Jorde, U.; Kremer, J.; MacGowan, G. , et al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10- Year Update. J Heart Lung Transplant 2023, 42, e1–e222. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K. , et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol 2022, 79, 933–946. [Google Scholar]
- Kittleson, M.M. Changing Role of Heart Transplantation. Heart Fail Clin 2016, 12, 411–421. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ Res 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Ahiante, B.O.; Smith, W.; Lammertyn, L.; Schutte, A.E. Leptin and the vasculature in young adults: The African-PREDICT study. Eur J Clin Invest 2019, 49, e13039. [Google Scholar] [CrossRef]
- Wojciechowska, C.; Jachec, W.; Romuk, E.; Nowalany-Kozielska, E.; Tomasik, A.; Sieminska, L. The effect of BMI, serum leptin, and adiponectin levels on prognosis in patients with non-ischaemic dilated cardiomyopathy. Endokrynol Pol 2017, 68, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.J.; Huang, Y.X.; Zhang, X.L.; Li, J.; Huang, J.; Zhang, H.; Hu, S.S. Apolipoprotein D as a novel marker in human end-stage heart failure: a preliminary study. Biomarkers 2008, 13, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Fyfe-Desmarais, G.; Desmarais, F.; Rassart, E.; Mounier, C. Apolipoprotein D in Oxidative Stress and Inflammation. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Wyler von Ballmoos, M.C.; Haring, B.; Sacks, F.M. The risk of cardiovascular events with increased apolipoprotein CIII: A systematic review and meta-analysis. J Clin Lipidol 2015, 9, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Rehues, P.; Girona, J.; Guardiola, M.; Plana, N.; Scicali, R.; Piro, S.; Muniz-Grijalvo, O.; Diaz-Diaz, J.L.; Recasens, L.; Pinyol, M. , et al. PCSK9 Inhibitors Have Apolipoprotein C-III-Related Anti-Inflammatory Activity, Assessed by 1H-NMR Glycoprotein Profile in Subjects at High or very High Cardiovascular Risk. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Contois, J.H.; McConnell, J.P.; Sethi, A.A.; Csako, G.; Devaraj, S.; Hoefner, D.M.; Warnick, G.R.; Lipoproteins, A.; Vascular Diseases Division Working Group on Best, P. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem 2009, 55, 407–419. [Google Scholar] [CrossRef]
- Sniderman, A.; Langlois, M.; Cobbaert, C. Update on apolipoprotein B. Curr Opin Lipidol 2021, 32, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Goldbourt, U.; Cohen, H.; Harats, D. Association between serum apolipoprotein C(II) concentration and coronary heart disease. Prev Med 2002, 35, 42–47. [Google Scholar] [CrossRef]
- Valenzuela, A.; Hougen, H.P.; Villanueva, E. Lipoproteins and apolipoproteins in pericardial fluid: new postmortem markers for coronary atherosclerosis. Forensic Sci Int 1994, 66, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Millan, O.; Brunet, M. Cytokine-based immune monitoring. Clin Biochem 2016, 49, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sayour, A.A.; Olah, A.; Ruppert, M.; Barta, B.A.; Horvath, E.M.; Benke, K.; Polos, M.; Hartyanszky, I.; Merkely, B.; Radovits, T. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol 2020, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.R.; Cheng, A.; Ising, M.; Lenneman, A.; Birks, E.; Slaughter, M.S. Heart Transplant Survival Based on Recipient and Donor Risk Scoring: A UNOS Database Analysis. ASAIO J 2016, 62, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Khush, K.; Colvin, M.; Acker, M.; Van Bakel, A.; Eisen, H.; Naka, Y.; Patel, J.; Baran, D.A.; Daun, T. , et al. Report From the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States. Am J Transplant 2017, 17, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancini, D.; Patel, J.; Razi, R.; Reichenspurner, H. , et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014, 33, 327–340. [Google Scholar] [CrossRef]
- Batchelor, R.J.; Wong, N.; Liu, D.H.; Chua, C.; William, J.; Tee, S.L.; Sata, Y.; Bergin, P.; Hare, J.; Leet, A. , et al. Vasoplegia Following Orthotopic Heart Transplantation: Prevalence, Predictors and Clinical Outcomes. J Card Fail 2022, 28, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Oba, K.; Matsui, Y.; Morimoto, Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018, 32, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A K-Means Clustering Algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics) 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Hunt, S.A.; Haddad, F. The changing face of heart transplantation. J Am Coll Cardiol 2008, 52, 587–598. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).