1. Introduction
Opioids (such as morphine) are the most effective drugs for treating severe pain. However, these treatments are accompanied by several adverse events, including itching, tolerance, dependence, nausea, constipation, sedation, and respiratory depression. Furthermore, the analgesic efficacy of opioids varies among individuals [
1,
2]. Therefore, effective pain treatment is often hampered by considerable differences in opioid sensitivity. Insufficient opioid doses can lead to inadequate pain relief, whereas unnecessarily high doses can result in adverse effects, both commonly observed in clinical settings [
3]. Thus, the proper administration of opioids is crucial to meet the needs of individual patients. However, the factors contributing to different inter-individual responses to opioids are not fully understood. Moreover, morphine has been used as a potent analgesic for the treatment of severe chronic pain. However, frequent long-term treatment results in the development of analgesic tolerance via two possible mechanisms. The first is involved in the signal transduction of opioid receptors, including receptor down-regulation, functional decoupling from G-proteins, and β-arrestin recruitment, which results in receptor desensitization and internalization and endocytosis [
4,
5] The second is characterized by alterations in primary drug-sensitive system adaptation, including the glutamatergic receptor system [
6] and glial cell activation with the release of proinflammatory cytokines [
7], thus inhibiting the analgesic effect of morphine. Tolerance to opioid analgesics is a major concern associated with these drugs; however, an effective solution is still needed.
Morphine produces analgesia via its activity at several levels of the nervous system; it inhibits neurotransmitter release from primary afferent terminals in the spinal cord and activates descending inhibitory controls in the midbrain [
8,
9]. However, the peripheral action of morphine in regulating pain transmission remains unclear [
10]. The endogenous opioid system is activated under pathological conditions. The ratio of morphine-induced partial antinociceptive activity at peripheral sites is more advantageous than that at the central site [
11]. Despite extensive investigation, the detailed mechanism underlying the analgesic action of morphine is not fully understood.
In a previous study, we showed a close correlation between time-course changes in morphine-induced itching and antinociceptive activity [
12]. Therefore, we suggest that the itch has a strong association with antinociceptive activity and focus on the measurement of the itch-associated scratching behavior. Itching elicits a strong desire to scratch; therefore, scratching behavior count is a useful index to evaluate itching [
13]. Using NC/Nga mice, an animal model for atopic dermatitis [
14], we designed a method to assess spontaneous scratching behavior [
15]. Current itch studies rely on human perception, and these nociceptive stimuli have no discernible differences. However, the sensory perception of foreign substances and true itching can be differentiated by dividing the scratching behavior of mice into long-lasting scratching (LLS; itch-associated scratching behavior) and short-lasting scratching (SLS; hygiene behavior) [
15]. The number of spontaneous scratches can be automatically detected and objectively evaluated using a computer. The study showed frequent LLS in NC/Nga mice with skin-lesions, and not in other mouse strains. SLS was frequently observed in skin-lesioned NC/Nga mice and other strains without skin-lesioned. These results indicate that SLS is a form of locomotor activity and/or hygienic behavior, whereas LLS represents a true itching response in mice. The study suggests differentiating genuine itch caused by foreign substance from contact itch. This can be achieved by objective mouse scratching as LLS and SLS behaviors [
16].
Interleukin-31 (IL-31) is a possible mediator of itching that induces severe pruritus and dermatitis in mice [
17]. In a previous NC/Nga mouse study, we found that IL-31mRNA is expressed only in scratching mice and not in those without scratching behavior [
18,
19]. We also observed that IL-31 pretreatment induces alloknesis (itching upon touching or brushing) and hyperemesis (itchiness upon pin pricking) itching sensations around an itching site in mice [
20,
21,
22,
23]. Furthermore, morphine caused analgesia and alloknesis simultaneously [
24]. Thus, in a recent study, we investigated the effect of morphine on nociception and the LLS in interleukin-31RA deficient (IL-31RA knock-in, IL-31RAKI) and wild-type mice [
25]. Morphine elicits both LLS and antinociception in wild-type mice. Additionally, a significant correlation was observed between LLS counts and antinociception, indicating a correlation between morphine-induced antinociception and itching [
12]. Morphine-induced antinociception is partially reduced in IL-31RAKI mice, providing the first evidence that IL-31RA may play an important role in morphine-induced antinociception. To confirm this hypothesis, we examined the effect of IL-31 pretreatment on the antinociceptive effect of morphine in mice. Furthermore, we highlighted that this effect of IL-31 resulted from an increase in the DRG neuronal level of IL-31RA by repeated administration of IL-31, thus elucidating the site of the peripheral antinociceptive effect of morphine. Recent findings have indicated that IL-31 is partially involved in peripheral analgesic mechanisms. As IL-31 and IL-31RA are not expressed in the CNS, the endogenous opioid system is activated under pathological conditions.
Previously, we demonstrated a close correlation between morphine-induced antinociceptive activity and scratching behaviors (LLS and SLS) in mice [
12]. Asntinociceptive activity can only be measured intermittently; however, scratching behavior is continually measurable. Therefore, in this study, we used an index of scratching behavior to elucidate the site of action of morphine. The purpose of the present study was to investigate the effect of morphine on itch-associated scratching behavior (LLS) and antinociceptive effects in IL-31RAKI mice compared with that in wild-type mice to elucidate the regulatory mechanism of morphine-induced antinociceptive activity and antinociceptive tolerance.
3. Discussion
Morphine has long been thought to act on the central nervous system, producing analgesia via its interaction with cerebral and peripheral opioid receptors [
27,
28]. Moreover, peripheral morphine exerts potent antinociceptive effects under inflammatory conditions [
29]. However, opioid drugs exert their analgesic effects by activating opioid receptors, which are targets of endogenously produced opioid peptides, including endorphins, enkephalins, and dynorphins. Based on pharmacological and molecular cloning studies, distinct-, μ-, δ-, and κ- opioid receptors have been identified. Several, researchers have studied the analgesic activity of opioids based on these receptors; however, the precise mechanism remains unknown.
Previously, we repeated that pretreatment with IL-31 resulted in significant antinociceptive action (25). Moreover, when combined with morphine, the analgesic effect was enhanced. We suggested that IL-31 played a role in the antinociceptive action of morphine [
12]. We examined the effect of IL-31 pretreatment on morphine-induced antinociception using a hot-plate test. There was a close correlation between morphine-induced LLS counts and antinociceptive effects. We investigated LLS as an indicator of morphine-induced antinociceptive activity. These data showed that cutaneous injected IL-31-induced LLS were enhanced by DRG neuronal IL-31RA expression [
26]. Morphine treatment significantly increased IL-31-induced LLS and antinociceptive activity. The subcutaneous injection of morphine induced LLS (itch-associated scratching behavior), SLS (hygiene behavior), and antinociception. The mechanism of morphine antinociception involve two sites of action: central and peripheral [
30,
31]. Previously, we have reported that IL-31 may play a more significant role in the modulation of peripheral morphine-induced antinociception via sensory neurons in IL-31RAKI mice than in wild-type mice [
12]. Antinociception and scratching behaviors (LLS and SLS) were observed simultaneously during the experimental period. In particular, a close correlation was observed between the development of scratching behavior (LLS and SLS) and morphine-induced antinociceptive action. These results showed that these behaviors mediate increased itching, locomotion, and antinociceptive activity. As LLS expression is not frequently observed in other pruritogen-induced scratching behaviors, IL-31RA may be involved in IL-31-induced LLS expression [
32]. Therefore, we generated IL-31RAKI mice and examined the effect of morphine on LLS (itching) and its antinociceptive activity in comparison with wild-type mice. We observed that morphine-induced LLS did not develop in IL-31RAKI mice and that morphine-induced antinociceptive effects were partially observed. Collectively, these results indicated that morphine-induced SLS and partial antinociception are central site actions, whereas morphine-induced LLS (itching) and partial antinociception are peripheral site actions. The peripheral site activity of morphine in the regulation of pain transmission is unclear; however, the endogenous opioid system is activated under pathological conditions. Therefore, the mechanism underlying the analgesic effects causing itching remains unclear.
Innocuous mechanical stimuli that do not normally induce a behavioral response in mice elicit bouts of scratching following intradermal administration of the pruritogen histamine, 5-HT, a PAR-4 agonist, and BAM8-22. Similarly, following the local application of histamine, 5-HT, or BAM8-22 in humans, innocuous mechanical stimuli induced itching, a phenomenon known as alloknesis [
33,
34,
35,
36]. In contrast, post-histamine mechanically induced scratching in mice was significantly attenuated by the μ-opioid antagonist naltrexone, which is consistent with the ability of another antagonist, naloxone, to reduce alloknesis in humans [
33]. Moreover, morphine potentiates histamine-induced itching in humans [
37] and chloroquine-induced scratching in rats [
38]. These results suggest that morphine (opioid) administration causes hyperknesis and alloknesis.
Recently, we reported that pretreatment with IL-31 cause alloknesis (LLS) and antinociceptive activity simultaneously in mice [
12]. In contrast, morphine-induced LLS and antinociceptive effects were closely correlated in wild-type mice. Repeated pre-administration of IL-31 marginally increased the morphine-induced antinociceptive effects in the hot-plate test. Morphine-induced antinociception enhanced by pretreatment with IL-31, increasing doses of morphine or IL-31. Moreover, repeated administration of IL-31 significantly increased LLS counts and the IL-31RA mRNA expression in the cervix and sacral plexus of DRG neuron cell bodies [
26]. These data suggest that IL-31 upregulates IL-31RA expression in DRG neuron cell bodies, enhancing topical cutaneous IL-31-induced LLS via IL-31RA levels [
39]. These results suggest that the individuality of analgesic sensitivity to morphine was due to differences in the interneural IL-31RA expression. Thus, LLS and antinociceptive activity depend on the IL-31RA expression in the DRG and the concentration of IL-31 in the blood [
39]. Our hypothesis links IL-31 and morphine via a potential site that promotes IL-31RA exocytosis, thus reinforcing the antinociceptive activity of morphine. Rapidly increasing IL-31 and IL-31RA synthetically is impossible, and the release of trace IL-31 into th blood remain consistent. Hence, we studied two morphine types under neural conditions to discern if the site of action was pre-synaptic or post-synaptic sensory nervous system.
Mite-infestation-induced itching in mice was caused by co-housing mice with skin-lesioned NC/Nga mice. Several strains of mice showed increased LLS counts after only a few days of co-housing with skin-lesioned NC/Nga mice [
40]. Mite-infestation significantly increased DRG neuronal IL-31RA expression and not IL-31 blood concentration (
Figure 11a, upside). Based on these data, we examined the site of action of morphine, considering the enhancement of morphine-induced LLS counts as an indicator of antinociceptive activity following pretreatment with IL-31. Mite-infested mice demonstrated a significantly increased LLS count; however, this effect was difficult to observe during the day as this reaction occurred at night [
23], Although morphine-induced LLS counts did not change in mite-infested mice, DRG neuronal IL-31RA increased (
Figure 11a, downside). These results suggest that morphine did not affect the release or production of endogenous IL-31.
In nomal BALB/c mice, pretreatment with IL-31 1 h before morphine administration did not increase the level of IL-31RA expression in the skin or neuronal DRG; however, it markedly increased the blood concentration of IL-31 (
Figure 11b upside). Morphine-induced LLS counts as an indicator of antinociceptive activities were enhanced compared with those in the PBS-treated group (
Figure 11b downside). These results suggest that morphine may accelerate the ability of IL-31RA to act on sensory nerves (e.g., promotion of the translocation of IL-31RA to the plasma membrane and exocytosis of IL-31RA). For example, intra neural catecholamines appear to be increased after reserpine administration for approximately 2 h, increases sympathetic nerve activity.
Analgesic sensitivity to opioids, such as morphine, can hinder positive effects and increase side effects associated with decreased quality of life. Genetic factors may also affect individual differences in opioid sensitivity [
1]. Previous reports have shown a reduced sensitivity to opioids in heterozygous μ-opioid receptor knockout mice with 50% μ-opioid receptor mRNA [
41,
42]. This suggested that the low amounts of μ-opioid receptor mRNA cause reduced sensitivity to opioids. Moreover, interindividual differences in opioid analgesia may be partly attributable to divergent μ-opioid receptor mRNA levels because of μ-opioid receptor gene differences [
43]. The mechanism by which they show inter-individual differences in morphine is regarded as a phenomenon occurring in the central nervous system. In contrast, this study showed that the inter-individual differences in morphine levels in the peripheral nerve depend on the difference in the levels of intraneural IL-31RA. Additionally, neuronal IL-31RA expression in the DRG may contribute to individual differences in morphine analgesic sensitivity. Repeated administration of IL-31 gradually increased the LLS counts, DRG neuronal IL-31RA mRNA expression, and morphine-induced antinociception. These findings suggest that neuronal IL-31RA expressed in the DRG is involved in the modulation of morphine-induced antinociception. Furthermore, morphine may induce exocytosis of IL-31RA at the end of the sensory nerve. Thus, IL-31 may be a useful adjunctive agent for pain relief in combination with morphine. Therefore, the combination treatment with IL-31 and morphine may reduce the required dose of morphine and suppress the onset of side effects. Furthermore, this combination may reduce individual differences in morphine sensitivity.
In this study, a single dose of morphine significantly decreased spontaneous scratching behavior over 6 h (dark phase, 19:00–24:00) after morphine administration. Moreover, nine times repeated administration of morphine also decreased the morphine-induced scratching behavior and antinociceptive activity, with every degree of morphine administration. Moreover, IL-31-induced LLS counts were significantly reduced after nine times repeated administration of morphine (every 3 h for 27 h). This phenomenon was regarded as the result of intraneural IL-31RA depletion caused by the repeated administration of morphine-induced exocytosis of IL-31RA. Previously, we have reported that IL-31RA may play a crucial role in sensory neurons in peripheral morphine-induced antinociception modulation in IL-31A deficient mice. In addition, they closely correlated with peripheral activity, morphine-induced SLS counts and central antinociceptive activity. Therefore, we suggest that morphine-induced antinociceptive tolerance was caused by both peripheral and central sites of action, as morphine decreased LLS and SLS counts after a single or repeated administration. Moreover, the recovery time of morphine-induced tolerance formation, the central site of action, was more than 24 h, and the peripheral site of action was more than 12 h after the last dose of nine-times repeated administration of morphine.
To date, several studies have investigated the mechanisms underlying morphine-induced antinociceptive tolerance. NMDA receptors [
44], cannabinoid receptors [
45], transient receptor potential vanilloid-1 [
46], serotonergic system, and noradrenergic system [
47,
48,
49], play a substantial role in morphine-induced tolerance. However, the exact mechanism underlying morphine-induced tolerance remains unknown. Previously we have reported that pretreatment with IL-31 significantly enhances morphine-induced antinociceptive activity, and that the site of action of morphine may promote exocytosis of IL-31RA in peripheral sensory nerve endings (
Figure 12a, upside). Therefore, repeated administration of morphine depletes IL-31RA in peripheral sensory nerves through recurrent promotion of IL-31RA exocytosis (
Figure 12a, middle side). Repeated morphine administration decreases morphine-induced LLS and antinociceptive activity (
Figure 12a, downside). For example, intraneural catecholamine depletion after reserpine administration for approximately 24 h, decreases sympathetic nerve activity.
Repeated doses of IL-31 administered every 12 h for 3 days significantly increased LLS counts and IL-31RA expression in the DRG neuron cell body, regardless of the IL-31 dosage [
39]. In contrast, repeated administration of morphine did not influence DRG neuronal IL-31RA expression (
Figure 12b, upside). These data suggest that IL-31 upregulates IL-31RA expression in DRG neurons, and that topical cutaneous IL-31-induced LLS (itching) is enhanced by IL-31RA in the same topically innervated DRG neuron (
Figure 12b, middle side). These data show that itching depends on the interaction between DRG neuronal IL-31RA and blood IL-31 concentrations [
32]. Therefore, combining IL-31 and morphine treatment could potentially address IL-31RA depletion in sensory nerves (
Figure 12b down side). Thus, we examined the effect of combined treatment with morphine and IL-31 on repeated administration of morphine to induce antinociceptive tolerance in mice, which resulted in decreased antinociceptive activity. The increase significantly decreased with increasing morphine doses. In contrast, repeated combined administration of IL-31 and morphine gradually decreased the antinociceptive activity; however the difference was not significant (
Figure 12b, down side). The antinociceptive tolerance of morphine involves dual actions; one shifts pain to itch via IL-31-induced alloknesis at the peripheral site, whereas the other triggers inhibitory controls at the central site. It has been suggested that IL-31 acts only at peripheral sites of morphine-induced analgesic tolerance. Moreover, clinical evidence has revealed that opioid tolerance does not develop frequently and that opioid treatments are not always problematic in terms of creating tolerance in patients with chronic pain [
50,
51,
52]. Recently, we reported that DRG neuronal IL-31RA expression increases in pain-induced model mice [
25]. The results explained that analgesic-tolerance relaxes in patients with chronic pain. These results suggest that morphine is united with IL-31RA, which contributes to antinociceptive activity rather than a former point of action for morphine and the relations of IL-31. These results suggest that repeated administration of IL-31 partially modulates nociception and tolerance to morphine antinociception. However, the function of opioid receptors and IL-31RA and the relationship between their existence are unknown. Therefore, further studies are warranted.
In conclusion, we suggest that IL-31RA may play a critical role in morphine-induced peripheral antinociception. In contrast, combination treatment with IL-31 and morphine significantly increased the morphine-induced antinociceptive activity. IL-31 upregulates IL-31RA expression in DRG neurons and morphine-induced antinociceptive activity is dependent on DRG neuronal IL-31RA expression. These results suggest that the individuality of analgesic sensitivity to morphine was caused by differences in the expression of intraneural IL-31RA. Therefore, pretreatment of IL-31 may reduce individual differences in the antinociceptive activity of morphine. The combination treatment with IL-31 and morphine may reduce the required dose of morphine and suppress the onset of its side effects. This site of action of morphine may induce exocytosis of IL-31RA in the sensory nervous system. Repeated administration of morphine decreased the morphine-induced scratching behavior (LLS and SLS) and antinociception (analgesic tolerance). Repeated administration of morphine completely inhibited IL-31-induced LLS (itching). These results suggest that repeated administration of morphine depletes inter-neuronal IL-31RA levels. Repeated administration of IL-31 increased IL-31RA expression in DRG. Furthermore, its combination with morphine enhanced its analgesic action. Collectively, the suppressive effect of IL-31 on morphine-induced antinociceptive tolerance may result, at least in part, from IL-31RA supplementation in sensory nerves. These results suggest that the combination of morphine and IL-31 reduces the analgesic tolerance of morphine.
Figure 1.
Effect of morphine on latency and antinociceptive activity in wild-type and IL-31RA KI mice. (a) Time-course change in latency following morphine (3 mg/kg, subcutaneous) administration in wild-type and IL-31RA KI mice. The blue line indicates the saline-administered group in wild-type mice; the green line indicates the saline-administered group in IL-31RAKI mice; the red line indicates the morphine-administered group in wild-type mice; the yellow line indicates the morphine-administered group in IL-31RAKI mice. Red arrows indicate saline or morphine administration point. Each value represents the mean ± S.E. of six mice. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of saline-administered groups # P < 0.05, and ## P < 0.01 compared with the values of morphine-administered groups in wild-type mice. (Student’s t-test with Bonferroni correction). (b) Antinociceptive activity from 15–120 min1 after morphine administration. The blue column indicates the saline-administered group in wild-type mice; the green column indicates the saline-administered group in IL-31RAKI mice; the red column indicates the morphine-administered group in wild-type mice; the yellow column indicates the morphine-administered group in IL-31RAKI mice. IL-31, interleukin-31; Wild, C57BL/6 mice; IL-31RAKI, IL-31RA deficient mice (C57BL/6 genetic background). Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with each value of the wild-type and IL-31RAKI mice group (Tukey’s multiple comparison test).
Figure 1.
Effect of morphine on latency and antinociceptive activity in wild-type and IL-31RA KI mice. (a) Time-course change in latency following morphine (3 mg/kg, subcutaneous) administration in wild-type and IL-31RA KI mice. The blue line indicates the saline-administered group in wild-type mice; the green line indicates the saline-administered group in IL-31RAKI mice; the red line indicates the morphine-administered group in wild-type mice; the yellow line indicates the morphine-administered group in IL-31RAKI mice. Red arrows indicate saline or morphine administration point. Each value represents the mean ± S.E. of six mice. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of saline-administered groups # P < 0.05, and ## P < 0.01 compared with the values of morphine-administered groups in wild-type mice. (Student’s t-test with Bonferroni correction). (b) Antinociceptive activity from 15–120 min1 after morphine administration. The blue column indicates the saline-administered group in wild-type mice; the green column indicates the saline-administered group in IL-31RAKI mice; the red column indicates the morphine-administered group in wild-type mice; the yellow column indicates the morphine-administered group in IL-31RAKI mice. IL-31, interleukin-31; Wild, C57BL/6 mice; IL-31RAKI, IL-31RA deficient mice (C57BL/6 genetic background). Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with each value of the wild-type and IL-31RAKI mice group (Tukey’s multiple comparison test).
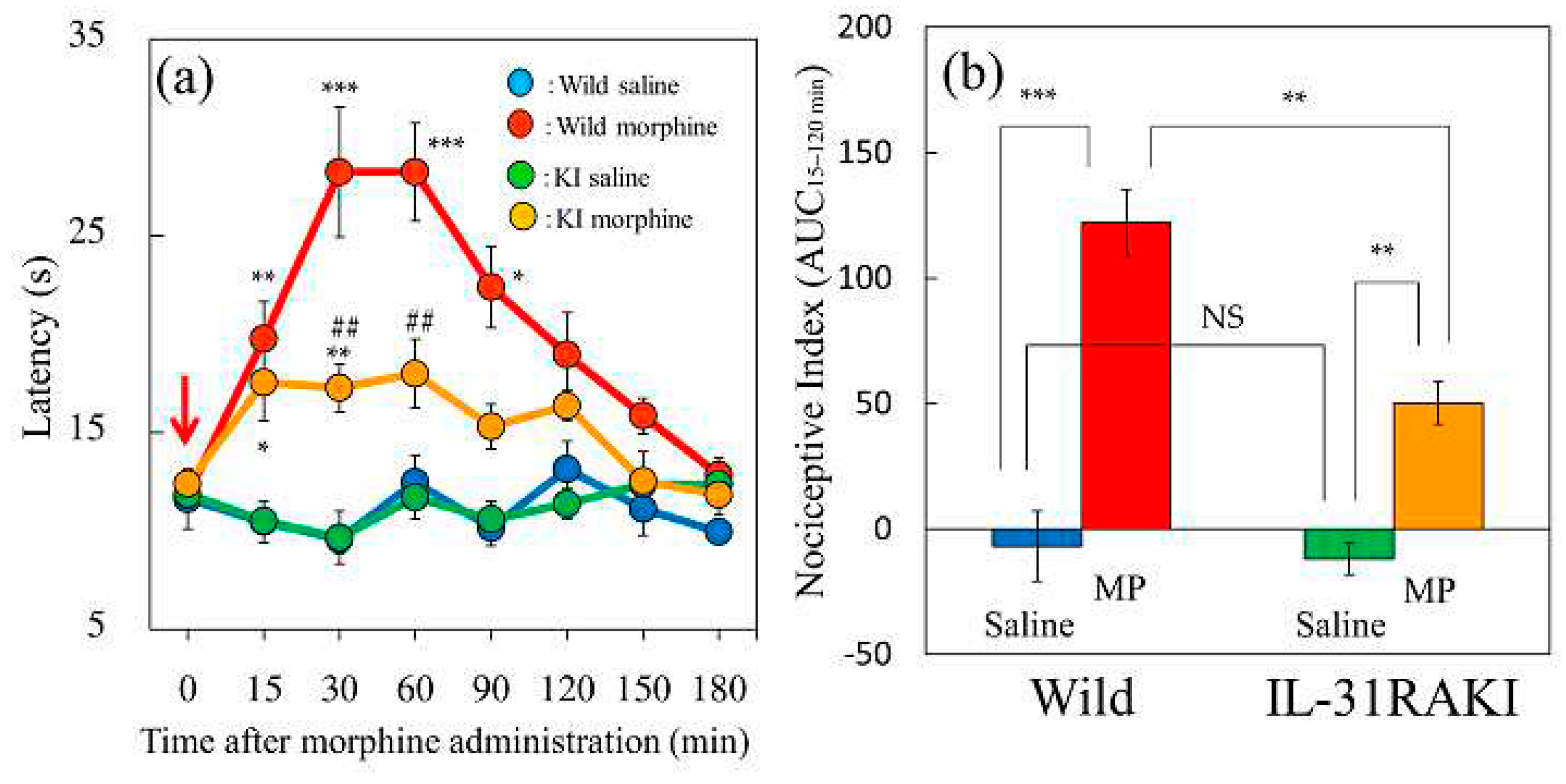
Figure 2.
Effects of single or repeated pretreatment with IL-31 on morphine-induced antinociceptive activity in mice. (a) Time-course change of morphine-induced (1 mg/kg, subcutaneous) antinociceptive activity in mice using the hot-plate test. The results are presented as the mean ± S.E. of six mice. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of the vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intraperitoneal) + saline-treated group (Student’s t-test with Bonferroni correction). (b) Total antinociceptive index (antinociceptive activity from 30 to 120 min (AUC30–120 min). sIL-31, single administration of IL-31 (50 mg/kg, intraperitoneal), reIL-31, repeated administration of IL-31 (50 μg/kg, intraperitoneal, every 12 h for 3 days). For both panels, the blue line and column indicates the PBS + saline-treated group; red line and column indicates the vehicle + morphine-treated group; green line and column indicates the single IL-31 + morphine treated-group; and yellow line and column indicates repeated IL-31 + morphine treated-group. The results are presented as the mean ± S.E. of six mice (total, 24 mice). # P < 0.05 compared with the PBS + saline-treated group (Student’s t-test). NS, not significant. ** P < 0.01 compared with the values of the PBS + morphine-treated group (Dunnett test).
Figure 2.
Effects of single or repeated pretreatment with IL-31 on morphine-induced antinociceptive activity in mice. (a) Time-course change of morphine-induced (1 mg/kg, subcutaneous) antinociceptive activity in mice using the hot-plate test. The results are presented as the mean ± S.E. of six mice. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of the vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intraperitoneal) + saline-treated group (Student’s t-test with Bonferroni correction). (b) Total antinociceptive index (antinociceptive activity from 30 to 120 min (AUC30–120 min). sIL-31, single administration of IL-31 (50 mg/kg, intraperitoneal), reIL-31, repeated administration of IL-31 (50 μg/kg, intraperitoneal, every 12 h for 3 days). For both panels, the blue line and column indicates the PBS + saline-treated group; red line and column indicates the vehicle + morphine-treated group; green line and column indicates the single IL-31 + morphine treated-group; and yellow line and column indicates repeated IL-31 + morphine treated-group. The results are presented as the mean ± S.E. of six mice (total, 24 mice). # P < 0.05 compared with the PBS + saline-treated group (Student’s t-test). NS, not significant. ** P < 0.01 compared with the values of the PBS + morphine-treated group (Dunnett test).
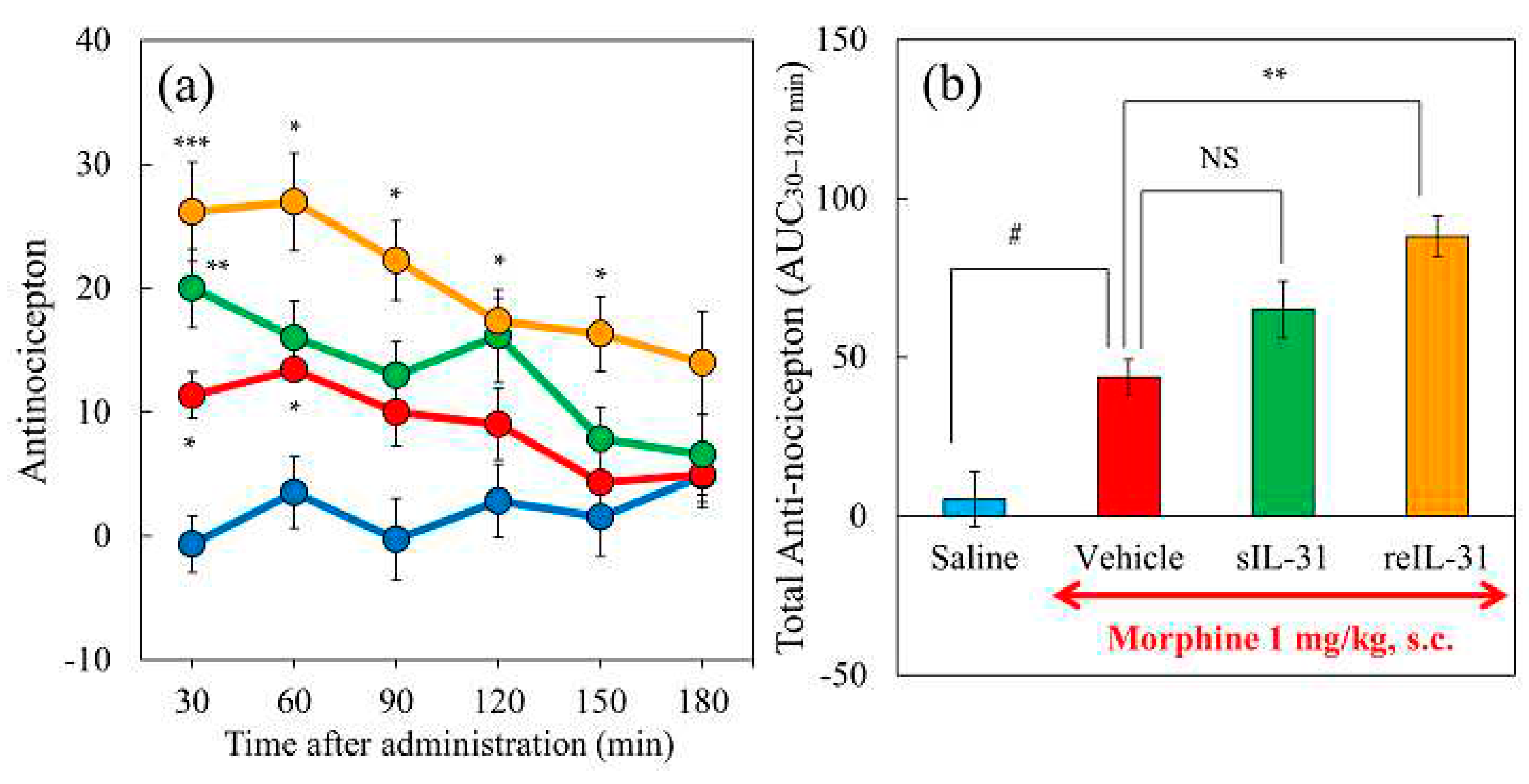
Figure 3.
Effect of IL-31 on morphine-induced antinociceptive activity in mice. (a) Time-course change of morphine-induced antinociception + vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intraperitoneally, every 12 h for 3 days) in hot-plate test. The blue line indicates the PBS + saline-treated group; the red line indicates the PBS + morphine 1 mg/kg-treated group; the green line indicates the PBS + morphine 3 mg/kg-treated group; the yellow line indicates the PBS + morphine 10 mg/kg-treated group. (b) Time-course change of three doses of morphine-induced antinociception with repeated administration of IL-31 (50 μg/kg, intraperitoneally, every 12 h for 3 days). The blue line indicates the repeated PBS + saline-treated group; the red line indicates the IL-31 + morphine (1 mg/kg, subcutaneous)-treated group; the green line indicates the repeated IL-31 + morphine (3 mg/kg, subcutaneous)-treated-group; and the yellow line indicates the repeated IL-31 + morphine (10 mg/kg, subcutaneous)-treated-group. The results are presented as the mean ± S.E. of six mice * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of the PBS + saline-treated group (Student’s t-test with Bonferroni correction). (c) Combined total antinociceptive index from 30 to 90 min (AUC30–90 min) of morphine and IL-31. The blue column indicates the vehicle + saline- or morphine-treated groups; the red column indicates the IL-31 + morphine-treated groups. The results are presented as the mean ± S.E. of six mice (total, 48 mice). NS, is not significant. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with the corresponding dose of PBS + morphine-treated group (Student’s t-test). # P < 0.05, and ### P < 0.001 compared with the corresponding values of the PBS + saline-treated or IL-31 + saline-treated group (Student’s t-test).
Figure 3.
Effect of IL-31 on morphine-induced antinociceptive activity in mice. (a) Time-course change of morphine-induced antinociception + vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intraperitoneally, every 12 h for 3 days) in hot-plate test. The blue line indicates the PBS + saline-treated group; the red line indicates the PBS + morphine 1 mg/kg-treated group; the green line indicates the PBS + morphine 3 mg/kg-treated group; the yellow line indicates the PBS + morphine 10 mg/kg-treated group. (b) Time-course change of three doses of morphine-induced antinociception with repeated administration of IL-31 (50 μg/kg, intraperitoneally, every 12 h for 3 days). The blue line indicates the repeated PBS + saline-treated group; the red line indicates the IL-31 + morphine (1 mg/kg, subcutaneous)-treated group; the green line indicates the repeated IL-31 + morphine (3 mg/kg, subcutaneous)-treated-group; and the yellow line indicates the repeated IL-31 + morphine (10 mg/kg, subcutaneous)-treated-group. The results are presented as the mean ± S.E. of six mice * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with corresponding values of the PBS + saline-treated group (Student’s t-test with Bonferroni correction). (c) Combined total antinociceptive index from 30 to 90 min (AUC30–90 min) of morphine and IL-31. The blue column indicates the vehicle + saline- or morphine-treated groups; the red column indicates the IL-31 + morphine-treated groups. The results are presented as the mean ± S.E. of six mice (total, 48 mice). NS, is not significant. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with the corresponding dose of PBS + morphine-treated group (Student’s t-test). # P < 0.05, and ### P < 0.001 compared with the corresponding values of the PBS + saline-treated or IL-31 + saline-treated group (Student’s t-test).
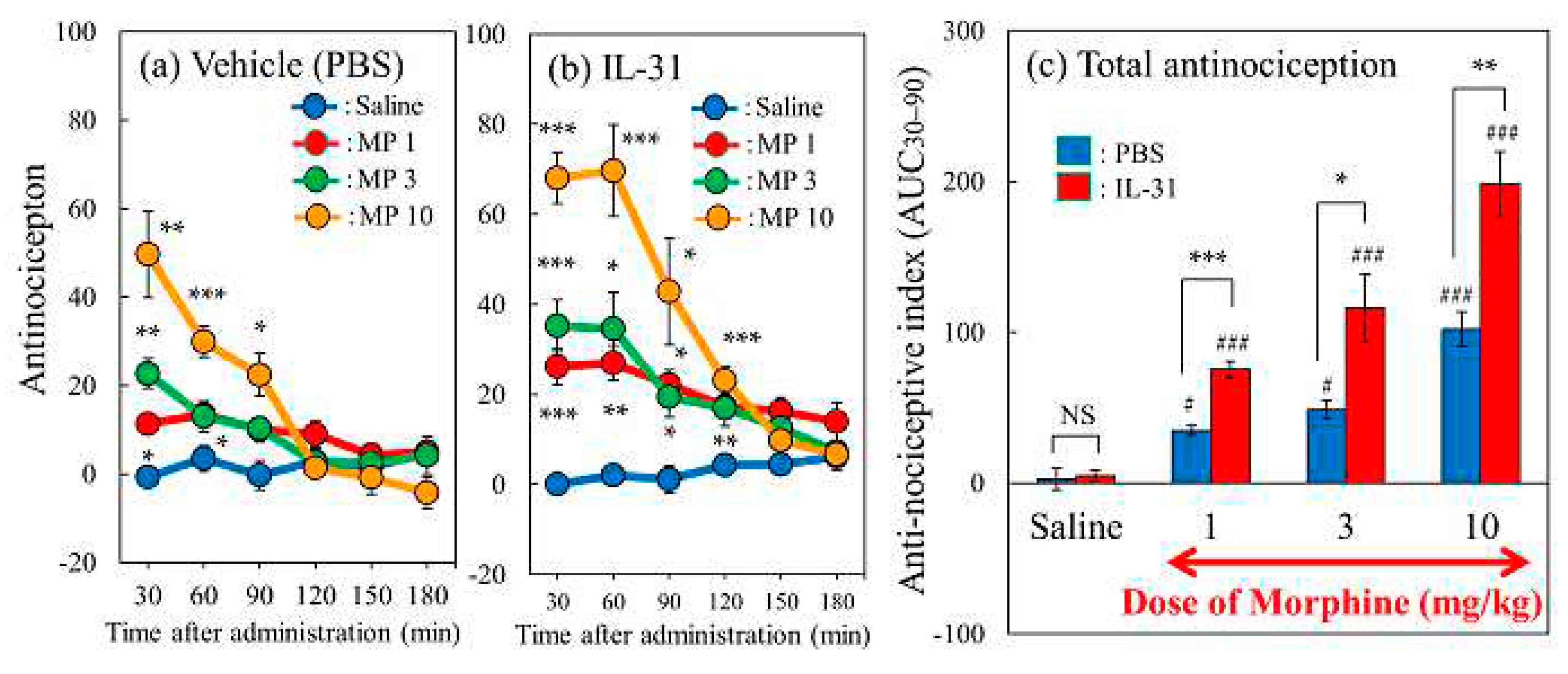
Figure 4.
Effect of repeated pretreatment with three doses of IL-31 on morphine-induced antinociception in mice. (a) Time-course change of morphine (3 mg/kg, subcutaneously)-induced antinociception with three doses of repeated administration of IL-31 (5, 15, and 50 μg/kg, intraperitoneal). The results are presented as the mean ± S.E. of six mice. # P < 0.05, ## P < 0.01 compared with corresponding values of the saline-treated group. *P < 0.05 compared with the values of the vehicle (phosphate buffered saline, PBS, 10 mL/kg, intraperitoneal) + morphine-treated group (Student’s t-test with Bonferroni correction). (b) Effects of pretreatment with repeated administration of IL-31 on morphine-induced total antinociception index from 30 to 90 min after morphine administration (AUC15–90 min) in the hot-plate test. The blue line and column indicate the repeated PBS + saline (10 mL/kg, subcutaneous)-treated group; the red line and column indicate the repeated PBS + morphine-treated group; the green line and column indicate the repeated IL-31 (5 μg/kg, intraperitoneal) + morphine-treated group; the yellow line and column indicate the repeated IL-31 (15 μg/kg, intraperitoneal) + morphine-treated group; and the purple line and column indicate the repeated IL-31 (50 μg/kg, intraperitoneal) + morphine-treated group. The results are presented as the mean ± S.E. of six mice (total, 30 mice). NS, not significant. # P < 0.05 compared with the PBS + saline-treated group (Student’s t-test). * P < 0.05, and ** P < 0.01 compared with the values of the PBS + morphine-treated group (Dunnett test).
Figure 4.
Effect of repeated pretreatment with three doses of IL-31 on morphine-induced antinociception in mice. (a) Time-course change of morphine (3 mg/kg, subcutaneously)-induced antinociception with three doses of repeated administration of IL-31 (5, 15, and 50 μg/kg, intraperitoneal). The results are presented as the mean ± S.E. of six mice. # P < 0.05, ## P < 0.01 compared with corresponding values of the saline-treated group. *P < 0.05 compared with the values of the vehicle (phosphate buffered saline, PBS, 10 mL/kg, intraperitoneal) + morphine-treated group (Student’s t-test with Bonferroni correction). (b) Effects of pretreatment with repeated administration of IL-31 on morphine-induced total antinociception index from 30 to 90 min after morphine administration (AUC15–90 min) in the hot-plate test. The blue line and column indicate the repeated PBS + saline (10 mL/kg, subcutaneous)-treated group; the red line and column indicate the repeated PBS + morphine-treated group; the green line and column indicate the repeated IL-31 (5 μg/kg, intraperitoneal) + morphine-treated group; the yellow line and column indicate the repeated IL-31 (15 μg/kg, intraperitoneal) + morphine-treated group; and the purple line and column indicate the repeated IL-31 (50 μg/kg, intraperitoneal) + morphine-treated group. The results are presented as the mean ± S.E. of six mice (total, 30 mice). NS, not significant. # P < 0.05 compared with the PBS + saline-treated group (Student’s t-test). * P < 0.05, and ** P < 0.01 compared with the values of the PBS + morphine-treated group (Dunnett test).

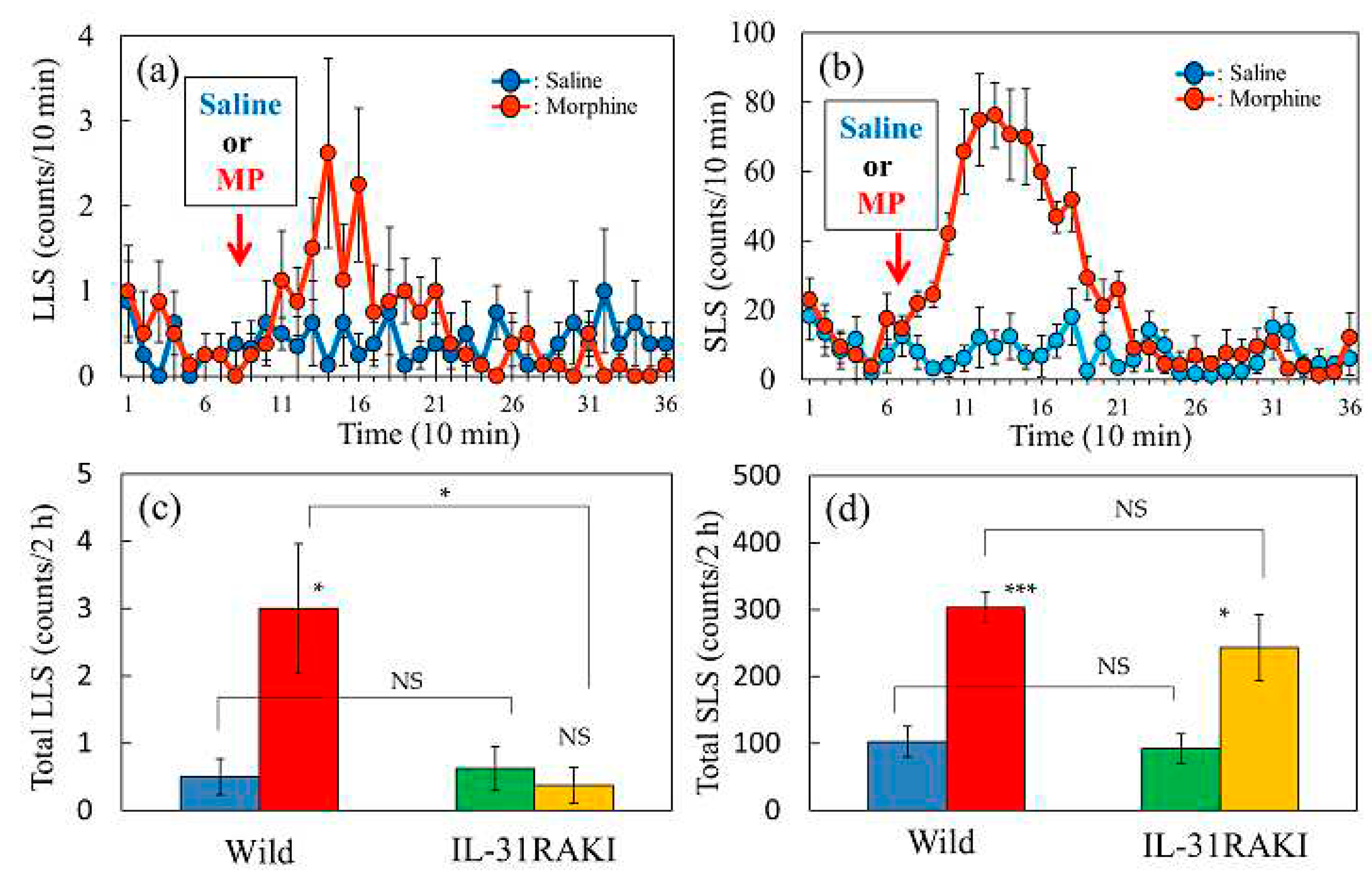
Figure 5.
Effect of morphine on LLS and SLS counts in wild-type and IL-31RAKI mice (a) Time-course changes in LLS after administration of morphine (MP, 3 mg/kg, subcutaneous) in wild-type mice. (b) Time-course changes in SLS after administration of morphine in wild-type mice. (c) Total LLS counts (counts/24 h) following saline- and morphine-administration in wild-type and IL-31RAKI mice. (d) Total SLS counts (counts/24 h) following saline and morphine administration in wild-type and IL-31RAKI mice. IL-31, interleukin-31; Wild, C57BL/6 mice; IL-31RAKI, IL-31RA deficient mice (C57BL/6 genetic background).The red arrows indicate the saline or morphine administration point. LLS, itch-associated scratching behavior; SLS, hygiene scratching. Values represent the mean ± S.E. of six mice (total 24 mice). NS, not significant, *P<0.05 and ***P<0.001 compared with each value of wild-type and IL-31KI mice (Tukey’s multiple comparison test).
Figure 5.
Effect of morphine on LLS and SLS counts in wild-type and IL-31RAKI mice (a) Time-course changes in LLS after administration of morphine (MP, 3 mg/kg, subcutaneous) in wild-type mice. (b) Time-course changes in SLS after administration of morphine in wild-type mice. (c) Total LLS counts (counts/24 h) following saline- and morphine-administration in wild-type and IL-31RAKI mice. (d) Total SLS counts (counts/24 h) following saline and morphine administration in wild-type and IL-31RAKI mice. IL-31, interleukin-31; Wild, C57BL/6 mice; IL-31RAKI, IL-31RA deficient mice (C57BL/6 genetic background).The red arrows indicate the saline or morphine administration point. LLS, itch-associated scratching behavior; SLS, hygiene scratching. Values represent the mean ± S.E. of six mice (total 24 mice). NS, not significant, *P<0.05 and ***P<0.001 compared with each value of wild-type and IL-31KI mice (Tukey’s multiple comparison test).
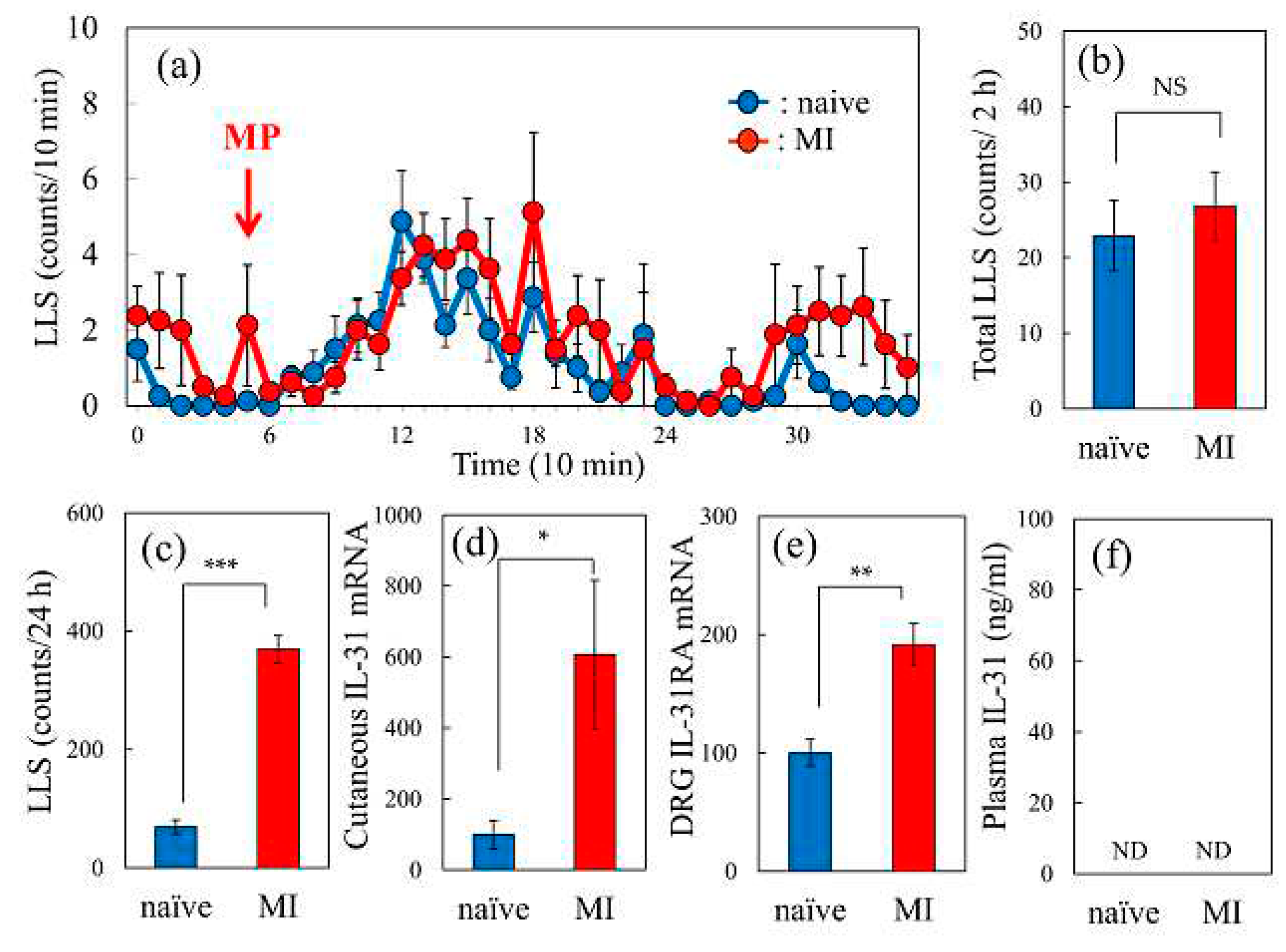
Figure 6.
Comparative effect of morphine-induced LLS counts in naïve and mite-infested mice. (a) Time-course change of morphine-induced LLS counts in naïve and mite-infested (MI) mice, (b) Total LLS counts for 2 h after morphine administration. (c) Total LLS counts for 24 h before morphine administration. (d) Cutaneous IL-31RA expression before morphine administration. (e) DRG neuronal IL-31RA expression levels before morphine administration. (f) Serum IL-31 concentration in naïve and mite-infested (MI) mice. The red arrows indicates the morphine (3 mg/kg, subcutaneous) administration point. The blue line indicate the LLS counts in naïve mice; the red line indicates LLS counts in mite-infested (MI) mice. Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. * P < 0.05, **P < 0.01, and ***P < 0.001 compared with the corresponding values of naïve mice (Student’s t-test).
Figure 6.
Comparative effect of morphine-induced LLS counts in naïve and mite-infested mice. (a) Time-course change of morphine-induced LLS counts in naïve and mite-infested (MI) mice, (b) Total LLS counts for 2 h after morphine administration. (c) Total LLS counts for 24 h before morphine administration. (d) Cutaneous IL-31RA expression before morphine administration. (e) DRG neuronal IL-31RA expression levels before morphine administration. (f) Serum IL-31 concentration in naïve and mite-infested (MI) mice. The red arrows indicates the morphine (3 mg/kg, subcutaneous) administration point. The blue line indicate the LLS counts in naïve mice; the red line indicates LLS counts in mite-infested (MI) mice. Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. * P < 0.05, **P < 0.01, and ***P < 0.001 compared with the corresponding values of naïve mice (Student’s t-test).
Figure 7.
Effect of an intravenous injection of IL-31 on morphine-induced LLS counts in BALB/c mice. (a) Time-course change of vehicle (phosphate buffer saline, PBS, 10 mL/kg, intravenous) or IL-31 (1 mg/kg, intravenous) on morphine-induced LLS counts in BALB/c mice. (b) Total LLS counts for 2 h after morphine administration. (c) Total LLS counts for 24 h before morphine administration. (d) Cutaneous IL-31RA expression levels before morphine administration. (e) DRG neuronal IL-31RA expression levels before morphine administration. (f) Serum IL-31 concentration 1 h after PBS or IL-31 administration. The red arrows indicate the morphine (3 mg/kg, subcutaneous) administration point. The blue arrows indicate the PBS or IL-31 (1 mg/kg, intravenous) administration point. The blue lines and columns indicate the values of the PBS-treated group, while the red lines and columns indicate the values of the IL-31-treated group. Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. *P < 0.05 compared with the value of the PBS-treated group (Student’s t-test).
Figure 7.
Effect of an intravenous injection of IL-31 on morphine-induced LLS counts in BALB/c mice. (a) Time-course change of vehicle (phosphate buffer saline, PBS, 10 mL/kg, intravenous) or IL-31 (1 mg/kg, intravenous) on morphine-induced LLS counts in BALB/c mice. (b) Total LLS counts for 2 h after morphine administration. (c) Total LLS counts for 24 h before morphine administration. (d) Cutaneous IL-31RA expression levels before morphine administration. (e) DRG neuronal IL-31RA expression levels before morphine administration. (f) Serum IL-31 concentration 1 h after PBS or IL-31 administration. The red arrows indicate the morphine (3 mg/kg, subcutaneous) administration point. The blue arrows indicate the PBS or IL-31 (1 mg/kg, intravenous) administration point. The blue lines and columns indicate the values of the PBS-treated group, while the red lines and columns indicate the values of the IL-31-treated group. Each value represents the mean ± S.E. of six mice (total, 24 mice). NS, not significant. *P < 0.05 compared with the value of the PBS-treated group (Student’s t-test).
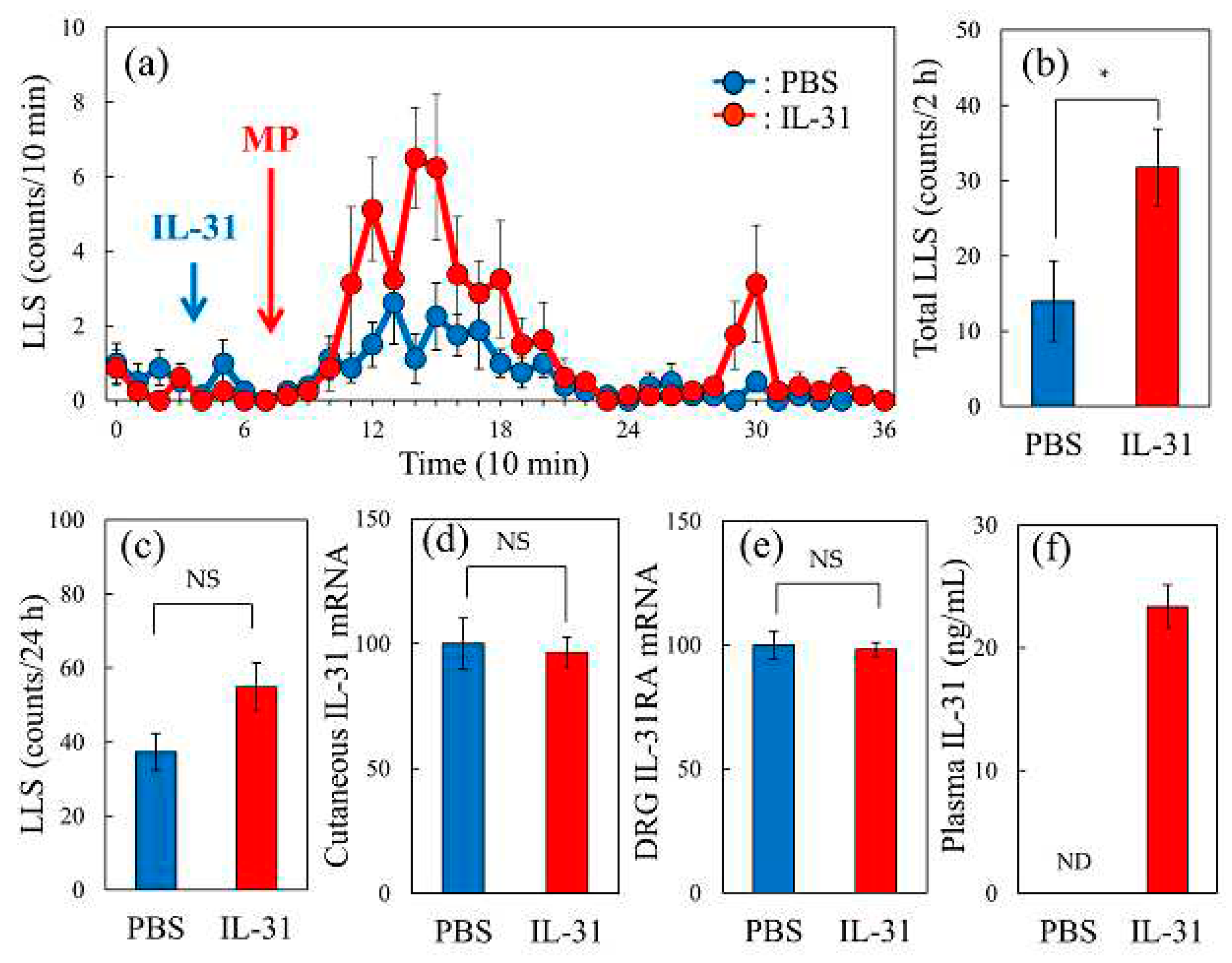
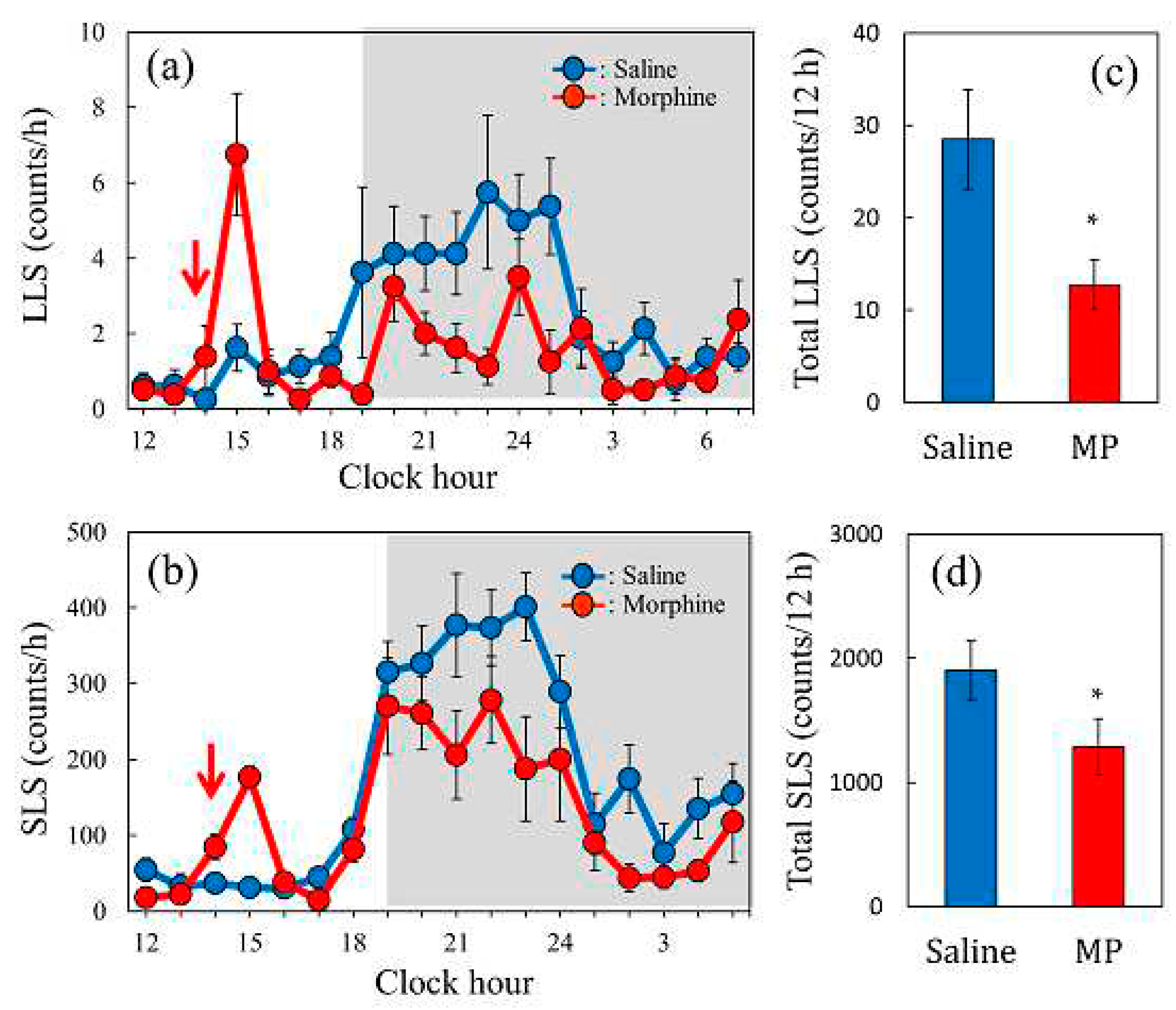
Figure 8.
Effect of a single dose of morphine on spontaneous scratching behavior in mice. (a) Time-course change of LLS (itch-associated scratching behavior) after a single dose of morphine (5 mg/kg, subcutaneous) administration in mice. (b) Time-course change of SLS (hygienic behavior) after morphine administration in mice. Each value represents the mean ± S.E. of six mice. The red arrow indicates saline or morphine administration. The lateral axis indicates the clock hour, and the shaded area represents dark phase (7:00 p.m. to 7:00 a.m.). (c) Total LLS counts for 12 h from 6 h after morphine administration. (d) Total SLS counts for 12 h from 6 h after morphine administration. Each value represents the mean ± S.E. of six mice (total 12 mice). *P < 0.05, **P < 0.01 compared with each value of the saline-treated group (Student’s t-test).
Figure 8.
Effect of a single dose of morphine on spontaneous scratching behavior in mice. (a) Time-course change of LLS (itch-associated scratching behavior) after a single dose of morphine (5 mg/kg, subcutaneous) administration in mice. (b) Time-course change of SLS (hygienic behavior) after morphine administration in mice. Each value represents the mean ± S.E. of six mice. The red arrow indicates saline or morphine administration. The lateral axis indicates the clock hour, and the shaded area represents dark phase (7:00 p.m. to 7:00 a.m.). (c) Total LLS counts for 12 h from 6 h after morphine administration. (d) Total SLS counts for 12 h from 6 h after morphine administration. Each value represents the mean ± S.E. of six mice (total 12 mice). *P < 0.05, **P < 0.01 compared with each value of the saline-treated group (Student’s t-test).
Figure 9.
Effect of repeated administration of morphine on morphine-induced scratching counts and antinociceptive activity in mice. (a) Time-course changes of repeated administration on morphine (5 mg/kg, subcutaneous)-induced LLS (itch-associated scratching behavior) in mice. The red arrows indicate vehicle (saline, 10 mL/kg, subcutaneous)or morphine (5 mg/kg, subcutaneous, every 3 h for 27 h) administration, the lateral axis indicates the clock hour, and the shaded area represents dark phase (19:00 to 7:00), The blue line indicates the saline-administered group; the red line indicate morphine-administration group. The red arrows indicate the saline or morphine administration points. The blue arrow indicates vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intravenous) or IL-31 (1 mg/kg, intravenous) administration point. (b) Total LLS (counts/3 h) of repeated administration of saline or morphine of the first, third or ninth administration group. (c) Total SLS (counts/3 h) of repeated administration of morphine of the first, third or ninth administration group. (d) Antinociception index of repeated administration of saline or morphine of the first, third or ninth administration group. The blue columns indicate the response of the first morphine-administration group; the red columns indicate the third morphine-administration group; and the green columns indicate the ninth morphine-administration group. The results are presented as the mean ± S.E. of 6 mice *P < 0.05, **P < 0.01 and ***P < 0.001 compared with those of the values of first morphine-administration group. Results are presented as the mean ± S.E. of 6 mice (total, 12 mice) (Dunnett test). (e) Effect of repeated administration of morphine on IL-31 (1 mg/kg, intravenous) induced LLS counts in mice. Each value represents the mean ± S.E. of six mice (total, 12 mice). *** P < 0.001 compared with the PBS-administration group (Student’s t-test).
Figure 9.
Effect of repeated administration of morphine on morphine-induced scratching counts and antinociceptive activity in mice. (a) Time-course changes of repeated administration on morphine (5 mg/kg, subcutaneous)-induced LLS (itch-associated scratching behavior) in mice. The red arrows indicate vehicle (saline, 10 mL/kg, subcutaneous)or morphine (5 mg/kg, subcutaneous, every 3 h for 27 h) administration, the lateral axis indicates the clock hour, and the shaded area represents dark phase (19:00 to 7:00), The blue line indicates the saline-administered group; the red line indicate morphine-administration group. The red arrows indicate the saline or morphine administration points. The blue arrow indicates vehicle (phosphate-buffered saline, PBS, 10 mL/kg, intravenous) or IL-31 (1 mg/kg, intravenous) administration point. (b) Total LLS (counts/3 h) of repeated administration of saline or morphine of the first, third or ninth administration group. (c) Total SLS (counts/3 h) of repeated administration of morphine of the first, third or ninth administration group. (d) Antinociception index of repeated administration of saline or morphine of the first, third or ninth administration group. The blue columns indicate the response of the first morphine-administration group; the red columns indicate the third morphine-administration group; and the green columns indicate the ninth morphine-administration group. The results are presented as the mean ± S.E. of 6 mice *P < 0.05, **P < 0.01 and ***P < 0.001 compared with those of the values of first morphine-administration group. Results are presented as the mean ± S.E. of 6 mice (total, 12 mice) (Dunnett test). (e) Effect of repeated administration of morphine on IL-31 (1 mg/kg, intravenous) induced LLS counts in mice. Each value represents the mean ± S.E. of six mice (total, 12 mice). *** P < 0.001 compared with the PBS-administration group (Student’s t-test).
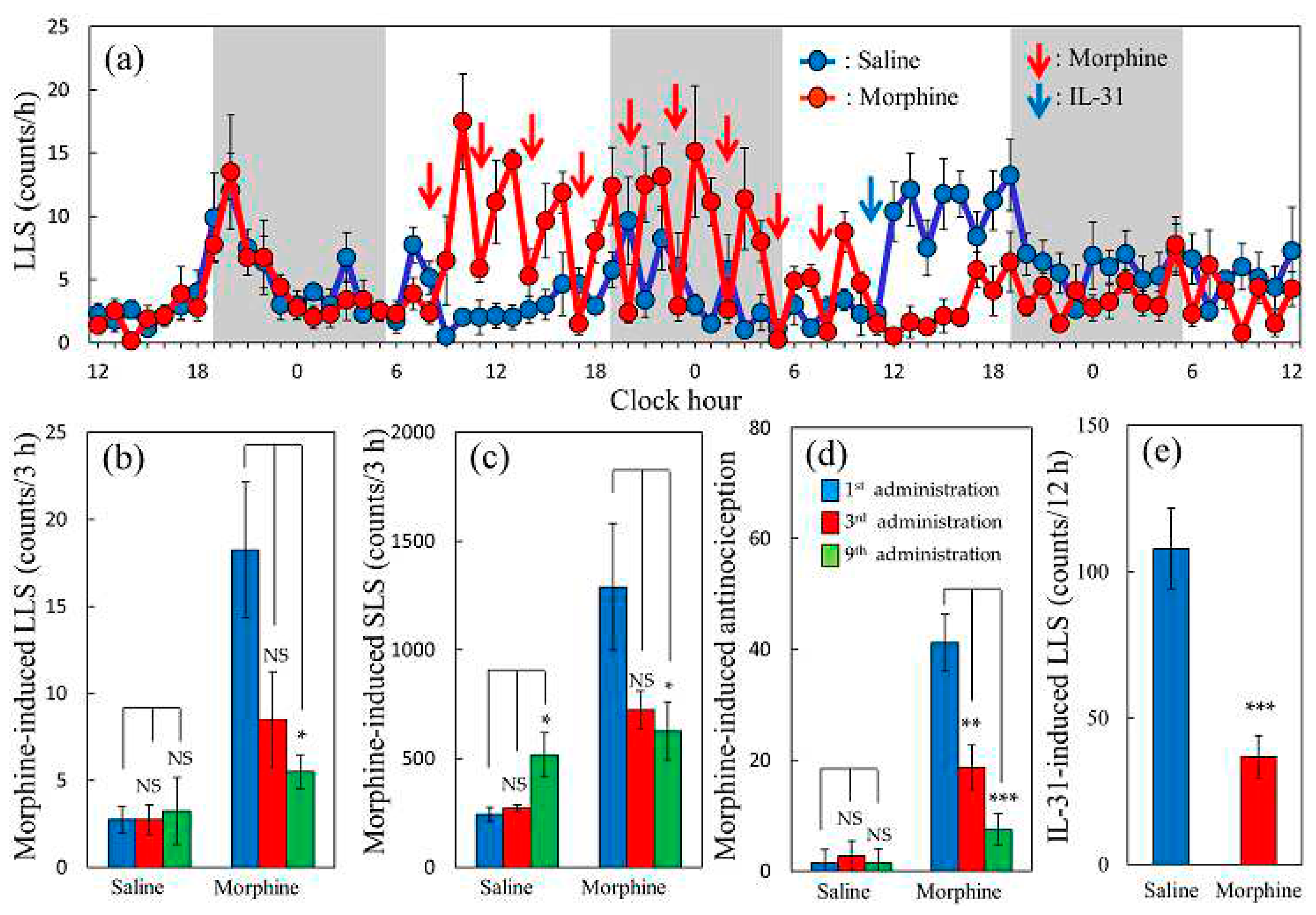
Figure 10.
Effects of combined administration of IL-31 and morphine on morphine-induced antinociceptive tolerance and DRG neuronal IL-31RA mRNA expression in mice. (a) Time-course change of combined administration of morphine (10 mg/kg, subcutaneous, once a day for 5 days) and vehicle (phosphate-buffered saline, PBS 10 mL/kg, intraperitoneal, every 12 h for 5 days) or IL-31 50 μg/kg, intraperitoneal, every 12 h for 5 days) on morphine-induced antinociceptive tolerance. Each column represents the mean ± S.E. of six mice (total, 12 mice) NS, not significant, ** P<0.01 and *** P<0.001 compared with the values of first morphine-administration of each PBS or IL-31 treated group (Dunnet-test). (b) Time-course change of combined administration of morphine and IL-31 on DRG neuronal IL-31RA expression. The blue line indicates the PBS administered group; the red line indicate IL-31 administration group. Each column represents the mean ± S.E. of six mice (total, 48 mice). NS, not significant, ** P < 0.01 and *** P < 0.001 compared with the corresponding values of each PBS + morphine-treated group (Student’s t-test with Bonferroni correction).
Figure 10.
Effects of combined administration of IL-31 and morphine on morphine-induced antinociceptive tolerance and DRG neuronal IL-31RA mRNA expression in mice. (a) Time-course change of combined administration of morphine (10 mg/kg, subcutaneous, once a day for 5 days) and vehicle (phosphate-buffered saline, PBS 10 mL/kg, intraperitoneal, every 12 h for 5 days) or IL-31 50 μg/kg, intraperitoneal, every 12 h for 5 days) on morphine-induced antinociceptive tolerance. Each column represents the mean ± S.E. of six mice (total, 12 mice) NS, not significant, ** P<0.01 and *** P<0.001 compared with the values of first morphine-administration of each PBS or IL-31 treated group (Dunnet-test). (b) Time-course change of combined administration of morphine and IL-31 on DRG neuronal IL-31RA expression. The blue line indicates the PBS administered group; the red line indicate IL-31 administration group. Each column represents the mean ± S.E. of six mice (total, 48 mice). NS, not significant, ** P < 0.01 and *** P < 0.001 compared with the corresponding values of each PBS + morphine-treated group (Student’s t-test with Bonferroni correction).
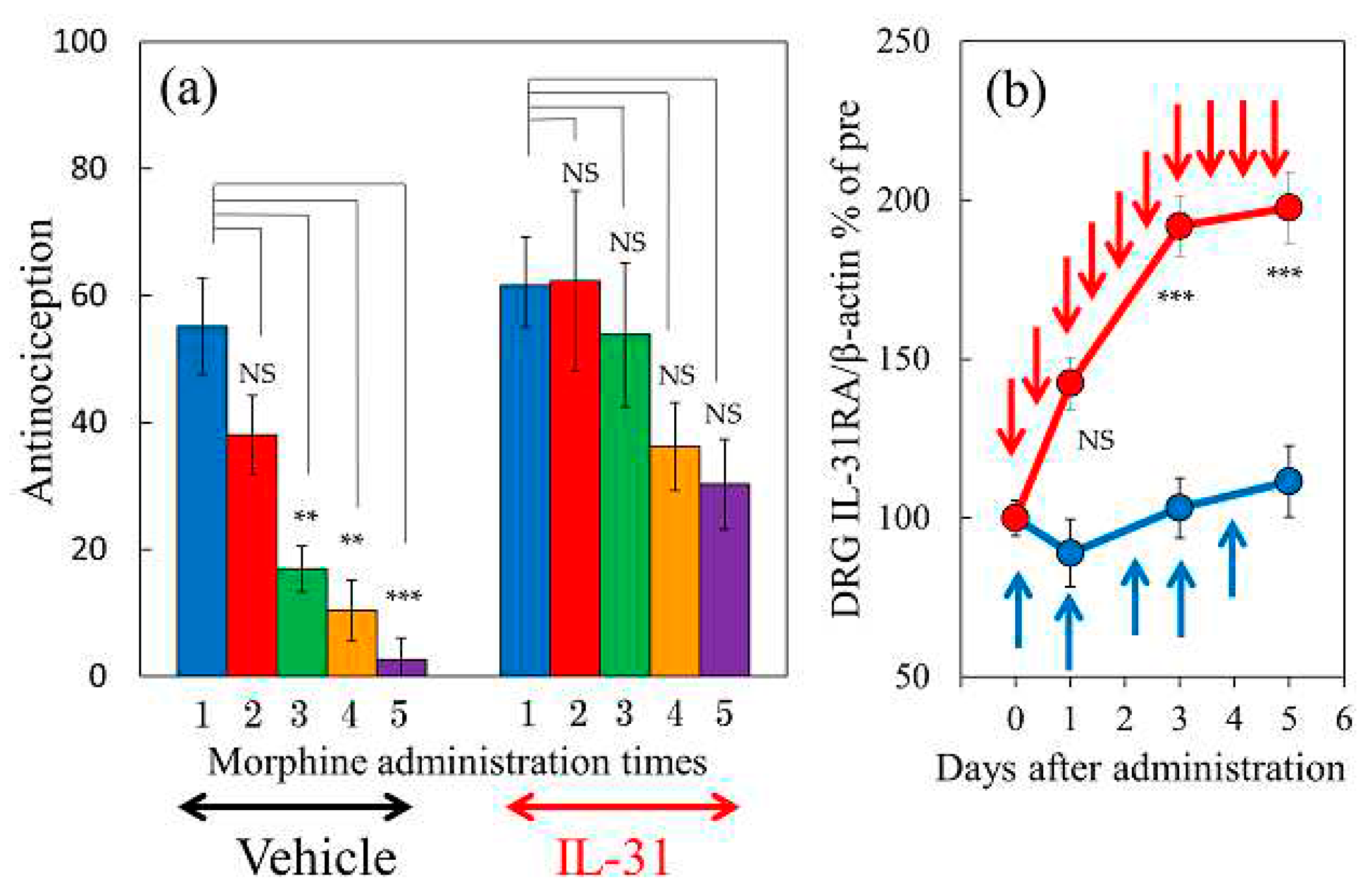
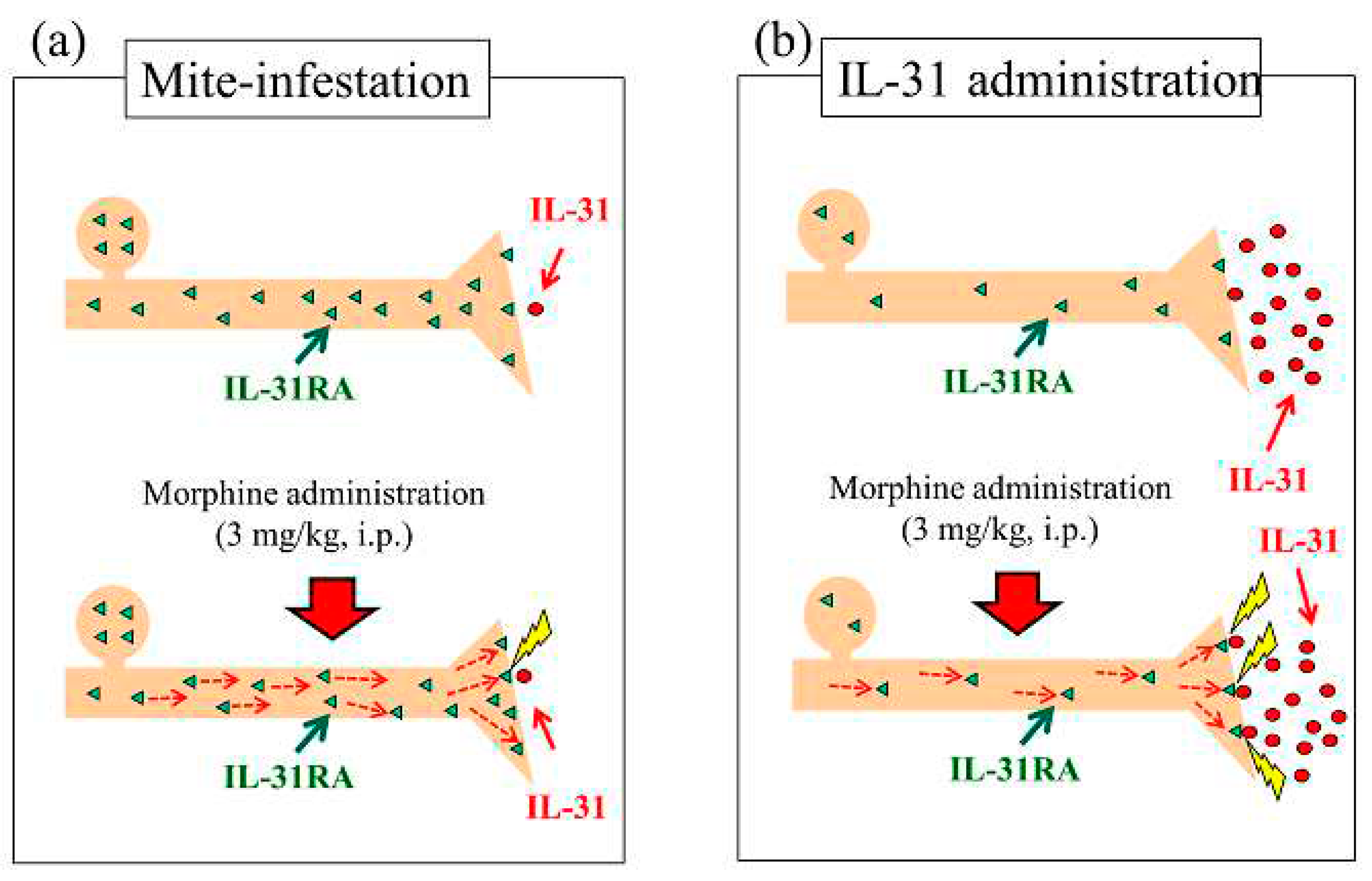
Figure 11.
Schematic of the association of intraneural IL-31RA and blood IL-31 concentration on naïve or mite-infested BALB/c mice. (a) Mite-infested mice were the result of co-housing mice with skin-lesioned NC/Nga mice. Mite-infestation significantly increased DRG neuronal IL-31RA expression and not the blood concentration of IL-31 (upside). The morphine-induced LLS counts did not change in mite-infested mice (downside). (b) IL-31 treatment (1 mg/kg, intravenous injection) 1 h before morphine administration; IL-31 administration does not increase IL-31RA expression in the skin and neuronal DRG; however, it markedly increases the blood concentration of IL-31 (upside). Morphine-induced LLS counts were significantly enhanced compared with those of the saline-treated group. These results suggest that morphine may promote the exocytosis of IL-31RA in peripheral sensory nerve endings.
Figure 11.
Schematic of the association of intraneural IL-31RA and blood IL-31 concentration on naïve or mite-infested BALB/c mice. (a) Mite-infested mice were the result of co-housing mice with skin-lesioned NC/Nga mice. Mite-infestation significantly increased DRG neuronal IL-31RA expression and not the blood concentration of IL-31 (upside). The morphine-induced LLS counts did not change in mite-infested mice (downside). (b) IL-31 treatment (1 mg/kg, intravenous injection) 1 h before morphine administration; IL-31 administration does not increase IL-31RA expression in the skin and neuronal DRG; however, it markedly increases the blood concentration of IL-31 (upside). Morphine-induced LLS counts were significantly enhanced compared with those of the saline-treated group. These results suggest that morphine may promote the exocytosis of IL-31RA in peripheral sensory nerve endings.
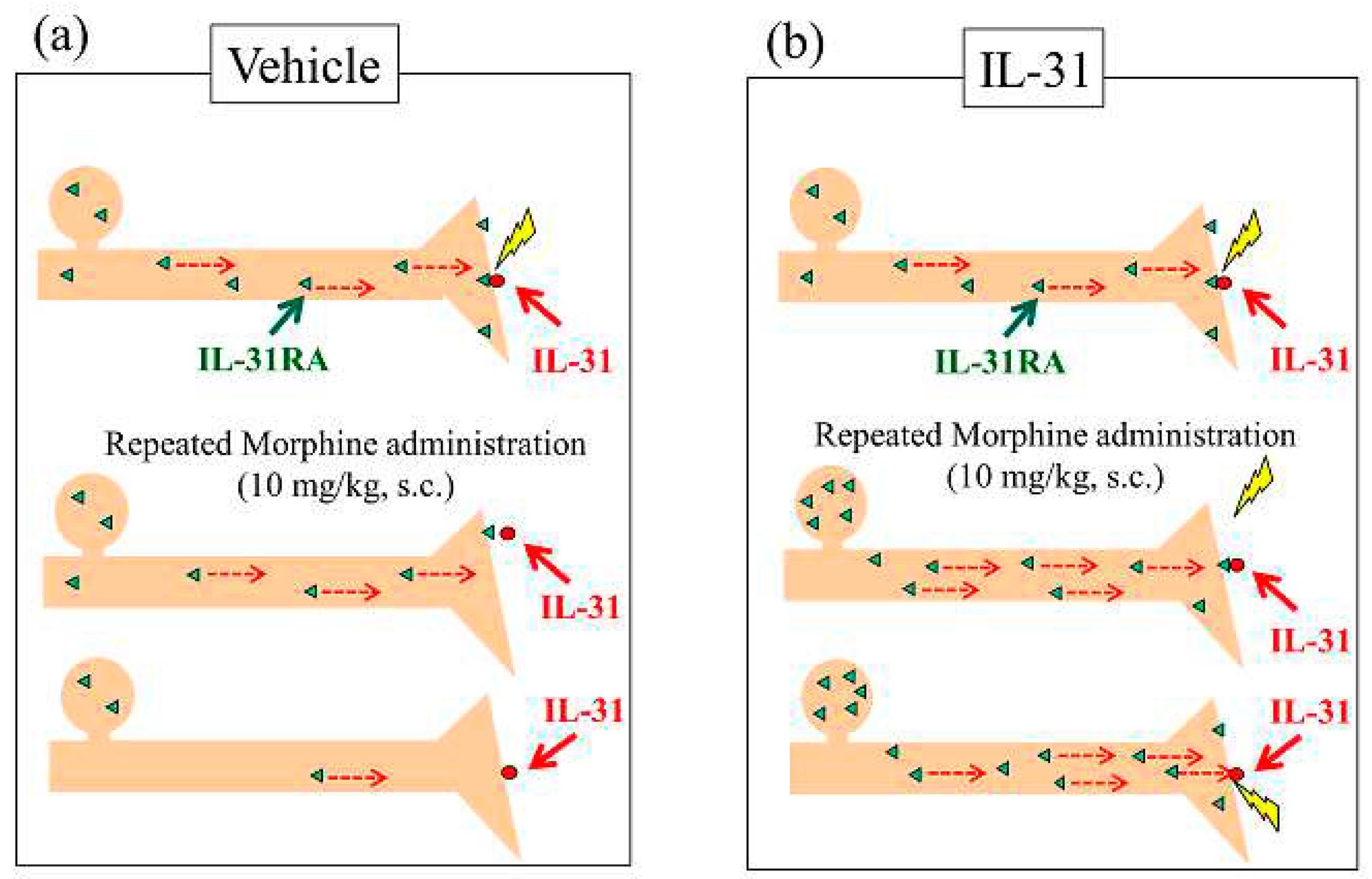
Figure 12.
Schematic of intraneural IL-31RA content upon repeated administration of morphine and the effect of combination treatment of IL-31 in mice. (a) Effect of repeated administration of morphine. IL-31RA content in the presynaptic nerve gradually decreases upon repeated morphine administrations (middle and down side). LLS counts and antinociceptive activity also decrease, and analgesic tolerance is formed. (b) Effect of combination treatment of repeated administration of morphine and IL-31. IL-31RA content in the presynaptic nerve gradually decreases upon repeated morphine administrations; however, IL-31RA decrease in the presynaptic nerve is controlled by the increase in IL-31RA expression by IL-31, and the analgesic tolerance formation relaxes (middle and down side).
Figure 12.
Schematic of intraneural IL-31RA content upon repeated administration of morphine and the effect of combination treatment of IL-31 in mice. (a) Effect of repeated administration of morphine. IL-31RA content in the presynaptic nerve gradually decreases upon repeated morphine administrations (middle and down side). LLS counts and antinociceptive activity also decrease, and analgesic tolerance is formed. (b) Effect of combination treatment of repeated administration of morphine and IL-31. IL-31RA content in the presynaptic nerve gradually decreases upon repeated morphine administrations; however, IL-31RA decrease in the presynaptic nerve is controlled by the increase in IL-31RA expression by IL-31, and the analgesic tolerance formation relaxes (middle and down side).