Submitted:
20 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
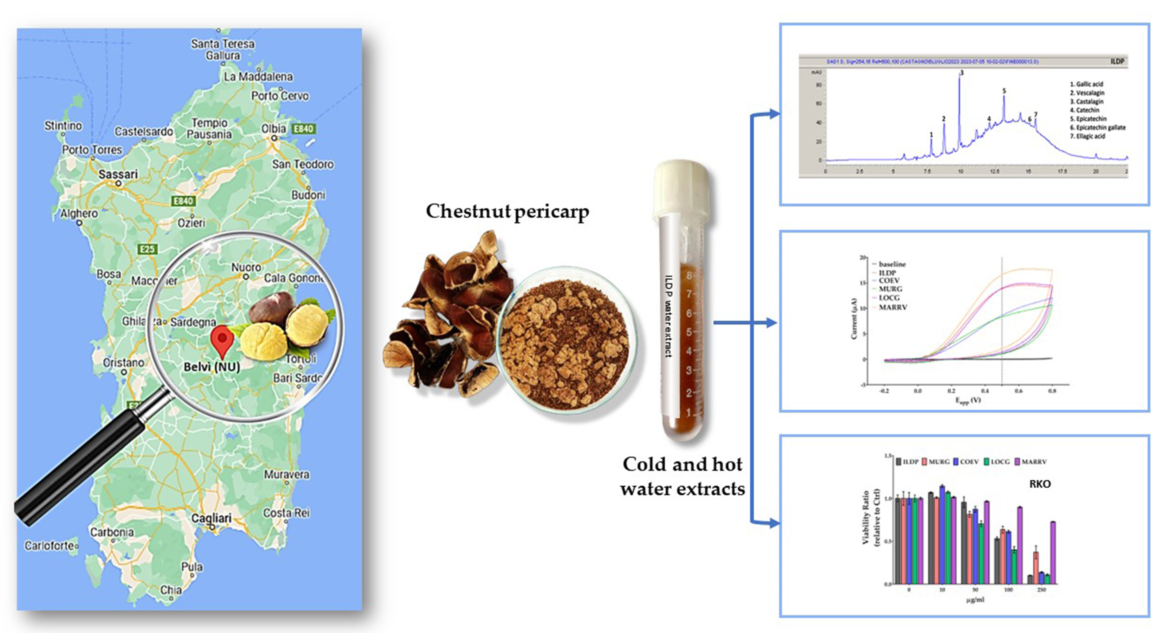
2.1. Phytochemical content of cold and hot water pericarp extracts
2.2. Antioxidant capacity determination by DPPH and ABTS tests
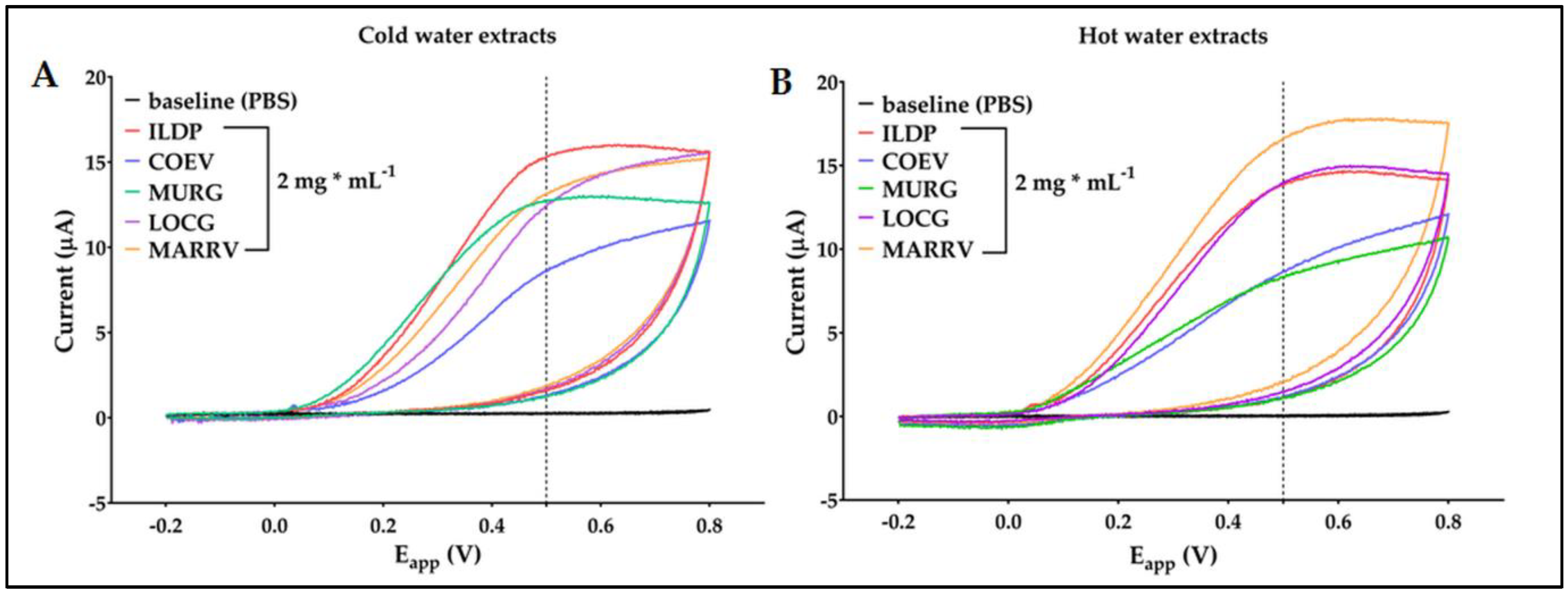
2.4. Electrochemical characterization of chestnut pericarp extracts
2.4. Chemical characterization of chestnut pericarp extracts
- -
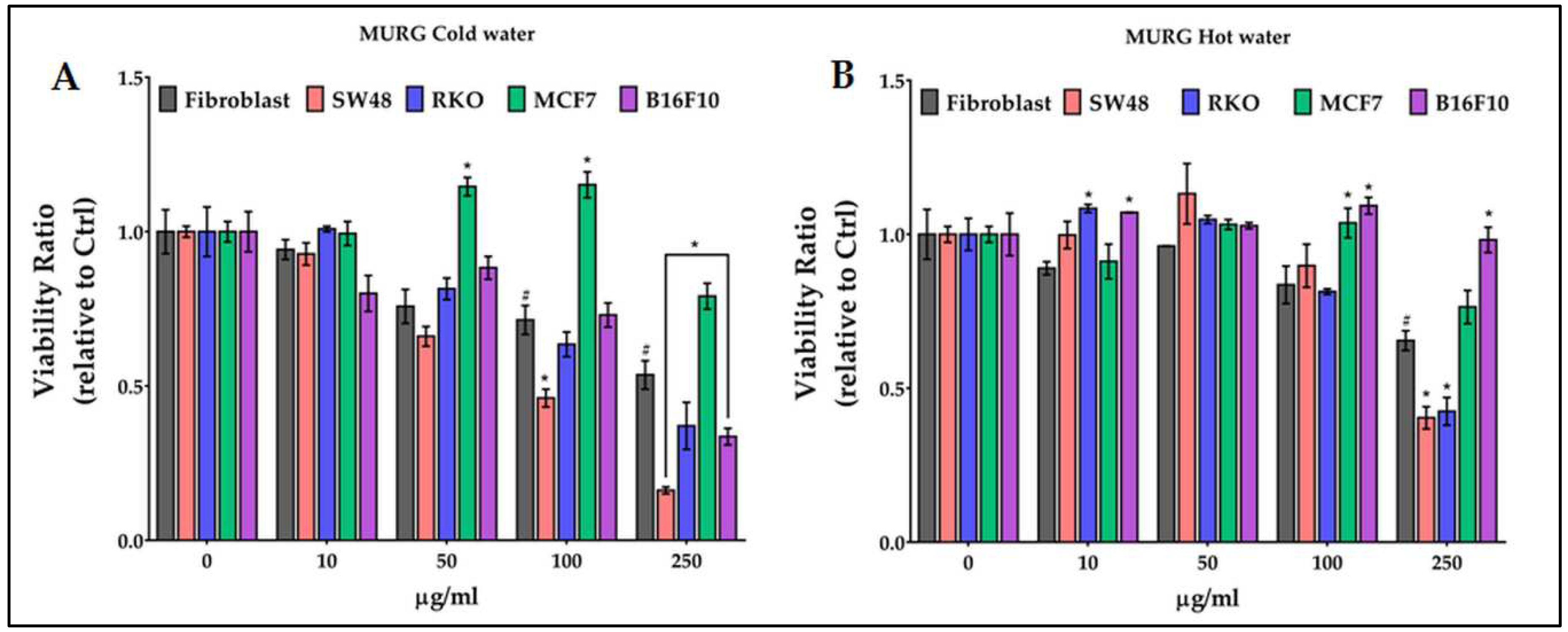
- vescalagin, castalagin and epicatechin characterize the MURG extracts, regardless of the temperature used for extraction;
- -
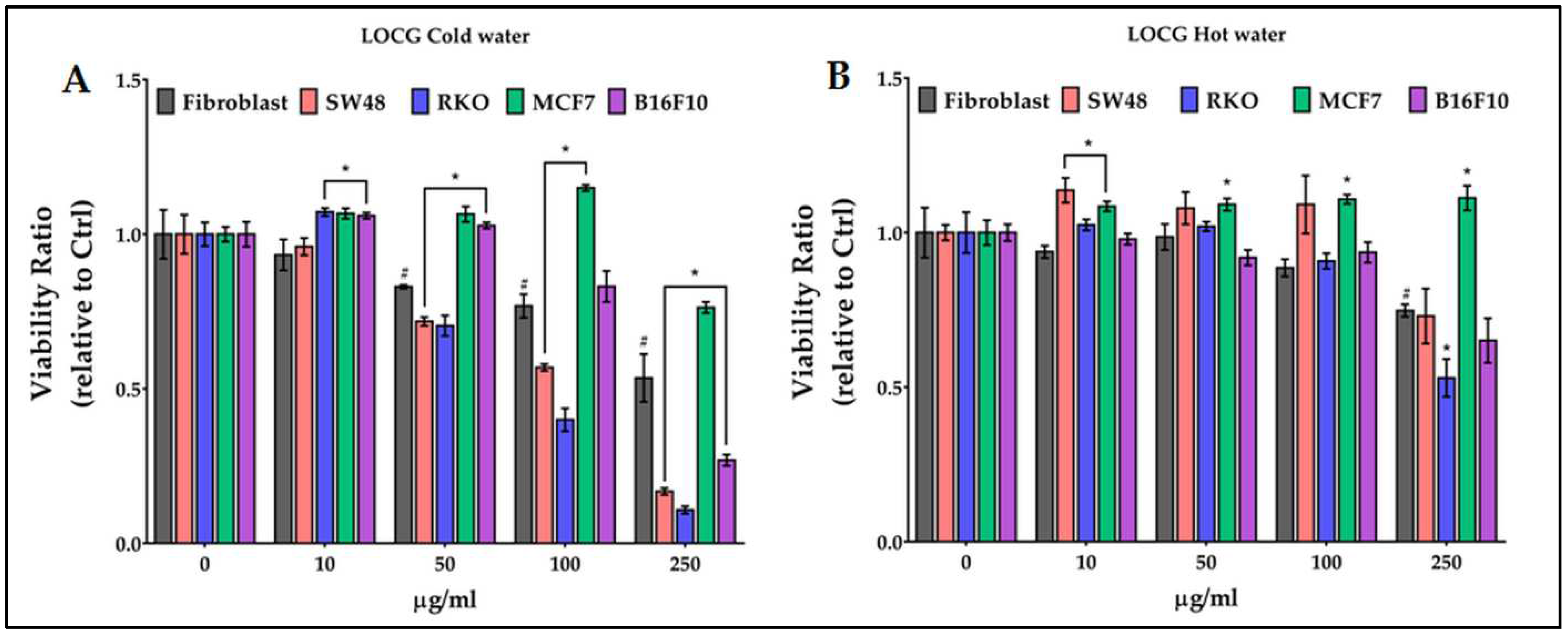
- LOCG cold extracts are characterized by gallic acid, vescalagin and castalagin, while the hot ones have a high content only of vescalagin, castalagin and, particularly, of epicatechin;
- -
- ILDP cold extracts can be distinguished by their content of gallic acid, catechin and epicatechin, whereas the hot extracts by vescalagin, castalagin and epicatechin;
- -
- COEV is distinguished by a low content of all compounds (thus confirming the results of the spectrophotometric assay) if the extraction takes place in cold water, and by a prevalence of castalagin and vescalagin when the extractant is hot water.
2.5. Contribution of main polyphenols to the total antioxidant activity of chestnut pericarp extracts
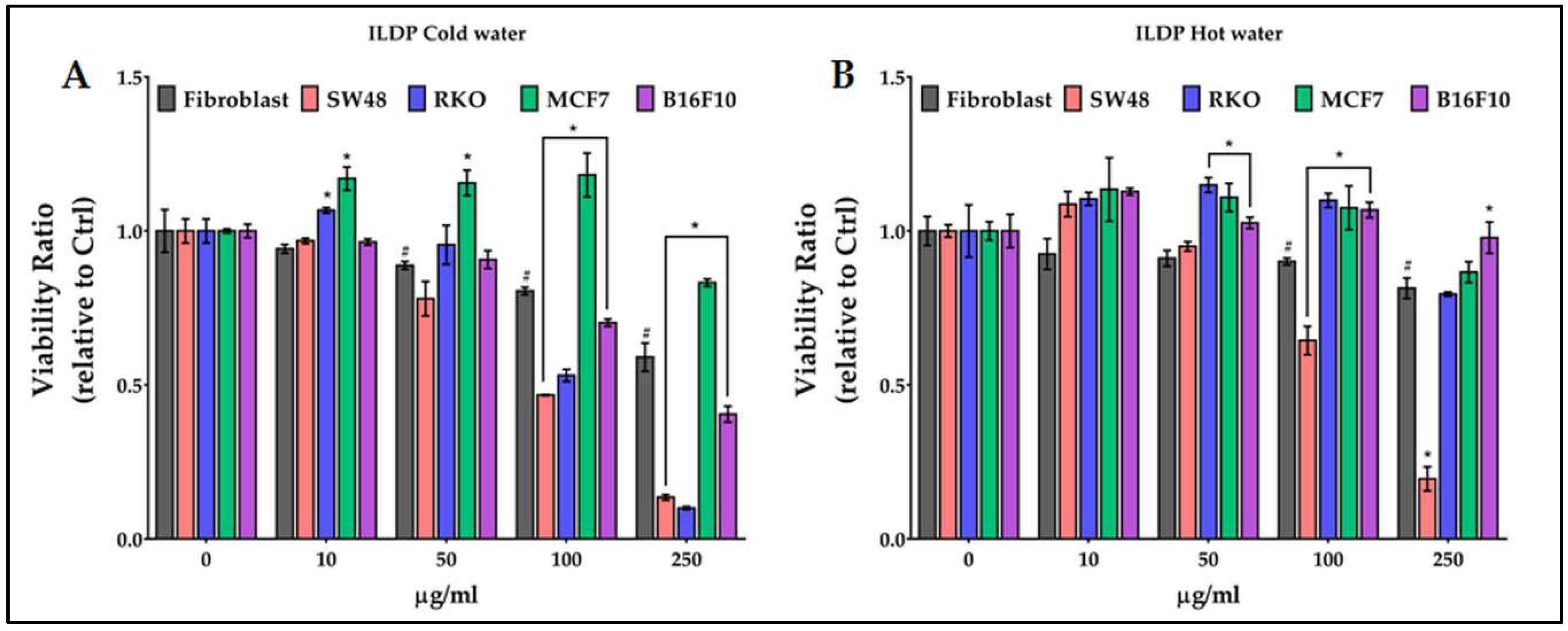
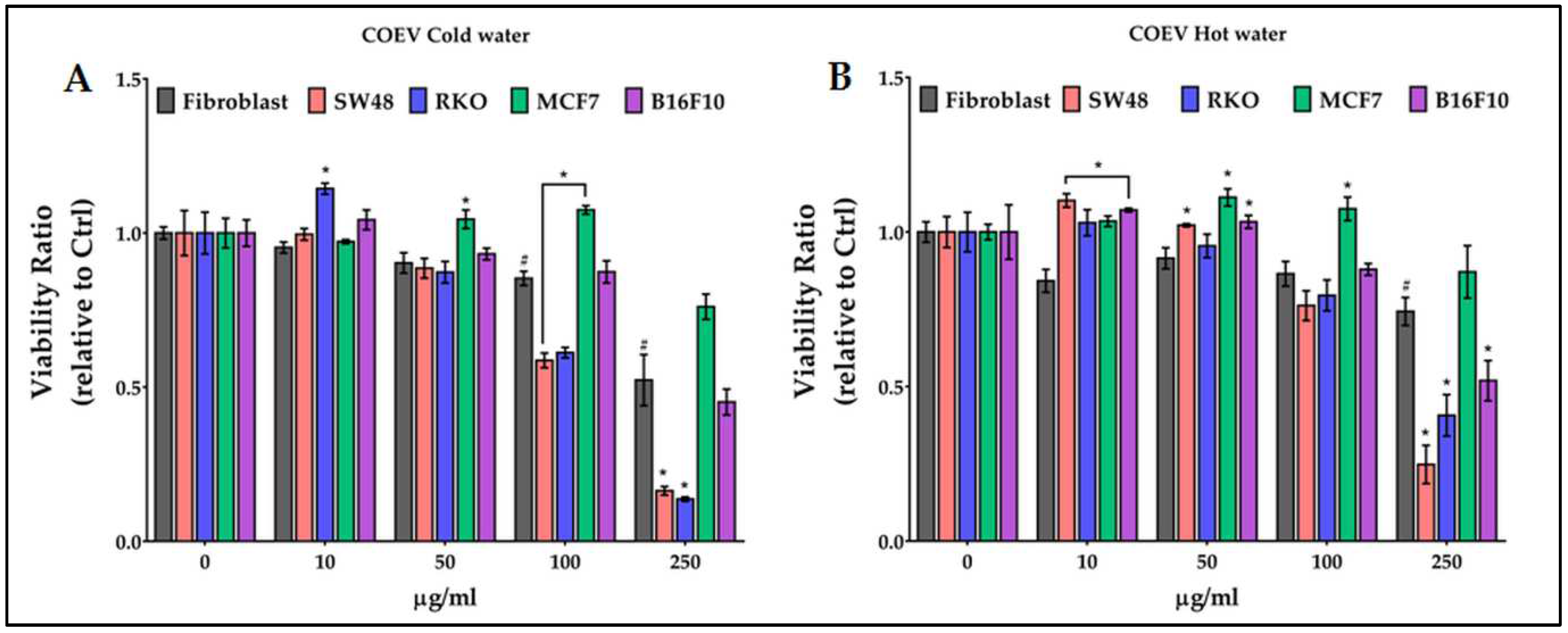
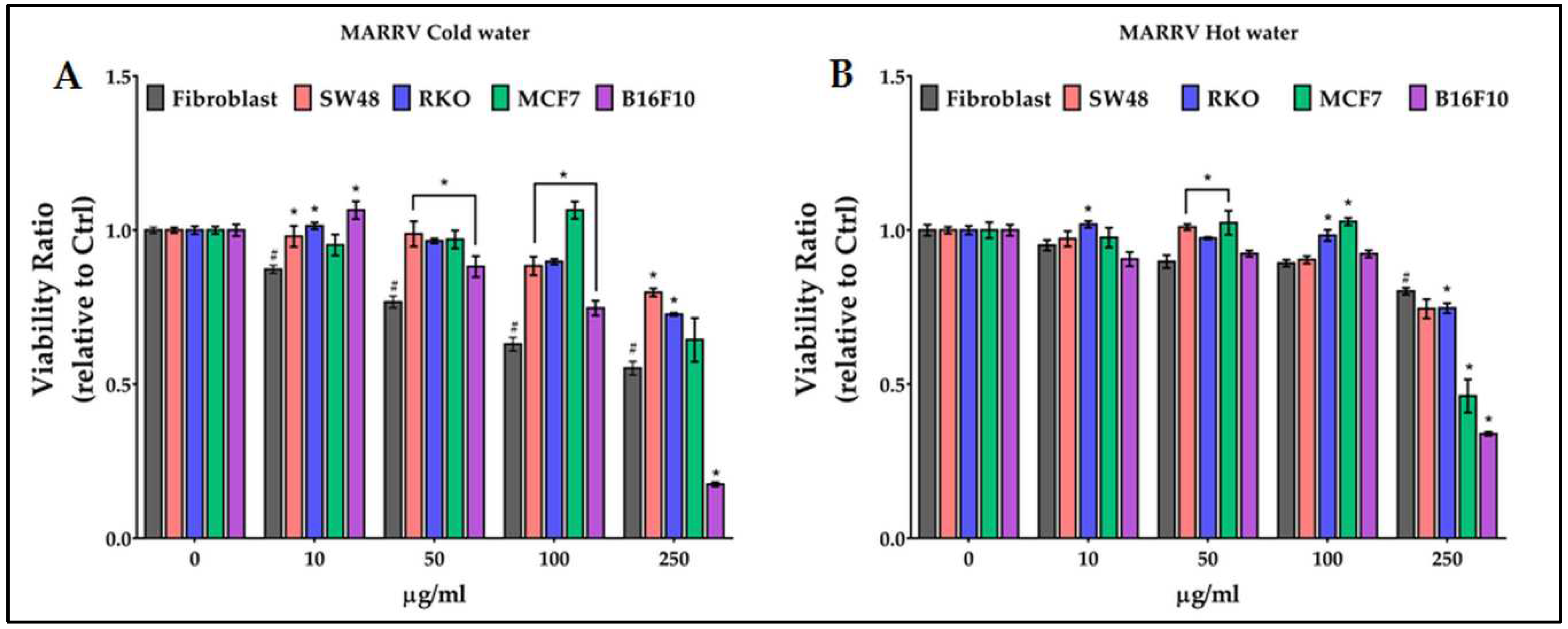
2.6. Antiproliferative activity of chestnut pericarp extracts on normal and cancer cells
3. Discussion
4. Materials and Methods
| Accession/variety | Origin | Elevation (m a.s.l) |
Harvest time | Acronym |
|---|---|---|---|---|
| MIGHELI URRU G | Belvì | 822 | October, second decade |
MURG |
| LOCCHEDDU G | Belvì | 812 | October, second decade |
LOCG |
| ILDUBBA P | Belvì | 849 | October, second decade |
ILDP |
| COESERRA V | Belvì | 686 | October, third decade |
COEV |
| MARRONE di Marradi V | Belvì | 780 | October, second decade |
MARRV |
4.1. Plant Material and Fruit Sampling
4.2. Extraction of phenolic compounds
4.3. Analytical tests
4.3.1. Determination of total phenolic content
4.3.2. Determination of total flavonoid content
4.3.3. Determination of condensed tannin content
4.3.4. Determination of antioxidant capacity by DPPH and ABTS assays
4.3.5. Determination of antioxidant capacity by electrochemical method
4.4. HPLC analysis of phenolic compounds
4.5. Cell culture and biological assays
4.5.1. MTT assay
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Poljak, I.; Idžojtić, M.; Šatović, Z.; Ježić, M.; Ćurković-Perica, M.; Simovski, B.; Acevski, J.; Liber, Z. Genetic Diversity of the Sweet Chestnut (Castanea Sativa Mill.) in Central Europe and the Western Part of the Balkan Peninsula and Evidence of Marron Genotype Introgression into Wild Populations. Tree Genetics & Genomes 2017, 13, 18–18. [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating Progress of Chestnut Quality: A Review of Recent Developments. Trends in Food Science & Technology 2021, 113, 245–254. [CrossRef]
- Piccolo, E.L.; Landi, M.; Ceccanti, C.; Mininni, A.N.; Marchetti, L.; Massai, R.; Guidi, L.; Remorini, D. Nutritional and Nutraceutical Properties of Raw and Traditionally Obtained Flour from Chestnut Fruit Grown in Tuscany. Eur Food Res Technol 2020, 246, 1867–1876. [CrossRef]
- De Vasconcelos, M.D.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the Potential of Chestnut (Castanea Sativa Mill.) Fruit Pericarp and Integument as a Source of Tocopherols, Pigments and Polyphenols. Industrial Crops and Products 2010, 31, 301–311. [CrossRef]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut Shells (Italian Cultivar “Marrone Di Roccadaspide” PGI): Antioxidant Activity and Chemical Investigation with in Depth LC-HRMS/MSn Rationalization of Tannins. Food Research International 2020, 129, 108787. [CrossRef]
- Braga, N.; Rodrigues, F.; P.P. Oliveira, M.B. Castanea Sativa by-Products: A Review on Added Value and Sustainable Application. Natural Product Research 2015, 29, 1–18. [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive Phenolic Components and Potential Health Effects of Chestnut Shell: A Review. J. Food Biochem. 2021, 45. [CrossRef]
- Zhu, T.; Shen, Q.; Xu, Y.; Li, C. Ionic Liquid and Ultrasound-Assisted Extraction of Chestnut Shell Pigment with Good Hair Dyeing Capability. Journal of Cleaner Production 2022, 335, 130195. [CrossRef]
- Santos, M.J.; Pinto, T.; Vilela, A. Sweet Chestnut (Castanea Sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology. Foods 2022, 11, 4052. [CrossRef]
- Pinto, D.; Moreira, M.M.; Vieira, E.F.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods 2023, 12, 640. [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; C. F. R. Ferreira, I.; et al. Evaluation of the Phenolic Profile of Castanea Sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [CrossRef]
- Cacciola, N.A.; Squillaci, G.; D’Apolito, M.; Petillo, O.; Veraldi, F.; La Cara, F.; Peluso, G.; Margarucci, S.; Morana, A. Castanea Sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects. Molecules 2019, 24, 3401. [CrossRef]
- Schirra, M.; Palma, A.; Barberis, A.; Angioni, A.; Garau, V.L.; Cabras, P.; D’Aquino, S. Postinfection Activity, Residue Levels, and Persistence of Azoxystrobin, Fludioxonil, and Pyrimethanil Applied Alone or in Combination with Heat and Imazalil for Green Mold Control on Inoculated Oranges. Journal of Agricultural and Food Chemistry 2010, 58, 3661–3666. [CrossRef]
- Fadda, A.; Barberis, A.; D’Aquino, S.; Palma, A.; Angioni, A.; Lai, F.; Schirra, M. Residue Levels and Performance of Potassium Sorbate and Thiabendazole and Their Co-Application against Blue Mold of Apples When Applied as Water Dip Treatments at 20 or 53 Degrees C. Postharvest Biology and Technology 2015, 106, 33–43. [CrossRef]
- Fadda, A.; Virdis, A.; Barberis, A.; Melito, S. Variation in Secondary Metabolites Contents of Spinoso Sardo Artichoke (Cynara Cardunculus l.) under Different Day Lengths. Turkish Journal of Agriculture and Forestry 2018, 42, 372–381. [CrossRef]
- Ramli, I.; Posadino, A.M.; Zerizer, S.; Spissu, Y.; Barberis, A.; Djeghim, H.; Azara, E.; Bensouici, C.; Kabouche, Z.; Rebbas, K.; et al. Low Concentrations of Ambrosia Maritima L. Phenolic Extract Protect Endothelial Cells from Oxidative Cell Death Induced by H2O2 and Sera from Crohn’s Disease Patients. Journal of Ethnopharmacology 2023, 300, 115722. [CrossRef]
- Spissu, Y.; Gil, K.A.; Dore, A.; Sanna, G.; Palmieri, G.; Sanna, A.; Cossu, M.; Belhadj, F.; Gharbi, B.; Pinna, M.B.; et al. Anti- and Pro-Oxidant Activity of Polyphenols Extracts of Syrah and Chardonnay Grapevine Pomaces on Melanoma Cancer Cells. Antioxidants 2023, 12. [CrossRef]
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M.G.; Santona, M.; Zara, S. Antimicrobial and Antibiofilm Activities of Pomegranate Peel Phenolic Compounds: Varietal Screening Through a Multivariate Approach. Journal of Bioresources and Bioproducts 2023, S2369969823000154. [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of Solid Wastes from Chestnut Industry Processing: Extraction and Optimization of Polyphenols, Tannins and Ellagitannins and Its Potential for Adhesives, Cosmetic and Pharmaceutical Industry. Waste Management 2016, 48, 457–464. [CrossRef]
- Fernández-Agulló, A.; Freire, M.S.; Antorrena, G.; Pereira, J.A.; González-Álvarez, J. Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from Chestnut ( Castanea Sativa ) Bur and Shell. Separation Science and Technology 2014, 49, 267–277. [CrossRef]
- Jung, B.S.; Lee, N.-K.; Na, D.S.; Yu, H.H.; Paik, H.-D. Comparative Analysis of the Antioxidant and Anticancer Activities of Chestnut Inner Shell Extracts Prepared with Various Solvents. Journal of the Science of Food and Agriculture 2016, 96, 2097–2102. [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut ( Castanea Sativa Mill.) Industrial Wastes as a Valued Bioresource for the Production of Active Ingredients. Process Biochemistry 2018, 64, 228–236. [CrossRef]
- Vella, F.M.; De Masi, L.; Calandrelli, R.; Morana, A.; Laratta, B. Valorization of the Agro-Forestry Wastes from Italian Chestnut Cultivars for the Recovery of Bioactive Compounds. Eur Food Res Technol 2019, 245, 2679–2686. [CrossRef]
- Galiñanes, C.; Freire, M.S.; González-Álvarez, J. Antioxidant Activity of Phenolic Extracts from Chestnut Fruit and Forest Industries Residues. Eur. J. Wood Prod. 2015, 73, 651–659. [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10. [CrossRef]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the Cyclic Voltammetry Measurements and DPPH• Spectrophotometric Assay to Determine the Antioxidant Capacity of Food-Grade Oenological Tannins. Molecules 2019, 24. [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11. [CrossRef]
- Zheng, Y.; Karimi-Maleh, H.; Fu, L. Evaluation of Antioxidants Using Electrochemical Sensors: A Bibliometric Analysis. Sensors 2022, 22. [CrossRef]
- Raja, A.N.; Annu; Singh, K.; Jain, R. Ultrasensitive Quantification of Ellagic Acid Using Gr/Bi2O3/GCE as Voltammetric Sensor. International Journal of Electrochemical Science 2020, 15, 10040–10057. [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Khayatkashani, M.; Motaghedifard, M.H. NEW APPLIED METHOD FOR SIMULTANEOUS DETERMINATION OF ELLAGIC AND TANNIC ACID BY MULTI-WALL CARBON NANOTUBE PASTE ELECTRODE: APPLICATION IN QUANTIFICATION PUNICA GRANATUM AND QUERCUS INFECTORIA. Digest Journal of Nanomaterials & Biostructures (DJNB) 2011, 6.
- Buratti, S.; Scampicchio, M.; Giovanelli, G.; Mannino, S. A Low-Cost and Low-Tech Electrochemical Flow System for the Evaluation of Total Phenolic Content and Antioxidant Power of Tea Infusions. Talanta 2008, 75, 312–316. [CrossRef]
- Spissu, Y.; Barberis, A.; D’hallewin, G.; Orrù, G.; Scano, A.; Serra, G.R.; Pinna, M.; Pinna, C.; Marceddu, S.; Serra, P.A. An Ascorbate Bluetooth© Analyzer for Quality Control of Fresh-Cut Parsley Supply Chain. Antioxidants 2021, 10. [CrossRef]
- Spissu, Y.; Barberis, A.; Bazzu, G.; D’hallewin, G.; Rocchitta, G.; Serra, P.A.; Marceddu, S.; Vineis, C.; Garroni, S.; Culeddu, N. Functionalization of Screen-Printed Sensors with a High Reactivity Carbonaceous Material for Ascorbic Acid Detection in Fresh-Cut Fruit with Low Vitamin C Content. Chemosensors 2021, 9. [CrossRef]
- Percevault, L.; Limanton, E.; Nicolas, P.; Paquin, L.; Lagrost, C. Electrochemical Determination and Antioxidant Capacity Modulation of Polyphenols in Deep Eutectic Solvents. ACS Sustainable Chemistry & Engineering 2021, 9, 776–784. [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Analytical Methods 2009, 2, 41–60. [CrossRef]
- Lee, H.S.; Kim, E.J.; Kim, S.H. Chestnut Extract Induces Apoptosis in AGS Human Gastric Cancer Cells. nrp 2011, 5, 185–191. [CrossRef]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Kong, A.-N.T. Dietary Phytochemicals and Cancer Prevention: Nrf2 Signaling, Epigenetics, and Cell Death Mechanisms in Blocking Cancer Initiation and Progression. Pharmacology & therapeutics 2013, 137, 153–171. [CrossRef]
- Dashwood, R.H. Frontiers in Polyphenols and Cancer Prevention. The Journal of nutrition 2007, 137, 267S-269S. [CrossRef]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [CrossRef]
- Beretta, G.; Moretti, R.M.; Nasti, R.; Cincinelli, R.; Dallavalle, S.; Montagnani Marelli, M. Apoptosis-Mediated Anticancer Activity in Prostate Cancer Cells of a Chestnut Honey (Castanea Sativa L.) Quinoline–Pyrrolidine Gamma-Lactam Alkaloid. Amino Acids 2021, 53, 869–880. [CrossRef]
- Santulli, C.; Brizi, C.; Durante, M.; Micucci, M.; Budriesi, R.; Chiarini, R.; Frosini, M. Apoptotic-Induced Effects of Castanea Sativa Bark Extract in Human SH-SY5Y Neuroblastoma Cells. NPC - Natural Product Communications 2018, 13, 887–890. [CrossRef]
- Sangiovanni, E.; Piazza, S.; Vrhovsek, U.; Fumagalli, M.; Khalilpour, S.; Masuero, D.; Di Lorenzo, C.; Colombo, L.; Mattivi, F.; De Fabiani, E.; et al. A Bio-Guided Approach for the Development of a Chestnut-Based Proanthocyanidin-Enriched Nutraceutical with Potential Anti-Gastritis Properties. Pharmacological Research 2018, 134, 145–155. [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous Amperometric Detection of Ascorbic Acid and Antioxidant Capacity in Orange, Blueberry and Kiwi Juice, by a Telemetric System Coupled with a Fullerene- or Nanotubes-Modified Ascorbate Subtractive Biosensor. Biosensors and Bioelectronics 2015, 67, 214–223. [CrossRef]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G.; et al. Antioxidant, Antimicrobial, and Other Biological Properties of Pompia Juice. Molecules (Basel, Switzerland) 2020, 25, 3186. [CrossRef]
- Barberis, A.; Spissu, Y.; Bazzu, G.; Fadda, A.; Azara, E.; Sanna, D.; Schirra, M.; Serra, P.A. Development and Characterization of an Ascorbate Oxidase-Based Sensor–Biosensor System for Telemetric Detection of AA and Antioxidant Capacity in Fresh Orange Juice. Analytical Chemistry 2014, 86, 8727–8734. [CrossRef]
- Barberis, A.; Bazzu, G.; Calia, G.; Puggioni, G.M.G.; Rocchitta, G.G.; Migheli, R.; Schirra, M.; Desole, M.S.; Serra, P.A. New Ultralow-Cost Telemetric System for a Rapid Electrochemical Detection of Vitamin C in Fresh Orange Juice. Analytical Chemistry 2010, 82, 5134–5140. [CrossRef]
- Pedotti, S.; Patti, A.; Dedola, S.; Barberis, A.; Fabbri, D.; Dettori, M.A.; Serra, P.A.; Delogu, G. Synthesis of New Ferrocenyl Dehydrozingerone Derivatives and Their Effects on Viability of PC12 Cells. Polyhedron 2016, 117, 80–89. [CrossRef]
- Ham, J.-S.; Kim, H.-Y.; Lim, S.-T. Antioxidant and Deodorizing Activities of Phenolic Components in Chestnut Inner Shell Extracts. Industrial Crops and Products 2015, 73, 99–105. [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Bioactive Compounds of Chestnut (Castanea Sativa Mill.) BT - Bioactive Compounds in Underutilized Fruits and Nuts. In; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, 2020; pp. 303–313 ISBN 978-3-030-30182-8.
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant Activity and Phenolic Content of Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus Globulus) Bark Extracts. Industrial Crops and Products 2008, 28, 279–285. [CrossRef]
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-Vicente, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11. [CrossRef]
- Fadda, A.; Sanna, D. Advantages and Pitfalls of the Methods for the Antioxidant Activity Evaluation. In Advances in Food Analysis Research; A. Haynes (Ed.), Ed.; Nova Science Publishers, 2015; pp. 65–88.
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chemical Papers 2014, 68. [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of Potential Applications for Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus Globulus) Bark Extracts. Industrial Crops and Products 2009, 29, 364–370. [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and Its Implication on Cancer Chemoprevention and Chemotherapy. Biochemical Pharmacology 2015, 98, 371–380. [CrossRef]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Current Drug Targets 2012, 13, 1738–1749. [CrossRef]
- Pasciu, V.; Posadino, A.M.; Cossu, A.; Sanna, B.; Tadolini, B.; Gaspa, L.; Marchisio, A.; Dessole, S.; Capobianco, G.; Pintus, G. Akt Downregulation by Flavin Oxidase–Induced ROS Generation Mediates Dose-Dependent Endothelial Cell Damage Elicited by Natural Antioxidants. Toxicological Sciences 2010, 114, 101–112. [CrossRef]
- Giordo, R.; Cossu, A.; Pasciu, V.; Hoa, P.T.; Posadino, A.M.; Pintus, G. Different Redox Response Elicited by Naturally Occurring Antioxidants in Human Endothelial Cells. The Open Biochemistry Journal 2013, 7, 44–53. [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9. [CrossRef]
- Floris, A.; Mazarei, M.; Yang, X.; Robinson, A.E.; Zhou, J.; Barberis, A.; D’hallewin, G.; Azara, E.; Spissu, Y.; Iglesias-Ara, A.; et al. SUMOylation Protects FASN Against Proteasomal Degradation in Breast Cancer Cells Treated with Grape Leaf Extract. Biomolecules 2020, 10. [CrossRef]
- Zhang, L.; Gao, H.; Baba, M.; Okada, Y.; Okuyama, T.; Wu, L.; Zhan, L. Extracts and Compounds with Anti-Diabetic Complications and Anti-Cancer Activity from Castanea Mollissina Blume (Chinese Chestnut). BMC Complementary and Alternative Medicine 2014, 14, 422–422. [CrossRef]
- Woo, Y.; Oh, J.; Kim, J.-S. Suppression of Nrf2 Activity by Chestnut Leaf Extract Increases Chemosensitivity of Breast Cancer Stem Cells to Paclitaxel. Nutrients 2017, 9. [CrossRef]
- Muroni, A.; D’hallewin, G.; Satta, D.; De Pau, L. La Biodiversità Castanicola Della Sardegna Centrale. In Proceedings of the VIII Convegno Nazionale del Castagno; Di Vaio, C., Cirillo, C., Eds.; Italian Society for Horticultural Sciences (SOI) ISBN: 978-88-32054-04-0: Portici (Napoli), 2023; pp. 46–46.
- Molinu, M.G.; Sulas, L.; Campesi, G.; Re, G.A.; Sanna, F.; Piluzza, G. Subterranean Clover and Sulla as Valuable and Complementary Sources of Bioactive Compounds for Rainfed Mediterranean Farming Systems. Plants 2023, 12, 417. [CrossRef]
- Piluzza, G.; Campesi, G.; D’hallewin, G.; Molinu, M.G.; Re, G.A.; Sanna, F.; Sulas, L. Antioxidants in Fruit Fractions of Mediterranean Ancient Pear Cultivars. Molecules 2023, 28, 3559. [CrossRef]
- José Jara-Palacios, M.; Hernanz, D.; Luisa Escudero-Gilete, M.; Heredia, F.J. Antioxidant Potential of White Grape Pomaces: Phenolic Composition and Antioxidant Capacity Measured by Spectrophotometric and Cyclic Voltammetry Methods. Food Research International 2014, 66, 150–157. [CrossRef]
| Accessions or variety |
T °C | Total Phenols | Total Flavonoids | Condensed Tannins | DPPH | ABTS | CV |
|---|---|---|---|---|---|---|---|
| mg GAE g-1 DW | mg CE g-1 DW | mg CE g-1 DW | mmol TEAC 100 g-1 DM | AUC (µC) | |||
| MURG | 20 95 |
39.58 ± 1.69 37.35 ± 0.64 |
18.69 ± 0.42* 16.52 ± 0.14* |
9.10 ± 0.72 9.20 ± 0.20 |
24.29 ± 1.16* 31.77 ± 2.40* |
33.33 ± 1.24 34.02 ± 0.67 |
3.02 ± 0.12* 2.04 ± 0.07* |
| LOCG | 20 95 |
44.33 ± 2.89 40.10 ± 2.16 |
12.92 ± 1.70* 16.44 ± 2.3* |
6.37 ± 1.17 7.55 ± 1.38 |
23.91 ± 1.34 27.52 ± 2.84 |
31.17 ± 0.31 29.57 ± 0.92 |
2.16 ± 0.13* 2.97 ± 0.19* |
| ILDP | 20 95 |
42.96 ± 1.25 38.92 ± 2.89 |
11.37 ± 1.14* 16.26 ± 1.62* |
7.05 ± 1.25 7.99 ± 0.96 |
27.20 ± 2.89 28.17 ± 2.39 |
35.71 ± 1.50 34.51 ± 0.91 |
3.15 ± 0.15 3.17 ± 0.31 |
| COEV | 20 95 |
25.06 ± 0.46 22.70 ± 0.43 |
6.38 ± 0.21* 9.29 ± 1.66* |
4.73 ± 0.59 4.02 ± 0.51 |
15.31 ± 0.36* 20.07 ± 2.94* |
21.03 ± 0.37* 25.11 ± 2.49* |
1.52 ± 0.37 1.87 ± 0.28 |
| MARRV | 20 95 |
15.04 ± 3.38* 31.47 ± 1.58* |
4.33 ± 1.73 3.47 ± 0.68 |
1.28 ± 0.11* 3.53 ± 0.09* |
9.12 ± 1.74* 14.22 ± 0.24* |
11.60 ± 0.20* 18.59 ± 0.05* |
2.61 ± 0.20* 3.78 ± 0.25* |
| A x T | # | # | n.s. | # | # | # | |
| Phenolic compound | T °C | MURG | LOCG | ILDP | COEV | MARRV |
|---|---|---|---|---|---|---|
| Gallic acid | 20 95 |
0.402 ± 0.003* 0.300 ± 0.007* |
0.553 ± 0.001* 0.390 ± 0.027* |
0.929 ± 0.125* 0.349 ± 0.025* |
0.299 ± 0.022 0.280 ± 0.029 |
0.131 ± 0.040* 0.255 ± 0.009* |
| Ellagic acid | 20 95 |
2.864 ± 0.032* 0.573 ± 0.019* |
4.732 ± 0.190* 0.727 ± 0.090* |
4.469 ± 0.492* 0.684 ± 0.075* |
3.395 ± 0.434* 0.363 ± 0.050* |
1.659 ± 0.068* 0.869 ± 0.033* |
| Vescalagin | 20 95 |
0.525 ± 0.058* 0.768 ± 0.007* |
0.406 ± 0.013* 0.998 ± 0.069* |
0.364 ± 0.037* 0.629 ± 0.012* |
0.237 ±0.001* 0.409 ±0.025* |
0.168 ± 0.029* 0.463 ± 0.007* |
| Castalagin | 20 95 |
0.631 ± 0.041 0.714 ± 0.015 |
0.449 ± 0.021* 0.980 ± 0.167* |
0.364 ± 0.037* 0.629 ± 0.012* |
0.246 ± 0.012* 0.419 ±0.064* |
0.238 ±0.060 0.374 ± 0.050 |
| Catechin | 20 90 |
0.219 ± 0.008 0.231 ± 0.009 |
0.261 ± 0.022 0.268 ± 0.016 |
0.564 ± 0.028* 0.248 ± 0.002* |
0.161 ± 0.005 0.180 ± 0.017 |
0.199 ± 0.027* 0.670 ± 0.013* |
| Epicatechin | 20 95 |
0.595 ± 0.077 0.665 ± 0.016 |
0.271 ± 0.033* 2.232 ± 0.279* |
0.732 ± 0.067 0.900 ± 0.009 |
0.287 ± 0.044 0.285 ± 0.065 |
0.204 ±0.011* 0.521 ± 0.008* |
| Epigallocatechin | 20 95 |
0.067 ± 0.012 0.052 ± 0.015 |
0.081 ± 0.011 0.063 ± 0.001 |
0.091 ± 0.020 0.075 ± 0.003 |
0.055 ± 0.001 0.041 ± 0.000 |
0.046 ± 0.004 0.056 ± 0.006 |
| Phenolic compound | Redox potential (V) | Reference |
|---|---|---|
| Gallic acid | + 0.391 + 0.180 |
[26,43] |
| Ellagic acid | + 0.367 | [26] |
| Vescalagin | + 0.384 | [26] |
| Castalagin | + 0.384 | [26] |
| Catechin | + 0.391 + 0.300 + 0.120 |
[26,43,44] |
| Epicatechin | + 0.120 | [44] |
| Epigallocatechin | + 0.080 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





