Submitted:
23 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
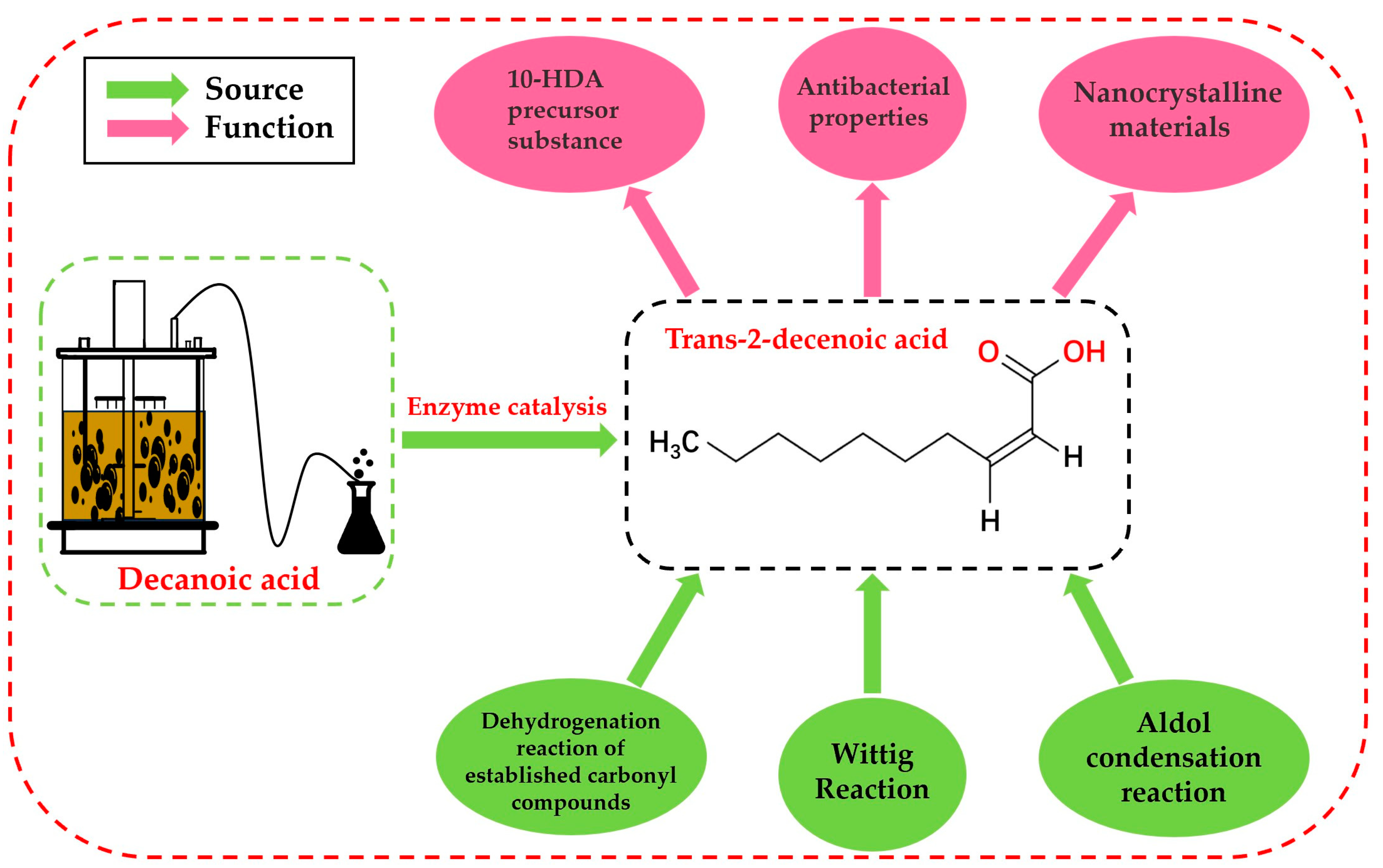
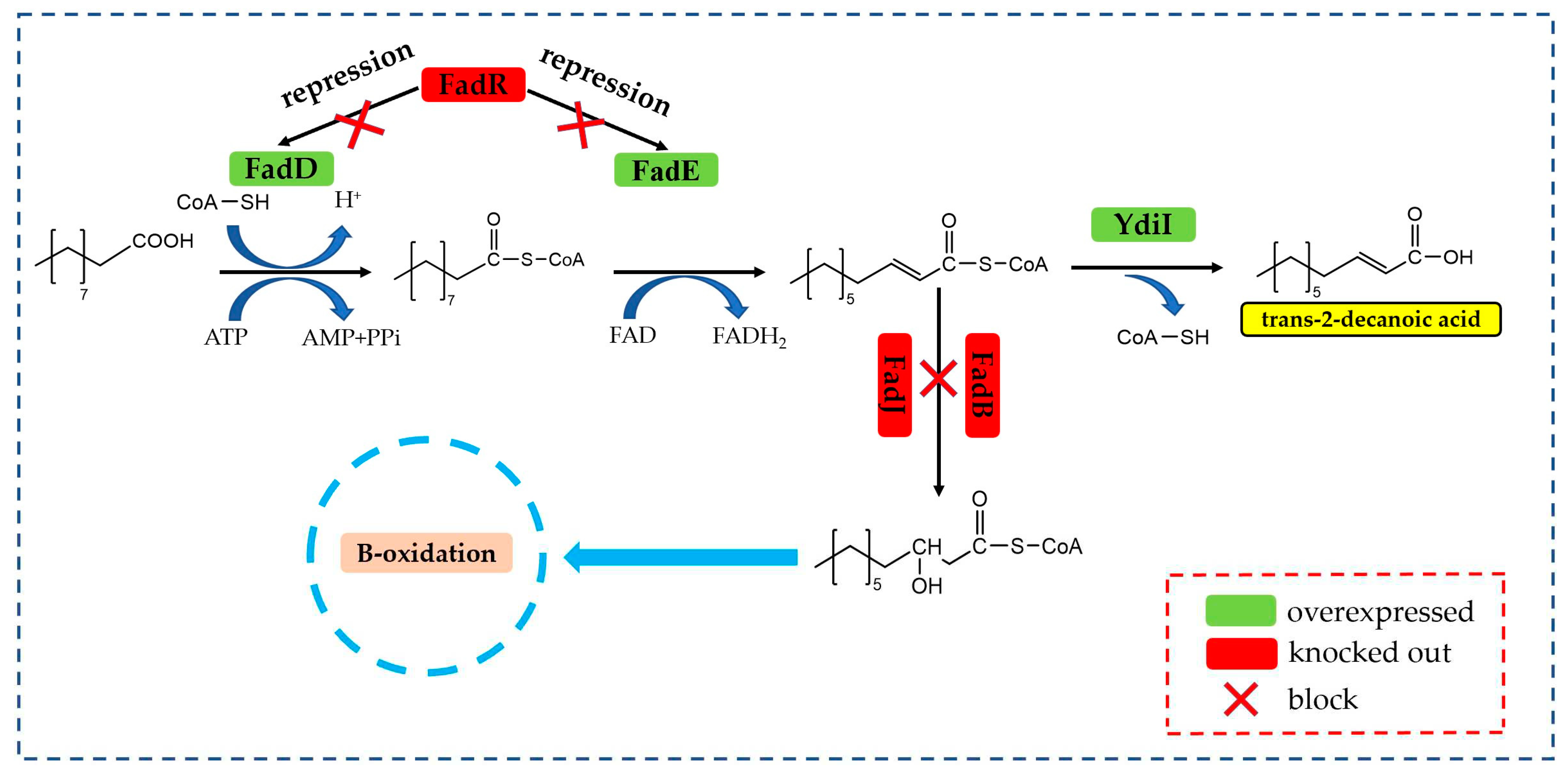
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Activation and culture of engineered Escherichia coli.
2.2.1. Strain activation
2.2.2. Strain culture and preservation.
2.3. Determination of substrates and target products based on Gas Chromatography.
2.3.1. Sample pretreatment
2.3.2. Gas Chromatography Detection
2.4. Single-factor analysis
2.5. Box–Behnken design
2.6. Comparison of expression of E. coli transporter proteins before and after optimization.
3. Results
3.1. Gas chromatography determination of substrate and product results
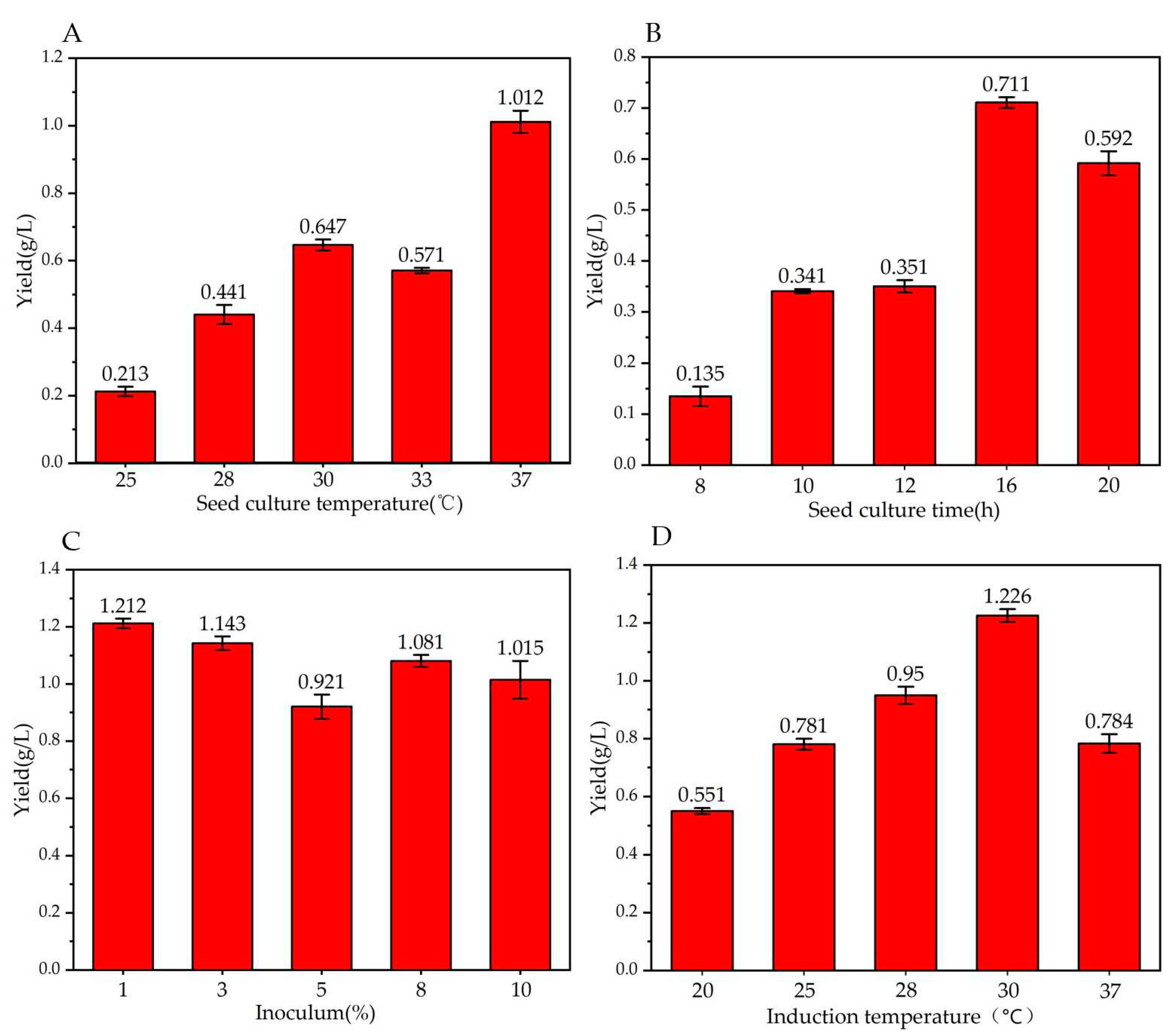
3.1.1. Effect of seed culture temperature on trans-2-decenoic acid production
3.1.2. Effect of seed culture time on trans-2-decenoic acid production
3.1.3. Effect of inoculation amount on trans-2-decenoic acid production
3.1.4. Effect of induction temperature on trans-2-decenoic acid production.
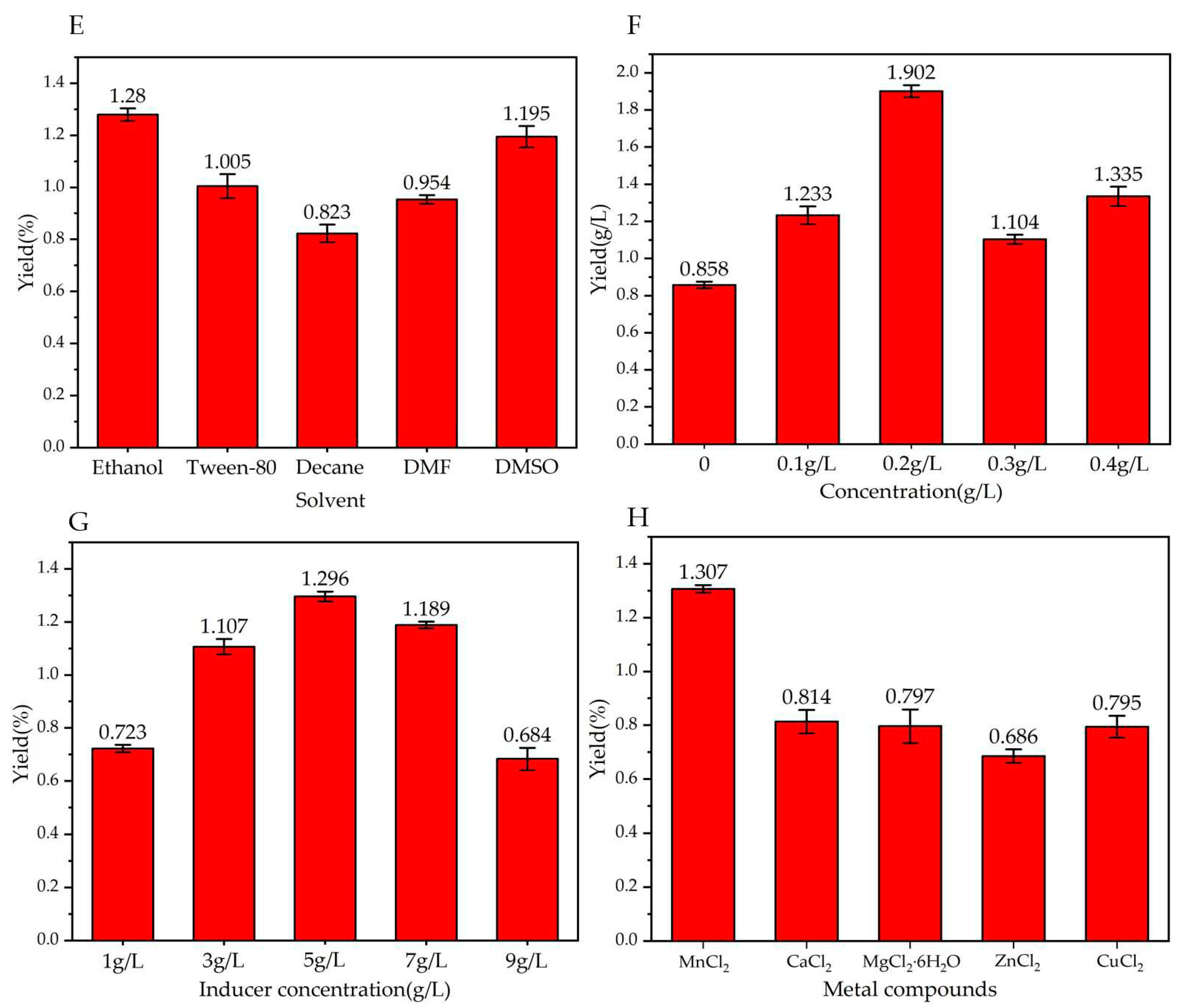
3.1.5. Effects of different solvents dissolving substrates on production of trans-2-decenoic acid
3.1.6. Effect of feeding different concentrations of substrate on production of trans-2-decenoic acid
3.1.7. Effect of inducer concentration on trans-2-decenoic acid production.
3.1.8. Effects of adding different metal ions to culture medium on production of trans-2-decenoic acid.
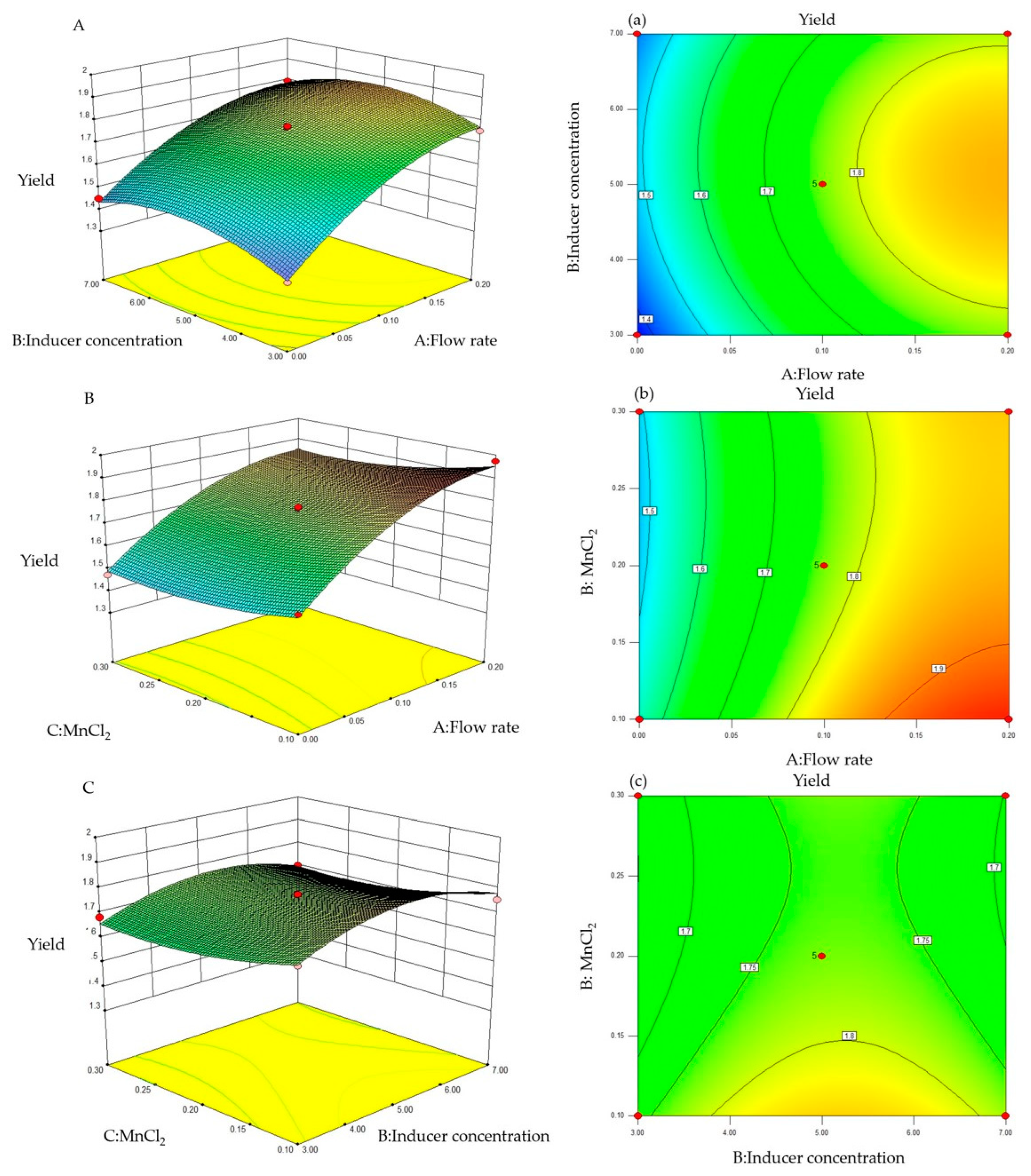
3.2. Response Surface Testing
3.3. Response Surface Interaction
3.4. Response Surface Results Optimization
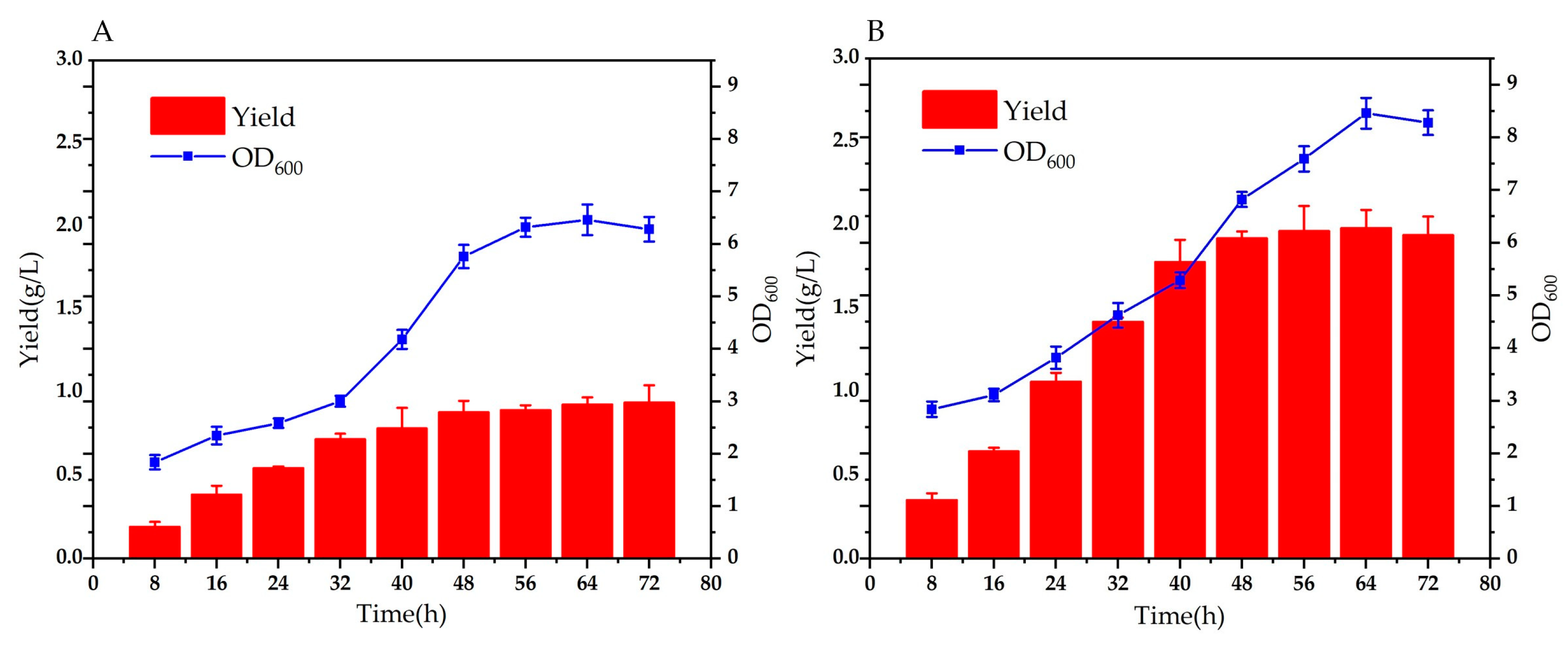
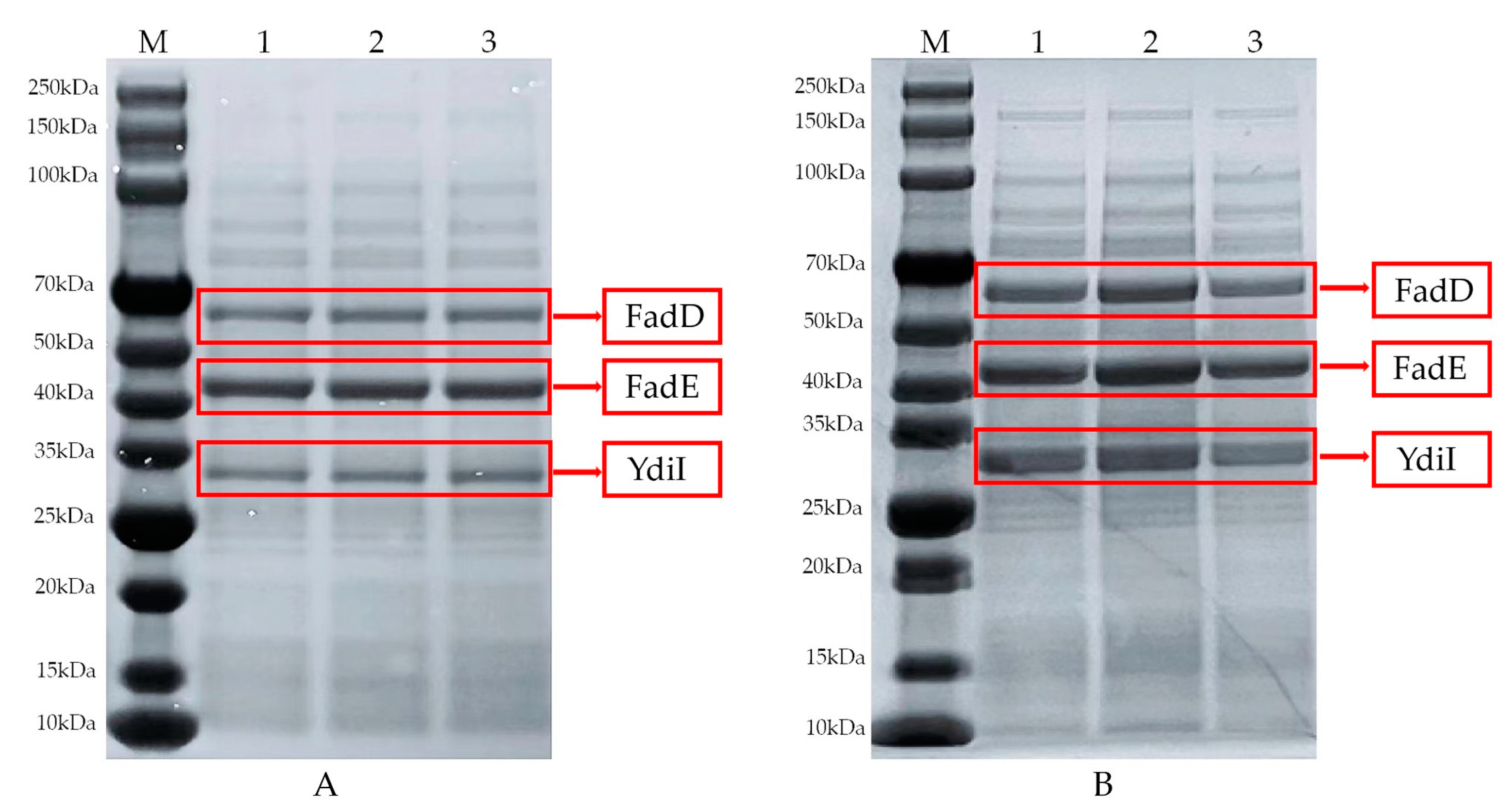
3.5. Comparison before and after optimization of SDS-PAGE protein expression of engineered E. coli.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirao, T. Synthetic strategy: palladium-catalyzed alpha,beta-dehydrogenation of carbonyl compounds. J. Org. Chem. 2019, 84(4), 1687-1692. [CrossRef]
- Perrin, C.L.; Chang, K.L. The complete mechanism of an aldol condensation. J. Org. Chem. 2016, 81(13), 5631–5635. [CrossRef]
- Farfan, P.; Gomez, S.; Restrepo, A. Dissection of the mechanism of the Wittig reaction. J. Org. Chem. 2019, 84(22), 14644–14658. [CrossRef]
- Bonnard, I.; Rolland, M.; Salmon, J. M.; Debiton, E; Barthomeuf, C.; Banaigs, B. Total structure and inhibition of tumor cell proliferation of laxaphycins. J. Med. Chem. 2017, 50(6), 1266-1279. [CrossRef]
- Wang, L.H.; He, Y.; Gao, Y.; Wu, J.E.; Dong, Y.H,; He, C.; Wang, S.X.; Weng, L.X.; Xu, J.L.; Tay, L.; Fang, R.X.; Zhang, L.H. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 2004, 51(3): 903-912. [CrossRef]
- Li, Y.; Wang, J.; Wang, F.; Wang, L.; Wang, L.; Xu, Z.; Yuan, H.; Yang, X.; Li, P.; Su, J.; & Wang, R. Production of 10-Hydroxy-2-Decenoic Acid from Decanoic Acid via Whole-Cell Catalysis in Engineered Escherichia coli. ChemSusChem. 2022, 15(9): e202102152. [CrossRef]
- Nascimento, A.P.; Moraes, L.R.; Ferreira, N.U.; Moreno, G.; Uahib, F.M.; Barizon, E.A.; Berretta, A.A. The lyophilization process maintains the chemical and biological characteristics of royal jelly. Evid Based Complement Alternat Med. 2015: 825068. [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Hazlett, B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukemia and ascitic tumours. Nature. 1959, 183(4670), 1270–1271. [CrossRef]
- Pavel, Al, C.I.; L.; Mărghitaş, O. Bobiş, & MN Mădaş. Lucrari Stiint. Zootehnie Biotecnol. 2011. Biological activities of royal jelly -review, 44 (2).
- Koh, W.K.; Bartnik, A.C.; Wise, F.W.; & Murray, C.B. Synthesis of monodisperse pbse nanorods: a case for oriented attachment. J Am Chem Soc. 2010, 132(11):3909-13. [CrossRef]
- Sarkar, A.; Middya, T.R.;Jana, A.D. A qsar study of radical scavenging antioxidant activity of a series of flavonoids using dft based quantum chemical descriptors - the importance of group frontier electron density. J Mol Model. 2012, (6):2621-31. [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A micro-ark for cells: highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv Mater. 2018, (31):e1802273. [CrossRef]
- Marques, C.N., Davies, D.G., & Sauer, K. Control of biofilms with the fatty acid signaling molecule cis-2-decenoic acid. Pharmaceuticals. 2015, 8(4), 816-835. [CrossRef]
- Cai, P.J.; Xiao, X.; He, Y.R.; Li, W.W.; Yu, L.; Yu, H.Q. Disintegration of aerobic granules induced by trans-2-decenoic acid. Bioresour. Technol. 2013, 128(Complete), 823-826. [CrossRef]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016, 7(1), 11709. [CrossRef]
- Liu, X.; Yu, H.; Jiang, X.; Ai, G.; Yu, B.; & Zhu, K. Biosynthesis of butenoic acid through fatty acid biosynthesis pathway in Escherichia coli. Appl Microbiol Biotechnol. 2015, 99(4), 1795-1804. [CrossRef]
- Kim, S.; Cheong, S.; & Gonzalez, R. Engineering Escherichia coli for the synthesis of short- and medium-chain α,β-unsaturated carboxylic acids. Metab Eng. 2016, 36, 90-98. [CrossRef]
- Cronan, J. E., Jr, & Rock, C. O. Biosynthesis of Membrane Lipids. EcoSal Plus. 2008, 3(1). [CrossRef]
- Jawed, K.; Mattam, A.J.; Fatma, Z.; Wajid, S.; Abdin, M.Z.; Yazdani, S.S. Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. Plos One. 2016, 11(7), e0160035. [CrossRef]
- Iram, S.H.; Cronan, J.E. The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J Bacteriol. 2006 Jan;188(2):599-608. [CrossRef]
- Lee, T.S.; Krupa, R.A.; Zhang, F.; Hajimorad, M.; Holtz, W. J.; Prasad, N.; Lee, S. K.; Keasling, J.D. Bglbrick vectors and datasheets: a synthetic biology platform for gene expression. J Biol Eng. 2011, 5(1), 12. [CrossRef]
- Morsczeck, C.; Berger, S.; Plum, G. The macrophage-induced gene (mig) of mycobacterium avium encodes a medium-chain acyl-coenzyme a synthetase - sciencedirect. Biochim Biophys Acta. 2001, 1521(1), 59-65. [CrossRef]
- Guzik, M.W.; Narancic, T.; Ilic-Tomic, T.; Vojnovic, S.; Kenny, S.T.; Casey, W.T.; Duane, G.F.; Casey, E.; Woods, T.; Babu, R.P.; Nikodinovic-Runic, J.; O'Connor, K.E. Identification and characterization of an acyl-CoA dehydrogenase from Pseudomonas putida KT2440 that shows preference towards medium to long chain length fatty acids. Microbiology. 2014, 160(8):1760-1771. [CrossRef]
- Campbell, J.W.; Cronan, J.E. The enigmatic Escherichia coli fade gene is yafh. J Bacteriol. 2002. 184(13):3759-64. [CrossRef]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal beta-oxidation--a metabolic pathway with multiple functions. Biochim Biophys Acta. 2006. 1763(12), 1413-26. [CrossRef]
- Yan, Q.; Simmons, T.R.; Cordell, W.T.; Hernández Lozada, N.J.; Breckner, C.J.; Chen, X., Jindra, M.A.; Pfleger, B.F. Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone, and 2-undecanone. Metab Eng. 2020. 61:335-343. [CrossRef]
- Tao, A.; Feng, X., Sheng, Y., et al. Optimization of the Artemisia polysaccharide fermentation process by Aspergillus niger. Front Nutr. 2022, 9, 842766. [CrossRef]
- Kuo, C.H.; Hsiao, F.W.; Chen, J.H.; Hsieh, C.W.; Liu, Y.C.; Shieh, C. J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason Sonochem. 2013, 20(1):546-52. [CrossRef]
- Gu, F.; Xu, F.; Tan, L.; Wu, H.; Chu, Z.; Wang, Q. Optimization of enzymatic process for vanillin extraction using response surface methodology. Molecules. 2012, 25;17(8):8753-61. [CrossRef]
- Wang, Y.H.; Xuan, Z.H.; Tian, S.; Du, G.H. Echinacoside Protects against 6-Hydroxydopamine-Induced Mitochondrial Dysfunction and Inflammatory Responses in PC12 Cells via Reducing ROS Production. Evid Based Complement Alternat Med. 2015:1-9. [CrossRef]
- Chen, W.; Lin, H.R.; Wei, C.M.; Luo, X.H.; Sun, M.L.; Yang, Z.Z.; Chen, X.Y.; Wang, H.B. Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans and protects from Aβ-induced toxicity." Biogerontology. 2018, 19(1): 47-65. [CrossRef]
- Peiran, L.; Ying, L.; Mingzhuo, Z.; Ye, Y.; Xiuming, C. The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells." Food Funct. 2017, 8. [CrossRef]
- Oh, H.J., Shin, K.C., Oh, D.K. Production of 10-hydroxy-12,15(z,z)-octadecadienoic acid from α-linolenic acid by permeabilized cells of recombinant escherichia coli expressing the oleate hydratase gene of stenotrophomonas maltophilia. Biotechnol Lett. 2013, Sep;35(9):1487-93. [CrossRef]
- Jung, D.H.; Jung, J.H.; Seo, D.H.; Ha, S.J.; Kweon, D.K.; Park, C.S. One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregates. Bioresour Technol. 2013, 130:801-4. [CrossRef]
- Rajendran V, Simab K, Aran I. Non-ionic surfactant integrated extraction of exopolysaccharides from engineered Synechocystis sp. PCC 6803 under fed-batch mode facilitates the sugar-rich syrup production for ethanol fermentation. Algal Research, 2022,66. [CrossRef]
- Mackenzie, S.; Zachary, W.; Lauren, W. Manganese Homeostasis in Bacteria: Interaction of the Small Protein MntS and Manganese Exporter MntP in E. coli. The FASEB Journal, 2022,36. [CrossRef]
- Letuta, U.G.; Binder, A. S; Tikhonova, T. A. (2020). Effect of magnesium isotopes on antibiotic sensitivity of e. coli. Microbiology, 89(3). [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: adaptation to changing environmental conditions and formation of volatile compounds. Molecules. 2021 Feb 16;26(4):1035. [CrossRef]
- Sónia Carneiro, Eugénio C. Ferreira, Rocha I. Metabolic responses to recombinant bioprocesses in Escherichia coli[J].Journal of Biotechnology, 2013, 164(3):396-408. [CrossRef]
- Wang, L.; Wang, L.; Wang, R.; Wang, Z.; Wang, J.; Yuan, H.; et al. Efficient biosynthesis of 10-hydroxy-2-decenoic acid using a nad(p)h regeneration p450 system and whole-cell catalytic biosynthesis. ACS Omega. 2022, 19;7(21):17774-17783. [CrossRef]
- Ford, T.J.; Way, J.C. Enhancement of e. coli acyl-coa synthetase fadd activity on medium chain fatty acids. PeerJ, 3, 2015, e1040. [CrossRef]
- Black, P.N.; Dirusso, C.C.; Metzger, A.K.; Heimert, T.L. Cloning, sequencing, and expression of the fadd gene of escherichia coli encoding acyl coenzyme a synthetase. J Biol Chem. 1992 Dec 15;267(35):25513-20.
- Kameda, K.; Nunn, W.D. Purification and characterization of acyl coenzyme a synthetase from escherichia coli. J Biol Chem. 1981 Jun 10;256(11):5702-7.
- Campbell, J.W.; Cronan, J. Jr. The enigmatic Escherichia coli fadE gene is yafH. J Bacteriol. 2002 Jul;184(13):3759-64. [CrossRef]
| Factor | Level | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A flow rate | 0 | 0.1 | 0.2 |
| B. Inducer concentration | 3 | 5 | 7 |
| C MnCl2 | 0.1 | 0.2 | 0.3 |
| Test number | Flow rate (g/L) | Inducer concentration (g/L) | MnCl2 (mM) | Yield (g/L) |
|---|---|---|---|---|
| 1 | 0.20 | 5.00 | 0.30 | 1.841 |
| 2 | 0.10 | 3.00 | 0.10 | 1.733 |
| 3 | 0.10 | 5.00 | 0.20 | 1.774 |
| 4 | 0.20 | 7.00 | 0.20 | 1.794 |
| 5 | 0.00 | 3.00 | 0.20 | 1.360 |
| 6 | 0.20 | 3.00 | 0.20 | 1.771 |
| 7 | 0.10 | 5.00 | 0.20 | 1.769 |
| 8 | 0.10 | 5.00 | 0.20 | 1.776 |
| 9 | 0.10 | 7.00 | 0.30 | 1.702 |
| 10 | 0.00 | 5.00 | 0.10 | 1.664 |
| 11 | 0.10 | 5.00 | 0.20 | 1.745 |
| 12 | 0.00 | 5.00 | 0.30 | 1.472 |
| 13 | 0.10 | 3.00 | 0.30 | 1.684 |
| 14 | 0.00 | 7.00 | 0.20 | 1.450 |
| 15 | 0.20 | 5.00 | 0.10 | 1.972 |
| 16 | 0.10 | 7.00 | 0.10 | 1.854 |
| 17 | 0.10 | 5.00 | 0.20 | 1.769 |
| Source | Sum of Squares | DF | Mean Square | F-value | P-value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.40 | 9 | 0.044 | 210.02 | <0.0001 | *** |
| A | 0.27 | 1 | 0.27 | 1304.41 | <0.0001 | *** |
| B | 7.938E-003 | 1 | 7.938E-003 | 37.72 | 0.0005 | *** |
| C | 0.028 | 1 | 0.028 | 133.44 | <0.0001 | *** |
| AB | 1.122E-003 | 1 | 1.122E-003 | 5.33 | 0.0543 | |
| AC | 3.080E-003 | 1 | 3.080E-003 | 14.64 | 0.0065 | |
| BC | 2.652E-003 | 1 | 2.652E-003 | 12.60 | 0.0093 | |
| A2 | 0.029 | 1 | 0.029 | 139.40 | <0.0001 | *** |
| B2 | 0.034 | 1 | 0.034 | 161.95 | <0.0001 | *** |
| C2 | 0.018 | 1 | 0.018 | 87.21 | <0.0001 | *** |
| Residual | 1.473E-003 | 7 | 2.105E-004 | |||
| Lack of Fit | 8.305E-004 | 3 | 2.768E-004 | 1.72 | 0.2998 | |
| Pure Error | 6.428E-004 | 4 | 1.607E-004 | |||
| Cor Total | 0.40 | 16 | ||||
| R2 = 0.9963 | R2adj = 0.9916 | R2pre = 0.9642 | ||||
| Adeq Precision | 54.485 |
| Flow rate (g/L) | Inducer concentration (g/L) | MnCl2 (mM) | Yield (g/L) |
|---|---|---|---|
| 0.14 | 5.63 | 0.11 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




